Synthesis, characterization and use of benzothioxanthene imide based dimers†
José María
Andrés Castán‡
a,
Clément
Dalinot‡
a,
Sergey
Dayneko
 b,
Laura
Abad Galan
c,
Pablo
Simón Marqués
a,
Olivier
Alévêque
a,
Magali
Allain
a,
Olivier
Maury
b,
Laura
Abad Galan
c,
Pablo
Simón Marqués
a,
Olivier
Alévêque
a,
Magali
Allain
a,
Olivier
Maury
 c,
Ludovic
Favereau
c,
Ludovic
Favereau
 d,
Philippe
Blanchard
d,
Philippe
Blanchard
 a,
Gregory C.
Welch
a,
Gregory C.
Welch
 *b and
Clément
Cabanetos
*b and
Clément
Cabanetos
 *a
*a
aCNRS UMR 6200, MOLTECH-Anjou, University of Angers, 2 Bd Lavoisier, 49045 Angers, France. E-mail: clement.cabanetos@univ-angers.fr
bDepartment of Chemistry, University of Calgary, 2500 University Drive N.W., Calgary, Alberta T2N 1N4, Canada
cUniv Lyon, ENS de Lyon, CNRS UMR 5182, Université Claude Bernard Lyon 1, F-69342 Lyon, France
dUniv Rennes, CNRS, ISCR – UMR 6226, ScanMAT – UMS 2001, F-35000 Rennes, France
First published on 10th August 2020
Abstract
The synthesis of benzothioxanthene imide based dimers is reported herein. Subtle chemical modifications were carried out and their impact on the optical and electrochemical properties was investigated for a better structure–property relationship analysis. The icing on the cake was that these new structures were used as light emitting materials for the fabrication and demonstration of the first BTXI-based OLEDs.
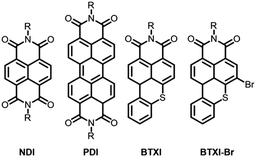
Over the years, imide containing rylenes have demonstrated their potential as strong candidates for a variety of organic electronic applications due to their excellent thermal, chemical and photochemical stabilities, as well as intriguing electronic and redox properties.1 Perylene and naphthalene diimides can be clearly identified as the beacons of this molecular family in terms of reported studies, chemical modifications and target applications (Fig. 1).2
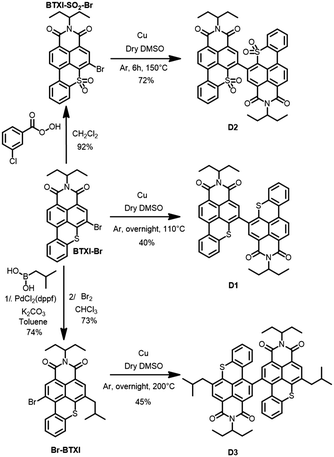
Motivated by the exploration and functionalization of original chemical structures, we have recently focused our attention on an overlooked and inexpensive vat dye, benzothioxanthene imide (BTXI). Never used for organic electronic purposes and exclusively functionalized on the nitrogen atom of the imide group, we have reported the selective and efficient mono-bromination of its rylene core, affording BTXI-Br (Fig. 1),3,4 and have opened the door to the design of new and original molecular architectures that have been successfully evaluated as active materials in organic photovoltaic (OPV) devices.5,6 Consequently, to further explore the potential of this dye, we report herein the synthesis and use of three dimers to prepare the first BTXI-based organic light-emitting diodes (OLEDs).
As depicted in Scheme 1, target molecules stemmed from the common BTXI-Br derivative. The later was indeed either sulfonated (BTXI-SO2-Br) or engaged in a Suzuki–Miyaura cross-coupling reaction in order to be subsequently and efficiently mono-brominated at the bay position (Br-BTXI).
Finally, dimers D1, D2 and D3 were prepared from the corresponding bromo derivatives, namely BTXI-Br, BTXI-SO2-Br and Br-BTXI, respectively, under copper-catalyzed Ullmann reaction conditions.7 In parallel, pallado-catalyzed cross-coupling conditions have been applied, in a further attempt to improve the synthetic yields, particularly for D1 and D3, but were not found to be very convincing (Scheme S1, ESI†).
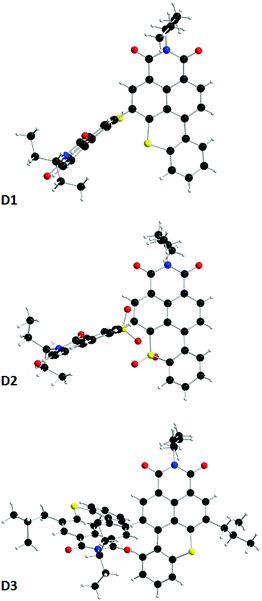
Single crystals of all the dimers were prepared by slow evaporation techniques. In addition to confirming the structures, the latter highlighted, as expected, large dihedral angles between the constituting BTXI units of ca. 75.9, 75.6 and 60.2 for D1, D2 and D3, respectively (Fig. 2). Such important twists might contribute to the good solubility of the derivatives and should profoundly modify the self-assembly properties compared to those of the BTXI monomer, and consequently tune the solid-state luminescence (vide supra). Comparison of crystal packing indicates that (i) BTXI forms a very regular head-to-tail stacking with strong cofacial π-interactions (d = 3.675 and 3.816 Å, Fig. S20, ESI†); (ii) D1 and D2 crystallized as dimers with strong and moderate intermolecular π-interactions, respectively (Fig. S21 and S22, ESI†); and D3 forms a more complex hexameric toroidal structure with circular slipped π-interactions between two vicinal molecules (Fig. S23–S25, ESI†).
In parallel, the photophysical properties have been investigated in both diluted dichloromethane solution and in the solid state. The results are listed in Table 1. In solution, it appears that oxidation into sulfones (D2) induces a significant blue shift (of ca. 50 nm) of the long-wavelength absorption band (π–π* transitions) initially centered around 450 nm (D1, D3) (Fig. S16 and S28, ESI†), in agreement with the previously reported BTXI-SO2 derivative.8 A similar effect is also observed in the emission. Importantly, compared to BTXI,8 the dimerization results in a significant decrease of the luminescence quantum yield in solution (Table 1). This effect is explained by a more favoured intersystem crossing process of a non-emissive triplet state (note that a detailed photophysical study will be published elsewhere). When moving to the solid state, the photophysics of the compounds are governed by the present intermolecular interactions. For instance, the emission of BTXI is red shifted and completely quenched due to aggregation quenching as a result of the formation of the strong π-stacking intermolecular interactions described above (Fig. S20, ESI†). This observation is also valid for dimer D1 with only a 0.06 quantum yield. D2 shows a larger quantum yield of 0.14 that can be explained by the distortion induced by the sulphur oxidation reducing the π-stacking intermolecular interactions. Finally, D3 presents a sharp emission at 580 nm strongly red-shifted (FWHM = 1442 cm−1 against 2348 cm−1 and 3145 cm−1 for D1 and D2, respectively), accompanied by the highest quantum yield of the series. This behaviour is in agreement with the slipped π-interactions observed in the crystal structure and suggests the formation of J-aggregates in the solid state that are frequently observed for the perylene diimide chromophore family (Fig. 3).9
| Dimer | λ abs (nm) | ε (M−1 cm−1) | λ em (nm) | Φ f | λ em (nm) | Φ f | E pa (V/Fc+/Fc) | E pc (V/Fc+/Fc) | E HOMO (eV) | E LUMO (eV) | ΔEelec (eV) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a In solution. b On powders. | |||||||||||
| BTXI | 455 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
502 | 0.99 | 579 | 0.02 | 0.94 | −1.82 | −5.63 | −3.10 | 2.53 |
| D1 | 455 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
509 | 0.19 | 534 | 0.06 | 1.00 | −1.74 | −5.64 | −3.16 | 2.48 |
| D2 | 384 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
450 | 0.29 | 460 | 0.14 | 1.47 | −1.18 | −6.07 | −3.73 | 2.35 |
| D3 | 473 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
530 | 0.34 | 580 | 0.18 | 0.86 | −1.70 | −5.54 | −3.21 | 2.32 |
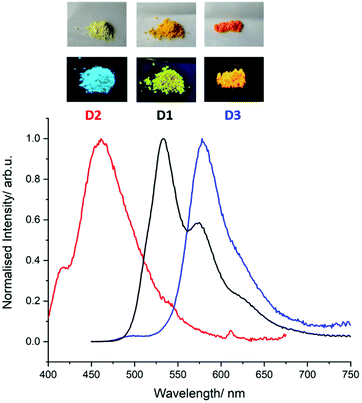 | ||
| Fig. 3 Photograph of powders of D1, D2 and D3 under white light (top) and 254 nm (bottom) and their respective emission spectra. | ||
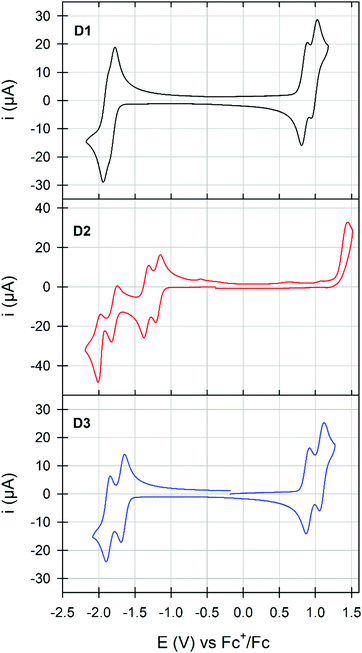
The cyclic voltammogram of D1, plotted in Fig. 4, revealed the presence of two reversible and successive processes in both the positive and negative regions attributed to the formation of stable cation/di-cation and anion/di-anion species, respectively.
While changing the grafting position (D3) induces a more visible splitting of the reversible processes with minor impact on their potentials (greater delocalization of charges), the conversion of the sulfurs into sulfones results in a drastic modification of the electrochemical signatures of D2. Indeed, on one side, the reversibility of the oxidation processes seems to be lost, while on the other side, two reversible and quasi-reversible split waves were recorded in the negative region. Thereafter, frontier molecular orbital energy levels were thus estimated from the onset of the first oxidation and reduction waves (Table 1). With almost similar LUMO levels, dimerization in the bay area (D3vs.D1) induces a slight destabilization of the HOMO level resulting in the reduction of the gap also highlighted during the UV-visible measurements. In stark contrast, oxidation of the sulfur into sulfone causes the stabilization of both frontier orbitals while increasing the band gap. Complementarily investigated by computational chemistry, simulated frontier energy levels and UV-visible spectra were also found to follow these experimental trends (Fig. S26–S28 and Table S2, ESI†).
Hence, and as a further step into the characterization of these dimers and for a deeper structure–property relationship investigation, solution-processed OLED devices, of architecture: glass/ITO/PEDOT:PSS/emitting layer (EML)/ZnO/Ag, were then fabricated.10 The luminescent and commercially available conjugated polymer PFO, also known as F8, was used in the EMLs as a host material to improve the performances of small molecule-based OLEDs.10,11
Tested under ambient conditions, it is noteworthy that all devices emitted light when connected to an external voltage, thus demonstrating, for the first time, up and running BTXI-based OLEDs. An overview of the main characteristics of the three BTXI-based OLEDs is summarized in Table 2 and their electroluminescence spectra (EL) and the Commission Internationale de l’Eclairage (CIE) color coordinates are shown in Fig. 5. In agreement with the optical properties of the three dimers, different colors of emitted lights, from blue to orange, were recorded. These results highlight, once again, the ease of tunability at different levels induced by simple chemical modifications. The redshift and changing of EL shape of BTXI-based OLEDs relative to the photoluminescence is a result of the aggregation of the dimers and various formation and relaxation of exciton mechanisms in the OLED structure (under voltage) and “clean” films (under light excitation).
| Emitting layer | Ratio | V on (V) | EQEmax (%) | LEmax (cd A−1) | PEmax (lm W−1) | L max (cd m−2) |
|---|---|---|---|---|---|---|
| PFO:D1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
4.8 | 0.04 | 0.26 | 0.30 | 608 (@9.9 V) |
| PFO:D2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.9 | 0.04 | 0.08 | 0.04 | 1336 (@6.9 V) |
| PFO:D3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
3.9 | 0.17 | 0.37 | 0.16 | 2860 (@10 V) |
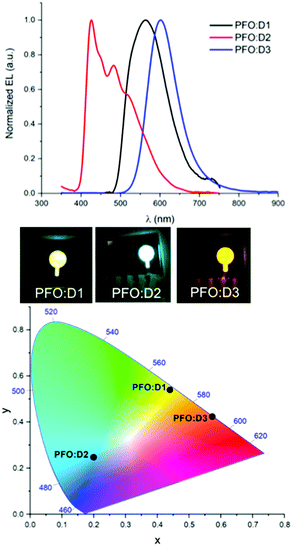 | ||
| Fig. 5 Electroluminescence spectra (top) and CIE color coordinate spectra (above) of the D1 (black), D2 (red) and D3 (blue) based devices under an external voltage. | ||
In addition, the EML layers were spin-casted from o-xylene, which indicates that these materials can be easily transferred to develop flexible OLEDs by roll-to-roll coating into industrial scale. Moreover, the performance of these BTXI-based OLEDs could be improved by using a multilayer OLED structure.12
The synthesis and characterization of three original BTXI-based dimers is reported herein. Subtle chemical modifications, namely the oxidation of the constituting sulphur into sulfone and the dimerization in the bay area, were investigated as well as their impact on the optical and electrochemical properties. Due to their good solubility and highly twisted structures, these dimers were finally and successfully used as emitting materials for the demonstration of the first BTXI-based OLEDs.
JMA: synthesis, characterization and writing of the ESI, CD: synthesis and characterization, SD: device fabrication and testing, LAG and PSM: computational chemistry, OA: electrochemical characterization, MA: crystallographic characterization, OM: LAG's PI and discussions, PB, JMA/PSM's co-PI and discussions, GW: SD's PI and discussions, CC: CD's PI and JMA/PSM's co-PI, writing of the article.
The authors thank the MATRIX SFR of the University of Angers. J. M. A. C. and P. S. M. thank the European Union's Horizon 2020 research and innovation program under Marie Sklodowska Curie Grant agreement No. 722651 (SEPOMO). The Région Pays de la Loire is also acknowledged for the grant of C. D. (Projet étoile montante SAMOA). The authors are also grateful to ANR (SADAM ANR-16-CE07-0015-01) for the financial support and PDF grant of L. A. G. S. D. thanks the MITACS program for financial support. G. C. W. thanks the University of Calgary.
Conflicts of interest
There are no conflicts to declare.Notes and references
- T. Weil, T. Vosch, J. Hofkens, K. Peneva and K. Müllen, Angew. Chem., Int. Ed., 2010, 49, 9068–9093 CrossRef CAS PubMed.
- F. Würthner, C. R. Saha-Möller, B. Fimmel, S. Ogi, P. Leowanawat and D. Schmidt, Chem. Rev., 2016, 116, 962–1052 CrossRef PubMed.
- P. Josse, S. Li, S. Dayneko, D. Joly, A. Labrunie, S. Dabos-Seignon, M. Allain, B. Siegler, R. Demadrille, G. C. Welch, C. Risko, P. Blanchard and C. Cabanetos, J. Mater. Chem. C, 2018, 6, 761–766 RSC.
- C. Dalinot, P. Simón Marqués, J. M. Andrés Castán, P. Josse, M. Allain, L. Abad Galán, C. Monnereau, O. Maury, P. Blanchard and C. Cabanetos, Eur. J. Org. Chem., 2020, 2140–2145 CrossRef CAS.
- S. V. Dayneko, A. D. Hendsbee, J. R. Cann, C. Cabanetos and G. C. Welch, New J. Chem., 2019, 43, 10442–10448 RSC.
- A.-J. Payne, N. A. Rice, S. M. McAfee, S. Li, P. Josse, C. Cabanetos, C. Risko, B. H. Lessard and G. C. Welch, ACS Appl. Energy Mater., 2018, 1, 4906–4916 CrossRef CAS.
- A. D. Hendsbee, J.-P. Sun, W. K. Law, H. Yan, I. G. Hill, D. M. Spasyuk and G. C. Welch, Chem. Mater., 2016, 28, 7098–7109 CrossRef CAS.
- L. A. Galán, J. M. Andrés Castán, C. Dalinot, P. S. Marqués, P. Blanchard, O. Maury, C. Cabanetos, T. Le Bahers and C. Monnereau, Phys. Chem. Chem. Phys., 2020, 22, 12373–12381 RSC.
- S. Herbst, B. Soberats, P. Leowanawat, M. Stolte, M. Lehmann and F. Würthner, Nat. Commun., 2018, 9, 2646 CrossRef PubMed.
- S. V. Dayneko, M. Rahmati, M. Pahlevani and G. C. Welch, J. Mater. Chem. C, 2020, 8, 2314–2319 RSC.
- J. H. Ahn, C. Wang, I. F. Perepichka, M. R. Bryce and M. C. Petty, J. Mater. Chem., 2007, 17, 2996–3001 RSC.
- M. Rahmati, S. V. Dayneko, M. Pahlevani and G. C. Welch, ACS Appl. Electron. Mater., 2020, 2, 48–55 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2012963 and 2012965–2012967. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0cc04556j |
| ‡ Both authors equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2020 |