Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3D printed O2 indicators†
Dilidaer
Yusufu
 ,
Ri
Han
,
Ri
Han
 and
Andrew
Mills
and
Andrew
Mills
 *
*
School of Chemistry and Chemical Engineering, Queens University Belfast, David Keir Building, Stranmillis Road, Belfast, BT95AG, UK. E-mail: andrew.mills@qub.ac.uk
First published on 26th May 2020
Abstract
A polymer filament, containing an O2-sensitive lumophore pigment, platinum(II) (pentafluorophenyl) porphyrin coated onto nanoparticulate silica particles, i.e. PtTFPP/SiO2, is produced and used in a filament which is then 3D printed as O2-sensitive indicator dots (1 cm diameter, 30 μm thick) on a polyethylene terephthalate (PET) supporting film substrate. Two different filaments, prepared using the polymers, low density polyethylene (LDPE) and polylactic acid (PLA), respectively, are used to produce 3D printed PtTFPP/SiO2/LDPE and PtTFPP/SiO2/PLA O2-sensitive indicator dots, with dynamic ranges of 0–30% and 0–400% O2, respectively. The O2 response characteristics of these two, very different, indicator dots, such as sensitivity, response time and temperature sensitivity, are measured and compared with those of a commercial O2 indicator, FOSPOR (OceanInsight) and other commercial O2 indicators. The potential of this method for mass manufacture of O2 indicator dots and the likelihood that it can be extended to produce other optical indicators, such as those for ammonia or CO2, are discussed briefly.
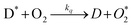
The measurement of O2 levels is important in a wide number of different fields, including chemical and clinical analysis, food packaging and environmental monitoring.1–3 Conventional O2 analysis usually involves common analytical techniques, such as gas chromatography or electrochemical analysis (e.g. using a Clark cell4 for example), that are expensive and often lab-based as they require bulky instrumentation and/or technical support. As a result, in recent years there has been a growing interest in the development of novel optical indicators for the detection of O2.5 Most optical indicators for O2 detection are based on the quenching of the luminescence of an electronically excited lumophoric dye, D*, by O2,5i.e.
 | (1) |
| I0/I = τ/τ0 = 1 + Ksv[O2] = 1 + kqτ0[O2] | (2) |
| I0/I = τ/τ0 = 1 + Ksv′%O2 | (3) |
Most commercial optical indicators for O2 are based on the luminescence quenching reaction (1), with quenching by O2 described by eqn (3), in which they often use a very lipophilic lumophore such as platinum(II) (pentafluorophenyl) porphyrin, PtTFPP, or Pt(II) tetrabenzoporphyrin, PtTBP, embedded in a hydrophobic polymer. Some examples of such commercial O2 indicators and their performance characteristics and current cost are listed in Table 1.
| Company | Product name | Operating range | Lower detection limits | Response time (s) | Lumophore | Price ($) | Ref. |
|---|---|---|---|---|---|---|---|
| Ocean insight | FOSPOR | 0–100% | 0.1% | <5 | PtTFPP | 36 | 10 and 11 |
| PreSens | Pst3 | 0–100% | 0.03% | <6 | PtTFPP | 33 | 12 and 13 |
| Mocon | OpTechTM-O2 platinum | 0–30% | 0.03% | <3 | PtTBP | 3 | 14 and 15 |
| PyroScience | OXSP5 | 0–100% | 0.02% | <7 | PtTBP | 33 | 16 |
As illustrated by the data in Table 1, a striking feature of all such commercial O2 indicators is the high cost of the indicator, which is usually in the form of a label and is either self-adhesive or needs to be stuck on. As noted by others, this high cost is because of the complexity of manufacture which is ‘slow and difficult to control and standardise’.17
As a consequence, although commonly used in many different areas, especially for research and product development, O2 optical indicator technology has not found widespread use in applications, such as in packaging, where they are likely to have significant impact but which would require their low-cost mass manufacture and subsequent large-scale utilisation. Indeed, Kelly et al., commenting on O2 optical indicators, notes that in order ‘to become viable in mass-scale packaging applications, indicator costs need to be reduced by at least ∼2 orders’.17
In recent years, 3D printing has been utilised by many industries, such as aeronautics, industrial tooling, healthcare (pills and medical devices), construction, robotics, and the automotive industry, for the large scale production of 3D printed parts and products.18 The advantages 3D printing has over most other methods of manufacture include speed of production of the final item, low initial capital investment cost, greater flexibility in product design and changing product design, less material waste and often a lower per item production cost, since it usually involves single step manufacture and near zero labour costs.19,20 It is not surprising, therefore, to find that 3D printing is used increasingly in the manufacture of indicators,21 but almost always to make a device, such as a micro-fluidic or electrochemical cell, which houses or utilises the key indicator chemical or biochemical elements, with the latter being added after the 3D printing process.21 Reports of polymer filaments incorporated with sensor materials are mostly related to conductive applications, such as conductive polymers incorporated with carbon black, carbon nanotube or graphene.22–24
To our knowledge, there have been no previous reports of a 3D printed optical gas indicator produced by printing a filament containing the indicator. Thus, in this paper we report the first examples of a 3D printable, optical O2 indicator-containing filament that is used to produce rapidly, easily and reproducibly O2 indicator dots that have performance characteristics on a par with those of current commercial O2 indicators, but that are likely to be produced at a fraction of the cost, due to the simplicity and scalability of their method of production.
In all this work the lumophore used was PtTFPP, from Inochem plc (Carnforth, UK), and the O2-sensitive pigment was made by coating the PtTFPP on hydrophilic fumed silica particles (Aerosil 130 V (Evonik Industries, Essen, Germany), particle size ∼20 nm). Briefly, 100 mg of PtTFPP were dissolved in the 100 mL acetone, after which 2 g of hydrophilic silica were added, the mixture stirred for 1 h and the acetone removed by rotary evaporation to leave a pink-coloured solid residue which was the 5 wt% PtTFPP/SiO2 pigment. This O2-sensitive lumophoric pigment was then used to make an O2 indicator-containing filament by mixing 1 g of the pigment with 19 g of LDPE pellets (Ultrapolymers Warrington, UK) and feeding this mixture into a Rondol (Staffordshire, UK) Microlab twin-screw extruder, thermostatted at 90 °C at the feed zone and with a temperature profile which gradually increased along its length to 140 °C at the exit die zone. The extruder screw speed was always run at 80 rpm. The extruded product, a 2 mm diameter filament, was withdrawn at a rate of 0.9 m min−1 and chopped up, using an inline pelletiser, into 3 mm long pellets to create the PtTFPP/SiO2/LDPE indicator masterbatch. The pellets of this masterbatch were then fed through the extruder 3 more times to ensure a homogeneous distribution of the pigment throughout each pellet. These fully-mixed, masterbatch pellets were then fed through the extruder one last time, but with an adjusted filament pulling speed so as to generate a 5 wt% (PtTFPP/SiO2) pigmented LDPE filament, i.e. PtTFPP/SiO2/LDPE filament, with a diameter of 1.75 ± 0.05 mm, as required for the 3D printer. A 3D printable 5 wt% (PtTFPP/SiO2) pigmented PLA filament, i.e. a PtTFPP/SiO2/PLA filament, was also produced using the same experimental procedure as above, but with an extruder temperature gradient of 100 to 180 °C.
The O2 indicator-loaded filament was then 3D printed as a 1.0 cm diameter, 30 μm thick, indicator dot on a 50 μm thick PET sheet supporting substrate placed on the glass platform of the printer heated to 80 °C (LDPE indicator) or 60 °C (PLA indicator). The 3D printer used was a ZMorph25 (Wroclaw, Poland) VX Full Set FDM, fitted with 0.3 mm nozzle and the LDPE indicator dots were printed at 135 °C, whereas the PLA printed dots were printed at 200 °C. Fig. 1 illustrates digital images of a typical, 3D printed PtTFPP/SiO2/LDPE indicator dot in air under daylight illumination and in Ar under UV illumination, provided by a 365 nm LED, 2 mW cm−2, thereby revealing its pink colour, λ(max) absorption 390, 506, 539 nm, and luminescent nature (λ(max) emission 642 nm), respectively. The adhesion of both the 3D printed LDPE and PLA indicator dots to the PET supporting substrate was sufficient to pass the 3 M Scotch™ Tape Adhesion test (ASTM D3359-017).26
 | ||
| Fig. 1 Photographs of a 30 μm thick 3D printed PtTFPP/SiO2/LDPE indicator dot with 1 cm diameter taken under daylight in air (left), and under UV light in an Ar atmosphere (right). | ||
Further details regarding the absorption and emission peaks exhibited by PtTFPP in solution,27 LDPE, PLA and polystyrene (PS)28 are given in section S1 in the Electronic Supplementary Information, i.e. ESI.† A comparison of this spectroscopic data for PtTFPP in solution, to that of PtTFPP embedded in the different polymers reveals very little difference in the values of λmax (absorption). The same striking similarity in λmax values is found for the emission spectrum of the dye, although in solution the main emission band at 647 nm has an additional shoulder band at 705 nm,27 which is absent when the dye in embedded in LDPE, PLA or PS.
A brief inspection of the daylight illuminated image of a typical O2 dot illustrated in Fig. 1 reveals it to have a reasonably uniform colour but with one or two flecks of dark red. These same features are also seen (although as white flecks) when the same dots are printed using non-pigmented LDPE and PLA and are due to imperfect printing. This feature is not too surprising given that the 3D printer was used to deposit films that are thinner (30 μm) than its specifications suggest is possible (50 μm).25 Smoother films will be produced if a higher specification 3D printer is used.
The 50 μm thick PET polymer supporting film substrate was chosen as this polymer is commonly used in food packaging, and so its use demonstrates that 3D printing could be used to print the indicator dot directly onto the packaging itself, if required. However, the supporting substrate is not limited just to PET, and other work showed that almost any polymer film could be used in its place, such as polypropylene, polyvinyl acetate and polystyrene, as well as ceramics, such as glass, and flexibles, such as paper and fabrics.
As indicated by eqn (3), it is possible to probe the sensitivity of any luminescence-based O2 indicator by monitoring either its luminescence intensity, I, or lifetime, as a function of %O2 and in this work we monitored I, using a PerkinElmer LS 45 Fluorescence Spectrometer, with an excitation wavelength of 390 nm.
In a typical experiment, an O2-sensitive dot on PET film was placed inside a plastic fluorescence cell, along the latter's diagonal, so that it made an angle of 45° to the excitation beam of the fluorimeter, which allowed its luminescence to be easily monitored. O2/Ar gas mixtures of known composition, i.e. known %O2 level, produced using a gas blender, were flowed into the cell and the emission spectrum of the dot recorded after 5 min gas purging with each different O2/Ar mix.
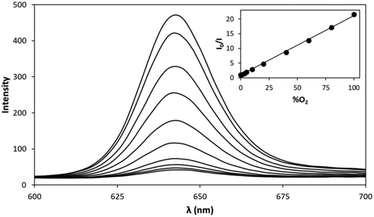
A typical set of emission spectra, recorded for the 3D printed PtTFPP/SiO2/LDPE O2 indicator dot, when exposed to a wide range of different O2/Ar gas mixtures at 18 °C, is illustrated in Fig. 2. As illustrated by the insert plot in Fig. 2, a Stern–Volmer plot of the intensity of luminescence at λ(max) emission (i.e. 642 nm) as a function of %O2 yielded a good straight line with a gradient (i.e. Ksv′ value) equal to (0.205 ± 0.002) %O2−1 and so a PO2(S = 1/2) value for the PtTFPP/SiO2/LDPE indicator dot of ca. 4.88%O2.
For comparison purposes, a similar set of experiments as those described above were conducted using a commercial OceanInsight FOSPOR O2 indicator. This work yielded a similar set of results to those illustrated in Fig. 2, and a linear Stern–Volmer plot with a gradient of (0.0586 ± 0.0007) %O2−1, i.e. a PO2(S = 1/2) value of 17.1%O2; see section S2, in the ESI.† Interestingly, the operating range of this indicator is reported by the manufacturer as 0–100%O2 (see Table 1), but, at a %O2 value of 100% it can be shown that the value of I0/I for the FOSPOR dot would be ca. 7. Following this guide as to how to estimate the maximum %O2 that defines the operating range of a typical commercial O2 indicator, i.e. when I0/I is ca. 7, it follows that the operating range of the PtTFPP/SiO2/LDPE indicator dot is 0–30%O2, i.e. ca. 3 times less than that of the FOSPOR dot.
From an initial consideration of the above results, it might not appear obvious why the PtTFPP/SiO2/LDPE indicator dot and the FOSPOR O2 indicator should be so very different in terms of O2 sensitivity, with Ksv′ values of 0.205 and 0.0586%O2−1, respectively, when both indicators utilise the same lumophore, namely, PtTFPP, see Table 1. However, a brief inspection of eqn (3) reveals that Ksv′ = 1/PO2(S = 1/2), depends upon τ0, kq and SO2, and while the value of τ0 will be largely independent of the polymer encapsulation medium, the values of kq and SO2 will not, since kq depends upon the diffusion coefficient, DO2; and so both DO2 and SO2 depend upon the encapsulation polymer. This situation is simplified somewhat in that the product of these two parameters, DO2 and SO2, is equal to the permeability of O2 in the encapsulation polymer, Pm. Thus, the difference in O2 sensitivity exhibited by the PtTFPP/SiO2/LDPE and FOSPOR O2 indicators is due to the difference in the values of Pm for the two different encapsulation polymers. An excellent review29 describing in more detail the effect of polymer permeability on the sensitivity of O2 sensors has been reported previously. Unfortunately, the identity of the encapsulation polymer used in the FOSPOR O2 indicator is not known, so all that can be said about the latter polymer is that it is likely to have a lower O2 permeability than that of LDPE (Pm = 22 × 10−18 m3 m m−1 s−1 Pa−1).30
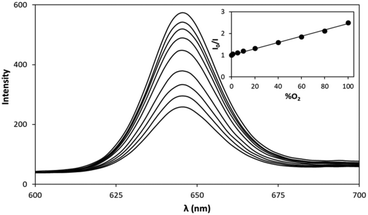
A more striking demonstration of the effect of Pm on O2-indicator sensitivity, is provided by a comparison of the O2 sensitivity of the PtTFPP/SiO2/LDPE indicator dot, illustrated in Fig. 2, with that of the PtTFPP/SiO2/PLA indicator dot, measured under identical conditions and illustrated in Fig. 3. A quick comparison of these two data sets, i.e.Fig. 2 and 3, reveals that the latter O2 indicator is much less sensitive than the former, which is not surprising given the O2 permeability of PLA is over 11.3 times less than that of LDPE, with Pm = 1.94 × 10−18 m3 m m−1 s−1 Pa−1.30 As illustrated by the insert plot in Fig. 3, a Stern–Volmer plot of the intensity of luminescence at λ(max) emission (i.e. 645 nm) as a function of %O2, for the PtTFPP/SiO2/PLA indicator dot, yielded a good straight line, which had a gradient (i.e. Ksv′ value) equal to (0.0142 ± 0.0003) %O2−1 and so a PO2(S = 1/2) value of 70.4%O2. The latter value shows that the PtTFPP/SiO2/PLA indicator dot is ca. 14.4 x's less sensitive than that of the PtTFPP/SiO2/LDPE indicator dot. Not surprisingly, this factor of 14.4 is very similar to the ratio of the O2 permeabilities of PLA and LDPE (i.e. 11.3), which in turn is consistent with the importance Pm plays in deciding the efficacy of the quenching reaction (1), viaeqn (3), and, therefore, the sensitivity of such O2 indicators. Once again, assuming that the maximum %O2 that defines the operating range of an O2 indicator is when I0/I = ca. 7, the operating range of the PtTFPP/SiO2/LDPE indicator dot is estimated to be 0–400%O2, via extrapolation of the Stern–Volmer plot in Fig. 3.
Another important characteristic of any optical O2 indicator is how rapidly it responds to a sudden change in %O2, and some manufacturer-reported response time values are given in Table 1. In contrast to film sensitivity, the response time exhibited by a film usually depends upon the rate of diffusion of the O2 into and out of the encapsulation medium, respectively, and so depends upon DO2.7,31 In this work, each of the three O2 indicators, i.e. PtTFPP/SiO2/LDPE, PtTFPP/SiO2/PLA and FOSPOR, were exposed to an alternating cycle of Ar and 100% O2 and the concomitant variation of the intensity of luminescence at λ(max) emission, I, recorded as a function of time, t; the results of this work are illustrated in S3 in the ESI.† An analysis of these results reveals values for their 90% response (i.e. Ar to O2, luminescence decreases) times, i.e. t↓90 values of 3, 41 and 3 s, respectively. The ratio of the t↓90 values for the PtTFPP/SiO2/PLA and PtTFPP/SiO2/LDPE indicators, is equal to 13.7, and so, as might be expected, similar to that of the O2 permeabilities of PLA and LDPE (i.e. 11.3), given that DO2 varies much more than SO2 for most polymers.32
As indicated by the response and recovery profiles illustrated in S3 in the ESI,† the degree of drift exhibited by all three O2 indicators is very small and similar, both in O2 and Ar. This is perhaps not too surprising given they all use the same lumophore, which is noted for its photostability.28,29 For reference, OceanInsight report the drifts of the FOSPOR indicator as 0.0002% and 0.015% in Ar and pure O2, respectively,33 and there appears no obvious reason why the 3D printed O2 dots shouldn't exhibit similar drift values.
As noted above most, if not all, O2 optical indicators exhibit a sensitivity, a measure of which is PO2(S = 1/2) or Ksv′, that depends on the O2 permeability of the encapsulation polymer, i.e. Pm. As noted earlier, Pm is the product of DO2 and SO2, both of which are temperature-sensitive parameters, with DO2 increasing, and SO2 decreasing, with increasing temperature. It follows that the sensitivity of all such O2 optical indicators varies with temperature, usually increasing with increasing temperature, which suggests that the positive activation energy value associated with DO2, is bigger than the negative activation energy value associated with SO2 for most encapsulation polymers.
Clearly, temperature sensitivity is an important characteristic of any O2 indicator and so was measured for each of the PtTFPP/SiO2/LDPE, PtTFPP/SiO2/PLA and FOSPOR O2 indicators by recording the variation of I as a function of %O2, at a series of different temperatures spanning the range 5–35 °C. The Stern–Volmer plots arising from this data generated a range of values of Ksv′ as a function of temperature, T. An Arrhenius plot of this data, i.e. ln(Ksv′) vs. T, for each of the O2 indicators, illustrated in S4 in the ESI,† yielded a good straight line, from the gradient of which a value of the activation energy, −ΔH, associated with Ksv′ was calculated. For the PtTFPP/SiO2/LDPE, PtTFPP/SiO2/PLA and FOSPOR O2 indicators the following values of −ΔH were determined 23.3, 16.5 and 13.1 kJ mol−1, respectively, a brief inspection of which reveals that the two 3D printed indicators are slightly more temperature sensitive than the commercial FOSPOR indicator. Using the above values for −ΔH it can be shown that at 18 °C, the temperature sensitivities of the PtTFPP/SiO2/LDPE, PtTFPP/SiO2/PLA and FOSPOR O2 indicators, as measured by Ksv′, when expressed as a percentage of Ksv′, i.e. %Ksv′/°C (see Table 2 for definition), are 3.2, 2.3 and 1.9% per °C, respectively. Because most optical O2 indicators, including the ones reported here, are temperature sensitive, all work with such indicators requires knowledge of the temperature of the system, and this is usually provided by using a contact thermocouple-based temperature probe or contactless IR temperature probe.1 Knowledge of the temperature allows these probes to operate typically over the temperature range 0–50 °C.1
| Indicators | 3D printed PtTFPP-LDPE dot | 3D printed PtTFPP-PLA dot | FOSPOR |
|---|---|---|---|
| a Top range value based on %O2 when I0/I = 7. b %Ksv′/°C = 100x(dKsv′/dT)/Ksv′ at 18 °C. c Calculated based on cost of chemicals. | |||
| Operating rangea | 0–30% | 0–400% | 0–100% |
| Lower detection limits | 0.05% | 0.1% | 0.1% |
| K sv (%O2−1) | 0.205 ± 0.002 | 0.0142 ± 0.0003 | 0.0586 ± 0.0007 |
| PO2(S = 1/2) (%O2) | 4.88 | 70.4 | 17.1 |
| t ↓90 (s) | 3 | 41 | 3 |
| ΔH (kJ mol−1) | 23.3 | 16.5 | 13.1 |
| %Ksv′/°Cb | 3.2% | 2.3% | 1.9% |
| Price ($) | 0.007c | 0.009c | 36 |
Although all of the above work was carried out using dry gas mixtures, other work, using the wet (i.e. 100% relative humidity) equivalent gas mixtures, showed no change in the performance characteristics of the PtTFPP/SiO2/LDPE, PtTFPP/SiO2/PLA and FOSPOR O2 indicators, a summary of which is given Table 2.
As noted earlier, the major problem with most commercial optical O2 indicators is the complex nature of their production which results in a high cost, typically $3-36 per indicator, see Table 1.3 This high cost has proved a major barrier to their routine and widespread use in food packaging for example.3 Calculations of the cost of the two different 1 cm diameter 3D printed O2-sensitive dots reported in this work were made based on the cost of chemicals used and, in both cases, was found to be <1 cent, see Table 2. Note, this cost would obviously be much less if a smaller dot was printed as is likely to be the case; for example, indicator dots with diameters of 0.5 cm or 0.25 cm would be 4 or 16 x's less expensive (in terms of chemical costs) than a 1 cm diameter dot. Obviously, the low chemical costs of the indicator dots given in Table 2 cannot be taken as a measure of their eventual retail cost. However, this feature and, more importantly, the simplicity and scalability of the method by which they are produced makes it likely that they will retail at a much lower price than their current commercial counterparts which, as others have noted, are ‘difficult to manufacture’.17
Other work carried out on the 3D printed O2 sensitive dots showed that large batches, i.e. arrays, can be easily printed on one sheet of PET and that the reproducibility of such batches, in terms of O2 sensitivity, within a batch (typically containing 9 indicator dots) and from batch to batch was >98.5%.
In conclusion, PtTFPP/SiO2/LDPE and PtTFPP/SiO2/PLA O2-sensitive indicator dots can be 3D printed from an appropriate O2-sensitive lumophore-containing filament prepared by extrusion. Since both polymer extrusion and 3D printing are common, relatively inexpensive, scalable processes, this method provides a route for the mass production of inexpensive O2 indicators. There also appears no reason why other optical indicators, such as those for ammonia,34 and CO235 cannot be produced in the same way. Thus, this work represents the first of a possible wide range of optical indicators that could be produced by 3D printing, which would in turn provide a route to their inexpensive mass manufacture and subsequent widespread utilisation in such diverse areas as: packaging, clinical analysis and environmental monitoring.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Mills, Chem. Soc. Rev., 2005, 34, 1003–1011 RSC.
- X.-D. Wang and O. S. Wolfbeis, Anal. Chem., 2013, 85, 487–508 CrossRef CAS PubMed.
- S. Banerjee, C. Kelly, J. P. Kerry and D. B. Papkovsky, Trends Food Sci. Tech., 2016, 50, 85–102 CrossRef CAS.
- L. C. Clark Jr., R. Wolf, D. Granger and Z. Taylor, J. Appl. Physiol., 1953, 6, 189–193 CrossRef CAS PubMed.
- X.-D. Wang and O. S. Wolfbeis, Chem. Soc. Rev., 2014, 43, 3666–3761 RSC.
- J. Lakowicz, Principles of Fluorescence Spectroscopy, Kluwer-Plenum, New York, USA, 2nd edn, 1999 Search PubMed.
- A. Mills and A. Lepre, Anal. Chem., 1997, 69, 4653–4659 CrossRef CAS.
- A. Mills, Platinum Met. Rev., 1997, 41, 115–127 CAS.
- A. Mills and A. Graham, Analyst, 2013, 138, 6488–6493 RSC.
- OceanInsight, https://www.oceaninsight.com/support/calibration-services/oxygen-sensor-calibration/, accessed May 2020.
- M. R. Shahriari, US Pat, 20080199360A1, 2007 Search PubMed.
- PreSens, https://www.presens.de/products/detail/oxygen-sensor-spot-sp-pst3-nau, accessed May 2020.
- I. Klimant, US Pat, 6770220B1, 1999 Search PubMed.
- Mocon, https://www.ametekmocon.com/products/headspacemapgasanalyzers/optech, accessed May 2020.
- D. W. Mayer, EU Pat, 2455746B1, 2010 Search PubMed.
- PyroScience, https://www.pyroscience.com/en/products/all-sensors/oxsp5#Compatible-meters, accessed May 2020.
- C. Kelly, D. Yusufu, I. Okkelman, S. Banerjee, J. P. Kerry, A. Mills and D. B. Papkovsky, Sens. Actuators, B, 2020, 304, 127357, DOI:10.1016/j.snb.2019.127357.
- R. Horne and K. Hausman, 3D Printing For Dummies, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2nd edn, 2017, pp. 33–49 Search PubMed.
- B. Redwood, F. Schoffer and B. Garret, The 3D Printing Handbook: Technologies, Design and Applications, 3D Hubs B.V., Amsterdam, The Netherlands, 2018, 241–279 Search PubMed.
- B. Berman, Bus. Horiz., 2012, 55, 155–162 CrossRef.
- T. Han, S. Kundu, A. Nag and Y. Xu, Sensors, 2019, 19, 1706, DOI:10.3390/s19071706.
- S. J. Leigh, R. J. Bradley, C. P. Purssell, D. R. Billson and D. A. Hutchins, PLoS One, 2012, 7, e49365, DOI:10.1371/journal.pone.0049365.
- J. F. Christ, N. Aliheidari, A. Ameli and P. Pötschke, Mater. Des., 2017, 131, 394–401 CrossRef CAS.
- S. Krachunov and A. J. Casson, Sensors, 2016, 16, 1635, DOI:10.3390/s16101635.
- ZMorph, https://www.zmorph3d.com, accessed May 2020.
- Standard Test Methods for Rating Adhesion by Tape Test, ASTM D3359 – 17 https://www.astm.org/Standards/D3359.htm, accessed May 2020.
- S.-W. Lai, Y.-J. Hou, C.-M. Che, H.-L. Pang, K.-Y. Wong, C. K. Chang and N. Zhu, Inorg. Chem., 2004, 43, 3724–3732 CrossRef CAS PubMed.
- S. K. Lee and I. Okura, Anal. Commun., 1997, 34, 185–188 RSC.
- Y. Amao, Microchim. Acta, 2003, 143, 1–12 CrossRef CAS.
- G. Colomines, V. Ducruet, C. Courgneau, A. Guinault and S. Domenek, Polym. Int., 2010, 59, 818–826 CAS.
- A. Mills and Q. Chang, Analyst, 1992, 117, 1461–1466 RSC.
- K. Yam, The Wiley Encyclopedia of Packaging Technology, John Wiley and Sons, Inc., New York, USA, 3rd edn, 2009, pp. 551–555 Search PubMed.
- Optical sensor catalogue, http://www.oemoptic.ru/docs/cat/catalogsensors.pdf, accessed May 2020.
- N. Wells, D. Yusufu and A. Mills, Talanta, 2019, 194, 830–836 CrossRef CAS PubMed.
- A. Mills, in Indicators and Environment, Health and Security, ed. M. I. Baraton, Springer Science, Dordrecht, Netherlands, 2009, pp. 347–370 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0an00809e |
| This journal is © The Royal Society of Chemistry 2020 |