Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Use of titanocalix[4]arenes in the ring opening polymerization of cyclic esters†
Ziyue
Sun
a,
Yanxia
Zhao
*a,
Orlando
Santoro
b,
Mark R. J.
Elsegood
 c,
Elizabeth V.
Bedwell
c,
Khadisha
Zahra
c,
Elizabeth V.
Bedwell
c,
Khadisha
Zahra
 d,
Alex
Walton
d,
Alex
Walton
 d and
Carl
Redshaw
d and
Carl
Redshaw
 *ab
*ab
aCollege of Chemistry and Material Science, Northwest University, 710069 Xi'an, China
bPlastics Collaboratory, Department of Chemistry and Biochemistry, The University of Hull, Cottingham Rd, Hull, HU6 7RX, UK. E-mail: c.redshaw@hull.ac.uk
cChemistry Department, Loughborough University, Loughborough, Leicestershire, LE11 3TU, UK
dDepartment of Chemistry and Photon Science Institute, University of Manchester, Oxford Road, Manchester, M13 9PL, UK
First published on 27th January 2020
Abstract
The known dichloride complexes [TiCl2L(O)2(OR)2] (type I: R = Me (1), n-Pr (2) and n-pentyl (3); L(OH)2(OR)2 = 1,3-dialkyloxy-p-tert-butylcalix[4]arene), together with the new complexes {[TiL(O)3(OR)]2(μ-Cl)2}·6MeCN (R = n-decyl (4·6MeCN)), and [Ti(NCMe)Cl(L(O)3(OR))]·MeCN (type II: R = Me, 5·MeCN) are reported. Attempts to prepare type II for R = n-Pr and n-pentyl using [TiCl4] resulted in the complexes {[TiL(O)3(On-propyl)]2(μ-Cl)(μ-OH)} 6·7MeCN and {[TiL(O)3(On-pentyl)]2(μ-Cl)(μ-OH)}·7.5MeCN (7·7.5MeCN), respectively; use of [TiCl4(THF)2] resulted in a co-crystallized THF ring-opened product [Ti(NCMe)(μ3-O)L(O)4TiCl(O(CH2)4Cl)]2–2[TiCl(NCMe)(L(O)3(On-Pr))]·11MeCN (8·11MeCN). The molecular structures of 2·2MeCN, 4·6MeCN, and 5·MeCN together with the hydrolysis products {[TiL(O)3(OR)]2(μ-Cl)(μ-OH)} (R = n-Pr 6·7MeCN; n-pentyl, 7·7.5MeCN, 9·9MeCN); R = n-decyl 10·8.5MeCN) and that of the ring opened product 8·11MeCN and the co-crystallized species [Ti2(OH)Cl(L(O)3(OR))][L(OH)2(OR)2]·2.85(C2H3N)·0.43(H2O) (R = n-pentyl, 11·2.85(C2H3N)·0.43(H2O)) are reported. Type I and II complexes have been screened for their ability to act as catalysts in the ring opening polymerization (ROP) of ε-caprolactone (ε-CL), δ-valerolactone (δ-VL), ω-pentadecalactone (ω-PDL) and rac-lactide (r-LA), both with and without benzyl alcohol present and either under N2 or in air. The copolymerization of ε-CL with δ-VL and with r-LA has also been investigated. For the ROP of ε-CL, all performed efficiently (>99% conversion) at 130 °C over 24 h both under N2 and in air, whilst over 1 h, for the type I complexes the trend was 3 > 2 > 1 but all were poor (≤12% conversion). By contrast, 5 over 1 h at 130 °C was highly active (85% conversion). At 80 °C, the activity trend followed the order 5 ≈ 4 > 3 > 2 > 1. For δ-VL, at 80 °C the activity trend 5 ≈ 4 > 1 > 2 > 3 was observed. ROP of the larger ω-PDL was only possible using 5 at 130 °C over 24 h with moderate activity (48% conversion). For r-LA, only low molecular weight products were obtained, whilst for the co-polymerization of ε-CL with δ-VL using 5, high activity was observed at 80 °C affording a polymer of molecular weight >23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and with equal incorporation of each monomer. In the case of ε-CL/r-LA co-polymerization using 5 either under N2 or air, the polymerization was more sluggish and only 65% conversion of CL was observed and the resultant co-polymer had 65
000 Da and with equal incorporation of each monomer. In the case of ε-CL/r-LA co-polymerization using 5 either under N2 or air, the polymerization was more sluggish and only 65% conversion of CL was observed and the resultant co-polymer had 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 incorporation. Complex 5 could also be supported on silica, however this system was not as active as its homogeneous counterpart. Finally, the activity of these complexes is compared with that of three benchmark species: a di-phenolate Ti compound {TiCl2(2,2′-CH3CH[4,6-(t-Bu)2C6H2O]2)} (12) and a previously reported NO2-containing titanocalix[4]arene catalyst, namely cone-5,17-bis-tert-butyl-11,23-dinitro-25,27-dipropyloxy-26,28-dioxo-calix[4]arene titanium dichloride (13), as well as [Ti(Oi-Pr)4]; the parent calixarenes were also screened.
35 incorporation. Complex 5 could also be supported on silica, however this system was not as active as its homogeneous counterpart. Finally, the activity of these complexes is compared with that of three benchmark species: a di-phenolate Ti compound {TiCl2(2,2′-CH3CH[4,6-(t-Bu)2C6H2O]2)} (12) and a previously reported NO2-containing titanocalix[4]arene catalyst, namely cone-5,17-bis-tert-butyl-11,23-dinitro-25,27-dipropyloxy-26,28-dioxo-calix[4]arene titanium dichloride (13), as well as [Ti(Oi-Pr)4]; the parent calixarenes were also screened.
Introduction
Metallocalixarenes have attracted a significant amount of interest in the catalysis field, and have found application for a variety of processes.1 In terms of titanocalixarenes, which date back to the 1980s,2 Frediani, Sémeril et al. employed the 1,3-di-n-propyloxycalix[4]arene, 1,3-L(OH)2(n-PrO)2, containing complex [1,3-L(O)2(n-PrO)2TiCl2], in conjunction with MAO (methylaluminoxane), to afford ultrahigh molecular weight polyethylene.3 The active species, as determined by 1H NMR spectroscopy, was determined to be a titanium methyl cation, as observed in earlier work by Proto et al.4 Subsequently, Taoufik, Bonnamour et al. extended these studies and investigated the effect of varying the 1,3-dialkyloxy R groups (for R = methyl, ethyl, n-propyl and i-butyl) on the catalytic activity during ethylene polymerization. In the same study, a couple of 1,2-dialkoxycalix[4]arene derivatives were also prepared, namely the methyloxy and a complex containing a chelating siloxide SiMe2, together with a number of depleted calix[4]arene complexes bearing 1,2- or 1,3-titanium dichloride motifs.5 Whilst the behavior of the 1,2- and 1,3-systems was different, it was determined that within each series, increasing the number of alkyloxy groups present was detrimental to the catalytic activity. On variation of the R group from methyl to isobutyl in the 1,3-systems, there was no clear structural activity trend. A number of other, less well-defined, titanium calix[n]arenes have also been employed for ethylene polymerization.6 We also note that this type of titanocalix[4]arene has been grafted onto silica and employed in olefin epoxidation by the group of Katz.7With regard to the ring opening polymerization (ROP) of cyclic esters, reports using titanocalix[n]arenes are scant. Frediani, Sémeril et al. employed the complex {[1,3-p-tert-butyl-2,4-(NO2)L(O)2(n-PrO)2-1,3]TiCl2}, under solvent-free conditions, for the well-controlled ROP of lactide.8 The resultant polymer was highly isotactic, whilst the addition of n-butanol led to an increase in both the rate of polymerization and transfer with the monomer. The same group also employed complex [1,3-L(O)2(n-PrO)2TiCl2] for the ROP of rac-lactide (r-LA), initiating the catalyst either by use of microwave radiation or heat.9 Although the rate of polymerization was enhanced by using microwaves, this was to the detriment of control. More recently, McIntosh et al. reported preliminary studies on the use of the complex [Ti4L2(O)8(On-Pr)8(THF)2] (where L2(OH)8 = p-tert-butylcalix[8]arene) as a catalyst for the ROP of r-LA at 130 °C.10 We have also investigated the use of metallocalix[n]arenes for the ROP of cyclic esters such as ε-caprolactone, and have reported how, for a series of tungstocalix[6 and 8]arenes, different sized rings and their associated conformations can drastically affect the catalytic activity.11 Molybdo- and tungstocalix[4]arenes were found to be less active.12 Herein, we have screened several titanocalix[4]arenes, namely [TiCl2L(O)2(OR)2] (type I: R = Me (1), n-Pr (2) and n-pentyl (3)), the dimeric compound {[TiL(O)3(OR)]2(μ-Cl)2} (R = n-decyl (4)), and [Ti(NCMe)Cl(L(O)3(OR))]·MeCN (type II, R = Me, 5·MeCN, R = n-propyl 6 and n-pentyl 7) for the ROP of ε-CL, δ-VL and r-LA (the copolymerization of ε-CL and r-LA was also investigated) (Chart 1).
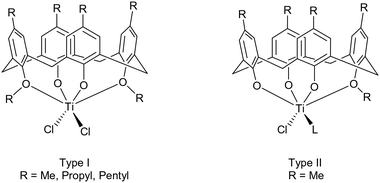 | ||
| Chart 1 Types I and II titanocalix[4]arenes complexes employed herein for the ROP of ε-CL, δ-VL and r-LA. | ||
Results and discussion
Interaction of 1,3-dialkoxycalix[4]arenes, L(OH)2(OR)2, with [TiCl4] or [TiCl4(THF)2] is known to readily afford titanocalix[4]arene dichlorides of the type [TiCl2L(O)2(OR)2].5,13 We have prepared herein the derivatives where R = Me (1), n-Pr (2) and n-Pent (3) as reported by Taoufik and Bonnamour, employing acetonitrile as the solvent of crystallization during the workup (Scheme 1).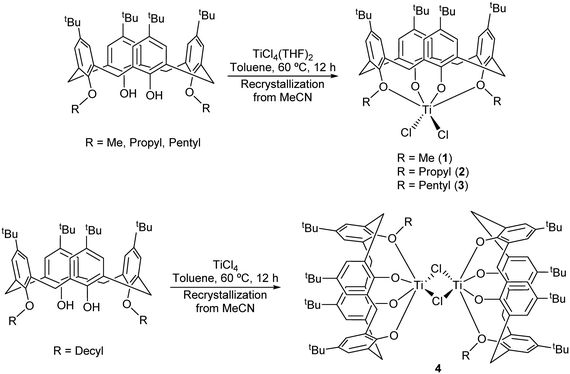 | ||
| Scheme 1 Synthesis of complexes 1–4.5 | ||
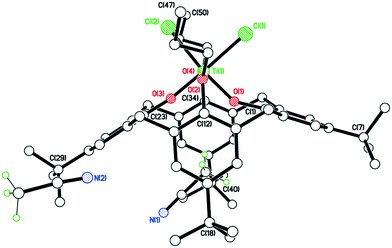
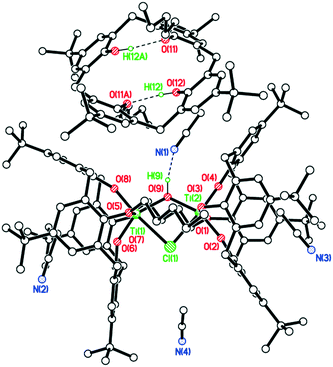
The structure of the n-Pr derivative as the MeCN solvate [TiCl2(L(O)2(On-Pr)2)]·2MeCN (2·2MeCN) (CCDC 1954692) is shown in Fig. 1, with selected bond lengths and angles given in the caption. The Ti(IV) center exhibits a slightly distorted octahedral geometry and bears cis chlorides. There is a pinched cone calix[4]arene conformation, but with the O(1) side more splayed out than the other at O(3). One MeCN resides inside the calix[4]arene cone with the methyl group most deeply embedded, the other lies exo. The molecules of [TiCl2(L(O)2(On-Pr)2)]·2MeCN (2·2MeCN) pack in anti-parallel layers with no significant intermolecular interactions (see Fig. S1, ESI†).
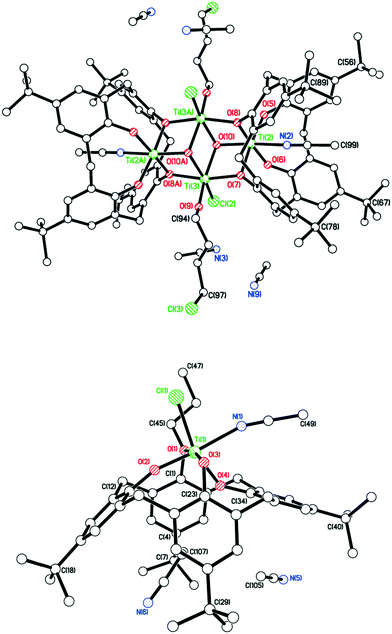
In the case of R = n-decyl, prolonged standing at 0 °C afforded large red prisms suitable for X-ray crystallography. The molecular structure (CCDC 1954693) is shown in Fig. 2, with selected bond lengths and angles given in the caption. The structure of 4 is a chloro-bridged dimer {[TiL(O)3(OR)]2(μ-Cl)2} (R = n-decyl), in which each titanium center is best described as distorted octahedral. The asymmetric unit contains two half molecules both on inversion centers as well as six molecules of crystallization (MeCN). There are weak C–H⋯O hydrogen bonds between the first CH2 group of the n-decyl chain and a phenolate oxygen on the symmetry related calix[4]arene. The two half molecules differ in two respects. Firstly, the n-decyl chains have different conformations at their tails and one is disordered here, while the other is not. Secondly, the number of MeCN molecules in the calixarene cavities is different.
For our subsequent catalytic studies, vide infra, we also employed a modification of the Floriani procedure for targeting monochloro titanium calix[4]arenes.13 In our work, LH2Me2 was refluxed with [TiCl4(THF)2] in toluene (60 h reflux) and then with acetonitrile (24 h) which afforded, on cooling, orange red prisms of [Ti(NCMe)Cl(L(O)3(OR))]·MeCN (5·MeCN) in good yield (Scheme 2a).
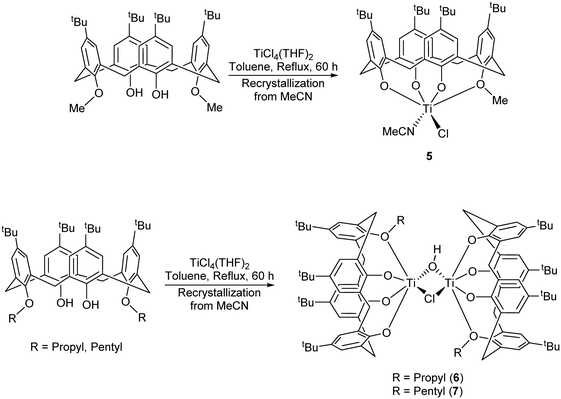 | ||
| Scheme 2 Synthesis of complexes 5–7.13 | ||
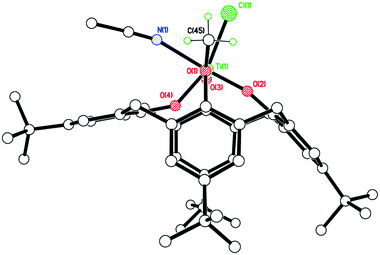
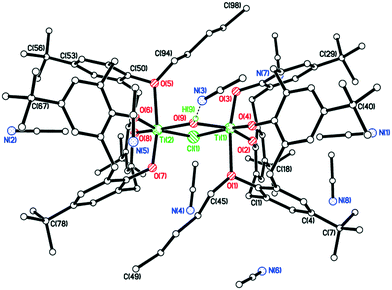
The structure (CCDC 1954694) is shown in Fig. 3, with selected bond lengths and angles given in the caption. The structure is non-merohedrally twinned via a 180° rotation about the direct axis (1 0 0). There are two titanocalix[4]arene complexes (related via mirror symmetry) and two acetonitrile molecules in the asymmetric cell. The coordination geometry about the distorted octahedral titanium center comprises cis chloride and acetonitrile ligands together with a monomethoxycalix[4]arene, the latter adopting a ‘down, down, down, out’ conformation. The acetonitriles of crystallization reside with their methyl groups inside the calix[4]arene cavities.
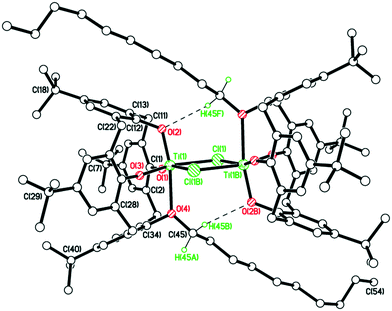
We note that a route to monofunctionalised calix[4]arenes via removal of an ether functionality using TiCl4 has been reported by Floriani and coworkers.13 Floriani et al. identified CH3Cl in their reaction and suggested this was the result of capture of the leaving group by the Cl− nucleophile. We also noted dealkylation when studying the air stability of the type I complex with R = n-pentyl.14 In the case of 5, this can be viewed as the acetonitrile trapped titanium intermediate en-route to the formation of the monoalkoxycalix[4]arene. Surprisingly, on extension of this methodology to the systems derived from L(OH)2(OR)2 (R = n-propyl or n-pentyl), the corresponding monochloride complexes [Ti(NCMe)Cl(L(O)3(OR))]·MeCN (R = n-propyl and n-pentyl) were not accessible (Scheme 2b). It was postulated that the de-alkylation of the calix[4]arenes bearing n-propyl and n-pentyl moieties does not proceed as per their methoxy analogue due to the nature of the elimination products, namely the non-volatile liquids n-propylchloride and n-pentylchloride versus gaseous CH3Cl. Instead, in the case of R = n-propyl, a dimeric Ti2 complex showing calix[4]arene moieties bearing only one n-propyl substituent each was isolated. Given the two Ti atoms are connected by Cl− and OH− bridges, it was hypothesized that the complex arose by adventitious exposure to air during work-up of the parental dichloride complex 2. The molecular structure of {[TiL(O)3(On-Pr)]2(μ-Cl)(μ-OH)}·7MeCN (6·7MeCN) (CCDC 1954695) is shown in Fig. 4 with selected bond lengths and angles given in the caption. The dimeric structure exhibits the previously seen bridging OH−/Cl− motif,14 linking the two distorted octahedral titanium centers. The calixarenes each adopt a ‘down, down, down, out’ conformation.
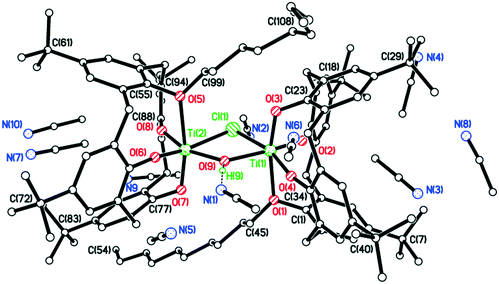
In the case of R = n-pentyl, the complex isolated was identified as {[TiL(O)3(On-pentyl)]2(μ-Cl)(μ-OH)}·7.5MeCN (7·7.5MeCN). The molecular structure (CCDC 1954696) is shown in Fig. 5, with selected bond lengths and angles given in the caption. Each titanium center is distorted octahedral with the coordinated provided by a mono-pentoxy calix[4]arene and bridging hydroxyl and chloro ligation. In the packing, the calixarene cavities approach each other forming fairly large solvent accessible volumes, leading to the observed disorder in the MeCN of crystallization.
This type of reaction was further complicated when [TiCl4(THF)2] was employed as the metal precursor.15 In this case, on attempting to isolate the n-propyl analogue of 5, the orange/red co-crystallized complex [Ti(NCMe)(μ3-O)L(O)4TiCl(O(CH2)4Cl)]2–2[TiCl(NCMe)(L(O)3(On-Pr))]·11MeCN (8·11MeCN) was isolated in moderate yield. The molecular structure (CCDC 1954697) is shown in Fig. 6, with selected bond lengths and angles given in the caption. The asymmetric unit comprises half a tetrametallic complex, one monometallic complex and 5.5 MeCNs. The tetrametallic complex lies on a centre of symmetry, with octahedral metal centres bound by either a calix[4]arene which adopts a cone conformation with a metal-bound MeCN in the cavity and a μ3-oxo {Ti(2) and Ti(2A)}, or in the case of Ti(3) and Ti(3A) by an oxygen from each L, the two μ3-oxos and a chlorinated butoxy ligand (O(CH2)4Cl). The calix[4]arenes have lost all of their lower rim bound n-propyl groups. By contrast, in the monometallic complex, the calixarene adopts a ‘down, down, down, out’ conformation and retains an n-propyl group.
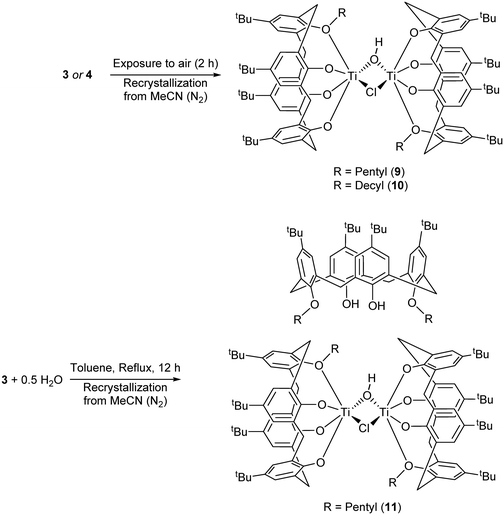
We have also investigated the air/water stability of a number of these systems, given that often during catalysis small amounts of water are present, which can lead to often unwanted transesterification processes. Furthermore, studies have revealed that the water content of hydrophilic monomers such as ε-CL can varying greatly with temperature and time.16 Thus, given the likely presence of water in our ROP studies, we re-visited the type I complex with R = n-pentyl, for which we had previously structurally characterized the complex {[TiL(O)3(OR)]2(μ-Cl)(μ-OH)}·71/4MeCN, as well as the and n-decyl system.14 Following initial exposure to air (2 h), work-up was conducted under an inert atmosphere of nitrogen (Scheme 3a). In the case of R = n-pentyl, the complex isolated, namely {[TiL(O)3(On-pentyl)]2(μ-Cl)(μ-OH)}·9MeCN 9·9MeCN, differed only in the degree of solvation from 7·7.5MeCN and from that which we reported previously, see ESI,† Fig. S2 (CCDC 1954689); all geometrical parameters were very similar.
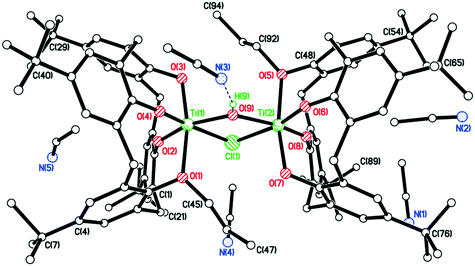
In the case of the n-decyl system, crystallization from acetonitrile again afforded a chloro/hydroxyl-bridged complex, namely {[TiL(O)3(On-decyl)]2(μ-Cl)(μ-OH)}·8.5MeCN (10·8.5MeCN). The molecular structure (CCDC 1954690) of 10·8.5MeCN is shown in Fig. 7, with selected bond lengths and angles given in the caption; alternative views are given in the ESI† (Fig. S3). Each titanium is distorted octahedral, and is bound by a calix[4]arene ligand bearing only one decyl chain. One decyl chain, on O(1), is fully extended, but the other, on O(5), is only straight until the 6th C atom, at which point it is somewhat folded. Molecules of 10 pack into layers in the b/c plane, but are generally well-separated with MeCNs of crystallization in crevices between molecules. MeCNs at N(3), N(7) and N(10) reside mostly with the calixarene cavities and with Me groups furthest inside, the others lie exo.
We observed that if the entire procedure above was conducted in air, then the result was precipitation of the parent calixarene ligand. However, we found that conducting a controlled hydrolysis reaction of the type I complex with R = n-pentyl, i.e. addition of 0.5 equivalents of H2O to a toluene solution of [TiCl2L(O)2(On-pentyl)2] under reflux, followed by extraction into acetonitrile and then crystallization (still under nitrogen) led to the isolation of orange, plate-like crystals in moderate yield (Scheme 3b). The molecular structure (see Fig. 8) revealed a co-crystallized species (11).
One Ti2 complex plus half the non-coordinated co-crystallized bis-n-pentyl ligand, plus the solvent of crystallization in the asymmetric unit (CCDC 1954691). Each Ti adopts a distorted octahedral conformation. The bis-n-pentyl ligand lies on a center of symmetry at the body center of the unit cell. The non-coordinated calix[4]arene molecule retains two phenol hydrogens which form intramolecular H-bonds to the neighbouring oxygens. This molecule adopts a 1,2-alternate conformation. The existence of the co-crystallized bis-n-pentyl ligand demonstrates that this is the species added to the reaction and that the loss of one n-pentyl group occurs upon coordination. The coordinated calix[4]arenes are essentially eclipsed when the complex molecule is viewed end-on (see Fig. S4, ESI†).
For catalytic comparison purposes, a titanium complex {TiCl2(2,2′-CH3CH[4,6-(t-Bu)2C6H2O]2)} (12) bearing a di-phenolate ligand derived from the diphenol 2,2′-CH3CH[4,6-(t-Bu)2C6H2OH]2 was synthesized according to the procedure reported by Aida et al. (Scheme 4).
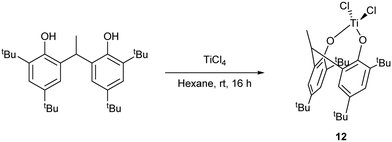 | ||
| Scheme 4 Synthesis of the di-phenolate complex 12.17 | ||
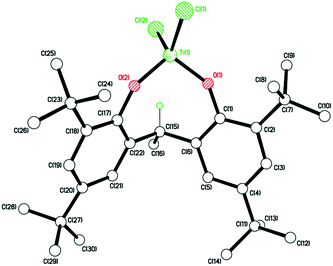
Plate-like, orange crystals suitable for X-ray analysis were obtained from a saturated hexane solution of the complex upon standing at room temperature for 2 days. The molecular structure of 12 (CCDC 969053) is shown in Fig. 9. A tetrahedral Ti4+ is coordinated to two chlorides and two di-phenolate oxygens. The complex is chiral but only one enantiomer is present. The fold angle between rings C(1) > C(6) and C(17) > C(22) is 64.63(7)°. No disorder or solvent of crystallization are observed.
ROP screening
ε-Caprolactone (ε-CL)
We have examined the ability of the complexes prepared herein to act as catalysts for the ROP of ε-CL both under nitrogen and in air; the ROP of ε-CL under air when using the titanium tetraalkoxides Ti(Oi-Pr)4 and Ti(On-Bu)4 has been reported.16Firstly, looking at the series [TiCl2L(O)2(OR)2] (type I: R = Me (1), n-Pr (2) and n-pentyl (3)), at 130 °C over 24 h with a ratio of 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and in the presence of two equivalents of benzyl alcohol, all three are efficient catalysts and afford >99% conversion (runs 1–4, Table 1). Complex 1 as its MeCN solvate exhibits the best control, whilst in all cases, observed molecular weights are far lower than calculated values suggesting extensive transesterification is occurring (Scheme 5).8 Over 1 h (runs 5–7), some differentiation is possible with highest conversion achieved using the pentyl catalyst system 3. Interestingly, if the ROP runs are conducted under air (runs 8–10), the catalysts remain efficient with conversions >99%, with comparable molecular weights for the polymers isolated using 1 and 2 as those obtained under N2, though that from 3 was somewhat lower.
1 and in the presence of two equivalents of benzyl alcohol, all three are efficient catalysts and afford >99% conversion (runs 1–4, Table 1). Complex 1 as its MeCN solvate exhibits the best control, whilst in all cases, observed molecular weights are far lower than calculated values suggesting extensive transesterification is occurring (Scheme 5).8 Over 1 h (runs 5–7), some differentiation is possible with highest conversion achieved using the pentyl catalyst system 3. Interestingly, if the ROP runs are conducted under air (runs 8–10), the catalysts remain efficient with conversions >99%, with comparable molecular weights for the polymers isolated using 1 and 2 as those obtained under N2, though that from 3 was somewhat lower.
| Run | Catalyst/solvent | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n(obs) | M n(corr) | M n(calc) | M w/Mnb |
|---|---|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [ε-CL] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture.b From GPC.c Values corrected considering Mark–Houwink factor (0.56) from polystyrene standards in THF.d Calculated from ([monomer]0/[OH]0) × conv. (%) × monomer molecular weight + molecular weight of BnOH.e Reaction performed in air. | |||||||||
| 1 | 1 Tol | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 5390 | 3020 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.90 |
| 2 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 7330 | 4100 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.30 |
| 3 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
7680 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.20 |
| 4 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 9280 | 5200 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.40 |
| 5 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 2 | nd | nd | nd | |
| 6 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 3 | nd | nd | nd | |
| 7 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 12 | nd | nd | nd | |
| 8e | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 6390 | 3580 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.80 |
| 9e | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 6060 | 3390 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.40 |
| 10e | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 2910 | 1630 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.50 |
| 11e | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.47 |
| 12e | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 8180 | 4580 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.08 |
| 13e | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 85 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.82 |
| 14 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 32 | 6510 | 3650 | 9240 | 1.10 |
| 15 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 33 | 650 | 360 | 9240 | 1.06 |
| 16 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 68 | 6310 | 3640 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
1.10 |
| 17 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | >99 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 990 990 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.55 |
| 18 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | >99 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
9260 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.48 |
| 19 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 24 | >99 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
8100 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.40 |
| 20 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | — | |
| 21 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | — | |
| 22 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | — | |
| 23 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | 11 | ||||
| 24 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | 37 | ||||
| 25 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 1 | 11 | ||||
| 26 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 1 | None | — | — | — | |
| 27 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 1 | None | — | — | — | |
| 28 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 1 | None | — | — | — | |
| 29 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | >99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
7690 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.40 |
| 30 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | >99 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
8300 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.90 |
| 31 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | >99 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.80 |
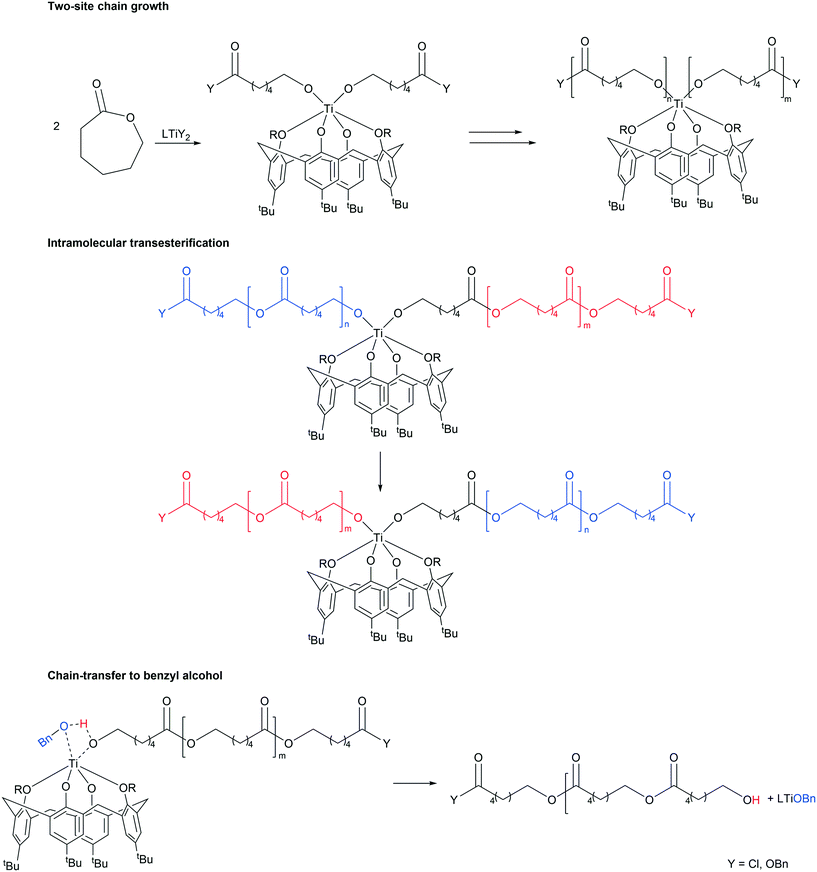 | ||
| Scheme 5 Proposed pathways for chain-growth, intramolecular transesterification and chain-transfer to co-catalyst [adapted from ref. 8]. | ||
The control in air was generally better than that observed under N2. On lowering the temperature to 80 °C, over 24 h (runs 14–16), complex 3 was again the most active, with both systems 1 and 3 affording polymers with good control (Mw/Mn = 1.1). In the case of system 2, only oily oligomers were isolated (360 Da). Over 1 h at 80 °C (runs 20–22), the systems were inactive. When runs were conducted in the absence of benzyl alcohol (runs 29–31), all systems were again active (>99% conversion), with observed molecular weight higher than for comparable runs in the presence of external alcohol. This could be ascribed to the lack of the chain-transfer to the co-activator (see Scheme 5). Over 1 h at 130 °C in the absence of alcohol, the systems were inactive (runs 26–28). It was noteworthy that complete conversion was achieved in the ROP conducted in air in the presence of complex 4·MeCN (run 11). The same outcome was achieved at 80 °C over 24 h (run 17). Although only 11% conversion was obtained after 1 h (run 17), the complex proved to be more active than its congeners 1–3.
Screening of the complex [Ti(NCMe)Cl(L(O)3(OMe))]·MeCN (5·MeCN) under the same conditions employed for 1–3 revealed that this system was more active, for example over 1 h, conversions were >85% in air (run 13), whilst at 80 °C over 24 h under N2 (runs 18, 19) conversions were >99% and over 1 h (run 24) conversions were >37%.
The analysis of the polymer terminal groups was performed by means of 1H NMR spectroscopy. The spectra of the PCLs synthesized in the presence of the co-activator, both under nitrogen and in air, exhibited signals for the aromatic and benzylic protons of the benzyloxy-end group (δ = 7.35 and 5.10 ppm, respectively) in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio (see the ESI,† Fig. S5 and S6, respectively). As expected, such signals were not observed in the spectrum of the polymer isolated in the absence of benzyl alcohol (see the ESI,† Fig. S7). In all cases, the triplet corresponding to the terminal –CH2OH group (δ = 3.65 ppm) was also observed. For the polymers isolated with BnOH, the ratio between the areas of the benzyl- and –CH2OH groups was found to be far from the theoretical value (5
2 ratio (see the ESI,† Fig. S5 and S6, respectively). As expected, such signals were not observed in the spectrum of the polymer isolated in the absence of benzyl alcohol (see the ESI,† Fig. S7). In all cases, the triplet corresponding to the terminal –CH2OH group (δ = 3.65 ppm) was also observed. For the polymers isolated with BnOH, the ratio between the areas of the benzyl- and –CH2OH groups was found to be far from the theoretical value (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 vs. 5
2 vs. 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 5
6 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for the run under N2 and air, respectively). This could be ascribed to the broad dispersities of the samples and/or to the formation of cyclic oligomers. Hence, further identification of end groups via MALDI-TOF mass spectrometry was undertaken. For the PCL isolated in the presence of BnOH under inert atmosphere (see the ESI,† Fig. S8), a main population compatible with benzyl- and CH2OH terminated polymers was observed; this was consistent with the 1H NMR spectrum of the sample. Nevertheless, a second distribution ascribable to cyclic compounds was also identified. In the case of the sample obtained in aerobic conditions, only cyclic species were observed for low Mn (20–30 repeating units, see the ESI,† Fig. S9a). For higher molecular weights (>35 repeating units), a second distribution accountable to α-hydroxyl-ω-(carboxylic acid) poly(ε-caprolactones) was detected (see the ESI,† Fig. S9b and c). For higher fractions, such distribution became predominant, although cyclic species are still present. The formation of α-hydroxyl-terminated chains could be explained considering that the insertion of the first monomer unit into a Ti–OH bond arises from the reaction of the pre-catalyst (or its parental benzyloxy-derivative) with adventitious water.18 Similarly, only cyclic species were observed in the polymer isolated in the absence of BnOH for low Mn (17–24 repeating units, see ESI,† Fig. S10a). Noteworthy, the formation of cyclic polyesters occurring in the absence of BnOH has recently been reported.19 A distribution of α-hydroxyl-ω-(carboxylic acid)-terminated polymers became predominant for higher fractions (see ESI,† Fig. S10b and c). Interestingly, no Cl-terminal groups were detected, suggesting the inability of the Ti–Cl species to promote ROP reactions.17,18 Also in this case, the active species was thought to be a Ti–OH compound derived from the hydrolysis of the pre-catalyst.
10 for the run under N2 and air, respectively). This could be ascribed to the broad dispersities of the samples and/or to the formation of cyclic oligomers. Hence, further identification of end groups via MALDI-TOF mass spectrometry was undertaken. For the PCL isolated in the presence of BnOH under inert atmosphere (see the ESI,† Fig. S8), a main population compatible with benzyl- and CH2OH terminated polymers was observed; this was consistent with the 1H NMR spectrum of the sample. Nevertheless, a second distribution ascribable to cyclic compounds was also identified. In the case of the sample obtained in aerobic conditions, only cyclic species were observed for low Mn (20–30 repeating units, see the ESI,† Fig. S9a). For higher molecular weights (>35 repeating units), a second distribution accountable to α-hydroxyl-ω-(carboxylic acid) poly(ε-caprolactones) was detected (see the ESI,† Fig. S9b and c). For higher fractions, such distribution became predominant, although cyclic species are still present. The formation of α-hydroxyl-terminated chains could be explained considering that the insertion of the first monomer unit into a Ti–OH bond arises from the reaction of the pre-catalyst (or its parental benzyloxy-derivative) with adventitious water.18 Similarly, only cyclic species were observed in the polymer isolated in the absence of BnOH for low Mn (17–24 repeating units, see ESI,† Fig. S10a). Noteworthy, the formation of cyclic polyesters occurring in the absence of BnOH has recently been reported.19 A distribution of α-hydroxyl-ω-(carboxylic acid)-terminated polymers became predominant for higher fractions (see ESI,† Fig. S10b and c). Interestingly, no Cl-terminal groups were detected, suggesting the inability of the Ti–Cl species to promote ROP reactions.17,18 Also in this case, the active species was thought to be a Ti–OH compound derived from the hydrolysis of the pre-catalyst.
δ-Valerolactone (δ-VL)
For the ROP of δ-VL (Table 2), using 1–3, at 80 °C over 24 h (runs 1–3) only moderate activity was observed with conversions ≤50% (activity followed the trend 1 > 2 > 3), but with good control and observed molecular weights much lower than calculated values. Higher conversion (78%) was obtained in the presence of 4 (run 4). Interestingly, the molecular weight observed was close to the calculated value. In the case of the reaction performed over 1 h at 80 °C, systems 1–3 were inactive, while only 4% conversion was observed with complex 4 (runs 7–9 and 10, respectively). In the case of [Ti(NCMe)Cl(L(O)3(OMe))]·MeCN (5·MeCN), good activity (81% conversion) was observed at 80 °C in the presence of either one or two equivalents of BnOH over 24 h (runs 5 and 6). Over 1 h, 5·MeCN exhibited low activity ≤14%. Moderate activity (62% conversion) was also observed on conducting the reaction under air over 24 h (run 7). Also in this case, the 1H NMR spectra of selected polymer samples highlighted the presence of benzyl- and –CH2OH terminal groups (see the ESI,† Fig. S11 and S12, for the run performed under N2 and in air, respectively).| Run | Catalyst/solvent | δ-VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n | M n(calc) | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [δ-VL] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture.b From GPC.c Calculated from ([monomer]0/[OH]0) × conv. (%) × monomer molecular weight + molecular weight of BnOH.d Reaction performed in air. | ||||||||
| 1 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 50 | 5310 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 620 620 |
1.10 |
| 2 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 45 | 6090 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.13 |
| 3 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 44 | 5700 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
1.10 |
| 4 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 78 | 18.830 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 630 |
1.60 |
| 5 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 81 | 9550 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
1.50 |
| 6 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 24 | 81 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.37 |
| 7d | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 62 | 9480 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.45 |
| 7 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | |
| 8 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | |
| 9 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | None | — | — | |
| 10 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | 4 | |||
| 11 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 1 | 14 | |||
| 12 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 1 | 6 | |||
rac-Lactide (r-LA)
Attempted polymerization of r-LA was less successful. At 80 °C over 24 h, none of the systems proved to be active (runs 1–4, Table 3). On increasing the temperature to 130 °C, almost complete conversion was achieved with complexes 1–3 and 5 (runs 5–7 and 9, respectively), while moderate activity was observed in the case of 4 (65% conversion, run 8). In the case of systems 1–3 and 5, the molecular mass observed was lower than the calculated values and there was poor control. It is noteworthy that a monodisperse polymer (Mw/Mn = 1.04) with molecular mass observed close to the calculated value was obtained with complex 4. It was hypothesized that in the presence of such catalysts, the rate of transesterification processes was lower than that of the chain growth allowing for the occurrence of a living polymerization. By contrast, 1–3 and 5 are subject to increased transesterification versus the other systems. Interestingly, complete conversion was achieved in air by using complex 5 (run 10). None of the systems was active over 1 h (runs 11–16). The syndiotactic bias was determined by 2D J-resolved 1H NMR spectroscopy, investigating the methine area (ca. 5.2 ppm) of the spectra (see ESI,† Fig. S13–S17).20 The peaks were assigned to the corresponding tetrads according to the literature reports.21 While complexes 1 and 2 afforded almost heterotactic polymers (Pr 0.46 and 0.42, respectively), isotactic materials were isolated in the case of systems 3, 4, and 5 (Chart 2).| Run | Catalyst/solvent |
r-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | P r | M n(corr) , | M n(calc) | M w/Mnc |
|---|---|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [r-LA] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture.b From 2D J-resolved 1H NMR spectroscopy.c From GPC.d Values corrected considering Mark–Houwink factor (0.58) from polystyrene standards in THF.e Calculated from ([monomer]0/[OH]0) × conv. (%) × monomer molecular weight + molecular weight of BnOH.f Reaction performed in air. | |||||||||
| 1 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | ||||
| 2 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | ||||
| 3 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | ||||
| 4 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | None | ||||
| 5 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 95 | 0.46 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
1.68 |
| 6 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 97 | 0.42 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 170 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
1.94 |
| 7 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 95 | 0.23 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 770 770 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
2.09 |
| 8 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 65 | 0.27 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
1.04 |
| 9 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 97 | 0.32 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
1.94 |
| 10f | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 94 | 0.25 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
1.73 |
| 11 | 1 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | — | — | ||
| 12 | 2 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | — | — | ||
| 13 | 3 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | — | — | ||
| 14 | 4 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | None | ||||
| 16 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | 5 | — | — | ||
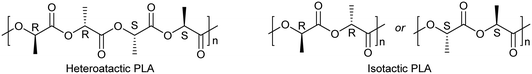 | ||
| Chart 2 Microstructure of heterotactic and isotactic poly-(rac-lactide).21 | ||
ω-pentadecalactone (ω-PDL)
In the case of the larger cyclic ester ω-PDL, only catalyst 5 was successful, achieving moderate activity at 130 °C over 24 h (run 1, Table 4). Low activity (11% conversion) was observed when the reaction was carried out in air (run 3, Table 4). In both cases, molecular weights lower than the expected values and compatible with oligomeric species (12–13 units) were observed. Also in this case, the benzyl end-group was observed by 1H NMR spectroscopy on the polymer sample (see the ESI,† Fig. S18).| Run | ω-PDL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n | M n(calc) |
|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [ω-PDL] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture.b From MALDI-TOF.c Calculated from ([monomer]0/[OH]0) × conv. (%) × monomer molecular weight + molecular weight of BnOH.d Reaction performed in air. | ||||||
| 1 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 53 | 2835 | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
| 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | — | — |
| 3d | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 11 | 2600 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 310 310 |
| 4d | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | None | — | — |
Interestingly, the triplet corresponding to the –CH2OH group was not observed. However, a rigorous analysis of the terminal groups could not be performed due the presence of the signals of residual monomer which could not be separated from the polymer.
Co-polymerization of ε-CL with δ-VL
At 80 °C using 5, high activity (99% conversion) was observed over 24 h affording a polymer of molecular weight >23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (Table 5, run 1). The ratio CL/VL was found to be 1
000 Da (Table 5, run 1). The ratio CL/VL was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as highlighted by 1H NMR spectroscopy (see the ESI,† Fig. S19). Interestingly, a similar outcome was observed in the case of the reaction performed in air at higher temperature (Table 5, entry 3). A broadening of the molecular weight distribution was also detected (2.26 vs. 1.30). The composition of the co-polymer was further investigated by 13C NMR spectroscopy. In fact, diagnostic resonances belonging to CL–VL, CL–CL, VL–VL and VL–CL dyads can be observed in the region between 63.7 and 64.5 (see ESI,† Fig. S20).22 Based in the integrations of these signals, the number-average sequence length was found to be 2.22 and 1.82 for CL and VL, respectively, compatible with a “random-type” co-polymer (randomness degree, R = 0.90, see ESI,† eqn (S1)–(S3)). The thermal properties of the co-polymers were studied by DSC (ESI,† Fig. S21). No transitions were observed between 23 and 100 °C, confirming the irregular co-monomer distribution within the polymer chain leading to lower melting points. This is in agreement with the observations recently disclosed by Hu et al.22a
1, as highlighted by 1H NMR spectroscopy (see the ESI,† Fig. S19). Interestingly, a similar outcome was observed in the case of the reaction performed in air at higher temperature (Table 5, entry 3). A broadening of the molecular weight distribution was also detected (2.26 vs. 1.30). The composition of the co-polymer was further investigated by 13C NMR spectroscopy. In fact, diagnostic resonances belonging to CL–VL, CL–CL, VL–VL and VL–CL dyads can be observed in the region between 63.7 and 64.5 (see ESI,† Fig. S20).22 Based in the integrations of these signals, the number-average sequence length was found to be 2.22 and 1.82 for CL and VL, respectively, compatible with a “random-type” co-polymer (randomness degree, R = 0.90, see ESI,† eqn (S1)–(S3)). The thermal properties of the co-polymers were studied by DSC (ESI,† Fig. S21). No transitions were observed between 23 and 100 °C, confirming the irregular co-monomer distribution within the polymer chain leading to lower melting points. This is in agreement with the observations recently disclosed by Hu et al.22a
| Run | Catalyst/solvent | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ-VL δ-VL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n , | M w/Mnb |
|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [ε-CL] = [δ-VL] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture based on ε-CL.b From GPC.c Values corrected considering Mark–Houwink factor (Mn × 0.56 + Mn) from polystyrene standards in THF.d Reaction performed in air. | |||||||
| 1 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 24 | >99 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.30 |
| 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 1 | 8.5 | — | — | |
| 3d | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | >99 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020 020 |
2.26 | |
Co-polymerization of ε-CL with r-LA
At 130 °C using 5 and a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of ε-CL/r-LA, high activity (99% conversion) was observed over 24 h affording a polymer of molecular weight >5000 Da (Table 6, run 1) which comprised mostly PCL (see the ESI,† Fig. S22). Use of a 1
1 ratio of ε-CL/r-LA, high activity (99% conversion) was observed over 24 h affording a polymer of molecular weight >5000 Da (Table 6, run 1) which comprised mostly PCL (see the ESI,† Fig. S22). Use of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of CL/r-LA afforded a copolymer of molecular weight >20
1 ratio of CL/r-LA afforded a copolymer of molecular weight >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Da (Table 6, entry 3), for which the 1H NMR spectra indicated only 65% of CL was converted (see the ESI,† Fig. S23). A similar outcome was obtained by performing the reaction in air (Table 6, run 5). The co-polymer compositions was determined by analyzing the carbonyl range of the 13C NMR spectrum.23 The LA/CL ratio was found to be 75
500 Da (Table 6, entry 3), for which the 1H NMR spectra indicated only 65% of CL was converted (see the ESI,† Fig. S23). A similar outcome was obtained by performing the reaction in air (Table 6, run 5). The co-polymer compositions was determined by analyzing the carbonyl range of the 13C NMR spectrum.23 The LA/CL ratio was found to be 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 and the average sequence length was 3.04 and 2.42 for CL and LA, respectively (ESI,† Fig. S24 and eqn (S3) and (S4)). Noteworthy, no peaks corresponding to the CL–LA–CL triad at 171.1 ppm was observed. Such signals arise from the transesterification of the cleavage of the lactyl–lactyl bond in the lactidyl unit.23
35 and the average sequence length was 3.04 and 2.42 for CL and LA, respectively (ESI,† Fig. S24 and eqn (S3) and (S4)). Noteworthy, no peaks corresponding to the CL–LA–CL triad at 171.1 ppm was observed. Such signals arise from the transesterification of the cleavage of the lactyl–lactyl bond in the lactidyl unit.23
| Run | Catalyst/solvent | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) r-LA r-LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n , | M w/Mnb |
|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [ε-CL] = [r-LA] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture based on ε-CL.b From GPC.c Mn values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factor (Mn, GPC × 0.56 × %PCL + Mn, GPC × 0.58 × %Pr-LA).d Reaction performed in air. | |||||||
| 1 | 5 MeCN | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | >99 (CL) | 5190 | 2.87 |
| 2 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | 12 (CL) | |||
| 3 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | >99 (LA) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.10 | |
| 4 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | 47(LA) | |||
| 5d | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | >99 (LA) | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150 150 |
2.17 | |
Concerning the thermal properties of the co-polymer, no transitions accountable to CL blocks were detected on the DSC curve of the sample (ESI,† Fig. S25). Nevertheless, rather intense endothermic peaks were observed between 150 and 170 °C, suggesting the existence of crystalline PLA-microdomains.23a
Comparison with other Ti-based catalysts
Since complex 5 proved to be the most performing catalyst for the ROP of cyclic esters, its activity was compared to that of other Ti-based species, namely the novel di-phenolate complex 12 and the nitro-containing titanocalix[4]arene species cone-5,17-bis-tert-butyl-11,23-dinitro-25,27-dipropyloxy-26,28-dioxo-calix[4]arene titanium dichloride 13, previously reported by Toupet et al. (Chart 3).8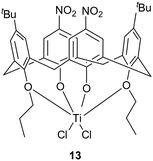 | ||
| Chart 3 Structure of the benchmark Ti-complex 13.8 | ||
These complexes, along with [Ti(Oi-Pr)4] were employed as catalysts in the ROP of different cyclic esters (Table 7). In all cases, 5 outperformed complexes 12 and 13, both in terms of conversion and polymer Mn (cf. for example runs 1–3 and 5–7). Although [Ti(Oi-Pr)4] was shown to promote the ROP of ε-CL under solvent-free conditions,16 it was found to be completely inactive under our reaction conditions (toluene, [monomer] = 0.9 M). Notably, only complex 5 proved to be active in the ROP of the larger ω-pentadecalactone.
| Run | Catalyst | Monomer | M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n(corr) , | M n(calc) | M w/Mnb |
|---|---|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [monomer] = 0.9 M, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture based on ε-CL.b From GPC.c Mn values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factor (0.56 for PCL and 0.58 for PLA).d Calculated from ([M]0/[OH]0 × Conv. × Mw(monomer) + Mw(BnOH)).e Determined by mass spectrometry. | |||||||||
| 1 | 5 | ε-CL | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 24 | >99 | 8100 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.40 |
| 2 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 53 | 6390 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
1.24 | |
| 3 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 24 | 2540 | 6950 | 1.13 | |
| 4 | [Ti(Oi-Pr) 4 ] | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
80 | 24 | None | — | — | — | |
| 5 | 5 | δ-VL | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 | 24 | 81 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.37 |
| 6 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 35 | 7490 | 8860 | 1.15 | |
| 7 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
80 | 24 | 33 | 5700 | 9500 | 1.13 | |
| 8 | [Ti(Oi-Pr) 4 ] | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
80 | 24 | None | — | — | — | |
| 9 | 5 | r-LA | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 97 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
1.94 |
| 10 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 80 | 8870 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.61 | |
| 11 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 77 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 830 830 |
1.20 | |
| 12 | [Ti(Oi-Pr) 4 ] | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | None | — | — | — | |
| 13 | 5 | ω-PDL | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | 53 | 2835e | 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 710 |
nd |
| 14 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | None | — | — | — | |
| 15 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | 12 | — | — | — | |
| 16 | [Ti(Oi-Pr) 4 ] | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | None | — | — | — | |
The polymerization of ε-CL under solvent-free conditions was next considered (Table 8). By performing the reaction in the presence of the monochloride titanocalix[4]arene complex 5, 50% conversion was achieved within 10 minutes at 80 °C (run 1). Under the same reaction conditions, no reaction was observed neither with the bi-phenolate complex 12 nor with the dichloro-species 13 (runs 2 and 3). Notably, 29% monomer conversion was obtained by using [Ti(Oi-Pr)4] (run 4). In order to explain the inactivity of 12 and 13, the inefficient activation of the dichloro-based species in the absence of the solvent was postulated. Differently, the presence of the labile acetonitrile ligand in complex 5 would allow for the prompt formation of the catalytically active species, even under solvent-free conditions. Moreover, higher performance of catalyst 5 over [Ti(Oi-Pr)4], both in terms of conversion and polymer Mn, highlighted the beneficial effect of the calix[4]arene ligand on the ROP process.
| Run | Catalyst | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (min) | Conv.a (%) | M n(corr) , | M n(calc) | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| Reaction conditions: no solvent, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture based on ε-CL.b From GPC.c Mn values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factor (0.56).d Calculated from ([M]0/[OH]0 × Conv. × Mw(monomer) + Mw(BnOH)). | ||||||||
| 1 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 10 | 50 | 8140 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.14 |
| 2 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 10 | None | — | — | — |
| 3 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
100 | 10 | None | — | — | — |
| 4 | [Ti(O i Pr) 4 ] | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
100 | 10 | 30 | 3270 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 530 530 |
1.26 |
Moreover, the performances of complexes 5, 12 and 13 were compared in the ROP of ε-CL under aerobic conditions (Table 9). At 130 °C in the presence of 5 and 12, good and moderate conversions were obtained within 1 h, respectively (runs 1 and 2). Interestingly, full monomer conversion was observed by performing the reaction with complex 13 (run 3). Nevertheless, higher polymer Mn were achieved in the presence of 5 (19 kDa vs. 11 kDa for 5 and 13, respectively). By carrying out the reaction for 24 h, complete monomer conversion was observed with both 5 and 13 (runs 4 and 5). The Mn of the samples were found to be in a rather narrow range (spanning from 4600 to 5400) albeit better control was exhibited by the monochloride titanocalix[4]arene complex.
| Run | Catalyst | ε-CL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n(corr) , | M n(calc) | M w/Mnb |
|---|---|---|---|---|---|---|---|---|
| Reaction conditions: toluene 5 mL, [ε-CL] = 0.9 M, air.a Determined by 1H NMR spectroscopy on crude reaction mixture based on ε-CL.b From GPC.c Mn values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factor (0.56).d Calculated from ([M]0/[OH]0 × Conv. × Mw(monomer) + Mw(BnOH)). | ||||||||
| 1 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 85 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
1.82 |
| 2 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | 40 | 4810 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.13 |
| 3 | 13 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 1 | >99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 550 550 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
1.69 |
| 4 | 5 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 4580 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
2.08 |
| 5 | 12 | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
130 | 24 | >99 | 5460 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 360 |
3.12 |
Finally, given the interest in metal-free ROP catalysts,20 the parent 1,3-dialkoxycalixarenes L(OH)2(OR)2 were also screened under the conditions herein. These metal-free species proved to be inactive.
Kinetics
From a kinetic study of the ROP of ε-CL using 1–5 and 12–13, it was observed that the polymerization rate exhibited first order dependence on the ε-CL concentration (Fig. 9, left), and the conversion of monomer achieved over 60 min was >75% (100% for 5 and 13). Fig. 10 indicates that the rate order is 13 > 5 > 12 > 3 > 2 > 1 > 4. This suggested that a labile acetonitrile ligand (for 5) or electron-withdrawing groups on the calixarene backbone (for 13) are beneficial. The induction period of ca. 10 min observed for complexes 1–4 could be ascribed to the longer time required for the formation of the catalytically active species (allegedly a Ti-bis(benzyloxide) compound) from a dichloro-precursor.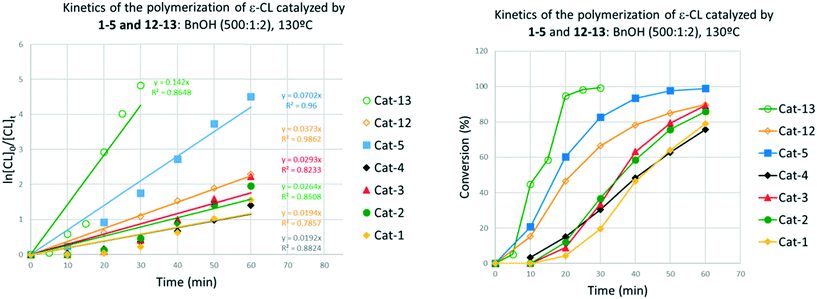 | ||
| Fig. 10 Left: Plot of ln[CL]0/[CL]t vs. time using complexes 1–5; right: relationship between conversion and time for the polymerization of ε-CL. | ||
A kinetic study of the ROP of δ-VL using 1–5 and 12–13, again revealed first order dependence on the monomer concentration (Fig. 11, left). Conversions over 60 min were lower than observed for ε-CL with all ≤60% (ca. 70% for 13). The activity trend in this case revealed that 13 was the most active and then 4 = 5 >3 > 1 > 2.
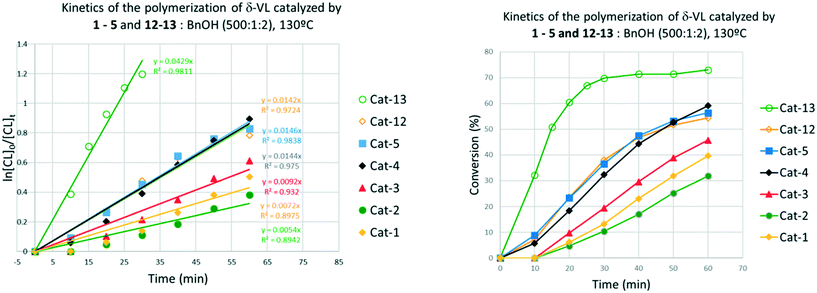 | ||
| Fig. 11 Left: Plot of ln[VL]0/[VL]t vs. time using complexes 1–5 and 12–13; right: relationship between conversion and time for the polymerization of δ-VL. | ||
The dependence of the Mn and molecular weight distribution on the monomer conversion in the reactions catalyzed by 5/BnOH was also investigated (Fig. 12). For the ROP of ε-CL, the polymer Mn was shown to increase linearly with the conversion, while the Mw/Mn was found to be rather constant at ca. 1.14 throughout the reaction (Fig. 12, left). A similar outcome was also observed in the reaction involving δ-VL (Fig. 12, right).
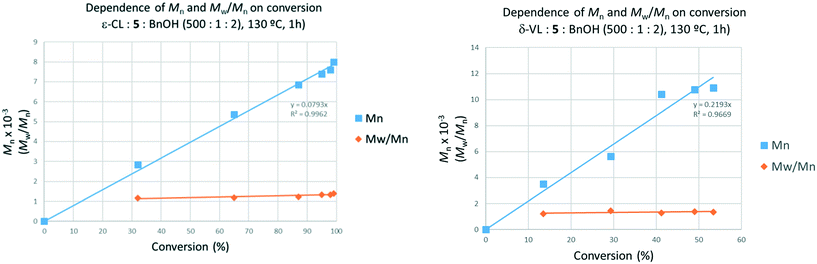 | ||
| Fig. 12 Dependence of Mn and polydispersity on conversion in the ROP of cyclic esters catalyzed by 5. Left: ε-CL; right: δ-VL. | ||
Finally, the kinetic behaviour of complex 5 in the ROP of ε-CL was compared to that of catalysts 12 and 13 both under inert and aerobic conditions (Fig. 13). In the presence of the calix[4]arene-based complexes 13 and 5, the reaction rate of the polymerization conducted in air was shown to be similar to that of the runs preformed under inert atmosphere. On the contrary, a detrimental effect was observed when the bi-phenolate complex 12 was employed in air compared to the reaction carried out under inert atmosphere.
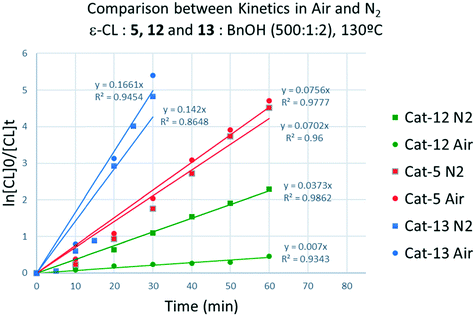 | ||
| Fig. 13 Comparison between the kinetic behaviour of catalysts 5, 12 and 13 in the ROP of ε-CL (inert and aerobic conditions). | ||
Silica immobilization of complex 5
Complex 5 was immobilized on pre-treated silica by refluxing in toluene affording structure Si-5; the silica was heated at 200 °C under dynamic vacuum for 2 h. X-ray photoelectron spectroscopy (XPS) analysis of the silica surface was performed (Fig. 14). The region area ratios of the C-1s to Ti-2p indicated a Ti content of 1.89% with respect to C. This was slightly lower than the calculated value (2.17%) and allegedly due to adventitious carbon bound to the surface. However, this evidence suggested that no decomposition occurred on binding the complex to the surface. Moreover, the Ti-2p peak was consistent with the presence of only one titanium species. | ||
| Fig. 14 Left: Full X-ray photoelectron survey spectrum of Si-5. Right: Titanium Ti-2p energy window 475–440 eV. | ||
Polymerization of cyclic esters catalyzed by Si-5
The supported version of complex 5 was tested in the ROP of cyclic esters (Table 10). For each run, 50 mg of catalyst were employed. Based on the Ti content found by XPS analysis, the monomer/Ti ratio was found to be 400. Si-5 proved to be very active in the reaction involving ε-CL. Complete conversion was observed after 24 h at 130 °C both in the presence and in the absence of 1 equiv. of alcohol (runs 1 and 2). Interestingly, higher molecular mass and better control was observed in the experiment conducted in the absence of the co-catalyst. While high conversions were achieved with the homogeneous complex 5 after 1 h (85%, cf.Table 1, entry 13), lower activity was observed in the case of its supported version (runs 3 and 4). The catalyst proved to be active also in the ROP of δ-VL and rac-LA albeit with moderate activity (runs 5 and 6). While low control was observed for the former, narrow polydispersity was achieved for the latter (2.48 vs. 1.15, respectively). Unlike its homogeneous analogues, system Si-5 was not active in the ROP of the larger monomer ω-pentadecalactone (run 7).| Run | Monomer | Monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BnOH BnOH |
T (°C) | t (h) | Conv.a (%) | M n , | M w/Mnb |
|---|---|---|---|---|---|---|---|
| Reaction conditions: ε-CL 4.5 mmol (400 equiv.), supported catalyst 50 mg, toluene 5 mL, 130 °C, 24 h, N2.a Determined by 1H NMR spectroscopy on crude reaction mixture.b From GPC.c Mn values were determined by GPC in THF vs. PS standards and were corrected with a Mark–Houwink factor (Mn,GPC × 0.56 for ε-CL, 0.58 for r-LA). | |||||||
| 1 | ε-Caprolactone | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | >99 | 9750 | 2.09 |
| 2 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 24 | >99 | 4840 | 2.77 | |
| 3 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 1 | 35 | — | — | |
| 4 | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
130 | 1 | 25 | — | — | |
| 5 | δ-Valerolactone | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | 61 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 260 260 |
2.48 |
| 6 | rac-Lactide | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | 78 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 340 |
1.15 |
| 7 | ω-pentadecalactone | 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
130 | 24 | 2 | — | — |
In conclusion, we have isolated and structurally characterized a number of titanocalix[4]arene complexes which are capable of the ROP of cyclic esters. In particular, we find that a mono-methoxy complex [Ti(NCMe)Cl(L(O)3(OR))]·MeCN exhibits superior behavior (Kobs 1.3 × 10−3 s−1 for CL; 2.5 × 10−4 s−1 for VL) compared with the dialkoxy-type complexes [TiCl2L(O)2(OR)2] (R = Me, n-Pr and n-pentyl) (Kobs 6.4 × 10−4 s−1 for CL; 1.8 × 10−4 s−1 for VL – complex 3), which is thought to be due to the lability of the metal-bound acetonitrile under ROP conditions. Importantly, the ROP of both ε-CL and δ-VL can be conducted under air without loss of activity. It also proved possible to ROP the larger cyclic ester ω-PDL with moderate conversation (ca. 50%). Complex 5 was able to co-polymerize ε-CL and δ-VL with the same activity, while higher poly-lactide content was observed in the ε-CL/rac-LA co-polymerization. We have also investigated the air and moisture stability of some of these titanocalix[4]arenes, and have isolated and structurally characterized bridged OH−/Cl− species.
Experimental
General
All manipulations were carried out under an atmosphere of dry nitrogen using conventional Schlenk and cannula techniques or in a conventional nitrogen-filled glove box. Hexane and toluene were refluxed over sodium. Acetonitrile was refluxed over calcium hydride. All solvents were distilled and degassed prior to use. IR spectra (nujol mulls, KBr windows) were recorded on a Nicolet Avatar 360 FT IR spectrometer; 1H NMR spectra were recorded at room temperature on a Varian VXR 400 S spectrometer at 400 MHz or a Gemini 300 NMR spectrometer or a Bruker Advance DPX-300 spectrometer at 300 MHz. The 1H NMR spectra were calibrated against the residual protio impurity of the deuterated solvent. Elemental analyses were performed by the elemental analysis service at the London Metropolitan University and in the Department of Chemistry, the University of Hull. The precursor [TiCl4(THF)2] was prepared by the literature method.24 The pro-ligands L(OH)2(OR)2 were prepared as described previously.25 Complex 13 was synthesized according to the literature procedures.8 All other chemicals were purchased from Sigma Aldrich or TCI UK/China.Synthesis of [TiCl2L(O)2(OR)2] (R = Me (1), n-Pr (2), n-pentyl (3)) and {[TiL(O)3(OR)]2(μ-Cl)2} (R = n-decyl (4))
The method employed here was a modification of that reported by Taoufik and Bonnamour,5 except for R = n-decyl, where [TiCl4] was employed. A representative example is [TiCl4(THF)2] (0.50 g, 1.50 mmol) was added to L1H2Me2 (1.00 g, 1.48 mmol) in toluene and stirred at 60 °C for 12 h. On cooling, volatiles were removed, and extraction into MeCN (40 mL) afforded on standing for 24 h at 0 °C the complex as the acetonitrile solvate.For 1 (0.62 g, 53%): C46H58Cl2O4Ti·(C2H3N) requires C 69.06, H 7.37, N 1.68% found C 68.91, H 7.52, N 1.41%. IR: 2246w, 1596w, 1414w, 1363m, 1306m, 1261s, 1207s, 1159 m, 1088s, 1019s, 994s, 948s, 939m, 918w, 869m, 796s, 747w, 706w, 680w. 1H NMR (C6D6, 298 K) δ: 7.14 (s, 4H, arylH), 6.81 (s, 4H, arylH), 4.61 (d, 4H, J = 13 Hz, endo-CH2), 4.18 (s, 6H, OCH3) 3.18 (d, 4H, J = 13 Hz, exo-CH2), 1.36 (s, 18H, C(CH3)3), 0.67 (s, 18H, C(CH3)3), 0.29 (s, 3H, CH3CN).
For 2, yield 0.71 g, 56%. C50H66Cl2O4Ti·2(C2H3N) requires C 69.60, H 7.79, 3.01 N% found C 68.89, H 8.32, N 2.83%. IR: 2245w, 1595w, 1413w, 1377m, 1309m, 1260s, 1200s, 1158w, 1117m, 1086s, 1024s, 960m, 937w, 874m, 858m, 795s, 744w, 702w, 677w. 1H NMR (CDCl3, 298 K) δ: 7.08 (s, 4H, arylH), 6.99 (s, 4H, arylH), 4.91 (m, 4H, OCH2), 4.54 (d, 4H, J = 13 Hz, endo-CH2), 3.36 (d, 4H, J = 13 Hz, exo-CH2), 2.04 (m, 4H, OCH2CH2), 1.9 (s, 6H, CH3CN), 1.33 (s, 18H, C(CH3)3), 1.15 (s, 18H, C(CH3)3), 0.85 (t, 6H, J = 7.6 Hz,CH3CH2).
For 3: yield 0.78 g, 57%. C54H74Cl2O4Ti·1.5(C2H3N) requires C 70.76, H 8.11, N 2.17% found C 70.47, H 8.39, N 2.30%. IR: 1601w, 1413w, 1377m, 1306m, 1260s, 1209m, 1158w, 1087s, 1020s, 937m, 873m, 863m, 795s, 746w, 677w. 1H NMR (CDCl3, 298 K) δ: 1H NMR (CDCl3, 298 K) δ: 7.09 (s, 4H, arylH), 7.00 (s, 4H, arylH), 4.91 (m, 4H, OCH2), 4.54 (d, 4H, J = 13 Hz, endo-CH2), 3.36 (d, 4H, J = 13 Hz, exo-CH2), 2.02 (m, 4H, OCH2CH2), 1.74 (s, 4.5H, CH3CN), 1.38 (s, 18H, C(CH3)3 overlapping with m, 4H, OCH2CH2CH2CH3), 1.16 (s, 18H, C(CH3)3 overlapping with m, 4H, OCH2CH2CH2CH3), 0.84 (t, 6H, J = 6.4 Hz,CH3CH2).
For 4: yield 0.88 g, 66%. C108H146Cl2O8Ti2(C2H3N) requires C 74.22, H 8.44, N 0.80%. Found C 74.70, H 8.92, N 0.67%. IR: 2249w, 1601w, 1580w, 1415w, 1393m, 1377s, 1363s, 1302m, 1256m, 1205s, 1172w, 1122m, 1113m, 1094m, 1022w, 974w, 944s, 925m, 876s, 864m, 831m, 798s, 761m, 723w, 678w. 1H NMR (CDCl3) δ: 7.08 (m, 4H, arylH), 7.03 (s, 2H, arylH), 7.02 (s, 2H, arylH), 4.91 (d, 2H, J = 12 Hz, endo-CH2), 4.23 (d, 4H, J = 12 Hz, endo-CH2), 4.38 (m, 4H, OCH2), 3.20 (m, 4H, exo-CH2), 2.05 (s, 1.5H, 0.5MeCN), 1.85 (bm, 4H, CH2), 1.33 (s, 18H C(CH3)3), 1.27–1.16 (overlapping m, 28H, CH2), 0.74 (s, 9H C(CH3)3), 0.88 (m, 6H, CH3), 0.66 (s, 9H C(CH3)3), 0.46 (s, 3H, MeCN).
Synthesis of [Ti(NCMe)Cl(LMe)]·MeCN·(5.MeCN)
As for 1, but the system was refluxed for 60 h, and then following removal of volatiles, the residue was stirred in MeCN (40 mL) for 24 h. Filtration, concentrated to half volume, and prolonged standing at 0 °C afforded orange/red crystals of 7·MeCN. Yield 0.82 g, 74%. C49H61N2ClO4Ti requires C 71.30, H 7.45, N 3.39%. Found C 71.00, H 7.68, N 3.65%. IR: 2306w, 2286w, 2245w, 1599w, 1413m, 1364s, 1307s, 1276m, 1256s, 1203s, 1167m, 1118m, 1095m, 1005s, 946m, 934s, 917m, 901w, 874s, 845m, 824m, 811w, 799s, 780m, 756m, 722m, 680m. MS (positive nanoelectrospray): 754.3 (MH+ –OMe). 1H NMR (C6D6) δ: 7.08 (m, 4H, arylH), 7.03 (s, 2H, arylH), 7.02 (s, 2H, arylH), 4.77 (d, 2H, J = 12 Hz, endo-CH2), 4.37 (d, 2H, J = 12 Hz, endo-CH2), 4.23 (s, 3H, OCH3), 3.34 (overlapping d, J = 12 Hz, 4H, exo-CH2), 1.85 (s, 3H, MeCN), 1.25 (s, 18H C(CH3)3), 1.19 (s, 9H C(CH3)3), 1.16 (s, 9H C(CH3)3).Synthesis of {[TiL(O)3(On-Pr)]2(μ-Cl)(μ-OH)}·7MeCN (6·7MeCN)
As for 5, but using L(O)2(n-PrO)2 (0.50 g, 0.68 mmol) and TiCl4·(THF)2 (0.23 g, 0.68 mmol) affording 6 as orange/red prisms. Yield: 0.53 g, 40%. C99H125N2ClO9Ti2 (sample dried in vacuo for 12 h, –5MeCN) requires C 73.27, H 7.84, N 1.74%. Found C 74.05, H 8.78, N 1.33%. IR: 3730w, 3698w, 3589w, 3335w, 3190w, 2730w, 1750w, 1645w, 1597w, 1410m, 1380m, 1305m, 1257s, 1200s, 1177w, 1093s, 1017s, 940m, 925m, 866w, 800s, 760w, 720w, 675w, 660w. 1H NMR (CDCl3) δ: 7.06–6.97 (m, 16H, ArylH), 4.80 (d, 2H, J = 13, endo-CH2), 4.54–4.44 (m, 8H, endo-CH2 overlapped with OCH2CH2CH3), 3.30 (m, 8H, overlapping exo-CH2), 1.92 (m, 4H, OCH2CH2CH3), 1.64 (s, 6H, 2CH3CN), 1.26–0.97 (m, 54H, C(CH3)3 overlapping with OCH2CH2CH3), OH signal not detected.Synthesis of {[TiL(O)3(On-pentyl)]2(μ-Cl)(μ-OH)}·7.5MeCN (7·7.5MeCN)
As for 5, but using L(O)2(n-pentylO)2 (1.00 g, 1.27 mmol) and TiCl4 (1.29 mL, 1 M in CH2Cl2, 1.29 mmol) affording 7 as orange/red prisms. Yield: 0.89 g, 74%. The sample was dried in vacuo for 12 h (−7.5 CH3CN). C98H127ClO9Ti2·requires C 74.49, H 8.09. Found C 73.76, H 8.57. IR: 2955w, 2920s, 2850m, 2724w, 1460s, 1378s, 1300w, 1260m, 1210w, 1155w, 1090m, 1020m, 970w, 940w, 920w, 890w, 875w, 800m, 720m, 560w. 1H NMR (CDCl3) δ: 7.00–6.76 (m, 16 H, ArylH), 5.10 (t, 4 H, J = 10 Hz, OCH2CH2CH2CH2CH3), 4.97 (d, 8 H, J = 13 Hz, endo-CH2), 3.27 (d, 8 H, J = 13, exo-CH2), 2.80 (m, 4 H, OCH2CH2CH2CH2CH3), 1.33 (s, 36 H, (C(CH3)3)), 0.90 (m, 8 H, OCH2CH2CH2CH2CH3), 0.83 (s, 36 H, (C(CH3)3)), 0.60 (6 H, t, J = 9, OCH2CH2CH2CH2CH3). OH signal not detected.Synthesis of [Ti(NCMe)(μ3-O)L(O)4TiCl(O(CH2)4Cl)]2–2[TiCl(NCMe)(L(O)3(On-Pr))]·11MeCN (8·11MeCN)
L(O)2(n-PrO)2 (1.00 g, 1.36 mmol) and TiCl4(THF)2 (0.46 g, 1.38 mmol) in toluene (30 mL) was refluxed for 60 h. On cooling, the volatiles were removed in vacuo, and the residue was extracted into MeCN (30 mL). On standing at 0 °C, large red prisms formed. Yield 0.75 g, 56%. C198H250N4Cl6O20Ti6 (sample dried in vacuo for 12 h) requires C 67.82, H 7.19, N 1.60%. Found C 68.81, H 8.32, N 2.83%.26 IR: 3650w, 3184w, 2728w, 1771w, 1603w, 1580w, 1417m, 1392s, 1314s, 1279s, 1262s, 1209s, 1115s, 1096s, 1029s, 988s, 939m, 921s, 874s, 862s, 823s, 796s, 754m, 679m. 1H NMR (CDCl3) δ: 7.19–6.95 (m, 16H arylH dimer overlapped with 8H arylH mono-Cl-complex), 4.81 (d, 4H, J = 13 Hz, endo-CH2 dimer), 4.56 (d, 4H, J = 13 Hz, endo-CH2 dimer), 4.45 (d, 2H, endo-CH2 mono-Cl complex) partially overlapped with 4.33 (m, 2H, OCH2CH2CH3 mono-Cl complex), 3.70 (m, 4H, ClCH2CH2CH2CH2O–), 3.58 (m, 4H, ClCH2CH2CH2CH2O–), 3.29 (m, 4H exo-CH2 dimer overlapped with 2H exo-CH2 mono-Cl complex), 3.05 (d, 2H, 13 Hz, exo-CH2 mono-Cl complex), 2.24 (m, 4H, ClCH2CH2CH2CH2O–), 1.98 (m, 4H, ClCH2CH2CH2CH2O–) partially overlapped with 1.86 (m, 2H, OCH2CH2CH3 mono-Cl complex), 1.56–1.23 (m, 72H C(CH3)3 dimer overlapped with 36H, C(CH3)3 mono-Cl complex), 0.99 (m, 3H, OCH2CH2CH3 mono-Cl complex).Synthesis of {[TiL(n-pentyl)]2(μ-Cl)(μ-OH)}·9MeCN (9·9MeCN)
Complex 3 (1.00 g, 1.10 mmol) in toluene (30 mL) was stirred under air for 1 h, the volatiles were removed in vacuo and the residue was extracted into warm (brief heating with a hot-gun) acetonitrile (30 mL). The solution was left to stand (3 to 4 days) affording on standing orange/red crystals of 9. Yield: 0.73 g, 68%. The sample was dried in vacuo for 12 h (−9 MeCN). C98H127ClO9Ti2 requires C 74.49, H 8.10. Found C 73.29, H 8.48. IR: 298w, 2919s, 2852m, 2726w, 1463s, 1380s, 1298w, 1258m, 1207w, 1157w, 1088m, 1015m, 967w, 943w, 925w, 893w, 878w, 803m, 725m, 561w. 1H NMR (CDCl3) δ: 7.10–6.80 (m, 16 H, ArylH), 5.13 (t, 4 H, J = 9 Hz, OCH2CH2CH2CH2CH3), 4.94 (d, 8 H, J = 12 Hz, endo-CH2), 3.22 (d, 8 H, J = 12, exo-CH2), 2.74 (m, 4 H, OCH2CH2CH2CH2CH3), 1.36 (s, 36 H, (C(CH3)3)), 0.87 (m, 8 H, OCH2CH2CH2CH2CH3), 0.80 (s, 36 H, (C(CH3)3)), 0.62 (6 H, t, J = 9, OCH2CH2CH2CH2CH3). OH signal not detected.{[TiL(n-decyl)]2(μ-Cl)(μ-OH)}·8.5MeCN (10·8.5MeCN)
As for 9, but using L(n-decyl)2 (1.00 g, 1.08 mmol) and TiCl4 (0.12 mL, 1.09 mmol) affording 10 as orange blocks. Yield: 0.54 g, 48%. C108H147ClO9Ti2·8.5MeCN. Sample dried in vacuo for 12 h (−7.5 MeCN) requires C 74.87, H 8.74, N 0.79%. Found C 74.70, H 8.92, N 0.67%. IR: 3728w, 3702w, 3596w, 3332w,3187w, 2727w, 1748w, 1647w, 1601w, 1416m, 1377m, 1301m, 1260s, 1205s, 1173w, 1095s, 1020s, 938m, 921m, 861w, 796s, 757w, 723w, 678w, 661w. 1H NMR (CDCl3) δ: 7.05 (m, 4H, ArylH), 6.99 (s, 4H, ArylH), 4.80 (d, 2H, J = 12, endo-CH2), 4.50–4.10 (m, 4H, endo-CH2 overlapped with OCH2CH2–), 4.23 (overlapping d, 6H, exo-CH2), 1.87 (s, 3H, MeCN), 1.25–1.16 (m, 52H, C(CH3)3 overlapping with CH2-decyl), 0.89 (m, 6H, CH3). OH signal not detected.[Ti2(μ-OH)(μ-Cl)(Ln-pentyl)]2[L(n-pentyl)2]·2.85(C2H3N)·0.43(H2O) (11·2.85(C2H3N)·0.43(H2O))
To 3 (1.00 g, 1.10 mmol) in toluene (30 mL) was added H2O (0.01 mL, 0.56 mmol) and the system was refluxed for 12 h. Volatiles were removed in vacuo, and the residue was extracted into warm MeCN (30 mL). On prolonged standing at ambient temperature, orange plate-like crystals of 11 formed. Yield: 0.79 g, 69%. The sample was dried under reduced pressure for 12 h (−2.85 CH3CN, −0.43 H2O). C112.5H165ClO11Ti2 requires C 74.05, H 9.11%. Found C 73.27, H 8.73, N <0.1%. IR: 3647w, 3344w, 3228w, 2726w, 2671w, 2379w, 2289w, 2249w, 1748w, 1600w, 1414s, 1393s, 1377s, 1363s, 1302m, 1281s, 1260s, 1207s, 1115m, 1095m, 1039m, 1019m, 974m, 938m, 921m, 874s, 827s, 810s, 798s, 768m, 758m, 723m, 679m. 1H NMR (C6D6) δ = 10.28 (s, 2H, ArylOH), 7.17 (m, 2H, ArylH), 7.02 (m, 2H, ArylH), 6.94 (m, 8H, ArylH) 6.74 (m, 8H, ArylH), 5.33 (m, 4H, OCH2CH2–), 4.93 (d, 4H J = 12 Hz, endo-CH2), 4.82 (d, 4H J = 12 Hz, endo-CH2) 4.47 (d, 4H J = 12 Hz, endo-CH2) 4.32 (m, 6H, exo-CH2 overlapped with OCH2CH2CH2CH2CH3), 3.22 (m, 8H, exo-CH2overlapped with OCH2CH2CH2CH2CH3), 2.05 (s, 3H, MeCH), 3.19–3.16 (m, 16 H, OCH2CH2CH2CH2CH3), 1.32 (s, 18 H, (C(CH3)3)), 1.30 (s, 18H, C(CH3)3), 1.06 (s, 18 H, C(CH3)3), 1.05 (s, 18 H, C(CH3)3), 1.01 (8 H, m, OCH2CH2CH2CH2CH3), 0.45 (m, 12 H, –OCH2CH2CH2CH2CH3). OH signal not detected.[Ti(Cl)2(6,6′-(ethane-1,1-diyl)bis(2,4-di-tert-butylphenolate))] (12)
To a suspension of 2,2′-CH3CH[4,6-(t-Bu)2C6H2O]2 (2.0 g, 5.9 mmol) in dry hexane (30 mL), [TiCl4] (0.65 mL, 5.9 mmol, 1 equiv.) was added dropwise. The mixture was stirred at room temperature for 16 h affording a deep-red solid which was collected by filtration and dried under reduced pressure at room temperature for 4 h. Yield 2.0 g (3.6 mmol, 61%). Requires C 64.87, H 7.98%. Found C 64.72, H 8.25%. IR: 1220 cm−1 m, 1200m, 1105w, 922m, 628w, 475m, 437w, 410m, 365w. 1H NMR (C6D6) δ 7.53 (d, J = 2.3 Hz, 2H, ArH), 7.23 (d, J = 2.3 Hz, 2H, ArH), 4.71 (q, J = 6.7 Hz, 1H, CH(CH3)), 1.37–1.61 (s, 18H, C(CH3)3 overlapping with d, 3H, CH3), 1.23 (s, 18H, C(CH3)3).Preparation of silica supported complex (Si-5)
SiO2 pretreatment: silica gel (60 Å, mesh 35–70 μm, 5.0 g) was heated at 200 °C under vacuum for 2 h.Immobilization of the complex: a solution of 5 in toluene (0.07 g in 10 mL) was added to a suspension of the pre-treated silica in toluene (500 mg in 10 mL). The mixture was stirred at reflux for 16 h and then allowed to cool down to room temperature. The mixture was filtered, and the solid residue was washed with warm toluene (2 × 10 mL) and dried under reduced pressure at room temperature for 2 h affording a yellow fine powder (0.46 g).
Ring open polymerization (ROP) procedures
ε-Caprolactone (ε-CL): typical polymerization procedures are as follows. A toluene solution of 1 (0.010 mmol, in 1.0 mL toluene) was added into a Schlenk tube in the glove-box at room temperature. The solution was stirred for 2 min, and then ε-caprolactone (2.5 mmol) along with 1.5 mL toluene was added to the solution. The reaction mixture was then placed into an oil bath pre-heated to the required temperature, and the solution was stirred for the prescribed time. The polymerization mixture was then quenched by addition of an excess of glacial acetic acid (0.2 mL) into the solution, and the resultant solution was then poured into methanol (200 mL). The resultant polymer was then collected on filter paper and was dried in vacuo.Kinetic studies
The polymerizations were carried out at 130 °C under N2 atmosphere. The molar ratio of monomer to catalyst to initiator was fixed at 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. At appropriate time intervals (10 minutes), 0.5 μL aliquots were removed and quenched with methanol. The solvent was removed in vacuo and the percent conversion was determined by 1H NMR spectroscopy in CDCl3.
2. At appropriate time intervals (10 minutes), 0.5 μL aliquots were removed and quenched with methanol. The solvent was removed in vacuo and the percent conversion was determined by 1H NMR spectroscopy in CDCl3.
X-ray photoelectron spectroscopy
A small amount (∼1 mg) of powdered sample was pressed onto adhesive carbon tape. High resolution XPS core level measurements were performed with a Specs NAP-XPS (operating under UHV conditions), equipped with a SPECS Phoibos 150 NAP hemispherical analyser. XPS experiments were carried out using a high intensity monochromated Al-Kα source (1486.6 eV) operated at 14.5 kV and 25 mA. Detailed spectra were recorded using a pass energy of 30 eV. The energy scale of the spectrometer was calibrated to the C-1s at 284.8 eV.Crystal structure determinations
Diffraction data for all crystal structures were collected at low temperature on modern diffractometers equipped with hybrid pixel array detectors and rotating anode X-ray sources.27 Full details are given in Table 11, in the ESI,† and in the deposited cif files. Data were corrected for absorption and Lp effects.27 The structures were solved using a dual-space, charge-flipping algorithm and refined on F2.28,29 In common with many calixarene crystal structures there are were often some difficulties to overcome during the refinement. Some tBu groups needed to be modelled with the methyl groups or the whole moiety split over two sets of positions. MeCNs of crystallization were often disordered and needed to be modelled with split positions, or as diffuse electron density via the Platon Squeeze procedure.30 The number of MeCN molecules of crystallization should only be taken as approximate. Where disorder has been modelled, restraints were applied to anisotropic displacement parameters and the geometry of the affected and immediately adjacent atoms. Hydrogen atoms were placed in geometrically determined positions, except for those on hetero atoms in structures with good data, where coordinates were refined. Exact details of the disorder modelling, etc. are given in the ESI† for each individual structure.| Compound | 2·2MeCN | 4·6MeCN | 5·MeCN | 6·7MeCN | 7·7.5MeCN | 8·11MeCN | 9·9MeCN | 10·8.5MeCN | 11·2.85MeCN·0.43(H2O) | 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Formula | C50H66Cl2O4Ti·2(C2H3N) | C108H146Cl2O8Ti2·6(C2H3N) | C47H58ClNO4Ti·(C2H3N) | C94H119ClO9Ti2·7(C2H3N) | C98H127ClO9Ti2·7.5(C2H3N) | C198H250Cl6N4O20Ti6·11(C2H3N) | C98H127ClO9Ti2·9(C2H3N) | C108H147ClO9Ti2·8.5(C2H3N) | C125H165ClO11Ti2·2.85(C2H3N)·0.43H2O | C30H44Cl2O2Ti |
| Formula weight | 931.93 | 1985.26 | 825.34 | 1811.51 | 1888.14 | 3957.70 | 1949.72 | 2069.45 | 2099.63 | 555.45 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Triclinic | Monoclinic | Triclinic | Monoclinic | Triclinic | Triclinic | Monoclinic |
| Space group | C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21 |
| a (Å) | 43.9803(4) | 12.5697(2) | 12.14575(13) | 13.9465(2) | 26.6007(4) | 17.6510(2) | 26.5998(4) | 17.4371(7) | 16.3253(4) | 11.0509(2) |
| b (Å) | 12.41683(17) | 18.1183(4) | 18.0271(2) | 17.4744(3) | 13.18235(14) | 18.2749(2) | 13.18144(15) | 17.7765(4) | 17.3213(3) | 9.69430(18) |
| c (Å) | 19.4608(2) | 27.5282(3) | 21.0909(2) | 23.5392(2) | 32.7812(5) | 19.8507(2) | 32.7719(4) | 21.1345(4) | 23.5069(3) | 14.8668(3) |
| α (°) | 90 | 104.5590(15) | 95.2564(9) | 85.6028(11) | 90 | 67.4531(11) | 90 | 100.630(2) | 102.481(2) | |
| β (°) | 94.9954(10) | 98.7810(13) | 105.3553(9) | 74.2347(12) | 104.6220(17) | 68.6833(11) | 104.6288(14) | 93.526(3) | 91.495(2) | 108.131(2) |
| γ (°) | 90 | 102.0930(18) | 90.2869(9) | 74.5316(14) | 90 | 73.3966(12) | 90 | 104.426(3) | 101.593(2) | |
| V (Å3) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 587.1(2) 587.1(2) |
5792.03(18) | 4432.27(8) | 5320.83(14) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 122.7(3) 122.7(3) |
5428.94(12) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 118.1(3) 118.1(3) |
6195.8(3) | 6340.9(2) | 1513.61(5) |
| Z | 8 | 2 | 4 | 2 | 4 | 1 | 4 | 2 | 2 | 2 |
| Temperature (K) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) | 100(2) |
| Wavelength (Å) | 1.54178 | 1.54178 | 1.54178 | 1.54178 | 0.71073 | 0.71075 | 0.71073 | 1.54178 | 0.71073 | 0.71073 |
| Calculated density (g cm−3) | 1.169 | 1.138 | 1.237 | 1.131 | 1.128 | 1.211 | 1.165 | 1.109 | 1.100 | 1.219 |
| Absorption coefficient (mm−1) | 2.63 | 2.03 | 2.54 | 1.95 | 0.22 | 0.35 | 0.23 | 1.73 | 0.20 | 0.48 |
| Transmission factors (min./max.) | 0.434, 1.000 | 0.788, 1.000 | 0.929, 1.000 | 0.822, 1.000 | 0.574, 1.000 | 0.832, 1.000 | 0.857, 1.000 | 0.720, 1.000 | 0.624, 1.000 | 0.432, 1.000 |
| Crystal size (mm3) | 0.20 × 0.15 × 0.03 | 0.21 × 0.13 × 0.02 | 0.27 × 0.24 × 0.13 | 0.13 × 0.05 × 0.04 | 0.90 × 0.13 × 0.02 | 0.22 × 0.14 × 0.08 | 0.18 × 0.09 × 0.05 | 0.15 × 0.05 × 0.03 | 0.18 × 0.12 × 0.02 | 0.18 × 0.10 × 0.04 |
| θ(max) (°) | 68.2 | 68.3 | 68.3 | 70.1 | 27.5 | 28.7 | 27.5 | 66.6 | 27.5 | 27.5 |
| Reflections measured | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 433 433 |
105![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 296 296 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 483 483 |
167![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 629 629 |
271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 747 747 |
133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
103![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 194 194 |
152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 246 246 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809 809 |
| Unique reflections | 9646 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 053 053 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744 744 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 826 826 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 491 491 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 021 021 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475 475 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 695 695 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 067 067 |
6818 |
| R int | 0.043 | 0.070 | 0.054 | 0.055 | 0.052 | 0.029 | 0.046 | 0.131 | 0.093 | 0.023 |
| Reflections with F2 > 2σ(F2) | 8677 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 916 916 |
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 425 425 |
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 665 665 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 319 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 736 736 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 105 105 |
6713 |
| Number of parameters | 609 | 1279 | 1058 | 1229 | 1323 | 1327 | 1241 | 1474 | 1504 | 329 |
| R 1 [F2 > 2σ(F2)] | 0.041 | 0.093 | 0.039 | 0.094 | 0.075 | 0.074 | 0.065 | 0.099 | 0.072 | 0.029 |
| wR2 (all data) | 0.118 | 0.260 | 0.111 | 0.265 | 0.204 | 0.231 | 0.168 | 0.293 | 0.208 | 0.077 |
| GOOF, S | 1.07 | 1.07 | 1.07 | 1.09 | 1.11 | 1.04 | 1.09 | 1.02 | 1.02 | 1.07 |
| Largest difference peak and hole (e Å−3) | 0.64 and −0.42 | 1.58 and −0.44 | 0.52 and −0.51 | 1.07 and −0.46 | 1.40 and −0.64 | 2.77 and −2.46 | 1.00 and −0.71 | 1.10 and −0.55 | 1.26 and −0.64 | 0.28 and −0.40 |
CCDC 1954689–97 and 1969053 contain the crystal data for structures 9·9MeCN, 10·8.5MeCN, 11·2.85MeCN·0.43H2O, 2·2MeCN, 4·6MeCN, 5·MeCN, 6·7MeCN, 7·7.5MeCN, 8·11MeCN and 12, respectively.
Conflicts of interest
There are no conflicts of interest.Acknowledgements
CR thanks the Shaanxi Province for the 100 Talents Award. We also thank the EPSRC National Crystallography Service at Southampton (UK), the EPSRC Mass Spectrometry Service, Swansea (UK) and the EPSRC for an Overseas Travel Grant (EP/R023816/1) and for the UKRI Creative Circular Plastic grant (EP/S025537/1).References
- (a) D. H. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086–5130 CrossRef; (b) Coordination Chemistry and Applications of Phenolic Calixarene–metal Complexes, Y. Li, K.-Q. Zhao, C. Redshaw, B. A. M. Ortega, A. Y. Nuñez and T. A. Hanna in Patai's Chemistry of Functional Groups, Wiley, 2014 Search PubMed.
- For early examples see (a) M. M. Olmstead, G. Sigel, H. Hope, X. Xu and P. P. Power, J. Am. Chem. Soc., 1985, 107, 8087–8091 CrossRef; (b) A. Zanotti-Gerosa, E. Solari, L. Giannini, C. Floriani, N. Re, A. Chiesi-Villa and C. Rizzoli, Inorg. Chimica Acta, 1998, 270, 298–311 CrossRef; (c) W. Clegg, M. R. J. Elsegood, V. C. Gibson and C. Redshaw, J. Chem. Soc., Dalton Trans., 1998, 3037–3039 RSC; (d) U. Radius, Inorg. Chem., 2001, 40, 6637–6642 CrossRef; (e) F. A. Cotton, E. V. Dikarev, C. A. Murillo and M. A. Petrukhina, Inorg. Chim. Acta, 2002, 332, 41–46 CrossRef.
- M. Frediani, D. Sémeril, A. Comucci, L. Bettucci, P. Frediani, L. Rosi, D. Matt, L. Toupet and W. Kaminsky, Macromol. Chem. Phys., 2007, 208, 938–945 CrossRef.
- C. Capacchione, P. Neri and A. Proto, Inorg. Chem. Commun., 2003, 6, 339–342 CrossRef.
- J. Espinas, U. Darbost, J. Pelletier, E. Jeanneau, C. Duchamp, F. Bayard, O. Boyron, J.-P. Broyer, J. Thivolle-Cazat, J.-M. Basset, M. Taoufik and I. Bonnamour, Eur. J. Inorg. Chem., 2010, 1349–1359 CrossRef.
- (a) Y. Li, Y. S. Zheng and G. H. Xie, Acta Polym. Sin., 1998, 101–103 Search PubMed; (b) Y. Chen, Y. Zhang, Z. Shen and W. Sun, Acta Polym. Sin., 2000, 2, 239–241 Search PubMed; (c) A. Diaz-Barrios, J. Liscano, M. Trujillo, G. Agrifoglio and J. O. Matos, Assignee: Intevep S.A., U.S. Pat. 5767034, 1998 Search PubMed; (d) J. O. Matos, A. Diaz-Barrios, J. Liscano, M. Trujillo and G. Agrifoglio, Assignee: Intevep. S.A, European Pat. EP1125951, 2001 Search PubMed.
- (a) J. M. Notestein, E. Iglesia and A. Katz, J. Am. Chem. Soc., 2004, 126, 16478–16486 CrossRef CAS; (b) J. M. Notestein, L. R. Andrini, V. I. Kalchenko, F. G. Requejo, A. Katz and E. Iglesia, J. Am. Chem. Soc., 2007, 129, 1122–1131 CrossRef CAS; (c) J. M. Notestein, L. R. Andrini, F. G. Requejo, A. Katz and E. Iglesia, J. Am. Chem. Soc., 2007, 129, 15585–15595 CrossRef CAS; (d) Y. Guo, A. Solovyov, N. A. Grosso-Giordano, S.-J. Hwang and A. Katz, ACS Catal., 2016, 6, 7760–7768 CrossRef CAS.
- M. Frediani, D. Sémeril, A. Marrioti, L. Rosi, P. Frediani, L. Rosi, D. Matt and L. Toupet, Macromol. Rapid Commun., 2008, 29, 1554–1560 CrossRef CAS.
- M. Frediani, D. Sémeril, D. Matt, L. Rosi, P. Frediani, F. Rizzolo and A. M. Papini, Int. J. Polym. Sci., 2010, 490724 Search PubMed.
- J. D. Ryan, K. J. Gagnon, S. J. Teat and R. D. McIntosh, Chem. Commun., 2016, 52, 9071–9073 RSC.
- Y. Li, K.-Q. Zhao, C. Feng, M. R. J. Elsegood, T. J. Prior, X. Sun and C. Redshaw, Dalton Trans., 2014, 43, 13612–13619 RSC.
- Z. Sun, Y. Zhao, T. J. Prior, M. R. J. Elsegood, K. Wang, T. Xing and C. Redshaw, Dalton Trans., 2019, 48, 1454–1466 RSC.
- A. Zanotti-Gerosa, E. Solari, L. Giannini, C. Floriani, N. Re, A. Chiesi-Villa and C. Rizzoli, Inorg. Chim. Acta, 1998, 270, 298–311 CrossRef CAS.
- D. M. Miller-Shakesby, S. Nigam, D. L. Hughes, E. Lopez-Estelles, M. R. J. Elsegood, C. J. Cawthorne, S. J. Archibald and C. Redshaw, Dalton Trans., 2018, 47, 8992–8999 RSC.
- (a) S. L. Borkowsky, R. F. Jordan and G. D. Hinch, Organometallics, 1991, 10, 1268–1274 CrossRef CAS; (b) T. L. Breen and D. W. Stephan, Inorg. Chem., 1992, 31, 4019–4022 CrossRef CAS; (c) J. P. Campbell and W. L. Gladfelter, Inorg. Chem., 1997, 36, 4094–4098 CrossRef CAS; (d) A. Mommertz, R. Leo, W. Massa, K. Harms and K. Dehnicke, Z. Anorg. Allg. Chem., 1998, 624, 1647–1652 CrossRef CAS; (e) B. Birkmann, T. Voss, S. J. Geier, M. Ullrich, G. Kehr, G. Erker and D. W. Stephan, Organometallics, 2010, 29, 5310–5319 CrossRef CAS; (f) A. Solovyev, E. Lacôte and D. P. Curran, Dalton Trans., 2013, 42, 695–700 RSC.
- A. Pärssinen, M. Kohlmayr, M. Leskelä, M. Lahcini and T. Repo, Polym. Chem., 2010, 1, 834–836 RSC.
- D. Takeuchi, T. Nakamura and T. Aida, Macromolecules, 2000, 33, 725–729 CrossRef CAS.
- Y. A. Piskun, I. V. Vasilenko, S. V. Kostjuk, K. V. Zaitsev, G. S. Zaitseva and S. S. Karlov, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 1230–1240 CrossRef CAS.
- D. Zhu, L. Guo, W. Zhang, X. Hu, K. Nomura, A. Vignesh, X. Hao, Q. Zhang and W.-H. Sun, Dalton Trans., 2019, 48, 4157–4167 RSC.
- (a) C. Ludwig and M. R. Viant, Phytochem. Anal., 2010, 21, 22–32 CrossRef CAS; (b) M. J. Walton, S. J. Lancaster and C. Redshaw, ChemCatChem, 2014, 6, 1892–1898 CrossRef CAS.
- (a) Z. Zhong, P. J. Dijkstra and J. Feijen, J. Am. Chem. Soc., 2003, 125, 11291–11298 CrossRef CAS; P. Hormnirun, E. L. Marshall, V. C. Gibson, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 2004, 126, 2688–2689 Search PubMed.
- (a) Q. Hu, S.-Y. Jie, P. Braunstein and B.-G. Lia, Chin. J. Polym. Sci., 2020, 38, 240–247 CrossRef CAS; (b) M. A. Woodruff and D. W. Hutmacher, Prog. Polym. Sci., 2010, 35, 1217–1256 CrossRef CAS; (c) T. Wu, Z. Wei, Y. Ren, Y. Yu, X. Leng and Y. Li, Polym. Degrad. Stab., 2018, 155, 173–182 CrossRef CAS; (d) M. T. Hunley, N. Sari and K. L. Beers, ACS Macro Lett., 2013, 2, 375–379 CrossRef CAS.
- (a) F. Della Monica, E. Luciano, A. Buonerba, A. Grassi, S. Milione and C. Capacchione, RSC Adv., 2014, 4, 51262–51267 RSC; (b) P. Vanhoorne, P. Dubois, R. Jerome and P. Teyssie, Macromolecules, 1992, 25, 37–44 CrossRef CAS; (c) J. Kasperczyk and M. Bero, Makromol. Chem., 1991, 192, 1777–1787 CrossRef CAS; (d) J. Kasperczyk and M. Bero, Makromol. Chem., 1993, 194, 913–925 CrossRef CAS; (e) N. Nomura, A. Akita, R. Ishii and M. Mizuno, J. Am. Chem. Soc., 2010, 132, 1750–1751 CrossRef CAS; (f) G. Li, M. Lamberti, D. Pappalardo and C. Pellecchia, Macromolecules, 2012, 45, 8614–8620 CrossRef CAS.
- D. Sun, W. Liu, M. Qiu, Y. Zhang and Z. Li, Chem. Commun., 2015, 51, 2056–2059 RSC.
- (a) A. Arduini and A. Casnati in Macrocycle Synthesis, ed. D. Parker, Oxford University Press, New York, 1996, ch. 7 Search PubMed; (b) S. Kim, J. S. Kim, O. J. Shon, S. S. Lee, K.-M. Park, S. O. Kang and J. Ko, Inorg. Chem., 2004, 43, 2906–2913 CrossRef CAS.
- Problems with the combustion of calixarene-based complexes have been noted previously, see for example W. Kuran, T. Listos, M. Abramczyk and A. Dawidek, J. Macromol. Sci., Part A: Pure Appl. Chem., 1998, 35, 427–437 CrossRef.
- CrysAlis PRO, Rigaku Oxford Diffraction, 2017–2018 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- (a) A. L. Spek, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 9–18 CrossRef CAS PubMed; (b) P. v. d. Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, 46, 194–201 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1954689–1954697 and 1969053. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cy02571e |
| This journal is © The Royal Society of Chemistry 2020 |