 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simultaneous ozonation of 90 organic micropollutants including illicit drugs and their metabolites in different water matrices†
Garyfalia A.
Zoumpouli
 abc,
Fernanda
Siqueira Souza
bcde,
Bruce
Petrie
abc,
Fernanda
Siqueira Souza
bcde,
Bruce
Petrie
 cfg,
Liliana Amaral
Féris
d,
Barbara
Kasprzyk-Hordern
cfg,
Liliana Amaral
Féris
d,
Barbara
Kasprzyk-Hordern
 cf and
Jannis
Wenk
cf and
Jannis
Wenk
 *bc
*bc
aCentre for Doctoral Training, Centre for Sustainable Chemical Technologies, University of Bath, BA2 7AY, UK
bDepartment of Chemical Engineering, University of Bath, Bath BA2 7AY, UK. E-mail: j.h.wenk@bath.ac.uk
cWater Innovation & Research Centre (WIRC), University of Bath, Bath BA2 7AY, UK
dFederal University of Rio Grande do Sul, Department of Chemical Engineering, Porto Alegre, Brazil
eLa Salle University, 2288 Victor Barreto Av, 92010-000 Canoas, Rio Grande do Sul, Brazil
fDepartment of Chemistry, University of Bath, Bath, BA2 7AY, Bath, UK
gSchool of Pharmacy and Life Sciences, Robert Gordon University, Aberdeen AB10 7GJ, UK
First published on 29th April 2020
Abstract
The ozonation of 90 chemically diverse organic micropollutants (OMPs) including four classes of illicit drugs and their metabolites was studied in pure buffered water, tap water and wastewater effluent at three specific ozone doses and three pH levels. The second order rate constants for the reaction of 40 OMPs with ozone were known and span across 8 orders of magnitude, from below 1 M−1 s−1 to above 107 M−1 s−1. 47 of the tested OMPs were removed to at least 90% at the highest specific ozone dose of 0.3 mM O3 per mM C at pH 7. However, most illicit drugs, including cocainics, amphetamines and ecstasy-group compounds, were ozone-resistant due to their lack of ozone-reactive functional groups. Exceptions included some opioids and the cocaine biomarker anhydroecgonine methylester which contain olefinic bonds and/or activated benzene rings. Different removal trends at different pH for OMPs were due to the combined effect of target compound speciation and ozone stability, leading to elimination of less than 70% for all OMPs at pH 11. In both tap water and wastewater effluent scavenging by matrix components led to lower ozone exposure compared to pure buffered water and consequently lower removal of OMPs. This multi-compound ozonation study utilised liquid chromatography-mass spectrometry to provide a large dataset on the removal of environmentally relevant OMPs, including those of interest for drinking water regulations. Besides including pharmaceutically active compounds that have not been studied with ozone before (e.g. gliclazide, anhydroecgonine methylester, quetiapine, 6-monoacetylmorphine), this study simultaneously shows ozonation data for a wide range of illicit drugs.
Water impactOzonation is a promising technology for the removal of organic micropollutants from water. Here, ozonation results for 90 chemically diverse micropollutants including illicit drugs are reported and interpreted based on compound chemical structure. The study provides a valuable ozonation database for a large variety of micropollutants with specific focus on occurrence and ozonation of illicit drugs in drinking water. |
1. Introduction
Many different organic micropollutants (OMPs) including pharmaceuticals, personal care products, hormones and their transformation products can be found at trace concentrations in surface water, groundwater and finished drinking water.1–4 OMPs may reach drinking water resources through numerous routes, with their main sources being the discharge of wastewater effluent and diffuse pollution, such as agricultural and urban runoff.5,6 OMPs have raised scientific and public concern regarding their impact on the environment and on human health, including short-term and long-term toxicity, endocrine disruption, antibiotic resistance of microorganisms and accumulation in soils, plants and animals.7,8 A group of OMPs of particular interest are illicit drugs and their metabolites,9–12 due to biological activity and largely unknown effects on the environment and on water quality.13,14Ozonation is among the most effective methods for the abatement of OMPs in full-scale water treatment applications.15 Ozone is a strong oxidant which reacts with organic compounds in water either directly, or indirectly through free radicals produced from ozone decomposition.16 The ozonation of single compounds has been extensively studied in terms of degradation, reaction kinetics and identification of transformation products.17–20 Analytical advancements have also enabled the investigation of the simultaneous ozonation of mixtures of OMPs. Multi-component ozonation studies have been performed at lab-, pilot- and full-scale and have included a wide range of compounds.21–25 However, the ozonation of some classes of OMPs, including illicit drugs and their metabolites, remains less conclusively studied.11,26–28
The reactivity of organic compounds with ozone depends on their chemical structure, with second order rate constants reaching across several orders of magnitude.29 Kinetic parameters of ozonation reactions can be determined experimentally or calculated through QSAR (quantitative structure–activity relationship) models.30 In complex water matrices, such as surface water, the properties of the matrix affect the stability of dissolved ozone, while matrix components act as oxidant scavengers, increasing the required ozone dose for a desired extent of OMP abatement. Therefore, the abatement of OMPs by ozonation can be related to kinetic parameters, operational parameters (e.g. ozone dose, temperature) and water quality parameters (e.g. organic carbon concentration, pH, alkalinity).31,32
The aim of this study was to gain insights into the simultaneous ozonation of 90 chemically diverse OMPs. The selection of the compounds was based on existing and proposed EU legislation, UK prescription data, metabolism and excretion from the human body, known environmental occurrence, persistence during wastewater treatment and toxicity to aquatic organisms.33 Ozonation experiments were conducted in three different water matrices (pure buffered water, tap water and wastewater effluent), at different ozone doses and pH levels. In contrast to the majority of previous ozonation studies, several illicit drugs and illicit drug metabolites were investigated. For some compounds, the reactivity with ozone in water is investigated for the first time, including the diabetes drug gliclazide, the cocaine biomarker anhydroecgonine methylester, the antipsychotic drug quetiapine and the heroin metabolite 6-monoacetylmorphine (O-6-MAM).
2. Materials and methods
Chemicals
OMPs were either purchased dissolved in 0.1 or 1.0 mg mL−1 solutions or as powder. Stock solutions from powders were prepared at 1 mg mL−1 in either acetonitrile or methanol and stored in the dark at −20 °C. All aqueous solutions were made in ultrapure water (Milli-Q, Millipore, USA). Chemicals and solvents (purity 95% or higher) were used as received from various commercial suppliers. Methanol, ammonium acetate (NH4OAc), ammonium fluoride (NH4F) and acetic acid (CH3COOH) for chromatographic analysis (all HPLC grade), phosphoric acid (H3PO4), disodium phosphate (Na2HPO4), monosodium phosphate (NaH2PO4) and sodium tetraborate (Na2B4O7) were obtained from either Sigma-Aldrich or Fisher Scientific, sodium thiosulfate (Na2S2O3) from Merck, sodium hydroxide (NaOH) from PanReac.Table 1 provides a list of the 90 OMPs studied, including information about their estimated or known ozone reactivity. The referenced studies consist of both mechanistic single-compound studies and multi-compound studies. Table S1† provides CAS number, molecular weight, formula, structure, and instrument detection and quantification limit for each compound. Table S2† provides second order rate constants for the reactions of the compounds with OH radicals, when available.
| Chemical | Mode of action/use | pKa (most acidic) | pKa (most basic) | Ozone-(non) reactive functional groups | k O3 (M−1 s−1) at pH 7 or estimated ozone reactivity | Literature |
|---|---|---|---|---|---|---|
| 1,7 Dimethylxanthine | Human indicator | 8.5 | 0.2 | Amide, imidazole | Medium | 34 |
| 10,11 Dihydro 10 hydroxycarbamazepine | Anti-epileptic metabolite | 13.8 | −0.5 | Amide, benzene ring | Low | |
| Acetaminophen | NSAID | 9.9 | 1.7 | Benzene ring | 4.1 × 106 | 35 |
| Amphetamine | Stimulant | — | 9.9 | (Protonated) amine, benzene ring | Low | 27 |
| Anhydroecgonine methylester | Stimulant metabolite | — | 8.0 | Olefin, (protonated) amine | High | |
| Atenolol | Beta-blocker | 13.9 | 9.4 | Amide, (protonated) amine, benzene ring | 1.7 × 103 | 36 |
| Atorvastatin | Lipid regulator | 4.3 | 0.4 | Benzene ring | Medium | |
| Azathioprine | Anti-cancer | — | 7.5 | (Deactivated) thioether, imidazole | Low | 37 |
| Azithromycin | Antibiotic | 13.3 | 8.6 | (Protonated) amine | 1.1 × 105 | 38 |
| Benzophenone-1 | UV filter | 7.7 | — | Benzene ring | High | |
| Benzophenone-2 | UV filter | 7.0 | — | Benzene ring | High | 39 |
| Benzophenone-3 | UV filter | 7.6 | — | Benzene ring | 6.9 × 105 | 40 |
| Benzophenone-4 | UV filter | −0.7 | — | Benzene ring | Medium | 41 |
| Benzoylecgonine | Stimulant metabolite | 3.4 | 10.8 | (Protonated) amine, deactivated benzene ring | Low | 11, 27 |
| Bezafibrate | Lipid regulator | 3.3 | −2.1 | Amide, benzene ring | 590 | 18, 42 |
| Bisphenol A | Plasticizer | 10.3 | — | Phenol | 1.1 × 106 | 43 |
| Butylparaben | Parabens | 8.2 | — | Phenol | 7.9 × 107 | 44 |
| Caffeine | Human indicator | — | 0.5 | Amide, imidazole | 673 | 23, 45 |
| Carbamazepine | Anti-epileptic | 13.9 | −0.5 | Olefin, amide, benzene ring | 3 × 105 | 18, 46 |
| Carbamazepine 10,11 epoxide | Anti-epileptic metabolite | 13.9 | −0.5 | Amide, benzene ring | Low | 24 |
| Cetirizine | Antihistamine | 3.5 | 6.7 | (Protonated) amine, benzene ring | 1.7 × 105 | 47 |
| Cimetidine | H2 receptor antagonists | 14.1 | 7.1 | Thioether, amidine, imidazole | High | |
| Citalopram | Anti-depressant | — | 9.6 | (Protonated) amine, deactivated benzene ring | Low | 48, 49 |
| Clarithromycin | Antibiotic | 13.1 | 8.2 | (Protonated) amine | 7 × 104 | 50 |
| Cocaethylene | Stimulant metabolite | — | 9.0 | (Protonated) amine, deactivated benzene ring | Low | |
| Cocaine | Stimulant | — | 9.0 | (Protonated) amine, deactivated benzene ring | Low | 11, 27 |
| Codeine | Analgesic | 13.4 | 8.2 | Olefin, (protonated) amine, benzene ring | High | 24 |
| Cotinine | Human indicator | — | 4.7 | Amide, pyridine | Low | 11, 24 |
| Creatinine | Human indicator | — | 6.9 | Amide, (protonated) amine | 2 | 51 |
| Desmethylcitalopram | Anti-depressant metabolite | — | 10.5 | (Protonated) amine, deactivated benzene ring | Low | 48 |
| Desmethylvenlafaxine | Anti-depressant metabolite | 10.0 | 9.3 | Phenol, (protonated) amine | High | 49 |
| Diclofenac | NSAID | 4.9 | −2.3 | Aniline | 1 × 106 | 18, 52 |
| Dihydrocodeine | Analgesic | 14.2 | 8.4 | (Protonated) amine, benzene ring | Medium | |
| Dihydromorphine | Analgesic metabolite | 9.6 | 8.4 | (Protonated) amine, benzene ring | High | |
| Diltiazem | Calcium channel blocker | — | 8.9 | (Protonated) amine, benzene ring | High | |
| E1 | Steroid estrogen | 10.3 | — | Phenol | 9.4 × 105 | 43, 53 |
| E2 | Steroid estrogen | 10.3 | — | Phenol | 2.2 × 106 | 43, 53 |
| EDDP | Analgesic metabolite | — | 7.7 | Olefin, (protonated) amine, benzene ring | High | 28 |
| EE2 | Steroid estrogen | 10.2 | — | Phenol | 2.3 × 106 | 43 |
| Ephedrine/pseudoephedrine | Drug precursor | 14.0 | 9.4 | (Protonated) amine, benzene ring | Low | |
| Ethylparaben | Parabens | 8.3 | — | Phenol | 5.5 × 107 | 44 |
| Fexofenadine | Antihistamine | 4.4 | 9.4 | (Protonated) amine, benzene ring | 9.0 × 103 | 47 |
| Fluoxetine | Anti-depressant | — | 10.1 | (Protonated) amine, benzene ring | 1.6 × 104 | 54 |
| Gliclazide | Diabetes | 6.1 | 3.9 | Amide, (protonated) amine, deactivated benzene ring | High | |
| Heroin | Opioid | — | 7.9 | Olefin, (protonated) amine, benzene ring | High | |
| Ibuprofen | NSAID | 4.4 | — | Benzene ring | 9.6 | 18 |
| Ifosfamide | Anti-cancer | — | 1.4 | Phosphamide | <1 (QSAR) | 55 |
| Iopromide | X-ray contrast media | 10.6 | −2.6 | Amide, deactivated benzene ring | <0.8 | 18 |
| Irbesartan | Hypertension | 4.2 | 2.6 | Amide, (protonated) amine, benzene ring | 24 | 25 |
| Ketamine | Anaesthetic | — | 6.5 | (Protonated) amine, deactivated benzene ring | Medium | 27 |
| Ketoprofen | NSAID | 4.2 | — | Deactivated benzene ring | 0.40 | 56 |
| Lisinopril | Hypertension | 2.2 | 10.5 | (Protonated) amine, benzene ring | Low | |
| MDA | Stimulant | — | 10.0 | (Protonated) amine, anisole | High | |
| MDMA | Stimulant | — | 10.3 | (Protonated) amine, anisole | High | 11, 27 |
| MDPV | Stimulant | — | 8.4 | (Protonated) amine, anisole | Medium | |
| Mephedrone | Stimulant | — | 7.4 | (Protonated) amine, deactivated benzene ring | Medium | |
| Metformin | Diabetes | — | 12.3 | (Protonated) amine | 1.2 | 19 |
| Methadone | Analgesic | — | 9.5 | (Protonated) amine, benzene ring | Low | 28 |
| Methamphetamine | Stimulant | — | 10.4 | (Protonated) amine, benzene ring | Low | 27 |
| Methotrexate | Anti-cancer | 3.5 | 5.6 | Aniline, amide, (protonated) amine | High | 57 |
| Methylparaben | Parabens | 8.3 | — | Phenol | 4.8 × 107 | 44 |
| Metoprolol | Beta-blocker | 13.9 | 9.4 | (Protonated) amine, benzene ring | 2.0 × 103 | 36 |
| Mirtazapine | Anti-depressant | — | 8.1 | (Protonated) amine, benzene ring, pyridine | Medium | 49 |
| Morphine | Analgesic | 9.5 | 8.3 | Olefin, (protonated) amine, benzene ring | 6.4 × 106 (QSAR) | 55 |
| Naproxen | NSAID | 4.8 | — | Naphthalene | 2 × 105 | 58 |
| N-Desmethyltramadol | Analgesic metabolite | 14.5 | 10.6 | (Protonated) amine, anisole | Medium | |
| Nicotine | Human indicator | — | 8.0 | (Protonated) amine, pyridine | Medium | 11, 24 |
| Norcodeine | Analgesic metabolite | 13.3 | 9.3 | Olefin, (protonated) amine, benzene ring | High | 26 |
| Norephedrine | Stimulant metabolite | 12.1 | 8.5 | (Protonated) amine, benzene ring | Low | |
| Norfluoxetine | Anti-depressant metabolite | — | 9.1 | (Protonated) amine, benzene ring | 65 | 54 |
| Norketamine | Anaesthetic metabolite | — | 6.3 | (Protonated) amine, deactivated benzene ring | Medium | |
| Normorphine | Analgesic metabolite | 9.2 | 9.5 | Olefin, (protonated) amine, benzene ring | High | |
| O-6-MAM | Opioid metabolite | 9.4 | 8.0 | Olefin, (protonated) amine, benzene ring | High | |
| O-Desmethyltramadol | Analgesic metabolite | 10.0 | 9.6 | (Protonated) amine, phenol | High | |
| Pholcodine | Cough suppressant | 13.4 | 8.2 | Olefin, (protonated) amine, benzene ring | High | |
| Propranolol | Beta-blocker | 13.8 | 9.5 | Naphthalene, (protonated) amine | 1 × 105 | 36 |
| Propylparaben | Parabens | 8.2 | — | Phenol | 7.0 × 107 | 44 |
| Quetiapine | Anti-psychotic | 14.4 | 6.7 | Amidine, benzene ring, thioether | High | |
| Ranitidine | H2 receptor antagonists | — | 8.4 | Amidine, furan, thioether | 2.1 × 106 | 59 |
| Sertraline | Anti-depressant | — | 9.5 | (Protonated) amine, benzene ring | Medium | 49 |
| Sulfamethoxazole | Antibiotic | 5.8 | 1.4 | Aniline, sulfonamide | 2.6 × 106 | 18, 60 |
| Sulfasalazine | Antibacterial | 2.7 | 0.9 | Benzene ring, sulfonamide | High | |
| Tamoxifen | Anti-cancer | — | 8.7 | Olefin, benzene ring | High | 61 |
| Temazepam | Hypnotic | 11.7 | 1.6 | Amide, deactivated benzene ring | Low | 62 |
| Tramadol | Analgesic | 14.5 | 9.6 | (Protonated) amine, anisole | 2.2 × 103 | 63 |
| Triclosan | Antibacterial | 7.8 | — | Benzene ring | 3.8 × 107 | 64, 65 |
| Trimethoprim | Antibiotic | — | 7.0 | (Protonated) amine, pyrimidine, benzene ring | 2.7 × 105 | 38, 66 |
| Tylosin | Veterinary | 13.1 | 7.4 | Olefin, (protonated) amine | 5.1 × 105 | 38 |
| Valsartan | Hypertension | 3.6 | 0.6 | Amide, benzene ring | 38 (QSAR) | 55, 67 |
| Venlafaxine | Anti-depressant | 14.8 | 9.3 | (Protonated) amine, anisole | 1.3 × 103 | 49, 68 |
Ozonation experiments
All reactions were conducted in 10 mL glass flasks. Freshly prepared methanol stock solution containing all 90 compounds at equal mass concentration was spiked into empty flasks. The solvent was evaporated under a gentle stream of nitrogen followed by re-dissolution with the aqueous phase, which consisted of either buffered ultrapure water at pH 3 (10 mM H3PO4/H2NaPO4), pH 7 (10 mM H2NaPO4/HNa2PO4) or pH 11 (10 mM H3BO3), tap water (total organic carbon (TOC) 1.5 mg C L−1, pH 7.5) or secondary wastewater effluent (TOC 7.1 mg C L−1, pH 7.8) from a wastewater treatment plant in the Southwest of England. The concentration of each OMP in the final reaction solution was approximately 100 μg L−1, which translated into a TOC of 6 mg C L−1 added to the TOC of the matrix. A high initial concentration of each OMP was chosen to avoid an analyte concentration step prior to LC-MS (liquid chromatography coupled with tandem mass spectrometry) analysis.Ozone was produced with a BMT 803 N ozone generator (BMT Messtechnik, Berlin, Germany). Stock solutions (1.3–1.5 mM, 62–72 mg L−1) were made by sparging ozone gas through ultrapure water (≤4 °C) that was cooled in an ice bath. The dissolved ozone concentration of stock solutions was quantified directly spectrophotometrically using a molar absorption coefficient of ε = 3000 M−1 cm−1 at an absorption wavelength of λ = 258 nm.69
The ozone stock solution was added under vigorous stirring to each flask to achieve ozone doses on a carbon basis of 0.05 mM O3 per mM C (0.2 g O3 per g C), 0.15 mM O3 per mM C (0.6 g O3 per g C) and 0.3 mM O3 per mM C (1.2 g O3 per g C), to cover the range used for water treatment. Specific ozone doses on a molar basis are hereafter used. After 5 min reaction time, the samples were quenched with 0.1 M sodium thiosulfate (Na2S2O3) and analysed within 24 h.
Analytical methods
A detailed description of the analytical method used for the OMPs can be found elsewhere.33 Briefly, the target compounds were analysed by liquid chromatography-tandem mass spectrometry (LC-MS) using a Waters Acquity UPLC system (Waters, Manchester, UK) coupled to a Xevo TQD (Triple Quadrupole Mass Spectrometer, Waters, Manchester, UK) equipped with an electrospray ionisation source. The determination of acidic and basic compounds was performed in negative and positive ionisation mode, respectively. Limits of quantification and detection for individual analytes are presented in Table S1.† Each sample was analysed in duplicate. Method performance is described in detail elsewhere.33Total organic carbon was analysed with a Shimadzu TOC-VCPN Analyzer (Shimadzu, Kyoto, Japan). Spectroscopic measurements were conducted with a Cary 100 UV–Vis Spectrometer (Agilent Technologies, Santa Clara, California, USA).
Ozone and OH radical exposures
The exposure (time-integrated concentration) of OH radicals was estimated from the elimination percentage of ketoprofen (KET). Ketoprofen was selected because it is the compound with the lowest ozonation second order rate constant (0.4 M−1 s−1) among the compounds included in this study (see Table 1). Additionally, ketoprofen has a known and high second order rate constant for its reaction with OH radicals (see Table S2). Therefore, its reaction with ozone can be considered negligible, while the OH radical exposure was calculated based on eqn (1):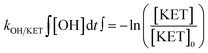 | (1) |
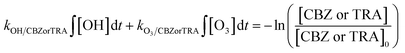 | (2) |
3. Results and discussion
Abatement by ozonation of organic micropollutants including illicit drugs added to pure water at pH 7
An overview of the elimination of the 90 OMPs by ozonation in pure buffered water at three different pH values and at three specific ozone doses is shown in Fig. 1. As expected by the chemical diversity of the OMPs (see Table 1 and Table S1†), the results range from no removal to complete removal. At the highest ozone dose of 0.3 mM O3 per mM C and at pH 7 almost half of all compounds were removed to below the limit of detection. The medium ozone dose of 0.15 mM O3 per mM C at pH 7 led to 80% or higher removal for more than a third of compounds. At the lowest ozone dose of 0.05 mM O3 per mM C at pH 7 partial removal occurred for most compounds.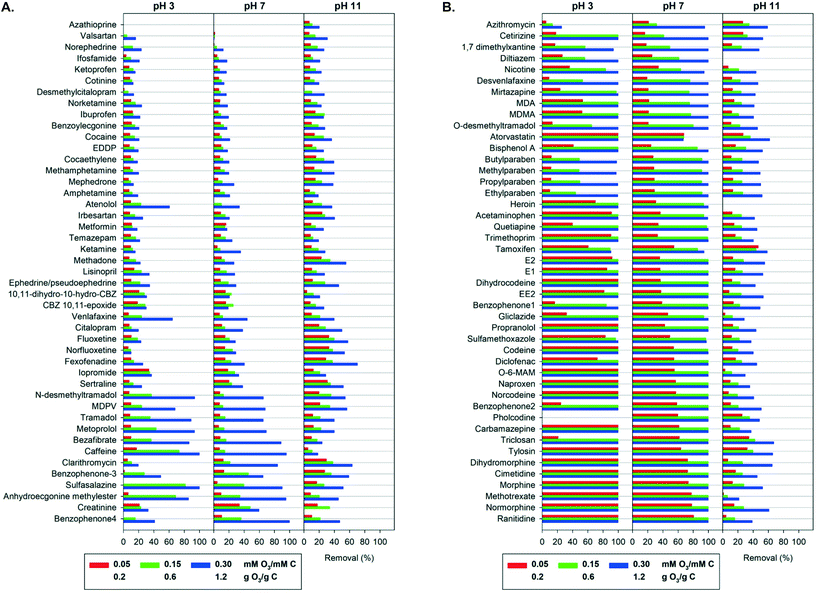 | ||
| Fig. 1 A. Simultaneous removal of 90 organic micropollutants added to pure buffered water as a function of the specific ozone dose and the pH (arranged with increasing average removal at pH 7). Error bars from duplicate analysis of samples were omitted for figure overview and are provided in the ESI† xlsx-data file. B. Simultaneous removal of 90 organic micropollutants added to pure buffered water as a function of the specific ozone dose and the pH (arranged with increasing average removal at pH 7). Error bars from duplicate analysis of samples were omitted for figure overview and are provided in the ESI† xlsx-data file. | ||
The OMPs may be classified into three groups according to their attenuation at the highest specific ozone dose at pH 7: group I compounds were readily removed by more than 90%, group II compounds had a moderate removal of 50 to 90% and group III compounds were hard to remove with less than 50% removal. Group I consisted of 47 (52%) of the tested compounds, 10 compounds (11%) were in group II, while 33 (37%) were in group III. Similar classifications of OMPs have been used in previous studies, with comparable elimination observed in municipal and hospital wastewater effluent at the same specific ozone doses.32,55 However, it should be noted that high concentrations of OMPs in waters with a low scavenger concentration (in this case pure buffered water) may affect the ozone and OH radical exposures,70 and therefore the observed OMP elimination (see also below discussion on ozone and OH radicals exposures).
Group III included most illicit stimulants, antidepressants and their metabolites. These compounds exhibit no functional groups that are readily reactive with ozone. As an electrophile, ozone reacts selectively with electron-rich moieties, such as neutral amines, activated benzene rings and olefins.16 Compounds in group III include deactivated benzene rings (e.g. ketoprofen, cocaine), amides (e.g. cotinine, ifosfamide) and protonated amines (e.g. citalopram, metformin), which have second order rate constants with ozone <10 M−1 s−1 (see Table 1). Their elimination can be attributed to reaction with less selective OH radicals. The OH radical second order rate constants (kOH) of most OMPs vary by only one order of magnitude, between 109 M−1 s−1 and diffusion-controlled values of 1010 M−1 s−1 (see Table S2). Group III compounds can be more effectively attenuated with advanced oxidation processes (AOPs) that aim to increase the concentration of OH radicals, such as the peroxone process (O3/H2O2) or ultraviolet (UV) light combined with hydrogen peroxide (UV/H2O2).15
Few compounds such as the carbamazepine metabolites carbamazepine-10,11-epoxide and 10,11-dihydro-10-hydroxycarbamazepine, exhibited unclear elimination trends with increasing ozone dose, which may be ascribed to simultaneous degradation and formation from the oxidation of structurally similar compounds. Azathioprine had the lowest removal of all compounds in this study, and there is only limited information about its ozone reactivity in the literature.37
Most antibacterial agents and antibiotics, analgesics and their metabolites, UV filters, parabens and steroid estrogens belong to Group I and exhibit high elimination with ozone. Group I compounds contain moieties known to react fast with ozone: activated benzene rings, such as phenols (e.g. methylparaben, estrone, bisphenol A) and anilines (e.g. methotrexate, diclofenac), amines (e.g. mirtazapine, gliclazide), olefins (e.g. morphine, pholcodine) and thioethers (e.g. ranitidine). Note that several compounds contain more than one ozone-reactive sites.
The illicit drugs and illicit drug metabolites included in this study fall into four categories: opioids (heroin, O-6-MAM, morphine, normorphine, dihydromorphine, methadone, EDDP), cocainics (cocaine, cocaethylene, benzoylecgonine, anhydroecgonine methylester), amphetamine-type (amphetamine, methamphetamine, mephedrone, norephedrine, ephedrine/pseudoephedrine [a precursor]) and ecstasy group (MDMA, MDA, MDPV). Fig. 2 provides an overview on the elimination of the four substance categories at five different specific ozone doses in pure buffered water at pH 7.
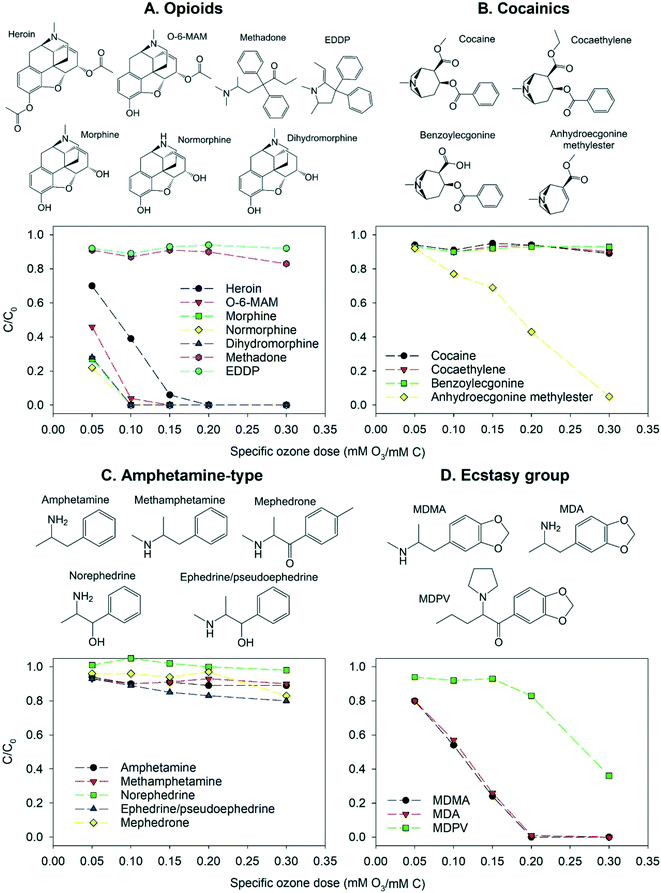 | ||
| Fig. 2 Abatement of illicit drugs and their metabolites as a function of the specific ozone dose in pure buffered water at pH 7. All compounds were added as a mixture of 90 OMPs in total. Error bars from duplicate analysis of samples were omitted for figure overview and are provided in the ESI† xlsx-data file. | ||
Five of the opioids (heroin, O-6-MAM, morphine, normorphine, dihydromorphine) have a similar molecular structure. They contain an activated benzene ring (phenol or anisole), a tertiary or secondary amine (pKa = 7.9–9.6) and, apart from dihydromorphine, a carbon double bond. These opioids are efficiently removed by ozonation at pH 7. Second order rate constants for reactions of opioids with ozone have not been determined experimentally, while for morphine the rate constant has been estimated with a QSAR approach as 6.4 × 106 M−1 s−1.55 Second order rate constants of other structurally similar opioids can be expected to be close to this value. Since dihydromorphine appears to have the same ozone reactivity as morphine, the primary site of ozone attack at pH 7 is likely the activated benzene ring rather than the olefinic bond. In contrast, methadone and its metabolite EDDP were both poorly removed by ozonation at pH 7, despite EDDP having a carbon double bond. Only partial removal of these two compounds has been observed in waterworks employing different treatment methods, while trace concentrations of both compounds have been detected in finished drinking water.28,71
Cocaine and two of its metabolites (cocaethylene and benzoylecgonine) have similar structures containing a deactivated benzene ring (carbonyl-substituted) and a protonated amine (pKa = 9–10.8). As a result, their reactivity with ozone is low and minimal removal at pH 7 was observed. Cocaine has been shown to be more ozone reactive than benzoylecgonine,27 which was not observed in this study, due to the very low removal of both compounds. These three cocainics have been found as traces in tap water of different countries.71,72 In contrast, anhydroecgonine methylester (a biomarker for the use of crack cocaine) contains an olefinic bond and has a lower pKa of 8. Accordingly, as shown in Fig. 2, this compound has a much higher ozonation removal than the other compounds in this category.
The amphetamine-type compounds contain a deactivated or slightly activated benzene ring and an amine (pKa = 7.4–10.4). Fig. 2 shows that all amphetamine-type compounds were ozone-resistant at pH 7. Mephedrone and methamphetamine have been detected in drinking water samples from the UK, which had undergone treatment including ozonation.12 Methamphetamine is reported to be more ozone-reactive than amphetamine due to the presence of a secondary rather than a primary amine.27 This was not observed in this study due to the very low removal of both compounds under the employed conditions. However, this effect could be observed for ephedrine/pseudoephedrine which had a higher elimination than norephedrine.
Drugs of the ecstasy group contain a benzene ring activated by two anisole substituents, and an amine with pKa of 8.4–10.3 (primary-MDA, secondary-MDMA, tertiary-MDPV). The main reactive site is expected to be the benzene ring leading to high removal. MDA and MDMA differ by only one methyl group attached to the amine and showed the same ozone reactivity, while MDPV contains an additional carbonyl substituent on the benzene ring, inducing partial deactivation and lower reactivity. MDMA has been detected in surface water and was only partly removed during the ozonation step of drinking water production.11
Effect of pH on micropollutant abatement by ozone in pure buffered water
Changes in pH strongly affect ozone chemistry in water. An elevated pH leads to faster ozone decay due to two phenomena: hydroxide ions initiate the chain reaction of ozone decomposition and at the same time electrophilic ozone reacts faster with deprotonated or dissociated species of the dissolved organic matter.73,74 In the experimental system of this study the latter phenomenon is expected to be more important due to the increased concentrations of OMPs. Deprotonated alkylamines (typical pKa = 9–11) have up to six orders of magnitude higher reactivity with ozone than the protonated species.29 The second order rate constant for the reaction of ozone with dissociated phenolic compounds is five orders of magnitude higher compared to the corresponding non-dissociated species.30 Despite lower ozone exposure at higher pH, the OH radical exposure remains roughly constant with pH in natural waters.73The estimated ozone and OH radical exposures in pure buffered water under each set of conditions are shown in Table 2 (tap water and wastewater effluent are discussed in the next section). At a given specific ozone dose, the ozone exposure increased by two orders of magnitude as the pH decreased by 4 units. The OH radical exposure remained roughly constant within the uncertainty of the employed estimation method (approximately accurate within an order of magnitude). The ozone exposure values at pH 3 and 7 were of the same order of magnitude as those measured in natural waters,73 while those at pH 11 were lower and accompanied by slightly higher OH radical exposures. It should be noted that samples were quenched of residual ozone after 5 minutes of reaction, which may have resulted in lower ozone exposure than the maximum possible. The ratio of OH radical exposure to ozone exposure, i.e. the Rct value,75 was in the range of 10−4 to 10−10 across the three pH levels.
| Specific ozone dose (mM O3 per mM C) | OH radical exposure (M s) | Ozone exposure (M s) | ||||
|---|---|---|---|---|---|---|
| 0.05 | 0.15 | 0.30 | 0.05 | 0.15 | 0.30 | |
| Buffered at pH 3 | 7 × 10−12 | 9 × 10−12 | 6 × 10−12 | 3 × 10−4 | 4 × 10−3 | 3 × 10−2 |
| Buffered at pH 7 | 4 × 10−12 | 3 × 10−12 | 8 × 10−12 | 3 × 10−6 | 4 × 10−5 | 4 × 10−4 |
| Buffered at pH 11 | 8 × 10−12 | 1 × 10−11 | 2 × 10−11 | 6 × 10−8 | 3 × 10−7 | 7 × 10−7 |
| Tap water | 1 × 10−13 | 6 × 10−12 | 1 × 10−11 | 5 × 10−7 | 1 × 10−6 | 5 × 10−6 |
| Wastewater effluent | 1 × 10−11 | 7 × 10−12 | 2 × 10−11 | 3 × 10−7 | 1 × 10−6 | 3 × 10−6 |
The combined effect of different ozone exposure and target compound speciation has led to different removal trends among the 90 OMPs (Fig. 1). The amines fluoxetine (pKa = 10.1) and sertraline (pKa = 9.5) were better removed at higher pH due to deprotonation. In contrast, the four parabens (phenols with pKa of 8.2 to 8.3) followed a distinct trend: their removal increased with a change of pH from 3 to 7 (due to increased dissociation of the phenols which enhanced their ozone reactivity) and then decreased at pH 11 (due to lower ozone exposure). The four benzophenones followed the same trend. However, the removal of the phenolic hormones E1, E2 and EE2 and the plasticizer bisphenol A decreased with higher pH, indicating that the increased reactivity of the dissociated form was outweighed by the lower ozone exposure. For olefins, such as carbamazepine and tamoxifen, a sharp drop of removal was observed at pH 11. In these cases, the effect of the pH is only due to the different ozone and OH radical exposures.
The effect of the pH on the ozonation of illicit drugs and their metabolites was also examined. Four of the opioids with structure similar to morphine have a phenolic moiety with pKa > 9. However, the effect of the pH change on their removal seems to be mainly due to the different ozone exposure rather than the dissociation of the phenolic moiety. Decreased elimination was observed with an increase of pH from 3 to 7 but only at the lowest ozone dose. At pH 11 removals were markedly lower than those at pH 3 and 7, with the highest one being 61% for dihydromorphine and the lowest being 21% for O-6-MAM. In contrast, methadone was better removed at higher pH due to deprotonation of its amine moiety (pKa = 9.5) and reached 50% removal at pH 11 with the highest ozone dose. The removal of EDDP also slightly increased with pH but remained poor (<20%) under all conditions.
Cocaine, cocaethylene and benzoylecgonine showed enhanced removal at pH 11, since their main ozone-reactive moiety is an amine (pKa = 9–10.8). Despite this increase, their removal was still below 35%. The fourth compound of the cocainics class, anhydroecgonine methylester, is an olefin and showed decreased elimination at pH 11 due to lower ozone exposure. The amphetamine-type compounds were ozone-resistant at all pH values (removal below 35%), but an increase of removal was observed at pH 11 due to deprotonation of the amine (pKa = 7.4–10.4). The removal of MDA and MDMA decreased at higher pH due to the lower ozone exposure, as their main ozone-reactive site is an activated benzene ring. The less reactive MDPV showed a slight increase of removal at pH 11, indicating that the amine (pKa = 8.4) plays a more important role in its reaction with ozone due to partial deactivation of its benzene ring.
An overview of the complete dataset is presented as box and whisker plots in Fig. 3. Since a similar broad range of compounds can be expected in real water matrices, such as river water,33 the box and whisker plots provide a rough estimation on ozonation performance for multi-compound mixtures. Overall, the optimal pH for the elimination of the selected OMPs was 3 and 7. At pH 3 higher removal compared to pH 7 was observed at the lowest ozone dose, while the removal was similar at the other two applied ozone doses. Ozonation at pH 11 was ineffective and would require higher ozone doses to yield results like those of the lower pH values. The only compounds whose removal improved at pH 11 were Group II and III compounds, including amines with pKa > 7. Typical pH for ozonation in treatment practice is 7 to 8.5.
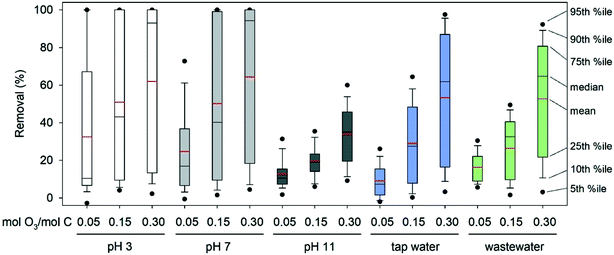 | ||
| Fig. 3 Box and whisker plots of the removal of the 90 OMPs under the different conditions used in this study. %ile: percentile. | ||
Removal in tap water and wastewater effluent
Although the ozone dose was normalised to the TOC concentration, the dissolved organic carbon in each water matrix used has different characteristics. In pure buffered water, the organic matter consists of the added OMPs, while in tap water and wastewater effluent it also includes the bulk organic matter. The bulk organic matter was 20% of the total TOC in tap water and 54% in wastewater effluent (on a mass basis). The ozone reactivity of bulk organic matter varies depending on the origin and characteristics of the sample, and typically covers a range of several orders of magnitude.76 Different fractions of dissolved organic matter promote or inhibit ozone decay and the production of OH radicals, leading to different ozone and OH radical exposures.16,77 The characteristics of the organic matrix, such as aromaticity, protein and humic acid content, were not determined in this study.As shown in Table 2, the ozone exposure in tap water (pH 7.5) and wastewater effluent (pH 7.8) was one to two orders of magnitude lower than the one in pure buffered water at pH 7, but higher than that at pH 11. For most of the compounds that react fast with ozone, the removal in tap water or wastewater effluent decreased compared to pure buffered water at pH 7 (see ESI† xlsx-data file). This matrix effect is also evident in Fig. 3, especially at the intermediate ozone dose (0.15 mM O3 per mM C) and can be attributed to partial ozone consumption by the bulk organic matter. With 0.15 mM O3 per mM C, no compound was removed by more than 90% in tap water or wastewater effluent. The maximum removal in tap water at this ozone dose was 79% (cimetidine), while in wastewater effluent it was 60% (triclosan). At the highest ozone dose (0.30 mM O3 per mM C) removal of cimetidine and normorphine to below the limit of detection was achieved in tap water, but removal was partial for all compounds in wastewater effluent.
The water matrix had a smaller effect on the OH radical exposure and the elimination of ozone-resistant compounds (Table 2 and ESI† xlsx-data file). Due to their high concentrations, the OMPs already reacted very fast with OH radicals in pure buffered water. Therefore, no additional scavenging of OH radicals by the bulk organic matter in tap water and wastewater effluent was observed. For a few compounds, such as citalopram, ibuprofen and valsartan, even an enhanced elimination in tap water or wastewater effluent was noticed as a result of a slightly increased OH radical exposure. The average Rct value was 2 × 10−5 in wastewater effluent and 2 × 10−6 in tap water, which was higher compared to previously reported values for wastewater effluent.78,79
Fig. 4 shows the elimination of 40 OMPs with known second order rate constants for their reaction with ozone, added in tap water and wastewater effluent. Data including compound names are provided in the ESI.† Overall, at the lowest specific ozone dose, ozone reactivity had a small effect on the removal of the OMPs in tap water or wastewater effluent, as all 40 compounds were poorly removed (<50% removal). The effect of ozone reactivity became obvious at the intermediate and the highest ozone dose.
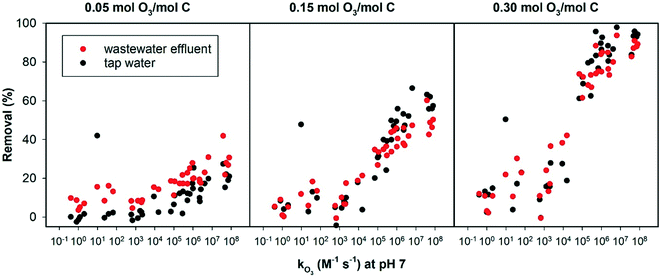 | ||
| Fig. 4 Removal of 40 OMPs in wastewater effluent and tap water versus their known from the literature ozonation second order rate constants. | ||
4. Conclusions
We conducted the simultaneous ozonation of 90 OMPs including illicit drugs and their metabolites in different aqueous matrices. Target compounds were chosen based on their relevance for current and future legislation and their environmental occurrence, persistence and toxicity. Forty-seven of the tested compounds were readily removed by ozone, including most antibacterials, antibiotics, analgesics, UV filters, parabens and steroids since these compounds contained moieties that are highly reactive with ozone. Compounds that were hard to remove with ozone contained deactivated benzene rings, amide and protonated amine moieties that are unreactive with ozone and included most illicit stimulants, antidepressants and their metabolites. This study provides a valuable database of both literature and experimental results on a wide range of OMPs, including some compounds not studied with ozone before. We specifically focused on discussing results for illicit drugs, including their occurrence in drinking water because ozonation of illicit drugs and their metabolites is significantly less studied compared to the pharmaceuticals and other compounds investigated here. The results of this study are important to predict the performance of ozonation for the removal of trace organic contaminants during water treatment.Conflicts of interest
There are no conflicts to declare.Note added after first publication
This article replaces the version published on 29th April 2020, which contained errors in the units of ozone dose.Acknowledgements
FSS and GAZ contributed equally to this study. FSS was supported by a scholarship of the PDSE/CAPES Sandwich PhD Program: Process PDSE 99999.006445/2015-02. The support of Wessex Water and the University of Bath's EPSRC Impact Acceleration Account; Project number: EP/K503897/1 and ZR-Z0248 is greatly appreciated. GAZ was supported by a University of Bath research scholarship and an EPSRC funded integrated PhD studentship in Sustainable Chemical Technologies: EP/L016354/1. JW research group was supported by a Royal Society equipment grant (RG2016-150544). Infrastructure and technical support by the Departments of Chemical Engineering and Chemistry and the Faculty of Engineering & Design is appreciated. We would like to thank Urs von Gunten for his valuable comments that helped to improve this manuscript. All data supporting this study is provided as supplementary information accompanying this paper.References
- B. Petrie, R. Barden and B. Kasprzyk-Hordern, A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring, Water Res., 2015, 72, 3–27 CrossRef CAS.
- M. J. Benotti, R. A. Trenholm, B. J. Vanderford, J. C. Holady, B. D. Stanford and S. A. Snyder, Pharmaceuticals and Endocrine Disrupting Compounds in U.S. Drinking Water, Environ. Sci. Technol., 2009, 43(3), 597–603 CrossRef CAS.
- E. Vulliet, C. Cren-Olivé and M.-F. Grenier-Loustalot, Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters, Environ. Chem. Lett., 2011, 9(1), 103–114 CrossRef CAS.
- M. S. Fram and K. Belitz, Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California, Sci. Total Environ., 2011, 409(18), 3409–3417 CrossRef CAS PubMed.
- B. Kasprzyk-Hordern, R. M. Dinsdale and A. J. Guwy, The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters, Water Res., 2009, 43(2), 363–380 CrossRef CAS PubMed.
- D. J. Lapworth, N. Baran, M. E. Stuart and R. S. Ward, Emerging organic contaminants in groundwater: A review of sources, fate and occurrence, Environ. Pollut., 2012, 163, 287–303 CrossRef CAS PubMed.
- C. Bouki, D. Venieri and E. Diamadopoulos, Detection and fate of antibiotic resistant bacteria in wastewater treatment plants: A review, Ecotoxicol. Environ. Saf., 2013, 91, 1–9 CrossRef CAS PubMed.
- S. Jobling, M. Nolan, C. R. Tyler, G. Brighty and J. P. Sumpter, Widespread Sexual Disruption in Wild Fish, Environ. Sci. Technol., 1998, 32(17), 2498–2506 CrossRef CAS.
- D. R. Baker and B. Kasprzyk-Hordern, Spatial and temporal occurrence of pharmaceuticals and illicit drugs in the aqueous environment and during wastewater treatment: New developments, Sci. Total Environ., 2013, 454–455, 442–456 CrossRef CAS.
- B. Subedi and K. Kannan, Mass Loading and Removal of Select Illicit Drugs in Two Wastewater Treatment Plants in New York State and Estimation of Illicit Drug Usage in Communities through Wastewater Analysis, Environ. Sci. Technol., 2014, 48(12), 6661–6670 CrossRef CAS PubMed.
- M. Huerta-Fontela, M. T. Galceran and F. Ventura, Stimulatory Drugs of Abuse in Surface Waters and Their Removal in a Conventional Drinking Water Treatment Plant, Environ. Sci. Technol., 2008, 42(18), 6809–6816 CrossRef CAS.
- Y. Peng, L. Gautam and S. W. Hall, The detection of drugs of abuse and pharmaceuticals in drinking water using solid-phase extraction and liquid chromatography-mass spectrometry, Chemosphere, 2019, 223, 438–447 CrossRef CAS.
- R. Pal, M. Megharaj, K. P. Kirkbride and R. Naidu, Illicit drugs and the environment — A review, Sci. Total Environ., 2013, 463-464, 1079–1092 CrossRef CAS.
- E. N. Evgenidou, I. K. Konstantinou and D. A. Lambropoulou, Occurrence and removal of transformation products of PPCPs and illicit drugs in wastewaters: a review, Sci. Total Environ., 2015, 505, 905–926 CrossRef CAS PubMed.
- U. von Gunten, Oxidation Processes in Water Treatment: Are We on Track?, Environ. Sci. Technol., 2018, 52(9), 5062–5075 CrossRef CAS.
- U. von Gunten, Ozonation of drinking water: Part I. Oxidation kinetics and product formation, Water Res., 2003, 37(7), 1443–1467 CrossRef CAS.
- K. Ikehata, N. Jodeiri Naghashkar and E.-D. M. Gamal, Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review, Ozone: Sci. Eng., 2006, 28(6), 353–414 CrossRef CAS.
- M. M. Huber, S. Canonica, G.-Y. Park and U. von Gunten, Oxidation of Pharmaceuticals during Ozonation and Advanced Oxidation Processes, Environ. Sci. Technol., 2003, 37(5), 1016–1024 CrossRef CAS PubMed.
- X. Jin, S. Peldszus and P. M. Huck, Reaction kinetics of selected micropollutants in ozonation and advanced oxidation processes, Water Res., 2012, 46(19), 6519–6530 CrossRef CAS.
- Y. Lee and U. von Gunten, Advances in predicting organic contaminant abatement during ozonation of municipal wastewater effluent: reaction kinetics, transformation products, and changes of biological effects, Environ. Sci.: Water Res. Technol., 2016, 2(3), 421–442 RSC.
- L. Kovalova, H. Siegrist, U. Von Gunten, J. Eugster, M. Hagenbuch and A. Wittmer, et al., Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV, Environ. Sci. Technol., 2013, 47(14), 7899–7908 CrossRef CAS.
- E. C. Wert, F. L. Rosario-Ortiz and S. A. Snyder, Effect of ozone exposure on the oxidation of trace organic contaminants in wastewater, Water Res., 2009, 43(4), 1005–1014 CrossRef CAS.
- R. Broséus, S. Vincent, K. Aboulfadl, A. Daneshvar, S. Sauvé and B. Barbeau, et al., Ozone oxidation of pharmaceuticals, endocrine disruptors and pesticides during drinking water treatment, Water Res., 2009, 43(18), 4707–4717 CrossRef PubMed.
- R. Rosal, A. Rodríguez, J. A. Perdigón-Melón, A. Petre, E. García-Calvo and M. J. Gómez, et al., Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation, Water Res., 2010, 44(2), 578–588 CrossRef CAS.
- M. Bourgin, B. Beck, M. Boehler, E. Borowska, J. Fleiner and E. Salhi, et al., Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products, Water Res., 2018, 129, 486–498 CrossRef CAS PubMed.
- M. R. Boleda, M. T. Galceran and F. Ventura, Behavior of pharmaceuticals and drugs of abuse in a drinking water treatment plant(DWTP) using combined conventional and ultrafiltration and reverse osmosis(UF/RO) treatments, Environ. Pollut., 2011, 159(6), 1584–1591 CrossRef CAS.
- A. Rodayan, P. A. Segura and V. Yargeau, Ozonation of wastewater: Removal and transformation products of drugs of abuse, Sci. Total Environ., 2014, 487, 763–770 CrossRef CAS PubMed.
- M. R. Boleda, M. T. Galceran and F. Ventura, Monitoring of opiates, cannabinoids and their metabolites in wastewater, surface water and finished water in Catalonia, Spain, Water Res., 2009, 43(4), 1126–1136 CrossRef CAS.
- C. Von Sonntag and U. Von Gunten, Chemistry of ozone in water and wastewater treatment, IWA publishing, 2012 Search PubMed.
- Y. Lee and U. von Gunten, Quantitative structure–activity relationships(QSARs) for the transformation of organic micropollutants during oxidative water treatment, Water Res., 2012, 46(19), 6177–6195 CrossRef CAS.
- M. Bourgin, E. Borowska, J. Helbing, J. Hollender, H. P. Kaiser and C. Kienle, et al., Effect of operational and water quality parameters on conventional ozonation and the advanced oxidation process O3/H2O2: Kinetics of micropollutant abatement, transformation product and bromate formation in a surface water, Water Res., 2017, 122, 234–245 CrossRef CAS.
- Y. Lee, D. Gerrity, M. Lee, A. E. Bogeat, E. Salhi and S. Gamage, et al., Prediction of Micropollutant Elimination during Ozonation of Municipal Wastewater Effluents: Use of Kinetic and Water Specific Information, Environ. Sci. Technol., 2013, 47(11), 5872–5881 CrossRef CAS.
- B. Petrie, J. Youdan, R. Barden and B. Kasprzyk-Hordern, Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry, J. Chromatogr. A, 2016, 1431, 64–78 CrossRef CAS.
- L. Prieto-Rodríguez, I. Oller, N. Klamerth, A. Agüera, E. M. Rodríguez and S. Malato, Application of solar AOPs and ozonation for elimination of micropollutants in municipal wastewater treatment plant effluents, Water Res., 2013, 47(4), 1521–1528 CrossRef PubMed.
- R. Andreozzi, V. Caprio, R. Marotta and D. Vogna, Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system, Water Res., 2003, 37(5), 993–1004 CrossRef CAS.
- J. Benner, E. Salhi, T. Ternes and U. von Gunten, Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation, Water Res., 2008, 42(12), 3003–3012 CrossRef CAS.
- R. Pérez Rey, A. S. Padrón, L. García León, M. Martínez Pozo and C. Baluja, Ozonation of Cytostatics in Water Medium, Nitrogen Bases, Ozone: Sci. Eng., 1999, 21(1), 69–77 CrossRef.
- M. C. Dodd, M.-O. Buffle and U. von Gunten, Oxidation of Antibacterial Molecules by Aqueous Ozone: Moiety-Specific Reaction Kinetics and Application to Ozone-Based Wastewater Treatment, Environ. Sci. Technol., 2006, 40(6), 1969–1977 CrossRef CAS PubMed.
- S. Wang, X. Wang, J. Chen, R. Qu and Z. Wang, Removal of the UV Filter Benzophenone-2 in Aqueous Solution by Ozonation: Kinetics, Intermediates, Pathways and Toxicity, Ozone: Sci. Eng., 2018, 40(2), 122–132 CrossRef CAS.
- Z. R. Hopkins, S. Snowberger and L. Blaney, Ozonation of the oxybenzone, octinoxate, and octocrylene UV-filters: Reaction kinetics, absorbance characteristics, and transformation products, J. Hazard. Mater., 2017, 338, 23–32 CrossRef CAS.
- H. Liu, P. Sun, Q. He, M. Feng, H. Liu and S. Yang, et al., Ozonation of the UV filter benzophenone-4 in aquatic environments: Intermediates and pathways, Chemosphere, 2016, 149, 76–83 CrossRef CAS.
- R. F. Dantas, M. Canterino, R. Marotta, C. Sans, S. Esplugas and R. Andreozzi, Bezafibrate removal by means of ozonation: Primary intermediates, kinetics, and toxicity assessment, Water Res., 2007, 41(12), 2525–2532 CrossRef CAS.
- M. Deborde, S. Rabouan, J.-P. Duguet and B. Legube, Kinetics of Aqueous Ozone-Induced Oxidation of Some Endocrine Disruptors, Environ. Sci. Technol., 2005, 39(16), 6086–6092 CrossRef CAS PubMed.
- K. S. Tay, N. A. Rahman and M. R. B. Abas, Ozonation of parabens in aqueous solution: Kinetics and mechanism of degradation, Chemosphere, 2010, 81(11), 1446–1453 CrossRef CAS.
- R. Rosal, A. Rodríguez, J. A. Perdigón-Melón, A. Petre, E. García-Calvo and M. J. Gómez, et al., Degradation of caffeine and identification of the transformation products generated by ozonation, Chemosphere, 2009, 74(6), 825–831 CrossRef CAS PubMed.
- D. C. McDowell, M. M. Huber, M. Wagner, U. von Gunten and T. A. Ternes, Ozonation of Carbamazepine in Drinking Water: Identification and Kinetic Study of Major Oxidation Products, Environ. Sci. Technol., 2005, 39(20), 8014–8022 CrossRef CAS PubMed.
- E. Borowska, M. Bourgin, J. Hollender, C. Kienle, C. S. McArdell and U. von Gunten, Oxidation of cetirizine, fexofenadine and hydrochlorothiazide during ozonation: Kinetics and formation of transformation products, Water Res., 2016, 94, 350–362 CrossRef CAS.
- M. Horsing, T. Kosjek, H. R. Andersen, E. Heath and A. Ledin, Fate of citalopram during water treatment with O3, ClO2, UV and Fenton oxidation, Chemosphere, 2012, 89(2), 129–135 CrossRef CAS.
- A. Lajeunesse, M. Blais, B. Barbeau, S. Sauvé and C. Gagnon, Ozone oxidation of antidepressants in wastewater–Treatment evaluation and characterization of new by-products by LC-QToFMS, Chem. Cent. J., 2013, 7(1), 15 CrossRef CAS.
- F. Lange, S. Cornelissen, D. Kubac, M. M. Sein, J. von Sonntag and C. B. Hannich, et al., Degradation of macrolide antibiotics by ozone: A mechanistic case study with clarithromycin, Chemosphere, 2006, 65(1), 17–23 CrossRef CAS PubMed.
- J. Hoigné and H. Bader, Rate constants of reactions of ozone with organic and inorganic compounds in water—II: Dissociating organic compounds, Water Res., 1983, 17(2), 185–194 CrossRef.
- M. M. Sein, M. Zedda, J. Tuerk, T. C. Schmidt and A. Golloch, Sonntag Cv. Oxidation of Diclofenac with Ozone in Aqueous Solution, Environ. Sci. Technol., 2008, 42(17), 6656–6662 CrossRef CAS.
- R. D. O. Pereira, M. L. de Alda, J. Joglar, L. A. Daniel and D. Barceló, Identification of new ozonation disinfection byproducts of 17β-estradiol and estrone in water, Chemosphere, 2011, 84(11), 1535–1541 CrossRef CAS.
- Y. Zhao, G. Yu, S. Chen, S. Zhang, B. Wang and J. Huang, et al., Ozonation of antidepressant fluoxetine and its metabolite product norfluoxetine: Kinetics, intermediates and toxicity, Chem. Eng. J., 2017, 316, 951–963 CrossRef CAS.
- Y. Lee, L. Kovalova, C. S. McArdell and U. von Gunten, Prediction of micropollutant elimination during ozonation of a hospital wastewater effluent, Water Res., 2014, 64, 134–148 CrossRef CAS PubMed.
- F. J. Real, F. J. Benitez, J. L. Acero, J. J. P. Sagasti and F. Casas, Kinetics of the Chemical Oxidation of the Pharmaceuticals Primidone, Ketoprofen, and Diatrizoate in Ultrapure and Natural Waters, Ind. Eng. Chem. Res., 2009, 48(7), 3380–3388 CrossRef CAS.
- A. Garcia-Ac, R. Broséus, S. Vincent, B. Barbeau, M. Prévost and S. Sauvé, Oxidation kinetics of cyclophosphamide and methotrexate by ozone in drinking water, Chemosphere, 2010, 79(11), 1056–1063 CrossRef CAS.
- M. M. Huber, A. GÖbel, A. Joss, N. Hermann, D. LÖffler and C. S. McArdell, et al., Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study, Environ. Sci. Technol., 2005, 39(11), 4290–4299 CrossRef CAS PubMed.
- D. Jeon, J. Kim, J. Shin, Z. R. Hidayat, S. Na and Y. Lee, Transformation of ranitidine during water chlorination and ozonation: Moiety-specific reaction kinetics and elimination efficiency of NDMA formation potential, J. Hazard. Mater., 2016, 318, 802–809 CrossRef CAS.
- M. M. Gómez-Ramos, M. Mezcua, A. Agüera, A. R. Fernández-Alba, S. Gonzalo and A. Rodríguez, et al., Chemical and toxicological evolution of the antibiotic sulfamethoxazole under ozone treatment in water solution, J. Hazard. Mater., 2011, 192(1), 18–25 Search PubMed.
- L. Ferrando-Climent, R. Gonzalez-Olmos, A. Anfruns, I. Aymerich, L. Corominas and D. Barceló, et al., Elimination study of the chemotherapy drug tamoxifen by different advanced oxidation processes: Transformation products and toxicity assessment, Chemosphere, 2017, 168, 284–292 CrossRef CAS.
- J. Blackbeard, J. Lloyd, M. Magyar, J. Mieog, K. G. Linden and Y. Lester, Demonstrating organic contaminant removal in an ozone-based water reuse process at full scale, Environ. Sci.: Water Res. Technol., 2016, 2(1), 213–222 RSC.
- S. G. Zimmermann, A. Schmukat, M. Schulz and J. Benner, Gunten Uv, Ternes TA. Kinetic and Mechanistic Investigations of the Oxidation of Tramadol by Ferrate and Ozone, Environ. Sci. Technol., 2012, 46(2), 876–884 CrossRef CAS PubMed.
- S. Suarez, M. C. Dodd, F. Omil and U. von Gunten, Kinetics of triclosan oxidation by aqueous ozone and consequent loss of antibacterial activity: Relevance to municipal wastewater ozonation, Water Res., 2007, 41(12), 2481–2490 CrossRef CAS PubMed.
- X. Chen, J. Richard, Y. Liu, E. Dopp, J. Tuerk and K. Bester, Ozonation products of triclosan in advanced wastewater treatment, Water Res., 2012, 46(7), 2247–2256 CrossRef CAS PubMed.
- J. Kuang, J. Huang, B. Wang, Q. Cao, S. Deng and G. Yu, Ozonation of trimethoprim in aqueous solution: Identification of reaction products and their toxicity, Water Res., 2013, 47(8), 2863–2872 CrossRef CAS.
- M. Diehle, W. Gebhardt, J. Pinnekamp, A. Schäffer and V. Linnemann, Ozonation of valsartan: Structural elucidation and environmental properties of transformation products, Chemosphere, 2019, 216, 437–448 CrossRef CAS PubMed.
- I. Zucker, H. Mamane, A. Riani, I. Gozlan and D. Avisar, Formation and degradation of N-oxide venlafaxine during ozonation and biological post-treatment, Sci. Total Environ., 2018, 619–620, 578–586 CrossRef CAS.
- C. Gottschalk, J. A. Libra and A. Saupe, Ozonation of water and waste water: A practical guide to understanding ozone and its applications, John Wiley & Sons, 2009 Search PubMed.
- Y. Pi, J. Schumacher and M. Jekel, The Use of para-Chlorobenzoic Acid(pCBA) as an Ozone/Hydroxyl Radical Probe Compound, Ozone: Sci. Eng., 2005, 27(6), 431–436 CrossRef CAS.
- A. Mendoza, B. Zonja, N. Mastroianni, N. Negreira, M. Lopez de Alda and S. Perez, et al., Drugs of abuse, cytostatic drugs and iodinated contrast media in tap water from the Madrid region(central Spain):A case study to analyse their occurrence and human health risk characterization, Environ. Int., 2016, 86, 107–118 CrossRef CAS.
- M. Rosa Boleda, M. Huerta-Fontela, F. Ventura and M. T. Galceran, Evaluation of the presence of drugs of abuse in tap waters, Chemosphere, 2011, 84(11), 1601–1607 CrossRef CAS.
- M. S. Elovitz, U. von Gunten and H.-P. Kaiser, Hydroxyl Radical/Ozone Ratios During Ozonation Processes. II. The Effect of Temperature, pH, Alkalinity, and DOM Properties, Ozone: Sci. Eng., 2000, 22(2), 123–150 CrossRef CAS.
- M.-O. Buffle, J. Schumacher, S. Meylan, M. Jekel and U. von Gunten, Ozonation and Advanced Oxidation of Wastewater: Effect of O3 Dose, pH, DOM and HO•-Scavengers on Ozone Decomposition and HO• Generation, Ozone: Sci. Eng., 2006, 28(4), 247–259 CrossRef CAS.
- M. S. Elovitz and U. von Gunten, Hydroxyl Radical/Ozone Ratios During Ozonation Processes. I. The RctConcept, Ozone: Sci. Eng., 1999, 21(3), 239–260 CrossRef CAS.
- P. Mandel, P. Roche and D. Wolbert, Large-Scale Experimental Validation of a Model for the Kinetics of Ozone and Hydroxyl Radicals with Natural Organic Matter, Ozone: Sci. Eng., 2014, 36(1), 73–85 CrossRef CAS.
- J. Staehelin and J. Hoigne, Decomposition of ozone in water in the presence of organic solutes acting as promoters and inhibitors of radical chain reactions, Environ. Sci. Technol., 1985, 19(12), 1206–1213 CrossRef CAS.
- J. Hollender, S. G. Zimmermann, S. Koepke, M. Krauss, C. S. McArdell and C. Ort, et al., Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration, Environ. Sci. Technol., 2009, 43(20), 7862–7869 CrossRef CAS PubMed.
- S. Gonzales, A. Peña and F. L. Rosario-Ortiz, Examining the Role of Effluent Organic Matter Components on the Decomposition of Ozone and Formation of Hydroxyl Radicals in Wastewater, Ozone: Sci. Eng., 2012, 34(1), 42–48 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ew00260g |
| This journal is © The Royal Society of Chemistry 2020 |
