Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Optimisation of pyruvate hyperpolarisation using SABRE by tuning the active magnetisation transfer catalyst†
Ben. J.
Tickner
 a,
Olga
Semenova‡
a,
Olga
Semenova‡
 a,
Wissam
Iali§
a,
Wissam
Iali§
 a,
Peter J.
Rayner
a,
Peter J.
Rayner
 a,
Adrian C.
Whitwood
a,
Adrian C.
Whitwood
 b and
Simon B.
Duckett
b and
Simon B.
Duckett
 *a
*a
aCentre for Hyperpolarization in Magnetic Resonance (CHyM), University of York, Heslington, YO10 5NY, UK. E-mail: simon.ducket@york.ac.uk
bDepartment of Chemistry, University of York, Heslington, YO10 5DD, UK
First published on 28th January 2020
Abstract
Hyperpolarisation techniques such as signal amplification by reversible exchange (SABRE) can deliver NMR signals several orders of magnitude larger than those derived under Boltzmann conditions. SABRE is able to catalytically transfer latent magnetisation from para-hydrogen to a substrate in reversible exchange via temporary associations with an iridium complex. SABRE has recently been applied to the hyperpolarisation of pyruvate, a substrate often used in many in vivo MRI studies. In this work, we seek to optimise the pyruvate-13C2 signal gains delivered through SABRE by fine tuning the properties of the active polarisation transfer catalyst. We present a detailed study of the effects of varying the carbene and sulfoxide ligands on the formation and behaviour of the active [Ir(H)2(η2-pyruvate)(sulfoxide)(NHC)] catalyst to produce a rationale for achieving high pyruvate signal gains in a cheap and refreshable manner. This optimisation approach allows us to achieve signal enhancements of 2140 and 2125-fold for the 1-13C and 2-13C sites respectively of sodium pyruvate-1,2-[13C2].
Introduction
Nuclear magnetic resonance (NMR) and magnetic resonance imaging (MRI) are some of the most widely used tools for the characterisation of molecules and the clinical diagnosis of disease. While these techniques are used widely in their fields, they remain insensitive as their signal strengths are derived from small Boltzmann population differences across the nuclear spin energy levels they probe. Recently, a growing number of researchers are turning their attention to hyperpolarisation to help address this problem.1–3 For example, dynamic nuclear polarisation (DNP) can achieve polarisation levels of 92% and 70% for 1H and 13C signals in times as short as 150 seconds and 20 minutes respectively.4,5 DNP transfers the inherent polarisation of an electron into target nuclei when both are located in a frozen glass matrix and subject to microwave irradiation at or near the resonance frequency of the electron at very low temperatures (1–2 K).4–6 Rapid heating of such solids then generates materials that yield MR signal enhancements in solution of up to 5 orders of magnitude.5,6 This approach has been applied to the production of hyperpolarised biomolecules such as pyruvate,7–14 succinate,15,16 and fumarate17,18 which are then injected and detected in vivo alongside their metabolic by-products. Imaging the formation of such metabolites provides a route to studying biochemical tissue function in real time with obvious benefits for disease diagnosis.7–17Para-Hydrogen induced polarisation (PHIP) methods are potentially a faster and cheaper alternative to DNP.19–21 The feedstock of PHIP is para-hydrogen (p-H2), which is the isomer of dihydrogen that exists as a nuclear spin singlet. In the first generation of PHIP studies, p-H2 was typically incorporated into a substrate via a hydrogenation reaction.22,23 The resulting product detection by NMR has since provided many significant observations in the field of catalysis wherein reaction intermediates are detected.24–26 The catalytic production of hyperpolarised probes suitable for in vivo study using this version of PHIP was therefore limited to biomolecules that have facile access to their dehydro-precursor.15,27,28 This limitation has been elegantly alleviated using a variant of PHIP, termed para-hydrogen induced polarisation by side arm hydrogenation (PHIP-SAH), which can produce aqueous solutions of hyperpolarised pyruvate and acetate.29 In the precursor, pyruvate is functionalised as an ester with an unsaturated side arm which, after hydrogenation by p-H2 and a magnetic field cycling step to transfer polarisation into the modified pyruvate, can be rapidly released through simultaneous hydrolysis and phase separation.29,30 The resulting pyruvate can then be detected by MRI through a much stronger, hyperpolarised, response. Hyperpolarised pyruvate prepared in this way is the result of a one-shot, irreversible batch synthesis.
In contrast, signal amplification by reversible exchange (SABRE) is an alternative non-hydrogenative PHIP based method that involves the transfer of spin polarisation from p-H2 to a substrate when both are concurrently bound to an iridium catalyst, as depicted in Fig. 1.31 As the ligands are in reversible exchange, a pool of hyperpolarized substrate is readily created in solution. Hence, the magnetisation transfer step is catalytic in nature, occurring via the temporary J-coupled network within the organometallic complex. Consequently, the process is completed without chemical change and is continuous and refreshable in nature.32 The identity of the ligands used in such SABRE magnetisation transfer catalysts are important in delivering high MR signal gains and controlling the type of substrates that can be hyperpolarised.33–35 SABRE has had the greatest success to date in polarising structures with N-heterocyclic motifs which have a simple and readily understandable binding mode.34,36–38 In these cases, polarisation transfer catalysts of the form [Ir(H)2(NHC)(Sub)3]Cl provide suitable substrate (Sub) and H2 exchange rates for significant polarisation build up in solution.
Until recently α-keto acids, such as pyruvate, were incompatible with SABRE because they were unable to form stable complexes due to their weak ligation to iridium.39 A related technique, SABRE-Relay, has allowed the hyperpolarisation of a wide range of non-ligating substrates that contain functional groups which can receive hyperpolarised protons through exchange from a suitable carrier.40,41 When applied to sodium pyruvate-1-[13C] this approach readily delivers 50-fold 13C enhancements.40 However, rapid in situ condensation of pyruvate with the amine carrier forms products of the type [Ir(H)2(η2-α-carboxyimine)(amine)(NHC)] which deactivate the system to further pyruvate polarisation.42
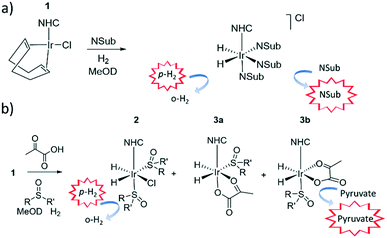
It has since been reported that by using appropriate stabilising ligands, SABRE can hyperpolarise pyruvate in a low cost, fast, and reversible fashion that does not involve the technologically demanding equipment of DNP, or the multiple steps of PHIP-SAH.39 This is possible due to the formation of the polarisation transfer catalyst [Ir(H)2(η2-pyruvate)(DMSO)(IMes)] (where IMes = 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene) when solutions of [IrCl(COD)(IMes)] (1a) (where COD = cis,cis-1,5-cyclooctadiene), DMSO and sodium pyruvate in methanol-d4 or 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
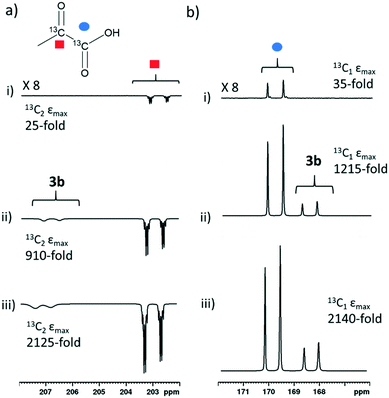
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 mixtures of D2O and ethanol-d6 are activated with 3 bar of H2. These sulfoxide based complexes exhibit significantly more elaborate catalysis than more commonly observed in SABRE with N-donor substrates as the Sub and H2 exchange pathways are no longer localised within a single inorganic species.43 This is because while [Ir(H)2(η2-pyruvate)(DMSO)(IMes)] reflects the active polarisation transfer catalyst for 13C pyruvate enhancement, it is [IrCl(H)2(DMSO)2(IMes)] (2) that mediates the necessary H2 exchange processes.43 This situation is complicated yet further by the fact [Ir(H)2(η2-pyruvate)(DMSO)(IMes)] (3) exists as two regioisomers that are differentiated by the geometry of η2-pyruvate coordination, as depicted in Fig. 1b. We have previously shown that the regioisomer where pyruvate binds in the same plane as the hydride ligands (3b) contains a spin topology that allows active polarisation transfer of singlet order from p-H2 derived hydride ligands to coordinated 13C pyruvate sites in the catalyst.39,43
30 mixtures of D2O and ethanol-d6 are activated with 3 bar of H2. These sulfoxide based complexes exhibit significantly more elaborate catalysis than more commonly observed in SABRE with N-donor substrates as the Sub and H2 exchange pathways are no longer localised within a single inorganic species.43 This is because while [Ir(H)2(η2-pyruvate)(DMSO)(IMes)] reflects the active polarisation transfer catalyst for 13C pyruvate enhancement, it is [IrCl(H)2(DMSO)2(IMes)] (2) that mediates the necessary H2 exchange processes.43 This situation is complicated yet further by the fact [Ir(H)2(η2-pyruvate)(DMSO)(IMes)] (3) exists as two regioisomers that are differentiated by the geometry of η2-pyruvate coordination, as depicted in Fig. 1b. We have previously shown that the regioisomer where pyruvate binds in the same plane as the hydride ligands (3b) contains a spin topology that allows active polarisation transfer of singlet order from p-H2 derived hydride ligands to coordinated 13C pyruvate sites in the catalyst.39,43
SABRE is dependent on the magnetic field experienced by the sample during polarisation transfer because a suitable matching condition for optimal polarisation transfer between p-H2 derived hydride ligands and the ligated target substrate must be achieved. For 13C-SABRE by complexes of this type, optimal transfer typically occurs at mG fields if direct transfer from the p-H2 derived hydride ligands into bound 13C sites is involved.39,43,44 For sodium pyruvate-1-[13C] and sodium pyruvate-2-[13C] this necessitates fields of ±9 and ±3 mG respectively.39 Interestingly, when sodium pyruvate-1,2-[13C2] is used, the resulting process leads to the spontaneous creation of long lived 13C2 singlet order in the product being detected whose decoherence lifetime exceeds that of T1.39 In such states, the underlying magnetisation involves two coupled spins and, in this case, its formation is independent of magnetic field. In the context of this paper, it is important to appreciate that p-H2 reflects another example of such a singlet state which is now not only very long lived, but NMR invisible. This singlet order becomes visible to NMR by a symmetry breaking reaction, such as the oxidative addition of p-H2 to the iridium centre.45 In contrast to p-H2, the two coupled 13C spins of sodium pyruvate-1,2-[13C2] are already magnetically distinct and consequently its singlet state is immediately accessible by NMR and evolves more quickly that than of p-H2.
In this paper we report on a series of rigorous catalytic studies that investigate the role that the [Ir(H)2(IMes)(η2-pyruvate)(sulfoxide)] and [IrCl(H)2(DMSO)2(IMes)] type species play in the 13C hyperpolarisation of pyruvate. Throughout this work we use sodium pyruvate-1,2-[13C2] as the target substrate because the longer lifetime of its hyperpolarised singlet state may have future benefits in reaction monitoring or medical imaging. It must be remembered that as we create the hyperpolarised molecule remote to the final point of observation there is a time delay between preparation and detection. Thus increased magnetic state lifetimes extend the timescale over which signal detection is possible and offer significant potential benefits for tracer analysis. We show here that optimisation of the hyperpolarisation level delivered by the catalyst is complex, with factors such as catalyst identity, concentration and temperature exhibiting non-trivial behaviour due to the complex interplay that exists between the roles of the different species present in solution. Consequently, we explore the properties of the active catalyst by varying the identity of both sulfoxide and NHC ligand to produce a rationale for achieving high 13C pyruvate NMR signal enhancements using SABRE.
Results and discussion
Formation of an active sulfoxide containing magnetisation transfer catalyst, [Ir(H)2(IMes)(η2-pyruvate)(DMSO)]
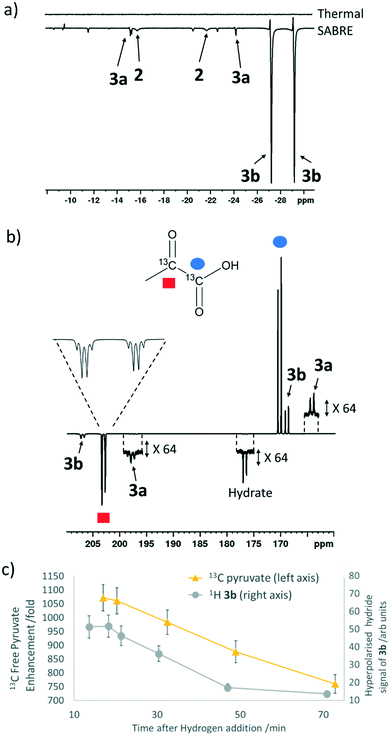
When sodium pyruvate-1-2-[13C2] (6 equivalents relative to iridium) is used as the substrate and added to 1 in the presence of dimethyl sulfoxide (DMSO) (4 equivalents) and 3 bar H2 in methanol-d4, an equilibrium mixture of [IrCl(H)2(sulfoxide)2(NHC)] (2) and [Ir(H)2(η2-pyruvate)(sulfoxide)(NHC)] (3) is formed (Fig. 1b).39,43 The regioisomer containing ligated pyruvate which lies trans to both hydride, and the NHC, is labelled 3a whereas the regioisomer where pyruvate lies in the same plane as the two hydride ligands is labelled 3b. Both of these structures are illustrated in Fig. 1b.When examining these solutions with a signal averaged 32 scan 1H NMR measurement at 298 K, the main hydride containing complex present is 3b. Resonances for 2 and 3a could not be discerned under these conditions, although, upon shaking this solution with 3 bar of p-H2 for 10 seconds at 65 G, hyperpolarised hydride responses for 2, 3a and 3b are immediately detected, as shown in Fig. 2a.39,43 Furthermore, upon shaking this sample for 10 seconds in a mu metal shield (ca. 300-fold shielding), hyperpolarised 13C resonances are observed, as shown in Fig. 2b. These correspond to those of free pyruvate at δ 169 and δ 203 (JCC = 62 Hz) and pyruvate bound in 3b at δ 168 and δ 207 (JCC = 60 Hz) and we quantify the 13C signal gains as 1215-fold and 910-fold for the [1-13C] and [2-13C] sites respectively in the free material. Additional resonances corresponding to pyruvate hydrate at δ 97 and δ 177 (JCC = 62 Hz) and pyruvate bound within 3a at δ 166 and δ 196 (JCC = 63 Hz) are also visible. A multi-scan thermally polarised 13C{1H} NMR measurement confirms these assignments for ligated pyruvate in both 3a and 3b in addition to those of the free material and its hydrate. 2D NMR characterisation data for these complexes has been previously reported.39 When we examine the signals of the [1-13C] and [2-13C] sites more closely we observe ∼2 Hz and ∼20 Hz resonance broadening upon pyruvate coordination respectively. We note that the [2-13C] resonance of the free material appears as a doublet of quartets with a 1JCC value of 62 Hz and a smaller 2JHC coupling between the adjacent methyl group protons of 6 Hz. This smaller 2JHC is not visible for pyruvate bound in 3b due to broadening effects. In order to explore how the efficiency of polarisation transfer changes with reaction time, this sample was shaken with fresh p-H2 at various time intervals after initial p-H2 addition. The hyperpolarised 13C and 1H signals that could be detected for pyruvate and 3b respectively were found to decrease with time, as shown in Fig. 2c, which is consistent with catalyst decomposition.
Variation of the [Ir(H)2(IMes)(η2-pyruvate)(L)] co-ligand, L
Polarisation transfer catalysts of the form [Ir(H)2(IMes)(η2-pyruvate)(L)] containing η2-ligated pyruvate and p-H2 derived hydride ligands are essential for catalytic polarisation transfer into bound pyruvate 13C sites and ultimately free pyruvate after ligand dissociation. A range of different co-ligands, L, (5 equivalents relative to iridium) were screened with [IrCl(COD)(IMes)] (1a) (5 mM), and sodium pyruvate-1,2-[13C2] (6 equivalents), and 3 bar p-H2 in methanol-d4 to identify if any other classes of co-ligands besides DMSO could form analogous complexes to 2 and 3.The use of 4-chlorobenzenemethanethiol as a co-ligand did not initially yield any hydride containing species. However, upon leaving the solution for a period of several months at 278 K, the growth of single crystals was observed. Upon examination by X-ray diffraction they were found to correspond to [Ir2(H)4(κ2-SCH2C6H4Cl)2(IMes)2] as detailed in the ESI.† We, and others, have reported structures of similar sulphur bridged iridium dimers26,46 and other products resulting from SH bond functionalisation.47 The other tested co-ligands, formaldehyde, triphenylphosphine (PPh3), ethylisothiocyanate and thiophene, all resulted in the formation of hydride complexes within 1 hour of H2 addition, but the corresponding solutions did not display any PHIP enhanced hydride signals upon shaking with p-H2. When a solution of 1a, PPh3 and sodium pyruvate-1,2-[13C2] with 3 bar H2 in methanol-d4 is left at 278 K for several months, the growth of single crystals was again observed. X-ray diffraction studies identified the product as [Ir(H)3(PPh3)3], as detailed in the ESI.† In contrast, the use of imidazole as a co-ligand did result in a hydride complex at δ −22.3 that exhibited PHIP, as detailed in the ESI.†48,49 However, in each of these cases no additional 13C pyruvate resonances were observed by NMR spectroscopy thereby suggesting that pyruvate coordination to iridium in these systems does not occur.
Of the co-ligands tested here, only sulfoxides supported pyruvate ligation to iridium. We expect this to be related to the optimum binding strength of the co-ligand which must be similar to that of pyruvate if its binding is not to be inhibited. For example, when the nitrogen based donor imidazole is used it seems to out compete pyruvate binding.31,34,50–52 In these cases [Ir(H)2(IMes)(NSub)3]Cl type complexes form as revealed by a single hydride signal around δ −22.3.47,48 The use of a sulfoxide based co-ligand is therefore a suitable compromise that leads to pyruvate binding and subsequent 13C signal gains and for this reason we explore how its identity affects this process.
Effect of sulfoxide identity on pyruvate 13C2signal enhancement
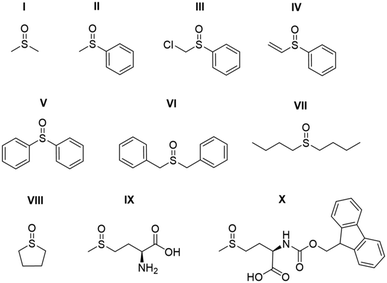
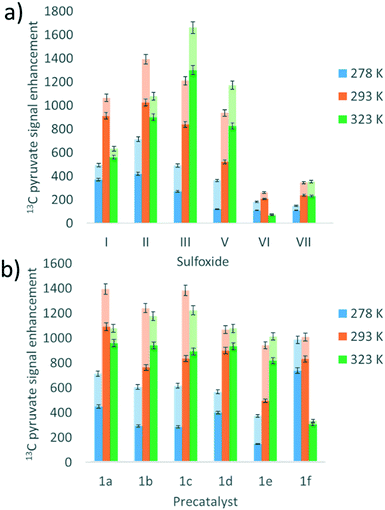
Studies on the effect of sulfoxide identity on the formation of [IrCl(H)2(sulfoxide)2(NHC)] (2) and [Ir(H)2(η2-pyruvate)(sulfoxide)(NHC)] (3) and the subsequent 13C pyruvate enhancement proved to be complex. For this work [IrCl(COD)(IMes)] (1a) was activated in methanol-d4 with 3 bar H2 in the presence of 6 equivalents of sodium pyruvate-1,2-[13C2] and 4 equivalents of one of the ten sulfoxides (I–X) of Fig. 3. The pyruvate 13C signal enhancement was then quantified after shaking the sample with fresh p-H2 several times over a 90 minute time period following H2 addition. In order to compare the performance efficiency as a function of sulfoxide identity we define several parameters. The first, εmax, describes the highest attained free 13C pyruvate signal enhancement for either the 1-[13C] or 2-[13C] site relative to the Boltzmann derived response. We observe that in most cases the signal gain on the 1-[13C] and 2-[13C] sites are the same within error, and we also quote averaged signal enhancements across the two sites. This is due to creation of 13C2 singlet order which must be shared equally amongst the two 13C sites. The second parameter, τ60, describes the percentage decrease in 13C pyruvate signal enhancement at the 60 minute reaction point when compared to the first measurement. R3b is the ratio of the 3b type product at the εmax point relative to the sum of all the hydride containing species and this should illustrate the stability of the sulfoxide–catalyst combination. The relative absolute integrals of the enhanced hydride 1H NMR signals of 3b after shaking at 65 G, S3b, was also determined during the reaction period and was found to exhibit similar behaviour. These values are presented for each of the sulfoxides I–X in Table 1.| Sulfoxide | 13C1εmax/folda | 13C2εmax/folda | τ 60/% | S 3b /arb. units | R 3b /% |
|---|---|---|---|---|---|
| a These reflect one shot measurements due to the change in signal over time. Errors are based on an average of three measurements for a sample containing II where the observed enhancement is relatively constant thereby allowing repeat measurement. b Rapid sample degradation prevented recording R3b at a similar time point to εmax. c No signals for a species analogous to 3b were evident. | |||||
| I | 1215 ± 40 | 910 ± 30 | 23 | 50 | 60 |
| II | 1090 ± 35 | 1035 ± 30 | 6 | 85 | 90 |
| III | 1090 ± 35 | 1040 ± 30 | 32 | 20 | 93 |
| IV | 115 ± 5 | 105 ± 5 | 92 | 5 | N/Ab |
| V | 555 ± 15 | 545 ± 15 | 3 | 60 | 90 |
| VI | 195 ± 5 | 180 ± 5 | 3 | 5 | 70 |
| VII | 400 ± 10 | 385 ± 10 | 9 | 25 | 45 |
| VIII | 1150 ± 35 | 1040 ± 30 | 60 | 70 | 95 |
| IX | 0 | 0 | N/A | N/A | 0 |
| X | 130 ± 5 | 115 ± 5 | 17 | N/Ac | N/Ac |
For sulfoxides I–VIII, hyperpolarised 13C pyruvate responses are observed immediately upon shaking the sample with 3 bar p-H2 in a mu metal shield. Over the next 90 minute time period, the resulting 13C pyruvate signal enhancements all gradually decrease (see ESI†). In all cases, the major dihydride complex present in solution proved to be of type 3b. Similarly, the hydride signals corresponding to 3b type products dominate the associated hyperpolarised hydride region of these 1H NMR spectra, and the intensity of their signals also decrease with increasing reaction time. Increasing the structural complexity of the co-ligand through the use of amino acid derived sulfoxide, IX, resulted in no 13C pyruvate signal enhancement, or detection of signals for species of type 3. While a hydride containing complex forms, which yields resonances at δ −12.3 and δ −27.3 that exhibit weak PHIP enhancement upon shaking with p-H2 (see ESI†), no evidence of pyruvate coordination was found. The poor performance of this sulfoxide could relate to the ready formation of an insoluble white precipitate, likely to be the corresponding [pyruvate-COO− + +NH3-IX] salt. When the protected co-ligand analogue, X, is used instead, pyruvate coordination and subsequent enhancement is again observed, but the resulting signal gains are just ∼120 fold. This is consistent with the lack of visible hydride signals for species of type 3. Hence, we link these low pyruvate enhancements to a low concentration of what we prove later to be the active magnetisation transfer catalyst.
Sulfoxides I–III and VIII delivered the highest levels of 13C signal enhancement for pyruvate across this series (εmax > 1000 fold) while IV–VII produced hyperpolarised 13C pyruvate signals of lower intensity (100–550 fold). These trends broadly matched those seen for the levels of hydride hyperpolarisation of the corresponding 3b derivatives. In the case of IV, the 13C pyruvate response rapidly decayed to zero as a consequence of hydrogenation of the original sulfoxide ligand and subsequent catalyst decomposition which has been observed in closely related systems.26 We have reported that C–S bond activation products result from this process which logically accounts for the low R3b and εmax values achieved by IV.26 In fact, we suggest that similar sulfoxide reactivity accounts for the loss of the 3b derivatives in all samples. We highlight that despite a high εmax being quantified for VIII, catalyst deactivation is extremely rapid. Hence, it is clear that SABRE efficiency is linked to the concentration of the 3b derivative in solution, which falls as the reaction time increases.
Sulfoxides VI and VII result in lower proportions of 3b (R3b of 70% and 45% in solution respectively) being present in these mixtures which will contribute to the lower pyruvate signal enhancements that are observed. In contrast, sulfoxides II–V commonly result in high proportions of 3b (R3b > 90%) and any differences in pyruvate 13C signal enhancement between these sulfoxides must now relate more closely to the efficiency of the polarisation transfer catalysis rather than catalyst concentration. For example, we note that I achieves a similar level of pyruvate enhancement to II, III and VIII despite the much lower ratio (60%) of 3b present in solution. In contrast, IV and V contain similar R3b values to II and III (90%) yet yield much lower pyruvate enhancements (<550-fold as compared to >1000 fold). This suggests that when sulfoxide I is utilized, a more effective catalytic system is created when compared to those derived from co-ligands IV and V.
We conclude from these data that the sulfoxide co-ligand identity plays a significant role in determining the concentration of the active SABRE catalyst in solution and the efficiency of the polarisation transfer process. Thus increasing the proportion of 3b present in solution is clearly one requirement for optimal SABRE. Of the four sulfoxides that gave pyruvate 13C NMR signal enhancements greater than 1000-fold, II appeared to be most stable to catalyst decomposition exhibiting only a 6% drop in signal intensity after 1 hour compared to 23, 32 and 60% for I, III and VIII respectively. These results show that H2 reaction time is also an important parameter that must be considered when optimising these pyruvate 13C NMR signal gains. This is often neglected when polarising N-donor substrates using SABRE as the associated magnetisation transfer catalysts are often more stable over longer reaction times.
Methylphenylsulfoxide, II, was identified as the best performing sulfoxide of this series as the associated complex gave some of the highest 13C pyruvate signal enhancements whilst also resulting in the highest hyperpolarised hydride ligand signal intensities for the isomer of type 3b. The concentration of methylphenylsulfoxide II was then varied to determine its effect on pyruvate enhancement. Similar behaviour is seen for both the 1H and 13C NMR signal enhancements of 3b when compared to those seen for free pyruvate. We find that using 10 equivalents of sulfoxide II relative to 1a provides the highest 13C pyruvate response, as shown in the ESI.† We expect that this is related to optimal ligand exchange processes at these effective reagent concentrations.
We have previously reported that complexes of the type [IrCl(H)2(NHC)(sulfoxide)2] (2) exchange hydrogen rapidly and are important in refreshing the p-H2 derived hydride ligands within the catalytic system.43 It is therefore likely that the rate of this process depends on sulfoxide identity and is reflected in the differing hydride signal intensities for the 3b isomer. The rate of H2 exchange within 2 was found to be independent of sulfoxide concentration when the sulfoxide is in excess (6–14 equivalents relative to iridium)43 in accordance with the first step of this process being the dissociative loss of sulfoxide. Therefore, we expect that changing sulfoxide concentration must have a greater effect on exchange between 2 and 3, however this process could not be quantified using EXSY methods. It is clear that the sulfoxide identity plays a role in p-H2 refreshment within 2 and likely the exchange between 2 and 3.
Effect of chloride ions on pyruvate 13C2signal enhancement
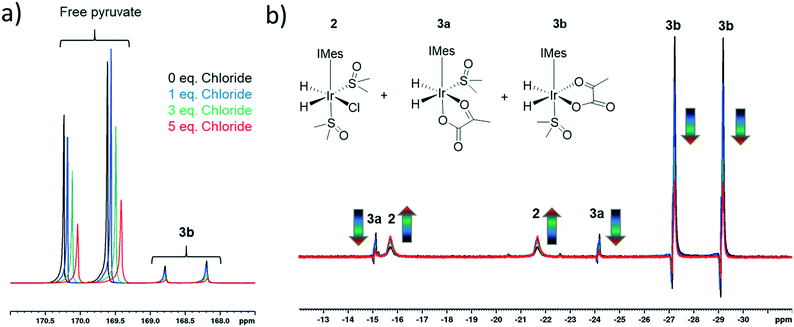
The rapid rate of H2 exchange in 2 in comparison to 3 indicates that 2 provides a clear route to refresh the p-H2 derived hydride ligands in 3. It is for this reason that the rate of exchange between 2 and 3 is proposed to play a significant role in determining the observed 13C pyruvate signal enhancement. As 2 contains a chloride ligand that is released into solution when 3 is formed, the concentration of available chloride might also be important in the formation of 3. We have already reported that there is a large decrease in the resulting pyruvate signal enhancement when chloride is replaced by bromide or acetonitrile.43 Here, we investigate the effect of changing the chloride concentration. To do this, solutions of 1a (5 mM), 10 equivalents of sulfoxide I and 5 equivalents of sodium pyruvate-1,2-[13C2] in 0.6 mL methanol-d4 containing 0–5 equivalents of NaCl in 5 μL of D2O were prepared. The resulting SABRE solutions were then activated with 3 bar H2 and their 13C NMR pyruvate signal enhancements monitored as a function of time. The associated signal intensity versus reaction time profiles are given in the ESI† and they all show an initial increase in 13C pyruvate signal enhancement over the first ∼30 minute period followed by a subsequent decrease as the reaction time increases. This change mirrors the associated change in concentration of 3b based on changes in hyperpolarised hydride resonance intensity. Upon increasing the chloride concentration from 0 to 1 equivalents, we observe a decrease in the average pyruvate enhancement across the two sites from 1000-fold to 920-fold. Further decreases to 715-fold and 570-fold are observed as the amount of NaCl is increased to 3 and 5 equivalents respectively. We note that greater chloride concentrations also result in higher proportions of free pyruvate signal relative to that seen for the associated bound resonances within 3b. These changes are accompanied by an increase in the size of the hydride signals seen for 2 relative to those of 3b, as shown in Fig. 4. These changes are therefore consistent with a shift in the equilibrium position towards 2 and the resulting fall in pyruvate signal gain is linked to a reduction in the amount of the active polarisation transfer catalyst, 3b present in these solutions.The effect of the reduction in active catalyst concentration was tested explicitly by increasing the initial amount of 1a and II so that a greater amount of 3b was present in solution. The resulting mixture with 1a (10 mM), II (100 mM) and sodium pyruvate-1,2-[13C2] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio yielded averaged 13C NMR signal enhancements of 705 and 255-fold for free and bound pyruvate respectively. The corresponding enhancements for the same solution containing 5 equivalents NaCl in 5 μL D2O were now much closer for the free pyruvate signal at 690-fold but the bound signal fell to 140 fold. These results confirm that the elevated chloride concentrations increase the proportion of free pyruvate enhancement relative to its bound counterpart. In addition they show that at the 10 mM catalyst concentration the free pyruvate signal enhancement remains comparable to that with the higher NaCl concentration. When the metal concentration was 5 mM, a reduction of pyruvate signal gain upon salt addition takes the averaged signal gain down from 1000-fold to 570-fold. As expected, this difference suggests the greater flux associated with improved efficiency in what would be a bimolecular H2 addition step can help offset the effect of increased chloride concentration.
6 ratio yielded averaged 13C NMR signal enhancements of 705 and 255-fold for free and bound pyruvate respectively. The corresponding enhancements for the same solution containing 5 equivalents NaCl in 5 μL D2O were now much closer for the free pyruvate signal at 690-fold but the bound signal fell to 140 fold. These results confirm that the elevated chloride concentrations increase the proportion of free pyruvate enhancement relative to its bound counterpart. In addition they show that at the 10 mM catalyst concentration the free pyruvate signal enhancement remains comparable to that with the higher NaCl concentration. When the metal concentration was 5 mM, a reduction of pyruvate signal gain upon salt addition takes the averaged signal gain down from 1000-fold to 570-fold. As expected, this difference suggests the greater flux associated with improved efficiency in what would be a bimolecular H2 addition step can help offset the effect of increased chloride concentration.
The equilibrium between 2 and 3 is also expected to be influenced by the concentration of pyruvate in solution. Therefore, samples containing 1a, 10 equivalents of II and 3, 6 or 8.5 equivalents of sodium pyruvate-1,2-[13C2] were shaken with 3 bar p-H2 in methanol-d4. Lowering the pyruvate concentration from 6 equivalents to 3 equivalents resulted in the averaged pyruvate signal enhancement reducing from 1085-fold and 515-fold for the free and bound pyruvate respectively to just 770-fold and 365-fold respectively. An increase in pyruvate concentration from 6 to 8.5 equivalents was accompanied by a similar drop in averaged signal gain to 630-fold and 180-fold respectively for the free and bound signals in comparative runs. Interestingly, as the ratio of pyruvate to iridium increases, the proportion of free pyruvate enhancement relative to the bound counterpart in 3b also increases. This is consistent with an increased likelihood of binding unpolarised pyruvate during SABRE as its concentration increases.
Effect of catalyst identity on pyruvate 13C2signal enhancement
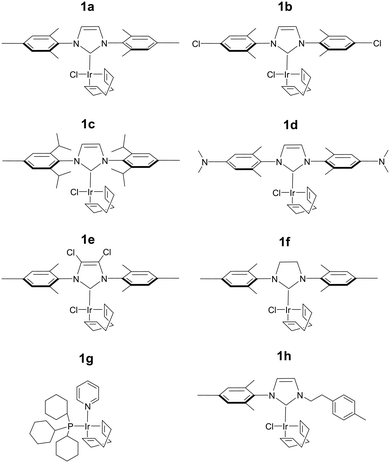
The efficiency of traditional [Ir(H)2(NHC)(Sub)3]Cl based SABRE catalysts is also influenced by the identity of the NHC ligand in the [IrCl(COD)(NHC)] precatalyst.34 Variation of this ligand has been used as a route to optimise signal enhancements by tuning substrate exchange rates.35,53–55 Changes to these ligands has also been used to synthesise water soluble SABRE catalysts.56–58 The effect of catalyst identity on the pyruvate signal enhancement was therefore probed by investigating the behaviour of the iridium precatalysts, 1a–h, of Fig. 5. These complexes were chosen to include symmetric N-heterocyclic carbenes with a range of Tolman electronic parameters and % buried volumes.55 Additionally, asymmetric N-heterocyclic carbenes59 and phosphine containing precatalysts,31,35 which have both been used previously for SABRE, were also included. Samples were prepared containing 6 equivalents of pyruvate and 10 equivalents of methylphenylsulfoxide (II) with 3 bar p-H2 in 0.6 mL methanol-d4. The 13C pyruvate signal enhancement, 1H hydride signal enhancement for the 3b type product, and its relative proportion in solution as measured in a 32 scan thermal 1H NMR spectrum, were monitored periodically over the first 90 minutes of reaction. These values are shown in Table 2 and are displayed graphically in the ESI.†| Precatalyst | 13C1εmax/folda | 13C2εmax/folda | R 3b /% |
|---|---|---|---|
| a These data reflect one shot measurements due to the change in signal over time. Errors are calculated based on an average of three measurements for a sample containing 1a where the observed enhancement is relatively constant thereby allowing repeat measurements. b No signals for the form corresponding to 3b were discerned. | |||
| 1a | 1085 ± 35 | 1085 ± 35 | 98 |
| 1b | 915 ± 25 | 905 ± 25 | 95 |
| 1c | 905 ± 30 | 885 ± 25 | 95 |
| 1d | 980 ± 30 | 980 ± 30 | 90 |
| 1e | 650 ± 20 | 660 ± 20 | 98 |
| 1f | 870 ± 25 | 860 ± 25 | 95 |
| 1g | 35 ± 2 | 25 ± 2 | 50 |
| 1h | 60 ± 3 | 55 ± 3 | N/Ab |
The identity of the precatalyst 1a–1f proved to have little effect on the proportion of the 3b type product in solution although there was an effect on 13C pyruvate signal enhancement. In all cases, a hyperpolarised 13C pyruvate response was observed after shaking with 3 bar p-H2. In contrast to 1a, precatalysts 1b–1h result in lower 13C pyruvate signal enhancements immediately after H2 addition. For some of these precatalysts, εmax, occurs at much longer reaction times. Hence the differing precatalysts exhibit different activation periods and subsequently, different time points where the maximum concentration of 3b is reached. This demonstrates how reaction time can be an important parameter that plays a large effect on the observed signal gain. For example, 1a activates very rapidly and the proportion of its 3b derivative is at a maximum shortly after the initial H2 addition step. The corresponding 13C pyruvate signal enhancement is also maximised at this time point. In contrast, 1c has one of the slowest rates of 3b derivative formation and hence the corresponding hyperpolarised pyruvate signal increases after the initial H2 addition step as a function of the growth in 3b concentration (see ESI†). Other precatalysts, such as 1b and 1d, also form 3b derivatives which is reflected in an initial increase in pyruvate enhancement followed by a slow decrease as the concentration of 3 falls over a longer timescale. The resulting 13C pyruvate signal enhancements can therefore be used as a route to effectively track the concentrations of 3b type products in solution and hence monitor the conversion of 1 to 3.
The phosphine based precatalyst 1g yields just 50% of the 3b type product in solution and the resulting pyruvate signal enhancements are now just ∼30-fold. In contrast, when precatalyst 1h, containing an asymmetric N-heterocyclic carbene ligand is used an isomer of type 3b no longer forms. Here, we form larger amounts of 3a which we expect to be due to reduced steric crowding associated with the smaller carbene ligand. Now, the hyperpolarised pyruvate signal shows a ∼60-fold enhancement which is an order of magnitude lower than that provided by the symmetric carbene 1a. This is consistent with the fact isomer 3b is essential for attaining high levels of pyruvate polarisation. Interestingly, when 3a is the more dominant species, the hyperpolarised 13C2 pyruvate profile no longer appears in the typical pattern diagnostic of 13C2 singlet order as created with catalysts 1a–1g. This is also consistent with previous theoretical modelling studies which suggest that the spin topology of 3a is incompatible with the easy retention of p-H2 derived singlet spin order in the product.39 It is clear that 3b, which is formed when the precatalysts 1a–f are used, reflects an active polarisation transfer catalysts with the necessary spin topology to mediate efficient polarisation transfer into bound 13C2 pyruvate. Some examples of representative NMR spectra are shown in Fig. 6.
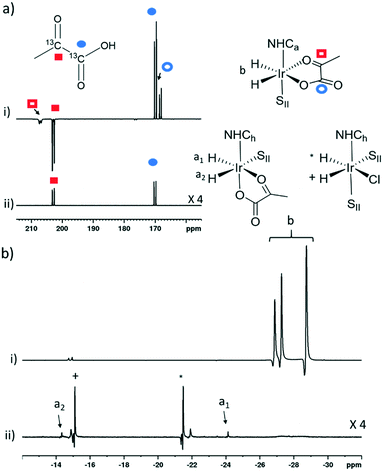 | ||
| Fig. 6 Partial hyperpolarised a) 13C and b) 1H NMR responses after samples of i) 1a and ii) 1h containing 6 equivalents of sodium pyruvate-1,2-[13C2], and 10 equivalents of II are shaken in methanol-d4 with 3 bar p-H2 for 10 seconds in a mu metal shield. SII and h in the structures refers to II and ligand h as shown in Fig. 3 and 5 respectively. | ||
Effect of temperature on pyruvate 13C2signal enhancement
Many studies have varied the temperature to achieve a substrate exchange rate optimum for SABRE magnetisation transfer.34,41,60 To this end, solutions of 1a, 6 equivalents of sodium pyruvate-1,2-[13C2], and 4 equivalents of the sulfoxides I–III, IV–VII, shown in Fig. 3, were shaken with 3 bar p-H2 in methanol-d4 at three different temperatures. These temperatures (278 K, 293 K and 323 K) were achieved by placing the NMR tube in a thermostatically controlled water bath for 60 seconds prior to shaking for 10 seconds under p-H2 at room temperature. Care was taken to record the NMR measurements at similar reaction times in order to compare these data. The effect of temperature upon the observed pyruvate 13C signal enhancement is shown in Fig. 7a. For I, II, VI, and VII the resulting free pyruvate 13C signal enhancement is maximised at room temperature while sulfoxides III and V perform better at the elevated temperature. Hence, whilst ligand exchange is slow and not detectable on the EXSY timescale, there must be an optimum rate for each complex as reported for other N-heterocyclic substrates.60When this study was expanded to include solutions of the iridium precatalysts 1a–f of Fig. 5, 6 equivalents of sodium pyruvate-1,2-[13C2], and 10 equivalents of methylphenylsulfoxide (II), similar temperature effects were seen as shown in Fig. 7b. For 1a, 293 K proved optimal with warming clearly moving away from the required exchange rate. For 1b and 1c, the proportion of bound substrate polarisation is much higher relative to the free material when compared to the other precatalysts which may suggest slower exchange. This is expected for 1b on account of the chloride substituent which decreases electron density on the metal relative to 1a and is therefore likely to result in stronger pyruvate binding and slower ligand exchange. 1c contains a sterically demanding NHC which might be expected to promote exchange, although this is clearly not the case. In contrast, the metal centre in 1d is electron rich thereby increasing exchange which agrees with the lower retained bound pyruvate polarisation level. Of the series, 1e is the most electron deficient with high proportions of bound pyruvate signal and low levels of pyruvate enhancement which increase at higher temperatures. This is consistent with slower pyruvate exchange in the 1e system.
We conclude that variation of the NHC ligand can have a large effect on the attained pyruvate enhancements. Understanding these effects is challenging and we expect that both steric and electronic effects associated with the ligands are important, as previously suggested for SABRE with N-heterocyclic substrates.53,55 Here though, the ligand effects are likely to be more complex as they will not only influence the rate of pyruvate exchange within 3, but also the rate of interconversion between 2 and 3 and the rate of H2 exchange in 2. Steric effects are important in determining the concentration of the active polarisation transfer catalyst, 3b, that forms in solution. Further optimisation of pyruvate signal gain is possible by subtle variation of the electronic effects of the active catalyst.
Effect of selective deuteration on pyruvate 13C2signal enhancement
Relaxation within the substrate when bound to the catalyst has been shown to limit its degree of 1H hyperpolarisation using SABRE. This effect has been reduced by the inclusion of deuterium labels within the active polarisation transfer catalyst whilst simultaneously reducing any polarisation leakage into the catalyst.31,34,61,62 Therefore, we examined the effect of deuterating the sulfoxide ligand and the IMes backbone of the precatalyst.Shaking a solution of 1a with 6 equivalents of sodium pyruvate-1,2-[13C2], 4 equivalents of I and 3 bar p-H2 in methanol-d4 for 10 seconds in a mu metal shield yielded averaged free and bound pyruvate 13C signal enhancements of 1070 and 200-fold respectively. When this process was repeated using the corresponding deuterium labelled sulfoxide I-d6 at the same time point after H2 addition these enhancements remained comparable at 1070 and 220-fold. This suggests that there is little polarisation leakage into this sulfoxide and that relaxation of hyperpolarised magnetisation via1H sites in its 3b derivative does not limit the efficiency of SABRE.
In contrast, when the results from shaking a solution of 1a with 6 equivalents of sodium pyruvate-1,2-[13C2], and 10 equivalents of II under 3 bar p-H2 in methanol-d4 are compared to those achieved with 1a-d22 an effect is seen; in this case all the protons in the NHC except the two in the imidazole ring are labelled with 2H. This is reflected in a fall in the averaged free 13C2 signal enhancement from 1085 to 875-fold although the bound pyruvate signals remain comparable at 515-fold and 535-fold respectively. When this measurement is repeated using 1a-d24 these signal gains further decrease to 675 and 465-fold for free and bound pyruvate respectively. This suggests that 2H labelling of the NHC is now detrimental to SABRE: a finding which is in direct contrast to commonly observed effects when [Ir(H)2(NHC)(NSub)3]Cl polarisation transfer catalysts are deuterated.31,34,61,62 We suggest this could be due to the effects of quadrupolar relaxation at the mG polarisation transfer field caused by the introduction of deuterium, whereas previous studies have typically employed G polarisation transfer fields.
Further optimisation of pyruvate 13C2signal enhancement by varying shaking time and hydrogen pressure
The effects of the p-H2 shaking time and hydrogen pressure on the 13C pyruvate signal enhancements were also investigated. This involved using a sample of 1a with two equivalents of II at 1, 2 and 3 bar of p-H2. The results revealed that there was a growth in averaged hyperpolarisation level for free pyruvate from 510 to 730-fold upon increasing the pressure from 1 bar to 3 bar, after which point the signal gain plateaus (see ESI†). This suggests that, in this case, hydrogen exchange is rate limiting at pressures lower than 3 bar but once this pressure is exceeded it is ligand exchange and the associated relaxation processes within the catalyst that become limiting. We have already investigated how hydrogen exchange within [IrCl(H)2(IMes)(DMSO)2] increases as H2 pressure is increased from 0.5–2 bar.43 We expect that the increase in pyruvate signal at these H2 pressures is related to increased p-H2 refreshment in the 2/3 system which does not become more efficient at pressures higher than 3 bar.Higher averaged 13C2 pyruvate signal enhancements result (1050 compared to 425-fold) when the shaking time in the mu metal shield is extended from 5 to 30 seconds in 5 second intervals, as detailed in the ESI.† This change allows polarisation to build up more effectively on both the bound and free 13C sites. The observed effect on the 1H signals of the hydride resonances in 3b is the opposite, with the visible signal gains decreasing. This implies more signal is transferred to the 13C centres at longer shaking times. We confirmed that these trends are also observed for a sample of 1a-d24 with ten equivalents of II.
Combining optimisation steps to achieve improved pyruvate 13C2signal enhancement
We were able to achieve a maximum averaged pyruvate 13C signal enhancement of 2135-fold (1.7% polarisation) for the free material alongside a 585-fold bound pyruvate signal gain. This averaged gain corresponds to signal enhancements of 2140 and 2125-fold for the 1-13C and 2-13C sites respectively. We can therefore increase pyruvate signal gains by two orders of magnitude (from 30-fold for catalyst 1g, Table 2, to 2135-fold) by careful optimisation of factors including temperature, shaking time, catalyst, sulfoxide and their concentrations. These optimum enhancements involved a sample containing 1a (5 mM), 10 equivalents II and 6 equivalents sodium pyruvate-1,2-[13C2] in 0.6 mL methanol-d4 that was shaken with 3 bar p-H2 for 30 seconds in a mu metal shield. The effect of these optimisation steps on improving the signal gain is depicted in Fig. 8. We failed to see further increases with deuterium labelling of the sulfoxide or the NHC ligands or by shaking with 4.5 bar p-H2. When all 13C species in this sample, including bound pyruvate and its hydrated form, are included a net 13C polarisation of 2845-fold (2.3% polarisation) is achieved which exceeds those previously reported.39 We also note that the preparations used here are stable for a greater time period after hydrogen addition, thereby allowing for more repeat measurements and improved sample examination.Conclusions
Optimising pyruvate signal gains is important for a range of applications that may include hyperpolarised reaction monitoring. For example, we have already demonstrated how the reaction of sodium pyruvate with hydrogen peroxide can be monitored using hyperpolarised 13C NMR spectroscopy.39 We have presented a detailed study that optimises the pyruvate signal gain by variation of sulfoxide and carbene ligand within [Ir(H)2(η2-pyruvate)(DMSO)(NHC)] polarisation transfer catalysts. We have shown that sulfoxide co-ligands are essential for the formation of these active species and the highest pyruvate signals can be achieved using a [IrCl(COD)(IMes)] precatalyst in conjunction with 10 equivalents of phenylmethylsulfoxide. Sterically large carbenes are required if the formation of the active isomer 3b is to be favoured, although electronic effects are important in fine tuning the ligand exchange processes. Hyperpolarised 13C2 pyruvate signal intensities are shown to be closely linked to the amount of the 3b isomer present in solution, although systems containing similar amounts but different ligands can result in very different pyruvate enhancements. These results highlight the tension between many different factors that influence the efficiency of polarisation transfer within this complex. In all cases we observe a decrease in both hyperpolarised 13C2 pyruvate level and the 1H hydride ligand signals of the 3b type isomer at longer reaction times which we associate with catalyst deactivation. By combining these effects we attained an averaged pyruvate 13C signal enhancement level of 2135-fold (1.7% polarisation) for free sodium pyruvate-1,2-[13C2].For biomedical applications, attaining high signal enhancements in aqueous, rather than methanolic solvents, is of more importance. When a solution of 1a and 10 eq. II are preactivated with 3 bar H2 in ethanol-d6 before adding sodium pyruvate-1,2-[13C2] in D2O and shaking in a mu metal shield for 30 seconds 13C signal gains are approximately an order of magnitude lower than those achieved in methanol-d4. In these 70/30 D2O/ethanol-d6 mixtures, which might reflect a system suitable for future in vivo studies, the proportion of bound pyruvate enhancement is significantly higher than those achieved in methanol-d4. It is therefore clear that in non-methanolic solvents pyruvate exchange is reduced which limits the attained polarisation levels. It is expected that the application of a similar optimisation approach would lead to improved pyruvate enhancements in this biocompatible solvent mixture via catalysts that exhibit faster exchange kinetics.
In summary, while SABRE provides a cheap, simple and reversible route to hyperpolarise pyruvate with time and cost advantages over alternative techniques such as DNP and PHIP-SAH, there is a limitation associated with the lower signal enhancements delivered here. Nevertheless, the formation and behaviour of these novel polarisation transfer catalysts and their applications to hyperpolarise pyruvate reflect an important step forward in para-hydrogen based hyperpolarisation. Future work is directed at gaining greater understanding of these catalyst effects, probing the ligand exchange processes that govern the attained polarisation levels and optimising them for use in conjunction with biocompatible solvents.
Experimental
All NMR measurements were carried out on a 400 MHz Bruker Avance III spectrometer using solutions at room temperature (298 K) unless otherwise stated. Para-Hydrogen (p-H2) was produced by passing hydrogen gas over a spin-exchange catalyst (Fe2O3) and used for all hyperpolarisation experiments. This method produces constant p-H2 with ca. 99% purity. 1H (400 MHz) and 13C (100.6 MHz) NMR spectra were recorded with an internal deuterium lock. Chemical shifts are quoted as parts per million and referenced to methanol-d4. 13C NMR spectra were recorded with broadband proton decoupling. Coupling constants (J) are quoted in Hertz. All commercial compounds listed were purchased from Sigma-Aldrich, Fluorochem, or Alfa-Aesar and used as supplied unless otherwise stated.Samples were prepared containing 2 mg iridium catalyst with 6 equivalents of sodium pyruvate-1,2-[13C2] and 4 equivalents of sulfoxide unless otherwise stated in 0.6 mL of methanol-d4 in a 5 mm NMR tube that was fitted with a J. Young's tap. Unless otherwise stated the iridium precatalyst used was [IrCl(COD)(IMes)] (where IMes = 1,3-bis(2,4,6-trimethyl-phenyl)imidazole-2-ylidene and COD = cis,cis-1,5-cyclooctadiene). Iridium precatalysts used in this work were synthesized in our laboratory according to literature procedures.63 The solutions were subsequently degassed by two freeze–pump–thaw cycles.
The shake & drop method was employed for recording hyperpolarised NMR spectra. NMR tubes were filled with p-H2 at 3 bar pressure and shaken vigorously for 10 seconds unless otherwise stated in the 65 G stray field next to a 9.4 T magnet for 1H polarisation or in a mu metal shield (ca. 300-fold shielding) for 13C polarisation and placing inside a 9.4 T spectrometer for NMR detection. Pyruvate 13C enhancements were calculated by reference to a more concentrated thermal sample as outlined in Shchepin et al.64 In cases where averaged 13C enhancements are given across both 1-[13C] and [2-13C] sites the sum of these integrals is referenced to the sum of these integrals in the corresponding thermal measurement. Data points usually reflect an average of three shake and drop measurements while those that monitor signal growth over time are single point measurements with typical errors as determined from averaged data.
Conflicts of interest
B. J. T., W. I., and S. B. D. (and others) are inventors on a patent application filed by the University of York related to this work (patent no. GB1818171.9, filed 7 November 2018).Acknowledgements
We thank Robin Brabham and Mark Dowsett for providing samples of sulfoxides IX and X. Financial support from the Wellcome Trust (Grants 092506 and 098335), the MRC (MR/M008991/1), the EPSRC (B. J. T. studentship and Impact Accelerator Award G0025101) and the University of York is gratefully acknowledged.Notes and references
- K. Golman, M. Lerche, R. Pehrson and J. H. Ardenkjaer-Larsen, Cancer Res., 2006, 66, 10855–10860 CrossRef CAS PubMed.
- K. Golman and M. Thaning, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 11270–11275 CrossRef CAS PubMed.
- S. E. Day, M. I. Kettunen, F. A. Gallagher, D.-E. Hu, M. Lerche, J. Wolber, K. Golman, J. H. Ardenkjaer-Larsen and K. M. Brindle, Nat. Med., 2007, 13, 1382 CrossRef CAS PubMed.
- S. Jannin, A. Bornet, R. Melzi and G. Bodenhausen, Chem. Phys. Lett., 2012, 549, 99–102 CrossRef CAS.
- J. H. Ardenkjær-Larsen, S. Bowen, J. R. Petersen, O. Rybalko, M. S. Vinding, M. Ullisch and N. C. Nielsen, Magn. Reson. Med., 2019, 81, 2184–2194 CrossRef PubMed.
- J. H. Ardenkjær-Larsen, B. Fridlund, A. Gram, G. Hansson, L. Hansson, M. H. Lerche, R. Servin, M. Thaning and K. Golman, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10158–10163 CrossRef PubMed.
- A. Z. Lau, A. P. Chen, N. R. Ghugre, V. Ramanan, W. W. Lam, K. A. Connelly, G. A. Wright and C. H. Cunningham, Magn. Reson. Med., 2010, 64, 1323–1331 CrossRef CAS PubMed.
- J. D. MacKenzie, Y.-F. Yen, D. Mayer, J. S. Tropp, R. E. Hurd and D. M. Spielman, Radiology, 2011, 259, 414–420 CrossRef PubMed.
- M. Pourfathi, Y. Xin, S. J. Kadlecek, M. F. Cereda, H. Profka, H. Hamedani, S. M. Siddiqui, K. Ruppert, N. A. Drachman and J. N. Rajaei, Magn. Reson. Med., 2017, 78, 2106–2115 CrossRef CAS PubMed.
- E. M. Serrao, M. I. Kettunen, T. B. Rodrigues, P. Dzien, A. J. Wright, A. Gopinathan, F. A. Gallagher, D. Y. Lewis, K. K. Frese and J. Almeida, Gut, 2016, 64, 465–475 CrossRef PubMed.
- M. Marjańska, I. Iltis, A. A. Shestov, D. K. Deelchand, C. Nelson, K. Uğurbil and P.-G. Henry, J. Magn. Reson., 2010, 206, 210–218 CrossRef PubMed.
- S. J. DeVience, X. Lu, J. Proctor, P. Rangghran, E. R. Melhem, R. Gullapalli, G. M. Fiskum and D. Mayer, Sci. Rep., 2017, 7, 1907 CrossRef PubMed.
- S. J. Nelson, J. Kurhanewicz, D. B. Vigneron, P. E. Larson, A. L. Harzstark, M. Ferrone, M. van Criekinge, J. W. Chang, R. Bok and I. Park, Sci. Transl. Med., 2013, 5, 198ra108 Search PubMed.
- T. Witney, M. Kettunen, D. Hu, F. Gallagher, S. Bohndiek, R. Napolitano and K. Brindle, Br. J. Cancer, 2010, 103, 1400 CrossRef CAS PubMed.
- N. M. Zacharias, H. R. Chan, N. Sailasuta, B. D. Ross and P. Bhattacharya, J. Am. Chem. Soc., 2011, 134, 934–943 CrossRef PubMed.
- P. C. Bhattacharya, E. Y, W. H. Perman, K. C. Harris, A. P. Lin, V. A. Norton, C. T. Tan, B. D. Ross and D. P. Weitekamp, J. Magn. Reson., 2007, 186, 150–155 CrossRef CAS PubMed.
- M. R. Clatworthy, M. I. Kettunen, D.-E. Hu, R. J. Mathews, T. H. Witney, B. W. Kennedy, S. E. Bohndiek, F. A. Gallagher, L. B. Jarvis and K. G. Smith, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 13374–13379 CrossRef CAS PubMed.
- J. Eills, J. Alonso-Valdesueiro, D. E. Salazar Marcano, J. Ferreira da Silva, S. Alom, G. J. Rees, J. V. Hanna, M. Carravetta and M. H. Levitt, ChemPhysChem, 2018, 19, 40–44 CrossRef CAS PubMed.
- T. C. Eisenschmid, R. U. Kirss, P. P. Deutsch, S. I. Hommeltoft, R. Eisenberg, J. Bargon, R. G. Lawler and A. L. Balch, J. Am. Chem. Soc., 1987, 109, 8089–8091 CrossRef CAS.
- R. Eisenberg, Acc. Chem. Res., 1991, 24, 110–116 CrossRef CAS.
- J. B. Hövener, A. N. Pravdivtsev, B. Kidd, C. R. Bowers, S. Glöggler, K. V. Kovtunov, M. Plaumann, R. Katz-Brull, K. Buckenmaier and A. Jerschow, Angew. Chem., Int. Ed., 2018, 57, 11140–11162 CrossRef PubMed.
- T. C. Eisenschmid, R. U. Kirss, P. P. Deutsch, S. I. Hommeltoft, R. Eisenberg, J. Bargon, R. G. Lawler and A. L. Balch, J. Am. Chem. Soc., 1987, 109, 8089–8091 CrossRef CAS.
- C. R. Bowers and D. P. Weitekamp, J. Am. Chem. Soc., 1987, 109, 5541–5542 CrossRef CAS.
- S. B. Duckett, C. L. Newell and R. Eisenberg, J. Am. Chem. Soc., 1994, 116, 10548–10556 CrossRef CAS.
- K. V. Kovtunov, I. E. Beck, V. I. Bukhtiyarov and I. V. Koptyug, Angew. Chem., Int. Ed., 2008, 47, 1492–1495 CrossRef CAS PubMed.
- B. J. Tickner, R. R. Parker, A. C. Whitwood and S. B. Duckett, Organometallics, 2019, 38, 4377–4382 CrossRef CAS PubMed.
- P. Bhattacharya, E. Y. Chekmenev, W. H. Perman, K. C. Harris, A. P. Lin, V. A. Norton, C. T. Tan, B. D. Ross and D. P. Weitekamp, J. Magn. Reson., 2007, 186, 150–155 CrossRef CAS PubMed.
- A. B. Schmidt, S. Berner, M. Braig, M. Zimmermann, J. Hennig, D. von Elverfeldt and J.-B. Hövener, PLoS One, 2018, 13, e0200141 CrossRef PubMed.
- F. Reineri, T. Boi and S. Aime, Nat. Commun., 2015, 6, 5858 CrossRef CAS PubMed.
- E. Cavallari, C. Carrera, S. Aime and F. Reineri, J. Magn. Reson., 2018, 289, 12–17 CrossRef CAS PubMed.
- R. W. Adams, J. A. Aguilar, K. D. Atkinson, M. J. Cowley, P. I. Elliott, S. B. Duckett, G. G. Green, I. G. Khazal, J. López-Serrano and D. C. Williamson, Science, 2009, 323, 1708–1711 CrossRef CAS PubMed.
- J.-B. Hövener, N. Schwaderlapp, T. Lickert, S. B. Duckett, R. E. Mewis, L. A. R. Highton, S. M. Kenny, G. G. R. Green, D. Leibfritz and J. G. Korvink, Nat. Commun., 2013, 4, 2946 CrossRef PubMed.
- M. J. Burns, P. J. Rayner, G. G. Green, L. A. Highton, R. E. Mewis and S. B. Duckett, J. Phys. Chem. B, 2015, 119, 5020–5027 CrossRef CAS PubMed.
- P. J. Rayner, M. J. Burns, A. M. Olaru, P. Norcott, M. Fekete, G. G. R. Green, L. A. R. Highton, R. E. Mewis and S. B. Duckett, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E3188–E3194 CrossRef CAS PubMed.
- M. Fekete, O. Bayfield, S. B. Duckett, S. Hart, R. E. Mewis, N. Pridmore, P. J. Rayner and A. Whitwood, Inorg. Chem., 2013, 52, 13453–13461 CrossRef CAS PubMed.
- P. Norcott, M. J. Burns, P. J. Rayner, R. E. Mewis and S. B. Duckett, Magn. Reson. Chem., 2018, 56, 663–671 CrossRef CAS PubMed.
- R. V. Shchepin, D. A. Barskiy, D. M. Mikhaylov and E. Y. Chekmenev, Bioconjugate Chem., 2016, 27, 878–882 CrossRef CAS PubMed.
- M. L. Truong, T. Theis, A. M. Coffey, R. V. Shchepin, K. W. Waddell, F. Shi, B. M. Goodson, W. S. Warren and E. Y. Chekmenev, J. Phys. Chem. C, 2015, 119, 8786–8797 CrossRef CAS PubMed.
- W. Iali, S. S. Roy, B. J. Tickner, F. Ahwal, A. J. Kennerley and S. B. Duckett, Angew. Chem., 2019, 131, 10377–10381 CrossRef.
- W. Iali, P. J. Rayner and S. B. Duckett, Sci. Adv., 2018, 4, eaao6250 CrossRef PubMed.
- P. J. Rayner, B. J. Tickner, W. Iali, M. Fekete, A. D. Robinson and S. B. Duckett, Chem. Sci., 2019, 10, 7709–7717 RSC.
- B. J. Tickner, W. Iali, S. S. Roy, A. C. Whitwood and S. B. Duckett, ChemPhysChem, 2019, 20, 241–245 CrossRef CAS PubMed.
- B. J. Tickner, J. S. Lewis, R. O. John, A. C. Whitwood and S. B. Duckett, Dalton Trans., 2019, 48, 15198–15206 RSC.
- M. Gemeinhardt, M. Limbach, T. Gebhardt, C. Eriksson, S. Eriksson, J. Lindale, E. Goodson, W. Warren, E. Chekmenev and B. Goodson, Angew. Chem., 2020, 132, 426–431 CrossRef.
- R. W. Adams, S. B. Duckett, R. A. Green, D. C. Williamson and G. G. R. Green, J. Chem. Phys., 2009, 131, 194505 CrossRef PubMed.
- W. D. Jones and R. M. Chin, J. Am. Chem. Soc., 1994, 116, 198–203 CrossRef CAS.
- B. J. Tickner, R. O. John, S. S. Roy, S. Hart, A. C. Whitwood and S. B. Duckett, Chem. Sci., 2019, 10, 5235–5245 RSC.
- R. V. Shchepin, D. A. Barskiy, A. M. Coffey, T. Theis, F. Shi, W. S. Warren, B. M. Goodson and E. Y. Chekmenev, ACS Sens., 2016, 1, 640–644 CrossRef CAS PubMed.
- M. Fekete, P. J. Rayner, G. G. R. Green and S. B. Duckett, Magn. Reson. Chem., 2017, 55, 944–957 CrossRef CAS PubMed.
- P. J. Rayner and S. Duckett, Angew. Chem., Int. Ed., 2018, 57, 6742–6753 CrossRef CAS PubMed.
- W. Iali, P. J. Rayner, A. Alshehri, A. J. Holmes, A. J. Ruddlesden and S. B. Duckett, Chem. Sci., 2018, 9, 3677–3684 RSC.
- M. J. Cowley, R. W. Adams, K. D. Atkinson, M. C. Cockett, S. B. Duckett, G. G. Green, J. A. Lohman, R. Kerssebaum, D. Kilgour and R. E. Mewis, J. Am. Chem. Soc., 2011, 133, 6134–6137 CrossRef CAS PubMed.
- B. J. A. van Weerdenburg, N. Eshuis, M. Tessari, F. P. J. T. Rutjes and M. C. Feiters, Dalton Trans., 2015, 44, 15387–15390 RSC.
- M. J. Cowley, R. W. Adams, K. D. Atkinson, M. C. R. Cockett, S. B. Duckett, G. G. R. Green, J. A. B. Lohman, R. Kerssebaum, D. Kilgour and R. E. Mewis, J. Am. Chem. Soc., 2011, 133, 6134–6137 CrossRef CAS PubMed.
- P. J. Rayner, P. Norcott, K. M. Appleby, W. Iali, R. O. John, S. J. Hart, A. C. Whitwood and S. B. Duckett, Nat. Commun., 2018, 9, 4251 CrossRef PubMed.
- P. Spannring, I. Reile, M. Emondts, P. P. M. Schleker, N. K. J. Hermkens, N. G. J. van der Zwaluw, B. J. A. van Weerdenburg, P. Tinnemans, M. Tessari, B. Blumich, F. Rutjes and M. C. Feiters, Chem. - Eur. J., 2016, 22, 9277–9282 CrossRef CAS PubMed.
- M. Fekete, C. Gibard, G. J. Dear, G. G. R. Green, A. J. J. Hooper, A. D. Roberts, F. Cisnetti and S. B. Duckett, Dalton Trans., 2015, 44, 7870–7880 RSC.
- F. Shi, P. He, Q. A. Best, K. Groome, M. L. Truong, A. M. Coffey, G. Zimay, R. V. Shchepin, K. W. Waddell and E. Y. Chekmenev, J. Phys. Chem. C, 2016, 120, 12149–12156 CrossRef CAS PubMed.
- C. M. Wong, M. Fekete, R. Nelson-Forde, M. R. D. Gatus, P. J. Rayner, A. C. Whitwood, S. B. Duckett and B. A. Messerle, Catal. Sci. Technol., 2018, 8, 4925–4933 RSC.
- D. A. Barskiy, A. N. Pravdivtsev, K. L. Ivanov, K. V. Kovtunov and I. V. Koptyug, Phys. Chem. Chem. Phys., 2016, 18, 89–93 RSC.
- H. Zeng, J. Xu, J. Gillen, M. T. McMahon, D. Artemov, J.-M. Tyburn, J. A. Lohman, R. E. Mewis, K. D. Atkinson and G. G. Green, J. Magn. Reson., 2013, 237, 73–78 CrossRef CAS PubMed.
- A. J. Holmes, P. J. Rayner, M. J. Cowley, G. G. Green, A. C. Whitwood and S. B. Duckett, Dalton Trans., 2015, 44, 1077–1083 RSC.
- L. D. Vazquez-Serrano, B. T. Owens and J. M. Buriak, Inorg. Chim. Acta, 2006, 359, 2786–2797 CrossRef CAS.
- R. V. Shchepin, L. Jaigirdar, T. Theis, W. S. Warren, B. M. Goodson and E. Y. Chekmenev, J. Phys. Chem. C, 2017, 121, 28425–28434 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1957542–1957543. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cy02498k |
| ‡ Present Address: Dr. O. Semenova, Drug Discovery Unit, School of Life Sciences, University of Dundee, Dundee, DD1 5EH, United Kingdom. |
| § Present Address: Dr. W. Iali, Department of Chemistry, King Fahd University of Petroleum and Minerals (KFUPM), Dhahran, 31261, Saudi Arabia. |
| This journal is © The Royal Society of Chemistry 2020 |