Thermal desorption part 1: introduction and instrumentation
Analytical Methods Committee AMCTB No. 97
First published on 1st July 2020
Abstract
The past 30 years have seen increasing availability of methods and equipment using thermal desorption for the measurement of airborne pollutants. These methods: offer greater sensitivity than methods using solvent desorption; are more amenable to automation; and, are better suited to mass spectrometry (MS)-based detection. The greater sensitivity offered by thermal desorption means it is well suited to the analysis of samples collected through diffusive sampling with the additional benefits that this offers. This Technical Brief informs both analysts and less technically aware users of the capabilities and limitations of thermal desorption equipment and measurement methods.
Exposure to chemical compounds in the air, in particular volatile organic compounds, is almost unavoidable in the modern world, whether at home, in the workplace or in the general environment. Some of these compounds have the potential to cause adverse health effects, both long term (chronic) and short term (acute), so it is important to be able to monitor their airborne concentration. In some cases this can be achieved using portable real-time instruments but this may not always be appropriate, in particular where complex mixtures are present. In these instances it is more usual to collect the target compounds on a solid sorbent held within a sampling tube which is then desorbed and analysed in the laboratory, generally by gas chromatography (GC).
Solvent desorption (SD) of air samples collected using sorbent tubes is a long established analytical procedure. However, in the 1980’s an alternative method, thermal desorption (TD), in which the sorbent is desorbed by heating and concentrated on a focusing trap (FT), began to become commercially available. Over the last 30 years the use of this technique, which allows measurement of airborne concentrations ranging from percent to parts per trillion has grown with advances in equipment and greater availability of validated methods. In addition to vapour phase organic compounds, TD can be used for the analysis of some inorganic gases such as nitrous oxide. TD is not suitable for the analysis of methane, compounds less volatile than C44 aliphatic hydrocarbons and most permanent inorganic gases (e.g. SO2, HCl, CO, CO2).
Thermal desorption
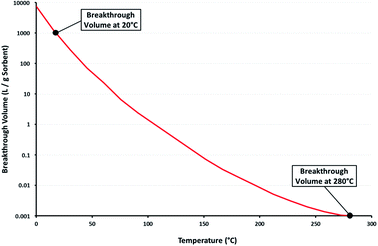
The desorption of analytes from a sample tube by TD is based on the principal that the breakthrough, or retention, volume of a given sorbent decreases significantly with increasing temperature. In the example shown in Fig. 1, the breakthrough volume is approximately 1000 L g−1 of sorbent at 20 °C but only 1 ml g−1 at 280 °C. Analytes collected in an air sample of many hundreds of litres at ambient temperature can be quantitatively desorbed with just a few ml of gas when heated to 280 °C.Thermal desorption equipment
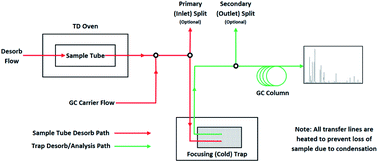
TD equipment is now available from a number of manufacturers; the schematic of a typical system is shown in Fig. 2. TD tubes are available in metal and glass and in various sizes, although the size compatible with most commercial equipment is 89 mm (3.5′′) long with an outside diameter of 6.2 mm (¼′′).During analysis, the sample tube is initially leak tested then heated in a flow of carrier gas (typically helium) to desorb the collected analytes. The desorption temperature needs to be high enough (typically 200–300 °C) to extract all the analytes, but not so high as to generate artefacts from thermal degradation of the sorbent. The desorption volume should be as small as possible but sufficient enough to extract all the analytes from the sorbent. The desorbed analytes are concentrated on a low mass focusing trap (FT); usually maintained at sub-ambient temperature and often referred to as the ‘cold’ trap. If dilution is required, an inlet split allows some of the desorbed sample to be vented to waste or, in some systems, into a clean sorbent tube for subsequent reanalysis. At the end of the desorption period the FT is rapidly heated and the collected analytes are flushed onto the GC system via an outlet split where, if required, a second dilution may be made. In most cases the FT is back-flushed, as shown in Fig. 2, i.e. the analytes exit the trap at the same end they entered. This is particularly important where the FT contains more than one sorbent in order to reduce the likelihood of low volatility analytes coming into contact with the stronger sorbent and not being fully extracted.
Benefits and drawbacks
TD offers several benefits over SD.(a) In a typical TD analysis around 2% of the desorbed analytes reach the GC detector compared with less than 0.1% for a 1 μl injection of a sample desorbed into 1 ml of solvent. Consequently, TD offers much greater sensitivity than SD.
(b) Chromatograms obtained using TD contain no solvent peak, the presence of which can mask the presence of some analytes and hinder the use of MS-based detection.
(c) Modern TD equipment sample tubes can be analysed with minimal sample handling thereby reducing labour costs and improving precision.
The primary drawback of TD is that analysis with some equipment is a “one-shot” process, i.e. should a problem occur during analysis there is no second opportunity. However, many TD systems now incorporate systems enabling partial recapture of the desorbed sample allowing repeat analysis, albeit of a slightly diluted sample.
Calibration
Calibration using TD differs from solvent-based methods in that calibration standards cannot simply be injected into the GC; they first have to be spiked on to the sorbent. This can be done using gas standards but is more usually done from solution. To avoid negating one of the main advantages of TD, namely the lack of a large solvent peak, the spiking solutions use a solvent with low affinity for the sorbent being used (typically methanol). Small volumes of these solutions (typically 1–5 μl) are spiked onto the tube in a flow of gas (typically nitrogen) removing most of the solvent but leaving known amounts of analyte on the sorbent. The same process can also be used to add internal standards to tubes prior to sampling. This is useful for MS-based analysis, which is more sensitive and selective than flame-ionisation detection, but can show greater drift.Sorbents
A wide variety of proprietary sorbents are available for use with TD. A good sorbent should: retain and desorb the compounds of interest; allow heating to temperatures in excess of that required for desorption; generate minimal artefacts on heating; have a low affinity for water; and, have a low metal content. Sorbents include porous polymers (e.g. Tenax® TA), graphitized carbon black (e.g. Carbopack™), carbon molecular sieves (e.g. Carboxen®), and inorganic adsorbents (e.g. silica gel, molecular sieves and glass wool). An essential consideration is the safe sampling volume (SSV) which is typically defined as half the breakthrough volume for the analytes of interest. Compounds that are more volatile tend to have lower SSVs and so require the use of stronger sorbents to avoid breakthrough during sampling. Less volatile compounds tend to have higher SSVs and require weaker sorbents to ensure full extraction of the analytes of interest. Tenax® TA is a widely recommended sorbent for measurement of most common vapour phase organic compounds, as indicated, for example, in ISO-16000-6:2011 Indoor air – Part 6.1Where the compounds of interest have a wide range of volatilities, multi-bed tubes containing two or more sorbents of differing strengths are sometimes used. When using multi-bed tubes it is important to orientate the sample tube so that the sample is collected through the weakest sorbent first (see Fig. 3). This is to avoid low volatility components coming into contact with the stronger sorbent and not being fully extracted during analysis. For the same reason, when desorbing a multi-bed tube the tube should be orientated with the flow passing through the strongest sorbent first, i.e. in the opposite direction to that during sampling.
The sorbent tubes used for TD are more expensive than those used with SD but can be reused after conditioning; typically more than 100 times. Conditioning involves heating the tube in a flow of gas to a temperature slightly above that used for desorption; typically 30–60 minutes with nitrogen.
Sampling
When measuring airborne concentrations using sorbent tubes the limit of detection (LoD) for any given analyte is dependent on the volume of air sampled; the greater the volume the lower the LoD.Pumped (active) sampling
Pumped (active) sampling uses a mechanical pump to draw a measured volume of air, ranging from a few ml to several hundred litres, through the sorbent tube.2 To minimize the possibility of breakthrough it is important not to exceed the SSV of the analytes of interest. The maximum flow rate that can reliably be used with a TD tube and portable sampling pump is typically around 200 ml min−1.Diffusive (passive) sampling
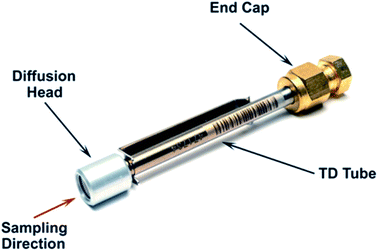
TD offers significantly better sensitivity than methods using SD, so for a given concentration a much smaller sample volume is required, lending itself to the use of diffusive (passive) sampling. A TD tube in diffusive sampling mode is shown in Fig. 4. Rather than having a pump drawing air through the tube, it has a diffusion head at the ‘sampling’ end and is sealed at the ‘non-sampling’ end.The dimensions of both the diffusion head and the sample tube are engineered precisely so that components diffuse onto the sorbent bed following Fick’s laws of diffusion.3 Different substances have different diffusive uptake rates (UR). In addition, the same substance will have a different UR on different sorbents. As diffusive sampling does not use a pump, the amount of sample taken cannot be expressed in terms of a flow, i.e. ml min−1. Instead UR is typically expressed in terms of the mass of analyte collected, in ng, per ppm of analyte in the air being sampled, per minute of sampling. Hence, the unit of a UR value is ng ppm−1 min−1. UR values for a range of analytes and sorbents are available from sources such as HSE Method MDHS 104.4
The main benefit of diffusive sampling is that no pump or flow rate measurement is required, making the devices simple, cheap and reliable. These devices are widely used for personal monitoring, where they are less obtrusive to the wearer, and in locations such as operating theatres where a larger pumped device may be unacceptable. However, diffusive sampling is not suitable for short-term sampling of less than 15 minutes. Diffusive sampling is intended for use with tubes containing a single sorbent. The use of multi-bed tubes offers no advantage as airborne compounds collected onto the sample tube by this technique are likely to be retained in the first few mm of the front sorbent bed making the other sorbent bed(s) redundant.
As well as the tube sampler shown in Fig. 4, radial and badge-type diffusive samplers are also available. These devices collect the air sample onto a TD-compatible sorbent which, after sampling, is transferred to an empty TD tube and analysed in the same manner as the tube samples. These devices have higher uptake rates than tube samples, but require more sample preparation prior to analysis and are less amenable to reuse.
Conclusions
Compared with solvent desorption, TD-based methods are more sensitive, more convenient to use and more amenable to automation and the use of MS-based analyses. A variety of sorbents are available and the greater sensitivity of TD-based methods allows the use of diffusive sampling with the additional benefits that offers. Drawbacks of TD are the requirement to purchase additional equipment and that the sample tubes are often expensive. However, the tubes are reusable so over the course of their life this additional up-front cost will be reduced or even eliminated.Ian Pengelly (Health and Safety Executive, HSE)
This Technical Brief was prepared for the Analytical Methods Committee (AMC), with contributions from members of the AMC Instrumental Analysis Expert Working Group, and approved by the AMC on 31 May 2020.
Further reading
- International Standards Organisation, ISO-16000-6:2011, Indoor air – Part 6: Determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID, 2011 Search PubMed.
- International Standards Organisation, ISO-16017-1:2000, Indoor, ambient and workplace air – Sampling and analysis of volatile organic compounds by sorbent tube/thermal desorption/capillary gas chromatography – Part 1: Pumped sampling, 2000 Search PubMed.
- International Standards Organisation, ISO-16017-2:2003, Indoor, ambient and workplace air – Sampling and analysis of volatile organic compounds by sorbent tube/thermal desorption/capillary gas chromatography – Part 2: Diffusive sampling, 2003 Search PubMed.
- Health and Safety Executive, Methods for the Determination of Hazardous Substances, MDHS 104. Volatile Organic Compounds in Air: Laboratory method using sorbent tubes, solvent desorption or thermal desorption and gas chromatography, 2016 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |