Evaluation of the OECD POV and LRTP screening tool for estimating the long-range transport of organophosphate esters†
Roxana
Sühring‡
 ab,
Martin
Scheringer
c,
Timothy F. M.
Rodgers
d,
Liisa M.
Jantunen
ae and
Miriam L.
Diamond
*ad
ab,
Martin
Scheringer
c,
Timothy F. M.
Rodgers
d,
Liisa M.
Jantunen
ae and
Miriam L.
Diamond
*ad
aUniversity of Toronto, Department of Earth Sciences, 22 Russell Street, Toronto, Canada M5S 3B1. E-mail: miriam.diamond@utoronto.ca
bLeuphana University Lüneburg, Institute of Sustainable and Environmental Chemistry, Scharnhorststraße 1, 21335 Lüneburg, Germany
cRECETOX, Masaryk University, 625 00 Brno, Czech Republic
dUniversity of Toronto, Department of Chemical Engineering and Applied Chemistry, 200 College Street, Toronto, Canada M5S 3E5
eAir Quality Processes Research Section, Environment, Egbert, ON L0L 1N0, Canada
First published on 13th December 2019
Abstract
Scientists and decision makers need accurate, accessible and fast tools to assess and prioritize the persistence (POV) and environmental long-range transport potential (LRTP) of chemicals. Here we evaluated the Organisation for Economic Co-operation and Development (OECD) POV and LRTP Screening Tool (“the Tool”) with respect to the POV and LRTP estimates that the Tool provides for organophosphate esters (OPEs). We found that the use of default parameter values could significantly underestimate POV and LRTP values of OPEs and, potentially, other Persistent Mobile Organic Compounds (PMOCs), by not accounting for episodic atmospheric transport and poleward river-based transport in the northern hemisphere. Specifically, sensitivity and Monte Carlo uncertainty analyses indicate that non-chlorinated OPEs could be subject to LRTP when uncertainties in gas-particle partitioning and its implications for atmospheric degradation are considered, and chlorinated OPEs when river-based transport is considered. Further, the analyses showed strong dependence of results on the accuracy of the environmental half-lives used as input parameters. We suggest that the Tool could be modified to include an optional “Arctic (PMOC) LRTP setting” that incorporates episodic atmospheric and river-based transport as well as decreased environmental half-lives due to cold temperatures.
Environmental significanceOrganophosphate esters (OPEs) are widely used as alternatives for brominated flame retardants (BFRs) because they are presumed to be less persistent (P) in the environment and don't undergo long-range transport (LRT). This presumption is supported by estimates made by the OECD POV and LRTP Screening Tool of low P and LRT for most OPEs. However measurements in remote regions show OPEs in concentrations exceeding those of BFRs. Here we evaluated the estimates of P and LRT of OPEs calculated by the OECD POV and LRTP Tool regarding uncertainties, transport pathways, and transport mechanism of OPEs. The results indicate the potential of some OPEs to undergo LRT by air and by water. The model supports the hypothesis that chlorinated OPEs are PMOCs – persistent mobile organic compounds. Further, we suggest changes to the Tool to account for transport mechanisms enabling PMOCs and other compounds to undergo LRT, such as episodic air and riverine transport. |
1. Introduction
Chemical pollution is a major threat to human and environmental health.1 Many chemicals enter the environment during and after use, cycle among environmental media, and can undergo long-range transport, resulting in local to global contamination of biota, human food and drinking water.Overall persistence (POV) and long-range transport potential (LRTP) are key criteria used to assess chemical hazards associated with the distribution of chemicals in the environment. For example, persistent organic pollutants (POPs) are typically defined as compounds that are persistent (P) in the environment, bioaccumulate (B) in biota, are toxic (T) to human or ecosystem health and have a potential for environmental long-range transport (LRT).2 A substance is considered persistent under the Stockholm Convention if it has a half-life of more than two months in water or more than six months in sediment or soil.2 Long-range environmental transport potential is indicated if a substance can be measured in locations distant from its sources and in concentrations that are of “potential concern”. Monitoring data and environmental fate properties used in transport models can show and evaluate, respectively, the potential for long-range transport.2
The PBT framework has proven to be effective for the assessment of “legacy” highly hydrophobic, neutral persistent organic pollutants (POPs) such as polychlorinated biphenyls (PCBs) under, for example, the Stockholm Convention.2 The convention has been updated to include newer compounds of concern, such as the polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane HBCDD.3–5 However, Reemtsma et al.,6 among others, have drawn attention to the gaps in current regulatory assessment tools for compounds that are persistent, mobile, and toxic but are not bioaccumulative, and so do not meet the PBT criteria. These persistent and mobile organic compounds (PMOCs) are typically more polar than legacy PBT compounds and so the concern is about accumulation in water cycles, rather than in biota,6 which is not yet a consideration under the Stockholm Convention.
To support hazard assessments of PBT-LRT compounds, an OECD/UNEP Workshop was held in Ottawa in 2001 on the use of multimedia models for estimating overall persistence and long-range transport in the context of PBTs/POPs.7 Participants agreed to create a multimedia model to assess LRTP that would be available for distribution to policymakers and to the public. This model, the OECD POV and LRTP Screening Tool (the Tool),8 was developed following detailed model comparison by the expert group from the OECD/UNEP Workshop on Multimedia Models. Since 2006, the Tool has been freely available as a downloadable file from the OECD website.9 The model has been used extensively within government agencies tasked with assessing the hazard of chemicals and by research groups.10
The Tool was designed to provide guidance for legacy non-ionizing and highly hydrophobic POPs such as PCBs, hexachlorobenzene (HCB) and other organochlorine pesticides.11 There are several reasons for this. The physical–chemical properties of these compounds are relatively well known and are well represented in the training sets of QSARs developed to estimate physical–chemical properties that are inputs to the Tool, such as those in the US EPA's EPI Suite.12 Second, gas-particle partitioning of these compounds can be estimated based on models using the octanol–air partition coefficient.13 Legacy POPs, by definition, tend to be highly persistent and as such, uncertainties in environmental degradation half-lives are less critical than for less persistent compounds (which also tend to be more polar or ionize).
Developers of the Tool drew attention to the model's sources of uncertainty. Scheringer et al. commented that one source of model uncertainty was the description of LRTP of particle-sorbed chemicals;14,15 in particular that the model is not able to capture episodic transport of particle-sorbed chemicals such as BDE-209.15 Other obvious sources of uncertainty come from uncertainty in input data, notably physical–chemical properties (the partition coefficients between octanol and water and air and water, KOW and KAW) and degradation half-lives in air, water and soil8 where the latter are notoriously uncertain. Despite these uncertainties and limitations, the Tool has been widely used for initial screening assessments of POV and LRTP of persistent and non-persistent compounds alike.
Organophosphate esters (OPEs) are high-production-volume chemicals with a global market volume of >600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes per year in 2013 (30% of the global flame retardant market volume).16 They are used in many applications, including as plasticizers and flame retardants.17 As flame retardants, they have been used as replacements for PBDEs including as components of FireMaster 550. Like PBDEs, OPEs are additive flame retardants and so can migrate from the treated polymer by volatilization, dissolution and abrasion.17 However, orders of magnitude higher air concentrations and emission estimates suggest a larger range of uses for OPEs compared to PBDEs at their peak usage, even when the higher vapour pressures of OPEs are considered.18,19 Urban areas, with high OPE concentrations in indoor and outdoor air as well as surface water,20–22 have been hypothesized as important OPE sources to remote environments. For example, Rodgers et al.18 estimated that OPE air emissions from Toronto, Canada in 2010 were 340 times higher than PBDE emissions in 2008, the year penta- and octa-BDEs were regulated in that country.
000 tonnes per year in 2013 (30% of the global flame retardant market volume).16 They are used in many applications, including as plasticizers and flame retardants.17 As flame retardants, they have been used as replacements for PBDEs including as components of FireMaster 550. Like PBDEs, OPEs are additive flame retardants and so can migrate from the treated polymer by volatilization, dissolution and abrasion.17 However, orders of magnitude higher air concentrations and emission estimates suggest a larger range of uses for OPEs compared to PBDEs at their peak usage, even when the higher vapour pressures of OPEs are considered.18,19 Urban areas, with high OPE concentrations in indoor and outdoor air as well as surface water,20–22 have been hypothesized as important OPE sources to remote environments. For example, Rodgers et al.18 estimated that OPE air emissions from Toronto, Canada in 2010 were 340 times higher than PBDE emissions in 2008, the year penta- and octa-BDEs were regulated in that country.
Furthermore, Sühring et al.,23 Jantunen et al.,24 Salamova et al.25 and Möller et al.26 have measured several OPEs in Arctic air at concentrations up to 1000 times that of current levels of PBDEs. OPE concentrations in Arctic air and water measured by Jantunen et al. (unpubl. data) are as high as 250 pg m−3 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg l−1, for TCEP, TCIPP and triphenyl phosphate (TPhP), respectively. McDonough et al.27 reported concentrations of dissolved OPEs from deep-water moorings in the Fram Strait of up to 440 pg l−1 (Σ11OPEs) and median concentrations of up to 4400 pg l−1 for chlorinated OPEs in Arctic surface water.
000 pg l−1, for TCEP, TCIPP and triphenyl phosphate (TPhP), respectively. McDonough et al.27 reported concentrations of dissolved OPEs from deep-water moorings in the Fram Strait of up to 440 pg l−1 (Σ11OPEs) and median concentrations of up to 4400 pg l−1 for chlorinated OPEs in Arctic surface water.
Despite the high solubility of OPEs in water, Arctic sediment concentrations for Σ7OPEs of 159–4658 pg g−1 dry weight have been observed.28
The Tool estimates low to medium LRTP for most OPEs.29,30 Sühring et al.23 hypothesized that some OPEs detected in the Canadian Arctic might have local sources, similarly to for the source of some PBDEs in the Antarctic reported earlier.31 Indications were also found that chlorinated OPEs could be primarily transported via water (including discharge of rivers) rather than by long-range atmospheric transport.23 Water-based transport could explain high observed atmospheric OPE concentrations close to river mouths in the Canadian Arctic either due to emissions of OPEs in sea-spray aerosols from breaking waves (similar to processes reported by Johansson et al. 2019 (ref. 32) for perfluoroalkyl acids) or possible from the “salting out” effect causing some increase in volatilization of OPEs discharged from the river when they reach the ocean. Rodgers et al.18 observed that the chlorinated OPEs, including tris(2-chloroethyl)phosphate (TCEP) and tris(1-chloro-2-propyl)phosphate (TCIPP), had very high (>95%) mobility in water and therefore fit the profile of PMOCs. Another explanation is that the Tool does not adequately capture gas-particle partitioning of some OPEs.29,30 Measurements of OPEs with low- and high-volume active air samplers have mostly characterized them as particle-phase compounds.33–35 In contrast, air measurements made using low density polyethylene passive samplers and models of gas-particle partitioning indicate that several OPEs, such as TCEP and TCIPP, are or should be mainly in the gas-phase.36–38 Okeme et al.36 posited that measurements of OPEs on the filters of active air-samplers could be due to a measurement artefact of gas-phase OPEs sorbing to the filter. They showed that the LRTP of chlorinated OPEs (estimated by the Tool) was dominated by water advection if gas-particle partitioning followed modelled KOA-based predictions rather than the assumption of particle-bound OPEs typically made in empirical studies. The results supported the hypothesis that water transport could be an important LRT mechanism for chlorinated OPEs (while there are uncertainties in these measurements).
Here we integrate our findings from measured and estimated results for the environmental behaviour and LRTP of OPEs in order to evaluate the factors in the Tool that determine whether an OPE is expected to have LRTP or not and we place these findings into the context of known LRTP mechanisms.
2. Methods
We used the OECD POV and LRTP Screening Tool (Version 2.2 (ref. 8)) to estimate the LRTP of the 35 OPEs that Sühring et al.39 assessed for gas-particle partitioning behaviour. LRTP in the Tool is expressed through the characteristic travel distance (CTD) of a compound in km. A complete list of all targeted OPEs including acronym, IUPAC name and CAS number is presented in the ESI Table S1.†Information on input data for OPEs and the methods used by the Tool to estimate gas-particle partitioning, CTD and persistence (POV) of contaminants have been described in detail by Sühring et al.39 In short, OPE data for input parameters were extracted from 67 publications (1979–2015). Data points below Q1 − 1.5IQR or above Q3 + 1.5IQR were removed from the input dataset as outliers, with Q1 = the first quartile or 25th percentile, Q3 = the third quartile or 75th percentile, and IQR the interquartile range defined as Q3 − Q1 (Table S2†).
Similar to Sühring et al.,39 a local sensitivity analysis was performed to assess the sensitivity of POV and CTD to variability in input data. The sensitivity analysis was performed as described by Morgan and Henrion.40 The sensitivity of POV and CTD to a specific input parameter was tested by changing the input parameters one at a time and calculating the impact on POV and CTD. A factor of 1.1 was chosen for the change of parameter values (Tables S3 and S4†). The sensitivity (S) of POV and CTD to an individual parameter was quantified using:
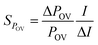 | (1) |
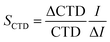 | (2) |
A particular focus was put on compounds that have been reported in the Arctic by Möller et al.,26 Salamova et al.25 and Sühring et al.23 For these compounds, additional tests were performed.
To test for potential combination effects of input data uncertainty and to quantify the overall uncertainty of the Tool, a Monte Carlo (MC) uncertainty analysis was performed. We used the Monte Carlo (MC) analysis function that is integrated in the Tool to perform the analysis. The built-in MC algorithm of the Tool assumes that the test values are distributed log-normally.8 For the MC, the Tool automatically varies all input parameters simultaneously (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW, log
KOW, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW, half-life air, half-life water, half-life soil) randomly within a defined range (the so-called “dispersion factor”). In this analysis a dispersion factor of 5 for log
KAW, half-life air, half-life water, half-life soil) randomly within a defined range (the so-called “dispersion factor”). In this analysis a dispersion factor of 5 for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW, log
KOW, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW, and a factor of 10 for the environmental half-lives was used.
KAW, and a factor of 10 for the environmental half-lives was used.
The MC analysis option in the Tool automatically generates a set of random values from the distribution of each input parameter, using the dispersion factor. We generated a set of 200 random input data sets based on the varied input parameters. POV and CTD were then calculated for these 200 input data sets. Thereby, the MC uncertainty analysis accounted for potential combined effects of input data uncertainty on the estimated POV and CTD (Tables S5–S7†).
The results of the MC analysis included information on the contribution of the different input parameters to the variance of POV and CTD as well as information on the estimated range of POV and CTD for each analysed OPE (95% confidence interval).
In addition, we tested the impact of increased atmospheric and water half-lives due to particle sorption, episodic atmospheric transport and episodic water-based transport (e.g. riverine) on the CTD and POV estimates. To this end, the default environmental parameters in the Tool source code were adjusted to simulate the following environmental conditions:
(a) Wind speed: episodic transport has been hypothesised as one of the reasons for the Tool to underestimate the LRTP of OPEs. Especially during the winter, transport from industrialized areas into the Arctic could be significantly enhanced.41 To simulate this effect, we compared the Tool results at the default wind speed of 4 m s−1 with the greater transport of 8 m s−1 (Table S8†).
(b) Water coverage: the Tool uses globally averaged environmental conditions to calculate the POV and CTD of contaminants.8 The default value for water coverage of the earth surface is set to 71%. For emissions in the northern hemisphere, the water coverage between emission sources and the Arctic is significantly less than this default value. To assess the impact on partitioning and subsequent transport behaviour of OPEs, we compared the default values in the Tool of 71% coverage and 100 m water depth (representing oceans) with reduced water coverage of 10% and 30 m water depth (representing lakes and rivers) (Table S9†).
(c) Sühring et al.23 hypothesized that chlorinated OPEs (Cl-OPEs), such as TCEP, tris(1,3-dichloroisopropyl)phosphate (TDCPP) and TCIPP, are transported into the Canadian Arctic through water-based transport, consistent with their classification as PMOCs. The Tool assesses water-based LRTP only based on oceanic transport, while potential transport by rivers is neglected. To assess the effect of river-based transport we compared the Tool's results for CTD at the default flow velocity of 0.02 m s−1 with an estimated enhanced river flow velocity of 0.04 m s−1. This value was based on average depth,42,43 annual discharge44,45 and a rough estimate of the average width of the Nelson and Churchill (MB) rivers,46,47 with average depth of 30 m and 18 m, respectively; average width = 2000 m and 1500 m,46,47 and average discharge 3486 m3 s−1, and 1200 m3 s−1.44,45 The Nelson and Churchill rivers are 644 km and 1609 km long46,47 and have watersheds of 1.1 × 106 and 2.8 × 105 km2, respectively. Both the Nelson and Churchill (MB) rivers discharge into Hudson Bay.
For these tests, we adjusted the default environmental parameters in the Tool (one at a time) at different environmental half-lives (from 0–500 h and 0–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h for half-life in air and half-life in water, respectively). For these tests log
000 h for half-life in air and half-life in water, respectively). For these tests log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW and log
KOW and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW were not changed and only one environmental half-life was changed at a time. The goal for this analysis was to investigate whether e.g. episodic transport could explain the LRT of OPEs at all and how slow the environmental half-lives would have to be to see a LRT for OPEs with the Tool.
KAW were not changed and only one environmental half-life was changed at a time. The goal for this analysis was to investigate whether e.g. episodic transport could explain the LRT of OPEs at all and how slow the environmental half-lives would have to be to see a LRT for OPEs with the Tool.
Stroebe et al. (2004) reported that transport in water and air were not coupled for most of the non-polar organic contaminants they investigated.48 We therefore hypothesized that the water- and air-based transport of OPEs in the Tool is independent of, and dominated by the transport in the receiving medium from the emission scenario (i.e. CTD from emission into water = CTD in water or CTDW; CTD from emission into air = CTD in air or CTDA). This hypothesis was supported by model results obtained from changing the water velocity and wind speed in the Tool, respectively: increasing the respective velocities only changed the CTD for the corresponding emission scenario (CTDA in the case of emission into air with increased wind speed and CTDW in the case of emission into water and increased flow velocity) (Table S10†).
3. Results and discussion
3.1 Tool results for POV and CTD of OPEs
Under the default settings of the Tool, the POV and CTD varied considerably between different OPEs as well as for different media (water and air) (Table 1). Triisobutyl phosphate (TIBP) had the overall lowest POV in air (PAOV) with 0.43 days (d). Tri-n-butyl phosphate (TnBP) had the lowest POV in water (PWOV) with 12 d (Table 1). The highest PAOV was 978 d for tris(2,3-dichloro-1-propyl)phosphate (MC 984) and 527 d in water for bis(1,3-dichloro-2-propyl)phosphate (BDCPP) (Table 2). BDCPP also displayed the highest CTDW with 897 km and the lowest CTDA with 2.2 km (Table 2). The lowest CTDW was estimated for TnBP with 21 km (Table 1). The highest CTDA was estimated for tetrakis(2,6-dimethylphenyl)-m-phenylene biphosphate (PBDMPP) at 2860 km (Table 1).| Non-Cl OPEs | P OV air | CTD air | P OV water | CTD water |
|---|---|---|---|---|
| EHDPP* | 45 | 234 | 47 | 80 |
| TBOEP* | 63 | 41 | 27 | 47 |
| TIBP* | 0.43 | 67 | 21 | 36 |
| TnBP* | 0.49 | 67 | 12 | 21 |
| TPhP* | 7.0 | 437 | 47 | 82 |
| BEHP | 58 | 346 | 399 | 601 |
| BMPPP | 28 | 562 | 55 | 93 |
| BPA-BDPP | 85 | 2010 | 87 | 112 |
| BPDP | 39 | 989 | 55 | 87 |
| DCP | 21 | 369 | 54 | 93 |
| DOPO | 27 | 536 | 54 | 93 |
| IDDPP | 56 | 231 | 134 | 215 |
| IPPP | 71 | 2750 | 87 | 79 |
| PBDMPP | 432 | 2860 | 260 | 121 |
| PBDPP | 78 | 2380 | 55 | 91 |
| TDMPP | 89 | 1970 | 55 | 72 |
| TEHP | 49 | 1480 | 14 | 22 |
| TEP | 0.70 | 70 | 22 | 37 |
| TiPP | 0.48 | 57 | 21 | 37 |
| TMP | 6.2 | 549 | 22 | 37 |
| TmCP | 55 | 977 | 55 | 92 |
| ToCP | 213 | 602 | 55 | 92 |
| TpCP | 106 | 977 | 55 | 92 |
| T2iPPP | 136 | 2740 | 55 | 62 |
| TTBPP | 340 | 2850 | 260 | 121 |
| TXP | 60 | 2412 | 87 | 87 |
| P OV air | CTD air | P OV water | CTD water | |
|---|---|---|---|---|
| Cl-OPEs | ||||
| TCEP* | 36 | 179 | 130 | 225 |
| TCIPP* | 61 | 117 | 196 | 338 |
| TDCIPP* | 575 | 107 | 260 | 445 |
| BCMP-BCEP | 495 | 8.8 | 260 | 446 |
| BDCPP | 515 | 2.2 | 527 | 897 |
| MC 984 | 978 | 1530 | 260 | 424 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Br-OPEs | ||||
| TDBPP | 112 | 139 | 87 | 149 |
| TTBNPP | 423 | 2850 | 260 | 139 |
In general, Cl-OPEs were estimated to be primarily transported in water, while non-Cl OPEs and Br-OPEs were estimated to be primarily transported in air (Tables 1 and 2). Exceptions were the chlorinated MC 984, which had a high CTDA of 1530 km and the non-Cl OPE bis(2-ethylhexyl)phosphate (BEHP) with CTDW > CTDA (Tables 1 and 2).
It is interesting that brominated Br-OPEs displayed behaviour similar to non-Cl-OPEs rather than to the chlorinated Cl-OPEs.
As noted by Liagkouridis et al.,30 Zhang & Sühring et al.29 as well as Sühring et al.,23 the Tool did not estimate POV or CTD sufficient for LRTP for OPEs that have been reported in the Arctic and other remote regions (Tables 1 and 2). None of the OPEs reported in the Arctic with detection frequencies >20%, namely ethylhexyldiphenyl phosphate (EHDPP), tris(2-butoxyethyl)phosphate (TBOEP), TIBP, TnBP, TPhP, TCEP, TCIPP and TDCIPP, had a CTD > 500 km in air or water (Tables 1 and 2).
To identify reasons for these differences in model results and observations we investigated several explanations.
3.2 Impact of input data uncertainty on tool results
A likely source of uncertainty and potential error in the model results comes from the uncertainty of physical–chemical properties (KOW and KAW) and degradation half-lives in air, water and soil used as input data.8 Sühring et al.39 found that the uncertainty of KOW and KAW did not significantly alter the predicted gas-particle partitioning of the OPEs. Physical–chemical properties of some of the OPEs can have uncertainties of up to five orders of magnitude, especially for KAW,39 although other properties were usually within two orders of magnitude variation.39 Degradation half-lives are particularly uncertain due to the number of mechanisms involved, natural variability under different conditions, and the challenges of both measuring and predicting “average” rates (or even defining what “average” environmental conditions are). CTD and POV are primarily related to the environmental half-lives of the tested compounds.Interestingly, our sensitivity analysis using the Tool's default environmental parameters found low sensitivities to individually changed input parameters by a factor of 1.1 for all OPEs (Tables S3 and S4†). POV had the highest sensitivity to changes in half-life in soil (t1/2 soil) with a median SPOV of 17%, but ranged from 7 × 10−4% (isopropylphenyl phosphate, IPPP) to 52% (1-propanol, 2,3-dibromo-, 1,1′,1′′-phosphate, TDBPP) (Table S3†). Half-life in water (t1/2 water) had a higher impact on the POV than t1/2 soil for six out of the 35 tested OPEs, namely BEHP, IPPP, TPhP, phenol-dimethyl-phosphate (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (TXP) and the Cl-OPEs BDCPP and TCEP with SPOV between 9% (TCEP) and 17% (BDCPP) (Table S3†).
1) (TXP) and the Cl-OPEs BDCPP and TCEP with SPOV between 9% (TCEP) and 17% (BDCPP) (Table S3†).
For CTD the sensitivity was even lower, with SCTD < 1% for most input parameters and none exceeding 10% (Table S4†). Neither Cl-/Br-OPEs nor non-Cl-OPEs were sensitive to changes in atmospheric half-lives (t1/2 air) even though Sühring et al.23 found indications that non-Cl OPEs are primarily transported via the atmosphere. Rodgers et al.18 similarly found that emissions of OPEs from Toronto were not highly sensitive to t1/2 air, although they did find that for OPEs modelled in the gas-phase such as TCEP and TCIPP.
Likewise, only 10 out of 35 OPEs showed a SCTD > 1% for t1/2 water (Table S4†).
The MC analysis resulted in dispersion factors for POV and CTD between 3.3 for the estimated POV of TCIPP and 8.1 for the estimated CTD of TnBP (Table S5†). The environmental half-lives contributed most (55–100%) of the total variance. The exception was the POV for TCIPP. Here, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW had the highest contribution to the variance with 44% (Table S6†).
KOW had the highest contribution to the variance with 44% (Table S6†).
According to the MC analysis results, TBOEP, TIBP, and TnBP had a CTD of less than 100 and 500 km at the median and 95th percentile, respectively. EHDPP, TCEP, and TCIPP had median CTDs below 500 km but CTDs of over 1000 km at the 95th percentile. TPhP and TDCIPP were the only OPEs with median CTDs of 500 km or more based on the MC analysis. Moreover, TDCIPP had a CTD of over 2000 km at a 95th percentile (Table S7†). These results indicate that the uncertainty of environmental half-lives could explain the lack of LRTP estimated by the Tool for, at least, TPhP and TDCIPP.
Considering the high uncertainty of environmental half-life data and its apparent impact on the model results for at least two of the tested OPEs (TPhP and TDCIPP), we decided to analyse the potential changes to the Tool estimates due to the inclusion of episodic transport, sorption to the particle phase and impact of water-mass in combination with uncertain environmental half-lives.
3.3 Episodic transport, particle-adsorption and impact of water mass on the Tool results
Under-estimation of LRTP for non-Cl OPEs with primary transport in air, such as TPhP, TIBP and potentially EHDPP, could be caused by the Tool underestimating their sorption to atmospheric particles. Sorption could reduce degradation and thereby substantially increase their atmospheric half-lives.49We assessed the impact of increased t1/2 air input data (assuming a reduced degradation of particle-bound OPEs) on the estimated CTD of eight OPEs that had been detected in the Arctic but for which the Tool estimated a low CTD, as well as bisphenol A bis(diphenyl phosphate) (BPA-BDPP) as a reference compound with a high CTD in air. The eight compounds investigated included three Cl-OPEs: TCEP, TCIPP and TDCIPP and five non-Cl OPEs: EHDPP, TBOEP, TIBP, TnBP and TPhP.
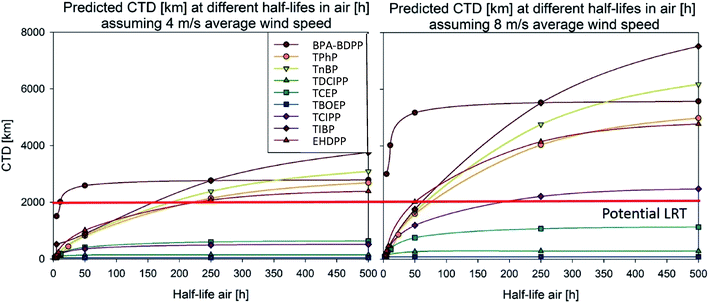
We observed that the Tool estimated long-range atmospheric transport of over 2000 km of non-Cl-OPEs when t1/2 air > 150 h (Fig. 1, left). For Cl-OPEs, t1/2 air did not have a significant impact on the estimated atmospheric long-range transport (Fig. 1, left). A higher particle-bound fraction could therefore be an explanation for the observed long-range transport of non-Cl-OPEs, but not for Cl-OPEs.
An alternative hypothesis could be that episodic atmospheric transport14,15 could increase the CTD of OPEs. To test this hypothesis, we assessed the impact of episodic transport on the CTD of the same eight OPEs detailed above with the default settings (wind speed = 4 m s−1 (Fig. 1, left)) and with an increased wind speed of 8 m s−1 (Fig. 1, right).
Similarly to the effect of increased t1/2 in air due to potential adsorption onto particles, an increased wind speed led to estimated CTDs of > 2000 km for non-Cl OPEs, whereas most Cl-OPEs were not found to have long-range atmospheric transport, even when high wind speed was combined with increased t1/2 in air (Fig. 1, right).
We conclude that increased wind speed representing episodic atmospheric transport and increased air degradation half lives representing greater sorption to particles could be relevant factors in the transport of non-Cl OPEs into the Canadian Arctic, while it could not explain the LRT of Cl-OPEs. The lack of atmospheric transport of Cl-OPEs could result from the Tool calculating that most of the Cl-OPEs primarily partition into the water, making them insensitive to changes in the air compartment.
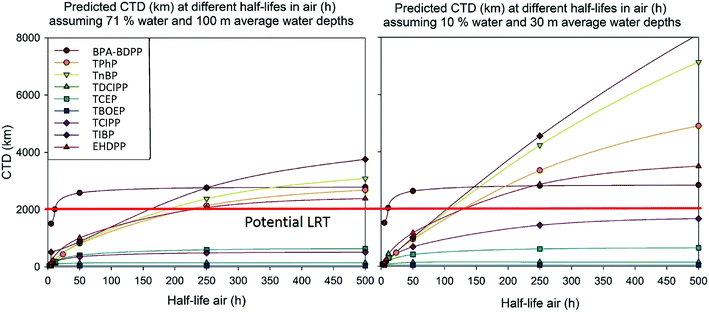
To assess the impact of distribution from air into water, we tested the LRTP of the eight compounds at a reduced water mass of 10% and 30 m depth (Fig. 2) to represent rivers and lakes, characteristic of the Canadian landmass, rather than the dominance of oceans as per the Tool's default values. The observed effect was similar to the impact of increased wind speed: the transport of non-Cl OPEs was accelerated with reduced water mass, while Cl-OPEs still reached their maximum CTD at below 2000 km (Fig. 2).
3.4 Water-based transport of OPEs
Wania et al.50 established a classification system for chemical transport into the Arctic based on physical–chemical properties. Most of the tested OPEs were outside the domain of this model for at least one parameter due to the narrow range of physical–chemical properties used by Wania et al.50 of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW = −4 to 3 and log
KAW = −4 to 3 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA = 3 to 12. However, the comparison with the classification system by Wania et al.50 still returned interesting results. Wania et al.50 noted that chemicals expected to undergo coupled transport in the atmosphere and oceans would have the highest tendency for transport to Polar regions. According to Wania et al.,50 these chemicals would typically have a log
KOA = 3 to 12. However, the comparison with the classification system by Wania et al.50 still returned interesting results. Wania et al.50 noted that chemicals expected to undergo coupled transport in the atmosphere and oceans would have the highest tendency for transport to Polar regions. According to Wania et al.,50 these chemicals would typically have a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW between −2 and −4 (and potentially lower) and a log
KAW between −2 and −4 (and potentially lower) and a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOA between 3 and 10. The non-Cl OPEs detected in highest concentrations or most frequently in the Arctic,23 TPhP and TnBP, fall into that category. This is consistent with the hypothesis of Sühring et al.23 that TPhP could have mixed water and atmospheric transport based on its CTD and POV estimates from the Tool. The Cl-OPEs, BDCPP, TCEP, TCIPP, and TDCIPP, on the other hand, have log
KOA between 3 and 10. The non-Cl OPEs detected in highest concentrations or most frequently in the Arctic,23 TPhP and TnBP, fall into that category. This is consistent with the hypothesis of Sühring et al.23 that TPhP could have mixed water and atmospheric transport based on its CTD and POV estimates from the Tool. The Cl-OPEs, BDCPP, TCEP, TCIPP, and TDCIPP, on the other hand, have log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KAW values of −8.82 to −6.04 and log
KAW values of −8.82 to −6.04 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW values of 1.63 to 3.89, which classified them as “swimmers” or “multimedia chemicals” at the transition between “swimmers” and particle-bound “single hoppers”, which matched the results from the Tool that water would be the primary transport pathway. As suggested by Rodgers et al., the “PMOC” nature of Cl-OPEs (e.g. high mobility in water) is consistent with the hypothesis of Sühring et al.23 that water-based transport could be an important pathway for Cl-OPEs into the Canadian Arctic.
KOW values of 1.63 to 3.89, which classified them as “swimmers” or “multimedia chemicals” at the transition between “swimmers” and particle-bound “single hoppers”, which matched the results from the Tool that water would be the primary transport pathway. As suggested by Rodgers et al., the “PMOC” nature of Cl-OPEs (e.g. high mobility in water) is consistent with the hypothesis of Sühring et al.23 that water-based transport could be an important pathway for Cl-OPEs into the Canadian Arctic.
However, even with water-based transport, the CTD and POV for Cl-OPEs was not high enough to explain their frequent detection in the Arctic (Table 2) under the default settings in the Tool, although their t1/2 in water are as high as 2900 h (TCEP) and 5100 h (TCIPP).
Similarly to the scenario of increased wind speed for non-Cl-OPEs, we were interested in whether increased water flow velocity representing river-based transport could help explain the observed long-range transport potential of Cl-OPEs.
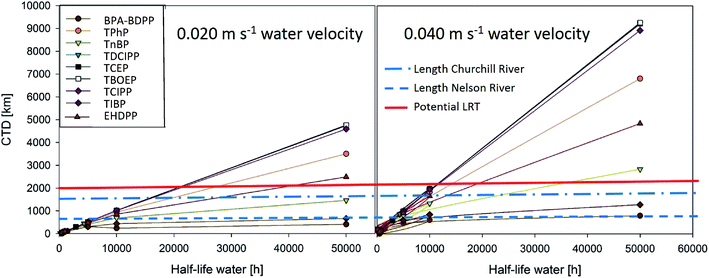
The Tool takes into account water-based transport only via oceanic currents that have a considerably lower flow velocity than rivers. To account for the higher flow velocities of rivers, we modelled the CTD of the eight OPEs detailed above at the default tool flow velocity of 0.02 m s−1 (Fig. 3, left) and an increased flow velocity of 0.04 m s−1 consistent with riverine transport (Fig. 3, right).
The results indicate that riverine transport (i.e. increased flow velocity) would substantially increase the LRTP of Cl-OPEs compared to the default oceanic transport scenario (Fig. 3). All OPEs reached CTDs of ≥2000 km at a t1/2 in water of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h assuming riverine transport, compared to at least 20
000 h assuming riverine transport, compared to at least 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h using the ocean-based transport scenario (Fig. 3). Furthermore, the results suggested that Cl-OPEs could travel the entire length of the Churchill and Nelson rivers at a t1/2 in water ≤5000 h, which is well within the expected uncertainty for t1/2 in water.
000 h using the ocean-based transport scenario (Fig. 3). Furthermore, the results suggested that Cl-OPEs could travel the entire length of the Churchill and Nelson rivers at a t1/2 in water ≤5000 h, which is well within the expected uncertainty for t1/2 in water.
The lack of temperature dependence of chemical properties in the Tool could additionally lead to an underestimation of POV of OPEs in water because one of the primary degradation processes in water would be hydrolysis. Hydrolysis is a temperature dependent process51 and can therefore be expected to be significantly reduced in cold Arctic waters compared to warmer waters in temperate zones. Beyer et al.52 reported the temperature dependence of CTD based on changes in e.g., mobility and deposition processes of chemicals.
3.5 Evaluation of the Tool's results and recommended improvements
Our results, along with measured and modelled occurrence of OPEs in the Arctic,23 lead us to conclude that the Tool is a suitable instrument to evaluate the transport pathways of OPEs. An accurate estimation of the LRTP of OPEs, on the other hand, appears to depend on highly uncertain environmental half-life data as well as variable environmental parameters such as particle concentration in air,39 wind speed and water flow velocity.The Tool was designed as a screening instrument for a first assessment of the LRTP and POV of chemicals.8 The advantage of this approach is that the Tool offers an easy and fast overview that can be used as a starting point for further assessments. However, the current default settings clearly lead to a significant underestimation of the POV and LRT of OPEs and, possibly, other PMOCs, as related to transport to the Arctic.
Ways of improving the POV and CTD results from the Tool could be allowing the user to adjust the default environmental parameters to address conditions related to Arctic transport or LRT of PMOCs and other emerging contaminants that do not meet the typical PBT criteria of POPs. The Tool could be adapted to include an optional “Arctic (PMOC) LRTP setting” or a separate optional setting that considers the results of this analysis for OPEs. This setting would include adjusted parameter values to reflect accelerated episodic atmospheric and riverine transport, as well as the potential increase in environmental half-lives of chemicals due to sorption to particles or decreased degradation kinetics at low temperatures. Additional features such as a temperature dependence for degradation could further improve the Tool results but this would come at the expense of added model complexity and data needs, where degradation half-lives even at standard temperatures are highly uncertain.
Additionally, it would be helpful to include information on the potential data range for the CTD and POV results that can be expected due to the uncertainty of input data. The Tool has the option of performing a Monte Carlo uncertainty analysis for individual compounds but could be improved by offering an automated solution that can be applied to an entire database. Providing a full Monte-Carlo uncertainty analysis for all compounds in a database would be a useful step towards assessing the confidence of the Tool's estimates for a given compound class.
Overall, we conclude that the Tool offers reasonable screening for “legacy POPs” but can underestimate LRTP and CTD of OPEs to the Arctic under conditions of e.g. episodic transport. Furthermore, the Tool can underestimate the LRTP of PMOCs where water-borne transport is likely to dominate. These results supported the hypothesis of Sühring et al.23 and Rodgers et al.18 that non-Cl OPEs are primarily transported via the atmosphere or could have some local sources, while Cl-OPEs act as PMOCs with water-based transport.
Despite these uncertainties, previous measurements in the Arctic show, and changes to the Tool confirm, that many Cl- and non-Cl OPEs have the capacity to reach remote areas. By varying key parameters in the Tool, we have shown that non-Cl-OPEs are likely mainly transported via air advection, whereas the Cl-OPEs, like other PMOCs, are likely mainly transported via water advection. The high estimated CTDs of >2000 km for non-Cl OPEs such as BPA-BDPP, PBDMPP, PBDPP, TDMPP, TEHP, TPPP and TTBPP, and high detection frequencies of Cl-OPEs in the Arctic indicate that these compounds cannot be considered “environmentally friendly” alternatives for brominated flame retardants.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by Environment Canada and Climate Change, Contract Number 3000597142.References
- M. L. Diamond, C. A. d. Wit, S. Molander, M. Scheringer, T. Backhaus, R. Lohmann, R. Arvidsson, Å. Bergman, M. Hauschild, I. Holoubek, L. Persson, N. Suzuki, M. Vighi and C. Zetzsch, Exploring the planetary boundary for chemical pollution, Environ. Int., 2015, 78, 8–15, DOI:10.1016/j.envint.2015.02.001.
- UNEP. United Nations Environment Programme, Stockholm Convention on Persistent Organic Pollutants, 2001 Search PubMed.
- UNEP, SC-4/14: Listing of hexabromodiphenyl ether and heptabromodiphenyl ether, 2009, vol. 1, pp. 3–4 Search PubMed.
- Stockholm Convention, Listing of tetrabromodiphenyl ether and pentabromodiphenyl ether, UNEP-POPS-COP.4-SC-4-18, http://chm.pops.int/Portals/0/download.aspx?d=UNEP-POPS-COP.4-SC-4-18.English.pdf Search PubMed.
- Stockholm Convention. Listing of decabromodiphenyl ether (commercial mixture, c-decaBDE), UNEP/POPS/COP.8/SC-8/10, 2017, pp. 4–5 Search PubMed.
- T. Reemtsma, et al., Mind the gap: persistent and mobile organic compounds—water contaminants that slip through, Environ. Sci. Technol., 2016, 50, 10308–10315 CrossRef CAS PubMed.
- K. Fenner, M. Scheringer, M. MacLeod, M. Matthies, T. McKone, M. Stroebe, A. Beyer, M. Bonnell, A. C. Le Gall, J. Klasmeier, D. Mackay, D. van de Meent, D. Pennington, B. Scharenberg, N. Suzuki and F. Wania, Comparing Estimates of Persistence and Long-Range Transport Potential among Multimedia Models, Environ. Sci. Technol., 2005, 39(7), 1932–1942, DOI:10.1021/es048917b.
- F. Wegmann, L. Cavin, M. MacLeod, M. Scheringer and K. Hungerbühler, The OECD software tool for screening chemicals for persistence and long-range transport potential, Environ. Model. Softw, 2009, 24(2), 228–237, DOI:10.1016/j.envsoft.2008.06.014.
- OECD website, http://www.oecd.org/chemicalsafety/risk-assessment/oecdpovandlrtpscreeningtool.htm#Features_of_the_tool, accessed May 2016.
- UNEP, Stockholm Convention on Persistent Organic Pollutants – Report on the assessment of chemical alternatives to endosulfan, UNEP/POPS/POPRC.8/INF/28, 2012 Search PubMed.
- J. Klasmeier, M. Matthies, M. MacLeod, K. Fenner, M. Scheringer, M. Stroebe, A. C. Le Gall, T. McKone, D. van de Meent and F. Wania, Application of Multimedia Models for Screening Assessment of Long-Range Transport Potential and Overall Persistence, Environ. Sci. Technol., 2006, 40(1), 53–60, DOI:10.1021/es0512024.
- USEPA, (EPI Suite) Estimation Programs Interface Suite, United States Environmental Protection Agency, Washington, D.C., USA, 2013, http://www.epa.gov/oppt/exposure/pubs/episuite.htm, last updated 2013-1-15 Search PubMed.
- T. Harner and T. F. Bidleman, Octanol-Air Partition Coefficient for Describing Particle/Gas Partitioning of Aromatic Compounds in Urban Air, Environ. Sci. Technol., 1998, 32, 1494–1502 CrossRef CAS.
- M. Scheringer, Long-range transport of organic chemicals in the environment, Environ. Toxicol. Chem., 2009, 28(4), 677–690, DOI:10.1897/08-324r.1.
- M. Scheringer, K. C. Jones, M. Matthies, S. Simonich and D. van de Meent, Multimedia partitioning, overall persistence, and long-range transport potential in the context of POPs and PBT chemical assessments, Integr. Environ. Assess. Manage., 2009, 5(4), 557–576, DOI:10.1897/ieam_2009-007.1.
- China Market Research Reports: Global and China Flame Retardant Industry Report, 2014–2016, http://www.chinamarketresearchreports.com/114859.html, accessed October 08, 2019 Search PubMed.
- I. van der Veen and J. de Boer, Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis, Chemosphere, 2012, 88, 1119–1153 CrossRef CAS PubMed.
- T. F. M. Rodgers, J. W. Truong, L. M. Jantunen, P. A. Helm and M. L. Diamond, Organophosphate Ester Transport, Fate, and Emissions in Toronto, Canada, Estimated Using an Updated Multimedia Urban Model, Environ. Sci. Technol., 2018, 52, 12465–12474 CrossRef CAS.
- A. Blum, M. Behl, L. S. Birnbaum, M. L. Diamond, A. Phillips, V. Singla and M. Venier, Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers?, Environ. Sci. Technol. Lett., 2019, 6(11), 638–649 CrossRef CAS.
- C. Yang, et al., Are cell phones an indicator of personal exposure to organophosphate flame retardants and plasticizers?, Environ. Int., 2019, 122, 104–116 CrossRef CAS.
- A. Saini, et al., Flame retardants in urban air: a case study in Toronto targeting distinct source sectors, Environ. Pollut., 2019, 247, 89–97 CrossRef CAS PubMed.
- U. E. Bollmann, A. Möller, Z. Xie, R. Ebinghaus and J. W. Einax, Occurrence and fate of organophosphorus flame retardants and plasticizers in coastal and marine surface waters, Water Res., 2012, 46, 531–538 CrossRef CAS PubMed.
- R. Sühring, M. L. Diamond, M. Scheringer, F. Wong, M. Pucko, G. Stern, A. Burt, H. Hung, P. Fellin, H. Li and L. M. Jantunen, Organophosphate Esters in Canadian Arctic Air: Occurrence, Levels and Trends, Environ. Sci. Technol., 2016, 50, 7409–7415 CrossRef PubMed.
- L. M. Jantunen, A. Gawor, F. Wong, T. Bidleman, F. Wania, G. A. Stern, M. Pucko and H. Hung, Organophosphate ester flame retardants and plasticizers in the Canadian Arctic: Workshop on Brominate & Other Flame Retardants, Indianapolis, USA, 2015 Search PubMed.
- A. Salamova, M. H. Hermanson and R. A. Hites, Organophosphate and halogenated flame retardants in atmospheric particles from a European Arctic site, Environ. Sci. Technol., 2014, 48(11), 6133–6140, DOI:10.1021/es500911d.
- A. Möller, R. Sturm, Z. Xie, M. Cai, J. He and R. Ebinghaus, Organophosphorus flame retardants and plasticizers in airborne particles over the Northern Pacific and Indian Ocean toward the Polar Regions: evidence for global occurrence, Environ. Sci. Technol., 2012, 46(6), 3127–3134, DOI:10.1021/es204272v.
- C. A. McDonough, A. O. De Silva, C. Sun, A. Cabrerizo, D. Adelman, T. Soltwedel, E. Bauerfeind, D. C. G. Muir and R. Lohmann, Dissolved Organophosphate Esters and Polybrominated Diphenyl Ethers in Remote Marine Environments: Arctic Surface Water Distributions and Net Transport Through Fram Strait, Environ. Sci. Technol., 2018, 52, 6208–6216 CrossRef CAS PubMed.
- Y. Ma, Z. Xie, R. Lohmann, W. Mi and G. Gao, Organophosphate Ester Flame Retardants and Plasticizers in Ocean Sediments from the North Pacific to the Arctic Ocean, Environ. Sci. Technol., 2017, 51, 3809–3815 CrossRef CAS PubMed.
- X. Zhang, R. Sühring, D. Serodio, M. Bonnell, N. Sundin and M. L. Diamond, Novel flame retardants: Estimating the physical-chemical properties and environmental fate of 94 halogenated and organophosphate PBDE replacements, Chemosphere, 2016, 144, 2401–2407, DOI:10.1016/j.chemosphere.2015.11.017.
- I. Liagkouridis, A. P. Cousins and I. T. Cousins, Physical-chemical properties and evaluative fate modelling of 'emerging' and 'novel' brominated and organophosphorus flame retardants in the indoor and outdoor environment, Sci. Total Environ., 2015, 524–525, 416–426, DOI:10.1016/j.scitotenv.2015.02.106.
- M. A. Khairy, J. L. Luek, R. Dickhut and R. Lohmann, Levels, sources and chemical fate of persistent organic pollutants in the atmosphere and snow along the western Antarctic Peninsula, Environ. Pollut., 2016, 216, 304–313 CrossRef CAS PubMed.
- J. H. Johansson, M. E. Salter, J. C. Acosta Navarro, C. Leck, E. D. Nilsson and I. T. Cousins, Global transport of perfluoroalkyl acids via sea spray aerosol, Environ. Sci.: Processes Impacts, 2019, 21, 635–649 RSC.
- A. Abdollahi, et al., Characterization of polyurethane foam (PUF) and sorbent impregnated PUF (SIP) disk passive air samplers for measuring organophosphate flame retardants, Chemosphere, 2017, 167, 212–219 CrossRef CAS PubMed.
- A. Möller, Z. Xie, A. Caba, R. Sturm and R. Ebinghaus, Organophosphorus flame retardants and plasticizers in the atmosphere of the North Sea, Environ. Pollut., 2011, 159, 3660–3665 CrossRef PubMed.
- H. Carlsson, U. Nilsson, G. Becker and C. Östman, Organophosphate Ester Flame Retardants and Plasticizers in the Indoor Environment: Analytical Methodology and Occurrence, Environ. Sci. Technol., 1997, 31, 2931–2936 CrossRef CAS.
- J. O. Okeme, T. F. M. Rodgers, L. M. Jantunen and M. L. Diamond, Examining the Gas-Particle Partitioning of Organophosphate Esters: How Reliable Are Air Measurements?, Environ. Sci. Technol., 2018, 52, 13834–13844 CrossRef CAS PubMed.
- S. Brommer, L. M. Jantunen, T. F. Bidleman, S. Harrad and M. L. Diamond, Determination of vapor pressures for organophosphate esters, J. Chem. Eng. Data, 2014, 59, 1441–1447 CrossRef CAS.
- M. A. Khairy and R. Lohmann, Organophosphate flame retardants in the indoor and outdoor dust and gas-phase of Alexandria, Egypt, Chemosphere, 2019, 220, 275–285 CrossRef CAS PubMed.
- R. Sühring, H. Wolschke, M. L. Diamond, L. M. Jantunen and M. Scheringer, Distribution of Organophosphate Esters between the Gas and Particle Phase–Model Predictions vs Measured Data, Environ. Sci. Technol., 2016, 50, 6644–6651 CrossRef PubMed.
- M. G. Morgan, M. Henrion and M. Small, Uncertainty. A guide to dealing with uncertainty in quantitative risk and policy analysis, Cambridge University Press, Cambridge, New York, 1992 Search PubMed.
- R. W. Macdonald, The Influence of global change on contaminant pathways to, within, and from the arctic, Chapter 3: Recent Change in the Arctic and the Arctic Oscillation; AMAP Assessment 2002; AMAP, Arctic Monitoring and Assessment Programme, Oslo, 2003 Search PubMed.
- Environment Canada. Real-Time Hydrometric Data Graph for CHURCHILL RIVER [MB] – WaterOffice – Environment Canada. https://wateroffice.ec.gc.ca, accessed January 18, 2016.
- Environment Canada. Real-Time Hydrometric Data Graph for NELSON [MB] – WaterOffice – Environment Canada. https://wateroffice.ec.gc.ca, accessed January 18, 2016.
- A. L. Beriault and D. J. Sauchyn, Tree-Ring Reconstructions of Streamflow in the Churchill River Basin, Northern Saskatchewan, Can. Water Resour. J., 2006, 31(4), 249–262, DOI:10.4296/cwrj3104249.
- S. St. George Hydrological dynamics in the Winnipeg River basin, Manitoba: In Report of Activities. Manitoba Science, Technology, Energy and Mines. Manitoba Geological Survey 2006, pp. 226–230 Search PubMed.
- Google maps, Nelson River (MB), https://www.google.ca/maps/place/Nelson+River,+Manitoba/@56.7178002,-94.6888139,8z/data=!3m1!4b1!4m2!3m1!1s0x5263450c29133615:0xe25de48adc9c9a7f, accessed January 2016 Search PubMed.
- Google maps, Churchill River (MB), https://www.google.ca/maps/place/Churchill+River/@57.9520239,-96.773467,8z/data=!3m1!4b1!4m2!3m1!1s0x5259d105e00d82c9:0x1e73fc352f9e63d2, accessed January 2016 Search PubMed.
- M. Stroebe, M. Scheringer, M. Held and K. Hungerbühler, Inter-comparison of multimedia modeling approaches: modes of transport, measures of long range transport potential and the spatial remote state, Sci. Total Environ., 2004, 321, 1–20, DOI:10.1016/j.scitotenv.2003.09.008.
- Y. Liu, J. Liggio, T. Harner, L. Jantunen, M. Shoeib and S.-M. Li, Heterogeneous OH initiated oxidation: a possible explanation for the persistence of organophosphate flame retardants in air, Environ. Sci. Technol., 2014, 48(2), 1041–1048, DOI:10.1021/es404515k.
- F. Wania, Potential of Degradable Organic Chemicals for Absolute and Relative Enrichment in the Arctic, Environ. Sci. Technol., 2006, 40(2), 569–577, DOI:10.1021/es051406k.
- L. T. Kanerva and E. K. Euranto, Temperature dependence of the neutral ester hydrolysis of chloromethyl dichloroacetate in 2-butoxyethanol-water mixtures over a wide temperature range, J. Am. Chem. Soc., 1982, 104(20), 5419–5421, DOI:10.1021/ja00384a029.
- A. Beyer, F. Wania, T. Gouin, D. Mackay and M. Matthies, Temperature Dependence of the Characteristic Travel Distance, Environ. Sci. Technol., 2003, 37(4), 766–771, DOI:10.1021/es025717w.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00410f |
| ‡ Current affiliation: Department of Environmental Science and Analytical Chemistry (ACES), Stockholm University, SE-106 91 Stockholm, Sweden. |
| This journal is © The Royal Society of Chemistry 2020 |