Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrophilic trace organic contaminants in urban stormwater: occurrence, toxicological relevance, and the need to enhance green stormwater infrastructure
Stephanie
Spahr†
 *ab,
Marc
Teixidó
*ab,
Marc
Teixidó
 bc,
David L.
Sedlak
bc,
David L.
Sedlak
 bc and
Richard G.
Luthy
bc and
Richard G.
Luthy
 *ab
*ab
aDepartment of Civil and Environmental Engineering, Stanford University, Stanford, California, USA. E-mail: stephanie.spahr@alumni.epfl.ch; luthy@stanford.edu
bNSF Engineering Research Center for Re-inventing the Nation's Urban Water Infrastructure (ReNUWIt), USA
cDepartment of Civil and Environmental Engineering, University of California at Berkeley, Berkeley, California, USA
First published on 22nd November 2019
Abstract
Hydrophilic trace organic contaminants (hyphil-TrOCs) are polar, often ionizable organic compounds of anthropogenic origin that have various applications in the urban environment e.g., as pesticides, plasticizers, and flame retardants. Hyphil-TrOCs can be washed off in storm events and enter surface waters via untreated urban stormwater discharges or combined sewer overflows. Though trace concentrations of these chemicals may pose a risk to ecosystem and human health, information on their presence in urban stormwater remains elusive. Monitoring and source apportionment of hyphil-TrOCs in urban stormwater is complicated by the vast number and sources of organic contaminants and the high variability in aqueous concentration over time and space. Here, we present the current state of knowledge on the occurrence and toxicological relevance of hyphil-TrOCs in urban stormwater. To mitigate negative impacts of contaminated surface runoff to receiving water bodies and to prevent sanitary or combined sewer overflows, many cities implement sustainable green stormwater infrastructure, also called best management practices (BMPs). Current knowledge suggests that conventional stormwater BMPs such as detention basins, constructed wetlands, and biofilters often fail to remove hyphil-TrOCs. We identify future research needs to enhance green stormwater infrastructure with respect to water quality and safe use of urban stormwater for non-potable applications or groundwater recharge and present potential benefits of geomedia amendments in BMPs (e.g., activated carbon or biochar-amended biofilters). We highlight the need to improve stormwater monitoring strategies by combining chemical and bioanalytical tools to better assess effects of complex chemical mixtures and the treatment performance of BMPs and assure safe stormwater use for water supply.
Water impactUrban stormwater contains diverse mixtures of hydrophilic trace organic contaminants (hyphil-TrOCs) that contribute to water quality impairments. To protect aquatic ecosystems and enable safe stormwater harvesting, we need cost-effective and reliable hyphil-TrOC removal strategies. This review discusses the occurrence of hyphil-TrOCs in urban stormwater and highlights future research needs for enhanced monitoring and abatement of hyphil-TrOCs in green stormwater infrastructure. |
1 Introduction
Hydrophilic trace organic contaminants (hyphil-TrOCs) are polar and often ionizable compounds of anthropogenic origin used e.g., as pesticides, plasticizers, flame retardants, corrosion inhibitors, personal care products, and pharmaceuticals.1,2 Hyphil-TrOCs are mobile in the aquatic environment and possess low sorption tendency due to their polarity and water solubility.1–3 Moreover, many hyphil-TrOCs are recalcitrant and poorly removed in traditional water treatment systems.1–3 Therefore, hyphil-TrOCs are ubiquitous in surface waters and groundwater and have been also detected in finished drinking water.1,3–8 Numerous hyphil-TrOCs cause biological effects such as endocrine disruption at trace concentrations, which pose risks to ecosystem and human health.9,10 One major point source of hyphil-TrOCs are effluents from conventional wastewater treatment plants.2,11 Also, hyphil-TrOCs can be washed off from urban areas during storm events and enter the aquatic environment through urban surface runoff or combined sewer overflow (CSO).12,13It has been known for decades that urban stormwater is a contributor to the impairment of water quality.14 Thus, monitoring and management programs have focused on conventional stormwater pollutants such as total suspended solids (TSS),15–17 nutrients,15,18 pathogens,19–21 metals,15–18,22,23 polycyclic aromatic hydrocarbons (PAHs),17,24–26 polychlorinated biphenyls,27 and certain pesticides.28,29 While stormwater research has long concentrated on particle-associated pollutants, a review article by LeFevre et al.18 highlighted the importance of dissolved stormwater pollutants (mainly nutrients, toxic metals, and hydrocarbons) and identified a major lack of knowledge about hyphil-TrOCs. Recently, urban stormwater runoff has received increased attention as an important but often overlooked input pathway of hyphil-TrOCs to receiving water bodies.12,30–32
In the face of global water scarcity, urban stormwater is increasingly valued as a currently underused freshwater resource.33–35 The capture, treatment, and recharge of stormwater runoff can augment urban water supplies and diversify urban water supply portfolios.34–36 However, safe non-potable and potable use of urban runoff requires water quality monitoring with respect to pathogens and potentially harmful chemical contaminants.35 In fact, infiltration of urban stormwater runoff may increase the concentrations of hyphil-TrOCs, such as bisphenol A (BPA), in groundwater.37 Data on the occurrence of hyphil-TrOCs in stormwater are necessary for the development of a regulatory framework for stormwater harvesting and its public acceptance, cost-effective stormwater treatment technologies, and risk-based water quality guidance.
This review article is the first to present the state-of-the-art knowledge on hyphil-TrOCs in urban stormwater. We use the octanol–water partition coefficient (KOW for neutral compounds) and the octanol–water distribution coefficient (DOW for ionizable compounds) as approximate indicator of aquatic mobility3 and focus predominantly on compounds with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW or log DOW values in the range of −1 to 4. This polarity range is well covered by reversed-phase liquid chromatography coupled to (high-resolution) mass spectrometry (LC-(HR)MS) which is the main analytical technique for the identification and quantification of hyphil-TrOCs in wastewater and stormwater.3,12,38 We highlight challenges associated with sampling and monitoring strategies of hyphil-TrOCs and discuss sources of these chemicals in the urban environment. We compile concentrations of hyphil-TrOCs reported in urban stormwater runoff, storm sewer discharge, and combined sewer overflow (CSO), and present current findings on stormwater toxicity. Moreover, we point out that conventional stormwater best management practices (BMPs) such as detention basins, constructed wetlands, and biofilters often fail to remove hyphil-TrOCs. Next generation green infrastructure elements such as biofilters amended with reactive geomedia (e.g., metal oxides) or adsorbents (e.g., activated carbon or biochar) are discussed that show promise to effectively remove hyphil-TrOCs from urban stormwater.
KOW or log DOW values in the range of −1 to 4. This polarity range is well covered by reversed-phase liquid chromatography coupled to (high-resolution) mass spectrometry (LC-(HR)MS) which is the main analytical technique for the identification and quantification of hyphil-TrOCs in wastewater and stormwater.3,12,38 We highlight challenges associated with sampling and monitoring strategies of hyphil-TrOCs and discuss sources of these chemicals in the urban environment. We compile concentrations of hyphil-TrOCs reported in urban stormwater runoff, storm sewer discharge, and combined sewer overflow (CSO), and present current findings on stormwater toxicity. Moreover, we point out that conventional stormwater best management practices (BMPs) such as detention basins, constructed wetlands, and biofilters often fail to remove hyphil-TrOCs. Next generation green infrastructure elements such as biofilters amended with reactive geomedia (e.g., metal oxides) or adsorbents (e.g., activated carbon or biochar) are discussed that show promise to effectively remove hyphil-TrOCs from urban stormwater.
2 Stormwater sampling and monitoring of hydrophilic TrOCs
Monitoring of hyphil-TrOCs in stormwater is essential to identify the need for stormwater treatment and to assess the efficiency of treatment measures.39 The types and concentrations of hyphil-TrOCs present in urban stormwater can be highly variable over time and space and depend on various factors including intensity of the storm event, catchment characteristics, and application patterns of chemicals.2.1 Metadata collection
The intensity of the storm event and the resulting volume of urban runoff impacts both the mobilization of hyphil-TrOCs from surfaces as well as their transport and spatio-temporal concentration patterns. In order to better understand the dynamics of hyphil-TrOC occurrence in samples collected during a storm event, the latter needs to be well characterized in terms of precipitation depth, duration, mean intensity over the rain event, and maximum intensity.26 When reporting hyphil-TrOCs in CSO, the overflow volume, duration, average overflow rate and maximum flow rate should be reported in addition to storm-related information.13Land use is a key driver affecting stormwater composition.25,40 Therefore, the catchment of interest needs to be well characterized in terms of geography, total catchment area, impervious area, runoff coefficient, land-use type, land slope, and traffic (e.g., vehicles per day).26,41,42 We identified 18 key peer-reviewed publications that report the occurrence of hyphil-TrOCs in urban surface runoff, separate storm sewers, and combined sewer overflows. Table 1 compiles metadata for the selected studies including country, catchment type and area, sampling site and period, number of storm events sampled, sampling method, as well as the number of analyzed and detected compounds. The catchments were characterized as urban, residential, commercial, or industrial (Table 1). The catchment areas varied significantly ranging from 1.3–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ha. Data on hyphil-TrOC occurrence are mainly reported in stormwater conveyance pipes, storm sewer outlets, and CSO (Table 1). Samples collected at these locations contain hyphil-TrOCs washed off from the entire catchment, with the concentrations being diluted by the precipitation volume. A higher spatial resolution is warranted including sampling locations near sources of contamination in order to link the presence of hyphil-TrOCs in urban stormwater to land-use patterns or specific applications of chemicals. To date, studies investigating hyphil-TrOCs in stormwater collected close to the source (e.g., next to highways or recreational sites43) are scarce.
000 ha. Data on hyphil-TrOC occurrence are mainly reported in stormwater conveyance pipes, storm sewer outlets, and CSO (Table 1). Samples collected at these locations contain hyphil-TrOCs washed off from the entire catchment, with the concentrations being diluted by the precipitation volume. A higher spatial resolution is warranted including sampling locations near sources of contamination in order to link the presence of hyphil-TrOCs in urban stormwater to land-use patterns or specific applications of chemicals. To date, studies investigating hyphil-TrOCs in stormwater collected close to the source (e.g., next to highways or recreational sites43) are scarce.
| Source | Country | Catchment type | Catchment area (ha) | Sampling site | Sampling period | Number of storm events sampled | Sampling method | Number of compounds | Data reporting | |
|---|---|---|---|---|---|---|---|---|---|---|
| analyzed | detected | |||||||||
a Dry weather.
b Stormwater.
c Flow-weighted automated sampling (AS).
d Includes hyphil-TrOCs and other pollutants (e.g., metals, PAHs, other hydrophobic organic compounds).
e Hyphil-TrOCs (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOW < 4).
f Dry season.
g Rain event.
h Time-based automated sampling.
i Feb: first winter snowmelt; Apr, May, Sept: rain events.
j 50 storm events sampled across 21 sites (each 1–4 events) in 17 US states.
k Manually collected time-weighted composites.
l Volume proportional under dry conditions; discharge proportional if discharge rate >30 L s−1.
m Volume proportional.
n Flow-weighted composite. DOW < 4).
f Dry season.
g Rain event.
h Time-based automated sampling.
i Feb: first winter snowmelt; Apr, May, Sept: rain events.
j 50 storm events sampled across 21 sites (each 1–4 events) in 17 US states.
k Manually collected time-weighted composites.
l Volume proportional under dry conditions; discharge proportional if discharge rate >30 L s−1.
m Volume proportional.
n Flow-weighted composite.
|
||||||||||
| Irrigation runoff | ||||||||||
| Gan et al.44 | CA, USA | Residential | — | Storm drain outfall | July 2006–Dec 2008 | DWa runoff | Grab | 4 | 4 | Conc |
| Surface runoff | ||||||||||
| Wilkinson et al.43 | England | Motorway | — | Street runoff | — | Various | Grab | 13 | 7 | Conc |
| Wilkinson et al.43 | England | Recreational | — | Grass field drainage | — | Various | Grab | 13 | 7 | Conc |
| Regnery and Püttmann56 | Germany | Urban | — | SWb holding tank | May 2008–Apr 2009 | — | Grab | 6 | 6 | Conc |
| Separate sewer system | ||||||||||
| Becouze-Lareure et al.42 | France | Industrial | 185 | Storm sewer outlet | Mar 2008–Sept 2009 | 14 | FW-ASc | 34d | 23d (6e) | EMC |
| Gasperi et al.26 | France | Residential | 228 | Catchment outlet | July 2011–May 2013 | 24 | FW-ASc | 77d | 42–48d (9e) | EMC |
| Gasperi et al.26 | France | Residential | 30 | Catchment outlet | July 2011–May 2013 | 18 | FW-ASc | 77d | 42–48d (8e) | EMC |
| Gasperi et al.26 | France | Industrial | 185 | Catchment outlet | July 2011–May 2013 | 7 | FW-ASc | 77d | 42–48d (9e) | EMC |
| Burant et al.40 | WI, USA | Residential | 8.5 | Storm sewer | Apr–Aug 2016 | 13 | FW-ASc | 81d | 34d (∼27e) | EMC |
| Burant et al.40 | WI, USA | Commercial | 18.4 | Storm sewer | June–Sept 2016 | 11 | FW-ASc | 81d | 30d (∼27e) | EMC |
| Ensminger et al.57 | CA, USA | Residential | Various | Storm drain outfalls | Apr 2008–June 2011 | 15 (DSf), 13 (REg) | Grab & AS | 64d | 32d (∼18e) | Conc |
| Rippy et al.58 | Australia | (Peri-)urban | Various | Storm drain outlets | 2011–2014 | Various | Grab & FW-ASc | 27 | 19 | Conc |
| Deffontis et al.45 | France | Downtown | 439 | Storm sewer outlet | Jan 2010–Feb 2011 | 12 (DWa), 8 (REg) | TB-ASh | 24d | ∼10d (∼4e) | Conc & AL |
| Deffontis et al.45 | France | Residential | 1428 | Storm sewer outlet | Jan 2010–Feb 2011 | 12 (DWa), 8 (REg) | TB-ASh | 24d | ∼9d (∼2e) | Conc & AL |
| Fairbairn et al.12 | MN, USA | Industrial, residential, university | 1380 | SWb conveyance pipe | Feb–Sept 2016 | 4i | Grab | 384d | 123d | Conc |
| Fairbairn et al.12 | MN, USA | Residential, industrial, commercial | 789 | SWb conveyance pipe | Feb–Sept 2016 | 4i | Grab | 384d | 123d | Conc |
| Fairbairn et al.12 | MN, USA | Residential | 187 | SWb conveyance pipe | Feb–Sept 2016 | 4i | Grab | 384d | 123d | Conc |
| Masoner et al.31 | USAj | Variousj | 10–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Culverts, canals, channels | Aug 2016–Dec 2017 | 1–4 (50j) | FW-ASc & TWCk | 438d | 215d | Conc, loads |
| Zgheib et al.59 | France | Residential | 261 | Storm sewer outlet | Feb 2008–Mar 2009 | 10 | AS | 88d | 55d (∼10e) | Conc |
| Zgheib et al.59 | France | Urban | 230 | Storm sewer outlet | Feb 2008–Mar 2009 | 6 | AS | 88d | 55d (∼10e) | Conc |
| Zgheib et al.59 | France | Residential, commercial | 130 | Storm sewer outlet | Feb 2008–Mar 2009 | 4 | AS | 88d | 55d (∼10e) | Conc |
| Beckers et al.30 | Germany | Residential | — | Storm sewer | Apr 2015–Apr 2016 | 12 (DWa), 6 (REg) | ASl | 149 | 67 | Loads |
| Bollmann et al.60 | Denmark | Residential | 21.5 | Storm sewer outlet | Oct 2011–June 2012 | 12 | FW-ASc | 11 | 11 | m event |
| Birch et al.46 | Denmark | Various | 1.3–60 | Storm sewer | Oct 2008–Sept 2009 | Various | Grab | >50d | ∼31d (∼6e) | Conc |
| Boyd et al.61 | LA, USA | Urban | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
Stormwater canals | Feb–July 2003 | 8 | Grab | 9 | 3 | Conc |
| Combined sewer system | ||||||||||
| Becouze-Lareure et al.42 | France | Residential | 245 | CSO | Mar 2008–June 2009 | 12 | FW-ASc | 34d | 23d (6e) | EMC |
| Birch et al.46 | Denmark | Residential | 1100 | CSO | Sept 2008 | 1 | Vol. prop.m | >50d | ∼27d (∼5e) | Conc |
| Phillips et al.62 | VT, USA | Urban | — | CSO bypass flow | Nov 2007–Dec 2008 | 10 | FWCn | 89 | 18 | Conc, loads |
| Launay et al.13 | Germany | Urban | 3500 | CSO outfall | July–Oct 2014 | 7 | AS | 69d | 60d (38e) | EMC |
To assess seasonal trends of hyphil-TrOCs in urban stormwater and to better understand concentration dynamics within one storm event as well as in-between events, many studies sample multiple storm events at the same site over a period of several months to years (Table 1). The site-specific number of rain events sampled in the 18 peer-reviewed publications range from 1 to 24 (Table 1). Dry weather periods in between consecutive rain events require reporting13,26 as chemicals may be predominantly applied during dry weather (e.g., urban-use pesticides) and accumulate on surfaces. Dry weather runoff, caused by e.g., extensive irrigation or car-washing, may reflect the seasonal use and application volume of chemicals that can be mobilized in the next storm event. However, few studies investigated hyphil-TrOCs in dry weather runoff30,44,45 and their mobilization patterns in first flush and peak flows.
2.2 Stormwater sampling methods
Stormwater quantity and quality is highly variable over time and space and the concentration of hyphil-TrOCs and other pollutants varies within one single storm event as well as between different storm events and different sampling locations.46,47 Owing to rapid fluctuations in pollutant concentrations and loads, stormwater sampling is often associated with many sources of uncertainty and bias.39 In principle, three different types of stormwater sampling methods are employed: (i) grab sampling, (ii) installation of automated samplers, and (iii) installation of passive samplers. Grab sampling is the most easy, cost-effective, and widely applied stormwater sampling strategy.41,46 Eight out of the 18 peer-reviewed publications summarized in Table 1 are based on grab samples taken at a specific location and time. However, grab samples are often not representative of the spatial and temporal variability of hyphil-TrOC concentrations in water streams such as stormwater runoff, CSOs, or receiving water bodies.12,46,48 Automated samplers provide time-weighted or flow-weighted sampling of stormwater at a specific location where samples are repeatedly collected at a pre-defined constant time interval or a constant incremental volume of discharge, respectively.14 Autosamplers can be used to collect discrete stormwater samples or composite samples that consist of multiple discrete samples collected in a common container.14 The sampling strategy depends on the objective of the sampling campaign, the regulatory requirements as well as logistic considerations and costs.47 The collection of numerous discrete samples during a storm event can enable a higher temporal resolution of variabilities in stormwater quality but is often associated with significant costs for sample analyses.14 Therefore, flow-weighted composite samples, that represent specific compositing periods, are often the method of choice.14 Flow-proportional and volume-proportional sampling are the most representative stormwater sampling methods that allow the calculation of event mean concentrations (EMC) as discussed below.46 A less accurate method is precipitation dependent sampling where a rain gauge determines when samples are taken.46 Flow-weighted stormwater samples are commonly collected using autosamplers (Table 1).14 However, automated stormwater sampling entails higher costs than grab sampling and is often associated with practical challenges (e.g., equipment installation and maintenance).14,39,41In order to avoid high costs of automated samplers and transport of large sample volumes, passive samplers might be a promising and cost-effective technique for the monitoring of hyphil-TrOCs in stormwater.39,49,50 Passive samplers have been developed for the monitoring of water quality in surface waters and sediments.51–54 The basic principle is passive diffusion of organic chemicals from water into a sorbing material placed in the sampler device.50 The mass sorbed is assumed to be in equilibrium with the time-averaged concentration in the water over the period of passive sampler deployment.50 Page et al.50 successfully deployed five different types of passive samplers for monitoring of hyphil-TrOCs in urban stormwater recycling systems that enabled the qualitative detection of a large number of individual chemicals. Moschet et al.52 found that styrenedivinylbenzene reverse phase sulfonate (SDB-RPS) disks covered by a polyether sulfone (PES) membrane are suited to qualitatively screen surface water for the presence of more than 300 hyphil-TrOCs. While compounds with relatively constant aqueous concentrations could be quantified accurately in the field, compounds with highly fluctuating aqueous concentrations were difficult to quantify with passive samplers.52 Concentrations of hyphil-TrOCs in stormwater are highly variable and may complicate uptake kinetics as well as potential desorption. Mutzner et al.49 confirmed that the quantification of hyphil-TrOCs with passive samplers is associated with uncertainty that needs to be assessed especially when sampling storm events or CSOs over a long duration. Still, SDB-RPS passive samplers provided a useful estimate of hyphil-TrOC concentrations in sewers and CSOs that can help to identify locations at which concentrations of hyphil-TrOCs comply with or exceed the environmental quality standards.49,55 Birch et al.39 discussed the limitation of conventional time-integrated passive samplers for highly dynamic stormwater discharges and proposed the use of velocity-dependent flow-through passive sampling that provides higher weighting of actual runoff events than no-flow periods. Thus, field evaluation of both time-integrated and velocity-dependent passive sampling for the assessment of hyphil-TrOCs in stormwater is warranted. To date, passive samplers cannot replace traditional stormwater sampling techniques that provide a more accurate quantification of TrOCs as well as higher temporal resolution.55
2.3 Hyphil-TrOC analyses and data reporting
Hyphil-TrOCs are predominantly analyzed using liquid-chromatography (high-resolution) mass spectrometry (LC-(HR)MS).12,13,31,40,55 The majority of peer-reviewed publications screened stormwater for the occurrence of various compound classes with the number of analyzed individual contaminants ranging from four to 438 (Table 1). However, these numbers often entail more hydrophobic organic contaminants such as PAHs.12,26,31,59 To date, Fairbairn et al.12 and Masoner et al.31 have screened urban stormwater for the broadest suite of organic contaminants including pesticides, pharmaceuticals, and wastewater indicator compounds. Fairbairn et al.12 detected 123 out of 384 analytes in stormwater with the median and maximum number of organic contaminants in individual samples being 35 and 54, respectively. Masoner et al.31 conducted a study across the United States and screened 50 stormwater samples collected at 21 sites in 17 states for the occurrence of 438 organic chemicals of which 215 were detected at least once. The median and maximum number of analytes detected in individual samples was 73 and 103, respectively.31The occurrence of hyphil-TrOCs and other stormwater contaminants is often reported as median or mean concentration derived from the analysis of samples collected during single or multiple rain events at one location (see Table 1). The measured hyphil-TrOC concentration in a sample can be used to calculate the mass load per storm event (mevent) as shown in eqn (1).
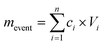 | (1) |
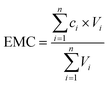 | (2) |
Site mean concentrations (SMCs) for a specific sampling site can be calculated using the EMCs and event volumes as shown in eqn (3).41
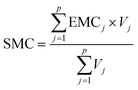 | (3) |
3 Sources and occurrence of hydrophilic TrOCs in urban stormwater
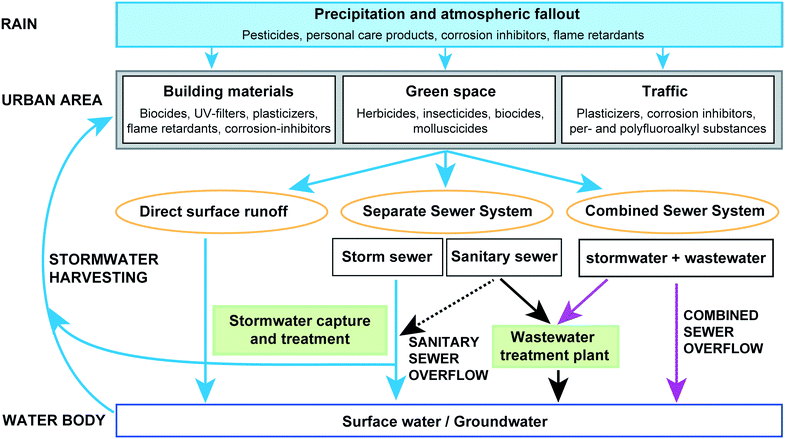
Hyphil-TrOCs are ubiquitous in urban stormwater but their sources are often difficult to elucidate. In the following section, we reflect on our current understanding of the occurrence of hyphil-TrOCs in urban stormwater runoff and discuss storm-related input pathways into surface waters and groundwater. As depicted in Fig. 1, hyphil-TrOCs have been detected in precipitation, they can leach from building materials, or are washed off from green spaces or motorways. Hyphil-TrOCs can then enter receiving water bodies through (i) direct surface runoff, (ii) separate storm sewer discharge, or (iii) CSO.3.1 Precipitation and atmospheric fallout
Knowledge on the occurrence of hyphil-TrOCs in precipitation and atmospheric fallout is scarce. Few studies report the presence of hyphil-TrOCs in rain water,63–65 snow,64,65 and atmospheric fallout.26,64 Asman et al.63 collected rain water in Denmark and found various pesticides including atrazine and isoproturon with maximum concentrations of 15 ng L−1 and 71 ng L−1, respectively. Certain pesticides (e.g., atrazine and 2,4-D) were detected in rain even though they were not sold in Denmark.63 Atmospheric transport distances for atrazine and 2,4-D were estimated as >280 km and >60 km, respectively.63 Atmospheric transport and deposition of agricultural-use pesticides such as atrazine in urban areas has been reported elsewhere.40 Ferrey et al.64 analyzed atmospheric aerosol, rain, and snow for the presence of 126 pharmaceuticals, personal care products, and other hyphil-TrOCs. Only a few compounds were detected (seven in air, eight in rain, and ten in snow) and the concentration levels were low.64N,N-Diethyl-m-toluamide (DEET) and benzothiazole were detected in rain with concentrations up to 14.5 ng L−1 and 70 ng L−1, respectively.64 BPA, 4- and 5-methyl-1H-benzotriazole, caffeine, ciprofloxacin, sulfamethoxazole, perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) were found in trace concentrations.64,65 In Germany and the US, organophosphorus flame retardants (OPs) have been detected in rain and snow samples with tris(2-chloro-1-methylethyl) phosphate (TCPP) being the most frequently detected species with concentrations up to 2659 ng L−1.56,66,67 Concentrations of OPs were significantly higher in urban than in rural precipitation indicating urban emissions to the atmosphere.56 Gasperi et al.26 determined organic contaminants in total atmospheric fallout that was collected on rooftops in three catchments in France. The contribution of total atmospheric fallout to the concentrations measured in stormwater from the catchment outlet was very low (<10% for BPA) indicating that atmospheric fallout is a minor contributor.26 Sources of hyphil-TrOCs in air and precipitation are likely traffic, buildings, manufacturing facilities, wastewater treatment plants, and anthropogenic activities.56,64,66 However, little is known about atmospheric transport, fate and wet deposition of hyphil-TrOCs.3.2 Structural materials and pest control
Building materials have been identified as major contributors to the occurrence of hyphil-TrOCs in stormwater. Building paints and coatings, plastics, and concrete contain numerous chemicals including biocides, flame retardants, UV-filters, corrosion inhibitors, and plasticizers that can be released and washed off during storm events.68 Biocides are the most frequently studied class of TrOCs leaching from construction materials.Biocides in materials are used to prevent the growth of fungi, algae, and bacteria e.g., on building façades or roofs. Commonly used biocides are triazines and phenylurea compounds as algaecides, isothiazolinones as bactericides, and carbamates as fungicides.60 To prevent growth of algae and fungi on façades, paints, coatings, and film preservatives often contain a mixture of three to eight different biocides with concentrations in the range of 0.1–2.0 g kg−1.69,68
Terbutryn, diuron, carbendazim, and irgarol 1051 are among the most frequently found and most persistent biocides in paints and renders.68,69 Other active ingredients are isothiazolinones and zinc pyrithione.69 Mecoprop is commonly used as root protection agent in bitumen sheets for water proofing.68–70
Burkhardt et al.68 investigated leaching of seven biocides from roofs, car parks, and façades of residential and commercial buildings in an urban catchment in Switzerland. Stormwater samples contained carbendazim and terbutryn at concentrations of up to a few hundred ng L−1.68 Much higher concentrations (in the range of 2 μg L−1) were found for mecoprop that stemmed from root resistant sealing membranes in foundations and from green roofs of underground parking.68 Bollmann et al.60 analyzed 191 stormwater samples from 12 rain events in Denmark for 11 target biocides. Carbendazim and terbutryn were detected in all analyzed samples with high median concentrations of 45 ng L−1 and 52 ng L−1, respectively (Table 2).60 Diuron, isoproturon, propiconazole, mecoprop, and iodocarb were frequently detected with significantly lower median concentrations of 2–7 ng L−1.60
| Contaminant group | Compound | AA-/MAC-EQSs (μg L−1) | Median conc (μg L−1) | Concentration range (μg L−1) | Country | Sample type | Detection frequency | Source |
|---|---|---|---|---|---|---|---|---|
| a Reported maximum concentration. b Calculated from data in the respective supporting information. c Northern CA. d Southern CA. e DS = dry season. f Reporting limit. g SE = storm event. h Mean value. | ||||||||
| Biocides | Carbendazim | 0.44*/0.7* | 0.045 | 0.306a | Denmark | SW | — | Bollmann et al.60 |
| 0.701 | 0.0098–9.58 | USA | SW | 94% | Masoner et al.31 | |||
| Terbutryn | 0.065/0.34 | 0.052 | 1.84a | Denmark | SW | — | Bollmann et al.60 | |
| 0.059b | 0.317a,b | Germany | SW | 100%b | Beckers et al.30 | |||
| 0.083 | 0.055–0.122 | Germany | CSO | — | Launay et al.13 | |||
| Insecticides | Dimethoate | 0.07*/0.98* | 0.0056b | 5.16a,b | Germany | SW | 94%b | Beckers et al.30 |
| Diazinon | 0.012*/0.02* | 0.80 | 1.1a | CA, USA | SW | 100% | Kratzer109 | |
| Fipronil | — | 0.033c; 0.076d | — | CA, USA | SW | 51%c; 83%d | Budd et al.74 | |
| 0.0031–0.131 | 2.05a,c; 10.0a,d | CA, USA | DWR | 66–100% | Gan et al.44 | |||
| Imidacloprid | 0.013*/0.1* | 0.013 | 0.0054–0.428 | WI, USA | SW | 100% | Burant et al.40 | |
| 0.023 | 0.0049–0.331 | USA | SW | 86% | Masoner et al.31 | |||
| 0.05 (DSe) | 0.16a (DSe) | CA, USA | SW | 50% | Ensminger et al.57 | |||
| <RLf (SEg) | 0.67a (SEg) | CA, USA | SW | 51% | Ensminger et al.57 | |||
| Herbicides | 2,4-D | 0.6*/4* | 0.008–2.0h | — | Australia | SW | 100%b | Rippy et al.58 |
| 0.39 | 11.6a | MN, USA | SW | 100% | Fairbairn et al.12 | |||
| 0.08 (DSe) | 11.5a (DSe) | CA, USA | SW | 84% | Ensminger et al.57 | |||
| 0.28 (SEg) | 10.4a (SEg) | CA, USA | SW | 66% | Ensminger et al.57 | |||
| 0.47 | 0.095–2.87 | WI, USA | SW | 100% | Burant et al.40 | |||
| Atrazine | 0.6/2.0 | 0.006–0.624h | — | Australia | SW | 70%b | Rippy et al.58 | |
| 0.040 | 0.787a | MN, USA | SW | 100% | Fairbairn et al.12 | |||
| Dicamba | 2.2*/52* | 0.01–0.241h | — | Australia | SW | 60%b | Rippy et al.58 | |
| <RLf (DSe) | 3.1a (DSe) | CA, USA | SW | 69% | Ensminger et al.57 | |||
| 0.06 (SEg) | 1.2a (SEg) | CA, USA | SW | 42% | Ensminger et al.57 | |||
| Diuron | 0.2/1.8 | 1.34h | 0.08–10.78 | CA, USA | SRO | — | Huang et al.110 | |
| 0.07*/0.25* | 0.37 | 0.03–1.75 | France | SW | 100% | Zgheib et al.59 | ||
| 1.213h | 0.025–0.795 | France | SW | 71–100% | Gasperi et al.26 | |||
| 0.009–0.895h | — | Australia | SW | 90%b | Rippy et al.58 | |||
| 0.020b | 0.107a,b | Germany | SW | 100%b | Beckers et al.30 | |||
| 0.0077 | 0.0045–0.014 | WI, USA | SW | 100% | Burant et al.40 | |||
| 0.019h | — | France | SW | 100% | Becouze-Lareure et al.42 | |||
| 0.072h | — | France | CSO | 100% | Becouze-Lareure et al.42 | |||
| 0.26 | 0.068–0.68 | Germany | CSO | — | Launay et al.13 | |||
| Glyphosate | 120*/360* | 2.69h | 1.36–9.44 | CA, USA | SRO | — | Huang et al.110 | |
| — | 0.043–1.2 | Denmark | SW | 100% | Birch et al.46 | |||
| 1.11 | <0.03–232 | France | SW | 93% | Zgheib et al.59 | |||
| 0.337h | 0.095–0.198 | France | SW | 40–75% | Gasperi et al.26 | |||
| Isoproturon | 0.3/1.0 | 0.09 | 0.05–0.2 | Germany | SRO | — | Stachel et al.89 | |
| 0.088h | 0.003–0.053 | France | SW | 29–100% | Gasperi et al.26 | |||
| Mecoprop | 3.6*/190* | 0.041b | 0.103a,b | Germany | SW | 100%b | Beckers et al.30 | |
| 0.151 | 0.031–1.062 | WI, USA | SW | 100% | Burant et al.40 | |||
| 0.140 | 0.100–0.378 | Germany | CSO | — | Launay et al.13 | |||
| Metolachlor | 0.69*/3.3* | 0.0179 | 0.489a | MN, USA | SW | 81% | Fairbairn et al.12 | |
| Oryzalin | — | 11.41h | 9.40–43.13 | CA, USA | SRO | — | Huang et al.110 | |
| 0.155 | 0.018–1.186 | WI, USA | SW | 100% | Burant et al.40 | |||
| Simazine | 1/4 | 0.13 | 0.24a | CA, USA | SW | 100% | Kratzer109 | |
| 0.0375h | — | France | SW | 100% | Becouze-Lareure et al.42 | |||
| 0.0017h | — | France | CSO | 100% | Becouze-Lareure et al.42 | |||
| Triclopyr | — | 0.010–0.31h | — | Australia | SW | 80%b | Rippy et al.58 | |
| 0.06 (DSe) | 1.5a (DSe) | CA, USA | SW | 40% | Ensminger et al.57 | |||
| 0.13 (SEg) | 6.8a (SEg) | CA, USA | SW | 64% | Ensminger et al.57 | |||
Biocides in stormwater show complex concentration patterns that depend on multiple factors such as rainfall intensity and duration, façade orientation, wind direction and speed, age of the building materials, dilution of the biocide load along the water pathway, and persistence of the biocides.68,71 Burkhardt et al.68 found that biocide concentrations measured in runoff from new buildings were at least 1000-times higher than for aged buildings. Laboratory leaching experiments showed that biocides from render were mobilized at the very beginning of simulated rain events showing that even short rain events can lead to significant loads in runoff.68 Biocides did not follow a typical first flush model, but were continuously released from the materials due to an underlying diffusion mechanism68,72 or a solubility or partition controlled release mechanism.71 This is in contrast to many conventional stormwater pollutants such as TSS that are transported according to the first-flush phenomenon i.e., 80% of the total pollutant mass is transported in the first 30% of volume discharge during storm events.73
Different pesticides are applied for structural pest control, i.e., the protection of buildings and other structures from pests of which some can carry and transmit diseases. Common active ingredients against ants and termites are the phenylpyrazole insecticide fipronil and the neonicotinoid insecticide imidacloprid, which were both detected with high frequency in urban stormwater (Table 2).40,57,74 Anticoagulant rodenticides such as bromadiolone, warfarin, and chlorophacinone are commonly applied to control mice and rats.75 One of the main applications of rodenticides in the urban environment is rodent control in sewer systems.75 Yet, anticoagulant rodenticides remain poorly studied in urban stormwater discharges and CSOs.75 While professional applicators are trained to minimize the release of pesticides into the environment by controlling application type, volume, and period, diffuse release of active ingredients as well as mishandling of chemicals (e.g., application shortly before rain events) can lead to increased pesticide concentrations in stormwater.
3.3 Urban green space
Urban green spaces such as gardens, parks, lawns, sports grounds, graveyards, and right of ways can be a source of herbicides, insecticides, fungicides, algaecides, molluscicides, and rodenticides to stormwater. While pesticides in surface and groundwater are believed to predominantly stem from agriculture, urban pesticides have been shown to contribute to the impairment of water quality.76,77 However, information on the types and volumes of pesticides applied in urban areas is often lacking.76 While agricultural pesticide application and urban pesticide use by licensed applicators can be assessed (e.g., in California), private use by households or owners of public or commercial properties is usually not reported. The California Department of Pesticide Regulation conducted a survey of pesticide products sold in retail stores in Northern California and found a total of 593 products with 168 active ingredients including 2,4-D, glyphosate, dicamba, and triclopyr.78 Interestingly, diuron and fipronil, which are often found in stormwater runoff, were absent in outdoor use products for residents, indicating that certain pesticides may be predominantly used by licensed applicators.78 While pesticide tracking through point-of-sale data is a first step to assess urban pesticide usage, it does not provide temporal and geographic resolution of actual application patterns and resulting pesticide concentrations in stormwater.79Few studies investigate the occurrence of pesticides and other hyphil-TrOCs in runoff from urban green spaces both during rain events as well as during dry periods with extensive irrigation. Gan et al.44 compared the occurrence of fipronil and its metabolites in dry weather urban residential runoff collected in Southern California (Orange County, US) and Northern California (Sacramento, US). Fipronil is used in a variety of products against pests on horticultural crops, lawns, and golf courses. Fipronil is also the active ingredient in flea and tick control sprays for dogs and cats and is frequently applied for structural pest control as discussed above. Median concentrations of fipronil in dry weather runoff were 3.1–5.6 ng L−1 and 79–131 ng L−1 in Northern and Southern CA, respectively, reflecting more frequent and long-term usage of this insecticide in the south.44 This in accordance with a survey of storm drains in which fipronil was detected at median concentrations of 33 ng L−1 in Northern California and 76 ng L−1 in Southern California (Table 2).74 Fipronil concentrations followed seasonal trends reflecting heavier use of fipronil from spring to fall.44 Lower fipronil concentrations in the winter season is likely due to lower applications from October to March and dilution effects during the rainy season.44
Urban green spaces with a stagnant or shallow pool of water (e.g., rain barrels, stormwater retention ponds, stormwater catch basins, and storm drains), can be potential mosquito breeding habitats. Hyphil-TrOCs applied for mosquito control include malathion, carbaryl, and imidacloprid. Neonicotinoids such as imidacloprid are among the most popular insecticides in the world to control a wide range of pests and disease vectors in soils, plants, and sewer systems. In California, imidacloprid was frequently found in storm drain outfalls and surface waters with maximum concentrations of 160 ng L−1 during the dry season and 670 ng L−1 during the rainy season (Table 2).57 After rain events, imidacloprid was detected at particularly high concentrations up to 1462 ng L−1 in an urban creek in California.80 Imidacloprid was also present in a Californian residential stormwater pond.81
In addition to pesticides, other chemicals can leach from urban green spaces but are much less studied. For instance, samples collected during rainfall from a grass field drainage in England contained bisphenol A (BPA) and bisphenol S (BPS) in the range of 100 ng L−1 (Table 3).43 Moreover, litter (packaging or cigarette butts) can contribute to hyphil-TrOC concentrations in urban stormwater runoff.12 Only one cigarette butt in 1 m3 of water may lead to nicotine concentrations above the predicted no effect concentration (2.4 μg L−1).82
| Contaminant group | Compound | AA-/MAC-EQSs (μg L−1) | Median conc (μg L−1) | Concentration range (μg L−1) | Country | Sample type | Detection frequency | Source |
|---|---|---|---|---|---|---|---|---|
| a Mean value. b Calculated from data in the respective supporting information. c Reported maximum concentration. d Benzothiazole-2-sulfonate. | ||||||||
| PFASs | PFOA | — | — | 0.0065–1.16 | England | SRO | 100% | Wilkinson et al.43 |
| 0.087a,b | 0.017–0.174 | Japan | SRO | 60%b | Murakami et al.83 | |||
| 0.0038 | 0.00051–0.029 | NY, USA | SRO, SW | 100% | Kim and Kannan65 | |||
| 0.0073 | 0.0023–0.0157 | CA, USA | SW | 100% | Houtz and Sedlak88 | |||
| PFOS | 0.00065/36 | — | 0.0090 | England | SRO | 50% | Wilkinson et al.43 | |
| 0.0105a,b | 0.0029–0.050 | Japan | SRO | 100%b | Murakami et al.83 | |||
| 0.00081 | 0.0146c | NY, USA | SRO, SW | 93%b | Kim and Kannan65 | |||
| 0.015 | 0.0026–0.0263 | CA, USA | SW | 100% | Houtz and Sedlak88 | |||
| PFNA | — | — | 0.0692–0.648 | England | SRO | 100% | Wilkinson et al.43 | |
| 0.030a,b | 0.0047–0.070 | Japan | SRO | 50%b | Murakami et al.83 | |||
| 0.00071 | 0.0059c | NY, USA | SRO, SW | 93%b | Kim and Kannan65 | |||
| — | 0.0003–0.0038 | CA, USA | SW | 100% | Houtz and Sedlak88 | |||
| Plasticizers | BPA | 0.24*/53* | — | 0.0456–0.101 | England | FD | 100% | Wilkinson et al.43 |
| — | 0.511–2.41 | England | SRO | 100% | Wilkinson et al.43 | |||
| 1.4 | 0.24–2.5 | Germany | SRO | — | Stachel et al.89 | |||
| 0.412 | 0.234–0.964 | France | SRO | — | Flanagan et al.111 | |||
| 0.552a | 0.207–0.817 | France | SW | >80% | Gasperi et al.26 | |||
| 0.056 | 0.158c | LA, USA | SW | 100% | Boyd et al.61 | |||
| 0.263 | 2.77c | USA | SW | 90% | Masoner et al.31 | |||
| — | 0.85–16.8 | Sweden | SW | 100% | Kalmykova et al.112 | |||
| — | 0.20 | Australia | SW | — | Tang et al.113 | |||
| 0.321 | 0.140–0.400 | Germany | CSO | — | Launay et al.13 | |||
| BPS | — | — | 0.00227–0.159 | England | FD | 100% | Wilkinson et al.43 | |
| — | 0.0406–0.0502 | England | SRO | 100% | Wilkinson et al.43 | |||
| Corrosion inhibitors | 1H-BT | 19*/160* | 0.36 | 0.075–1.906 | WI, USA | SW | 100% | Burant et al.40 |
| 0.403b | 2.65b,c | Germany | SW | 100%b | Beckers et al.30 | |||
| 0.727 | 0.358–1.793 | Germany | CSO | — | Launay et al.13 | |||
| Methyl-1H-BT | 20*/430* | 0.806 | 5.55c | MN, USA | SW | 100% | Fairbairn et al.12 | |
| 0.861 | 6.79c | USA | SW | 92% | Masoner et al.31 | |||
| 5-MeBT | — | 0.135 | 13.4c | MN, USA | SW | 56% | Fairbairn et al.12 | |
| 0.285 | 0.190–1.058 | Germany | CSO | — | Launay et al.13 | |||
| Benzothiazole | 240*/250* | — | 1.210c | RI, USA | SRO | 100% | Reddy and Quinn94 | |
| 0.410 | 0.282–1.037 | Germany | CSO | — | Launay et al.13 | |||
| Benzo. sulf.d | — | — | 55c | Germany | SRO | — | Kloepfer et al.95 | |
| Organophosphates | TCEP | — | 0.077 | 0.033–0.275 | Germany | SRO | 100% | Regnery and Püttmann56 |
| 0.431 | 0.056–0.660 | WI, USA | SW | 100% | Burant et al.40 | |||
| 0.270 | 0.041–0.340 | Germany | CSO | — | Launay et al.13 | |||
| TCPP | — | 0.880 | 0.016–5.79 | Germany | SRO | 100% | Regnery and Püttmann56 | |
| 0.854 | 0.275–2.074 | WI, USA | SW | 100% | Burant et al.40 | |||
| 0.692 | 0.516–2.700 | Germany | CSO | — | Launay et al.13 | |||
| TBEP | — | 0.077 | 1.616c | Germany | SRO | — | Regnery and Püttmann56 | |
| 0.055 | 5.93c | MN, USA | SW | 50% | Fairbairn et al.12 | |||
| 1.060 | 2.750c | WI, USA | SW | 54% | Burant et al.40 | |||
| 1.300 | 0.078–4.100 | Germany | CSO | — | Launay et al.13 | |||
| TiBP | — | 0.117 | 0.002–1.478 | Germany | SRO | 100% | Regnery and Püttmann56 | |
3.4 Traffic
Street runoff is known to carry significant concentrations of per- and polyfluoroalkyl substances (PFASs).43,83 PFASs are frequently detected in the environment and include perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) used in fluoropolymers such as Teflon, and perfluorooctane sulfonate (PFOS) a fluorosurfactant used in stain repellents and fire-fighting foams.84 PFASs are persistent in the environment, difficult to remove in water treatment, and pose a health risk to biota and humans.85 Concentrations of PFASs are usually <100 ng L−1 in surface waters but high levels of PFOA up to 3640 ng L−1 have been reported in a German river.86 Street runoff and urban stormwater are reported to contain high levels of PFOA, PFOS, and PFNA with concentrations being as high as 1160 ng L−1,43 50 ng L−1,83 and 648 ng L−1,43 respectively (Table 3). These PFNA and PFOA concentrations were 3.0 and 8.5 times higher in street runoff than in wastewater treatment plant effluent.43 Zushi and Masunaga87 reported elevated PFAS concentrations in urban runoff from catchments with transport-related land use, especially in the presence of train stations. Thus, stormwater runoff from streets or other transportation-related structures can be a significant non-point source of PFASs in receiving waters.43,65,83,88Street runoff may also show high levels of the plasticizers bisphenol A (BPA) and bisphenol S (BPS).43,89 BPA and BPS are high production volume chemicals used in the production of plastic materials, epoxy resins, food packaging and many other products. BPA is frequently detected in surface waters with median and maximum concentrations of 140 and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1, respectively.90 BPA and BPS have been detected in street runoff in England with concentrations as high as 2410 ng L−1 and 50 ng L−1, respectively (Table 3).43 The concentration of BPA in street runoff was 2.7 times higher than the highest BPA levels in wastewater treatment plant effluents.43 Similar findings were made in Germany where median and maximum BPA concentrations in highway runoff were in the range of 1400 ng L−1 and 2500 ng L−1, respectively (Table 3).89 Urban groundwater in Germany was found to contain comparable or even higher BPA concentrations than wastewater treatment plant effluents what is likely due to urban stormwater infiltration.37
000 ng L−1, respectively.90 BPA and BPS have been detected in street runoff in England with concentrations as high as 2410 ng L−1 and 50 ng L−1, respectively (Table 3).43 The concentration of BPA in street runoff was 2.7 times higher than the highest BPA levels in wastewater treatment plant effluents.43 Similar findings were made in Germany where median and maximum BPA concentrations in highway runoff were in the range of 1400 ng L−1 and 2500 ng L−1, respectively (Table 3).89 Urban groundwater in Germany was found to contain comparable or even higher BPA concentrations than wastewater treatment plant effluents what is likely due to urban stormwater infiltration.37
1H-Benzotriazole (1H-BT), 4-methyl-1H-benzotriazole (4-MeBT), and 5-methyl-1H-benzotriazole (5-MeBT) are corrosion inhibitors that are widely used in aircraft deicing and antiicing fluids, cooling and brake fluids, and dishwasher detergents.91 Cancilla et al.92 analyzed stormwater runoff from a US airport during a deicer application event and found remarkably high concentrations of up to 1.67 mg L−1 and 2.16 mg L−1 of 4-MeBT and 5-MeBT, respectively. While airports and wastewater treatment plant effluents are believed to be the main sources of benzotriazoles to the environment, vehicular emissions can also contribute to their loads in surface waters.93 During rain events and snowmelt, 1H-BT, 4-MeBT, and 5-MeBT were detected in two Canadian stormwater-fed creeks that were not impacted by wastewater treatment plant effluents.93 Total time-weighted average benzotriazole concentrations were 1.3–110 times higher in a creek located in a highly urbanized watershed (up to approximately 1800 ng L−1) compared to a creek in an agricultural, suburban watershed (up to approximately 280 ng L−1).93 Vehicle fluids are likely a main source of benzotriazoles in both watersheds with heavy vehicle traffic leading to elevated baseflow concentrations.93
Street runoff can also contain benzothiazoles which are used as corrosion-inhibitors in antifreeze liquids and as vulcanization accelerators in rubber production.94–96 Tire abrasion from roads is a known stormwater-related input pathway of benzothiazoles into surface waters.94 The total concentration of five benzothiazoles in German street runoff was in the range of 20–74 μg L−1 which was about 1 order of magnitude higher than concentrations found in untreated municipal wastewater.95 Benzothiazole-2-sulfonate accounted for 60% of the total benzothiazole concentration and 2-hydroxybenzothiazole and benzothiazole for 25–30% and 8–13%, respectively.95
Traffic-impacted stormwater runoff can contain numerous other hyphil-TrOCs such as organophosphate (OP) flame retardants. Six OP flame retardants were detected in urban surface runoff in Germany with TCPP exhibiting highest concentration levels (median and maximum concentrations of 0.88 μg L−1 and 5.79 μg L−1, respectively).56 Also frequently detected were tris(2-chloroethyl) phosphate (TCEP, up to 0.275 μg L−1), tri-iso-butyl phosphate (TiBP, up to 1.478 μg L−1), and tris(2-butoxyethyl) phosphate (TBEP, up to 1.616 μg L−1, Table 3).56
3.5 Sanitary sewer overflow
The occurrence of hyphil-TrOCs in urban stormwater has been predominantly studied in separate sewer systems (Table 1) in which storm sewers drain untreated urban stormwater into surface waters. Even though stormwater and sewage are conveyed in separate conduits, frequent detection of wastewater-derived hyphil-TrOCs in storm drains (e.g., lifestyle compounds, pharmaceuticals, and personal care products) indicate intrusion of raw sewage.12,31,40,61 For instance, Burant et al.40 found the wastewater indicator caffeine in all samples collected at storm sewer outlets in a residential and commercial area. Masoner et al.31 frequently detected the prescription pharmaceutical metformin in urban stormwater. Sewage can enter storm sewers through (i) illicit connections of sewage pipes to storm sewers, (ii) sanitary sewer overflow (SSO), and (iii) cross-flow between sanitary and storm systems due to broken sewer pipes.12,30 Illicit connections are a continuous source of wastewater into storm drains and can lead to discharge of raw sewage also during dry weather conditions.30 SSOs can be caused by blockage of the sanitary sewer or stormwater entering sewer lines. SSO causes raw sewage to overflow onto streets where it can intrude into storm sewers. Many sewer connections have been installed decades ago and a lack of repair or renewal can lead to leakages. Raw sewage has been found to contaminate stormwater drainage and receiving water bodies in the city of New Orleans (USA) due to aging infrastructure.61 In two stormwater canals, pharmaceuticals (naproxen, ibuprofen) and endocrine disrupting chemicals (BPA) were present with maximum concentrations in the range of several hundred ng L−1 (Tables 3 and 4).61 Wastewater-derived compounds from low-flow sources such as leaky sanitary systems have been observed to display greater concentrations in winter.12| Contaminant group | Compound | AA-/MAC-EQSs (μg L−1) | Median conc (μg L−1) | Concentration range (μg L−1) | Country | Sample type | Detection frequency | Source |
|---|---|---|---|---|---|---|---|---|
| a N,N-Diethyl-m-toluamide. b Reported maximum concentration. c Calculated from data in the respective supporting information. d Northern CA. e Southern CA. f Desethylatrazine. g Mean value. h Deisopropylatrazine. | ||||||||
| Personal care products | DEETa | 88*/410* | 0.12 | 0.49b | MN, USA | SW | 97% | Fairbairn et al.12 |
| — | 0.11 | Australia | SW | — | Tang et al.113 | |||
| 0.078 | 0.013–0.114 | Germany | CSO | — | Launay et al.13 | |||
| Artificial sweeteners | Acesulfame | — | 2.370c | 19.9b,c | Germany | SW | 100%c | Beckers et al.30 |
| 3.200 | 0.812–5.314 | Germany | CSO | — | Launay et al.13 | |||
| Cyclamate | — | 3.1c | 32.3b,c | Germany | SW | 100%c | Beckers et al.30 | |
| Pharmaceuticals | Lidocaine | — | 0.00394 | 0.0199b | MN, USA | SW | 89% | Fairbairn et al.12 |
| 0.0048c | 0.027b,c | Germany | SW | 100%c | Beckers et al.30 | |||
| 0.00925 | 0.242b | USA | SW | 69% | Masoner et al.31 | |||
| Metformin | 160*/640* | 7.74c | 89.6b,c | Germany | SW | 100%c | Beckers et al.30 | |
| 0.0149 | 0.247b | MN, USA | SW | 64% | Fairbairn et al.12 | |||
| 0.1016 | 1.26b | USA | SW | 73% | Masoner et al.31 | |||
| Metoprolol | 8.6*/75* | 0.30c | 3.84b,c | Germany | SW | 100%c | Beckers et al.30 | |
| 0.178 | 0.089–0.365 | Germany | CSO | — | Launay et al.13 | |||
| Ibuprofen | 0.011*/1700* | 0.038 | 0.674b | LA, USA | SW | 66%c | Boyd et al.61 | |
| 1.200 | 0.576–2.25 | Germany | CSO | — | Launay et al.13 | |||
| Naproxen | — | 0.0078 | 0.145b | LA, USA | SW | 86%c | Boyd et al.61 | |
| 0.130 | 0.033–0.238 | Germany | CSO | — | Launay et al.13 | |||
| Lifestyle compounds | Caffeine | — | 0.207 | 1.71b | MN, USA | SW | 92% | Fairbairn et al.12 |
| 0.942 | 32.2b | USA | SW | 96% | Masoner et al.31 | |||
| 1.06 | 0.235–5.40 | WI, USA | SW | 100% | Burant et al.40 | |||
| 1.07 | 0.0029–53.0 | Canada | SW | — | Sauvé et al.98 | |||
| 7.600 | 3.495–18.54 | Germany | CSO | — | Launay et al.13 | |||
| Nicotine | — | 0.205 | 3.89b | MN, USA | SW | 94% | Fairbairn et al.12 | |
| 0.782 | 18.3b | USA | SW | 98% | Masoner et al.31 | |||
| Cotinine | — | 0.054 | 0.54b | MN, USA | SW | 100% | Fairbairn et al.12 | |
| 0.045 | 0.55b | USA | SW | 92% | Masoner et al.31 | |||
| 0.18c | 0.85b,c | Germany | SW | 100%c | Beckers et al.30 | |||
| Transformation products | Fipronil sulfone | — | 0.09 | 0.55b | CA, USA | SW | — | Ensminger et al.57 |
| 0.026d; 0.077e | — | CA, USA | SW | 36%d; 89%e | Budd et al.74 | |||
| 0.0047–0.145 | 0.391b,d; 1.96b,e | CA, USA | DWR | 64–100% | Gan et al.44 | |||
| Fipronil sulfide | — | 0.004d; 0.009e | — | CA, USA | SW | 2%d; 5%e | Budd et al.74 | |
| 0.0011–0.0198 | 0.054b,d; 0.33b,e | CA, USA | DWR | 47–100% | Gan et al.44 | |||
| Fipronil desulfinyl | — | 0.015d; 0.041e | — | CA, USA | SW | 23%d; 68%e | Budd et al.74 | |
| 0.0016–0.077 | 0.034b,d; 1.12b,e | CA, USA | DWR | 72–100% | Gan et al.44 | |||
| DESf | — | 0.007–0.036g | — | Australia | SW | 40%c | Rippy et al.58 | |
| DIAh | — | 0.013 | 0.144b | WI, USA | SW | 92% | Burant et al.40 | |
| AMPA | 1500*/1500* | 0.64 | 0.14–9.37 | France | SW | 93% | Zgheib et al.59 | |
| 0.824g | 0.016–0.469 | France | SW | 40–75% | Gasperi et al.26 | |||
| — | 0.06–1.3 | Denmark | SW, CSO | 100% | Birch et al.46 | |||
Wastewater-associated contaminants may serve as indicator for human waste in separate storm sewers.61,97,98 Sauvé et al.98 found that caffeine concentrations of >400 ng L−1 were indicative of coliform counts >200 colony-forming units per 100 mL of water. As shown in Table 4, median caffeine concentrations reported in separate storm sewers in the US and Canada range from 0.207–1.07 μg L−1 with maximum concentrations up to 53 μg L−1 indicating a widespread and often overlooked impact of sewage intrusion into storm sewers.12,31,40,98 However, the use of hyphil-TrOCs as indicator for fecal contamination in stormwater has limitations and should be carefully employed because fecal contamination can be also present in the absence of anthropogenic contaminants, e.g., when stemming from encampments or agriculture.98
3.6 Hyphil-TrOC mixtures in separate storm sewers
Urban storm sewer discharges can contain complex mixtures of hundreds of individual chemicals stemming from the many sources discussed above. Fairbairn et al.12 collected 36 grab samples from stormwater conveyance pipes in the US and screened them for the occurrence of 384 organic contaminants of which 123 different individual compounds were detected (Table 1). 31 compounds were found with a frequency of ≥50% and 8 compounds with a frequency of ≥90%.12 Most frequently detected were the herbicides 2,4-D (median concentration 0.390 μg L−1) and atrazine (0.040 μg L−1), the corrosion inhibitor methyl-1H-benzotriazole (methyl-1H-BT, 0.806 μg L−1), the insect repellent DEET (0.120 μg L−1), and the lifestyle compounds nicotine (0.205 μg L−1) and caffeine (0.207 μg L−1) (see Tables 2–4).12 Masoner et al.31 screened 50 stormwater samples collected across the US for the occurrence of 438 different organic chemicals of which 215 were detected (Table 1). 69 compounds were frequently detected in ≥50% of the samples.31 Among the most pervasive hyphil-TrOCs were DEET, nicotine, caffeine, carbendazim, and methyl-1H-benzotriazole which is consistent with the findings of Fairbairn et al.12,31Stormwater conveyance pipes demonstrate diverse pollutant profiles that depend on (i) catchment type and land use, (ii) seasonal application of chemicals, (iii) weather dynamics, and (iv) intrusion of raw sewage. Current literature suggests no clear correlation between land use and the occurrence of specific hyphil-TrOCs. Gasperi et al.26 analyzed hyphil-TrOCs in storm sewer outlets located in three different catchments (industrial, residential, and residential with heavy traffic) in France and did not find any significant difference between the sites. Six herbicides (glyphosate, glufosinate, AMPA, diuron, isoproturon, and 2,4-MCPA), one biocide (carbendazim) and BPA were systematically detected in stormwater from all sites.26 Burant et al.40 found that land use seems to affect different contaminant groups to different extents. With few exceptions, pesticide concentrations were equivalent in stormwater from a residential area and a commercial site.40 In contrast, significant differences were observed for OP flame retardants and benzotriazoles which had higher concentrations at the residential site.40 Future research is needed to understand how land use affects the leaching and transport of hyphil-TrOCs and whether this information can be used to predict the occurrence of hyphil-TrOCs in urban runoff.
Seasonal use patterns of chemicals such as urban-use pesticides are reflected in stormwater.44 The common application periods of pesticides are usually during late spring, summer, and early fall when pest pressure is high.44 Indeed, Wittmer et al.76 found that mecoprop concentrations in urban storm sewers were higher from May to September (up to 32 μg L−1) compared to October and November (below 100 ng L−1). For compounds with multiple applications in the urban environment (e.g., mecoprop as pesticide on lawns and in bitumen sheets on roofs), such seasonal concentrations patterns can hint to predominant sources.76 Similar findings were made by Fairbairn et al.12 who detected 1–2 orders of magnitude greater herbicide concentrations in spring and early summer than late summer and winter. However, compared to agricultural herbicides, urban and mixed-use pesticides (e.g., diuron and triclopyr) showed more frequent late summer or winter detections indicating seasonally independent sources.12
Beckers et al.30 studied seasonal and weather dynamics of hyphil-TrOCs in a separate sewer system in Germany (Table 1). Storm sewer effluent was sampled under dry and wet weather conditions over one year.30 Out of 149 target compounds, 67 were detected.30 No clear seasonal dynamics could be observed, which is in contrast to Fairbairn et al.12,30 Instead, concentrations of hyphil-TrOCs in stormwater were mainly driven by wet and dry weather conditions.30 Urban pesticides (e.g., diuron), biocides (e.g., terbutryn, carbendazim), and insecticides (e.g., fipronil) were predominant during storm events.30 In contrast, legacy pesticides and pharmaceuticals dominated storm sewer effluents during dry weather conditions indicating wastewater as a source.30
3.7 Hyphil-TrOC mixtures in combined sewer systems
Combined sewers transport stormwater as well as domestic and industrial wastewater to wastewater treatment plants (WWTPs) where the water is treated prior to discharge into the receiving water body. Mixing of stormwater with sewage complicates the study of stormwater-derived hyphil-TrOCs due to heavy dilution and potential co-occurrence of the same contaminants in raw sewage.Frequent detections of hyphil-TrOCs in urban surface runoff suggest that stormwater contributes to the overall hyphil-TrOC load in WWTPs. Conventional WWTPs are not designed to remove hyphil-TrOCs and therefore act as major point sources of these chemicals to receiving water bodies. Approximately half of the overall TrOC load in wastewater is eliminated either by sorption (e.g., hydrophobic organic compounds like certain personal care products) or degradation (e.g., surfactants and hormones).2,4,11 Hyphil-TrOCs that are poorly biodegradable are poorly removed.2,11 Broad-scale implementations of advanced wastewater treatment technologies such as ozonation or powdered activated carbon have the potential to significantly enhance the removal of hyphil-TrOCs from wastewater.11,99,100 Yet, intense storm events will continue to challenge combined sewer systems and WWTPs. High flows reduce the hydraulic residence time of wastewater in the treatment system and reduce the treatment efficiency of biodegradable compounds.62 Moreover, high volumes of rainwater and sewage can exceed the capacity of combined sewers and WWTPs leading to discharges of untreated storm- and wastewater as bypass flow or CSO.
Bypass flows and CSOs have been shown to significantly contribute to the hyphil-TrOC load in surface waters.13,48,62,101,102 Bypass flows and CSOs can lead to short peaks in discharge with high loads of hyphil-TrOCs48,76 and subsequent water quality degradation and ecotoxicological effects.103 Phillips et al.62 compared the relative contribution of WWTP bypass flow and treated wastewater to the load of hormones and other hyphil-TrOCs to a lake. The total volume of water discharged as bypass flow represented only 10% of the total annual water discharged (i.e., bypass flow plus treated wastewater).62 Yet, discharges of the bypass flow accounted for 40–90% of the annual load for certain hormones and wastewater-derived hyphil-TrOCs.62 Compounds with the highest elimination efficiencies in WWTPs (>90%, e.g., caffeine and hormones) had the highest portion of total annual load from bypass discharge.62 At the studied WWTP, >80% of the caffeine load was attributable to bypass flow discharge.62 In contrast, for compounds with low removal efficiency in WWTPs (e.g., BPA) the contribution of bypass flow to the overall pollutant load was not significant (<10%).62 The concentration of hyphil-TrOCs that are poorly removed in WWTPs, generally decreased during intense rain events due to dilution of untreated wastewater with stormwater.62,104
4 Occurrence of transformation products
Few studies have investigated transformations of hyphil-TrOCs in urban stormwater. While we know that certain hyphil-TrOCs can undergo photolysis, oxidation, reduction, or biotransformation, knowledge on the occurrence of transformation products in urban stormwater is scarce.Stormwater samples collected in an urban catchment in Switzerland contained the terbutryn phototransformation product desethyl-terbutryn.68 Indeed, it is known that terbutryn is degradable by UV-irradiation.105,106 Also octylisothiazolinone can undergo photodegradation and is likely the reason for its absence in stormwater samples collected in an urban catchment in Switzerland.68,114 Burkhardt et al.71 analyzed façade runoff water for N-(3,4-dichlorophenyl)-N-methylurea (DCPMU) and N-tert-butyl-6-(methylsulfanyl)-1,3,5-triazine-2,4-diamine, which are major transformation products of diuron and terbutryn or irgarol 1051, respectively. They found that concentrations of transformation products can exceed the concentration of the parent biocides especially after longer periods of façade exposure to rain.71 However, they were not able to close the mass balance indicating that undetected transformation products or other unknown loss mechanisms play an important role in both the activity and longevity of biocides for material protection and their release into stormwater.71 Also the insecticide fipronil is subject to photolysis leading to fipronil desulfinyl, which was frequently detected in dry weather residential runoff (median concentration up to 77 ng L−1)44 and urban stormwater (up to 41 ng L−1) in California (Table 4).74 The presence of fipronil desulfinyl in dry weather urban runoff collected at the outfall of an underground storm sewer suggested rapid fipronil photolysis on concrete or soil.44 Also 4-keto molinate, a photodegradation product of the herbicide molinate, has been reported in the literature.115
In addition to photolysis, fipronil can undergo oxidation and reduction. The oxidation product fipronil sulfone was found in residential urban runoff and stormwater with median concentrations as high as 145 ng L−1 (Table 4).44,57,74 The fipronil reduction product fipronil sulfide was frequently detected in residential runoff, but in much lower concentrations (<20 ng L−1) than the parent compound.44 One of the most frequently studied transformation products in stormwater is aminomethylphosphonic acid (AMPA), the primary transformation product of glyphosate. AMPA is frequently found in stormwater runoff and CSOs.26,46,59,116,117 Other reported transformation products include desethylatrazine,30,40,58 deisopropylatrazine,30,40 2-hydroxyatrazine,116 and 2-hydroxysimazine116 as well as diazinon-oxon, which is more toxic than the parent diazinon.115
Current knowledge suggests that transformation products can show temporally delayed concentration profiles compared to their parent compounds, but the same seasonal trends.44 Transformation products are often reported with high detection frequencies (Table 4), indicating their so far overlooked importance. Comprehensive screenings of transformation products in urban stormwater are needed to understand their formation mechanisms, (trans)formation kinetics, transportation patterns, and contribution to stormwater toxicity.
5 Toxicological relevance of hydrophilic TrOCs in urban stormwater
While chemical analyses provide the opportunity to identify and quantify individual contaminants, they can not reveal the toxicity of complex chemical mixtures in stormwater.118 Various studies indicate that urban stormwater runoff can be a significant contributor to increased toxicity of surface waters.119–122 For instance, urban stormwater has been found to be acutely lethal to coho salmon (Oncorhynchus kisutch) and other aquatic species in the Pacific Northwest of the United States.119,120 Coho mortality could not be linked to conventional water quality parameters such as dissolved oxygen or dissolved solids and was not correlated with pathogen-associated disease or exposure to metals, PAHs, and common pesticides.123,124 Exposure of healthy coho spawners to untreated highway runoff collected during storm events reproduced the mortality syndrome indicating that coho salmon are vulnerable to one or more chemicals present in runoff.124,123 Using high-resolution mass spectrometry (HRMS), Peter et al.125 developed a chemical signature for the coho mortality by isolating HRMS features (i.e., exact mass-retention time pairs) that co-occurred in road runoff and field samples that caused symptomatic coho. This “coho mortality signature” was then compared to chemical signatures of several motorvehicle fluids and tire wear particle leachate.125 The chemical signature of tire wear particle leachate displayed most similarity to waters that caused coho mortality.125 Prominent chemicals identified in the coho mortality signature were octylphenol ethoxylates, glycols, bicyclic amines, and (methoxymethyl)melamine compounds.125 However, the presence of individual chemicals could not be linked to observed coho mortality.125 Given that stormwater runoff may contain hundreds to thousands of different organic compounds,119 the identified chemicals likely represent a small fraction of the overall cocktail. It remains unknown whether or not the actual toxicants were represented by HRMS features in the coho mortality signature because sample preparation and analysis inherently exclude a fraction of the chemicals present in field samples.125 Notwithstanding, HRMS based approaches have great potential to reveal chemical patterns and similarities that may indicate (storm)water toxicity.125 Compared to conventional LC-MS analysis, suspect and non-target HRMS screenings also provide more comprehensive insights into the occurrence of hyphil-TrOCs and allow for the identification of overlooked or unknown chemicals.Acute coho salmon mortality is a severe manifestation of stormwater toxicity that raises public awareness and regulatory concern. Lethal effects of stormwater runoff have been reported elsewhere for other organisms including water fleas, rainbow trout, and fathead minnows.121,122 Apart from lethal effects, stormwater can also cause sub-lethal or chronic effects that are less apparent, yet detrimental to long-term organismal fitness.126 Zebrafish embryos exposed to urban highway runoff displayed various sub-lethal effects including delayed hatching, reduced growth, abnormal swim bladder inflation, and lateral line defects.127,126 How these observed effects relate to the presence of complex stormwater pollutant mixtures is currently unknown.
While early studies on stormwater toxicity tried to link adverse effects to the occurrence of conventional stormwater pollutants (e.g., TSS, metals, and PAHs),121,122,128,129 there is increasing awareness of the importance of hyphil-TrOCs as shown in the case of coho salmon mortality. As discussed in sections 3 and 4, stormwater can carry numerous hyphil-TrOCs of which many are bioactive and persistent and, thus, of high concern. In a US-wide survey of trace organic contaminants in surface waters, bioactive compounds including pesticides, antimicrobials, and pharmaceuticals comprised 57% of 406 compounds that were detected at least once.5 Designed bioactivity can lead to ecosystem concern and specific interactions with aquatic organisms.5 Beckers et al.30 reported that four out of seven risk drivers in storm sewer effluent were biocides. Biocide concentrations in urban stormwater often exceeded the predicted no effect concentration values as well as Swiss water regulations of 0.1 μg L−1.68 Fipronil detected in dry weather urban runoff in CA often exceeded the LC50 values for mysid shrimp and grass shrimp (0.14 and 0.32 μg L−1, respectively), which are important trophic links in aquatic food webs.44 Spills or incorrect disposal of chemicals such as the neuroactive insecticide dimethoate can significantly increase the acute toxicity of stormwater.30 Cancilla et al.92 reported increased toxicity of airport runoff during intensive application of aircraft deicing and antiicing fluids as well as the presence of 4-MeBT and 5-MeBT in whole-tissue extracts from minnows that were exposed to airport runoff. While this review article cannot provide a comprehensive compilation of TrOC-related toxicity studies, these selected examples highlight the potential impact of hyphil-TrOCs in stormwater runoff on observed acute or chronic effects as well as the need to identify relevant drivers of toxicity.
In risk assessment, analytically determined concentrations of priority pollutants are compared to environmental quality standards.130 In Tables 2–4, we report long-term environmental quality standards expressed as annual average concentration (AA-EQS) as well as short-term environmental quality standards expressed as maximum allowable concentration (MAC-EQS) for priority substances in inland surface waters as proposed by the EU Water Framework Directive.107 For compounds not yet under EU regulation, we list chronic and acute quality standards proposed by the Swiss Ecotox Center.108 For certain hyphil-TrOCs (e.g., imidacloprid, diuron, PFOS, and BPA), the reported median concentrations in stormwater were oftentimes greater than the chronic quality standards (Tables 2 and 3). For various pesticides (e.g., carbendazim, terbutryn, dimethoate, imidacloprid, and 2,4-D), the reported maximum concentrations in stormwater oftentimes exceeded the acute quality standards (Table 2). Such observations may help to select priority contaminants in stormwater and identify candidate drivers of toxicity. However, risk assessments which are purely based on target chemical analyses may overlook analytically undetected but toxicologically relevant chemicals, transformation products of unknown toxicity and persistence, and complex mixture effects.130
First evidence suggests that seasonal and inter-event variations of urban stormwater composition and quality may be reflected in stormwater toxicity.121 However, the assessment of linkages between stormwater contaminants and toxicity is complicated by (i) the vast number of chemicals (many of them unidentified), (ii) the great variability in stormwater quality over time and space, and (iii) different, predominantly unknown effects of individual chemicals and chemical mixtures on different organisms.113,119 Conventional stormwater toxicity assessment techniques follow standardized protocols to expose different test organisms (e.g., fathead minnow, Ceriodaphnia, or green algae) to stormwater for a predefined amount of time prior to reporting the species response to a specific endpoint (e.g., survival, growth, reproduction, or biomass production).131 The traditional approach to combine in vivo toxicity testings with target chemical analyses of stormwater is, however, not well suited to reveal and monitor the toxicity of rapidly changing chemical mixtures in stormwater runoff.132–134 Little is known about the toxicity of complex chemical mixtures that may exert synergistic or additive effects, which complicates the identification of toxicants and their role in observed toxicity.113,121
Recently, cell-based (in vitro) high-throughput screening assays have been developed as effect-based method for water monitoring.130,134 Cell-based bioassays target health-relevant biological endpoints and can serve as effect-based screening tool to assess the toxicity of stormwater and complement chemical water quality analyses.133,134 In a given bioassay, all organic chemicals with a common mode of toxic action act together.133 Tang et al.133 applied a battery of six bioassays to characterize stormwater samples from urban, residential, and industrial areas in Australian cities in terms of their baseline toxicity, phytotoxicity, dioxin-like activity, estrogenicity, genotoxicity, and oxidative stress. The overall toxicity of stormwater samples was similar to secondary treated wastewater effluent.133 Stormwater displayed higher effect levels than tertiary treated water, surface water, and drinking water, indicating the need for stormwater treatment prior to discharge or stormwater harvesting.133 Some stormwater samples displayed toxicity similar to primary wastewater treatment plant effluent, which is likely due to sewage-impacted storm sewers.133 Industrial and urban commercial sites had higher baseline toxicity compared to residential sites.133 Road runoff displayed especially high baseline toxicity, genotoxicity, and oxidative stress response.133 The baseline toxicity of the stormwater samples correlated significantly with their dissolved organic carbon (DOC) concentration suggesting that DOC could be used as indicator of baseline toxicity.133 Using the combined algae test, herbicides were shown to dominate the baseline toxicity in most samples.133 Typically, a few herbicides (including diuron, terbutryn, bromacil, atrazine, and simazine) can explain the majority of the bioassay-derived phytotoxicity.133 Estrogenic activity was negligible in 95% of the collected stormwater samples.133 However, some samples showed estrogenic effects similar to raw sewage suggesting that the presence of high estrogenic activity could serve as indicator of sewage contamination.133
Collectively, results from in vivo and in vitro studies highlight a possible significant contribution of urban stormwater runoff to acute and chronic toxicity of receiving water bodies. Toxic hyphil-TrOC mixtures can pose a threat in drinking water, especially when coupling the capture of stormwater to potable water supply via aquifer recharge.35 Hyphil-TrOCs in drinking water supplies are of concern because (i) they can pose a risk to human health and (ii) they may be transformed to potentially more harmful byproducts during the production of safe drinking water. For instance, the herbicide diuron, which is frequently found in stormwater, is classified as “known/likely” human carcinogen.135 During drinking water chlorination and chloramination, diuron can be transformed to N-nitrosodimethylamine (NDMA), which is mutagenic, probably carcinogenic to humans, and frequently detected in drinking water.135–137 Indeed, stormwater runoff has been shown to be a source of N-nitrosamines and their precursor compounds.138
In order to choose appropriate monitoring strategies and management options for hyphil-TrOCs in stormwater, it is essential to identify the most relevant toxicants. Therefore, chemical screening tools can be complemented with cell-based bioassays to assess which of the detected chemicals drive the biological effects and which fraction of effect remains unexplained by detected chemicals.130,139 Subsequently, an approach called effect-directed analysis can be applied to isolate and identify so-far unknown but relevant toxicants.130,140 Monitoring of such priority substances will improve the development of efficient and cost-effective stormwater treatment technologies, which are needed to protect aquatic ecosystems and enable safe use of urban stormwater for groundwater recharge and potable water supply.
6 Fate of hydrophilic TrOCs in green stormwater infrastructure
Green stormwater infrastructure is designed to capture and treat stormwater while also providing multiple ecological and societal benefits.141 Green stormwater infrastructure components (also called best management practices, BMPs) include permeable pavement, green roofs, detention and retention basins, constructed wetlands, and biofilters.141 BMPs complement gray infrastructure (i.e., storm drains and pipes), reduce runoff volumes, provide cost-effective stormwater treatment, and expand green spaces in highly urbanized areas.141The outflow of BMPs is either discharged into receiving water bodies or infiltrated into the ground for aquifer recharge. The type and design of BMPs can significantly impact the removal of contaminants from urban stormwater. Current knowledge suggests that green stormwater infrastructure can reduce the toxicity associated with stormwater.120,124,142 Stormwater quality improvements through BMPs are, however, only assessed by monitoring the removal of traditional stormwater pollutants such as TSS,143 nitrate,144–146 phosphates,147 metals,143,148 microorganisms,145 and specific organic legacy compounds such as PAHs and certain pesticides.14,149 Little is known about the fate of hyphil-TrOCs in stormwater BMPs which are not designed to remove highly mobile and persistent organic pollutants. In the following section, we present the current knowledge on the removal of hyphil-TrOCs in detention basins, constructed wetlands, and biofilters.
6.1 Detention basins
Stormwater detention basins (also called dry ponds) are among the most adopted urban BMPs.150 While the main purpose of detention basins is to prevent flooding, they can provide a water quality benefit when the hydraulic residence time allows for sedimentation of suspended solids and other particle-associated contaminants. However, hyphil-TrOCs with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOW values <4 (e.g., atrazine, diuron, chlorfenvinphos, isoproturon, and simazine) are predominantly present in the dissolved fraction of stormwater.26,42,59,112 As the tendency of hyphil-TrOCs to bind to particles is low, they are poorly removed through sedimentation.3,59,150,151 In fact, Sébastian et al.151,152 observed that a number of pesticides including diuron, isoproturon, simazine, atrazine, carbendazim, mecoprop, and chlorfenvinphos were not trapped in a stormwater detention basin. In two monitoring campaigns (out of three studied), high removal efficiencies of >50% were observed for 2,4-MCPA, glyphosate, and ammonium glyphosate probably due to a combination of mechanisms including transformation.151 The more hydrophobic alkylphenols and alkylphenol ethoxylates, which were present in the dissolved and particulate phase, showed proven removal efficiencies of around 40%.151 In fact, alkylphenols, alkylphenol ethoxylates, and BPA were also found in sediments of detention ponds.150
KOW values <4 (e.g., atrazine, diuron, chlorfenvinphos, isoproturon, and simazine) are predominantly present in the dissolved fraction of stormwater.26,42,59,112 As the tendency of hyphil-TrOCs to bind to particles is low, they are poorly removed through sedimentation.3,59,150,151 In fact, Sébastian et al.151,152 observed that a number of pesticides including diuron, isoproturon, simazine, atrazine, carbendazim, mecoprop, and chlorfenvinphos were not trapped in a stormwater detention basin. In two monitoring campaigns (out of three studied), high removal efficiencies of >50% were observed for 2,4-MCPA, glyphosate, and ammonium glyphosate probably due to a combination of mechanisms including transformation.151 The more hydrophobic alkylphenols and alkylphenol ethoxylates, which were present in the dissolved and particulate phase, showed proven removal efficiencies of around 40%.151 In fact, alkylphenols, alkylphenol ethoxylates, and BPA were also found in sediments of detention ponds.150
6.2 Constructed wetlands
Constructed wetlands are engineered, low-energy water treatment systems in which stormwater or CSO is directed through an open marsh land. In addition to water treatment, constructed wetlands provide volume control, ecosystem and recreational benefits. The two basic designs of constructed wetlands are: (i) subsurface flow wetlands in which water moves horizontally or vertically through the planted substrate and (ii) surface flow wetlands in which water flows mainly above the soil surface.153 As discussed by Imfeld et al.153 and Jasper et al.,154 constructed wetlands can remove organic contaminants through natural (a)biotic processes including sedimentation, hydrolysis, photolysis, sorption, oxidation, biotransformation, plant uptake, and phytotransformation (Fig. 2). Removal mechanisms depend on the hyphil-TrOC being treated, the wetland type and operational design, the environmental conditions, vegetation, and the soil matrix.153,155 Our knowledge on removal mechanisms of hyphil-TrOCs in constructed wetlands remains scarce because the majority of studies focus on removal efficiencies.Jasper et al.154 highlight that constructed wetlands provide only partial and highly variable removal of numerous pharmaceuticals and personal care products (e.g., carbamazepine and clofibric acid) from wastewater effluent. Few studies report removal efficiencies of hyphil-TrOCs in constructed wetlands for stormwater treatment. Page et al.156 investigated a constructed wetland receiving stormwater from a residential and light industrial catchment and found mean removal efficiencies of 33–51% for diuron and 20–60% for simazine. Low to moderate removal of hyphil-TrOCs from a vineyard catchment was found by Maillard et al.157 who reported load reductions of 36–60% for simazine, 57–72% for diuron, 77–90% for glyphosate, and 10–59% for AMPA in a surface flow stormwater wetland. Other compounds (e.g., cymoxanil and terbuthylazine) were completely eliminated.157 Plant roots and fine sediments were primary contributors to the retention of glyphosate, AMPA, and dithiocarbamates in the studied wetland.158 Under reducing conditions in summer, glyphosate was degraded into AMPA, which was more persistent than its parent compound.158 AMPA accumulated in the fine sediments posing ecotoxicological risks from accumulation, remobilization, and the release of not yet identified transformation products.158 Dithiocarbamates were found to be degraded under oxic conditions in spring.158 Complex seasonal changes in the wetlands source/sink functions are likely driven by climate conditions, storm duration and frequency, vegetative cover, root structure, and the resulting hydrochemical conditions.157,158
Tondera et al.159 investigated the elimination of hyphil-TrOCs from CSO in a subsurface flow constructed wetland after seven years of operation. Low to medium removal rates were found for sulfamethoxazole (23 ± 10%), carbamazepine (30 ± 9%), 1H-BT (40 ± 8%), and TCPP (43 ± 8%).159 Better removal was achieved for metoprolol (60 ± 6%), BPA (69 ± 5%), and diclofenac (73 ± 3%).159 After 10 years of operation, the studied wetland lost its efficiency to remove chemicals with no or slow biological degradability (e.g., sulfamethoxazole or TCPP) likely due to exhausted adsorption capacity.160 The abatement of BPA exhibited seasonal variations with warmer temperatures potentially promoting biotransformation.160 More research is needed to assess the long-term functionality of constructed wetlands including changes in their hydrochemistry and underlying hyphil-TrOC removal mechanisms.
Highly fluctuating flows and pollutant loads, seasonal variability in performance, and emergence of short-circuited sections may contribute to incomplete removal of hyphil-TrOCs in constructed stormwater wetlands. Novel wetland designs may consist of a sequence of unit process cells that target the removal of specific contaminants.154 Moreover, wetland effluents may be polished using a post-treatment unit containing engineered reactive geomedia (e.g., pyrogenic carbonaceous adsorbents) as depicted in Fig. 2 and described in detail in section 7.
6.3 Biofilters
Stormwater biofilters, also known as bioretention cells, rain gardens, or bioswales, are low-energy treatment systems consisting of a planted soil-based filter media, which commonly has a high content of sand.161 Stormwater percolates through the biofilter before it is discharged through an underdrain or infiltrated into the subsoil for aquifer recharge. Biofilters are among the most promising stormwater treatment technologies as they are designed to remove traditional stormwater pollutants (TSS, nutrients, and metals).161,162 Indeed, biofilters can enhance water quality and reduce stormwater toxicity.120,124,142 However, biofilters may fail to remove hyphil-TrOCs. Few studies have investigated the fate of hyphil-TrOCs in biofilters, which depends on the filter media, the physicochemical properties of the contaminants, the percolation rate through the filter, and the environmental conditions.161Zhang et al.161 challenged two full-scale biofiltration cells with stormwater to which a mixture of organic contaminants was dosed. Hydrophobic contaminants such as petroleum hydrocarbons and phthalates were well removed in the biofilters of which one had no submerged zone and consisted of loamy sand and the other contained a submerged zone and used sand.161 Also glyphosate showed good removal (>80%) probably due to a combination of adsorption and transformation.161 However, triazine herbicides (atrazine and simazine) were poorly eliminated with 20–50% load removal in the cell without a submerged zone and <20% in the cell with a submerged zone.161 Prolonged dry periods of the filter as well as warmer temperatures in summer promoted atrazine and simazine abatement, likely due to biodegradation of the adsorbed herbicides.161 While the validation of the treatment performance of stormwater biofilters is needed, such full-scale field challenge tests are difficult to conduct.163 Therefore, Zhang et al.163 developed an alternative validation method using in situ columns inserted in the biofilter media which attempted to reproduce the performance of the full-scale system.
To date, few other studies have evaluated hyphil-TrOC removal in full scale biofilters with field or in situ methods due to large biofilter sizes and highly fluctuating stormwater flows. For instance, bioswales have been shown to effectively remove TSS, metals, PAHs, and pyrethroid pesticides, but reduction of the more hydrophilic pesticide fipronil was highly inconsistent.142 In a vegetative filter strip and a biofiltration swale treating heavily loaded road runoff, removal of dissolved organic pollutants was generally less effective than that of particles.111,164 While BPA was relatively well removed in the vegetated filter strip (86% removal), it was less well retained in the bioswale (57% removal).111 Other compounds such as nonylphenol monocarboxylate were poorly removed (32%) in the filter strip.111 In the bioswale, release of nonylphenol monocarboxylate increased over time indicating leaching of this compound from biofilter construction materials (asphalt, drains, geomembranes).111,164
Ex situ approaches, i.e., laboratory batch and column experiments under controlled conditions, prevail to study removal mechanisms of hyphil-TrOCs in biofilters. Bester et al.165,166 conducted a laboratory study to investigate the removal of biocides and other TrOCs in planted biofilters containing peat, sand, and gravel. While high removal of biocides (82–100%) was achieved under low flow conditions, pulses with high loads and low retention times of <1 h resulted in a significant drop in removal efficiency.165 Other hyphil-TrOCs such as methylthiobenzothiazole were removed to a large extent independent of the flow through the biofilters.166 Triclosan-methyl, a transformation product of triclosan, was detected in the effluent of the biofilter indicating biotransformation of certain organic contaminants.166 While removal processes of dissolved pollutants (especially nutrients, metals, and hydrocarbons) in bioretention cells have been intensively discussed elsewhere,18,167 information about the fate of hyphil-TrOCs in biofilters remains elusive. Current knowledge confirms that sand is not an adequate adsorbent material to remove hyphil-TrOCs. Therefore, sand-based stormwater treatment systems need modification, for instance through amendments with carbonaceous adsorbents or other reactive geomedia.168
7 Enhanced removal of hydrophilic TrOCs in geomedia-amended BMPs
Potential stormwater geomedia for next generation BMPs include metal oxide materials (e.g., iron filings and manganese oxide-coated sand) and pyrogenic carbonaceous materials (e.g., activated carbon or biochar).7.1 Metal oxide materials
Iron-enhanced sand filters, consisting of sand with approximately 5% iron filings (w/w), have been shown to effectively remove particulate and dissolved phosphorus from stormwater12,147 and may also retain hyphil-TrOCs due to polar and electrostatic interactions with iron oxide surface functional groups.169 Fairbairn et al.12 studied the removal of hyphil-TrOCs from urban stormwater in full-scale iron-enhanced sand filters in Minnesota, USA. Hydrophobic organic contaminants such as PAHs and sterols were effectively removed.12 High removal (>60%) was also observed for several lifestyle compounds (e.g., caffeine and nicotine) and BPA.12 However, removal of other hyphil-TrOCs was poor or negligible. Removal efficiencies were 36% for DEET, 23% for 2,4-D, 19% for diuron, 11% for carbendazim and metolachlor, and 4% for 5-MeBT.12 While iron-enhanced sand filters efficiently attenuated phosphate (61% removal), the competition between different chemical species in stormwater may reduce the removal of organic contaminants through sorption.12Manganese oxides (MnO2) are natural oxidants in soils and sediments and can enhance the removal and transformation of organic contaminants through direct oxidation, adsorption, and surface catalysis.170–172 MnO2 can oxidize a variety of hyphil-TrOCs including antibacterial agents,173,174 sulfonamide antibiotics,171 glyphosate,175 steroid hormones (estrone and 17α-ethinylestradiol),176 BPA,177 and β-blockers (betaxolol, atenolol, and metoprolol).178 Compared to iron oxides, manganese oxides have a higher redox potential resulting in greater oxidation possibilities for organic contaminants.179 Zhang et al.180 studied manganese oxide-containing biofilters as a polishing treatment step for secondary wastewater. While diclofenac and sulfamethoxazole were effectively removed (>70%), carbamazepine was not abated.180 Recently, manganese oxide-coated sand has been proposed as cost-effective and regenerative geomedia for stormwater treatment to target the removal of hyphil-TrOCs.181,182 Grebel et al.181 observed that birnessite (a layered MnO2) was highly reactive towards aromatic compounds with electron-donating moieties (e.g., BPA and 2-mercaptobenzothiazole) and moderately reactive towards compounds with electron-withdrawing functional groups (e.g., diuron) or steric hindrance at the most likely reaction site (e.g., prometon). However, birnessite was unreactive towards numerous other hyphil-TrOCs (e.g., TCPP, benzotriazole, and fipronil).181 Stormwater constituents such as dissolved organic matter or inorganic ions (e.g., calcium or carbonate) can contribute to exhaustion of the redox reactivity of manganese oxide-coated sands.181 Charbonnet et al.182 found that exhausted manganese oxide-coated sand could be regenerated with HOCl leading to similar reactivity and longevity than the pristine MnO2. Geomedia regeneration in the field could be a main advantage as it prevents excavation of the exhausted material, reduces disposal costs, and helps to overcome implementation barriers. To the best of our knowledge, there are no current studies that assess the field performance and field regeneration of manganese oxide-coated sand in BMPs for stormwater treatment. As iron and manganese oxides are redox-sensitive materials, careful design and operation of the biofilter is required to minimize mobilization of Fe2+ and Mn2+ from the filter.
7.2 Pyrogenic carbonaceous materials
Engineered pyrogenic carbonaceous materials such as (regenerated) activated carbon and biochar exhibit a high specific surface area and strong affinity to adsorb hyphil-TrOCs.183,184 Similar to activated carbon, biochar is the solid product of heating biomass under oxygen-limited conditions. However, biochar is usually not subject to energy-intensive thermal or chemical activation and is produced from renewable, locally available materials, such as wood, crop residues, and manure.185 Biochar has recently received increased attention as a cost-effective and sustainable alternative to activated carbon for the treatment of wastewater and stormwater.186–193The adsorption of hyphil-TrOCs onto biochar is mainly driven by diffusion into char pores. The presence of polar functional groups at the edges of the graphene-like layers facilitate electrostatic interactions and other forces (e.g., van der Waals and H-bonding).189 The polyaromatic surface of biochars can also enhance sorbent–sorbate π-electron interactions.184,194 Moreover, biochars contain a variety of surface functional groups that likely play a key role for reactive transformation processes of organic contaminants.195,196 For instance, it is known that biochars are redox-active and reversibly accept and donate electrons.196–201
Numerous studies investigated sorption of hyphil-TrOCs on activated carbon and biochar in controlled laboratory batch experiments.191,194,202,203 Ulrich et al.202 compared activated carbon and 18 different types of biochars in terms of their performance to remove hyphil-TrOCs (atrazine, benzotriazole, 2,4-D, diuron, fipronil, oryzalin, prometon, and TCPP) from synthetic stormwater. While activated carbon best removed hyphil-TrOCs, several biochars were also effective in sorbing these compounds (i.e., logarithmic solid–water distribution coefficients mostly ranged from 4 to 7 L kg−1).202
Biochar-amended stormwater infiltration basins or biofilters have been simulated in column experiments to study the fate of hyphil-TrOCs under controlled flow regimes.191,192,202,204 Ulrich et al.202 filled columns of 15 cm length with sand – activated carbon or sand – biochar mixtures, equilibrated the filter materials over night with synthetic stormwater, and spiked the columns for four days with a mixture of hyphil-TrOCs including prometon, atrazine, TCPP, benzotriazole, and diuron. Breakthrough of all compounds was observed within less than 500 pore volumes, except diuron which was efficiently retained.202 Breakthrough curves were used to verify a forward-prediction intraparticle diffusion model.202 This model was applied to predict sorption-controlled atrazine breakthrough times of 54 years and 5.8 years for a full-scale infiltration basin (103 m2) amended with 12.3 wt% activated carbon or 4.5 wt% biochar, respectively, at 1 inch per hour infiltration rate.202 Water-shed scale simulations by Wolfand et al.205 show that biochar-amended biofilters are also effective at reducing urban runoff concentrations and loads of fipronil and bifenthrin.
Ashoori et al.191 combined biochar with woodchips for enhanced nitrate and hyphil-TrOC removal from urban stormwater. Woodchip bioreactors of 50 cm length were amended with 33 wt% biochar and aged for eight months with urban runoff from a creek in Sonoma, CA.191 Subsequently, the bioreactors were challenged for five months with synthetic stormwater containing six hyphil-TrOCs (fipronil, diuron, 1H-benzotriazole, atrazine, TCEP, and 2,4-D).191 While conventional woodchip bioreactors exhibited rapid breakthrough of hyphil-TrOCs, the compounds were effectively retained by biochar.191 The aforementioned intraparticle diffusion model was applied to predict a breakthrough time of 26 years for the anionic herbicide 2,4-D in biochar-amended woodchip bioreactors assuming that 100% of the annual rainfall volume in the watershed is treated.191 Ulrich et al.206 show that biochar amendments are also effective for hyphil-TrOC removal in vegetated biofilters containing a sand-compost planting layer. It is postulated that the presence of dissolved organic carbon (DOC) can reduce sorption of hyphil-TrOCs onto biochar due to pore blockage and competitive sorption effects.202 However, the presence of labile DOC in biochar systems may also stimulate the evolution of beneficial microbial communities that facilitate biotransformation of hyphil-TrOCs.207 The type of DOC seems to play a critical role as the attenuation of hyphil-TrOCs was more pronounced when biochar-amended columns were fed with DOC extracted from compost than from straw.207 Additional research is needed to elucidate the interplay of biochar, DOC, and microbial communities and their impact on the fate of hyphil-TrOCs in biochar-amended stormwater treatment systems.
Compared to other novel stormwater filtration media such as functionalized polymer-clay composites, biochar showed superior performance for the removal of hyphil-TrOCs.204 While polycation-clay sorbents have been shown to successfully remove hyphil-TrOCs (e.g., atrazine) from water,208 biochar exhibited similar performance for the removal of perfluoroalkyl substances (i.e., PFOA and PFOS) and superior performance for the removal of other hyphil-TrOCs including 2,4-D and TCEP.204
Results from controlled laboratory batch and column experiments provide evidence that biochar-amendments to conventional stormwater filters can significantly enhance the removal of hyphil-TrOCs from stormwater.191,192,202,206,207 Moreover, biochar amendments to conventional green infrastructure can have co-benefits such as the removal of other stormwater pollutants (e.g., fecal indicator bacteria,145,209 or nitrate191,210) and increased water holding capacity.210,211 Biochar-amendments to vegetated stormwater infrastructure may, thus, expand the selection of plants and may enhance the removal of hyphil-TrOCs through sorption and plant uptake.211
The effectiveness and longevity of (regenerated) activated carbon or biochar-amended stormwater treatment systems remain to be evaluated in actual field experiments. One major challenge is the selection of biochar, for which properties are highly dependent on the charring conditions, the feedstock, and the post-treatment handling. Standards to assess the quality of biochar are currently lacking but required to ensure a safe and effective (storm)water treatment. Moreover, we need to develop management strategies for exhausted biochar. While activated carbon is frequently regenerated at the end of its service life, very few studies have attempted biochar regeneration.212
8 Conclusions and future research needs
Research on the occurrence of hyphil-TrOCs in urban stormwater and their impact on stormwater toxicity is emerging. Sources of hyphil-TrOCs in the urban environment are manifold and include structural materials, pest control, urban green spaces, traffic, as well as raw sewage. Urban stormwater can carry numerous hyphil-TrOCs many of which are bioactive, persistent, and of toxicological concern. Conventional green stormwater infrastructure is not designed to eliminate hyphil-TrOCs and often fails to remove polar and mobile organic compounds. Cost-effective amendments of conventional green stormwater infrastructure with reactive geomedia such as metal oxide-coated sands, regenerated activated carbon, or biochar show promise to enhance the removal of hyphil-TrOCs and, thus, protect aquatic ecosystems and enable safe use of urban stormwater for water supply. In the following section, we summarize specific findings and highlight future research needs to advance our understanding of the occurrence, toxicity, and treatment of hyphil-TrOCs in stormwater.Monitoring of hyphil-TrOCs in stormwater
• The majority of studies dealing with hyphil-TrOCs in urban stormwater has been based on a small number of samples collected at few locations during a limited number of storm events. Long-term monitoring of hyphil-TrOCs in urban stormwater is needed at high temporal and spatial resolution to better understand sources as well as regional and seasonal occurrence patterns of these chemicals. Little is known about the frequency and relevance of single, high-intensity peaks due to spills or improper disposal of chemicals in comparison to background concentration levels of hyphil-TrOCs.• The use of passive samplers for urban stormwater monitoring may cost-effectively complement traditional grab and automated sampling strategies. However, more research is needed to validate time-integrated and velocity-dependent passive samplers in the field during storm events with highly variable hyphil-TrOC loads. These research efforts should result in standardized protocols and guidance documents for the consistent use of passive sampling methods in stormwater monitoring.
• Assessment tools exist to predict runoff concentrations of sediments, nutrients, and metals in non-point stormwater runoff based on land use data.213 More extensive monitoring data of hyphil-TrOCs in urban stormwater will help to evaluate whether such prediction tools can be developed for organic contaminants.
• Source apportionment of hyphil-TrOCs is currently difficult to accomplish. New analytical techniques such as compound-specific isotope analysis (CSIA) may be applied to identify predominant sources of relevant chemicals and develop elimination strategies.214
• Increasing evidence suggests that separate storm sewers can be impacted by raw sewage leading to discharge of elevated levels of hyphil-TrOCs and other pollutants. Little is known about the input pathways and the nationwide extent of storm sewer cross-contamination with wastewater.
• Stormwater samples have been predominantly analyzed for a finite set of target analytes. More than 100 different hyphil-TrOCs have been reported in stormwater indicating their frequent and divers occurrence. Suspect and non-target screenings using LC-HRMS are increasingly applied to detect unknown contaminants in wastewater,38 natural or finished water.215–217 Such screening approaches have been rarely applied for urban stormwater119 but may significantly advance our knowledge on the presence and fate of hyphil-TrOCs.
• Not all hyphil-TrOCs are easy to detect and quantify using reversed-phase LC-HRMS. For instance, illicit disposal or spills of antifreeze from automobiles may lead to high concentrations of N-nitrosodimethylamine (NDMA) in stormwater.218 As highlighted in Reemtsma et al.,3 an analytical gap persists for highly polar compounds with negative log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOW values. Future monitoring studies should consider the use of novel analytical approaches such as hydrophilic interaction LC (HILIC) to complement screening efforts.3
DOW values. Future monitoring studies should consider the use of novel analytical approaches such as hydrophilic interaction LC (HILIC) to complement screening efforts.3
• Research on the significance of microplastics in urban stormwater is emerging. While microplastics have been recently found in stormwater retention ponds,219 their role as source or sink for hyphil-TrOCs in urban stormwater remains elusive.
Assessment of stormwater toxicity and priority contaminants
• A comprehensive analysis of the occurrence of hyphil-TrOCs should be accompanied with stormwater toxicity assessments using a combination of classical laboratory in vivo studies as well as high-throughput cell-based bioassays.• More research is needed to assess the toxicity of complex chemical mixtures in stormwater e.g., by developing effect-based water quality trigger values.113,130 Studies should also focus on evaluating the effects of combined exposure to hyphil-TrOCs and other pollutants, especially metals.220
• Research is warranted to assess the impact of urban runoff and BMP effluents on ecological health. Chemical analyses combined with cell-based bioassays may help to detect potential adverse effects from complex contaminant mixtures on freshwater aquatic life and community compositions.
• In order to develop a regulatory framework for stormwater harvesting, risk-based water quality guidance is needed. Therefore, criteria for the selection of stormwater priority contaminants need to be established based on their acute toxicity, chronic effects, concentration levels, persistence, and widespread occurrence.
Fate and removal of hyphil-TrOCs in stormwater BMPs
• To enhance our understanding of the removal, accumulation, and release of hyphil-TrOCs in conventional BMPs, a broad set of hyphil-TrOCs should be included in existing contaminant monitoring lists that so far mostly include traditional stormwater contaminants (e.g., TSS, metals, PAHs). Chemical analyses should be complemented with bioanalytical tools to assess the impact of BMPs on stormwater toxicity. Knowledge on the performance of conventional BMPs can then be used to design next generation BMPs that target the removal of hyphil-TrOCs as well as traditional stormwater contaminants.• New cost-efficient and sustainable materials need to be developed that are able to effectively remove hyphil-TrOCs from stormwater runoff and CSO. While these materials will be designed and tested on the laboratory scale, the field performance and longevity of stormwater treatment materials are of utmost importance for their actual implementation. Geomedia-amended BMPs require optimized construction and operational parameters to avoid premature treatment failure through clogging or loss of geomedia functionality. Studies should especially focus on the impact of hydraulic conductivity and residence time on the removal of hyphil-TrOCs. Research is needed to evaluate possible retrofitting strategies for existing BMPs with geomedia. Moreover, the impact of geomedia-amended BMPs on stormwater toxicity reduction has to be assessed.
• Removal mechanisms of hyphil-TrOCs in conventional and geomedia-amended BMPs remain largely unstudied. Research is needed to understand the impact of various BMP components (e.g., plants, roots, soil, engineered reactive geomedia) on hyphil-TrOC removal and elucidate potential transformation pathways. Suspect and non-target analyses can help identify transformation products which may, however, be transient or difficult to detect. Compound-specific isotope analysis (CSIA) might be a promising complementary approach to investigate natural transformation processes and elucidate reaction mechanisms of hyphil-TrOCs and their transformation products in BMPs.221,222
• Layered or mixed-media filters containing multiple geomedia such as carbonaceous adsorbents (regenerated activated carbon, biochar), zeolite, or manganese oxide-coated sand need testing under aging conditions in the field to assess longevity of performance. More research is needed to develop cost-effective and sustainable management strategies for exhausted stormwater treatment materials, e.g., regeneration of MnO2 or biochar replacement schedules.182,212
• As stormwater biofilters often face prolonged dry periods and, thus, present underused water treatment potential, dual-mode biofilters for treatment of stormwater in wet seasons and greywater in dry seasons have been proposed.143 The fate of greywater-derived hyphil-TrOCs in conventional and geomedia-amended biofilters needs to be investigated especially for infiltrating systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation Engineering Research Center: Re-inventing the Nation's Urban Water Infrastructure (NSF ERC ReNUWIt 1028968) and the US Department of Defense Strategic Environmental Research and Development Program, project ER18-1145.References
- R. P. Schwarzenbach, B. I. Escher, K. Fenner, T. B. Hofstetter, A. C. Johnson, U. von Gunten and B. Wehrli, The challenge of micropollutants in aquatic systems, Science, 2006, 313, 1072–1077 CrossRef CAS.
- J. Margot, L. Rossi, D. A. Barry and C. Holliger, A review of the fate of micropollutants in wastewater treatment plants, Wiley Interdiscip. Rev.: Water, 2015, 2, 457–487 CrossRef CAS.
- T. Reemtsma, U. Berger, H. P. H. Arp, H. Gallard, T. P. Knepper, M. Neumann, J. B. Quintana and P. de Voogt, Mind the gap: Persistent and mobile organic compounds – Water contaminants that slip through, Environ. Sci. Technol., 2016, 50, 10308–10315 CrossRef CAS.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment, Sci. Total Environ., 2014, 473-474, 619–641 CrossRef CAS PubMed.
- P. M. Bradley, C. A. Journey, K. M. Romanok, L. B. Barber, H. T. Buxton, W. T. Foreman, E. T. Furlong, S. T. Glassmeyer, M. L. Hladik, L. R. Iwanowicz, D. K. Jones, D. W. Kolpin, K. M. Kuivila, K. A. Loftin, M. A. Mills, M. T. Meyer, J. L. Orlando, T. J. Reilly, K. L. Smalling and D. L. Villeneuve, Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. streams, Environ. Sci. Technol., 2017, 51, 4792–4802 CrossRef CAS PubMed.
- D. J. Fairbairn, M. E. Karpuzcu, W. A. Arnold, B. L. Barber, E. F. Kaufenberg, W. C. Koskinen, P. J. Novak, P. J. Rice and D. L. Swackhamer, Sources and transport of contaminants of emerging concern: A two-year study of occurrence and spatiotemporal variation in a mixed land use watershed, Sci. Total Environ., 2016, 551–552, 605–613 CrossRef CAS.
- S. M. Elliott, M. L. Erickson, A. L. Krall and B. A. Adams, Concentrations of pharmaceuticals and other micropollutants in groundwater downgradient from large on-site wastewater discharges, PLoS One, 2018, 13, 1–17 CrossRef PubMed.
- D. J. Lapworth, N. Baran, M. E. Stuart and R. S. Ward, Emerging organic contaminants in groundwater: A review of sources, fate and occurrence, Environ. Pollut., 2012, 163, 287–303 CrossRef CAS.
- J. O. Tijani, O. O. Fatoba, O. O. Babajide and L. F. Petrik, Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: A review, Environ. Chem. Lett., 2016, 14, 27–49 CrossRef CAS.
- R. P. Schwarzenbach, T. Egli, T. B. Hofstetter, U. von Gunten and B. Wehrli, Global water pollution and human health, Annu. Rev. Environ. Resour., 2010, 35, 109–136 CrossRef.
- R. I. L. Eggen, J. Hollender, A. Joss, M. Schärer and C. Stamm, Reducing the discharge of micropollutants in the aquatic environment: The benefits of upgrading wastewater treatment plants, Environ. Sci. Technol., 2014, 48, 7683–7689 CrossRef CAS PubMed.
- D. J. Fairbairn, S. M. Elliott, R. L. Kiesling, H. L. Schoenfuss, M. L. Ferrey and B. M. Westerhoff, Contaminants of emerging concern in urban stormwater: Spatiotemporal patterns and removal by iron-enhanced sand filters (IESFs), Water Res., 2018, 145, 332–345 CrossRef CAS PubMed.
- M. A. Launay, U. Dittmer and H. Steinmetz, Organic micro-pollutants discharged by combined sewer overflows – Characterisation of pollutant sources and stormwater-related processes, Water Res., 2016, 104, 82–92 CrossRef CAS.
- A. J. Erickson, P. T. Weiss and J. S. Gulliver, Optimizing Stormwater Treatment Practices, Springer, New York, 2013 Search PubMed.
- J. H. Lee and K. W. Bang, Characterization of urban stormwater runoff, Water Res., 2000, 34, 1773–1780 CrossRef CAS.
- M. C. Gromaire, S. Garnaud, M. Saad and G. Chebbo, Contribution of different sources to the pollution of wet weather flows in combined sewers, Water Res., 2001, 35, 521–533 CrossRef CAS.
- L. Lundy, J. B. Ellis and D. M. Revitt, Risk prioritisation of stormwater pollutant sources, Water Res., 2012, 46, 6589–6600 CrossRef CAS.
- G. H. LeFevre, K. H. Paus, P. Natarajan, J. S. Gulliver, P. J. Novak and R. M. Hozalski, Review of dissolved pollutants in urban storm water and their removal and fate in bioretention cells, J. Environ. Eng., 2014, 141, 04014050 CrossRef.
- H. M. Murphy, Z. Meng, R. Henry, A. Deletic and D. T. McCarthy, Current stormwater harvesting guidelines are inadequate for mitigating risk from Campylobacter during nonpotable reuse activities, Environ. Sci. Technol., 2017, 51, 12498–12507 CrossRef CAS.
- J. A. Steele, A. D. Blackwood, J. F. Griffith, R. T. Noble and K. C. Schiff, Quantification of pathogens and markers of fecal contamination during storm events along popular surfing beaches in San Diego, California, Water Res., 2018, 136, 137–149 CrossRef CAS.
- J. M. Wolfand, C. D. Bell, A. B. Boehm, T. S. Hogue and R. G. Luthy, Multiple pathways to bacterial load reduction by stormwater best management practices: Trade-offs in performance, volume, and treated area, Environ. Sci. Technol., 2018, 52, 6370–6379 CrossRef CAS PubMed.
- M. Huber and B. Helmreich, Stormwater management: Calculation of traffic area runoff loads and traffic related emissions, Water, 2016, 8, 294 CrossRef.
- M. Huber, H. Hilbig, S. C. Badenberg, J. Fassnacht, J. E. Drewes and B. Helmreich, Heavy metal removal mechanisms of sorptive filter materials for road runoff treatment and remobilization under de-icing salt applications, Water Res., 2016, 102, 453–463 CrossRef CAS.
- J. N. Brown and B. M. Peake, Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff, Sci. Total Environ., 2006, 359, 145–155 CrossRef CAS.
- A. E. Barbosa, J. N. Fernandes and L. M. David, Key issues for sustainable urban stormwater management, Water Res., 2012, 46, 6787–6798 CrossRef CAS.
- J. Gasperi, C. Sebastian, V. Ruban, M. Delamain, S. Percot, L. Wiest, C. Mirande, E. Caupos, D. Demare, M. D. K. Kessoo, M. Saad, J. J. Schwartz, P. Dubois, C. Fratta, H. Wolff, R. Moilleron, G. Chebbo, C. Cren, M. Millet, S. Barraud and M. C. Gromaire, Micropollutants in urban stormwater: Occurrence, concentrations, and atmospheric contributions for a wide range of contaminants in three French catchments, Environ. Sci. Pollut. Res., 2014, 21, 5267–5281 CrossRef CAS.
- L. Rossi, L. de Alencastro, T. Kupper and J. Tarradellas, Urban stormwater contamination by polychlorinated biphenyls (PCBs) and its importance for urban water systems in Switzerland, Sci. Total Environ., 2004, 322, 179–189 CrossRef CAS.
- E. Eriksson, A. Baun, L. Scholes, A. Ledin, S. Ahlman, M. Revitt, C. Noutsopoulos and P. S. Mikkelsen, Selected stormwater priority pollutants – A European perspective, Sci. Total Environ., 2007, 383, 41–51 CrossRef CAS.
- J. Soller, J. Stephenson, K. Olivieri, J. Downing and A. W. Olivieri, Evaluation of seasonal scale first flush pollutant loading and implications for urban runoff management, J. Environ. Manage., 2005, 76, 309–318 CrossRef CAS.
- L.-M. Beckers, W. Busch, M. Krauss, T. Schulze and W. Brack, Characterization and risk assessment of seasonal and weather dynamics in organic pollutant mixtures from discharge of a separate sewer system, Water Res., 2018, 135, 122–133 CrossRef CAS.
- J. R. Masoner, D. W. Kolpin, I. M. Cozzarelli, L. B. Barber, D. S. Burden, W. T. Foreman, K. J. Forshay, E. T. Furlong, J. F. Groves, M. L. Hladik, M. E. Hopton, J. B. Jaeschke, S. H. Keefe, D. P. Krabbenhoft, R. Lowrance, K. M. Romanok, D. L. Rus, W. R. Selbig, B. H. Williams and P. M. Bradley, Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the United States, Environ. Sci. Technol., 2019, 53, 10070–10081 CrossRef CAS.
- L. You, V. T. Nguyen, A. Pal, H. Chen, Y. He, M. Reinhard and K. Y.-H. Gin, Investigation of pharmaceuticals, personal care products and endocrine disrupting chemicals in a tropical urban catchment and the influence of environmental factors, Sci. Total Environ., 2015, 536, 955–963 CrossRef CAS.
- J. L. Bradshaw, M. Osorio, T. G. Schmitt and R. G. Luthy, System modeling, optimization, and analysis of recycled water and dynamic storm water deliveries to spreading basins for urban groundwater recharge, Water Resour. Res., 2019, 55, 2446–2463 CrossRef.
- R. G. Luthy and D. L. Sedlak, Urban Water-Supply Reinvention, Daedalus, 2015, 144, 72–82 CrossRef.
- R. G. Luthy, S. Sharvelle and P. Dillon, Urban stormwater to enhance water supply, Environ. Sci. Technol., 2019, 53, 5534–5542 CrossRef CAS PubMed.
- National Academies of Sciences, Engineering, and Medicine, Using Graywater and Stormwater to Enhance Local Water Supplies: An Assessment of Risks, Costs, and Benefits, The National Academies Press, Washington, DC, 2016 Search PubMed.
- K. Osenbrück, H.-R. Gläser, K. Knöller, S. M. Weise, M. Möder, R. Wennrich, M. Schirmer, F. Reinstorf, W. Busch and G. Strauch, Sources and transport of selected organic micropollutants in urban groundwater underlying the city of Halle (Saale), Germany, Water Res., 2007, 41, 3259–3270 CrossRef.
- E. L. Schymanski, H. P. Singer, P. Longrée, M. Loos, M. Ruff, M. A. Stravs, C. Ripollés Vidal and J. Hollender, Strategies to characterize polar organic contamination in wastewater: Exploring the capability of high resolution mass spectrometry, Environ. Sci. Technol., 2014, 48, 1811–1818 CrossRef CAS PubMed.
- H. Birch, A. K. Sharma, L. Vezzaro, H.-C. H. Lützhøft and P. S. Mikkelsen, Velocity dependent passive sampling for monitoring of micropollutants in dynamic stormwater discharges, Environ. Sci. Technol., 2013, 47, 12958–12965 CrossRef CAS PubMed.
- A. Burant, W. Selbig, E. T. Furlong and C. P. Higgins, Trace organic contaminants in urban runoff: Associations with urban land-use, Environ. Pollut., 2018, 242, 2068–2077 CrossRef CAS.
- D. T. McCarthy, K. Zhang, C. Westerlund, M. Viklander, J.-L. Bertrand-Krajewski, T. D. Fletcher and A. Deletic, Assessment of sampling strategies for estimation of site mean concentrations of stormwater pollutants, Water Res., 2018, 129, 297–304 CrossRef CAS.
- C. Becouze-Lareure, A. Dembélé, M. Coquery, C. Cren-Olivé and J.-L. Bertrand-Krajewski, Assessment of 34 dissolved and particulate organic and metallic micropollutants discharged at the outlet of two contrasted urban catchments, Sci. Total Environ., 2019, 651, 1810–1818 CrossRef CAS.
- J. L. Wilkinson, J. Swinden, P. S. Hooda, J. Barker and S. Barton, Markers of anthropogenic contamination: A validated method for quantification of pharmaceuticals, illicit drug metabolites, perfluorinated compounds, and plasticisers in sewage treatment effluent and rain runoff, Chemosphere, 2016, 159, 638–646 CrossRef CAS PubMed.
- J. Gan, S. Bondarenko, L. Oki, D. Haver and J. X. Li, Occurrence of fipronil and its biologically active derivatives in urban residential runoff, Environ. Sci. Technol., 2012, 46, 1489–1495 CrossRef CAS PubMed.
- S. Deffontis, A. Breton, C. Vialle, M. Montréjaud-Vignoles, C. Vignoles and C. Sablayrolles, Impact of dry weather discharges on annual pollution from a separate storm sewer in Toulouse, France, Sci. Total Environ., 2013, 452-453, 394–403 CrossRef CAS PubMed.
- H. Birch, P. S. Mikkelsen, J. K. Jensen and H.-C. H. Lützhøft, Micropollutants in stormwater runoff and combined sewer overflow in the Copenhagen area, Denmark, Water Sci. Technol., 2011, 64, 485–493 CrossRef CAS PubMed.
- D. Ackerman, E. D. Stein and K. J. Ritter, Evaluating performance of stormwater sampling approaches using a dynamic watershed model, Environ. Monit. Assess., 2011, 180, 283–302 CrossRef.
- A. Musolff, S. Leschik, M. Möder, G. Strauch, F. Reinstorf and M. Schirmer, Temporal and spatial patterns of micropollutants in urban receiving waters, Environ. Pollut., 2009, 157, 3069–3077 CrossRef CAS.
- L. Mutzner, E. L. M. Vermeirssen and C. Ort, Passive samplers in sewers and rivers with highly fluctuating micropollutant concentrations – Better than we thought, J. Hazard. Mater., 2019, 361, 312–320 CrossRef CAS PubMed.
- D. Page, K. Miotliński, D. Gonzalez, K. Barry, P. Dillon and C. Gallen, Environmental monitoring of selected pesticides and organic chemicals in urban stormwater recycling systems using passive sampling techniques, J. Contam. Hydrol., 2014, 158, 65–77 CrossRef CAS PubMed.
- B. Vrana, I. J. Allan, R. Greenwood, G. A. Mills, E. Dominiak, K. Svensson, J. Knutsson and G. Morrison, Passive sampling techniques for monitoring pollutants in water, TrAC, Trends Anal. Chem., 2005, 24, 845–868 CrossRef CAS.
- C. Moschet, E. L. M. Vermeirssen, H. Singer, C. Stamm and J. Hollender, Evaluation of in-situ calibration of Chemcatcher passive samplers for 322 micropollutants in agricultural and urban affected rivers, Water Res., 2015, 71, 306–317 CrossRef CAS PubMed.
- J. E. Tomaszewski and R. G. Luthy, Field deployment of polyethylene devices to measure PCB concentrations in pore water of contaminated sediment, Environ. Sci. Technol., 2008, 42, 6086–6091 CrossRef CAS PubMed.
- Y. Choi, Y.-M. Cho and R. G. Luthy, Polyethylene-water partitioning coefficients for parent- and alkylated-polycyclic aromatic hydrocarbons and polychlorinated biphenyls, Environ. Sci. Technol., 2013, 47, 6943–6950 CrossRef CAS PubMed.
- L. Mutzner, E. L. M. Vermeirssen, S. Mangold, M. Maurer, A. Scheidegger, H. Singer, K. Booij and C. Ort, Passive samplers to quantify micropollutants in sewer overflows: Accumulation behaviour and field validation for short pollution events, Water Res., 2019, 160, 350–360 CrossRef CAS PubMed.
- J. Regnery and W. Püttmann, Seasonal fluctuations of organophosphate concentrations in precipitation and storm water runoff, Chemosphere, 2010, 78, 958–964 CrossRef CAS PubMed.
- M. P. Ensminger, R. Budd, K. C. Kelley and K. S. Goh, Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008-2011, Environ. Monit. Assess., 2013, 185, 3697–3710 CrossRef CAS.
- M. A. Rippy, A. Deletic, J. Black, R. Aryal, J.-L. Lampard, J. Y.-M. Tang, D. McCarthy, P. Kolotelo, J. Sidhu and W. Gernjak, Pesticide occurrence and spatio-temporal variability in urban run-off across Australia, Water Res., 2017, 115, 245–255 CrossRef CAS PubMed.
- S. Zgheib, R. Moilleron and G. Chebbo, Priority pollutants in urban stormwater: Part 1 – Case of separate storm sewers, Water Res., 2012, 46, 6683–6692 CrossRef CAS PubMed.
- U. E. Bollmann, J. Vollertsen, J. Carmeliet and K. Bester, Dynamics of biocide emissions from buildings in a suburban stormwater catchment – Concentrations, mass loads and emission processes, Water Res., 2014, 56, 66–76 CrossRef CAS.
- G. R. Boyd, J. M. Palmeri, S. Zhang and D. A. Grimm, Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA, Sci. Total Environ., 2004, 333, 137–148 CrossRef CAS.
- P. J. Phillips, A. T. Chalmers, J. L. Gray, D. W. Kolpin, W. T. Foreman and G. R. Wall, Combined sewer overflows: An environmental source of hormones and wastewater micropollutants, Environ. Sci. Technol., 2012, 46, 5336–5343 CrossRef CAS.
- W. A. Asman, A. Jørgensen, R. Bossi, K. V. Vejrup, B. B. Mogensen and M. Glasius, Wet deposition of pesticides and nitrophenols at two sites in Denmark: Measurements and contributions from regional sources, Chemosphere, 2005, 59, 1023–1031 CrossRef CAS.
- M. L. Ferrey, M. C. Hamilton, W. J. Backe and K. E. Anderson, Pharmaceuticals and other anthropogenic chemicals in atmospheric particulates and precipitation, Sci. Total Environ., 2018, 612, 1488–1497 CrossRef CAS PubMed.
- S.-K. Kim and K. Kannan, Perfluorinated acids in air, rain, snow, surface runoff, and lakes: Relative importance of pathways to contamination of urban lakes, Environ. Sci. Technol., 2007, 41, 8328–8334 CrossRef CAS PubMed.
- J. Regnery and W. Püttmann, Organophosphorus flame retardants and plasticizers in rain and snow from middle Germany, Clean, 2009, 37, 334–342 CAS.
- U.-J. Kim and K. Kannan, Occurrence and distribution of organophosphate flame retardants/plasticizers in surface waters, tap water, and rainwater: Implications for human exposure, Environ. Sci. Technol., 2018, 52, 5625–5633 CrossRef CAS PubMed.
- M. Burkhardt, S. Zuleeg, R. Vonbank, P. Schmid, S. Hean, X. Lamani, K. Bester and M. Boller, Leaching of additives from construction materials to urban storm water runoff, Water Sci. Technol., 2011, 63, 1974–1982 CrossRef CAS PubMed.
- M. Burkhardt, T. Kupper, S. Hean, R. Haag, P. Schmid, M. Kohler and M. Boller, Biocides used in building materials and their leaching behavior to sewer systems, Water Sci. Technol., 2007, 56, 63–67 CrossRef CAS.
- T. D. Bucheli, S. R. Müller, A. Voegelin and R. P. Schwarzenbach, Bituminous roof sealing membranes as major sources of the herbicide (R,S)-mecoprop in roof runoff waters: Potential contamination of groundwater and surface waters, Environ. Sci. Technol., 1998, 32, 3465–3471 CrossRef CAS.
- M. Burkhardt, S. Zuleeg, R. Vonbank, K. Bester, J. Carmeliet, M. Boller and T. Wangler, Leaching of biocides from façades under natural weather conditions, Environ. Sci. Technol., 2012, 46, 5497–5503 CrossRef CAS.
- U. Schoknecht, J. Gruycheva, H. Mathies, H. Bergmann and M. Burkhardt, Leaching of biocides used in façade coatings under laboratory test conditions, Environ. Sci. Technol., 2009, 43, 9321–9328 CrossRef CAS PubMed.
- J.-L. Bertrand-Krajewski, G. Chebbo and A. Saget, Distribution of pollutant mass vs volume in stormwater discharges and the first flush phenomenon, Water Res., 1998, 32, 2341–2356 CrossRef CAS.
- R. Budd, M. Ensminger, D. Wang and K. S. Goh, Monitoring fipronil and degradates in California surface waters, 2008-2013, J. Environ. Qual., 2015, 44, 1233–1240 CrossRef CAS PubMed.
- J. Regnery, A. Friesen, A. Geduhn, B. Göckener, M. Kotthoff, P. Parrhysius, E. Petersohn, G. Reifferscheid, E. Schmolz, R. S. Schulz, J. Schwarzbauer and M. Brinke, Rating the risks of anticoagulant rodenticides in the aquatic environment: A review, Environ. Chem. Lett., 2019, 17, 215–240 CrossRef CAS.
- I. K. Wittmer, H.-P. Bader, R. Scheidegger, H. Singer, A. Lück, I. Hanke, C. Carlsson and C. Stamm, Significance of urban and agricultural land use for biocide and pesticide dynamics in surface waters, Water Res., 2010, 44, 2850–2862 CrossRef CAS.
- I. Hanke, I. Wittmer, S. Bischofberger, C. Stamm and H. Singer, Relevance of urban glyphosate use for surface water quality, Chemosphere, 2010, 81, 422–429 CrossRef CAS.
- R. Budd and K. Peters, Survey of Pesticide Products Sold in Retail Stores in Northern California, 2017, (accessed August 2019), https://www.cdpr.ca.gov/docs/emon/pubs/ehapreps/analysis_memos/pesticide_product_survey_120718.pdf Search PubMed.
- N. Bekarian, D. Payne-Sturges, S. Edmondson, B. Chism and T. J. Woodruff, Use of point-of-sale data to track usage patterns of residential pesticides: Methodology development, Environ. Health, 2006, 5, 15 CrossRef.
- D. P. Weston, D. Chen and M. J. Lydy, Stormwater-related transport of the insecticides bifenthrin, fipronil, imidacloprid, and chlorpyrifos into a tidal wetland, San Francisco Bay, California, Sci. Total Environ., 2015, 527-528, 18–25 CrossRef CAS.
- M. E. DeLorenzo, B. Thompson, E. Cooper, J. Moore and M. H. Fulton, A long-term monitoring study of chlorophyll, microbial contaminants, and pesticides in a coastal residential stormwater pond and its adjacent tidal creek, Environ. Monit. Assess., 2012, 184, 343–359 CrossRef CAS.
- A. L. R. Green, A. Putschew and T. Nehls, Littered cigarette butts as a source of nicotine in urban waters, J. Hydrol., 2014, 519, 3466–3474 CrossRef.
- M. Murakami, H. Shinohara and H. Takada, Evaluation of wastewater and street runoff as sources of perfluorinated surfactants (PFSs), Chemosphere, 2009, 74, 487–493 CrossRef CAS.
- J. Wilkinson, P. S. Hooda, J. Barker, S. Barton and J. Swinden, Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field, Environ. Pollut., 2017, 231, 954–970 CrossRef CAS PubMed.
- M. F. Rahman, S. Peldszus and W. B. Anderson, Behaviour and fate of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in drinking water treatment: A review, Water Res., 2014, 50, 318–340 CrossRef CAS PubMed.
- M. Exner and H. Färber, Perfluorinated surfactants in surface and drinking waters, Environ. Sci. Pollut. Res., 2006, 13, 299–307 CrossRef PubMed.
- Y. Zushi and S. Masunaga, Identifying the nonpoint source of perfluorinated compounds using a geographic information system based approach, Environ. Toxicol. Chem., 2009, 28, 691–700 CrossRef CAS.
- E. F. Houtz and D. L. Sedlak, Oxidative conversion as a means of detecting precursors to perfluoroalkyl acids in urban runoff, Environ. Sci. Technol., 2012, 46, 9342–9349 CrossRef CAS.
- B. Stachel, J.-U. Holthuis, W. Schulz, W. Seitz, W. H. Weber, K.-T. Tegge and I. Dobner, in Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies, ed. D. Fatta-Kassinos, K. Bester and K. Kümmerer, Springer Science, Dordrecht, 2010, ch. 24, vol. 16, pp. 445–461 Search PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS.
- M. D. Alotaibi, A. J. McKinley, B. M. Patterson and A. Y. Reeder, Benzotriazoles in the aquatic environment: A review of their occurrence, toxicity, degradation and analysis, Water, Air, Soil Pollut., 2015, 226, 226 CrossRef.
- D. A. Cancilla, J. C. Baird, S. W. Geis and S. R. Corsi, Studies of the environmental fate and effect of aircraft deicing fluids: Detection of 5-methyl-1H-benzotriazole in the fathead minnow (Pimephales promelas), Environ. Toxicol. Chem., 2003, 22, 134–140 CrossRef CAS PubMed.
- A. Parajulee, Y. D. Lei, A. O. De Silva, X. Cao, C. P. J. Mitchell and F. Wania, Assessing the source-to-stream transport of benzotriazoles during rainfall and snowmelt in urban and agricultural watersheds, Environ. Sci. Technol., 2017, 51, 4191–4198 CrossRef CAS PubMed.
- C. M. Reddy and J. G. Quinn, Environmental chemistry of benzothiazoles derived from rubber, Environ. Sci. Technol., 1997, 31, 2847–2853 CrossRef CAS.
- A. Kloepfer, M. Jekel and T. Reemtsma, Occurrence, sources, and fate of benzothiazoles in municipal wastewater treatment plants, Environ. Sci. Technol., 2005, 39, 3792–3798 CrossRef CAS PubMed.
- R. B. Spies, B. D. Andresen and D. W. Rice Jr, Benzthiazoles in estuarine sediments as indicators of street runoff, Nature, 1987, 327, 697–699 CrossRef CAS.
- Y. Xu, F. Luo, A. Pal, K. Y.-H. Gin and M. Reinhard, Occurrence of emerging organic contaminants in a tropical urban catchment in Singapore, Chemosphere, 2011, 83, 963–969 CrossRef CAS PubMed.
- S. Sauvé, K. Aboulfadl, S. Dorner, P. Payment, G. Deschamps and M. Prévost, Fecal coliforms, caffeine and carbamazepine in stormwater collection systems in a large urban area, Chemosphere, 2012, 86, 118–123 CrossRef PubMed.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell, C. Ort, H. Singer, U. von Gunten and H. Siegrist, Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration, Environ. Sci. Technol., 2009, 43, 7862–7869 CrossRef CAS PubMed.
- J. Margot, C. Kienle, A. Magnet, M. Weil, L. Rossi, L. F. de Alencastro, C. Abegglen, D. Thonney, N. Chèvre, M. Schärer and D. Barry, Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon?, Sci. Total Environ., 2013, 461–462, 480–498 CrossRef CAS.
- A.-S. Madoux-Humery, S. M. Dorner, S. Sauvé, K. Aboulfadl, M. Galarneau, P. Servais and M. Prévost, Temporal analysis of E. coli, TSS and wastewater micropollutant loads from combined sewer overflows: Implications for management, Environ. Sci.: Processes Impacts, 2015, 17, 965–974 RSC.
- L. J. Fono and D. L. Sedlak, Use of the chiral pharmaceutical propranolol to identify sewage discharges into surface waters, Environ. Sci. Technol., 2005, 39, 9244–9252 CrossRef CAS PubMed.
- B. Heinz, S. Birk, R. Liedl, T. Geyer, K. L. Straub, J. Andresen, K. Bester and A. Kappler, Water quality deterioration at a karst spring (Gallusquelle, Germany) due to combined sewer overflow: Evidence of bacterial and micro-pollutant contamination, Environ. Geol., 2009, 57, 797–808 CrossRef CAS.
- M. J. Benotti and B. J. Brownawell, Distributions of pharmaceuticals in an urban estuary during both dry- and wet-weather conditions, Environ. Sci. Technol., 2007, 41, 5795–5802 CrossRef CAS PubMed.
- G. Minelgaite, A. H. Nielsen, M. L. Pedersen and J. Vollertsen, Photodegradation of three stormwater biocides, Urban Water J., 2017, 14, 53–60 CrossRef CAS.
- U. E. Bollmann, G. Minelgaite, M. Schlüsener, T. Ternes, J. Vollertsen and K. Bester, Leaching of terbutryn and its photodegradation products from artificial walls under natural weather conditions, Environ. Sci. Technol., 2016, 50, 4289–4295 CrossRef CAS PubMed.
- European Commission. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy, 2013.
- Ecotox Centre Switzerland. Proposals for Acute and Chronic Quality Standards, (accessed August 2019), https://www.ecotoxcentre.ch/expert-service/quality-standards.
- C. R. Kratzer, Pesticides in storm runoff from agricultural and urban areas in the Tuolumne River Basin in the vicinity of Modesto, California, US Geological Survey; Branch of Information Services technical report, 1998 Search PubMed.
- X. Huang, T. Pedersen, M. Fischer, R. White and T. M. Young, Herbicide runoff along highways. 1. Field observations, Environ. Sci. Technol., 2004, 38, 3263–3271 CrossRef CAS PubMed.
- K. Flanagan, P. Branchu, L. Boudahmane, E. Caupos, D. Demare, S. Deshayes, P. Dubois, L. Meffray, C. Partibane, M. Saad and M.-C. Gromaire, Field performance of two biofiltration systems treating micropollutants from road runoff, Water Res., 2018, 145, 562–578 CrossRef CAS PubMed.
- Y. Kalmykova, K. Björklund, A.-M. Strömvall and L. Blom, Partitioning of polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachates and stormwater, Water Res., 2013, 47, 1317–1328 CrossRef CAS PubMed.
- J. Y. Tang, S. McCarty, E. Glenn, P. A. Neale, M. S. J. Warne and B. I. Escher, Mixture effects of organic micropollutants present in water: Towards the development of effect-based water quality trigger values for baseline toxicity, Water Res., 2013, 47, 3300–3314 CrossRef CAS PubMed.
- U. E. Bollmann, G. Minelgaite, M. Schlüsener, T. A. Ternes, J. Vollertsen and K. Bester, Photodegradation of octylisothiazolinone and semi-field emissions from facade coatings, Sci. Rep., 2017, 7, 41501 CrossRef PubMed.
- J. Domagalski, Pesticides and pesticide degradation products in stormwater runoff: Sacramento River Basin, California, J. Am. Water Resour. Assoc., 1996, 32, 953–964 CrossRef CAS.
- E. Eriksson, A. Baun, P. S. Mikkelsen and A. Ledin, Risk assessment of xenobiotics in stormwater discharged to Harrestrup Å, Denmark, Desalination, 2007, 215, 187–197 CrossRef CAS.
- F. Mauffrey, P.-Y. Baccara, C. Gruffaz, S. Vuilleumier and G. Imfeld, Bacterial community composition and genes for herbicide degradation in a stormwater wetland collecting herbicide runoff, Water, Air, Soil Pollut., 2017, 228, 452 CrossRef.
- L. Scholes, A. Baun, M. Seidl, E. Eriksson, M. Revitt and J.-M. Mouchel, in Highway and Urban Environment, ed. G. M. Morrison and S. Rauch, Springer, Dordrecht, 2007, vol. 12, pp. 399–410 Search PubMed.
- B. Du, J. M. Lofton, K. T. Peter, A. D. Gipe, C. A. James, J. K. McIntyre, N. L. Scholz, J. E. Baker and E. P. Kolodziej, Development of suspect and non-target screening methods for detection of organic contaminants in highway runoff and fish tissue with high-resolution time-of-flight mass spectrometry, Environ. Sci.: Processes Impacts, 2017, 19, 1185–1196 RSC.
- J. K. McIntyre, J. W. Davis, C. Hinman, K. H. Macneale, B. F. Anulacion, N. L. Scholz and J. D. Stark, Soil bioretention protects juvenile salmon and their prey from the toxic impacts of urban stormwater runoff, Chemosphere, 2015, 132, 213–219 CrossRef CAS PubMed.
- T. Mayer, Q. Rochfort, J. Marsalek, J. Parrott, M. Servos, M. Baker, R. McInnis, A. Jurkovic and I. Scott, Environmental characterization of surface runoff from three highway sites in Southern Ontario, Canada: 2. Toxicology, Water Qual. Res. J. Can., 2011, 46, 121–136 CrossRef CAS.
- B. J. Mahler, C. G. Ingersoll, P. C. Van Metre, J. L. Kunz and E. E. Little, Acute toxicity of runoff from sealcoated pavement to Ceriodaphnia dubia and Pimephales promelas, Environ. Sci. Technol., 2015, 49, 5060–5069 CrossRef CAS PubMed.
- N. L. Scholz, M. S. Myers, S. G. McCarthy, J. S. Labenia, J. K. McIntyre, G. M. Ylitalo, L. D. Rhodes, C. A. Laetz, C. M. Stehr, B. L. French, B. McMillan, D. Wilson, L. Reed, K. D. Lynch, S. Damm, J. W. Davis and T. K. Collier, Recurrent die-offs of adult coho salmon returning to spawn in Puget Sound lowland urban streams, PLoS One, 2011, 6, 1–12 CrossRef PubMed.
- J. A. Spromberg, D. H. Baldwin, S. E. Damm, J. K. McIntyre, M. Huff, C. A. Sloan, B. F. Anulacion, J. W. Davis and N. L. Scholz, Coho salmon spawner mortality in western US urban watersheds: bioinfiltration prevents lethal storm water impacts, J. Appl. Ecol., 2016, 53, 398–407 CrossRef CAS PubMed.
- K. T. Peter, Z. Tian, C. Wu, P. Lin, S. White, B. Du, J. K. McIntyre, N. L. Scholz and E. P. Kolodziej, Using high-resolution mass spectrometry to identify organic contaminants linked to urban stormwater mortality syndrome in coho salmon, Environ. Sci. Technol., 2018, 52, 10317–10327 CrossRef CAS PubMed.
- A. Young, V. Kochenkov, J. K. McIntyre, J. D. Stark and A. B. Coffin, Urban stormwater runoff negatively impacts lateral line development in larval zebrafish and salmon embryos, Sci. Rep., 2018, 8, 2830 CrossRef PubMed.
- J. K. McIntyre, J. W. Davis, J. P. Incardona, J. D. Stark, B. F. Anulacion and N. L. Scholz, Zebrafish and clean water technology: Assessing soil bioretention as a protective treatment for toxic urban runoff, Sci. Total Environ., 2014, 500–501, 173–180 CrossRef CAS PubMed.
- D. Barałkiewicz, M. Chudzińska, B. Szpakowska, D. Świerk, R. Gołdyn and R. Dondajewska, Storm water contamination and its effect on the quality of urban surface waters, Environ. Monit. Assess., 2014, 186, 6789–6803 CrossRef PubMed.
- S. Brudler, M. Rygaard, K. Arnbjerg-Nielsen, M. Z. Hauschild, C. Ammitsøe and L. Vezzaro, Pollution levels of stormwater discharges and resulting environmental impacts, Sci. Total Environ., 2019, 663, 754–763 CrossRef CAS PubMed.
- R. Altenburger, W. Brack, R. M. Burgess, W. Busch, B. I. Escher, A. Focks, L. Mark Hewitt, B. N. Jacobsen, M. L. de Alda, S. Ait-Aissa, T. Backhaus, A. Ginebreda, K. Hilscherová, J. Hollender, H. Hollert, P. A. Neale, T. Schulze, E. L. Schymanski, I. Teodorovic, A. J. Tindall, G. de Aragão Umbuzeiro, B. Vrana, B. Zonja and M. Krauss, Future water quality monitoring: Improving the balance between exposure and toxicity assessments of real-world pollutant mixtures, Environ. Sci. Eur., 2019, 31, 12 CrossRef.
- K. C. Schiff and D. Greenstein, Stormwater Monitoring Coalition: Toxicity Testing Laboratory Guidance Document, Southern California Coastal Water Research Project Technical Report 956, 2016 Search PubMed.
- K. A. Maruya, N. G. Dodder, A. C. Mehinto, N. D. Denslow, D. Schlenk, S. A. Snyder and S. B. Weisberg, A tiered, integrated biological and chemical monitoring framework for contaminants of emerging concern in aquatic ecosystems, Integr. Environ. Assess. Manage., 2016, 12, 540–547 CrossRef CAS PubMed.
- J. Y. Tang, R. Aryal, A. Deletic, W. Gernjak, E. Glenn, D. McCarthy and B. I. Escher, Toxicity characterization of urban stormwater with bioanalytical tools, Water Res., 2013, 47, 5594–5606 CrossRef CAS PubMed.
- B. I. Escher, M. Allinson, R. Altenburger, P. A. Bain, P. Balaguer, W. Busch, J. Crago, N. D. Denslow, E. Dopp, K. Hilscherova, A. R. Humpage, A. Kumar, M. Grimaldi, B. S. Jayasinghe, B. Jarosova, A. Jia, S. Makarov, K. A. Maruya, A. Medvedev, A. C. Mehinto, J. E. Mendez, A. Poulsen, E. Prochazka, J. Richard, A. Schifferli, D. Schlenk, S. Scholz, F. Shiraishi, S. Snyder, G. Su, J. Y. M. Tang, B. van der Burg, S. C. van der Linden, I. Werner, S. D. Westerheide, C. K. C. Wong, M. Yang, B. H. Y. Yeung, X. Zhang and F. D. L. Leusch, Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays, Environ. Sci. Technol., 2014, 48, 1940–1956 CrossRef CAS PubMed.
- W.-H. Chen and T. M. Young, NDMA formation during chlorination and chloramination of aqueous diuron solutions, Environ. Sci. Technol., 2008, 42, 1072–1077 CrossRef CAS PubMed.
- W.-H. Chen and T. M. Young, Influence of nitrogen source on NDMA formation during chlorination of diuron, Water Res., 2009, 43, 3047–3056 CrossRef CAS PubMed.
- S. W. Krasner, W. A. Mitch, D. L. McCurry, D. Hanigan and P. Westerhoff, Formation, precursors, control, and occurrence of nitrosamines in drinking water: A review, Water Res., 2013, 47, 4433–4450 CrossRef CAS PubMed.
- T. Zeng, C. M. Glover, E. J. Marti, G. C. Woods-Chabane, T. Karanfil, W. A. Mitch and E. R. V. Dickenson, Relative importance of different water categories as sources of N-nitrosamine precursors, Environ. Sci. Technol., 2016, 50, 13239–13248 CrossRef CAS PubMed.
- J. Y. Tang, F. Busetti, J. W. Charrois and B. I. Escher, Which chemicals drive biological effects in wastewater and recycled water?, Water Res., 2014, 60, 289–299 CrossRef CAS PubMed.
- W. Brack, S. Ait-Aissa, R. M. Burgess, W. Busch, N. Creusot, C. Di Paolo, B. I. Escher, L. M. Hewitt, K. Hilscherova, J. Hollender, H. Hollert, W. Jonker, J. Kool, M. Lamoree, M. Muschket, S. Neumann, P. Rostkowski, C. Ruttkies, J. Schollee, E. L. Schymanski, T. Schulze, T.-B. Seiler, A. J. Tindall, G. de Aragão Umbuzeiro, B. Vrana and M. Krauss, Effect-directed analysis supporting monitoring of aquatic environments – An in-depth overview, Sci. Total Environ., 2016, 544, 1073–1118 CrossRef CAS PubMed.
- A. R. McFarland, L. Larsen, K. Yeshitela, A. N. Engida and N. G. Love, Guide for using green infrastructure in urban environments for stormwater management, Environ. Sci.: Water Res. Technol., 2019, 5, 643–659 RSC.
- B. S. Anderson, B. M. Phillips, J. P. Voorhees, K. Siegler and R. Tjeerdema, Bioswales reduce contaminants associated with toxicity in urban storm water, Environ. Toxicol. Chem., 2016, 35, 3124–3134 CrossRef CAS PubMed.
- N. J. Barron, A. Deletic, J. Jung, H. Fowdar, Y. Chen and B. E. Hatt, Dual-mode stormwater-greywater biofilters: The impact of alternating water sources on treatment performance, Water Res., 2019, 159, 521–537 CrossRef CAS PubMed.
- A. J. Erickson, J. S. Gulliver, W. A. Arnold, C. Brekke and M. Bredal, Abiotic capture of stormwater nitrates with granular activated carbon, Environ. Eng. Sci., 2016, 33, 354–363 CrossRef CAS.
- A. R. M. N. Afrooz and A. B. Boehm, Effects of submerged zone, media aging, and antecedent dry period on the performance of biochar-amended biofilters in removing fecal indicators and nutrients from natural stormwater, Ecol. Eng., 2017, 102, 320–330 CrossRef.
- B. J. Halaburka, G. H. LeFevre and R. G. Luthy, Evaluation of mechanistic models for nitrate removal in woodchip bioreactors, Environ. Sci. Technol., 2017, 51, 5156–5164 CrossRef CAS PubMed.
- A. J. Erickson, J. S. Gulliver and P. T. Weiss, Capturing phosphates with iron enhanced sand filtration, Water Res., 2012, 46, 3032–3042 CrossRef CAS PubMed.
- G.-T. Blecken, Y. Zinger, A. Deletić, T. D. Fletcher and M. Viklander, Influence of intermittent wetting and drying conditions on heavy metal removal by stormwater biofilters, Water Res., 2009, 43, 4590–4598 CrossRef CAS PubMed.
- S. E. Clark and R. Pitt, Targeting treatment technologies to address specific stormwater pollutants and numeric discharge limits, Water Res., 2012, 46, 6715–6730 CrossRef CAS PubMed.
- L. Wiest, R. Baudot, F. Lafay, E. Bonjour, C. Becouze-Lareure, J.-B. Aubin, P. Jame, S. Barraud, G. L. Kouyi, C. Sébastian and E. Vulliet, Priority substances in accumulated sediments in a stormwater detention basin from an industrial area, Environ. Pollut., 2018, 243, 1669–1678 CrossRef CAS PubMed.
- C. Sébastian, C. Becouze-Lareure, G. L. Kouyi and S. Barraud, Event-based quantification of emerging pollutant removal for an open stormwater retention basin – Loads, efficiency and importance of uncertainties, Water Res., 2015, 72, 239–250 CrossRef PubMed.
- C. Sébastian, S. Barraud, C. Gonzalez-Merchan, Y. Perrodin and R. Visiedo, Stormwater retention basin efficiency regarding micropollutant loads and ecotoxicity, Water Sci. Technol., 2014, 69, 974–981 CrossRef PubMed.
- G. Imfeld, M. Braeckevelt, P. Kuschk and H. H. Richnow, Monitoring and assessing processes of organic chemicals removal in constructed wetlands, Chemosphere, 2009, 74, 349–362 CrossRef CAS PubMed.
- J. T. Jasper, M. T. Nguyen, Z. L. Jones, N. S. Ismail, D. L. Sedlak, J. O. Sharp, R. G. Luthy, A. J. Horne and K. L. Nelson, Unit process wetlands for removal of trace organic contaminants and pathogens from municipal wastewater effluents, Environ. Eng. Sci., 2013, 30, 421–436 CrossRef CAS PubMed.
- D. Zhang, R. M. Gersberg, W. J. Ng and S. K. Tan, Removal of pharmaceuticals and personal care products in aquatic plant-based systems: A review, Environ. Pollut., 2014, 184, 620–639 CrossRef CAS PubMed.
- D. Page, P. Dillon, J. Mueller and M. Bartkow, Quantification of herbicide removal in a constructed wetland using passive samplers and composite water quality monitoring, Chemosphere, 2010, 81, 394–399 CrossRef CAS PubMed.
- E. Maillard, S. Payraudeau, E. Faivre, C. Grégoire, S. Gangloff and G. Imfeld, Removal of pesticide mixtures in a stormwater wetland collecting runoff from a vineyard catchment, Sci. Total Environ., 2011, 409, 2317–2324 CrossRef CAS PubMed.
- E. Maillard and G. Imfeld, Pesticide mass budget in a stormwater wetland, Environ. Sci. Technol., 2014, 48, 8603–8611 CrossRef CAS PubMed.
- K. Tondera, S. Koenen and J. Pinnekamp, Survey monitoring results on the reduction of micropollutants, bacteria, bacteriophages and TSS in retention soil filters, Water Sci. Technol., 2013, 68, 1004–1012 CrossRef CAS PubMed.
- K. Tondera, J. P. Ruppelt, J. Pinnekamp, T. Kistemann and C. Schreiber, Reduction of micropollutants and bacteria in a constructed wetland for combined sewer overflow treatment after 7 and 10 years of operation, Sci. Total Environ., 2019, 651, 917–927 CrossRef CAS PubMed.
- K. Zhang, A. Randelovic, D. Page, D. T. McCarthy and A. Deletic, The validation of stormwater biofilters for micropollutant removal using in situ challenge tests, Ecol. Eng., 2014, 67, 1–10 CrossRef.
- W. Feng, B. E. Hatt, D. T. McCarthy, T. D. Fletcher and A. Deletic, Biofilters for stormwater harvesting: Understanding the treatment performance of key metals that pose a risk for water use, Environ. Sci. Technol., 2012, 46, 5100–5108 CrossRef CAS PubMed.
- K. Zhang, V. Valognes, D. Page, A. Deletic and D. McCarthy, Validation of stormwater biofilters using in-situ columns, Sci. Total Environ., 2016, 544, 48–55 CrossRef CAS PubMed.
- K. Flanagan, P. Branchu, L. Boudahmane, E. Caupos, D. Demare, S. Deshayes, P. Dubois, L. Meffray, C. Partibane, M. Saad and M.-C. Gromaire, Retention and transport processes of particulate and dissolved micropollutants in stormwater biofilters treating road runoff, Sci. Total Environ., 2019, 656, 1178–1190 CrossRef CAS PubMed.
- K. Bester, S. Banzhaf, M. Burkhardt, N. Janzen, B. Niederstrasser and T. Scheytt, Activated soil filters for removal of biocides from contaminated run-off and waste-waters, Chemosphere, 2011, 85, 1233–1240 CrossRef CAS PubMed.
- K. Bester and D. Schäfer, Activated soil filters (bio filters) for the elimination of xenobiotics (micro-pollutants) from storm- and waste waters, Water Res., 2009, 43, 2639–2646 CrossRef CAS PubMed.
- C. P. Muerdter, C. K. Wong and G. H. LeFevre, Emerging investigator series: The role of vegetation in bioretention for stormwater treatment in the built environment: Pollutant removal, hydrologic function, and ancillary benefits, Environ. Sci.: Water Res. Technol., 2018, 4, 592–612 RSC.
- L. Paredes, E. Fernandez-Fontaina, J. M. Lema, F. Omil and M. Carballa, Understanding the fate of organic micropollutants in sand and granular activated carbon biofiltration systems, Sci. Total Environ., 2016, 551-552, 640–648 CrossRef CAS PubMed.
- L. Clausen and I. Fabricius, Atrazine, isoproturon, mecoprop, 2,4-D, and bentazone adsorption onto iron oxides, J. Environ. Qual., 2001, 30, 858–869 CrossRef CAS PubMed.
- S. Laha and R. G. Luthy, Oxidation of aniline and other primary aromatic amines by manganese dioxide, Environ. Sci. Technol., 1990, 24, 363–373 CrossRef CAS.
- Y. Song, J. Jiang, J. Ma, Y. Zhou and U. von Gunten, Enhanced transformation of sulfonamide antibiotics by manganese(IV) oxide in the presence of model humic constituents, Water Res., 2019, 153, 200–207 CrossRef CAS PubMed.
- D. Wang, J. Y. Shin, M. A. Cheney, G. Sposito and T. G. Spiro, Manganese dioxide as a catalyst for oxygen-independent atrazine dealkylation, Environ. Sci. Technol., 1999, 33, 3160–3165 CrossRef CAS.
- H. Zhang and C.-H. Huang, Oxidative transformation of fluoroquinolone antibacterial agents and structurally related amines by manganese oxide, Environ. Sci. Technol., 2005, 39, 4474–4483 CrossRef CAS PubMed.
- H. Zhang, W.-R. Chen and C.-H. Huang, Kinetic modeling of oxidation of antibacterial agents by manganese oxide, Environ. Sci. Technol., 2008, 42, 5548–5554 CrossRef CAS PubMed.
- K. A. Barrett and M. B. McBride, Oxidative degradation of glyphosate and aminomethylphosphonate by manganese oxide, Environ. Sci. Technol., 2005, 39, 9223–9228 CrossRef CAS PubMed.
- K. M. Furgal, R. L. Meyer and K. Bester, Removing selected steroid hormones, biocides and pharmaceuticals from water by means of biogenic manganese oxide nanoparticles in situ at ppb levels, Chemosphere, 2015, 136, 321–326 CrossRef CAS PubMed.
- S. Balgooyen, P. J. Alaimo, C. K. Remucal and M. Ginder-Vogel, Structural transformation of MnO2 during the oxidation of bisphenol A, Environ. Sci. Technol., 2017, 51, 6053–6062 CrossRef CAS PubMed.
- Y. Chen, X. Lu, L. Liu, D. Wan, H. Chen, D. Zhou and V. K. Sharma, Oxidation of β-blockers by birnessite: Kinetics, mechanism and effect of metal ions, Chemosphere, 2018, 194, 588–594 CrossRef CAS PubMed.
- C. K. Remucal and M. Ginder-Vogel, A critical review of the reactivity of manganese oxides with organic contaminants, Environ. Sci.: Processes Impacts, 2014, 16, 1247–1266 RSC.
- Y. Zhang, H. Zhu, U. Szewzyk and S. U. Geissen, Removal of pharmaceuticals in aerated biofilters with manganese feeding, Water Res., 2015, 72, 218–226 CrossRef PubMed.
- J. E. Grebel, J. A. Charbonnet and D. L. Sedlak, Oxidation of organic contaminants by manganese oxide geomedia for passive urban stormwater treatment systems, Water Res., 2016, 88, 481–491 CrossRef CAS PubMed.
- J. A. Charbonnet, Y. Duan, C. M. van Genuchten and D. L. Sedlak, Chemical regeneration of manganese oxide-coated sand for oxidation of organic stormwater contaminants, Environ. Sci. Technol., 2018, 52, 10728–10736 CrossRef CAS PubMed.
- Y. Tong, P. J. McNamara and B. K. Mayer, Adsorption of organic micropollutants onto biochar: A review of relevant kinetics, mechanisms and equilibrium, Environ. Sci.: Water Res. Technol., 2019, 5, 821–838 RSC.
- J. J. Pignatello, W. A. Mitch and W. Xu, Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds, Environ. Sci. Technol., 2017, 51, 8893–8908 CrossRef CAS PubMed.
- Biochar for Environmental Management: Science, Technology and Implementation, ed. J. Lehmann and S. Joseph, Routledge, London, 2015 Search PubMed.
- K. A. Thompson, K. K. Shimabuku, J. P. Kearns, D. R. U. Knappe, R. S. Summers and S. M. Cook, Environmental comparison of biochar and activated carbon for tertiary wastewater treatment, Environ. Sci. Technol., 2016, 50, 11253–11262 CrossRef CAS PubMed.
- N. A. Qambrani, M. M. Rahman, S. Won, S. Shim and C. Ra, Biochar properties and eco-friendly applications for climate change mitigation, waste management, and waste-water treatment: A review, Renewable Sustainable Energy Rev., 2017, 79, 255–273 CrossRef CAS.
- W. Gwenzi, N. Chaukura, C. Noubactep and F. N. Mukome, Biochar-based water treatment systems as a potential low-cost and sustainable technology for clean water provision, J. Environ. Manage., 2017, 197, 732–749 CrossRef PubMed.
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Biochar as a sorbent for contaminant management in soil and water: A review, Chemosphere, 2014, 99, 19–33 CrossRef CAS PubMed.
- T. M. Huggins, A. Haeger, J. C. Biffinger and Z. J. Ren, Granular biochar compared with activated carbon for wastewater treatment and resource recovery, Water Res., 2016, 94, 225–232 CrossRef CAS PubMed.
- N. Ashoori, M. Teixido, S. Spahr, G. H. LeFevre, D. L. Sedlak and R. G. Luthy, Evaluation of pilot-scale biochar-amended woodchip bioreactors to remove nitrate, metals, and trace organic contaminants from urban stormwater runoff, Water Res., 2019, 154, 1–11 CrossRef CAS PubMed.
- L. Lu and B. Chen, Enhanced bisphenol A removal from stormwater in biochar-amended biofilters: Combined with batch sorption and fixed-bed column studies, Environ. Pollut., 2018, 243, 1539–1549 CrossRef CAS PubMed.
- S. K. Mohanty, R. Valenca, A. W. Berger, I. K. M. Yu, X. Xiong, T. M. Saunders and D. C. W. Tsang, Plenty of room for carbon on the ground: Potential applications of biochar for stormwater treatment, Sci. Total Environ., 2018, 625, 1644–1658 CrossRef CAS PubMed.
- M. Teixidó, J. J. Pignatello, J. L. Beltrán, M. Granados and J. Peccia, Speciation of the ionizable antibiotic sulfa-methazine on black carbon (biochar), Environ. Sci. Technol., 2011, 45, 10020–10027 CrossRef PubMed.
- W.-J. Liu, H. Jiang and H.-Q. Yu, Development of biochar-based functional materials: Toward a sustainable platform carbon material, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS PubMed.
- L. Klüpfel, M. Keiluweit, M. Kleber and M. Sander, Redox properties of plant biomass-derived black carbon (biochar), Environ. Sci. Technol., 2014, 48, 5601–5611 CrossRef PubMed.
- A. Prévoteau, F. Ronsse, I. Cid, P. Boeckx and K. Rabaey, The electron donating capacity of biochar is dramatically underestimated, Sci. Rep., 2016, 6, 32870 CrossRef PubMed.
- A. Kappler, M. L. Wuestner, A. Ruecker, J. Harter, M. Halama and S. Behrens, Biochar as an electron shuttle between bacteria and Fe(III) minerals, Environ. Sci. Technol. Lett., 2014, 1, 339–344 CrossRef CAS.
- F. J. Chacón, M. L. Cayuela, A. Roig and M. A. Sánchez-Monedero, Understanding, measuring and tuning the electrochemical properties of biochar for environmental applications, Rev. Environ. Sci. Bio/Technol., 2017, 16, 695–715 CrossRef.
- T. Sun, B. D. A. Levin, J. J. L. Guzman, A. Enders, D. A. Muller, L. T. Angenent and J. Lehmann, Rapid electron transfer by the carbon matrix in natural pyrogenic carbon, Nat. Commun., 2017, 8, 14873 CrossRef CAS PubMed.
- P. Quin, S. Joseph, O. Husson, S. Donne, D. Mitchell, P. Munroe, D. Phelan, A. Cowie and L. Van Zwieten, Lowering N2O emissions from soils using eucalypt biochar: The importance of redox reactions, Sci. Rep., 2015, 5, 16773 CrossRef CAS PubMed.
- B. A. Ulrich, E. A. Im, D. Werner and C. P. Higgins, Biochar and activated carbon for enhanced trace organic contaminant retention in stormwater infiltration systems, Environ. Sci. Technol., 2015, 49, 6222–6230 CrossRef CAS PubMed.
- J. P. Kearns, L. S. Wellborn, R. S. Summers and D. R. U. Knappe, 2,4-D adsorption to biochars: Effect of preparation conditions on equilibrium adsorption capacity and comparison with commercial activated carbon literature data, Water Res., 2014, 62, 20–28 CrossRef CAS PubMed.
- J. R. Ray, I. A. Shabtai, M. Teixidó, Y. G. Mishael and D. L. Sedlak, Polymer-clay composite geomedia for sorptive removal of trace organic compounds and metals in urban stormwater, Water Res., 2019, 157, 454–462 CrossRef CAS PubMed.
- J. M. Wolfand, C. Seller, C. D. Bell, Y.-M. Cho, K. Oetjen, T. S. Hogue and R. G. Luthy, Occurrence of urban-use pesticides and management with enhanced stormwater control measures at the watershed scale, Environ. Sci. Technol., 2019, 53, 3634–3644 CrossRef CAS PubMed.
- B. A. Ulrich, M. Loehnert and C. P. Higgins, Improved contaminant removal in vegetated stormwater biofilters amended with biochar, Environ. Sci.: Water Res. Technol., 2017, 3, 726–734 RSC.
- B. A. Ulrich, M. Vignola, K. Edgehouse, D. Werner and C. P. Higgins, Organic carbon amendments for enhanced biological attenuation of trace organic contaminants in biochar-amended stormwater biofilters, Environ. Sci. Technol., 2017, 51, 9184–9193 CrossRef CAS PubMed.
- D. Zadaka, S. Nir, A. Radian and Y. G. Mishael, Atrazine removal from water by polycation-clay composites: Effect of dissolved organic matter and comparison to activated carbon, Water Res., 2009, 43, 677–683 CrossRef CAS PubMed.
- S. K. Mohanty, K. B. Cantrell, K. L. Nelson and A. B. Boehm, Efficacy of biochar to remove Escherichia coli from stormwater under steady and intermittent flow, Water Res., 2014, 61, 288–296 CrossRef CAS PubMed.
- J. Tian, J. Jin, P. C. Chiu, D. K. Cha, M. Guo and P. T. Imhoff, A pilot-scale, bi-layer bioretention system with biochar and zero-valent iron for enhanced nitrate removal from stormwater, Water Res., 2019, 148, 378–387 CrossRef CAS PubMed.
- C. T. Cao, C. Farrell, P. E. Kristiansen and J. P. Rayner, Biochar makes green roof substrates lighter and improves water supply to plants, Ecol. Eng., 2014, 71, 368–374 CrossRef.
- B. G. Greiner, K. K. Shimabuku and R. S. Summers, Influence of biochar thermal regeneration on sulfamethoxazole and dissolved organic matter adsorption, Environ. Sci.: Water Res. Technol., 2018, 4, 169–174 RSC.
- B. R. Zivkovich and D. C. Mays, Predicting nonpoint stormwater runoff quality from land use, PLoS One, 2018, 13, 1–17 CrossRef PubMed.
- S. Spahr, S. Huntscha, J. Bolotin, M. P. Maier, M. Elsner, J. Hollender and T. B. Hofstetter, Compound-specific isotope analysis of benzotriazole and its derivatives, Anal. Bioanal. Chem., 2013, 405, 2843–2856 CrossRef CAS PubMed.
- J. Hollender, E. L. Schymanski, H. P. Singer and P. L. Ferguson, Nontarget screening with high resolution mass spectrometry in the environment: Ready to go?, Environ. Sci. Technol., 2017, 51, 11505–11512 CrossRef CAS PubMed.
- C. M. G. Carpenter, L. Y. J. Wong, C. A. Johnson and D. E. Helbling, Fall Creek Monitoring Station: Highly resolved temporal sampling to prioritize the identification of nontarget micropollutants in a small stream, Environ. Sci. Technol., 2019, 53, 77–87 CrossRef CAS PubMed.
- C. M. G. Carpenter and D. E. Helbling, Widespread micro-pollutant monitoring in the Hudson River Estuary reveals spatio-temporal micropollutant clusters and their sources, Environ. Sci. Technol., 2018, 52, 6187–6196 CrossRef CAS PubMed.
- D. L. Sedlak, R. A. Deeb, E. L. Hawley, W. A. Mitch, T. D. Durbin, S. Mowbray and S. Carr, Sources and fate of nitrosodimethylamine and its precursors in municipal wastewater treatment plants, Water Environ. Res., 2005, 77, 32–39 CrossRef CAS PubMed.
- F. Liu, K. B. Olesen, A. R. Borregaard and J. Vollertsen, Microplastics in urban and highway stormwater retention ponds, Sci. Total Environ., 2019, 671, 992–1000 CrossRef CAS.
- Z. Duan, Y. Xing, Z. Feng, H. Zhang, C. Li, Z. Gong, L. Wang and H. Sun, Hepatotoxicity of benzotriazole and its effect on the cadmium induced toxicity in zebrafish Danio rerio, Environ. Pollut., 2017, 224, 706–713 CrossRef CAS PubMed.
- S. Huntscha, T. B. Hofstetter, E. L. Schymanski, S. Spahr and J. Hollender, Biotransformation of benzotriazoles: Insights from transformation product identification and compound-specific isotope analysis, Environ. Sci. Technol., 2014, 48, 4435–4443 CrossRef CAS PubMed.
- O. F. Elsayed, E. Maillard, S. Vuilleumier, I. Nijenhuis, H. H. Richnow and G. Imfeld, Using compound-specific isotope analysis to assess the degradation of chloroacetanilide herbicides in lab-scale wetlands, Chemosphere, 2014, 99, 89–95 CrossRef CAS PubMed.
Footnote |
| † Current address: Center for Applied Geoscience, University of Tübingen, Tübingen, Germany. |
| This journal is © The Royal Society of Chemistry 2020 |