Open Access Article
Open Access ArticleStrategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health
Ian T.
Cousins
 *a,
Jamie C.
DeWitt
*a,
Jamie C.
DeWitt
 b,
Juliane
Glüge
b,
Juliane
Glüge
 c,
Gretta
Goldenman
d,
Dorte
Herzke
c,
Gretta
Goldenman
d,
Dorte
Herzke
 ef,
Rainer
Lohmann
ef,
Rainer
Lohmann
 g,
Mark
Miller
h,
Carla A.
Ng
g,
Mark
Miller
h,
Carla A.
Ng
 i,
Martin
Scheringer
i,
Martin
Scheringer
 c,
Lena
Vierke
j and
Zhanyun
Wang
c,
Lena
Vierke
j and
Zhanyun
Wang
 k
k
aDepartment of Environmental Science, Stockholm University, SE-10691 Stockholm, Sweden. E-mail: ian.cousins@aces.su.se
bDepartment of Pharmacology & Toxicology, Brody School of Medicine, East Carolina University, Greenville, NC, USA
cInstitute of Biogeochemistry and Pollutant Dynamics, ETH Zürich, 8092 Zürich, Switzerland
dMilieu Consulting SPRL, Brussels, Belgium
eNorwegian Institute for Air Research (NILU), Fram Centre, N-9296 Tromsø, Norway
fInstitute for Arctic and Marine Biology, UiT The Arctic University of Norway, N-9037 Tromsø, Norway
gGraduate School of Oceanography, University of Rhode Island, Narragansett, RI 02882, USA
hNational Institute of Environmental Health Sciences, U.S. Public Health Service, Research Triangle Park, NC, USA
iDepartment of Civil & Environmental Engineering and Environmental and Occupational Health, University of Pittsburgh, Pittsburgh, PA 15261, USA
jGerman Environment Agency (UBA), Dessau-Roßlau, Germany
kChair of Ecological Systems Design, Institute of Environmental Engineering, ETH Zürich, 8093 Zürich, Switzerland
First published on 4th June 2020
Abstract
Grouping strategies are needed for per- and polyfluoroalkyl substances (PFAS), in part, because it would be time and resource intensive to test and evaluate the more than 4700 PFAS on the global market on a chemical-by-chemical basis. In this paper we review various grouping strategies that could be used to inform actions on these chemicals and outline the motivations, advantages and disadvantages for each. Grouping strategies are subdivided into (1) those based on the intrinsic properties of the PFAS (e.g. persistence, bioaccumulation potential, toxicity, mobility, molecular size) and (2) those that inform risk assessment through estimation of cumulative exposure and/or effects. The most precautionary grouping approach of those reviewed within this article suggests phasing out PFAS based on their high persistence alone (the so-called “P-sufficient” approach). The least precautionary grouping approach reviewed advocates only grouping PFAS for risk assessment that have the same toxicological effects, modes and mechanisms of action, and elimination kinetics, which would need to be well documented across different PFAS. It is recognised that, given jurisdictional differences in chemical assessment philosophies and methodologies, no one strategy will be generally acceptable. The guiding question we apply to the reviewed grouping strategies is: grouping for what purpose? The motivation behind the grouping (e.g. determining use in products vs. setting guideline levels for contaminated environments) may lead to different grouping decisions. This assessment provides the necessary context for grouping strategies such that they can be adopted as they are, or built on further, to protect human and environmental health from potential PFAS-related effects.
Environmental significancePFAS comprise more than 4700 individual substances that are used in many, highly diverse applications in society. All PFAS are very persistent (if PFAS with persistent transformation products are considered as persistent substances, as is the case under REACH) and several PFAS are also known to be bioaccumulative and toxic. However, for most PFAS there are insufficient data to facilitate chemical assessments. Generating these missing data on a chemical-by-chemical basis is too resource intensive and it is therefore essential to identify groups of similar PFAS that can be assessed together. Here we discuss various grouping approaches and their advantages and limitations. The structural diversity of PFAS poses a challenge to grouping. However, some kind of grouping approach, or a combination of several different approaches, will be needed for the future assessment and management of PFAS. |
Introduction
Buck et al.1 provided the first class definition of per- and polyfluoroalkyl substances (PFAS) as “the highly fluorinated aliphatic substances that contain 1 or more C atoms on which all the H substituents… have been replaced by F atoms, in such a manner that they contain the perfluoroalkyl moiety CnF2n+1–” (where n is equal to or greater than 1, i.e. the structure must contain at least one CF3– group). A more recent and broader definition by the Organisation for Economic Co-operation and Development (OECD)/United Nations Environment Programme (UNEP) Global PFC Group2 defined PFAS as chemicals with at least one perfluorocarbon moiety (–CnF2n–). PFAS therefore comprise a diverse group of chemistries with the common feature of the fully or “per”-fluorinated carbon chain.Structurally diverse PFAS are used in a wide variety of commercial products and industrial applications. In the 2018 OECD PFAS list2 over 4700 CAS numbers were identified for PFAS on the global market. For the majority of PFAS, little or no data on uses, properties and effects are available to determine how these chemicals may impact the health of living organisms.3–6 Our current understanding of biological impact is based primarily on studies of four PFAS, perfluorooctane sulfonic acid (PFOS), perfluorooctanoic acid (PFOA), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA).7 Epidemiological studies of human populations suggest that PFAS may act as endocrine and metabolic disruptors, increase cholesterol levels, adversely impact the immune system, and cause cancer.7 These data are supported by studies in laboratory animals showing changes in liver, thyroid, immune and pancreatic function.7
But researching individual chemicals is both expensive and time consuming. It can take many years to gather the evidence needed under regulatory regimes to restrict harmful chemicals. It is becoming increasingly apparent that to effectively protect the public and environment from the wide range of possible PFAS-related environmental and human health effects, strategies should be sought to group PFAS for action, e.g. for guiding regulatory and voluntary phase-out actions, etc., rather than to address them chemical-by-chemical. For example, in the recent Zurich Statement,8 the authors recommended “that actions need to address groups of PFAS rather than individual chemicals and that such a grouping approach needs to be scientifically sound.” It was further recognized “that a grouping approach requires a better mechanistic understanding of the physicochemical and toxicological properties of PFAS as well as additional data that can be used to support grouping approaches for PFAS.”
Between 2000 and 2002,9 after about 50 years of continuous manufacture, 3M phased out all PFAS products derived from perfluorooctane sulfonyl fluoride (POSF; C-8) and its C-6 and C-10 homologues, which represented the first large-scale grouping of hundreds of PFAS for voluntary phase-out. Shortly thereafter, in 2006, eight major PFAS manufacturers committed to eliminating the global use and emissions of PFOA, its longer-chain homologues, and their precursors by 2015 through the PFOA Stewardship program10 agreement with the US Environmental Protection Agency (US EPA).
In conjunction with these phase-outs, the fluorochemical industry introduced another grouping approach, namely the concept of “long-chain” and “short-chain” perfluoroalkyl acids (PFAAs),11 defining long-chain PFAAs as only perfluoroalkyl carboxylic acids (PFCAs) with ≥7 perfluorinated carbons and perfluoroalkane sulfonic acids (PFSAs) with ≥6 perfluorinated carbons. While emerging evidence showed long-chain PFAAs are bioaccumulative and toxic, the PFAS manufacturing industry held that short-chain PFAAs were not, and thus one of the strategies of the PFAS manufacturing industry was to replace long-chain PFAAs with their short-chain homologues.12 Another substitution strategy is to replace long-chain PFAAs with substances containing perfluoroalkyl ether moieties (e.g. per- and polyfluoroalkyl ether carboxylic and sulfonic acids (PFECAs and PFESAs)).12
It is now apparent that this industry substitution strategy for long-chain PFAAs requires reconsideration given (1) the widespread environmental contamination (including drinking water sources) by short-chain PFAAs13 and perfluoroalkyl ether acids14 due to their high environmental mobility and (2) the listing of both hexafluoropropylene oxide dimer acid (HFPO-DA, sometimes referred to as GenX), a PFOA-replacement introduced by DuPont in 2009 that contains perfluoroalkyl ether moieties, and perfluorobutane sulfonic acid (PFBS), a short-chain PFAA that is the ultimate degradation product of 3M's replacement chemistry (introduced in 2003), as Substances of Very High Concern (SVHCs) under the EU REACH Regulation.15
Given the number of substitutions of long-chain PFAAs with other PFAS that are now also considered to be problematic, there is a need for more effective grouping strategies for the regulation of PFAS than the current approach of regulating only long-chain PFAAs and related substances. In the Madrid Statement,16 more than 200 scientists and regulators suggested that PFAS should be managed as a class, and that production and use should be limited. This grouping of all PFAS for phase-out is based on concerns regarding the high persistence of PFAS, the lack of knowledge on chemical structures, properties, uses, and toxicological profiles of most PFAS currently in use, and the need for informed substitutions of problematic PFAS chemistries.16 A counterpoint to regulating PFAS as a class, authored by the FluoroCouncil17 in response to the Madrid Statement, stated (among other things) that PFAS are a structurally diverse group exhibiting “important differences between the health and environmental impacts”, and that “fluorotechnology is essential technology for many aspects of modern life”.
The Montreal Protocol's concept of essential use has been put forward as an approach for reducing exposure to PFAS, by phasing out all non-essential uses of PFAS.18 While such a phase-out of PFAS is likely not feasible in the short term, it is not an insurmountable challenge in the longer term. Indeed, within the European Union (EU), there are already discussions underway for a restriction proposal for all non-essential uses of PFAS,19,20 although it is not yet known how “essential use” will be defined. Innovation, in conjunction with regulation and economic incentives for the development of new technologies, should in time provide functional alternatives to even current essential uses of PFAS.18 In cases where the uses of PFAS are seen as “necessary for health, safety or is critical for the functioning of society”16 but no functional alternatives with favourable hazard properties are currently available, certain uses of PFAS will probably continue, at least in the short term.18 However, the use of the grouping strategies presented here could provide opportunities for market adjustment, and spark more voluntary efforts to reduce non-essential uses.18
The aims of this paper are to discuss (1) current and potential grouping strategies that inform PFAS assessment for various control actions, with advantages and disadvantages for each, (2) highlight motivations for action that could guide use of specific grouping approaches and (3) outline the way forward and remaining challenges in advancing these grouping approaches.
Motivations for grouping
The method used to group PFAS depends on the type of action intended. Grouping PFAS may have benefits, for example: (1) to more efficiently protect human and environmental health, (2) to avoid animal testing through read across,21 (3) for product labelling and consumer education (e.g. for interpretation of a label such as “PFAS free”), or (4) to manage clean-up of contaminated sites.Most existing grouping approaches have been developed to protect human and environmental health from potential adverse effects resulting from exposure to the multiple PFAS in commerce. Moreover, further motivations for grouping of PFAS are based on their environmental and biological persistence, the high number of individual PFAS, and the lessons learned from recent industrial substitution strategies.
Proactive strategies concerning new or continued use of PFAS may benefit from more precautionary grouping approaches because these decisions will directly impact future exposures and because their implementation – at least avoidance of non-essential uses – will always be less costly than retrospective risk assessment and remediation. On the other hand, decisions for how to group already emitted PFAS for the establishment of drinking water guidelines or environmental cleanup levels will have profound impacts on enforcement including costs and resource needs. It may therefore be necessary, in resource-constrained settings, to more strictly prioritize cleanup levels on the basis of established toxicological risk.
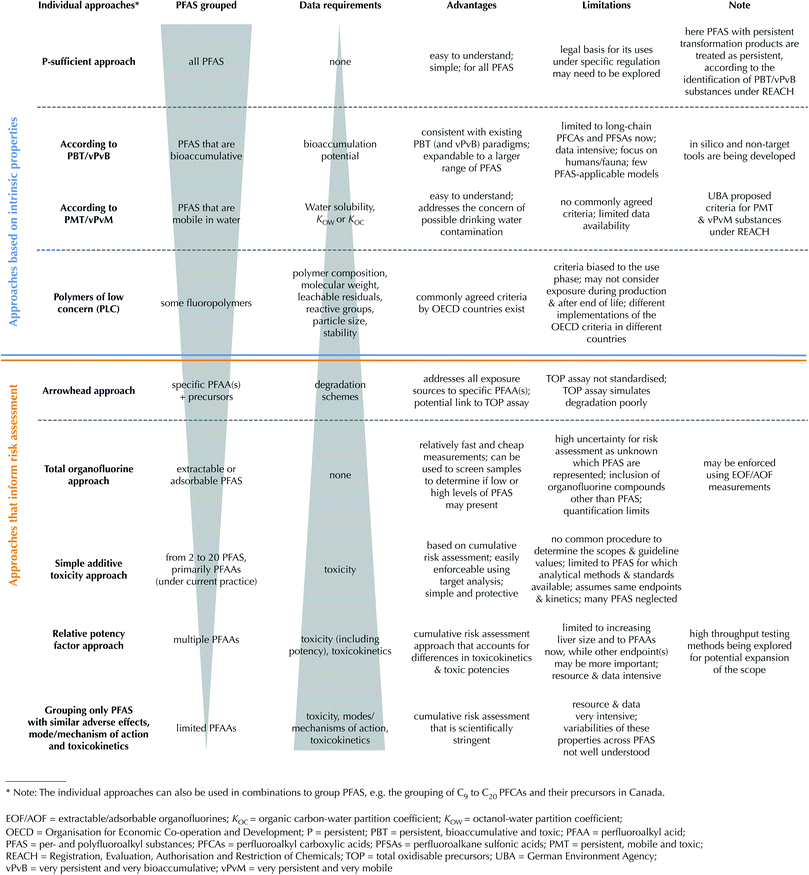
Grouping approaches
Here existing grouping approaches to protecting human and environmental health are subdivided into (1) those based on the intrinsic properties of the PFAS and (2) those that inform risk assessment through estimation of cumulative exposure and/or effects (see Fig. 1). National or international chemical assessments rely on intrinsic properties of the chemical, including its persistence (P), bioaccumulation (B) and toxicity (T). This “PBT approach” can be found for example in the EU REACH Regulation.22 Under REACH, substances can also be identified as “Substances of Very High Concern” (SVHC) if they are very persistent (vP) and very bioaccumulative (vB) meaning that if these criteria can be met, toxicity does not require consideration.The approaches that inform risk assessment, on the other hand, consider anticipated exposure when determining whether or not an adverse effect to human health or the environment may occur. For example, the point of departure for establishing acceptable risk could be the no observed adverse effect level (NOAEL) for a critical toxicological endpoint. The NOAEL can then be compared to either the external dose or exposure (e.g. concentration in exposure medium) or internal dose or exposure (e.g. serum or tissue concentration) to determine the risks.
Risk assessment has typically been performed on a chemical-by-chemical basis, but there is some current focus on developing methods for combined risk assessment through estimation of cumulative exposure (e.g. total organofluorine (TOF) or extractable or adsorbable organofluorine (EOF/AOF)) and/or effects (e.g. additive).23 Such combined risk assessment is challenging for multiple PFAS, given that sufficient toxicity data are only available for relatively few (<20) substances.7 Measurement of exposure can be achieved for more substances, but may be constrained by the lack of knowledge of what/how to measure and also lack of analytical standards.
Each individual approach is discussed in more detail in the following sections. It is important to note that the individual grouping approaches were developed for different purposes, have different data needs, and therefore cannot always be directly compared to each other. The selection of the grouping approach needs to account for the specific protection goal, data requirements and enforcement techniques.
Grouping approaches based on intrinsic properties
Grouping according to the “P-sufficient” approach
The continuous release of persistent chemicals will lead to widespread, long-lasting, and increasing contamination, which will inevitably result in increasing probabilities of known and unknown adverse effects on human health and the environment.24 The perfluoroalkyl (CnF2n+1–) and perfluoroether (CnF2n+1–O–CmF2m–) moieties are highly persistent under environmental conditions.4 Although some polyfluoroalkyl substances (so called “precursors”) may degrade in the environment and biota, they all ultimately (partially) transform into highly stable end products, which are usually the persistent PFAAs.3 This view is consistent with the REACH Regulation that all chemicals with persistent transformation products should be classified as persistent.22 Based on this definition, all PFAS are therefore considered to be very persistent in environmental media, and under the proposed “P-sufficient” approach all PFAS would be managed as a single group.An advantage of this approach is that it is easily implementable to all PFAS for non-experts, i.e. non-experts will not need to ask if a (new) PFAS belongs to the group or not. A disadvantage of the “P-sufficient approach” is that no legal precedent has been made in any jurisdiction, although the idea of regulating highly persistent chemicals and microplastics is being explored within the EU.25,26
Grouping according to the PBT/vPvB approach
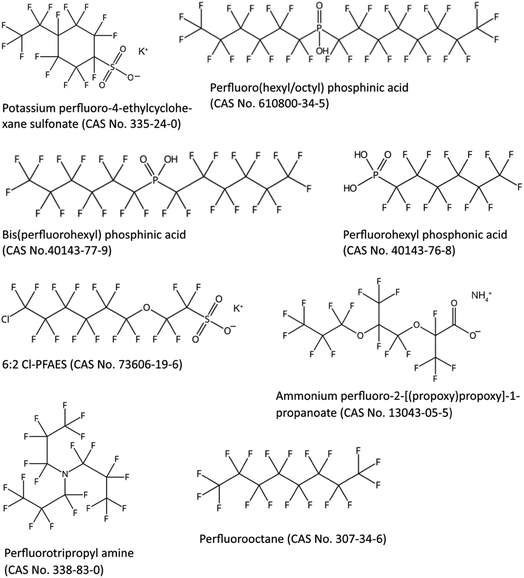
As mentioned in the introduction, PFAAs have been grouped into long-chain and short-chain PFAAs, where long-chain PFAAs are considered bioaccumulative in animals and short-chain PFAAs are not.11 A major disadvantage in the current grouping of long- versus short-chain PFAAs to determine if PFAS are bioaccumulative is that the definitions of long- and short-chain PFAAs only apply to PFCAs and PFSAs;11 however, it has been suggested that there are other PFAS that are bioaccumulative. To more accurately define those PFAS that are bioaccumulative, new grouping approaches would be required; a few suggestions are provided below.There are already a number of PFAS that are suggested to be bioaccumulative according to observations from bioaccumulation experiments. For example, certain perfluoroalkyl phosphonic and phosphinic acids (PFPAs and PFPIAs) can only be slowly eliminated from rainbow trout27 and rats,28 similarly to long-chain PFCAs and PFSAs.29 There is also evidence that perfluorotripropyl amine is bioaccumulative based on the long elimination half-lives observed in the liver and spleen of rats.30 Perfluorooctane is also potentially bioaccumulative based on bioconcentration factor (BCF) measurements in European carp (BCF up to 3200 L kg−1) and rice fish (BCF up to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 L kg−1).31 Finally, chlorinated PFESAs, predominately the so-called 6:2 Cl-PFASA (often called F-53B, CAS no. 73606-19-6), and a novel PFECA, perfluoro-2-[(propoxy)propoxy]-1-propanoate have been shown to bioaccumulate in biota and human serum.32–35
600 L kg−1).31 Finally, chlorinated PFESAs, predominately the so-called 6:2 Cl-PFASA (often called F-53B, CAS no. 73606-19-6), and a novel PFECA, perfluoro-2-[(propoxy)propoxy]-1-propanoate have been shown to bioaccumulate in biota and human serum.32–35
Indications of bioaccumulation that need further evaluation are the observations of a number of emerging and novel PFAS in top predators including humans. For example, perfluoro-4-ethylcyclohexane sulfonate has been detected in top predator fish in the Great Lakes36 and in crucian carp in China.37 PFPIAs, predominately 6:8 PFPIA (cormorants and pike) and 6:6 PFPIA (dolphins), have been observed in biota in North American inland and coastal waters.38 PFPAs, predominately perfluorohexyl phosphonate (PFHxPA), have been detected in a Norwegian human cohort.39
Fig. 2 illustrates the structures of some PFAS suggested to be bioaccumulative. A common feature of the PFAS in Fig. 2 is that they contain at least six perfluorinated carbons. The head group of PFAAs is also known to influence their bioaccumulation potential; for example, it is well known that PFSAs are more bioaccumulative than PFCAs with the same perfluorinated carbon chain length.11
Both computational and empirical methods have been explored to estimate protein binding affinity. In vitro methods include, among others, equilibrium dialysis40 and fluorescence displacement.41,42 In a recent paper, Yang et al.43 used a non-target screening approach to identify novel PFAS present in aqueous film forming foams (AFFF) that bind to human liver fatty acid binding protein. Computational methods are based on structure–property relationships and could potentially be used to estimate the bioaccumulation potential of novel and emerging PFAS. For example, the protein affinity of certain legacy and novel PFAS was recently estimated using molecular dynamic approaches,44 and protein affinity is a key determinant of bioaccumulation potential. Such structure–property relationships may also aid in estimating the elimination half-lives of PFAS, which is another important factor in determining bioaccumulation potential. Predictive approaches for bioaccumulation potential will be especially important for informing grouping, as they are proactive and resource-efficient in comparison to biomonitoring and laboratory testing (in vitro or in vivo testing).
Short-chain PFAAs have not been reported to bioaccumulate in animals,11 but are known to bioaccumulate in above-ground plant tissues (shoots, leaves and fruit).45–48 An inverse relationship has been observed between perfluoroalkyl chain length and BCFs of PFAAs in above-ground plant tissues for edible crops grown in sludge-amended soils.47 In regions where the soil is highly contaminated with short-chain PFAAs, human exposure from consumption of crops can become an important pathway.49
A fundamental limitation of grouping according to bioaccumulation potential (B) is that for highly persistent chemicals, B may become less relevant if a high exposure is achieved via other pathways than uptake and accumulation within the body. It has been argued50 that B is not a sufficient criterion for protecting against poorly reversible effects because the residence time of highly persistent chemicals in the environment is often much greater than their residence time in humans and biota, which means that levels in organisms will be poorly reversible regardless of the magnitude of B. The limitations of the PBT and vPvB assessment criteria were the motivation for the development of other complementary chemical management approaches such as the “P-sufficient” and the “PMT/vPvM” approaches. On the other hand, the PBT/vPvB approach is a well-established regulatory framework.
Grouping according to the PMT/vPvM approach
The German Environment Agency (UBA) has recently proposed a PMT/vPvM approach for identifying substances that may pose a threat to sources of drinking water.51 The approach presents and discusses updated guidelines for using the REACH registration process to identify persistent, mobile, and toxic (PMT) substances as well as very persistent and very mobile (vPvM) substances. The motivation for this approach is to pinpoint substances that might require control to protect waters used as sources for drinking water or food production. The PMT approach classifies substances considered persistent in the environment (P), mobile in the aquatic environment (M) and toxic (T). For substances identified as very persistent (vP) and very mobile (vM), it is not necessary to consider toxicity data.51 Under this concept, the short-chain PFAAs and many other replacements of long-chain PFAAs such as HFPO-DA, which are both vP and vM, would be identified.A consequence of introducing the PMT/vPvM approach is that, in combination with the existing PBT/vPvB approach under REACH, a wide range of substances that are vP would be covered. Hydrophobic substances with a high octanol–water partition coefficient (KOW) (e.g. KOW cutoff of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW > 5 under the Stockholm Convention on Persistent Organic Pollutants) would be covered by the vPvB approach, and hydrophilic substances with low KOW (a cutoff of log
KOW > 5 under the Stockholm Convention on Persistent Organic Pollutants) would be covered by the vPvB approach, and hydrophilic substances with low KOW (a cutoff of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW < 4 under the proposed PMT/vPvM approach) would be covered by the vPvM approach. Therefore, the authors of the “P-sufficient” approach argue that partitioning properties such as KOW, KOC (organic-carbon-water partition coefficient) and the BCF are irrelevant and that PFAS should be managed according to their high persistence alone.24 Similar to the “P-sufficient approach” the PMT/vPvM approach is still a proposal and not currently broadly implemented under REACH.
KOW < 4 under the proposed PMT/vPvM approach) would be covered by the vPvM approach. Therefore, the authors of the “P-sufficient” approach argue that partitioning properties such as KOW, KOC (organic-carbon-water partition coefficient) and the BCF are irrelevant and that PFAS should be managed according to their high persistence alone.24 Similar to the “P-sufficient approach” the PMT/vPvM approach is still a proposal and not currently broadly implemented under REACH.
Grouping some fluoropolymers as “polymers of low concern”
PFAS are broadly subdivided into low molecular weight substances and fluorinated polymers.1 There are three subclasses of fluorinated polymers that meet the PFAS structural definition and these are termed: fluoropolymers, perfluoropolyethers and side-chain fluorinated polymers.1 According to Buck et al.,1 fluoropolymers are a distinct subset of fluorinated polymers made by (co)polymerization of olefinic monomers, at least one of which contains fluorine bound to one or both of the olefinic carbon atoms, to form a carbon-only polymer backbone with fluorine atoms directly attached to it, e.g., polytetrafluoroethylene (PTFE).It was recently suggested that a subset of fluoropolymers should be considered distinct from other fluorinated polymers based on international criteria for “polymers of low concern” (PLC) due to (among other things) their high molecular weight, narrow molecular weight distribution, negligible oligomer content and organic and inorganic leachables.52 Classification as PLC may exempt the manufacturers of certain fluoropolymers from certain regulatory notification requirements. Integration of the PLC criteria into a risk management framework may differ from country to country according to individual regulatory mandate.52 Although a recent framework for polymer risk assessment recommended consideration of impacts throughout the lifecycle of a polymeric product,53 Henry et al.52 limited their assessment of fluoropolymers to the use phase. However, there are serious concerns regarding the environmental impacts of fluoropolymers during manufacture (“beginning of life”) and waste management (“end of life”) that need to be addressed. Specifically: (i) some fluoropolymers (e.g. PTFE fine powder) are still manufactured in Asia using processing aids containing hazardous long-chain PFAAs (e.g. PFOA), which are widely distributed in the Asian environment54 and can undergo long-range global transport,55,56 (ii) there are concerns among scientists and regulators regarding the substitute processing aids used (e.g. HFPO-DA is now an SVHC under the EU REACH regulation),15 (iii) a wide range of potentially hazardous byproducts have been observed in the environment near fluoropolymer manufacturing sites,14,57,58 (iv) environmental emissions of these persistent polymers during use and at end of life are problematic given the current concern regarding persistent microplastics in the environment (even if fluoropolymer plastic waste is of relatively low volume),59 and (v) the best available technology for treatment of solid wastes is currently incineration, from which emissions of harmful chemicals including certain PFAS could occur if incineration is not operated according to international guidelines.60 The PLC criteria should be applied on a product-by-product basis because individual fluoropolymer products (e.g. due to different impurity levels) may not meet the PLC criteria.
Grouping approaches that inform risk assessment
Arrowhead approach: grouping PFAAs together with their precursors
The so-called “arrowhead approach” is defined as when a representative PFAS (usually a PFAA) is managed together with its salts and precursors. The approach represents the dominant current approach to grouping PFAS for risk assessment and risk management globally. Industry have used the approach in voluntary phase-out actions (e.g. 3M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) of PFAS chemistries and it is applied globally in PFAS regulations. For example, precursors to long-chain PFAAs have been grouped together with specific PFAAs in risk management (e.g. under REACH,61,62 in the Stockholm Convention,63,64 see Table 1, or are currently under discussion, see Table 2) given that these precursor substances will transform to an “arrowhead substance of concern” (i.e. the long-chain PFAAs that have PBT properties) in the environment, in biota, or in humans. There is no indication of how many substances, past or present, are covered by definitions such as, “PFOA, its salts and PFOA-related compounds”. There are thousands of substances that can theoretically be broken down into PFOA, but it is not clear which of them are or have been used.
9) of PFAS chemistries and it is applied globally in PFAS regulations. For example, precursors to long-chain PFAAs have been grouped together with specific PFAAs in risk management (e.g. under REACH,61,62 in the Stockholm Convention,63,64 see Table 1, or are currently under discussion, see Table 2) given that these precursor substances will transform to an “arrowhead substance of concern” (i.e. the long-chain PFAAs that have PBT properties) in the environment, in biota, or in humans. There is no indication of how many substances, past or present, are covered by definitions such as, “PFOA, its salts and PFOA-related compounds”. There are thousands of substances that can theoretically be broken down into PFOA, but it is not clear which of them are or have been used.
| Substances | What is included | Context |
|---|---|---|
| PFOA, its salts and PFOA-related compounds63 | Perfluorooctanoic acid (PFOA), its salts and PFOA-related compounds means the following: (i) perfluorooctanoic acid (PFOA; CAS no. 335-67-1), including any of its branched isomers; (ii) its salts; (iii) PFOA-related compounds which, for the purposes of the convention, are any substances that degrade to PFOA, including any substances (including salts and polymers) having a linear or branched perfluoroheptyl group with the moiety (C7F15)C as one of the structural elements | Stockholm Convention on Persistent Organic Pollutants (POPs) |
| The following compounds are not included as PFOA-related compounds: (i) C8F17–X, where X = F, Cl, Br; (ii) fluoropolymers that are covered by CF3[CF2]n–R′, where R′ = any group, n > 16; (iii) perfluoroalkyl carboxylic and phosphonic acids (including their salts, esters, halides and anhydrides) with ≥8 perfluorinated carbons; (iv) perfluoroalkane sulfonic acids (including their salts, esters, halides and anhydrides) with ≥9 perfluorinated carbons; (v) perfluorooctane sulfonic acid (PFOS), its salts and perfluorooctane sulfonyl fluoride (PFOSF), as listed in Annex B to the Convention | ||
| PFOA, its salts and PFOA related compounds62 | Any related substance (including its salts and polymers) having a linear or branched perfluoroheptyl group with the formula C7F15– directly attached to another carbon atom, as one of the structural elements. Any related substance (including its salts and polymers) having a linear or branched perfluorooctyl group with the formula C8F17– as one of the structural elements. The following substances are excluded from this designation: C8F17–X, where X = F, Cl, Br – C8F17–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OH, C8F17–C( O)OH, C8F17–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O–X′ or C8F17–CF2–X′ (where X′ = any group, including salts) O)O–X′ or C8F17–CF2–X′ (where X′ = any group, including salts) |
EU REACH restriction (REACH Annex XVII entry 68) |
| PFOA, its salts and precursors as well as long-chain (C9–C20) PFCAs, their salts and precursors66 | PFOA, its salts and precursors as well as long-chain (C9–C20) PFCAs, their salts and precursors | Order Adding Toxic Substances to Schedule 1 of the Canadian Environmental Protection Act, 1999 |
| Substances | Context |
|---|---|
| Undecafluorohexanoic acid (PFHxA), its salts and related substances67 | EU REACH restriction proposal |
| Perfluorononan-1-oic acid (PFNA); nonadecafluorodecanoic acid (PFDA); henicosafluoroundecanoic acid (PFUnDA); tricosafluorododecanoic acid (PFDoDA); pentacosafluorotridecanoic acid (PFTrDA); heptacosafluoro-tetradecanoic acid (PFTDA) including their salts and precursors68 | EU REACH restriction proposal |
| PFHxS, its salts and PFHxS-related compounds as well as polymers and mixtures64 | Proposed for listing under the Stockholm Convention on Persistent Organic Pollutants |
| PFHxS, its salts and related substances69 | EU REACH restriction proposal |
Although the arrowhead approach is an efficient way of assessing and regulating large groups of chemicals simultaneously there are some limitations. One limitation is that the approach may overlook the risks from the parent PFAS themselves, or intermediate degradation products that are formed along the pathway to the presumed arrowhead degradation products. For example, a recent study demonstrated that 6:2 fluorotelomer alcohol (6:2 FTOH) is significantly more toxic to rodents than perfluorohexanoic acid (PFHxA).65 The authors concluded that the use of toxicological studies conducted with PFHxA to assess 6:2 FTOH exposure may significantly underestimate human health risk.
Challenges with the above groups are the lack of an exhaustive list of present precursors and analytical methods for individually measuring all relevant precursors to a specific PFAA in a certain medium. Although it was primarily developed as a research tool,70 the total oxidizable precursor (TOP) assay is a potential solution to quantifying PFAAs and their precursors. The TOP assay has been primarily applied to quantify precursors that can be oxidized to PFAAs in water samples,70 although it has further been developed and applied to a wider range of sample types, e.g. soils,71 paper and textiles.72
Application of the TOP assay usually involves quantifying PFAAs in samples using targeted analysis before and after treatment with powerful oxidizing agents.70 The difference between the levels of PFAAs before and after treatment is considered to be an indicator of the total concentration of the oxidizable PFAA precursors, because PFCAs and PFSAs that were present in the original sample remain mostly intact under the conditions of the assay. Currently it is not possible to apply the TOP assay to enforce the PFOA restriction under REACH in Table 1 because, for example, PFOA might be formed during TOP assay oxidation from a precursor which is not within the restriction scope.
Levels of PFAAs in drinking water samples could be compared to drinking water guidelines after the samples have been treated with the TOP assay. An advantage of this approach is that precursors would be included that could be transformed in the water or metabolized to PFAAs inside the body after intake. On the other hand, the TOP assay may not simulate environmental transformation and metabolic processes accurately. The assay is an aggressive oxidation process that generates shorter-chain PFAAs than natural environmental oxidation processes, and even degrades polyfluoroalkyl ether acids with –O–CFH– moieties.73 Furthermore, it may overestimate the contribution of some precursors to PFAA body burdens, and underestimate others and, thus, inaccurately estimate the risks. For example, the TOP assay transforms perfluorooctane sulfonamide (FOSA) to PFOA,70 whereas FOSA is likely metabolized to PFOS in vivo in humans.74 An enzyme-based assay would be preferable to simulate biological transformations, but is not yet broadly available. Finally, the TOP assay has not to date been standardized so results from different laboratories may be inconsistent.75
Total fluorine and extractable/adsorbable organofluorine approaches
Driven by the need for fast and inexpensive analytical methods to determine the presence or absence of PFAS in a given sample and by the lack of analytical standards for most known and unknown PFAS, total fluorine (TF) and extractable/adsorbable organofluorine measurements have been put forward.72,76–79 These methods could also be used in screening-level exposure assessments, e.g. to determine if the level of total extractable/adsorbable organofluorine in a sample is below or above a pre-defined limit, which would trigger further chemical assessment and management measures including more in-depth targeted analysis.TF comprises the sum of all fluorine as a surrogate for all inorganic and organic fluorinated substances in a sample.76 TF can be measured through particle-induced gamma(γ)-ray emission (PIGE) spectroscopy, X-ray photoelectron spectroscopy (XPS) and combustion ion chromatography (CIC). PIGE spectroscopy is an ion beam technique used for the analysis of fluorine in solid materials, and liquids after solid-phase extraction.72 XPS has also recently been used for fluorine mass balance experiments in consumer products.77 CIC involves combusting samples or extracts, collecting fluoride ions in water and then separating them on an ion exchange column, and has also been applied to consumer products.78
Today, TF is used in Denmark with an official indicator value of 0.1 μg cm−2 for food packaging.80 The indicator value can help industry and regulators assess whether organic fluorinated substances have been added to paper and cardboard. Furthermore, it can inform if PFAS levels are increasing over time. If the indicator level is exceeded, this can justify further analyses needed for risk assessment. The fast application of TF methods and relatively simple evaluation of results (yes and no for presence of fluorine) is appealing. The relatively high detection limits of TF methods and lack of specificity (cannot specify if TF is PFAS) are drawbacks. Assuming a 10 mg sample size, detection limits for TF were recently reported as 0.8 and 38 μg g−1 for CIC and PIGE in paper samples, respectively, which is at least 1000 times higher compared to modern PFAS analysis by liquid chromatograph-tandem mass spectrometry (LC-MS/MS).78
Depending on the sample type, a certain fraction of the TF can be extracted using organic solvents (extractable organic fluorine, EOF). Alternatively, the PFAS in aqueous samples can be extracted using a sorbent, which is then analyzed for TF (adsorbable organic fluorine, AOF). The EOF/AOF fraction in a sample can be assumed to contain primarily synthetic organofluorine substances given the low abundance of naturally occurring ones, rarely exceeding more than one fluorine per molecule.79 By comparing the concentration of EOF/AOF with the total PFAS measured in a sample by targeted analysis, the fractions of known and unknown organofluorine substances can be determined. If the unknown fraction of organofluorine substances is large in a given sample, then this can be probed using non-targeted analytical methods.14,57,81 As shown in recent literature, the explainable contributions of EOF to the TF in, e.g., cosmetics,82 seawater,83 food packaging,78 contaminated water83 and human blood84 may be 0.1–3%, 2%, 5.5%, 30% and 80%, respectively. Fig. 3 illustrates fluorine-containing chemicals covered by available analytical methods.
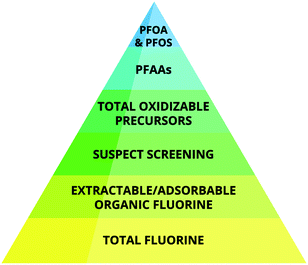 | ||
| Fig. 3 Schematic of increasing resolution in information detail of analytical methods used for PFAS analyses. | ||
For estimating the drinking water exposure to total PFAS, EOF/AOF could be potentially used instead of targeted analysis for groups of PFAS. For example, in the EU very likely a ‘PFAS total’ limit of 500 ng L−1 will be provisionally set in a recast of the Drinking Water Directive85 and EOF/AOF could potentially be used to relatively rapidly determine if a sample is below this 500 ng L−1 limit. An advantage of the EOF/AOF approach is that all PFAS would be captured in a single measurement that is relatively inexpensive compared to targeted LC-MS/MS methods for individual PFAS. EOF/AOF measurement approaches may further help to determine if unknown PFAS are released to the environment from production sites and are present in drinking water or a particular product (e.g. ski waxes or food contact materials). They are therefore good screening approaches that can be followed up with non- or suspect-targeted analytical methods to identify substances in the unknown PFAS fraction.14,57,81 A disadvantage, however, would be uncertainties in translating the EOF/AOF measurements into risk-based guidelines. A “worst case” assumption could be that the EOF/AOF concentration is equal to the concentration of the most toxic PFAS known (e.g. typically PFOS or PFOA, see Table 1). This approach may be considered precautionary and protective, but on the other hand, humans are exposed to a lot of unknown PFAS with unknown risks, which may be more toxic than the currently known ones. Another disadvantage of this approach in its application to PFAS is that it will likely capture organofluorine substances that are currently not considered as PFAS (e.g. fluorinated substances used as pharmaceuticals and pesticides). Finally, a common problem with TF, EOF/AOF and the TOP assay is that these methods require further development before they can be considered sufficiently reliable for regulatory applications. Efforts are underway to assess, further develop and standardize methods as well as to conduct inter-laboratory comparison studies.
Simple additive toxicity approach: application to drinking water standards
Regulatory agencies worldwide have developed guidelines or advisories for acceptable levels of PFAS in drinking water. Because there are so many PFAS and only limited toxicological and toxicokinetic data for most of them, it is challenging to generate guidelines for individual PFAS, let alone robust grouping strategies. Some regulatory agencies have grouped multiple PFAS together and set one limit for the combined (sum of) concentrations of these chemicals (Table 3). A simple example is the combined drinking water health advisory of 70 ng L−1 set by the US EPA for the sum of PFOA and PFOS.86 The assumptions made in this grouping are that the critical toxicological endpoint is the same for the two substances (i.e., developmental toxicity) and that the margin of safety (MOS, i.e. the ratio of NOAEL obtained from animal toxicology studies to the predicted or estimated human exposure level or dose) is similar. In Sweden, 11 different PFAS87 are grouped with the limit of 90 ng L−1 for the sum of these 11 PFAS, above which consumption of drinking water is not recommended.| Entity | Date | Conc. (ng L−1) | Sum of which PFAS? | Background |
|---|---|---|---|---|
| a Many US States have simply adopted US EPA's recommended Lifetime Health Advisory (LHA) of 70 ppt for PFOA and PFOS in drinking water. Several states have passed or proposed compound-specific MCLs or health advisories, including California, Michigan, Minnesota, New Hampshire, New Jersey, North Carolina, Ohio. Some states have recommendations for ground water that are separate from drinking water. Only sum of PFAS parameters are included. b Cumulative toxicity estimated by adding the ratio of the monitoring result for PFOS to its maximum acceptable concentration (MAC) with the ratio of the monitoring result for PFOA to its MAC; if the result is below or equal to one, then the water is considered safe for drinking. According to the Canadian assessment, “science currently does not justify the use of this approach for other PFAS”.93 | ||||
| EU85 | 2020 (pending final adoption) | 100; 500 | 100 ng L−1 for sum of 20 PFAS (C4–C13 PFSAs and C4–C13 PFCAs) | Politically agreed parameter (not based on risk assessment) based on a precautionary approach |
| 500 ng L−1 for ‘PFAS Total’ – the total of all PFAS | ‘PFAS Total’ suggested to be enforced through measurement of EOF/AOF | |||
| Denmark91 | 2015 | 100 | C4–C10 PFCAs, PFBS, PFHxS, PFOS, PFOSA, and 6:2 FTS | Assumes all 12 PFAS are similarly toxic to PFOS |
| Sweden87 | 2014 | 90 | C4–C10 PFCAs, PFBS, PFHxS, PFOS and 6:2 FTS | Assumes all 11 PFAS are similarly toxic to PFOS |
| Australia92 | 2017 | 70 | PFOS and PFHxS combined, if both present | Assumes PFHxS is similarly toxic to PFOS |
| Canada93 | 2018 | 200, 600 | PFOA and PFOS | When PFOS and PFOA are found together in drinking water, a cumulative toxicity approach is appliedb |
| US EPAa86 | 2016 | 70 | PFOA and PFOS | Lifetime health advisory level. Assumes additive toxicity of PFOA and PFOS |
| Connecticut (USA)94 | 2017 | 70 | PFHpA, PFOA, PFNA, PFHxS and PFOS | Application of US EPA lifetime health advisory level to the sum of five PFAS; assumes toxicity similar to that of PFOS and PFOA |
| Maine (USA)95 | 2020 | 70 | PFHxS, PFNA, PFHpA, PFOA and PFOS | Application of US EPA lifetime health advisory level to the sum of five PFAS; assumes toxicity similar to that of PFOS and PFOA |
| Massachusetts (USA)96 | 2018/19 | 20 | PFHpA, PFOA, PFNA, PFDA, PFHxS and PFOS | Proposed maximum contaminant level (MCL) based on similarities in chemical structure and toxicities of six PFAS to PFOS and PFOA. Same approach as US EPA lifetime health advisory level, but includes an additional uncertainty factor to account for evidence of toxicities in experimental animals at lower levels of exposure than those used by US EPA |
| Vermont (USA)97 | 2019 | 20 | PFHpA, PFOA, PFNA, PFHxS and PFOS | Interim drinking water standard based on similar health risks of five PFAS. Difference to US EPA advisory is due to Vermont's calculation being based on infant consumption rates |
The simple additive toxicity approach has the advantage that it is easy to understand and environmental or health-based guidelines can be evaluated with current analytical methods. Furthermore, it is thought to be protective for humans and the environment in that the additive toxicity is based on the most toxic PFAS in the group. Scientific shortcomings of the simple additive toxicity approach that sums multiple PFAS are that (1) it assumes an external dose-additive model88,89 whereas elimination kinetics vary largely among individual PFAS,90 (2) the identified critical adverse effects, as well as modes and mechanisms of action, may vary for individual PFAS,7 (3) mixture toxicity may not be simply additive even if the critical adverse effects are the same88,89 and (4) although multiple PFAS are included in these drinking water standards, many more PFAS are neglected. Some possible solutions to the highlighted issues are discussed in the remaining approaches reviewed, below.
Relative potency factor approach
The Dutch National Institute for Public Health and the Environment (RIVM) recently developed a mixture toxicity approach for a number of PFAS termed Relative Potency Factors (RPFs).98 RIVM's RPF approach builds on the assumption that the combined toxicity of two or more substances can be calculated based on the concept of dose addition, whereby the substances have the same effect, but differ only in their toxic potencies. Liver toxicity data were available for a number of PFAS for rats and mice from which RPFs could be derived. PFOA was the reference substance and assigned an RPF of 1.0. RPFs were estimated for 18 other PFAS with values ranging from 0.001 for PFBS up to 10 for PFDA. Environmental concentrations can be converted into PFOA equivalents by multiplying the RPFs by specific PFAS concentrations. However, questions surrounding potential synergism of toxic effects remain;99 while observations for many endpoints have been largely additive, there is some evidence from in vivo animal studies on specific endpoints and in vitro studies, for some higher doses, that PFAS impacts may be synergistic.100 Thus, a successful grouping strategy may need to be endpoint-specific, in which the additivity of impact for the most sensitive endpoint will need to be carefully considered.101,102The RPFs derived by RIVM were defined using external exposures in rodents, i.e. based on the administered dose. Gomis et al.90 demonstrated that the differences in RPF in rats can be largely explained by differences in the elimination rates of PFAS. When potencies of PFAS were compared on an internal dose basis, the differences in potencies disappeared and the various PFAS were equally potent. This suggests that relative external potency is in fact largely a measure of accumulation potential, and that it may be possible to set a single internal dose for a particular endpoint and sum across all PFAS. Further confirmation is needed that this observation holds across a wider variety of PFAS structures, as Gomis et al.90 considered primarily PFAAs. Moreover, the application of simple addition of effective internal dose across many PFAS, in the absence of effects data linked to internal dose, would require more toxicokinetic data than are currently available. Elimination half-lives can vary by PFAS structure (chain length and degree of branching), across species, and by sex. Because of this, grouping for the purpose of wildlife protection should be based on first identifying the most sensitive species and sex. For humans, translation of animal data would require two key pieces of information: first, whether the internal dose effect level is the same, and, second, the toxicokinetic data and associated model required to translate the effective internal dose in the human back to an external dose that can be associated with an exposure medium (e.g. drinking water).
Finally, the RPF approach may be difficult to reconcile for substances that have the potential to biotransform; should the parent compound, the metabolite, or both be considered in the calculation? In each case, is there a temporal component that needs to be taken into account, in addition to the toxicokinetic considerations suggested above? For example, cellular assays suggest that reactive intermediate degradation products of fluorotelomer alcohols, such as short-chain saturated and unsaturated fluorotelomer aldehydes, are more toxic than either the parent compound or the terminal PFCA transformation products.103,104
The specific RPF approach suggested by RIVM is sound if it can be argued that liver hypertrophy is a sensitive and reliable endpoint for all PFAAs; a problem here is that many regulatory jurisdictions disagree with that assessment. However, a similar additive toxicity approach could potentially be applied for those other endpoints. The RPF approach is currently limited by the database of toxicity data available for PFAS. Expanding this knowledge base would require a large number of animal experiments and associated ethical considerations, time and money.
Grouping only PFAAs with the same adverse effect, modes and mechanisms of action, and toxicokinetics
The most demanding grouping approach would be to only group PFAS that have the same adverse effects, modes and mechanisms of action, and toxicokinetics for risk assessment. The clear disadvantages with an approach of this kind are that (1) very few substances are likely to be grouped together given that there is currently no agreement on a single mode and mechanism of action for even the well-studied PFCAs and PFSAs,7 (2) modes and mechanisms of action may be tissue or system-specific, requiring a determination of the most sensitive or reliable effect for grouping, (3) detailed effect and kinetic data are needed for each PFAS, such that individual chemicals would still need extensive toxicological profiles and (4) many groups will be required. Such a grouping approach can be considered only a marginal improvement on conducting risk assessments on a chemical-by-chemical basis.Remaining challenges and the way forward
There are a number of challenges if the PFAS grouping approaches summarized in this article are to be integrated into chemical regulation and company policies, namely; (1) the universe of PFAS2 has not been fully mapped and divided into subcategories, (2) only for a few PFAS (e.g. certain PFAAs and their precursors) is there sufficient information available to conduct detailed hazard and risk assessments, whereas little or no information exists on production volumes, properties and toxic effects for the vast majority of PFAS,3,8 and (3) no single grouping strategy may be adequate for all decision contexts. Each of these challenges will be discussed in turn below.Within the universe of PFAS, most research to date has focused on the occurrence and effects of certain PFAAs and their precursors due to the availability of analytical methods and standards for these substances. Expanding beyond this domain has been challenging because the chemical composition of most remaining commercial products is unknown. These factors are slowly becoming less of a barrier for identifying overlooked and unknown PFAS due to the recent advancement of non- and suspect-targeted screening techniques.14,57,81 However, these screening analytical methods are extremely challenging to apply, even by experts, and the lack of methods and analytical standards for a wider range of PFAS will remain a barrier for regulatory purposes.
Depending on the grouping strategies to be taken by individual regulatory agencies and companies, there will inevitably be efforts in the coming years to generate the missing data for some of the thousands of PFAS. To address these data issues, the US EPA in partnership with the US National Toxicology Program (NTP) has recently selected 150 PFAS (expanded from 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10,86,105) for high-throughput toxicity testing (e.g. in vitro assays) for multiple endpoints.106 Selection criteria for this subset of 150 PFAS included maximizing information to support read-across within structure-based groupings and capturing the structural diversity of the PFAS landscape. The new toxicity and toxicokinetic data generated from this initiative will support the development of quantitative structure–activity relationships (QSARs) that could facilitate filling data gaps, as well as further grouping and prioritization of the universe of PFAS. There are clearly relationships between PFAS structural elements and properties and behaviour (e.g. number of fluorinated carbons in the perfluoroalkyl(ether) chain, protein binding affinities, bioaccumulation potential, elimination rates, bioactivities within the PFAA/perfluoroalkylether acid subclasses),11,44,90,107 but on the other hand, critical toxic endpoints, as well as modes and mechanisms of action vary within the PFAS and such inconsistencies could limit the applicability of QSARs and thus reliability of computational tools.
10,86,105) for high-throughput toxicity testing (e.g. in vitro assays) for multiple endpoints.106 Selection criteria for this subset of 150 PFAS included maximizing information to support read-across within structure-based groupings and capturing the structural diversity of the PFAS landscape. The new toxicity and toxicokinetic data generated from this initiative will support the development of quantitative structure–activity relationships (QSARs) that could facilitate filling data gaps, as well as further grouping and prioritization of the universe of PFAS. There are clearly relationships between PFAS structural elements and properties and behaviour (e.g. number of fluorinated carbons in the perfluoroalkyl(ether) chain, protein binding affinities, bioaccumulation potential, elimination rates, bioactivities within the PFAA/perfluoroalkylether acid subclasses),11,44,90,107 but on the other hand, critical toxic endpoints, as well as modes and mechanisms of action vary within the PFAS and such inconsistencies could limit the applicability of QSARs and thus reliability of computational tools.
Within the EU, there is already discussion to phase out all non-essential uses of PFAS based on concerns of the chemical class as a whole.19 Within the US, as discussed above, the focus of the US EPA is on developing high-throughput testing methods for PFAS,106 but otherwise adhering to the traditional risk assessment paradigm. These differences in approaches are inevitable given the differences in chemical management philosophies around the world and motivations to group PFAS. It is expected that many of the approaches reviewed in this paper will be taken in parallel by regulatory agencies in the different countries. In addition, some of the reviewed grouping approaches could even be combined (e.g. the newly identified bioaccumulative PFAS could be regulated together with potential precursors).
An advantage of the precautionary grouping approaches based on intrinsic properties is that relatively few data are needed to group PFAS and regulate them. Conversely, traditional testing and regulation of PFAS on a chemical-by-chemical basis would require huge resources and the information required to perform risk assessments would take many years or decades to generate. Arguably, regulation could never catch up given that new PFAS continue to be invented and produced. Regulation is not the only way to reduce the use of harmful PFAS in society. Since PFAS have come under pressure in society, there has been much innovation to produce a new generation of alternative chemical products that aim to provide healthier, safer, and more sustainable solutions.18,108 It should be possible for manufacturers to make chemical products that provide the function required in modern society while limiting or eliminating hazardous impacts over a chemical product's life-cycle.
Some product manufacturers and retailers continue to take proactive voluntary measures to phase out PFAS from their supply chains especially where they are non-essential or where functional non-fluorinated alternatives are available. Examples of retailers who have phased out PFAS from their supply chains include IKEA,109 Lindex,110 and H&M111 in Sweden, Coop112 in Denmark, and Vaude113 and Jack Wolfskin114 in Germany. In some jurisdictions and even internationally, PFAS are also being phased out from certain use categories, for example, PFAS will be phased out of use in ski waxes in international competitions from the winter season of 2020–2021,115 multiple global manufacturers moved to phase out PFAS from cosmetics by 2020,116 Denmark will ban PFAS in food contact materials in 2020,117 South Australia will transition away from the use of PFAS in fire-fighting foams by 2020118 and California designated all PFAS used in carpets and rugs as “Chemicals of Concern”.119 However, given the complexity of supply chains and ignorance of the full range of PFAS in society, these phase-outs may in some use cases only be partially successful, and largely focus on a few well known PFAS.
Given that PFAS will continue to be used in society until alternatives are developed, scientists should work to identify the groups and applications of PFAS among those still in use that have unfavorable properties which make them particular threats to human and environmental health. However, there is a justifiable concern that approaches requiring multiple grouping approaches would result in a similarly large usage of resources as a chemical-by-chemical regulatory approach. Investing additional public funds for scientists to identify all troublesome PFAS, their environmental behaviour and effects could delay broader regulatory action on PFAS. A precautionary approach with the aim of phasing out the “non-essential” uses of PFAS18 would reduce future exposures and the high costs of research, regulation and cleanup of contaminated sites, while having minimal impacts on daily life and the economy.
Conflicts of interest
This paper does not necessarily reflect the opinions or the policies of the German Environment Agency. Ian Cousins has provided expert reports in three separate class actions related to PFAS in the Federal Court of Australia. Jamie DeWitt is serving as a plaintiff's expert witness in several cases related to PFAS. No other authors declare any conflicts of interest.Acknowledgements
The authors thank the Global PFAS Science Panel (GPSP) and the Tides Foundation for supporting this cooperation (grant 1806-52683). The University of Rhode Island thanks the US National Institute of Environmental Health Sciences (grant P42ES027706). NILU acknowledges the support of the Strategic Institute Program CleanArctic (grant N117031). Dr Juliane Glüge acknowledges the support of the Swiss Federal Office for the Environment (FOEN, grant 1-004496-000). The authors appreciate the contributions of Dr Xenia Trier of the European Environment Agency, Dr Andrew Lindstrom of the U.S. Environmental Protection Agency, and Dr Robin Vestergren of IVL Swedish Environmental Research Institute.References
- R. C. Buck, J. Franklin, U. Berger, J. M. Conder, I. T. Cousins, P. de Voogt, A. A. Jensen, K. Kannan, S. A. Mabury and S. P. J. van Leeuwen, Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins, Integrated Environ. Assess. Manag., 2011, 7(4), 513–541 CrossRef CAS PubMed.
- OECD/UNEP, Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs), Organisation for Economic Co-operation and Development; 2018 Search PubMed.
- Z. Y. Wang, J. C. DeWitt, C. P. Higgins and I. T. Cousins, A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)?, Environ. Sci. Technol., 2017, 51(5), 2508–2518 CrossRef CAS PubMed.
- Z. Y. Wang, I. T. Cousins, M. Scheringer and K. Hungerbuehler, Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions, Environ. Int., 2015, 75, 172–179 CrossRef CAS PubMed.
- M. Scheringer, X. Trier, I. T. Cousins, P. de Voogt, T. Fletcher, Z. Y. Wang and T. F. Webster, Helsingor Statement on poly- and perfluorinated alkyl substances (PFASs), Chemosphere, 2014, 114, 337–339 CrossRef CAS PubMed.
- Z. Y. Wang, M. MacLeod, I. T. Cousins, M. Scheringer and K. Hungerbuhler, Using COSMOtherm to predict physicochemical properties of poly- and perfluorinated alkyl substances (PFASs), Environ. Chem., 2011, 8(4), 389–398 CrossRef CAS.
- ATSDR Toxicological profile for Perfluoroalkyls. (Draft for Public Comment), U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA, 2018, https://www.atsdr.cdc.gov/ToxProfiles/tp.asp?id=1117%26tid=237, last updated September 26, 2019, accessed 11-05-2020 Search PubMed.
- A. Ritscher, Z. Y. Wang, M. Scheringer, J. M. Boucher, L. Ahrens, U. Berger, S. Bintein, S. K. Bopp, D. Borg, A. M. Buser, I. Cousins, J. DeWitt, T. Fletcher, C. Green, D. Herzke, C. Higgins, J. Huang, H. Hung, T. Knepper, C. S. Lau, E. Leinala, A. B. Lindstrom, J. X. Liu, M. Miller, K. Ohno, N. Perkola, Y. L. Shi, L. S. Haug, X. Trier, S. Valsecchi, K. van der Jagt and L. Vierke, Zurich Statement on Future Actions on Per- and Polyfluoroalkyl Substances (PFASs), Environ. Health Perspect., 2018, 126(8), 5 CrossRef PubMed.
- 3M Press Release: 3M Phasing Out Some of its Specialty Materials, 2000 Search PubMed.
- US EPA Factsheet, https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/fact-sheet-20102015-pfoa-stewardship-program, accessed 30-01-2020.
- J. M. Conder, R. A. Hoke, W. De Wolf, M. H. Russell and R. C. Buck, Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds, Environ. Sci. Technol., 2008, 42(4), 995–1003 CrossRef CAS PubMed.
- Z. Y. Wang, I. T. Cousins, M. Scheringer and K. Hungerbuhler, Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors, Environ. Int., 2013, 60, 242–248 CrossRef CAS PubMed.
- S. Brendel, E. Fetter, C. Staude, L. Vierke and A. Biegel-Engler, Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH, Environ. Sci. Eur., 2018, 30, 11 CrossRef PubMed.
- W. A. Gebbink, L. van Asseldonk and S. P. J. van Leeuwen, Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands, Environ. Sci. Technol., 2017, 51(19), 11057–11065 CrossRef CAS PubMed.
- ECHA European Chemicals Agency, Candidate List of substances of very high concern for Authorisation, https://echa.europa.eu/de/candidate-list-table, accessed 11-02-2020 Search PubMed.
- A. Blum, S. A. Balan, M. Scheringer, X. Trier, G. Goldenman, I. T. Cousins, M. Diamond, T. Fletcher, C. Higgins, A. E. Lindeman, G. Peaslee, P. de Voogt, Z. Y. Wang and R. Weber, The Madrid Statement on Poly- and Perfluoroalkyl Substances (PFASs), Environ. Health Perspect., 2015, 123(5), A107–A111 CrossRef CAS PubMed.
- J. S. Bowman, Fluorotechnology Is Critical to Modern Life: The FluoroCouncil Counterpoint to the Madrid Statement, Environ. Health Perspect., 2015, 123(5), A112–A113 CrossRef CAS PubMed.
- I. T. Cousins, G. Goldenman, D. Herzke, R. Lohmann, M. Miller, C. A. Ng, S. Patton, M. Scheringer, X. Trier, L. Vierke, Z. Y. Wang and J. C. DeWitt, The concept of essential use for determining when uses of PFASs can be phased out, Environ. Sci.: Process. Impacts, 2019, 21(11), 1803–1815 CAS.
- Elements for an EU-strategy for PFASs, December 2019, https://www.documentcloud.org/documents/6586418-EU-Strategy-for-PFASs-FINAL-VERSION-December-19.html, accessed 11-02-2020 Search PubMed.
- ECHA European Chemicals Agency, Five European states call for evidence on broad PFAS restriction, http://https://echa.europa.eu/de/-/five-european-states-call-for-evidence-on-broad-pfas-restriction, accessed 28-05-2020 Search PubMed.
- ECHA European Chemicals Agency, Grouping of substances and read-across, https://echa.europa.eu/support/registration/how-to-avoid-unnecessary-testing-on-animals/grouping-of-substances-and-read-across, accessed 11-02-2020 Search PubMed.
- ECHA European Chemicals Agency, Management of PBT/vPvB substances under REACH, https://echa.europa.eu/management-of-pbt-vpvb-substances, accessed 11-02-2020 Search PubMed.
- D. Borg, B. O. Lund, N. G. Lindquist and H. Hakansson, Cumulative health risk assessment of 17 perfluoroalkylated and polyfluoroalkylated substances (PFASs) in the Swedish population, Environ. Int., 2013, 59, 112–123 CrossRef CAS PubMed.
- I. T. Cousins, C. A. Ng, Z. Y. Wang and M. Scheringer, Why is High Persistence Alone a Major Cause of Concern?, Environ. Sci.: Process. Impacts, 2019, 21(5), 781–792 CAS.
- G. Goldenman, R. Pedersen, H. Bradley, M. Fernandez, R. Weber, M. Scheringer and P. Fantke, Study for the strategy for a non-toxic environment of the 7th EAP. Sub-study d: Very Persistent Chemicals, Milieu Ltd, Brussels, 2017 Search PubMed.
- ECHA European Chemicals Agency, Restriction proposal for intentionally added microplastics in the EU – update. ECHA/NR/19/28, https://echa.europa.eu/de/-/restriction-proposal-for-intentionally-added-microplastics-in-the-eu-update, accessed 08-05-2020 Search PubMed.
- H. Lee, A. O. De Siva and S. A. Mabury, Dietary Bioaccumulation of Perfluorophosphonates and Perfluorophosphinates in Juvenile Rainbow Trout: Evidence of Metabolism of Perfluorophosphinates, Environ. Sci. Technol., 2012, 46(6), 3489–3497 CrossRef CAS PubMed.
- J. C. D'Eon and S. A. Mabury, Uptake and Elimination of Perfluorinated Phosphinic Acids in the Rat, Environ. Toxicol. Chem., 2010, 29(6), 1319–1329 Search PubMed.
- Z. Y. Wang, I. T. Cousins, U. Berger, K. Hungerbuhler and M. Scheringer, Comparative assessment of the environmental hazards of and exposure to perfluoroalkyl phosphonic and phosphinic acids (PFPAs and PFPiAs): Current knowledge, gaps, challenges and research needs, Environ. Int., 2016, 89–90, 235–247 CrossRef CAS PubMed.
- K. L. Meyer, M. J. Carvlin, B. Mukherji, H. A. Sloviter and P. M. Joseph, Fluorinated Blood Substitute Retention in the Rat Measured by F-19 Magnetic-Resonance-Imaging, Investig. Radiol., 1992, 27(8), 620–627 CrossRef CAS PubMed.
- J-CHECK Japan CHEmicals Collaborative Knowledge Database, Substance Data. Perfluorooctane, https://www.nite.go.jp/chem/jcheck/template.action?ano=22714%26mno=2-2366%26cno=307-34-6%26request_locale=en, accessed 11-02-2020 Search PubMed.
- Y. L. Shi, R. Vestergren, Z. Zhou, L. Xu, C. X. Li, Y. Liang and Y. Q. Cai, First discovery of chlorinated perfluoroalkyl ether sulfonic acids (Cl PFAESs) in humans and estimation of elimination kinetics, Abstr. Pap. Am. Chem. Soc., 2016, 251, 2 Search PubMed.
- Y. T. Pan, H. X. Zhang, Q. Q. Cui, N. Sheng, L. W. Y. Yeung, Y. Guo, Y. Sun and J. Y. Dai, First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern, Environ. Sci. Technol., 2017, 51(17), 9553–9560 CrossRef CAS PubMed.
- X. W. Song, R. Vestergren, Y. L. Shi and Y. Q. Cai, A Matrix-Correction Approach to Estimate the Bioaccumulation Potential of Emerging PFASs, Environ. Sci. Technol., 2020, 54(2), 1005–1013 CrossRef CAS PubMed.
- Y. L. Shi, R. Vestergren, Z. Zhou, X. W. Song, L. Xu, Y. Liang and Y. Q. Cai, Tissue Distribution and Whole Body Burden of the Chlorinated Polyfluoroalkyl Ether Sulfonic Acid F-53B in Crucian Carp (Carassius carassius): Evidence for a Highly Bioaccumulative Contaminant of Emerging Concern, Environ. Sci. Technol., 2015, 49(24), 14156–14165 CrossRef CAS PubMed.
- A. O. De Silva, C. Spencer, B. F. Scott, S. Backus and D. C. G. Muir, Detection of a Cyclic Perfluorinated Acid, Perfluoroethylcyclohexane Sulfonate, in the Great Lakes of North America, Environ. Sci. Technol., 2011, 45(19), 8060–8066 CrossRef CAS PubMed.
- Y. Wang, R. Vestergren, Y. L. Shi, D. Cao, L. Xu, Y. Q. Cai, X. L. Zhao and F. C. Wu, Identification, Tissue Distribution, and Bioaccumulation Potential of Cyclic Perfluorinated Sulfonic Acids Isomers in an Airport Impacted Ecosystem, Environ. Sci. Technol., 2016, 50(20), 10923–10932 CrossRef CAS PubMed.
- A. O. De Silva, C. Spencer, K. C. D. Ho, M. Al Tarhuni, C. Go, M. Houde, S. R. de Solla, R. A. Lavoie, L. E. King, D. C. G. Muir, P. A. Fair, R. S. Wells and G. D. Bossart, Perfluoroalkylphosphinic Acids in Northern Pike (Esox lucius), Double-Crested Cormorants (Phalacrocorax auritus), and Bottlenose Dolphins (Tursiops truncatus) in Relation to Other Perfluoroalkyl Acids, Environ. Sci. Technol., 2016, 50(20), 10903–10913 CrossRef CAS PubMed.
- S. Poothong, C. Thomsen, J. A. Padilla-Sanchez, E. Papadopoulou and L. S. Haug, Distribution of Novel and Well-Known Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood, Environ. Sci. Technol., 2017, 51(22), 13388–13396 CrossRef CAS PubMed.
- H. N. Bischel, L. A. MacManus-Spencer and R. G. Luthy, Noncovalent Interactions of Long-Chain Perfluoroalkyl Acids with Serum Albumin, Environ. Sci. Technol., 2010, 44(13), 5263–5269 CrossRef CAS PubMed.
- Y. M. Chen and L. H. Guo, Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin, Arch. Toxicol., 2009, 83(3), 255–261 CrossRef CAS PubMed.
- N. Sheng, J. Li, H. Liu, A. Q. Zhang and J. Y. Dai, Interaction of perfluoroalkyl acids with human liver fatty acid-binding protein, Arch. Toxicol., 2016, 90(1), 217–227 CrossRef CAS PubMed.
- D. Yang, J. Han, D. R. Hall, J. Sun, J. Fu, S. Kutarna, K. A. Houck, C. A. LaLone, J. A. Doering, C. A. Ng and H. Peng, Nontarget Screening of Per- and Polyfluoroalkyl Substances Binding to Human Liver Fatty Acid Binding Protein, Environ. Sci. Technol., 2020, 54(9), 5676–5686 CrossRef CAS PubMed.
- W. X. Cheng and C. A. Ng, Predicting Relative Protein Affinity of Novel Per- and Polyfluoroalkyl Substances (PFASs) by An Efficient Molecular Dynamics Approach, Environ. Sci. Technol., 2018, 52(14), 7972–7980 CrossRef CAS PubMed.
- S. Felizeter, M. S. McLachlan and P. de Voogt, Uptake of Perfluorinated Alkyl Acids by Hydroponically Grown Lettuce (Lactuca sativa), Environ. Sci. Technol., 2012, 46(21), 11735–11743 CrossRef CAS PubMed.
- S. Felizeter, M. S. McLachlan and P. De Voogt, Root Uptake and Translocation of Perfluorinated Alkyl Acids by Three Hydroponically Grown Crops, J. Agric. Food Chem., 2014, 62(15), 3334–3342 CrossRef CAS PubMed.
- A. C. Blaine, C. D. Rich, L. S. Hundal, C. Lau, M. A. Mills, K. M. Harris and C. P. Higgins, Uptake of perfluoroalkyl acids into edible crops via land applied biosolids: field and greenhouse studies, Environ. Sci. Technol., 2013, 47(24), 14062–14069 CrossRef CAS PubMed.
- A. C. Blaine, C. D. Rich, E. M. Sedlacko, L. S. Hundal, K. Kumar, C. Lau, M. A. Mills, K. M. Harris and C. P. Higgins, Perfluoroalkyl Acid Distribution in Various Plant Compartments of Edible Crops Grown in Biosolids-Amended soils, Environ. Sci. Technol., 2014, 48(14), 7858–7865 CrossRef CAS PubMed.
- H. Y. Zhang, R. Vestergren, T. Wang, J. C. Yu, G. B. Jiang and D. Herzke, Geographical Differences in Dietary Exposure to Perfluoroalkyl Acids between Manufacturing and Application Regions in China, Environ. Sci. Technol., 2017, 51(10), 5747–5755 CrossRef CAS PubMed.
- I. T. Cousins, R. Vestergren, Z. Y. Wang, M. Scheringer and M. S. McLachlan, The precautionary principle and chemicals management: the example of perfluoroalkyl acids in groundwater, Environ. Int., 2016, 94, 331–340 CrossRef CAS PubMed.
- M. Neumann and I. Schliebner, Protecting the Sources of Our Drinking Water–A Revised Proposal for Implementing Criteria and an Assessment Procedure to Identify Persistent, Mobile and Toxic (PMT) and Very Persistent, Very Mobile (vPvM) Substances Registered Under REACH, German Environment Agency, Dessau-Rosslau, 2017, p. 16, ISSN 2363-8273 Search PubMed.
- B. J. Henry, J. P. Carlin, J. A. Hammerschmidt, R. C. Buck, L. W. Buxton, H. Fiedler, J. Seed and O. Hernandez, A critical review of the application of polymer of low concern and regulatory criteria to fluoropolymers, Integrated Environ. Assess. Manag., 2018, 14(3), 316–334 CrossRef CAS PubMed.
- ECETOC, The ECETOC Conceptual Framework for Polymer Risk Assessment: (CF4 Polymers). Technical Report No. 133-1. ISSN-2079-1526-133-1 (online), 2019, http://www.ecetoc.org/wp-content/uploads/2019/05/ECETOC-TR133-1CF4Polymers.pdf Search PubMed.
- T. Wang, R. Vestergren, D. Herzke, J. C. Yu and I. T. Cousins, Levels, Isomer Profiles, and Estimated Riverine Mass Discharges of Perfluoroalkyl Acids and Fluorinated Alternatives at the Mouths of Chinese Rivers, Environ. Sci. Technol., 2016, 50(21), 11584–11592 CrossRef CAS PubMed.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, Fate and Transport of Perfluorocarboxylates, Environ. Sci. Technol., 2006, 40(1), 32–44 CrossRef CAS PubMed.
- J. Armitage, I. T. Cousins, R. C. Buck, K. Prevedouros, M. H. Russell, M. MacLeod and S. H. Korzeniowski, Modeling Global-Scale Fate and Transport of Perfluorooctanoate Emitted from Direct Sources, Environ. Sci. Technol., 2006, 40(22), 6969–6975 CrossRef CAS PubMed.
- J. McCord and M. Strynar, Identification of Per- and Polyfluoroalkyl Substances in the Cape Fear River by High Resolution Mass Spectrometry and Nontargeted Screening, Environ. Sci. Technol., 2019, 53(9), 4717–4727 CrossRef CAS PubMed.
- A. L. Myers, K. J. Jobst, S. A. Mabury and E. J. Reiner, Using mass defect plots as a discovery tool to identify novel fluoropolymer thermal decomposition products, J. Mass Spectrom., 2014, 49(4), 291–296 CrossRef CAS PubMed.
- P. Villarrubia-Gomez, S. E. Cornell and J. Fabres, Marine plastic pollution as a planetary boundary threat - The drifting piece in the sustainability puzzle, Mar. Pol., 2018, 96, 213–220 CrossRef.
- Z. Y. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbuhler, Global emission inventories for C-4-C-14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, part II: the remaining pieces of the puzzle, Environ. Int., 2014, 69, 166–176 CrossRef CAS PubMed.
- L. Vierke, C. Staude, A. Biegel-Engler, W. Drost and C. Schulte, Perfluorooctanoic acid (PFOA) — main concerns and regulatory developments in Europe from an environmental point of view, Environ. Sci. Eur., 2012, 24(1), 16 CrossRef.
- ECHA European Chemicals Agency, ANNEX XVII TO REACH – Conditions of restriction. Entry 68. Perfluorooctanoic acid (PFOA), https://echa.europa.eu/documents/10162/7a04b630-e00a-a9c5-bc85-0de793f6643c, accessed 17-02-2020 Search PubMed.
- Stockholm Convention, PFOA, its salts and PFOA-related compounds draft risk profile, 2015, http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC11/POPRC11Followup/tabid/4723/Default.aspx, accessed 23-02-2020 Search PubMed.
- Stockholm Convention, PFHxS, its salts and PFHxS-related compounds as well as polymers and mixtures, 2018, http://chm.pops.int/TheConvention/POPsReviewCommittee/Meetings/POPRC15/Overview/tabid/8052/Default.aspxFile:UNEP/POPS/POPRC.15/7/Add.1, accessed 23-02-2020 Search PubMed.
- P. A. Rice, J. Aungst, J. Cooper, O. Bandele and S. V. Kabadi, Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHxA), Food Chem. Toxicol., 2020, 138, 111210 CrossRef CAS PubMed.
- Order Adding Toxic Substances to Schedule 1 to the Canadian Environmental Protection Act, 1999. PFOA, its salts and precursors as well as long-chain (C9-C20) PFCAs, their salts and precursors, http://www.gazette.gc.ca/rp-pr/p2/2013/2013-11-06/html/sor-dors188-eng.html, accessed 28-04-2020 Search PubMed.
- ECHA European Chemicals Agency, Annex XV Restriction Report, Proposal for a Restriction. Substance names: undecafluorohexanoic acid (PFHxA), its salts and related substances, https://echa.europa.eu/documents/10162/c4e04484-c989-733d-33ed-0f023e2a200e, accessed 17-02-2020 Search PubMed.
- ECHA European Chemicals Agency, Public activities coordination tool. EU REACH Restriction Proposal for: perfluorononan-1-oic acid (PFNA); nonadecafluorodecanoic acid (PFDA); henicosafluoroundecanoic acid (PFUnDA); tricosafluorododecanoic acid (PFDoDA); pentacosafluorotridecanoic acid (PFTrDA); heptacosafluorotetradecanoic acid (PFTDA); including their salts and precursors, https://echa.europa.eu/de/pact?p_p_id=disspact_WAR_disspactportlet%26p_p_lifecycle=0%26_disspact_WAR_disspactportlet_substanceId=100.256.331%26_disspact_WAR_disspactportlet_jspPage=%2Fhtml%2Fportlet%2Fdisspact%2FdetailsPage%2Fview_detailsPage.jsp, accessed 17-02-2020 Search PubMed.
- ECHA European Chemicals Agency, Registry of restriction intentions until outcome for Perfluorohexane-1-sulphonic acid, its salts and related substances. https://echa.europa.eu/de/registry-of-restriction-intentions/-/dislist/details/0b0236e1827f87da, accessed 17-02-2020 Search PubMed.
- E. F. Houtz and D. L. Sedlak, Oxidative Conversion as a Means of Detecting Precursors to Perfluoroalkyl Acids in Urban Runoff, Environ. Sci. Technol., 2012, 46(17), 9342–9349 CrossRef CAS PubMed.
- J. Janda, K. Nodler, M. Scheurer, O. Happel, G. Nurenberg, C. Zwiener and F. T. Lange, Closing the gap - inclusion of ultrashort-chain perfluoroalkyl carboxylic acids in the total oxidizable precursor (TOP) assay protocol, Environ. Sci.: Process. Impacts, 2019, 21(11), 1926–1935 CAS.
- A. E. Robel, K. Marshall, M. Dickinson, D. Lunderberg, C. Butt, G. Peaslee, H. M. Stapleton and J. A. Field, Closing the Mass Balance on Fluorine on Papers and Textiles, Environ. Sci. Technol., 2017, 51(16), 9022–9032 CrossRef CAS PubMed.
- C. H. Zhang, Z. R. Hopkins, J. McCord, M. J. Strynar and D. R. U. Knappe, Fate of Per- and Polyfluoroalkyl Ether Acids in the Total Oxidizable Precursor Assay and Implications for the Analysis of Impacted Water, Environ. Sci. Technol. Lett., 2019, 6(11), 662–668 CrossRef CAS PubMed.
- R. Vestergren, I. T. Cousins, D. Trudel, M. Wormuth and M. Scheringer, Estimating the contribution of precursor compounds in consumer exposure to PFOS and PFOA, Chemosphere, 2008, 73(10), 1617–1624 CrossRef CAS PubMed.
- D. Martin, G. Munoz, S. Mejia-Avendano, S. V. Duy, Y. Yao, K. Volchek, C. E. Brown, J. X. Liu and S. Sauve, Zwitterionic, cationic, and anionic perfluoroalkyl and polyfluoroalkyl substances integrated into total oxidizable precursor assay of contaminated groundwater, Talanta, 2019, 195, 533–542 CrossRef CAS PubMed.
- C. A. McDonough, J. L. Guelfo and C. P. Higgins, Measuring total PFASs in water: the tradeoff between selectivity and inclusivity, Curr. Opin. Environ. Sci. Health, 2019, 7, 13–18 CrossRef.
- A. K. Tokranov, N. Nishizawa, C. A. Amadei, J. E. Zenobio, H. M. Pickard, J. G. Allen, C. D. Vecitis and E. M. Sunderland, How Do We Measure Poly- and Perfluoroalkyl Substances (PFASs) at the Surface of Consumer Products?, Environ. Sci. Technol. Lett., 2019, 6(1), 38–43 CrossRef CAS.
- L. Schultes, G. F. Peaslee, J. D. Brockman, A. Majumdar, S. R. McGuinness, J. T. Wilkinson, O. Sandblom, R. A. Ngwenyama and J. P. Benskin, Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform?, Environ. Sci. Technol. Lett., 2019, 6(2), 73–78 CrossRef CAS.
- B. D. Key, R. D. Howell and C. S. Criddle, Fluorinated organics in the biosphere, Environ. Sci. Technol., 1997, 31(9), 2445–2454 CrossRef CAS.
- Ministry of Environment and Food of Denmark: Danish Vetenary and Food Administration, Fluorinated substances in paper and cardboard food contact materials (FCM). Fact sheet, May 2018, https://www.foedevarestyrelsen.dk/english/SiteCollectionDocuments/Kemiogfoedevarekvalitet/UK-Fact-sheet-fluorinated-substances.pdf, accessed 23-03-2020 Search PubMed.
- M. Strynar, S. Dagnino, R. McMahen, S. Liang, A. Lindstrom, E. Andersen, L. McMillan, M. Thurman, I. Ferrer and C. Ball, Identification of Novel Perfluoroalkyl Ether Carboxylic Acids (PFECAs) and Sulfonic Acids (PFESAs) in Natural Waters Using Accurate Mass Time-of-Flight Mass Spectrometry (TOFMS), Environ. Sci. Technol., 2015, 49(19), 11622–11630 CrossRef CAS PubMed.
- L. Schultes, R. Vestergren, K. Volkova, E. Westberg, T. Jacobson and J. P. Benskin, Per- and polyfluoroalkyl substances and fluorine mass balance in cosmetic products from the Swedish market: implications for environmental emissions and human exposure, Environ. Sci.: Process. Impacts, 2018, 20(12), 1680–1690 CAS.
- Y. Miyake, N. Yamashita, P. Rostkowski, M. K. So, S. Taniyasu, P. K. S. Lam and K. Kannan, Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: a mass balance approach to determine individual perfluorinated chemicals in water, J. Chromatogr. A, 2007, 1143(1), 98–104 CrossRef CAS PubMed.
- Y. Miyake, N. Yamashita, M. K. So, P. Rostkowski, S. Taniyasu, P. K. S. Lam and K. Kannan, Trace analysis of total fluorine in human blood using combustion ion chromatography for fluorine: a mass balance approach for the determination of known and unknown organofluorine compounds, J. Chromatogr. A, 2007, 1154(1), 214–221 CrossRef CAS PubMed.
- European Commission, New Rules for PFAS. Recast of the Drinking Water Directive (DWD) Proposal: COM(2017) 753 final of 01.02.2018. Provisional Agreement Council+EP: 18.12.2019, text under review Search PubMed.
- US EPA, United States Environmental Protection Agency, Drinking Water Health Advisories for PFOA and PFOS, https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos, accessed 31-01-2020 Search PubMed.
- SFA, Swedish Food Agency, PFAS in drinking water and fish - risk management, Sweden, 2013, https://www.livsmedelsverket.se/en/food-and-content/oonskade-amnen/miljogifter/pfas-in-drinking-water-fish-risk-management/#SuitablePFAStoanalyzeindrinkingwater, accessed 31-01-2020 Search PubMed.
- L. S. McCarty and C. J. Borgert, Review of the toxicity of chemical mixtures: theory, policy, and regulatory practice, Regul. Toxicol. Pharmacol., 2006, 45(2), 119–143 CrossRef CAS PubMed.
- M. E. Meek, A. R. Boobis, K. M. Crofton, G. Heinemeyer, M. Van Raaij and C. Vickers, Risk assessment of combined exposure to multiple chemicals: a WHO/IPCS framework, Regul. Toxicol. Pharmacol., 2011, 60(2), S1–S14 CrossRef CAS PubMed.
- M. I. Gomis, R. Vestergren, D. Borg and I. T. Cousins, Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives, Environ. Int., 2018, 113, 1–9 CrossRef CAS PubMed.
- Danish Environmental Protection Agency, Perfluoroalkylated substances: PFOA, PFOS and PFOSA Evaluation of health hazards and proposal of a health based quality criterion for drinking water, soil and ground water. Environmental project No. 1665, 2015, available online https://www2.mst.dk/Udgiv/publications/2015/04/978-87-93283-01-5.pdf, accessed 13-03-2020 Search PubMed.
- Australian Government, National Health and Medical Research Council (NHMRC), Australian Drinking Water Guidelines, https://www.nhmrc.gov.au/about-us/publications/australian-drinking-water-guidelines, accessed 13-03-2020 Search PubMed.
- Government of Canada, Water Talk - Perfluoroalkylated substances in drinking water, April 2019, https://www.canada.ca/en/services/health/publications/healthy-living/water-talk-drinking-water-screening-values-perfluoroalkylated-substances.html, accessed 13-03-2020 Search PubMed.
- Connecticut Department of Public Health, Perfluoroalkyl Substances (PFAS) in Drinking Water: Health Concerns, Environmental & Occupational Health Assessment Program, October 2017, https://www.ecos.org/wp-content/uploads/2018/06/CT-PFAS-in-drinking-water.pdf, accessed 13-03-2020 Search PubMed.
- Managing PFAS in Maine. Final Report from the Maine PFAS Task Force January 2020, https://www1.maine.gov/pfastaskforce/materials/report/PFAS-Task-Force-Report-FINAL-Jan2020.pdf, accessed 13-03-2020 Search PubMed.
- Massachusetts Department of Environmental Protection, Development of a PFAS Drinking Water Standard (MCL) Information on MassDEP efforts to establish a drinking water standard for Per- and Polyfluoroalkyl Substances (PFAS), January 2019, https://www.mass.gov/lists/development-of-a-pfas-drinking-water-standard-mcl, accessed 13-03-2020 Search PubMed.
- Vermont Department of Health, PFAS in Public Drinking Water, July 2019, https://www.healthvermont.gov/sites/default/files/documents/pdf/ENV_DW_PFAS.pdf, accessed 13-03-2020 Search PubMed.
- M. J. Zeilmaker, S. Fragki, M. J. Verbruggen, S. B. G. H. Bokkers and J. P. A. Lijzen, Mixture exposure to PFAS: A Relative Potency Factor approach. RIVM Report 2018-0070, National Institute for Public Health and the Environment, P.O. Box 1, 3720 BA Bilthoven, The Netherlands, 2018, https://www.rivm.nl/bibliotheek/rapporten/2018-0070.pdf Search PubMed.
- J. L. Guelfo, T. Marlow, D. M. Klein, D. A. Savitz, S. Frickel, M. Crimi and E. M. Suuberg, Evaluation and Management Strategies for Per- and Polyfluoroalkyl Substances (PFASs) in Drinking Water Aquifers: Perspectives from Impacted U.S. Northeast Communities, Environ. Health Perspect., 2018, 126(6), 065001 CrossRef PubMed.
- G. Ding, J. Zhang, Y. Chen, L. Wang, M. Wang, D. Xiong and Y. Sun, Combined Effects of PFOS and PFOA on Zebrafish (Danio rerio) Embryos, Arch. Environ. Contam. Toxicol., 2013, 64(4), 668–675 CrossRef CAS PubMed.
- R. W. Flynn, M. F. Chislock, M. E. Gannon, S. J. Bauer, B. J. Tornabene, J. T. Hoverman and M. S. Sepúlveda, Acute and chronic effects of perfluoroalkyl substance mixtures on larval American bullfrogs (Rana catesbeiana), Chemosphere, 2019, 236, 124350 CrossRef CAS PubMed.
- C. J. Wolf, C. V. Rider, C. Lau and B. D. Abbott, Evaluating the additivity of perfluoroalkyl acids in binary combinations on peroxisome proliferator-activated receptor-α activation, Toxicol, 2014, 316, 43–54 CrossRef CAS PubMed.
- A. A. Rand, J. P. Rooney, C. M. Butt, J. N. Meyer and S. A. Mabury, Cellular Toxicity Associated with Exposure to Perfluorinated Carboxylates (PFCAs) and Their Metabolic Precursors, Chem. Res. Toxicol., 2014, 27(1), 42–50 Search PubMed.
- A. A. Rand and S. A. Mabury, Is there a human health risk associated with indirect exposure to perfluoroalkyl carboxylates (PFCAs)?, Toxicol, 2017, 375, 28–36 CrossRef CAS PubMed.
- US EPA, PFAS Chemical Lists and Tiered Testing Methods Descriptions, https://www.epa.gov/chemical-research/pfas-chemical-lists-and-tiered-testing-methods-descriptions, accessed 28-04-2020 Search PubMed.
- G. Patlewicz, A. M. Richard, A. J. Williams, C. M. Grulke, R. Sams, J. Lambert, P. D. Noyes, M. J. DeVito, R. N. Hines, M. Strynar, A. Guiseppi-Elie and R. S. Thomas, A Chemical Category-Based Prioritization Approach for Selecting 75 Per- and Polyfluoroalkyl Substances (PFAS) for Tiered Toxicity and Toxicokinetic Testing, Environ. Health Perspect., 2019, 127(1), 5 CrossRef PubMed.
- W. X. Cheng and C. A. Ng, Using Machine Learning to Classify Bioactivity for 3486 Per- and Polyfluoroalkyl Substances (PFASs) from the OECD List, Environ. Sci. Technol., 2019, 53(23), 13970–13980 CrossRef CAS PubMed.
- H. Holmquist, S. Schellenberger, I. van der Veen, G. M. Peters, P. E. G. Leonards and I. T. Cousins, Properties, performance and associated hazards of state-of-the-art durable water repellent (DWR) chemistry for textile finishing, Environ. Int., 2016, 91, 251–264 CrossRef CAS PubMed.
- IKEA FAQ HIGHLY FLUORINATED CHEMICALS, https://www.ikea.com/ms/en_US/pdf/reports-downloads/product_safety/IKEA_FAQ_highly_fluorinated_chemicals.pdf, accessed 17-02-2020 Search PubMed.
- Lindex Sustainability Report, 2016. https://about.lindex.com/files/documents/lindex-sustainability-report-2016.pdf, accessed 17-02-2020 Search PubMed.
- H&M Group, Phasing out PFAS, https://hmgroup.com/media/Our-stories/PhasingoutPFAS.html, accessed 17-02-2020 Search PubMed.
- Coop, The Danish Coop Bans Fluorinated Compounds in All Cosmetics. Press release 09-03-2019, https://www.businesswire.com/news/home/20190309005001/en/Danish-Coop-Bans-Fluorinated-Compounds-Cosmetics, accessed 17-02-2020 Search PubMed.
- Vaude, Sustainability Report. Waterproof without fluorocarbons, 2018, https://csr-report.vaude.com/gri-en/product/water-repellent-materials.php, accessed 17-02-2020 Search PubMed.
- Jack Wolfskin, GOAL ACHIEVED - OUR CLOTHES AND ALL OUR BAGS ARE COMPLETELY PFC-FREE, https://www.jack-wolfskin.com/information-pfc/, accessed 17-02-2020 Search PubMed.
- FIS Moves to Ban Fluorinated Ski Waxes for the 2020/2021 Season, https://fasterskier.com/fsarticle/fis-moves-to-ban-fluorinated-ski-waxes-for-the-2020-2021-season/, accessed 17-02-2020 Search PubMed.
- ChemSec. Interrnational Chemicals Secretariat, News Article from 11-07-18. The world's biggest cosmetics brands say NO to PFCs, https://chemsec.org/the-worlds-biggest-cosmetics-brands-say-no-to-pfcs/, accessed 11-05-2020 Search PubMed.
- Food Packaging Forum, News: Denmark to ban PFAS in paper & board in 2020, https://www.foodpackagingforum.org/news/denmark-to-ban-pfas-in-paper-board-in-2020, accessed 17-02-2020 Search PubMed.
- EPA South Australia, Per- and poly-fluoroalkyl substances (PFAS): Transitioning to fluorine-free firefighting foam. https://www.epa.sa.gov.au/environmental_info/perfluorinated-compounds, accessed 17-02-2020 Search PubMed.
- DTSC. Department of Toxic Substances Control, Proposed Priority Product: Carpets and Rugs with Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs), State of California, US, https://dtsc.ca.gov/scp/carpets-and-rugs-with-perfluoroalkyl-and-polyfluoroalkyl-substances-pfass/, accessed 17-02-2020 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |