Direct enantioselective Mannich reactions of α-azido cyclic ketones: asymmetric construction of chiral azides possessing an α-quaternary stereocenter†
Xueqian
Ye
ab,
Yongkai
Pan
a and
Xiaoyu
Yang
 *a
*a
aSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China. E-mail: yangxy1@shanghaitech.edu.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 25th November 2019
Abstract
Direct enantioselective Mannich reactions of α-azido cyclic ketones with aldimines are realized through chiral phosphoric acid catalysis, which generate chiral azides possessing an α-quanternary stereocenter with complete regioselectivities and high diastereoselectivities and enantioselectivities.
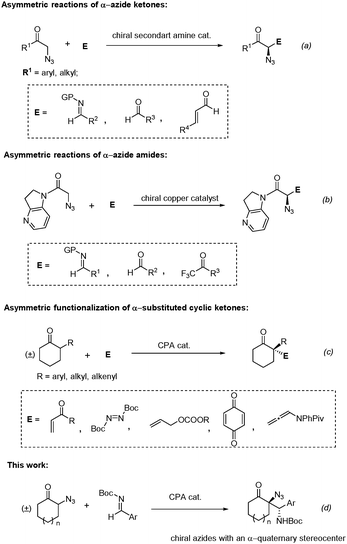
α-Chiral azides represent a special type of masked amines, which not only exist in some bioactive compounds,1 but also act as important building blocks in the synthesis of a variety of value-added chiral products, due to the fact that they can undergo a range of stereospecific transformations, such as reduction, cycloaddition, aza ylide formation and so on.1,2 Owing to their significance, numerous elegant catalytic asymmetric methods have been developed for the construction of α-chiral azides, which could be categorized into three types of strategies: nucleophilic azidation reactions, electrophilic azidation reactions and functionalizations of organic azides.3 Although the enantioselective nucleophilic4 and electrophilic azidation reactions5 have provided straightforward methods to produce α-chiral azides, the alternative strategy based on asymmetric functionalizations of organic azides also turns out to be fruitful, which has the advantage of more expanded reaction types and avoiding the use of some unreadily available azidation reagents. Among these, the asymmetric nucleophilic additions of α-azido carbonyl compounds6 to various electrophiles represent the most general ones.7 For example, Barbas and co-workers developed the asymmetric Mannich reactions of α-azido ketones catalysed by a chiral secondary amine catalyst.8 Amo and co-workers achieved asymmetric aldol reactions of α-azido ketones enabled by proline catalysis.9 The McNulty group reported the asymmetric Michael reaction of α-azido ketones, which was further adopted in the asymmetric synthesis of an anticancer natural product (Fig. 1a).10 On the other hand, Shibasaki and co-workers developed a series of asymmetric reactions of α-azido amides catalysed by a chiral copper catalyst, including aldol reactions with aldehydes11 and trifluoromethyl ketones12 and Mannich reactions (Fig. 1b).13 However, among the aforementioned examples, only chiral azides possessing an α-tertiary stereocenter were generated. To the best of our knowledge, asymmetric synthesis of chiral azides with an α-quaternary stereocenter through asymmetric functionalizations of organic azides has not been realized to date.
Recently, the chiral phosphoric acid (CPA)14 catalysed asymmetric functionalizations of α-substituted cyclic ketones have become a powerful protocol for the asymmetric construction of α-chiral quaternary stereocenters for cyclic ketones, which were developed by the List15 and Toste group,16 respectively (Fig. 1c). A range of different electrophiles were utilised in these reactions, affording products with chiral all-carbon and hetero-atom containing quaternary stereocenters regiospecifically with high enantioselectivities, which was attributed to the formation of the thermodynamically favoured more-substituted enol intermediate enabled by CPA catalysis. Herein, we report the asymmetric synthesis of chiral azides possessing an α-quaternary stereocenter through enantioselective direct Mannich reactions17 of racemic α-azido cyclic ketones with aldimines under chiral phosphoric acid catalysis (Fig. 1d).
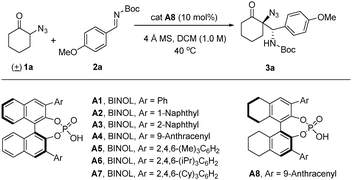
We initiated our study by choosing racemic α-azido cyclohexanone (1a) and N-Boc aldimine 2a as model substrates and BINOL/H8-BINOL-derived chiral phosphoric acids as catalysts. Upon exploring a range of conditions, we determined the reaction between 1a (1.0 equiv.) and 2a (2.0 equiv.) under the catalysis of CPA catalyst cat. A8 (10 mol%) in DCM (0.1 mL) at 40 °C for 16 h as the optimal reaction conditions, under which the desired Mannich product 3a was obtained in 93% yield, with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 diastereomeric ratio (dr) and 95% ee (Table 1, entry 1). Switching the catalysts from cat. A8 to other chiral phosphoric acid catalysts led to either an erosion of enantioselectivities (entries 2, 4, and 6–8) or a decrease of yields (entries 3 and 5). Performing this reaction in toluene and CHCl3 both led to decreased yields and diastereoselectivities (entries 9 and 10), while conducting the reaction in THF couldn’t provide the desired Mannich product (entry 11). The role of molecular sieves was also studied, which provided diminished stereoselectivities without the presence of 4 Å MS. It is worth mentioning that the reaction under the standard conditions generated a solvent-free mixture after the reaction, due to the low boiling point of DCM. Thus, the reaction under exactly solvent-free conditions was also performed; however, it afforded the product in diminished yield, demonstrating the importance of predissolving the substrates and catalysts in DCM (entry 13). Conducting the reaction at room temperature (25 °C) resulted in diminished reactivity, affording the product with poor yield (42%, entry 14). Interestingly, conducting the reaction with 1.0 equiv. aldimine 2a in toluene (0.25 M) for 2 h resulted in the isolation of the product 3a in 60% yield with the same stereoselectivity, while the α-azido cyclohexenone (1a) was recovered with 70% ee, which indicated the presence of kinetic resolution of the racemic starting material under these conditions (entry 15, for more information see ESI†).16a
10 diastereomeric ratio (dr) and 95% ee (Table 1, entry 1). Switching the catalysts from cat. A8 to other chiral phosphoric acid catalysts led to either an erosion of enantioselectivities (entries 2, 4, and 6–8) or a decrease of yields (entries 3 and 5). Performing this reaction in toluene and CHCl3 both led to decreased yields and diastereoselectivities (entries 9 and 10), while conducting the reaction in THF couldn’t provide the desired Mannich product (entry 11). The role of molecular sieves was also studied, which provided diminished stereoselectivities without the presence of 4 Å MS. It is worth mentioning that the reaction under the standard conditions generated a solvent-free mixture after the reaction, due to the low boiling point of DCM. Thus, the reaction under exactly solvent-free conditions was also performed; however, it afforded the product in diminished yield, demonstrating the importance of predissolving the substrates and catalysts in DCM (entry 13). Conducting the reaction at room temperature (25 °C) resulted in diminished reactivity, affording the product with poor yield (42%, entry 14). Interestingly, conducting the reaction with 1.0 equiv. aldimine 2a in toluene (0.25 M) for 2 h resulted in the isolation of the product 3a in 60% yield with the same stereoselectivity, while the α-azido cyclohexenone (1a) was recovered with 70% ee, which indicated the presence of kinetic resolution of the racemic starting material under these conditions (entry 15, for more information see ESI†).16a
| Entry | Variation from the standard conditions | Yieldb (%) | drc | eed (%) |
|---|---|---|---|---|
| a Reactions were performed with 1a (0.1 mmol), 2a (0.2 mmol), catalyst (10 mol%), 4 Å molecular sieves (150 mg) in DCM (0.1 mL) at 40 °C for 16 h. b Yields were isolated yields. c dr was determined by 1H NMR. d ee was determined by chiral HPLC analysis. e With 1.0 equiv. 2a. | ||||
| 1 | None | 93 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
95 |
| 2 | A1 instead A8 | 86 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
55 |
| 3 | A2 instead A8 | 49 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
92 |
| 4 | A3 instead A8 | 92 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
72 |
| 5 | A4 instead A8 | 71 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
96 |
| 6 | A5 instead A8 | 65 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
86 |
| 7 | A6 instead A8 | 50 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54 54 |
60 |
| 8 | A7 instead A8 | 29 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
81 |
| 9 | In toluene | 86 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
96 |
| 10 | In CHCl3 | 70 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
95 |
| 11 | In THF | NR | — | — |
| 12 | Without 4 Å MS | 88 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
92 |
| 13 | Without solvents | 64 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
93 |
| 14 | 25 °C instead of 40 °C | 42 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
95 |
| 15e | In toluene (0.25 M) for 2 h | 60 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
95 |
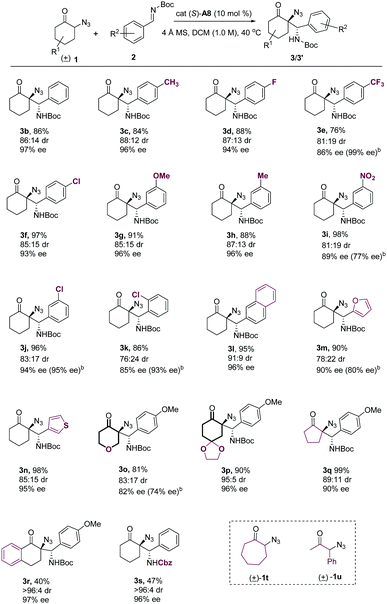
With the optimal conditions in hand, we set out to explore the substrate scope of this reaction. A series of substitutions (including both electron-donating and electro-withdrawing groups) at the para- and meta-positions of benzaldimine substrates were well tolerated under the standard conditions, producing products in high yields, with good diastereoselectivities and excellent enantioselectivities (Table 2, 3b–3j).18 An ortho-substituted benzaldimine was also examined under the optimal conditions, which provided the chiral azide product with a slightly diminished enantioselectivity (3k). 2-Naphthyl (2l) and heteroaryl substituted aldimines (2m, 2n) had good compatibility with the optimal conditions as well. With regard to the cyclic ketone scope, switching the cyclohexanone moiety to other six-membered cyclic ketones (2o, 2p) and five-membered cyclopentanone (2q) was all well amenable to the standard conditions, producing the desired Mannich products with good stereoselectivities. A benzo-fused α-azido cyclohexanone substrate was also compatible with the optimal conditions, providing the product with excellent stereoselectivities, albeit with moderate yield (3r). Switching the N-protecting group on aldimine to Cbz was also explored, which generated the product in moderate yield with high stereoselectivities (3s). However, expanding the cyclic ketone moiety to cycloheptanone or using an acyclic α-azido ketone as the substrate resulted in no desired products under the standard conditions (1t and 1u).
| a Reactions were performed with 1 (0.1 mmol), 2 (0.2 mmol), cat. (S)-A8 (10 mol%), 4 Å molecular sieves (150 mg) in DCM (0.1 mL) at 40 °C for 16 h. Yields were isolated. dr was determined by 1H NMR. ee was determined by chiral HPLC analysis. b The ee values of the minor diastereomers are provided in the parentheses. |
|---|
|
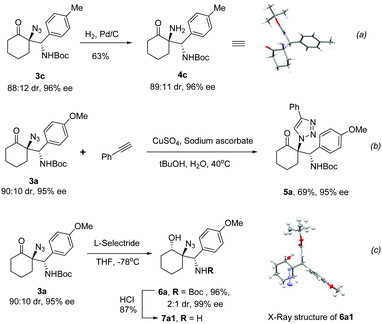
To demonstrate the synthetic applicability of these reactions, the derivatizations of the chiral products were studied. Reduction of the azido group in 3c by catalytic hydrogenation using Pd/C as the catalyst afforded the free amine product 4c in 63% yield with the completely retained enantioselectivity (Scheme 1a), whose absolute configuration was unambiguously assigned by X-ray crystallography.19 Cycloaddition of 3a with phenylacetylene in the presence of CuSO4 and sodium ascorbate20 proceeded smoothly to provide the triazole containing product 5a in 69% yield, without the erosion of the chiral information (Scheme 1b). Stereoselective reduction of the ketone moiety in 3a with L-selectride in THF at −78 °C gave alcohol 6a in 96% yield with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity, which could be easily separated by flash column chromatography. The stereochemistry of the newly generated alcohol of the major diastereomer (6a1) was determined as trans to the azido group via X-ray crystallography.19 Further deprotection of the Boc group of the major diastereomer 6a1 by treatment with HCl/EA solution facilely generated the γ-amino alcohol product 7a1 in good yield (Scheme 1c).
1 diastereoselectivity, which could be easily separated by flash column chromatography. The stereochemistry of the newly generated alcohol of the major diastereomer (6a1) was determined as trans to the azido group via X-ray crystallography.19 Further deprotection of the Boc group of the major diastereomer 6a1 by treatment with HCl/EA solution facilely generated the γ-amino alcohol product 7a1 in good yield (Scheme 1c).
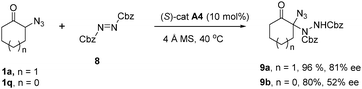
To further demonstrate the versatility of this method in the asymmetric synthesis of chiral organic azides, other electrophiles apart from aldimines were also attempted in the reactions with α-azido cyclic ketones under the catalysis of CPA catalyst. Encouragingly, we found that the reaction between α-azido cyclohexanone 1a with azodicarboxylate 8 in the presence of CPA catalyst A4 afforded the chiral amination product 9a in 96% yield with 81% ee (Scheme 2), which represents a rare example of the asymmetric synthesis of the acyclic N,N-aminal-type product.21 However, using α-azido cyclopentanone 1q as the substrate generated the product 9b in 80% yield with 52% ee under these conditions.
In summary, we have described the direct asymmetric Mannich reactions of α-azido cyclic ketones with aldimines enabled by chiral phosphoric acid catalysis, which provide access to chiral azides with an α-quaternary stereocenter regiospecifically with good diastereoselectivities and high enantioselectivities. The asymmetric catalytic system is further extended to asymmetric amination with azodicarboxylates, which broadens the applications of this method in the enantioselective synthesis of chiral organic azides with an α-quaternary stereocenter.
We gratefully acknowledge NSFC (Grant No. 21702138), “Thousand Talents Plan” Youth program and ShanghaiTech University start-up funding for the financial support. We thank Analytical Instrumentation Centre of ShanghaiTech for facilities and services of characterization of compounds.
Conflicts of interest
There are no conflicts to declare.References
- Organic Azides: Syntheses and Applications, ed. S. Bräse and K. Banert, Wiley, 2009 Search PubMed.
- (a) S. Bräse, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188–5240 CrossRef; (b) S. Chiba, Synlett, 2012, 21–44 CrossRef CAS.
- P.-G. Ding, X.-S. Hu, F. Zhou and J. Zhou, Org. Chem. Front., 2018, 5, 1542–1559 RSC.
- (a) W. A. Nugent, J. Am. Chem. Soc., 1992, 114, 2768–2769 CrossRef CAS; (b) L. E. Martinez, J. L. Leighton, D. H. Carsten and E. N. Jacobsen, J. Am. Chem. Soc., 1995, 117, 5897–5898 CrossRef CAS; (c) J. K. Myers and E. N. Jacobsen, J. Am. Chem. Soc., 1999, 121, 8959–8960 CrossRef CAS; (d) D. J. Guerin and S. J. Miller, J. Am. Chem. Soc., 2002, 124, 2134–2136 CrossRef CAS; (e) M. S. Taylor, D. N. Zalatan, A. M. Lerchner and E. N. Jacobsen, J. Am. Chem. Soc., 2005, 127, 1313–1317 CrossRef CAS; (f) Y. Fukuta, T. Mita, N. Fukuda, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 6312–6313 CrossRef CAS; (g) E. B. Rowland, G. B. Rowland, E. Rivera-Otero and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 12084–12085 CrossRef CAS PubMed; (h) B. Wu, J. C. Gallucci, J. R. Parquette and T. V. RajanBabu, Angew. Chem., Int. Ed., 2009, 48, 1126–1129 CrossRef CAS; (i) D. A. Khrakovsky, C. Tao, M. W. Johnson, R. T. Thornbury, S. L. Shevick and F. D. Toste, Angew. Chem., Int. Ed., 2016, 55, 6079–6083 CrossRef CAS; (j) D. Meyer and P. Renaud, Angew. Chem., Int. Ed., 2017, 56, 10858–10861 CrossRef CAS; (k) P. Zhou, L. Lin, L. Chen, X. Zhong, X. Liu and X. Feng, J. Am. Chem. Soc., 2017, 139, 13414–13419 CrossRef CAS; (l) Y. Liang and X. Zhao, ACS Catal., 2019, 9, 6896–6902 CrossRef CAS; (m) P. Zhou, X. Liu, W. Wu, C. Xu and X. Feng, Org. Lett., 2019, 21, 1170–1175 CrossRef CAS; (n) F. J. Seidl, C. Min, J. A. Lopez and N. Z. Burns, J. Am. Chem. Soc., 2018, 140, 15646–45650 CrossRef CAS PubMed; (o) X. Zhang, J. Ren, S. M. Tan, D. Tan, R. Lee and C.-H. Tan, Science, 2019, 363, 400 CrossRef CAS.
- (a) Q.-H. Deng, T. Bleith, H. Wadepohl and L. H. Gade, J. Am. Chem. Soc., 2013, 135, 5356–5359 CrossRef CAS; (b) C.-J. Wang, J. Sun, W. Zhou, J. Xue, B.-T. Ren, G.-Y. Zhang, Y.-L. Mei and Q.-H. Deng, Org. Lett., 2019, 21, 7315–7319 CrossRef CAS.
- (a) T. Patonay, K. Konya and E. Juhasz-Toth, Chem. Soc. Rev., 2011, 40, 2797–2847 RSC; (b) S. Faiz, A. Zahoor, N. Rasool, M. Yousaf, A. Mansha, M. Zia-Ul-Haq and H. Jaafar, Molecules, 2015, 20, 14699 CrossRef CAS.
- For other selected examples of asymmetric synthesis of chiral α-azides through asymmetric functionalizations of organic azides: (a) X. Yang and V. B. Birman, Chem. – Eur. J., 2011, 17, 11296–11304 CrossRef CAS; (b) A. A. Ott, C. S. Goshey and J. J. Topczewski, J. Am. Chem. Soc., 2017, 139, 7737–7740 CrossRef CAS PubMed; (c) J.-B. Qiao, Y.-M. Zhao and P. Gu, Org. Lett., 2016, 18, 1984–1987 CrossRef CAS; (d) N. Thirupathi, F. Wei, C.-H. Tung and Z. Xu, Nat. Commun., 2019, 10, 3158 CrossRef.
- N. S. Chowdari, M. Ahmad, K. Albertshofer, F. Tanaka and C. F. Barbas, Org. Lett., 2006, 8, 2839–2842 CrossRef CAS.
- A. Martinez-Castaneda, K. Kedziora, I. Lavandera, H. Rodriguez-Solla, C. Concellon and V. del Amo, Chem. Commun., 2014, 50, 2598–2600 RSC.
- J. McNulty and C. Zepeda-Velázquez, Angew. Chem., Int. Ed., 2014, 53, 8450–8454 CrossRef CAS.
- K. Weidner, Z. Sun, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2015, 54, 6236–6240 CrossRef CAS.
- H. Noda, F. Amemiya, K. Weidner, N. Kumagai and M. Shibasaki, Chem. Sci., 2017, 8, 3260–3269 RSC.
- Z. Sun, K. Weidner, N. Kumagai and M. Shibasaki, Chem. – Eur. J., 2015, 21, 17574–17577 CrossRef CAS.
- For pioneer works on chiral phosphoric acids catalysis: (a) T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566–1568 CrossRef CAS; (b) D. Uraguchi and M. Terada, J. Am. Chem. Soc., 2004, 126, 5356–5357 CrossRef CAS ; For recent reviews on chiral Brønsted acids catalysis: ; (c) T. Akiyama, Chem. Rev., 2007, 107, 5744–5758 CrossRef CAS; (d) M. Terada, Synthesis, 2010, 1929–1982 CrossRef CAS; (e) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS; (f) T. Akiyama and K. Mori, Chem. Rev., 2015, 115, 9277–9306 CrossRef CAS; (g) X. Li and Q. Song, Chin. Chem. Lett., 2018, 29, 1181–1192 CrossRef CAS.
- (a) I. Felker, G. Pupo, P. Kraft and B. List, Angew. Chem., Int. Ed., 2015, 54, 1960–1964 CrossRef CAS; (b) G. Pupo, R. Properzi and B. List, Angew. Chem., Int. Ed., 2016, 55, 6099–6102 CrossRef CAS; (c) G. A. Shevchenko, B. Oppelaar and B. List, Angew. Chem., Int. Ed., 2018, 57, 10756–10759 CrossRef CAS; (d) G. A. Shevchenko, G. Pupo and B. List, Synlett, 2019, 49–53 CAS.
- (a) X. Yang and F. D. Toste, J. Am. Chem. Soc., 2015, 137, 3205–3208 CrossRef CAS PubMed; (b) X. Yang and F. D. Toste, Chem. Sci., 2016, 7, 2653–2656 RSC.
- For other selected examples of direct asymmetric Mannich reactions of ketones by CPA catalysis: (a) Q.-X. Guo, H. Liu, C. Guo, S.-W. Luo, Y. Gu and L.-Z. Gong, J. Am. Chem. Soc., 2007, 129, 3790–3791 CrossRef CAS PubMed; (b) S. Tong, Q. Wang, M.-X. Wang and J. Zhu, Tetrahedron, 2018, 74, 5143–5149 CrossRef CAS; (c) Y.-Y. Chen, Y.-J. Jiang, Y.-S. Fan, D. Sha, Q. Wang, G. Zhang, L. Zheng and S. Zhang, Tetrahedron: Asymmetry, 2012, 23, 904–909 CrossRef CAS.
- The absolute structures of the products were assigned by analogy to the derivatized product 4c and 6a1.
- For details see ESI†.
- K. Wright, P. Quinodoz, B. Drouillat and F. Couty, Chem. Commun., 2017, 53, 321–323 RSC.
- (a) B. Ma, W. Luo, L. Lin, X. Liu and X. Feng, Chem. Commun., 2017, 53, 4077–4079 RSC; (b) Q. Shao, J. Chen, M. Tu, D. W. Piotrowski and Y. Huang, Chem. Commun., 2013, 49, 11098–11100 RSC; (c) M.-X. Zhao, H.-L. Bi, H. Zhou, H. Yang and M. Shi, J. Org. Chem., 2013, 78, 9377–9382 CrossRef CAS PubMed; (d) S. Yarlagadda, B. Ramesh, C. Ravikumar Reddy, L. Srinivas, B. Sridhar and B. V. Subba Reddy, Org. Lett., 2017, 19, 170–173 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1968655 and 1965952. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc08000g |
| This journal is © The Royal Society of Chemistry 2020 |