Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The carbon footprint of the carbon feedstock CO2†
Leonard Jan
Müller
 a,
Arne
Kätelhön
a,
Arne
Kätelhön
 ab,
Stefan
Bringezu
ab,
Stefan
Bringezu
 c,
Sean
McCoy
c,
Sean
McCoy
 d,
Sangwon
Suh
e,
Robert
Edwards
f,
Volker
Sick
d,
Sangwon
Suh
e,
Robert
Edwards
f,
Volker
Sick
 g,
Simon
Kaiser
c,
Rosa
Cuéllar-Franca
h,
Aïcha
El Khamlichi
i,
Jay H.
Lee
j,
Niklas
von der Assen
a and
André
Bardow
g,
Simon
Kaiser
c,
Rosa
Cuéllar-Franca
h,
Aïcha
El Khamlichi
i,
Jay H.
Lee
j,
Niklas
von der Assen
a and
André
Bardow
 *akl
*akl
aInstitute of Technical Thermodynamics, RWTH Aachen University, Schinkelstr. 8, 52062 Aachen, Germany
bCarbon Minds GmbH, Eupener Str. 165, 50933 Cologne, Germany
cCenter for Environmental Systems Research (CESR), Universität Kassel, Wilhelmshöher Allee 47, 34109 Kassel, Germany
dDepartment of Chemical and Petroleum Engineering, University of Calgary, 750 Campus Dr NW, Calgary, AB T2N 4H9, Canada
eBren School of Environmental Science and Management, University of California, Santa Barbara, Bren Hall, 2400 University of California, Santa Barbara, CA 93117, USA
fEuropean Commission – Joint Research Centre, Via Enrico Fermi 2749, 21027 Ispra (VA), Italy
gDepartment of Mechanical Engineering, University of Michigan, 2007 W. E. Lay Automotive Laboratory, 1231 Beal Avenue, Ann Arbor, MI 48109-2133, USA
hDepartment of Chemical Engineering and Analytical Science, The Mill, Sackville Street, The University of Manchester, Manchester M13 9PL, UK
iAdeme Industries, Department avenue du Grésillé, BP 90406, 49004 Angers Cedex 01, France
jKorea Advanced Institute of Science and Technology, Department of Chemical & Biomolecular Engineering, 291 Daehak-ro (373-1 Guseong-dong), Yuseong-gu, Daejeon 305-338, Republic of Korea
kInstitute of Energy and Climate Research – Energy Systems Engineering (IEK-10), Forschungszentrum Jülich GmbH, Jülich, Germany
lEnergy & Process System Engineering, Department of Mechanical and Process Engineering, ETH Zurich, Switzerland. E-mail: abardow@ethz.ch
First published on 24th July 2020
Abstract
Capturing and utilizing CO2 as carbon feedstock for chemicals, fuels, or polymers is frequently discussed to replace fossil carbon and thereby help mitigate climate change. Emission reductions by Carbon Capture and Utilization (CCU) depend strongly on the choice of the CO2 source because CO2 sources differ in CO2 concentration and the resulting energy demand for capture. From a climate-change perspective, CO2 should be captured at the CO2 source with the lowest CO2 emissions from capture. However, reported carbon footprints differ widely for CO2 captured, from strongly negative to strongly positive for the same source. The differences are due to methodological ambiguity in the treatment of multifunctionality in current assessment practice. This paper reviews methodological approaches for determining the carbon footprint of captured CO2 as carbon feedstock, and shows why some approaches lead to suboptimal choices of CO2 sources and that increased consistency in life cycle assessment (LCA) studies on CCU is needed. Based on strict application of Life Cycle Assessment (LCA) standards and guidelines, it is shown that substitution should be applied to avoid suboptimal choices of CO2 sources. The resulting methodological recommendations are applied to estimate the carbon footprint of feedstock CO2 for current CO2 sources in Europe and for future CO2 sources in a scenario for a low carbon economy. For all CO2 sources, the cradle-to-gate footprint of captured CO2 is negative ranging from −0.95 to −0.59 kg CO2 eq. per kg of feedstock CO2 today and from −0.99 to −0.98 kg CO2 eq. in a low carbon economy. The carbon footprints of different CO2 sources differ mainly due to their energy demands. The presented assessment method and the carbon footprints of the CO2 feedstocks CO2 provide the basis for future assessments of carbon capture and utilization processes.
Broader contextAnthropogenic CO2 emissions are a major driver of global warming. One potential way to reduce these emissions is capturing CO2 from industrial point sources or from ambient air, and utilizing the captured CO2 as carbon feedstock for value-added products. Today, the amount of CO2 produced by industrial point sources is far larger than the current CO2 demand by carbon capture and utilization (CCU). Therefore, the environmentally most beneficial CO2 sources should be prioritized for CCU. However, since CO2 from point sources is generated together with other co-products such as cement and steel, there is a debate in literature how to prioritize CO2 sources for CCU considering the emissions and benefits attributable to co-products. Due to the differing views on this issue, available values for the carbon footprint of CO2 vary from strongly negative to strongly positive. In this work, we propose a consistent methodology for prioritizing CO2 sources for CCU based on Life Cycle Assessment. Based on the method developed, we determine the optimal choices of CO2 sources today and for a low carbon future. |
1. Introduction
The greenhouse gas carbon dioxide (CO2) can be converted into value-added products by carbon capture and utilization (CCU).1 CCU aims at reducing the use of fossil resources and emissions of greenhouse gases (GHG).2–4 Importantly, CCU is a potential key enabler for deep de-fossilization of industries that currently rely on fossil feedstocks not only for energy but also a source of carbon, such as the chemical industry.5 Available analyses suggest that CCU could utilize CO2 up to the gigatonne scale.6However, utilizing CO2 does not necessarily reduce climate change impacts. In fact, GHG emissions may even be higher compared to conventional technologies depending on the specific CCU technology, its supply chain, and the nature of the product.7 The development of environmentally beneficial CCU technologies thus requires a proper understanding of the underlying supply chains, the context in which the technology will be used, and at what scale the CCU technology will replace an existing service/technology in the market.
A central part of all CCU supply chains is the capture and supply of CO2 as carbon feedstock. CO2 can be captured and supplied from fossil point sources such as power or cement plants,8–11 from biogenic point sources such as biogas and wastewater treatment plants12–14 and even directly from the air.15–17 Some point sources already supply almost pure CO2 streams, while the vast majority of CO2 sources have concentrations between 5 to 35%.18 Ambient air has the lowest concentration of around 400 ppm.19 Lower CO2 concentrations increase the energy demand for capture.20 Since the provision of this energy usually also leads to CO2 emissions, CO2 sources with high concentrations should be prioritized in general for CCU to maximize carbon mitigation given energy resource availability limitations. Since reductions of climate change impacts is a main driver for CCU development, a sound environmental assessment is critical.
The carbon footprint of CO2 supply can be properly accounted for by Life Cycle Assessment (LCA). LCA is a well-established holistic method taking the entire life cycle into account, from the extraction of raw materials to the final disposal of wastes for multiple environmental impacts. LCA is standardized according to ISO 14040, 14044, and 14067.21–23 The generic ISO standards have recently been adapted for CO2 utilization in LCA guidelines developed by the Global CO2 Initiative24 and the U.S. Department of Energy, National Energy Technology Laboratory.25 These LCA guidelines have been linked to techno-economic assessment (TEA)26 enabling harmonized assessment of LCA and TEA for CO2 utilization.27
Despite these LCA standards and guidelines, however, in current practice, several accounting approaches are applied leading to a wide range for greenhouse gas emissions (in the following called ‘carbon footprint’). In some studies, for example, it is assumed that a concentrated CO2 flow is simply available and that consuming this flow leads to negative emissions of −1 kg CO2 eq. emissions per kg CO2 captured (see SI 1, ESI†).28–33 In other studies, a flue gas stream with a defined composition is available but the source of this stream is not included in the study.34–38 Assuming a more or less concentrated CO2 flow is simply available, neglects the main principle of LCA to consider all relevant parts of the life cycle. The resulting carbon footprint is negative and depending on the needed make up for the assumed CO2 flow between −1 and 0 kg CO2 eq. emissions per kg CO2 captured. Other papers include the source of the CO2 stream and split the entire emissions between the carbon feedstock CO2 and other products of the CO2 source.39–46 As a consequence, the carbon footprint of the feedstock CO2 is positive. In summary, carbon footprints of the carbon feedstock CO2 range from positive – implying that CO2 capture is harmful to the climate – to negative which suggests benefits. These differences can substantially impact the selection of environmentally beneficial CO2 sources in industry and policy-making, and even the perception of CCU in general. Therefore, a consistent determination of the carbon feedstock CO2 is needed. Due to these major discrepancies in the literature for the carbon footprint of the carbon feedstock CO2 and its importance for the sound assessment of the advantages and disadvantages of CCU, the authors feel the need to offer a clarifying perspective. The initiative to the current work originated in expert workshops as part of the development of LCA guidelines on CCU where the carbon footprint of capture CO2 emerged repeatedly as a matter of confusion. The authors of the present paper, therefore, came together to present their joint analysis and consensus.
In this paper, we show why certain methodological options in LCA lead to suboptimal choices for CO2 sources and why it is important to adhere to physical relationships as much as possible. The term ‘physical relationships’ here refers to the marginal changes needed for adding CO2 capture and transportation to existing operations and associated GHG emissions. These physical relationships are reflected by the LCA methods of system expansion and substitution. The resulting assessment of the carbon footprint of the carbon feedstock CO2 corresponds to a strict application of current LCA standards and guidelines. From this analysis, we provide recommendations on how to avoid sub-optimal decisions and select environmentally optimal CO2 sources, as illustrated for Europe. Here, we study two scenarios in which: (1) current CO2 sources in Europe are available for CCU, and (2) future CO2 sources are available that would still exist in a low carbon European economy, in which all currently envisioned technologies for carbon reduction are applied to the full extent.
In Section 2, we explain the multifunctionality problem in LCA, the existing solution methods, and how these methods should be applied to CO2 sources. Since the present work also addresses non-LCA experts that approach CCU on a technological and political level, we apply these methods in an illustrative example to select a CO2 source at an industrial production site. Based on this illustrative example, we recommend a method to avoid sub-optimal selection of CO2 sources based on the carbon footprint of the carbon feedstock CO2. In Section 4, the recommended assessment method is applied to select the optimal CO2 sources in Europe today and in a low carbon future. Section 5 presents the overall conclusions.
2. LCA of CO2 supply: the multifunctionality problem
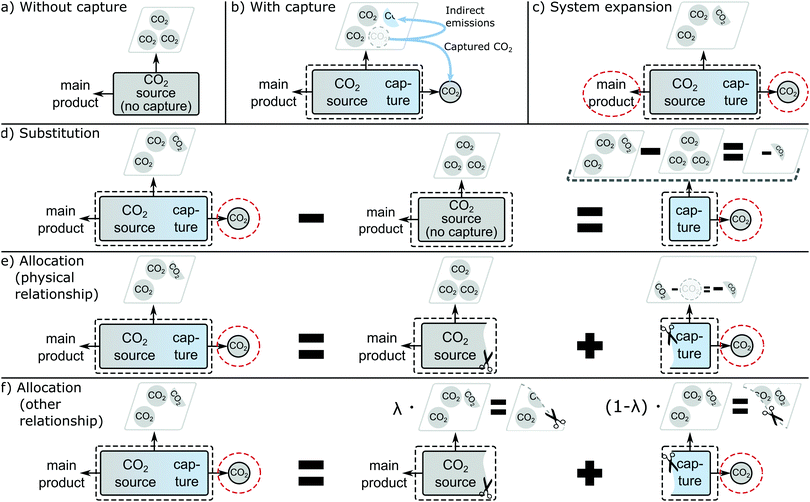
Greenhouse gas emissions and more generally, environmental impacts can vary among CO2 sources, due to the different CO2 concentrations, impurities, and carbon capture methods available leading to different energy and material requirements.14 Therefore, LCA is needed to determine the carbon footprint of the feedstock CO2 from each CO2 source. If LCA is used to select CO2 sources, we expect that LCA reflects the actual changes in emissions due to the installation of CO2 capture.CO2 supply is mostly considered from point sources. An existing point source without capture emits CO2 and all environmental burdens associated with the operation of the CO2 source are assigned to its products (Fig. 1a). What changes if we decide to co-produce the feedstock CO2? CO2 capture is added to the point source. As a result, direct CO2 emissions at the point source are lowered. However, additional indirect greenhouse gas emissions are caused by the demand for energy and materials to run the capture process and potential market-mediated effects (Fig. 1b). Consequently, the co-production of the feedstock CO2 is solely responsible for any additional indirect emissions, but also avoided direct emissions. Thus, the marginal emissions of feedstock CO2 are the difference in increased emissions and avoided emissions. These relationships should be reflected in the LCA analysis.
In LCA, capturing CO2 as a co-product turns the elementary flow “CO2 emission” into a technical flow “CO2 feedstock”. This supply of the technical flow “CO2 feedstock” typically introduces the problem of multifunctionality because the main product of the point source is now jointly produced with feedstock CO2. In LCA terminology, the process has two functions: producing the main product and supplying feedstock CO2. This multifunctionality causes ambiguity in the determination of product-specific environmental impacts for both the main product and feedstock CO2 because environmental impacts occur due to the joint production of the two products. Several methods for solving the multifunctionality problem have been proposed in the literature.
Here, we review the methods for solving the multifunctionality problem and recommendations provided by the ISO standard and leading LCA guidelines on how to address multifunctionality. Since the aim is to determine the carbon footprint of one product, CO2, we focus on LCA guidelines which consider product-specific life cycle assessment.21,22,47–51
2.1. Methods for solving the multifunctionality problem
In LCA literature and standards, four methods have been proposed to address multifunctionality: (1) sub-division, (2) system expansion, (3) substitution, and (4) allocation.21–23,47–51 In the following, the four methods are explained with their application to CO2 capture.Sub-division solves the problem of multifunctionality by sub-dividing a process with multiple products or services into independent sub-processes with one product or service each. For example, a process representing an entire factory with multiple production lines can be sub-divided into sub-processes representing the individual production lines. In this case, the multifunctionality problem is not of technical nature, but only a problem of data aggregation that can be solved by collecting additional data for the sub-processes. For CO2 point sources, however, sub-division is not applicable since the main product and CO2 are jointly produced in one process. Consequently, CO2 sources cannot be sub-divided into separate sub-processes producing the main product and feedstock CO2.
System expansion can be applied to CO2 sources by accounting for the main product and the feedstock CO2 simultaneously (Fig. 1c). The expanded functional unit is then “production of the main product and supply of feedstock CO2”. However, system expansion does not yield separate product-specific environmental impacts for the main product and CO2, which are often desirable to study further supply chains or for product declarations. In particular, the carbon footprint of captured CO2 cannot be resolved.
Substitution assumes that a co-product avoids its marginal production elsewhere.52,53 Substitution is applied by subtracting the avoided, marginal production system from the production system where the co-product is produced. Thereby, a hypothetical production system is built that does not produce the co-product. The resulting environmental impacts are product-specific and represent the impacts of the joint production minus the impacts of the avoided, marginal production system.
For the case of CCU, the avoided, marginal production system refers to those technologies that are displaced due to the introduction of the CCU technology. The identification of the marginal production system can be complex in markets with many different production technologies since it depends on market-mediated effects such as changes in supply and demand due to potential price changes.54 While different models have been proposed to account for market-mediated effects, there has been a debate on how useful these models are for LCA in practice, e.g., because the required data for such models is rarely available.55,56
Here, we assume that the operation of a plant with CO2 capture directly and fully avoids the operation of the same plant without capture. This assumption neglects more complex market-mediated effects, e.g., potential changes in production output due to changes in cost competitiveness. A direct 100% substitution can be applied by subtracting the environmental impacts of the CO2 source without capture from the CO2 source with capture. In consequence, a hypothetical process is generated with feedstock CO2 as the only product (Fig. 1d). The resulting environmental impacts of supplying feedstock CO2 are the difference between the environmental impacts of the process with and without capture. This approach is, in fact, the basis for the widely used “cost of CO2 avoided” metric to evaluate and compare the performance of CO2 capture systems and other emissions-mitigation approaches.57
For comparative LCA studies, substitution is mathematically equivalent to system expansion.49 System expansion for a comparison between an ammonia plant with a carbon capture unit and a direct air capture plant, for example, would lead to a functional unit that includes both ammonia and feedstock CO2. To provide the same functional unit, the product system of the direct air capture plant needs to also produce ammonia. For this purpose, the system is expanded by adding the marginal production of ammonia, in our case, an ammonia plant without capture. Thus, the environmental impacts of the expanded direct air capture system, eiCC@DAC, for providing ammonia and CO2 are given by the sum of the environmental impacts from the ammonia plant without capture, eiAP, and the direct air capture process, eiDAC. For substitution, the functional unit only includes feedstock CO2. In this case, the ammonia plant without capture and its environmental impacts, eiAP, are subtracted from the ammonia plant with carbon capture. In consequence, the differences in environmental impacts in comparative LCA studies are identical for both system expansion (ΔeiSE = eiCC@AP − eiSECC@DAC = eiCC@AP − (eiAP + eiDAC)) and substitution (ΔeiSUB = eiSUBCC@AP − eiCC@DAC = (eiCC@AP − eiAP) − eiDAC).
In reality, in particular, power plants retrofitted with a carbon capture unit may or may not displace the same power plant without capture. As a direct consequence of retrofitting, steam is taken from the power plant to drive the capture process. Since less steam can be used at the turbines, less electricity is generated and the efficiency of the power plant drops. Other power plants are needed to compensate for the electricity deficit. Which power plants are affected by the operation of the retrofitted power plant carbon capture process also depends on market-mediated effects.
Substitution typically leads to negative carbon footprints for feedstock CO2, because the amount of CO2 captured is usually higher than the greenhouse gas emissions caused by the capture process itself. However, the sound interpretation of this negative value is important: a negative carbon footprint shows that greenhouse gas emissions are reduced in comparison to the process without capture and not that CO2 capture physically removes greenhouse gas from the atmosphere over the entire life cycle.58 In contrast, all other environmental impacts usually have positive values since only CO2 emissions are avoided by the capture process.18
Allocation artificially sub-divides the multi-functional process into several hypothetical processes with exactly one function, each. The inputs and outputs of the multi-functional process are then distributed among the single functions. For this purpose, an underlying physical relationship should be used if available. Following ISO 14044,22 an underlying physical relationship can be established if it is possible to quantitatively change the relative amounts of the products of a process and to observe how the inputs of the process are affected.59–61 Then, the inputs of the process are allocated according to this physical relationship.
When a clear physical relationship cannot be found, ISO 14044 recommends applying allocation according to another relationship. Following the ILCD Handbook, this relationship “may be an economic relationship or a relationship between some other (e.g. non-causal) properties of the co-function”.49 In other words, the inputs are allocated according to a product property, the assigned allocation criterion.
In the case of retrofitting a CO2 source with a capture unit, a physical underlying relationship can be established: it is usually possible to quantitatively change the amount of CO2 captured without affecting the amount of main product. If CO2 is completely vented, the CO2 source produces only the main product. In this case, there is no demand for energy or materials for capture. With increasing CO2 capture, CO2 emissions drop but the demand for energy and materials for capture increases, while the amount of main product remains constant. Alternatively, less main product could be produced, reducing also CO2 formation and the amount of CO2 available for capture. Consequently, there is a physical relationship between the production of the feedstock CO2 and the CO2 emission reductions due to CO2 capture, but also to the indirect emissions caused by the energy demand and the materials needed for capture (Fig. 1e).
If a physical relationship for CO2 sources can be established, allocation using other underlying relationships is not needed for CO2 sources according to the order of methods in the ISO standard.22 Still, allocation using other relationships is commonly applied in practice. Allocation using other relationships distributes the environmental impacts of the CO2 source according to an allocation criterion λie.g. produced mass mi (λi = mi/∑mi), energy mi·ei(λi = mi·ei/∑(mi·ei)) or economic value mi·pi(λi = mi·pi/∑(mi·pi)) (Fig. 1f). E.g. with mass as allocation criterion, all in- and outputs are allocated among the sub-processes according to the share of produced masses of their functions (products). In literature, it has been argued to use economic value as allocation criterion for CO2 sources as economic allocation is always applicable, easy to understand, and was sometimes found to be a proxy for an underlying physical relationship.60 One practical drawback of this approach is that the relative economic value of products (as measured in their market price) can vary substantially in time and between regions, leading to results that may only be narrowly applicable. Usually, more than one criterion can be applied and the selection of the criterion depends on the LCA author.
For this reason, the ILCD handbook requires a sensitivity analysis of the allocation criteria.49
2.2. Hierarchy of methods to solve the multifunctionality problem
Not all methods to solve the multifunctionality problem can be applied in all situations. For this reason, the life cycle assessment standard ISO 14044 and other standards and guidelines set priorities for the selection of methods as summarized in Table 1.| Order of allocation methods | DIN ISO 14044 and 14067, BPX 30-323, | ILCD handbook situation A and B | BSI PAS 2050 | GHG-protocol product life cycle accounting | PCR basic chemicals | PEF guide |
|---|---|---|---|---|---|---|
| 1 | Sub-division | Sub-division | Sub-division | Sub-division | Sub-division | Sub-division or system expansion |
| 2 | System expansion | System expansion | System expansion | System expansion | System expansion not applicable | Allocation following underlying physical relationship or direct substitution |
| 3 | Substitution (not explicit) | Substitution | Substitution | Substitution | Substitution not applicable | System expansion via substitution (indirect) or allocation following underlying other relationship or indirect substitution |
| 4 | Allocation following underlying physical relationship | Allocation following underlying physical relationship | Allocation following underlying physical relationship | Allocation following underlying physical relationship | Allocation following underlying physical relationship | |
| 5 | Allocation following underlying other relationship | Allocation following underlying other relationship | Allocation in proportion to the economic value | Allocation following underlying other relationship | Allocation following underlying other relationship | |
| Ref. | 22, 23 and 47 | 49 | 48 | 51 | 50 | 62 |
All guidelines and standards propose the same order of methods to be employed. Thus, there should be no ambiguity in solving the multifunctionality problem once it is clarified which method is applicable. The preferred order of methods is as follows: sub-division is preferred whenever possible. If sub-division is not possible, system expansion is recommended. However, system expansion cannot be applied if product-specific environmental impacts are required (cf. Section 2.1). In this case, substitution should be applied. Finally, allocation should be used following a physical relationship and then allocation following other relationships. Product Category Rules account for exactly one product and thus, only consider sub-division and allocation since these methods yield product-specific footprints.
3. Impact on the selection of CO2 sources
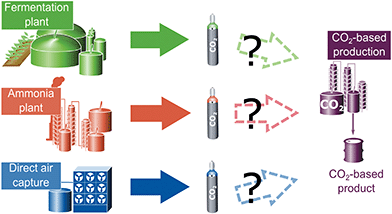
The methods to solve multifunctionality shown in Table 1 are applied in an illustrative example. The sole purpose of this example is to illustrate the effect of methods to solve multifunctionality on the LCA results and the selection of CO2 sources. A full quantitative LCA study should consider more aspects such as the potential variation of process parameters (e.g. heat integration to use waste heat).The example considers a new plant for CO2-based products that require CO2 as feedstock. We examine three potential sources for the CO2: a plant producing ammonia from fossil resources, a fermentation plant producing ethanol from glucose via fermentation, and a direct air capture facility (Fig. 2).
The aim is to select the CO2 source to minimize the greenhouse gas emissions of the entire production site. All investigated supply options shall provide the same amount of feedstock CO2 with the same quality and thus, the functional unit is defined as “Provision of 1 kg CO2 at 10 MPa pressure as carbon feedstock for further processing”. In practice, the specifications of the feedstock CO2 would be further refined based on the requirements for the specific utilization process.
Ammonia is produced via the Haber–Bosch process from hydrogen and nitrogen. The hydrogen is produced by steam methane reforming within the plant.44 Steam methane reforming produces CO2 which is separated before ammonia formation. An average amount of 1.26 kg CO2 per kg ammonia is assumed.63–66 A stream of humid CO2 containing 2.5 vol% of water at 0.17 MPa is produced. Using this CO2 stream as carbon feedstock requires drying and compression.67 This CO2 treatment uses 0.401 MJ of electricity and 0.008 MJ of heat per kg of CO2.63
The fermentation plant produces ethanol from glucose obtained from corn.68 The plant produces approximately 0.96 kg of CO2 per kg ethanol.69 As in the steam reformer at the ammonia plant, the CO2 stream needs to be dried and compressed, requiring 0.432 MJ of electricity per kg of feedstock CO2.70 Absorbed CO2 from plant growth is modelled as removal of CO2 from the atmosphere and thus, leads to negative greenhouse gas emissions from cradle-to-gate. The illustrative example neglects emissions from land-use change.
Direct air capture is based on a commercial-scale plant (∼1 Mt of CO2 captured from the air per year) from Carbon Engineering.16 The continuous process uses an aqueous KOH sorbent coupled to a calcium caustic recovery loop. The energy demand per kg of feedstock CO2 supplied is 4.04 MJ of natural gas supply and 1.01 MJ of electricity.16
The economic values of the products are assumed to be 60 € per ton for CO2,71 380 € per ton for ammonia,72 and 425 € per ton for bioethanol.73 In all plants, electricity is supplied from the European grid and heat is supplied by the combustion of natural gas (both EU-28 mixes from GaBi database).45
3.1. Selecting CO2 sources using system expansion
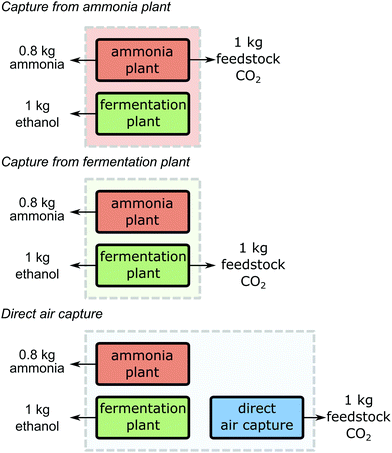
In all guidelines where it is applicable, system expansion is the preferred approach (cf. Section 2.2). System expansion allows identifying the CO2 source leading to the lowest greenhouse gas emissions for the supply of all products of the industrial production site. Using system expansion, the following three scenarios are compared: (1) CO2 is supplied from the ammonia plant (“Capture from ammonia plant”) while the fermentation plant continues production without capture, (2) CO2 supply from the fermentation plant (“Capture from fermentation plant”) while the ammonia plant continues production without capture, and (3) CO2 is captured from air using direct air capture (“Direct air capture”) while both the ammonia and the fermentation plant continue production without capture (Fig. 3). To make the scenarios comparable, all scenarios need to serve the same function. Therefore, the functional unit is expanded from “production of 1 kg feedstock CO2 at 10 MPa” to “the production of 1 kg feedstock CO2 at 10 MPa, 0.8 kg ammonia and 1 kg ethanol”. The amounts of ammonia and ethanol in the functional unit represent the amounts that are co-produced with 1 kg feedstock CO2 in the case of capture.Further processing of the products, use phase, and end-of-life treatment is identical for all considered production systems and thus, cancel in the comparison. In consequence, the system boundaries span from cradle-to-gate.
For the production system “Capture from ammonia plant”, the overall carbon footprint is 0.132 kg CO2 eq. (Fig. 4). Here, the ammonia plant emits 0.525 kg CO2 eq. per 1 kg of feedstock CO2 and 0.8 kg of ammonia (see SI 2.1, ESI†). The fermentation plant causes negative emissions of around −0.393 kg CO2 eq. from cradle-to-gate for the production of 1 kg ethanol (see SI 2.2, ESI†). These negative emissions result from the removal of atmospheric CO2 during the growth of the biomass. However, the removed atmospheric CO2 will usually be re-emitted during the use of ethanol at the end-of-life (e.g. combustion) which is not included in this example following the cradle-to-gate perspective. For the scenario “Capture from fermentation plant”, the overall carbon footprint is 0.135 kg CO2 eq. The ammonia plant emits around 1.471 kg CO2 eq. and the fermentation plant emits −1.336 kg CO2 eq. from cradle-to-gate. For the last option, the production system “Direct air capture”, the carbon footprint is 0.486 kg CO2 eq. Here, the ammonia plant and the fermentation plant have no capture units and thus, emit 1.471 kg CO2 eq. and −0.393 kg CO2 eq. respectively. The direct air capture process emits −0.592 kg CO2 eq. from cradle-to-gate for each kg of feedstock CO2 (see SI 2.3, ESI†). Recently, Jonge et al.74 reported −0.62 kg CO2 eq. for a similar DAC process with slightly different assumptions but this difference does not impact our illustrative analysis.
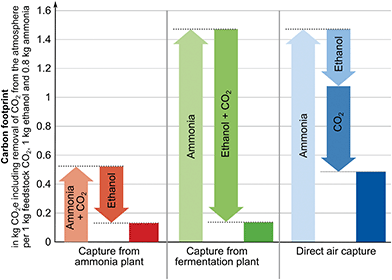 | ||
| Fig. 4 Contribution analysis of the carbon footprint of the production systems (Fig. 3) to produce 1 kg feedstock CO2, 1 kg ethanol and 0.8 kg ammonia. | ||
A comparison shows that the scenario “Capture from ammonia plant” leads to the lowest carbon footprint for the production of 1 kg feedstock CO2, 0.8 kg ammonia, and 1 kg ethanol. “Capture from fermentation plant” leads to a slightly higher carbon footprint. Considering uncertainty, both plants could most likely be considered as equally beneficial CO2 sources from a global warming perspective. The least beneficial scenario in this illustrative example is “Direct air capture” since it leads to a substantially larger carbon footprint from a system-wide perspective.
Consequently, direct air capture should be utilized only if the CO2 supply capacities of first the ammonia plant and second the fermentation plant are exceeded. In conclusion, we obtain the following environmental merit-order for the CO2 sources of our illustrative example: (1) ammonia plant, (2) fermentation plant, and (3) direct air capture. Again, these results serve illustrative purposes only and are not meant as a general statement about these CO2 sources.
3.2. Carbon footprints of feedstock CO2
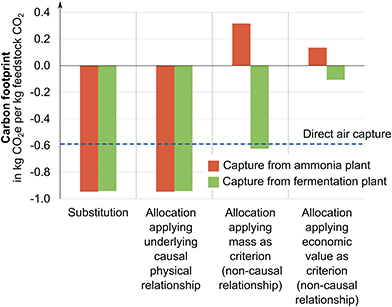
For the illustrative example in Section 3.1, we have identified the CO2 source leading to the lowest overall carbon footprint for the product bundle of feedstock CO2, ammonia, and ethanol via system expansion. However, system expansion only yields the carbon footprint for the product bundle of ammonia, ethanol, and CO2 and does not provide the carbon footprint of the carbon feedstock CO2. To obtain the carbon footprint of CO2, we use substitution, allocation using an underlying physical relationship, and allocation using other relationships namely economic value and mass as criteria (cf. Section 2.1).The methods to obtain product-specific results lead to substantially different carbon footprints for the feedstock CO2 (Fig. 5): as explained in Section 2.1, substitution and allocation using an underlying physical relationship lead to nearly identical negative carbon footprints of −0.95 kg CO2 eq. per kg of feedstock CO2 from the ammonia plant and −0.94 kg CO2 eq. per kg of feedstock CO2 from the fermentation plant.
In contrast, positive carbon footprints are obtained for capture from the ammonia plant when applying allocation using other relationships: using mass as allocation criterion results in 0.32 kg CO2 eq. per kg of feedstock CO2 and economic value of the products as criterion leads to 0.13 kg CO2 eq. per kg of feedstock CO2.
For capture from the fermentation plant, negative cradle-to-gate GHG emissions are allocated and thus also obtained for CO2, specifically, −0.62 kg CO2 eq. per kg of feedstock CO2 for mass as criterion and −0.11 kg CO2 eq. per kg of feedstock CO2 for economic value as criterion. Since CO2 has a high share of the total produced mass, allocation by mass assigns a higher fraction of the GHG emissions to the feedstock CO2. Using the economic value of the products as underlying relationship distributes a smaller fraction of the total GHG emissions to the feedstock CO2 since the assumed economic value of CO2 (60 € per ton) is far lower than economic values of ammonia (380 € per ton) or bioethanol (425 € per ton).
3.3. Recommended methods to calculate product-specific impacts
We have shown that methodological choices to obtain product-specific carbon footprints can substantially alter the selection of CO2-sources compared to a system-wide assessment based on system expansion, and thus can lead to sub-optimal decisions. System expansion provides the full view on the changes in the physical reality and should be applied to avoid sub-optimal decisions, wherever possible (cf. Section 2.1).However, system expansion cannot provide the carbon footprint of the feedstock CO2 from point sources which requires a product-specific assessment. Based on the present analysis (Section 3.2), the carbon footprint should be calculated using either the mathematical equivalent of the system expansion approach, substitution, or allocation using an underlying physical relationship. Both substitution and allocation using an underlying physical relationship lead to the same preference of CO2 sources as systems expansion.
In fact, the results of substitution and allocation using an underlying physical relationship are identical. However, this only holds for a direct 100% substitution of a plant without CO2 capture. Furthermore, an underlying physical relationship for CO2 capture may not found at all CO2 sources, because the relative amounts of products cannot be quantitatively changed. For example, an oxyfuel process produces the main product and feedstock CO2 in a fixed ratio. Consequently, substitution should be applied instead of allocation which is also in line with standards and guidelines.
In contrast, allocation using other relationships leads to sub-optimal choices of CO2 sources and should not be employed which is also in line with the hierarchy of LCA standards (cf. Section 2.1).
Calculating the carbon footprint of carbon feedstock CO2 using substitution keeps the carbon footprint of the main product of the CO2 source unchanged. In this case, all emissions reductions are credited to the feedstock CO2. As a result, the feedstock CO2 has negative carbon footprints even though fossil carbon is used, e.g. in the case of ammonia plants. It is important to keep in mind that LCA results showing negative carbon footprints from cradle-to-gate should not be misinterpreted as physical removal of greenhouse gases from the atmosphere being carbon negative from cradle-to-grave.58 According to the ILCD handbook, these results simply mean that emissions are reduced due to capturing CO2.49 For this reason, the greenhouse gas protocol requires that avoided environmental impacts and the avoided process should be reported separately to circumvent confusion with the removal of atmospheric greenhouse gases.51 Furthermore, it should be reported how much of the CO2 emitted is currently captured at a point source to avoid claiming reductions that are beyond the supply capability of the point source. In summary, life cycle inventories specific for feedstock CO2 should be documented particularly well to avoid misinterpretation of results.
In this study, feedstock CO2 is treated as a product and not as a waste because CO2 has a positive market price. However, even if CO2 was considered to be a waste, the hierarchy of allocation methods applies as defined by ISO 14044. In this case, the CO2-using process would be considered to be multifunctional with the functions “production of a CO2based product and treatment of waste CO2”. By treating waste CO2, the CO2-using process avoids the emissions of CO2 to the atmosphere, but usually requires a pre-treatment step by a capture process. Consequently, the CO2-based product is credited for the avoided CO2 emissions but carries the burdens of the capture process. As a result, the carbon footprint of the functions “the treatment of waste CO2” and “supply of feedstock CO2” are identical, whether CO2 is considered a waste or a valuable product. The LCA literature, e.g. in the product-environmental-footprint category rules, provides other waste-specific methods to solve multifunctionality.51 However, these methods have been shown to be non-causal56 and thus, should not be applied if a physical relationship is available.
4. Environmentally optimal selection of CO2 sources
The carbon footprint of the feedstock CO2 can be used to select the CO2 sources that reduce greenhouse gas emissions the most. Here, we select environmentally optimal CO2 sources for Europe today as a maximum CO2 supply scenario and a low carbon future as a minimum supply scenario. As shown before, the carbon footprint should be calculated by substitution. Here, we assume that installing CO2 capture substitutes the same CO2 source without capture (direct 100% substitution). The carbon footprint of CO2 is then the difference between the CO2 emissions from the CO2 source with and without capture. Since direct air capture does not yield another product besides feedstock CO2, substitution is neither applicable, nor needed.4.1. Current CO2 sources
The selection is based on the database of von der Assen et al. for CO2 sources based on the “European Pollutant Release and Transfer Register” and a comprehensive literature study.18,37 Von der Assen et al. used the database to map and environmentally rank CO2 sources in Europe. However, biogas plants, fermentation plants, and waste incineration plants had not been included. These plants are added here since biogenic point sources are currently discussed as an opportunity for negative emissions12 and utilizing CO2 from waste incineration could lead to closed carbon cycles.75In 2015, 228 TW h of biogas have been generated and combusted in combined-heat-and-power plants (CHP) in Europe.76 It is assumed that on average the biogas is composed of 60 wt% methane and 40 wt% CO2 with a lower heating value of 13 kW h kg−1,77 has an energy demand for capture of 2.30 MJ of electricity per kg of feedstock CO2 and a capture rate of 90%.12 In consequence, 72 Mt of feedstock CO2 could potentially be captured annually from biogas CHP plants in Europe. Fermentation has produced 5300 million liters of ethanol in 2015.78 Fermentation of glucose produces 0.76 kg of CO2 per liter ethanol. In consequence, 4 Mt of feedstock CO2 could potentially be supplied from fermentation plants.69
Waste incineration plants have treated 64 Mt of waste in Europe. The corresponding CO2 emissions depend on various parameters, e.g., the carbon content and the heating value of the waste. In consequence, the amount of CO2 in the exhaust gases varies over time and region.79 According to several studies, between 0.7 and 1.2 (average 0.95) kg of CO2 are generated per kg of waste.80–82 Tang et al. report a capture rate of 0.9 kg feedstock CO2 per kg CO2 emitted and a reduction in electricity output due to the capture from waste incineration plants by about 1.11 MJel per kg of feedstock CO2.83 As this electricity is no longer available to the power grid, it is assumed that other power plants will generate electricity instead of the waste incineration plant. In consequence, the capture of 1 kg feedstock CO2 causes the emissions related to the generation of 1.11 MJel from the average power plants in Europe.
4.2. CO2 sources in a low carbon economy
Most current CO2 sources provide fossil carbon. Since current policy goals aim to reduce fossil CO2 emissions, we assume that the current CO2 supply is the maximum value. To estimate the minimum CO2 supply available in the future, we study a low carbon economy in Europe. For this purpose, we assume that no more fossil fuels are employed and that all technologies currently available to reduce CO2 emissions are deployed to the potential maximum or latest available projection. We do not asses the likelihood or timing of achieving such a low carbon economy or whether it would lead to undesirable side-effects, but use it only to determine a lower bound for CO2 supply. In the following, our assumptions are described. More details are provided in the ESI.†The basis for our minimum CO2 supply scenario is the replacement of all fossil fuel for power by renewable energies and sufficient energy storage to guarantee grid stability. A power sector based on renewables enables emission reductions of most other industrial sectors by “Power-to-x” technologies.84 First of all, power-to-heat technologies can substitute fossil boilers for heating in households and industrial applications. We assume that heat pumps will be used for low-temperature applications (up to 120 °C), e.g., household heating.85 Process steam can be generated via electrode vessels up to 230 °C at 30 bar.86 Higher temperatures require other electrical technologies, e.g. resistance furnaces, induction heating, microwave heating or non-fossil fuels.87
The production of hydrogen is assumed to switch from steam methane reforming to water splitting via electrolysis. Furthermore, ammonia is no longer produced by the Haber–Bosch but by electrochemical conversion, rendering the steam methane reforming step in ammonia synthesis obsolete.88 Other chemical processes, including the direct oxidation of ethylene to ethylene oxide can be substituted by carbon capture and utilization technologies or bio-based processes. In consequence, steam crackers with naphtha as a major fossil feedstock for the chemical industry can be decommissioned.7,89–92
Furthermore, refineries producing fossil fuels for mobility and transportation application can be decommissioned if battery electric vehicles and bio- or CO2-based fuels are fully deployed, e.g. for heavy duty application and aircrafts.93–96
Biogenic point sources can provide additional CO2 supply. For biogas production, the maximum technical potential reported in the literature is 780 TW h in Europe.77 The potential seems large and full deployment may lead to unwanted side-effects. More conservative projections report a potential for biogas production from 233 TW h97 to 324 TW h98 (see SI 3.1, ESI†). We use the average of both studies, 279 TW h, and use both studies as upper and lower bound for biogas generation. The entire biogas production can be upgraded and fed into the natural gas grid and then burned in existing natural gas combined cycle (NGCC) power plants. In consequence, 39 Mt of feedstock CO2 can be supplied from biogas upgrading and 59 Mt can be supplied from the NGCC power plants. Furthermore, fermentation plants could increase their production from around 5300 million liters to 9285 million liters corresponding to an increase in CO2 supply from 4 to 7 Mt CO2.99
Steel and iron mills currently consist of blast oven furnaces. After pretreatment, the iron ore is reduced with coke and coal as reduction agents and energy carries to pig iron. In a subsequent step, the carbon content is reduced in an oxygen blast oven furnace, producing high levels of CO2 emissions. To reduce CO2 emissions, hydrogen can be used as an alternative reduction agent, such as the Circored process.100 The product of the Circored process is not pig iron but sponge iron, which cannot be processed in a blast oven furnace but in an electrical arc furnace.101 The combination of the Circored process with electrical arc furnaces allows the integration of renewable energy into the steel and iron industry by using electrical power, hydrogen, and synthetic methane.102 The combustion of synthetic methane is the only source of CO2 left. Assuming a constant annual steel production capacity of 169 Mt,103 the potential CO2 supply drops from 159 to 46 Mt of feedstock CO2 (see SI 3.2, ESI†). In a sensitivity analysis, we consider that the primary production of steel can be avoided by recycling and material efficiency. In this case, the potential CO2 supply from steel drops to 15 Mt of feedstock CO2.
The CO2 emissions from cement plants are generated from the combustion of fuels and during calcination, the thermal decomposition of calcium carbonate into calcium oxide and CO2. CO2 emissions from combustion can be reduced by using synthetic methane instead of coal or other fuels but calcination emissions remain unchanged. Therefore, the potential CO2 supply from cement plants shows only a slight decrease from 122 Mt to 103 Mt of feedstock CO2 (see SI 3.3, ESI†).
Note that both cement plants as well as iron and steel mills could also use their CO2 emissions to produce their own synthetic methane and consequently, no CO2 could be supplied to other purposes.
Like power-to-x technologies, pulp and paper mills could reduce CO2 emissions by co-producing chemicals: instead of burning black liqueur it could be converted into syngas via gasification. In a subsequent step, several chemicals, e.g. methanol or DME, can be synthesized.104,105 This process forms 47.8% less CO2 but offers an almost pure CO2 stream since CO2 has to be separated from the synthesis gas before subsequent conversion (see SI 3.4, ESI†). For supplying feedstock CO2, only compression is needed.
Waste incineration will decrease as the EU Commission has set the goal to recycle 65% of the municipal waste and to limit the share of landfilling to a maximum of 10% for 2030.106 Assuming a constant share of 17% for composting and other treatments at 3%, only 25% of the municipal waste will be burned according to the latest available forecast, corresponding to 48.5 Mt of waste. In the future, the composition may change to higher biogenic fractions, however, it is assumed that these higher fractions do not change the CO2 formation.107 Therefore, it is assumed that the CO2 formation remains at 0.95 kg of CO2 per kg of waste and the capture rate is 0.9 kg feedstock CO2 per kg CO2 emitted. Furthermore, we assume that the capture process reduces the electricity output of the plant by about 1.11 MJel per kg of feedstock CO2 supplied, and in consequence, the average power supply has to compensate for the loss of energy generation. In total, waste incineration plants could supply 41.6 Mt CO2.
For the energy supply of the capture processes in a low carbon economy, we assume that electricity is generated by 100% by the current mix of renewables energies in Europe.45 Heat is supplied by electrode vessels with an efficiency of 99%85 and fuel demand is satisfied by synthetic methane.102
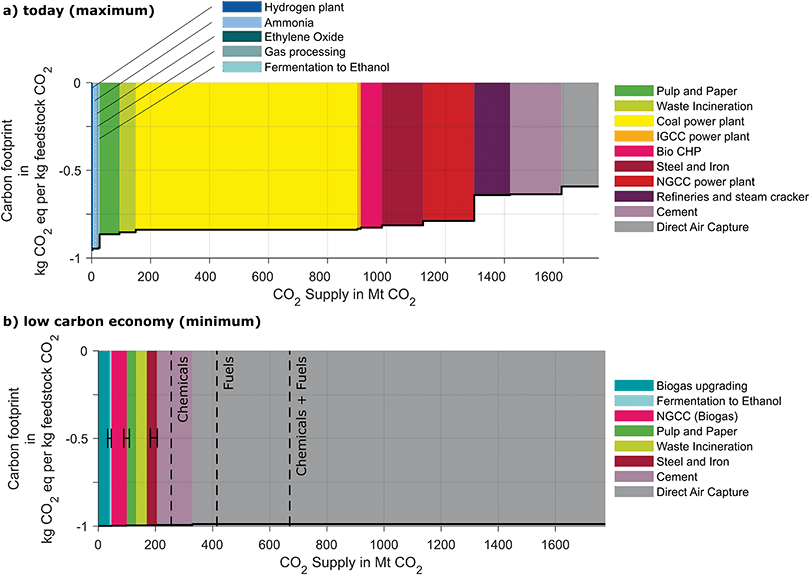
4.3. Environmental merit order curves today and in a low carbon economy
Today, the potential CO2 supply from European point sources is approximately 1550 Mt per year (Fig. 6). All sources offer the potential for negative carbon footprints from cradle-to-gate but do not reach the maximum reduction of −1 kg CO2 eq. per kg feedstock CO2 due to the energy and materials needed for the capture process.The negative carbon footprint from cradle-to-gate shows that all CO2 sources could effectively reduce GHG emissions. However, carbon footprints show significant variation between the CO2 sources: the CO2 sources leading to the lowest carbon footprints are hydrogen, ammonia, ethylene oxide production, natural gas processing, and fermentation to ethanol (all −0.95 kg CO2 eq. per kg feedstock CO2). Carbon footprints are between −0.88 and −0.78 kg CO2 eq. per kg feedstock CO2 for CO2 from pulp and paper mills, waste incineration plants, coal and integrated gasification combined cycle plants, biogas power plants, steel, and iron mills and natural gas combined power plants. Average carbon footprints from refineries, steam crackers, and cement plants are −0.64 respectively −0.63 kg CO2 eq. per kg feedstock CO2. Refineries and stream crackers show a strong variation since the energy demands are very site-specific. The direct air capture process has the highest carbon footprint of all sources with −0.592 kg CO2 eq. per kg feedstock CO2. Therefore, selecting an ammonia plant as CO2 source instead of a direct air capture plant could reduce the carbon footprint by 63%.
In a low carbon economy, the potential supply of feedstock CO2 from point sources will reduce by almost 80% compared to today with 330 Mt of feedstock CO2 per year compared to 1477 Mt available today. At the same time, the carbon footprints of feedstock CO2 are significantly lower and the range is significantly lower than today with footprints spanning from −0.98 to −0.99 kg CO2 eq. per kg CO2 supplied since renewable energy is assumed to drive the capture processes. The CO2 sources with the lowest carbon footprints are then fermentation plants, pulp, and paper mills equipped with black liqueur gasification and biogas upgrading plants since they offer almost pure CO2 streams. Other sources are waste incineration plants, cement plants, steel and iron mills, biogas power plants, and direct air capture in the order of ascending carbon footprints.
In a maximum future projection for Europe, Bazzanella et al. project a demand for CO2 of 255 Mt as feedstock for the chemical industry and additional 415 Mt if fuels are produced from CO2.89 Therefore, a low carbon economy could still provide sufficient feedstock CO2 from point sources for the production of chemicals but not for if fuels are produced from CO2. However, since the carbon footprints of all CO2 sources will significantly improve due to renewable energy use, even direct air capture processes can supply CO2 with a low carbon footprint. In consequence, differences in carbon footprints become small, and thus, also the importance of the environmental merit order is reduced for the climate change perspective. However, the differences in energy demand of capture processes remain and thus, the overall energy consumption for CO2 capture can be significantly reduced by following the environmental merit order: to supply the maximum demand of 670 Mt of feedstock CO2 for chemicals and fuels would require 1531 TW h of renewably generated electricity to drive the direct air capture processes. The same amount of feedstock CO2 could be provided only using 1053 TW h or 69% of renewably generated electricity by selecting sources that have inevitable CO2 emissions following the environmental order (see SI 4, ESI†). As a result, it is expected that the order of CO2 sources will still play an important role for the deployment of CCU in a low carbon economy.
5. Conclusions
The carbon footprint of feedstock CO2 is one important but not the only criterion to select CO2 sources. However, the carbon footprint of feedstock CO2 strongly depends on the method used to solve the multifunctionality problem at the CO2 source in a life cycle assessment. This ambiguity can potentially lead to suboptimal decisions for the climate. This paper proposes the methodology of how to suboptimal decisions by either determining system-wide environmental impacts using the method of system expansion or by product-specific environmental impacts using the substitution approach. Allocation following other relationships, e.g., for CO2 sources mass or economic value as allocation criterion, can result in a sub-optimal selection of CO2 sources and should, therefore, be avoided. These findings are in line with the ISO 14044 standard22,23,47,48 and the major LCA guidelines.49,51The presented method allows decision-makers to choose where installing a CO2 capture unit would reduce greenhouse gas emissions the most. To apply the method, no distinction is required between sources that supply biogenic, fossil, or CO2 captured from ambient air. However, we only determine the reduction in greenhouse gas emissions and not whether or not a source should exist at all from a climate perspective. It is noteworthy that our method does not provide any incentives for installing novel or retaining fossil-based processes to co-supply CO2.
Applying substitution, we have determined merit-order curves for the selection of CO2 sources in Europe for two scenarios. (1) All currently available CO2 sources in Europe and (2) all potential CO2 sources still available in a low carbon European economy. These curves illustrate that around one fifth of today's potential CO2 supply (1550 Mt of feedstock CO2) could still be available in a low carbon economy (346 Mt of feedstock CO2). Furthermore, the carbon footprint of feedstock CO2 will significantly drop in the future from an average of around −0.80 to −0.98 kg CO2 eq. per kg CO2 as more low carbon electricity becomes available. Another result of low carbon electricity is that differences in the carbon footprints of feedstock CO2 from different sources will vanish. Consequently, the importance of the selection of CO2 sources with respect to climate change will shrink. However, the demand for renewable energy to provide the feedstock CO2 can still be reduced by 31% by following the environmental merit-order obtained by substitution.
The importance to apply substitution for the calculation of the carbon footprint of CO2 remains even in a low carbon future, in particular, if clean energy is not available abundantly. The shortage of renewable electricity will become more critical when considering the processes for converting the captured CO2 further to chemicals and fuels. Those processes require even much higher amounts of clean energy.5 As the enormous requirements for CO2 utilization technologies are associated with higher energy and material requirements, the analysis of the life-cycle-wide resource footprints should complement the carbon footprint.91
The present contribution aims to provide a common basis and starting point for the assessment of CO2 as a carbon footprint. Depending on the real-world situation, the analysis might need to be adapted and further refined. For example, our analysis assumed that a plant with CO2 capture directly substitutes a plant without CO2 capture to 100%. The substitution might differ due to market-mediated effects. Therefore, further development to model these market-mediated effects is needed when the scale of the CO2 capture is projected to affect the current market for the main product(s) of the CO2 sources. In the absence of known market effects, assessing the difference between existing operations with and without carbon capture by implying a direct 100% market substitution creates a consistent and comparable approach for determining the carbon footprint of CO2.
Carbon capture has been shown to reduce greenhouse gas emissions at the CO2 source. Still, it is important to realize that CO2 reductions from carbon capture are not always included in carbon pricing schemes. In the EU ETS, for example, captured CO2 from processes is counted as emitted, as long as CO2 is not permanently stored, and capture from ambient air is not considered at all. Thus, the EU ETS does not account for the climate benefits of CCU. In contrast, the current draft of the Renewable Energy Directive RED II takes climate benefits from CO2 utilization into account and the proposed accounting scheme corresponds to the approach proposed in this work. Accounting for the climate benefits of utilizing CO2 as carbon feedstock needs to be integrated further into new LCA-based regulations and monitoring standards. The present work supports this development by proper life-cycle assessment of the carbon footprint of the carbon feedstock CO2.
Author contributions
L. J. M. conceived the idea, worked on data research and processing, developed the first draft of the methodology, and wrote the manuscript. A. K. conceived the idea, developed the first draft of the methodology, and assisted in writing the manuscript. A. B. supervised the project, conceived the idea, and assisted in writing the manuscript. All authors contributed to discussions, data research, and to finalizing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research has been funded by the Federal Ministry of Education and Research of Germany in the project Dream Resource (033RC002). V. Sick acknowledges support from the Global CO2 Initiative. STM contribution is supported by the Canada First Research Excellence Fund (CFREF). CESR contribution supported by BMBF project CO2-WIN-Connect (033RC016B). The authors would like to thank Timothy J. Skone, P. E., U.S. Department of Energy, National Energy Technology Laboratory for his review and comments on the paper's methodological approach. All recommendations and findings are attributed to the authors only. Neither the methodology in this paper nor the input data are necessarily endorsed by the European Commission for use in future legislation.References
- M. Aresta, Carbon dioxide as chemical feedstock, Wiley-VCH, Weinheim, 2010 Search PubMed.
- N. von der Assen, P. Voll, M. Peters and A. Bardow, Chem. Soc. Rev., 2014, 43, 7982–7994 RSC.
- IPCC, Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva, Switzerland.
- P. Markewitz, W. Kuckshinrichs, W. Leitner, J. Linssen, P. Zapp, R. Bongartz, A. Schreiber and T. E. Müller, Energy Environ. Sci., 2012, 5, 7281 RSC.
- A. Kätelhön, R. Meys, S. Deutz, S. Suh and A. Bardow, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 11187–11194 CrossRef PubMed.
- C. Hepburn, E. Adlen, J. Beddington, E. A. Carter, S. Fuss, N. Mac Dowell, J. C. Minx, P. Smith and C. K. Williams, Nature, 2019, 575, 87–97 CrossRef CAS PubMed.
- J. Artz, J. Kleinekorte, R. Meys, T. E. Müller, A. Sternberg, K. Thenert, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- T. Kuramochi, A. Ramírez, W. Turkenburg and A. Faaij, Prog. Energy Combust. Sci., 2012, 38, 87–112 CrossRef CAS.
- H. S. Eggleston, 2006 IPCC guidelines for national greenhouse gas inventories, Institute for Global Environmental Strategies, Hayama, Japan, 2006 Search PubMed.
- A. Schreiber, P. Zapp and W. Kuckshinrichs, Int. J. Life Cycle Assess., 2009, 14, 547–559 CrossRef CAS.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- M. Fajardy and N. Mac Dowell, Energy Environ. Sci., 2017, 10, 1389–1426 RSC.
- J. Kemper, Int. J. Greenhouse Gas Control, 2015, 40, 401–430 CrossRef CAS.
- I. Dimitriou, P. García-Gutiérrez, R. H. Elder, R. M. Cuéllar-Franca, A. Azapagic and R. W. K. Allen, Energy Environ. Sci., 2015, 8, 1775–1789 RSC.
- M. Mazzotti, R. Baciocchi, M. J. Desmond and R. H. Socolow, Clim. Change, 2013, 118, 119–135 CrossRef CAS.
- D. W. Keith, G. Holmes, D. St. Angelo and K. Heidel, Joule, 2018, 2, 1573–1594 CrossRef CAS.
- R. Baciocchi, G. Storti and M. Mazzotti, Chem. Eng. Process., 2006, 45, 1047–1058 CrossRef CAS.
- N. von der Assen, L. J. Müller, A. Steingrube, P. Voll and A. Bardow, Environ. Sci. Technol., 2016, 50, 1093–1101 CrossRef CAS PubMed.
- I. P. o. C. Change, ed., Climate Change 2013 – The Physical Science Basis. The physical science basis: Working Group I contribution to the Fifth assessment report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, 2014.
- K. S. Lackner, Energy, 2013, 50, 38–46 CrossRef CAS.
- ISO 14040:2006, Environmental management – Life cycle assessment – Principles and framework, European Committee for Standardisation, Brussels, 2016, accessed 11 January 2017.
- ISO 14044:2018, Environmental management – Life cycle assessment – Requirements and guidelines, European Committee for Standardisation, Brussels, accessed 11 January 2017.
- ISO/DIS 14067.2:2012, Greenhouse gases – Carbon footprint of products – Requirements and guidelines for quantification and communication, European Committee for Standardisation, Brussels, 2012.
- L. J. Müller, A. Kätelhön, M. Bachmann, A. Zimmermann, A. Sternberg and A. Bardow, Front. Energy Res., 2020, 8, 1 CrossRef.
- T. J. Skone, M. Mutchek, M. Krynock, G. Conney, A. Pegallapati, S. Rai, J. Chou, D. Carlson, M. Jamieson, A. Venkatesh, J. Littledield, G. G. Zaimes, S. Roman-White and E. Dale, Carbon Dioxide Utilization Life Cycle Analysis Guidance For the U.S. DOE Office of Fossil Energy, National Energy Technology Laboratory, Pittsburgh, 2019 Search PubMed.
- A. W. Zimmermann, J. Wunderlich, L. Müller, G. A. Buchner, A. Marxen, S. Michailos, K. Armstrong, H. Naims, S. McCord, P. Styring, V. Sick and R. Schomäcker, Front. Energy Res., 2020, 8, 52 CrossRef.
- V. Sick, K. Armstrong, G. Cooney, L. Cremonese, A. Eggleston, G. Faber, G. Hackett, A. Kätelhön, G. Keoleian, J. Marano, J. Marriott, S. McCord, S. A. Miller, M. Mutchek, B. Olfe-Kräutlein, D. Ravikumar, L. K. Roper, J. Schaidle, T. Skone, L. Smith, T. Strunge, P. Styring, L. Tao, S. Völker and A. Zimmermann, Energy Technol., 2019, 1901034 CrossRef.
- J. Kim, C. A. Henao, T. A. Johnson, D. E. Dedrick, J. E. Miller, E. B. Stechel and C. T. Maravelias, Energy Environ. Sci., 2011, 4, 3122 RSC.
- P. Kongpanna, V. Pavarajarn, R. Gani and S. Assabumrungrat, Chem. Eng. Res. Des., 2015, 93, 496–510 CrossRef CAS.
- M. T. Luu, D. Milani, A. Bahadori and A. Abbas, J. CO2 Util., 2015, 12, 62–76 CrossRef CAS.
- L. F. S. Souza, P. R. R. Ferreira, J. L. de Medeiros, R. M. B. Alves and O. Q. F. Araújo, ACS Sustainable Chem. Eng., 2014, 2, 62–69 CrossRef CAS.
- C. van der Giesen, R. Kleijn and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 7111–7121 CrossRef CAS PubMed.
- W. Wu, H.-T. Yang and J.-J. Hwang, Energy, 2014, 74, 753–761 CrossRef CAS.
- H. Al-Kalbani, J. Xuan, S. García and H. Wang, Appl. Energy, 2016, 165, 1–13 CrossRef CAS.
- D. Parra, X. Zhang, C. Bauer and M. K. Patel, Appl. Energy, 2017, 193, 440–454 CrossRef.
- M. Pérez-Fortes, J. C. Schöneberger, A. Boulamanti, G. Harrison and E. Tzimas, Int. J. Hydrogen Energy, 2016, 41, 16444–16462 CrossRef.
- G. Reiter and J. Lindorfer, Int. J. Life Cycle Assess., 2015, 20, 477–489 CrossRef CAS.
- V. Uusitalo, S. Väisänen, E. Inkeri and R. Soukka, Energy Convers. Manage., 2017, 134, 125–134 CrossRef CAS.
- M. Aresta and M. Galatola, J. Cleaner Prod., 1999, 7, 181–193 CrossRef.
- A. F. Clarens, J. B. Zimmerman, G. A. Keoleian, K. F. Hayes and S. J. Skerlos, Environ. Sci. Technol., 2008, 42, 8534–8540 CrossRef CAS PubMed.
- M. Matzen and Y. Demirel, J. Cleaner Prod., 2016, 139, 1068–1077 CrossRef CAS.
- M. Overcash, Y. Li, E. Griffing and G. Rice, J. Chem. Technol. Biotechnol., 2007, 82, 1023–1038 CrossRef CAS.
- X. Zhang, C. Bauer, C. L. Mutel and K. Volkart, Appl. Energy, 2017, 190, 326–338 CrossRef CAS.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- thinkstep AG, GaBi Software-System and Database for Life Cycle Engineering, thinkstep AG, Leinfelden-Echterdingen, 1992–2016 Search PubMed.
- S. D. Supekar and S. J. Skerlos, Environ. Sci. Technol., 2014, 48, 14615–14623 CrossRef CAS PubMed.
- AFNOR BPX 30-323-0, General principles for an environmental communication on mass market products. Part 0: General principles and methodological framework, AFNOR, 2015.
- BSI PAS 2050:2011, Specification for the assessment of the life cycle greenhouse gas emissions of goods and services, BSI, London, 2011.
- JRC, ILCD handbook. General guide for life cycle assessment: detailed guidance, Publications Office of the European Union, Luxembourg, 2010, EUR 24708.
- The international EPD system®, Product Category Rule: Basic Chemicals, 2015.
- WRI/WBCSD, Greenhouse gas protocol. Product life cycle accounting and reporting standard, World Resources Institute; World Business Council for Sustainable Development, Washington, DC, Geneva, Switzerland, 2011.
- S. Suh, B. Weidema, J. H. Schmidt and R. Heijungs, J. Ind. Ecol., 2010, 14, 335–353 CrossRef.
- B. Weidema, J. Ind. Ecol., 2000, 4, 11–33 CrossRef CAS.
- J. Palazzo, R. Geyer and S. Suh, J. Ind. Ecol., 2020, 31, 165 Search PubMed.
- R. J. Plevin, M. A. Delucchi and F. Creutzig, J. Ind. Ecol., 2014, 18, 73–83 CrossRef.
- S. Suh and Y. Yang, Int. J. Life Cycle Assess., 2014, 19, 1179–1184 CrossRef.
- E. S. Rubin, Int. J. Greenhouse Gas Control, 2012, 10, 181–190 CrossRef.
- S. E. Tanzer and A. Ramírez, Energy Environ. Sci., 2019, 12, 1210–1218 RSC.
- C. Moretti, A. Moro, R. Edwards, M. V. Rocco and E. Colombo, Appl. Energy, 2017, 206, 372–381 CrossRef CAS.
- A. Azapagic and R. Clift, Int. J. Life Cycle Assess., 1998, 3, 305–316 CrossRef CAS.
- A. Azapagic and R. Clift, Comput. Chem. Eng., 1995, 19, 229–234 CrossRef.
- S. Manfredi, K. Allacker, K. Chomkhamsri, N. Pelletier and D. Maia de Souza, Product Environmental Footprint (PEF) Guide, Italy, 2012 Search PubMed.
- J. C. M. Farla, C. A. Hendriks and K. Blok, Energy Convers. Manage., 1995, 36, 827–830 CrossRef CAS.
- F. Ullmann, Ullmann's encyclopedia of industrial chemistry, Wiley, Chichester, 7th edn, 2010 Search PubMed.
- M. Weiss, M. Neelis and M. Patel, Non-energy use and related CO2 emissions in Germany: a carbon flow analysis with the NEAT model for the period of 1990-2003, Utrecht University, Copernicus Institute, Utrecht, 2007 Search PubMed.
- European Commission – Integrated pollution prevention and control, Reference Document on best Available Techniques for the Manufacture of large Volume Inorganic Chemicals – Ammonia, Acids and Fertilisers, available at: http://eippcb.jrc.ec.europa.eu/reference/lvic-aaf.html.
- H. A. Haugen, N. H. Eldrup, A. M. Fatnes and E. Leren, Energy Procedia, 2017, 114, 6133–6140 CrossRef CAS.
- N. Kaliyan, R. V. Morey and D. G. Tiffany, Biomass Bioenergy, 2011, 35, 1103–1113 CrossRef CAS.
- H. S. Kheshgi and R. C. Prince, Energy, 2005, 30, 1865–1871 CrossRef CAS.
- K. Möllersten, J. Yan and J. R. Moreira, Biomass Bioenergy, 2003, 25, 273–285 CrossRef.
- E. A. Quadrelli, G. Centi, J.-L. Duplan and S. Perathoner, ChemSusChem, 2011, 4, 1194–1215 CrossRef CAS PubMed.
- Thomson Reuters Eikon, 2018, https://eikon.thomsonreuters.com/index.html.
- Food and Agricultural Policy Research Institute, FAPRI U.S. and World Agricultural Outlook, 2012.
- M. M. J. de Jonge, J. Daemen, J. M. Loriaux, Z. J. N. Steinmann and M. A. J. Huijbregts, Int. J. Greenhouse Gas Control, 2019, 80, 25–31 CrossRef CAS.
- S. Bringezu, J. Ind. Ecol., 2014, 18, 327–340 CrossRef CAS.
- Eurostat, the Statistical Office of the European Union, Energy statistics – supply, transformation and consumption (nrg_10). Primary production nrg_109a, 2017.
- The European biomass association, A biogas road map for europe, Brussels, 2009 Search PubMed.
- The Hague, GAIN Report, 2017.
- I. Aouini, A. Ledoux, L. Estel and S. Mary, Oil Gas Sci. Technol., 2014, 69, 1091–1104 CrossRef.
- B. Johnke, Good Practice Guidance and Uncertainty Management in National Greenhouse Gas Inventories, Emissions from waste incineration, Hayama, Japan, 2002 Search PubMed.
- S. Ödberg, Design of Partial CO2 Capture from Waste Fired CHP Plants, Chalmers University of Technology, Gothenburg, 2017 Search PubMed.
- N. Pour, P. A. Webley and P. J. Cook, Int. J. Greenhouse Gas Control, 2018, 68, 1–15 CrossRef CAS.
- Y. Tang and F. You, ACS Sustainable Chem. Eng., 2018, 6, 937–956 CrossRef CAS.
- A. Sternberg and A. Bardow, Energy Environ. Sci., 2015, 8, 389–400 RSC.
- Forschungsstelle für Energiewirtschaft e.V., Kurzstudie Power-to-X. Ermittlung des Potenzials von PtX-Anwendungen für die Netzplanung der deutschen ÜNB, Munich, 2017.
- M. Kuprat, M. Bendig and K. Pfeiffer, Front. Energy, 2017, 11, 135–145 CrossRef.
- D. Sandalow, J. Friedman, R. Aines, C. McCormick, S. McCoy and J. Stolaroff, ICEF Industrial heat decarbonization roadmap. Draft for comment, 2019.
- V. Kyriakou, I. Garagounis, E. Vasileiou, A. Vourros and M. Stoukides, Catal. Today, 2017, 286, 2–13 CrossRef CAS.
- A. Bazzanella and F. Ausfelder, Low carbon energy and feedstock for the European chemical industry, Frankfurt am Main, 2017 Search PubMed.
- A. Sternberg, C. M. Jens and A. Bardow, Green Chem., 2017, 19, 2244–2259 RSC.
- W. Hoppe, N. Thonemann and S. Bringezu, J. Ind. Ecol., 2018, 22, 327–340 CrossRef CAS.
- A. Sternberg and A. Bardow, ACS Sustainable Chem. Eng., 2016, 4, 4156–4165 CrossRef CAS.
- L. K. Rihko-Struckmann, A. Peschel, R. Hanke-Rauschenbach and K. Sundmacher, Ind. Eng. Chem. Res., 2010, 49, 11073–11078 CrossRef CAS.
- G. A. Olah, A. Goeppert and G. K. S. Prakash, J. Org. Chem., 2009, 74, 487–498 CrossRef CAS.
- S. Deutz, D. Bongartz, B. Heuser, A. Kätelhön, L. Schulze Langenhorst, A. Omari, M. Walters, J. Klankermayer, W. Leitner, A. Mitsos, S. Pischinger and A. Bardow, Energy Environ. Sci., 2018, 11, 331–343 RSC.
- P. Schmidt, V. Batteiger, A. Roth, W. Weindorf and T. Raksha, Chem. Ing. Tech., 2018, 90, 127–140 CrossRef CAS.
- European Environmental Agency, EU bioenergy potential from a resource-efficiency perspective. EEA Report No. 6/2013, 2013.
- ICCT, What is the role for renewable methane in European decarbonization?, Beijing, 2018 Search PubMed.
- OECD-FAO Agricultural Outlook 2016–2025. Special focus: Sub-Saharan Africa, OECD, Paris, France, 2016.
- D. Nuber, H. Eichberger and B. Rollinger, Millenium Steel, 2006, 37–40 Search PubMed.
- K. Schumacher and R. D. Sands, Energy Economics, 2007, 29, 799–825 CrossRef.
- A. Otto, M. Robinius, T. Grube, S. Schiebahn, A. Praktiknjo and D. Stolten, Energies, 2017, 10, 451 CrossRef.
- Worldsteel association, Steel Statistical Yearbook, 2013.
- T. Ekbom, M. Lindblom, N. Berglin and P. Ahlvik, Technical and Commercial Feasibility Study of Black Liquor Gasification with Methanol/DME Production as Motor Fuels for Automotive Uses - BLGMF, Nykomb Synergetics AB, Stockholm, 2003 Search PubMed.
- J. M. Joelsson and L. Gustavsson, Energy, 2012, 39, 363–374 CrossRef CAS.
- Review of Waste Policy and Legislation, 2016.
- T. Schwarzböck, H. Rechberger, O. Cencic and J. Fellner, Österr Wasser- und Abfallw, 2016, 68, 415–427 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ee01530j |
| This journal is © The Royal Society of Chemistry 2020 |