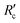Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Coagulation/flocculation prior to low pressure membranes in drinking water treatment: a review
Tyler A.
Malkoske
 *a,
Pierre R.
Bérubé
b and
Robert C.
Andrews
a
*a,
Pierre R.
Bérubé
b and
Robert C.
Andrews
a
aDepartment of Civil and Mineral Engineering, University of Toronto, Toronto, ON M5S 1A4, Canada. E-mail: tyler.malkoske@mail.utoronto.ca
bDepartment of Civil Engineering, University of British Columbia, Vancouver, BC V6T 1Z4, Canada
First published on 8th September 2020
Abstract
Microfiltration (MF) and ultrafiltration (UF) consistently remove suspended material and pathogens from drinking water; however, membrane fouling inhibits their application by increasing operation and maintenance costs. Coagulation/flocculation is a commonly used pretreatment method for the reduction of membrane fouling; in this review it has been grouped into three typical configuration types: Type 1: coagulation + no/incidental flocculation, Type 2: coagulation + flocculation, and Type 3: conventional coagulation, based on operational conditions. The impact of each configuration on floc properties, membrane fouling, and organics removal has been reviewed in detail. Due to relatively high membrane resistance and low NOM reductions, configuration Type 1 may not be optimal for fouling control and organics removal when compared to Types 2 and 3. Configuration Type 2 led to the lowest cake layer and specific cake layer resistance for both MF and UF, while there is evidence that Type 3 results in the greatest reduction in fouling rate by reducing mass flux towards the membrane surface. As expected, with no coagulant results indicate that UF achieves greater organics removal when compared to MF, but with the addition of coagulant performance is similar for all configuration types. By highlighting the connection between coagulation/flocculation configuration types and membrane performance, the review provides insight for the design and operation of pretreatment for low pressure membrane filtration. In addition, understanding the impact of configuration types on floc properties aids in revealing the fouling mechanisms that dictate membrane performance. Knowledge gaps have been identified for guidance on future research.
Tyler Malkoske, PhD Candidate, Department of Civil and Mineral Engineering, University of Toronto. |
Dr. Pierre R. Bérubé, Professor, Department of Civil Engineering, University of British Columbia. |
Dr. Robert C. Andrews, Professor and Senior NSERC Industrial Chair in Drinking Water Research, Department of Civil and Mineral Engineering, University of Toronto. |
Water impactMembrane fouling inhibits the application of low pressure membranes by increasing operation and maintenance costs. Coagulation/flocculation is a commonly used pretreatment method to reduce fouling, which may be grouped into three typical configuration types. This review provides insight for the design and operation of coagulation/flocculation by highlighting the impact of each configuration on floc properties, membrane fouling, and organics removal. |
1. Introduction
Microfiltration (MF) and ultrafiltration (UF) membranes, commonly referred to as low pressure membranes, are widely employed due to their ability to consistently remove suspended material and pathogens from drinking water.1 However, membrane fouling remains a challenge, causing increased transmembrane pressure (TMP), flux deterioration, and greater frequency of required backwashing and chemical cleaning. Thus fouling reduces operating efficiency and membrane life, and ultimately increases the operating and maintenance costs of membrane filtration.2 Membrane fouling is typically characterized as reversible or irreversible based on the impacts of cleaning practices. Hydraulically reversible fouling can be addressed hydraulically (e.g. backwashing), while hydraulically irreversible fouling can be addressed chemically (e.g. chemical cleaning). Chemically irreversible fouling cannot be removed and its gradual increase contributes to membrane ‘ageing’, or irreversible changes to membrane performance and characteristics associated with long-term foulant and cleaning agent exposure.3Membrane fouling is highly impacted by natural organic matter (NOM).4 Historically, hydrophobic humic substances (i.e. humic and fulvic acids) which constitute the majority of NOM present in surface waters5 have been identified as the predominant NOM foulants.6,7 However, there is increasing evidence that hydrophilic biopolymers (i.e. protein- and polysaccharide-like macromolecules) are the main contributor to membrane fouling.8–12 As NOM is ubiquitous in source waters, identification of the NOM fractions responsible for membrane fouling is a primary concern for development of mitigation methods for drinking water treatment using low pressure membranes.
Coagulation/flocculation is commonly used prior to low pressure membrane filtration to reduce fouling, and has been reported to reduce pore blocking, decrease cake layer resistance, and increase backwash efficiency.13 Previously published reviews regarding pretreatment,1,14 as well as fouling and cleaning15 for low pressure membranes, and an overview of coagulation/flocculation pretreatment for membrane treatment of drinking water and wastewater16 can be found in the literature. Gao1 summarized pretreatment methods (e.g. coagulation, adsorption, peroxidation, prefiltration) and operational conditions (e.g. running modes, rinsing modes, chemical cleaning, air scouring) for reducing fouling, while Huang14 also reviewed pretreatment methods to address membrane fouling concluding that coagulation had been most successful. Shi15 provided a summary of both conventional and non-conventional (e.g. electrical cleaning, ultrasonic) cleaning methods, as well as their impact on membrane materials though no results quantifying cleaning performance were included. Thus, the available review studies do not provide insight on the impact of coagulation/flocculation prior to low pressure membranes on floc properties, membrane fouling, and NOM removal. In particular, no comprehensive review exists on the impact of coagulation/flocculation configuration types on membrane performance.
The present review classified coagulation/flocculation pretreatment configurations from all of the reviewed literature into three typical types: Type 1: coagulation + no/incidental flocculation (i.e. coagulation with direct membrane filtration), Type 2: coagulation + flocculation (i.e. coagulation, flocculation, with direct membrane filtration), and Type 3: conventional coagulation (i.e. coagulation, flocculation, sedimentation, and membrane filtration). The impact of each configuration type on floc properties and membrane performance in terms of fouling and NOM removal was assessed. The number of studies considered in reviewing the impact of configuration type on floc properties, fouling performance, and DOC/TOC removal is summarized in Fig. 1. Published studies that counted towards fouling performance include at least one of total resistance, cake layer/specific cake layer resistance, specific hydraulic resistance, mean rate of TMP increase/flux decline, and flux recovery after hydraulic and chemical cleaning. This review highlights the connection between coagulation/flocculation configuration types and membrane performance, providing insight for the design and operation of pretreatment for low pressure membrane filtration. Summarized results are used to understand the impact of configuration types on floc properties, which dictate fouling mechanisms and membrane performance. Knowledge gaps have also been identified to provide guidance for future research.
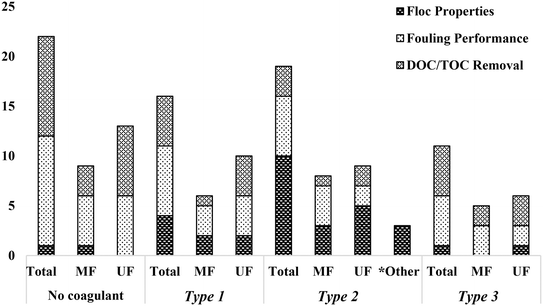 | ||
| Fig. 1 Number of published studies considered for floc properties, fouling performance, and DOC/TOC removal based on configuration type.* Studies did not include membrane filtration. | ||
2. Coagulation/flocculation-low pressure membranes
2.1. Overview of coagulation/flocculation theory
Coagulation and flocculation typically occur sequentially, governing the formation and properties of floc, and impacting the performance of downstream membranes. Initial aggregation of particles/NOM occurs by destabilization during coagulation, where mechanisms include charge neutralization, interparticle bridging, and sweep flocculation.17 As particles in natural waters, including NOM, are negatively charged (pH 6.0 to 8.0), charge neutralization occurs when sufficient cationic metal hydroxides are adsorbed to reduce zeta potential to zero.18,19 Sweep flocculation occurs when the concentration of a coagulant exceeds its solubility limit, precipitates, and enmeshes particles/NOM.20 The preferred destabilization mechanism for membrane pretreatment is dependent on water quality, and may be different for each configuration type.Following destabilization, particle-particle interactions (i.e. collisions) result in floc formation.17,19 Fluid shear induced by mixing is the dominant flocculation mechanism when two colliding particles are >1 μm in diameter, while Brownian motion dominates when at least one particle is small (i.e. <1 μm in diameter) and differential sedimentation in all other cases.21 Aggregation rates are highest when particles have been fully destabilized by coagulation, and are lower in the case of partial destabilization. The hydrodynamic conditions of coagulation/flocculation are typically described by mean velocity gradient, ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) , and contact time, t, and will be different for each configuration type. In some of the published studies impeller speed (rpm) rather than
, and contact time, t, and will be different for each configuration type. In some of the published studies impeller speed (rpm) rather than ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value is used to describe hydrodynamic conditions; however, despite the limitations of
value is used to describe hydrodynamic conditions; however, despite the limitations of ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value, impeller speed is inadequate for quantifying the forces being applied to water during mixing, and hinders the reproducibility of study results. Finally, mass flux of floc towards the membrane surface results in fouling, where floc properties may be associated with the type of membrane fouling that predominates.
value, impeller speed is inadequate for quantifying the forces being applied to water during mixing, and hinders the reproducibility of study results. Finally, mass flux of floc towards the membrane surface results in fouling, where floc properties may be associated with the type of membrane fouling that predominates.
2.2. Typical configuration types
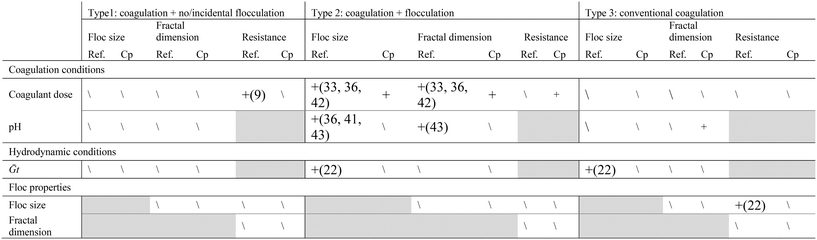
Coagulation/flocculation pretreatment for all of the reviewed literature could be classified into three typical configuration types: Type 1: coagulation + no/incidental flocculation; Type 2: coagulation + flocculation; and Type 3: conventional coagulation (Fig. 2). In this review, coagulation without flocculation/sedimentation is considered as coagulation + no/incidental flocculation. Coagulation followed by flocculation in the absence of sedimentation is commonly referred to as direct filtration,22,23 while here it is characterized as coagulation + flocculation. Conventional coagulation, which includes sedimentation, has been applied prior to membrane filtration to remove aquatic constituents that cause fouling.14 However, it has been observed that this may not be effective in removing the NOM fractions which contribute to irreversible fouling,24 and that similar fouling performance may be realized without sedimentation.25 In coagulation pretreatment without sedimentation, floc size is only required to grow beyond that of membrane pores (i.e. sub-micron), thus reduced coagulant dosages may be applicable.26 Coagulation pretreatment without flocculation/sedimentation has been investigated for its potential to significantly reduce flocculation times and water treatment plant footprint.27 A summary of coagulation/flocculation conditions for configuration Types 1, 2, and 3 from the literature is provided in Table 1.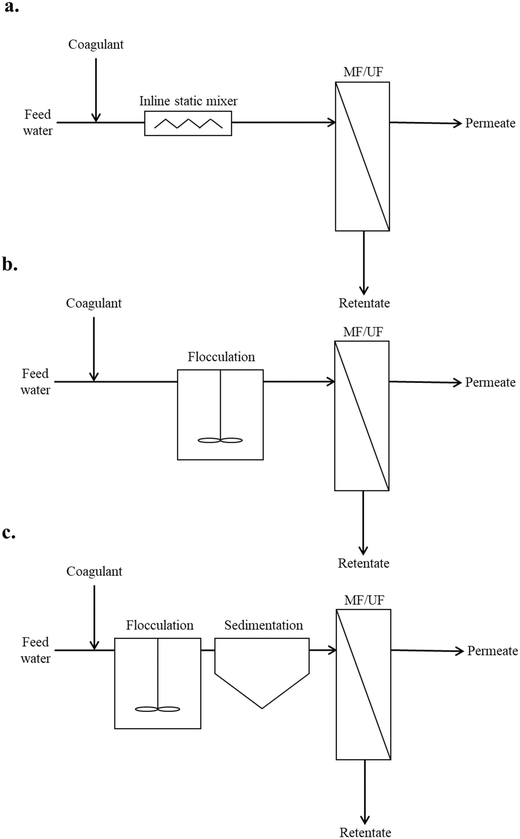 | ||
| Fig. 2 Typical configuration types: a. Type 1: coagulation + no/incidental flocculation, b. Type 2: coagulation + flocculation, c. Type 3: conventional coagulation. | ||
| Configuration | Coagulation conditions | Flocculation conditions | Sedimentation | Ref. | |||
|---|---|---|---|---|---|---|---|
| Dose (mg L−1 Me+) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
t (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
t (s) | t (s) | ||
| a N/A = not available. | |||||||
| Type 1: coagulation + no/incidental flocculation | 0.34–10.00 | N/Aa | N/A | N/A | 20–120 | N/A | 9, 22, 25, 27–32 |
| 100–150 | 180 | 0–5.25 | 360–720 | ||||
| Type 2: coagulation + flocculation | 6.75 × 10−4–39.96 | 100–184 | 60–180 | 14.85–80 | 600–1800 | N/A | 22, 26, 28, 29, 33–43 |
| Type 3: conventional coagulation | 0.04–9.23 | 100–300 rpm | 60–180 | 5.25–60 | 360–1740 | 900–3600 | 8, 22–24, 39, 44–48 |
No standardized method for the optimization of coagulant dosage for configuration Type 1 has been presented in the literature. Coagulant dosages that have been considered range from 0.59 to 5.68 mg L−1 Al and 0.34 to 10.00 mg L−1 Fe. Several studies have examined the impact of coagulant dose on the basis of turbidity and NOM removal by charge neutralization and sweep flocculation.22,29,30,32 Coagulant dosage has also been optimized for reducing membrane fouling. Pronk9 reported increased fouling at an FeCl3 dosage of 5 ppm (1.70 mg L−1 Fe) compared to 1 and 2 ppm (0.34 and 0.68 mg L−1 Fe), while Judd and Hillis27 observed that at coagulant dosages <0.035 mM Fe3+ (1.96 mg L−1 Fe) fouling increased when compared to no coagulant addition. These results suggest there may be dose thresholds, above or below which fouling is exacerbated by the application of coagulant. Choi and Dempsey32 examined the effect of alum and aluminum chlorohydrate (ACH) dosages ranging from 0.59 to 2.93 mg L−1 Al on membrane fouling. The authors suggested that low dose conditions (1.17 mg L−1 Al, pH 4.81), below those required for charge neutralization, could simultaneously reduce membrane fouling and coagulant costs. Konieczny30 observed that FeCl3 and Al2(SO4)3 doses which were 20% lower than those determined by jar testing (3.0 mg L−1 Fe and 3.6 mg L−1 Al, respectively) resulted in the lowest drop in permeate flux.
Where mechanical mixing or inline mixing were applied, mean velocity gradient (![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ) and contact time (t) after coagulation for Type 1 have been reported to range from 0 to 5.25 s−1, and 360 to 720 s, respectively. Studies that did not include mixing did not report a
) and contact time (t) after coagulation for Type 1 have been reported to range from 0 to 5.25 s−1, and 360 to 720 s, respectively. Studies that did not include mixing did not report a ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value but contact time ranged from 20 to 120 s. Hydrodynamic conditions for coagulation generally are not well described in studies where static mixing is used.27 For incidental flocculation conditions, it is difficult to characterize mixing in terms of
value but contact time ranged from 20 to 120 s. Hydrodynamic conditions for coagulation generally are not well described in studies where static mixing is used.27 For incidental flocculation conditions, it is difficult to characterize mixing in terms of ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) and t, and where these values are provided justification for their selection is not always stated directly.
and t, and where these values are provided justification for their selection is not always stated directly.
For configuration Type 2, ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) and t for coagulation have been reported to range from 100 to 184 s−1, and 60 to 180 s, respectively. Flocculation
and t for coagulation have been reported to range from 100 to 184 s−1, and 60 to 180 s, respectively. Flocculation ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) and t ranged from 14.85 to 80 s−1, and 600 to 1800 s, respectively. Coagulation conditions have frequently been simulated using a jar test, where high mean velocity gradients (
and t ranged from 14.85 to 80 s−1, and 600 to 1800 s, respectively. Coagulation conditions have frequently been simulated using a jar test, where high mean velocity gradients (![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ranging from 100 to 150 s−1) were applied for a short duration (t ranging from 60 to 180 s).22,23,28,41,42 Compared to configuration Type 1,
ranging from 100 to 150 s−1) were applied for a short duration (t ranging from 60 to 180 s).22,23,28,41,42 Compared to configuration Type 1, ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) values during flocculation are approximately 3 to 15 times greater and contact times generally longer. Howe and Clark23 incorporated a shorter contact time (240 s) to promote the development of pin-floc, which are floc with relatively small size when compared to those typically formed to promote sedimentation.
values during flocculation are approximately 3 to 15 times greater and contact times generally longer. Howe and Clark23 incorporated a shorter contact time (240 s) to promote the development of pin-floc, which are floc with relatively small size when compared to those typically formed to promote sedimentation.
For configuration Type 3, ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) and t after coagulation have been reported to range from 5.25 to 60 s−1, and 360 to 1740 s, respectively. Mixing intensities for coagulation were not reported in terms of
and t after coagulation have been reported to range from 5.25 to 60 s−1, and 360 to 1740 s, respectively. Mixing intensities for coagulation were not reported in terms of ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) , but impeller speeds ranged from 100 to 300 rpm, and t from 60 to 180 s. While the maximum flocculation time for Type 2 was 1800 s (30 min) that for Type 3 is slightly lower, but still expected to be relatively long to enhance floc development for subsequent settling. Settling times prior to membrane filtration ranged from 900 to 3600 s (15 to 60 min).23,46,47 For Type 3, it was also demonstrated that tapered flocculation, or gradual reductions in mixing speed (65, 40, and 25 rpm for 17 min each), could promote the formation of larger floc size and greater removal of turbidity prior to membranes.23
, but impeller speeds ranged from 100 to 300 rpm, and t from 60 to 180 s. While the maximum flocculation time for Type 2 was 1800 s (30 min) that for Type 3 is slightly lower, but still expected to be relatively long to enhance floc development for subsequent settling. Settling times prior to membrane filtration ranged from 900 to 3600 s (15 to 60 min).23,46,47 For Type 3, it was also demonstrated that tapered flocculation, or gradual reductions in mixing speed (65, 40, and 25 rpm for 17 min each), could promote the formation of larger floc size and greater removal of turbidity prior to membranes.23
2.3. Operational variations
While the conditions for each configuration described in section 2.2 provide an overview of the main alternatives, there are several operational variations that may be applied in each case. Yu52 examined the addition of polyacrylamide (PAM) as a coagulant aid to reduce UF fouling, while Xu53 investigated the use of titanium sulfate (Ti(SO4)2) and UF for treatment of waters containing algal organic matter. The application of natural coagulants in drinking water treatment, such as chitosan,54,55 seed extract,56 and starches,57 has been investigated in order to address the sustainability of coagulation/flocculation. Encouraging results were obtained with respect to turbidity removal, as well as in reducing membrane fouling when applied in combination with Al-based coagulants. It was suggested that pre-coating metal hydroxides prior to a permeation cycle may reduce fouling and enhance the removal of organic matter by forming a permeable, easily removable fouling layer.29,58 Pronk9 suggested establishing a protective fouling layer would result in longer permeation cycle times, and examined the application of phased coagulation as an alternative. By applying 1 to 2 ppm FeCl3 during the first 30 min of a 60 min permeation cycle, the authors observed similar total membrane fouling when compared to continuous coagulant addition. Phased coagulation would have the added benefit of significantly reducing coagulant costs.3. Impact of configuration types on floc properties
3.1. Summary of results
Coagulation/flocculation, including coagulation conditions (i.e. coagulant dose, coagulant type, pH) and hydrodynamic conditions (i.e.![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value, contact time) can impact floc properties such as floc size, growth rate, surface charge, structure, and strength25,59 (Table 2). As the coagulation/flocculation conditions of each configuration type differ, it is anticipated that floc properties will also vary. Potential relationships between coagulation/flocculation conditions and floc properties for Types 2 and 3 have been highlighted for results compiled from several studies (Table 8 and Fig. 7 and 8). Due to lack of data, similar figures were not included for Type 1.
value, contact time) can impact floc properties such as floc size, growth rate, surface charge, structure, and strength25,59 (Table 2). As the coagulation/flocculation conditions of each configuration type differ, it is anticipated that floc properties will also vary. Potential relationships between coagulation/flocculation conditions and floc properties for Types 2 and 3 have been highlighted for results compiled from several studies (Table 8 and Fig. 7 and 8). Due to lack of data, similar figures were not included for Type 1.
| Coagulant/dosage | Feedwater | Coagulation | Flocculation | Floc properties | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
Size (μm) | D f | |||
| a N/A = not available. b Particle size reported as percent of particles ranging from 2–5 μm at each coagulant dosage. c Given as range of highest volume averages across several dosage concentrations. d Lower values in range typical of Type 2, upper values Type 1; not distinguished in study, therefore data only presented for Type 2. e Reported as particle size distribution peak. f Floc sizes after flocculation, but before settling. | ||||||||||
| Type 1: coagulation + no/incidental flocculation | ||||||||||
| Alum/70 mg L−1 | Synthetic (HA) | N/Aa | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5.25 | 360 | 1890 | ∼1 | 1.95–2.12 | 22 |
| 10 mg L−1 TOC | ||||||||||
| pH 4.8–5.5 | ||||||||||
| Fe-Based/0–0.072 mM (Fe) | Reservoir | 230 rpm | 20 | N/A | N/A | N/A | N/A | 2–5 (5–65%)b | N/A | 27 |
| 2.4 mg L−1 TOC | ||||||||||
| pH ∼5.4 | ||||||||||
| 3.18 mg L−1 (Al2O3) | River | 150 | 20 min–8 h | N/A | 0 | N/A | N/A | 49–63 | 1.92–2.30 | 28 |
| 2–3 mg L−1 DOC | ||||||||||
| pH N/A | ||||||||||
| PACl/4.1/10.0 ppm (Al2O3) | River | N/A | N/A | N/A | 0 | 720 | N/A | 12–30 | N/A | 29 |
| 2.3–2.9 ppm DOC | ||||||||||
| pH 7.2–7.8 | ||||||||||
| No coagulant | Latex particles | 140 | 10–220 min | N/A | N/A | N/A | N/A | 77–371 | 2.13–2.64 | 63 |
| 1–16 mg L−1 | ||||||||||
| pH 3–12 | ||||||||||
| Type 2: coagulation + flocculation | ||||||||||
| AC/8 mg L−1 (Al) | Synthetic (HA) | 200 rpm | 90 | N/A | 40 rpm | 900 | N/A | 235–295 | 2.509–2.533 | 41 |
| PACb/8 mg L−1 (Al) | 5.35 mg L−1 DOC | 130–250 | 2.493–2.534 | |||||||
| PACc/8 mg L−1 (Al) | pH 4–8 | 130–210 | 2.448–2.482 | |||||||
| FeCl3/22 mg L−1 (Fe) | Synthetic (HA) | 200 rpm | 90 | N/A | 40 rpm | 900 | N/A | 380–740 | 2.54–2.61 | 43 |
| PFC10/22 mg L−1 (Fe) | 4.67 mg L−1 DOC | 300–450 | 2.50–2.60 | |||||||
| PFC22/22 mg L−1 (Fe) | pH 4–9 | 225–450 | 2.54–2.54 | |||||||
| Alum/1.7 mg L−1 (Al) | Synthetic (HA) | 100 | 60 | 6000 | 25 | 1200 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
20c | 1.70 | 42 |
| 3.4–8.5 mg L−1 (Al) | 7.5 mg L−1 | 35–40 | 2.70–2.79 | |||||||
| PACl/2.6 mg L−1 (Al) | pH 8.3 | 10 | 1.80 | |||||||
| 3.4–8.5 mg L−1 (Al) | 60–105 | 2.81–2.85 | ||||||||
| ACH/3.4 mg L−1 (Al) | 40 | 1.90 | ||||||||
| 3.4–8.5 mg L−1 (Al) | 55–105 | 2.83–2.92 | ||||||||
| Alum/70 mg L−1 | Synthetic (HA) | N/A | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
14.85 | 1200 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
222 | 2.46–2.73 | 22 |
| 10 mg L−1 TOC | ||||||||||
| 42.00 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
303 | 2.56–2.72 | |||||||
| pH 4.8–5.5 | ||||||||||
| PACl/3.18 mg L−1 (Al2O3) | River | 150 | 3 min | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45 | 20 min–8 h | N/A | 90–447 | 1.89–2.29 | 28 |
| 2–3 mg L−1 DOC | ||||||||||
| pH N/A | ||||||||||
| PACl/0.025 mM (Al) | Synthetic (HA) | 175 | 90 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
20 | 900 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
120 | 1.81 | 33 |
| 0.1 mM (Al) | 5 mg L−1 | 160 | 1.97 | |||||||
| pH 7.5 | ||||||||||
| AlCl3/7 mg L−1 | Synthetic (HA) | 200 rpm | 30 | N/A | 40 rpm | 600 | N/A | 410 | 2.13–2.27 | 64 |
| PAC-1/13 mg L−1 | 3.56–4.38 mg L−1 TOC | 316 | 1.93–2.17 | |||||||
| PAC-2/8 mg L−1 | pH 7.7 | 279 | 1.88–2.03 | |||||||
| PACla/0.08 mM (Al) | Synthetic | 200 rpm | 1 min | N/A | 40 rpm | 15 min | N/A | 375 | 2.37 | 34 |
| PAClb/0.08 mM (Al) | 3.0–3.13 mg L−1 DOC | 255 | 2.35 | |||||||
| PAClp/0.08 mM (Al) | pH 7.8–7.9 | 360 | 2.37 | |||||||
| PACl/4.1/10.0 ppm (Al2O3) | River | N/A | N/A | N/A | 58–350d | 1800-3600 | N/A | 30–40 | N/A | 29 |
| 2.3–2.9 ppm DOC | ||||||||||
| pH 7.2–7.8 | ||||||||||
| Alum/0.1 mmol L−1 | Synthetic (kaolin) | 200 rpm | 60 | N/A | 40 rpm | 1500 | N/A | 595.7 | 2.45 | 35 |
| 50 mg L−1 | 60 rpm | 380.1 | 2.52 | |||||||
| pH 7.50 | ||||||||||
| Alum/0.01 mM (Al) | Synthetic (kaolin) | 184 | 60 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040 040 |
23 | 1800 | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
140 | 1.90–2.17 | 36 |
| 0.02 mM (Al) | 57.8 g L−1 | 170 | 1.92–2.42 | |||||||
| pH 4.4–7.0 | ||||||||||
| Alum/5.33 mg L−1 | Latex particles | 100 | 60 | 6000 | 20 | 600 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14 | 1.83e | 37 |
| 366 L−1/pH 6.5 | 80 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14 | 1.82 | ||||||
| 3.33 mg L−1 | 220 L−1 | 20 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14 | 1.91 | |||||
| 80 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
14 | 1.94 | |||||||
| 5.33 mg L−1 | 220 L−1 | 20 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18 | 1.72 | |||||
| 80 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
18 | 1.75 | |||||||
| 3.33 mg L−1 | 366 L−1 | 20 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
12 | 1.84 | |||||
| 80 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
16 | 1.91 | |||||||
| Type 3: conventional coagulation | ||||||||||
| Alum/70 mg L−1 | Synthetic (HA) | N/A | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5.25 | 360 | 1890 | ∼1f | 2.06–2.16 | 22 |
| 10 mg L−1 TOC | 14.85 | 1200 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
222f | 2.53–2.71 | |||||
| pH 4.8–5.5 | 42.00 | 1200 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
303f | 2.42–2.67 | |||||
Floc structure is commonly characterized by fractal dimension, Df, which may range from 1 to 3.60 A Df value of one represents a linear aggregate having a mass proportional to length, whereas a Df value of three represents a uniform aggregate with mass approximately equal to the size cubed.61 It has been suggested that as the value of Df increases the number of particle–particle bonds within the floc also increases along with strength.62 However, while floc formed under sweep flocculation conditions are compact, they are less dense and may be more susceptible to compression under pressure.44
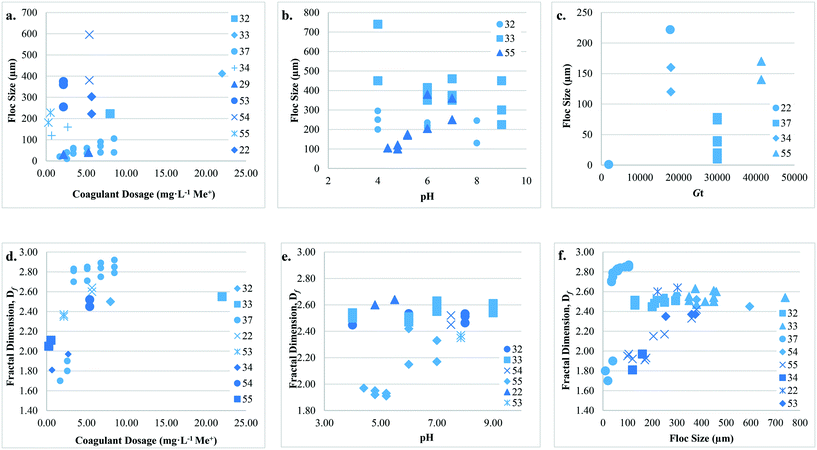
3.2. Type 1: coagulation + no/incidental flocculation
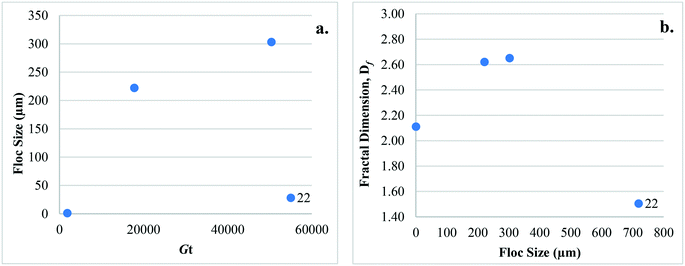
Studies that have considered configuration Type 1 have investigated the impact of coagulant dose, type, and hydrodynamic conditions on floc size, surface charge, and Df (Table 2). In general, floc sizes reported were smallest (∼1 to 8 μm) when compared to other configuration types. The size range reported by Cho28 (49 ± 5 to 63 ± 5 μm) is an exception since contact time (20 min to 8 h) was extended in an effort to determine the effects of hydrodynamic conditions on floc properties. After 20 s of rapid mixing (230 rpm) and applying low (1.12 to 1.67 mg L−1 Fe) and high (4.02 mg L−1 Fe) coagulant doses, Judd and Hillis27 reported particle aggregation into 2 to 5 μm floc of <10% and 65%, respectively. Similar observations were made by Cho28 following 3 min of rapid mixing where zeta potential was near zero, suggesting that charge properties develop early in the coagulation process.For Type 1 hydrodynamic conditions, Amjad22 reported that floc formed at low ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t (1890) were at least an order of magnitude smaller, and Df lower when compared to those at higher
t (1890) were at least an order of magnitude smaller, and Df lower when compared to those at higher ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t values (17
t values (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 and 50
820 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400). Cho28 observed that Df decreased over time (2.30 ± 0.02 to 1.92 ± 0.01) as floc structure became less dense. While this was with rapid mixing only, the contact time was relatively long (3 min to 8 h) and it is unknown if similar results would be observed at shorter times. As it has been suggested that floc strength is related to Df, and since Df increases with increasing particle collisions,62 it is expected that floc formed by configuration Type 1 will have relatively low Df values and may also have low strength.
400). Cho28 observed that Df decreased over time (2.30 ± 0.02 to 1.92 ± 0.01) as floc structure became less dense. While this was with rapid mixing only, the contact time was relatively long (3 min to 8 h) and it is unknown if similar results would be observed at shorter times. As it has been suggested that floc strength is related to Df, and since Df increases with increasing particle collisions,62 it is expected that floc formed by configuration Type 1 will have relatively low Df values and may also have low strength.
3.3. Type 2: coagulation + flocculation
Studies that have considered configuration Type 2 examined the effect of coagulant dose, type, pH, and hydrodynamic conditions on floc size, growth rate, Df, and strength (Table 2) (Fig. 7). The reported floc sizes (10 to 740 μm) generally increase with increasing coagulant dose. Coagulant doses below those required for charge neutralization may lead to insufficient hydrolytic coagulant species and poor floc formation.33,42 Chakraborti37 suggested that at a low alum dose (3.33 mg L−1) that there was insufficient coagulant present to cause significant floc growth within the first 10 min (600 s) of flocculation. It has also been reported that at coagulant doses below those optimized for NOM removal by sweep flocculation, Df values are also lower.33,42With respect to coagulant type, Feng41 observed that monomeric AlCl3 resulted in larger floc size at pH 6.0 than polymeric coagulants. Similarly, at pH 6.0 and optimum dosages for humic acid removal (7 to 13 mg L−1), Wang64 reported that floc size was largest for aluminum chloride (AlCl3) followed by polyaluminum chloride (PAC-1) and purified polyaluminum chloride (PAC-2), and that the corresponding growth rates were 0.59, 0.50, and 0.64 μm s−1, respectively. The authors identified a lag time in floc formation where minimal growth occurred over the first 70 to 140 s of flocculation, followed by a growth period from 420 to 665 s. This lag is longer than that reported by Judd and Hillis,27 where floc growth was observed within 20 s using an Fe-based coagulant. Wang42 suggested that amorphous aluminum hydroxide (Al(OH)3) produced by alum resulted in the formation of porous floc (i.e. low Df), while aluminum polymers formed clusters and chains of small spheres with higher Df. At pH 6.0, Feng41 reported that AlCl3 produced floc with higher Df than PAC-1 and PAC-2; however, the variance in Df values was <5%. Dong43 also reported that monomeric coagulant species form the highest density floc around neutral pH.
The predominant coagulation destabilization mechanism at various pH levels affects floc size, growth rate, and Df. Results indicate that at acidic pH (4.0) larger steady-state floc size can generally be achieved when compared to more neutral or alkaline pH (6.0 to 8.0), while growth rates followed an opposite trend.41,43 Low growth rates at acidic pH (4.0) were attributed to the predominance of charge neutralization by monomeric coagulant species, while at pH 6.0 and 8.0 monomeric coagulant species were rapidly transformed into polymers and solid precipitates. It was suggested that larger floc formed at lower pH because of charge neutralization and complexation, which involves stronger forces than sweep flocculation. Compared to more neutral and alkaline pH (>6.0), observations indicate that Df is typically lower at acidic pH (4.0).36,41,43 The formation of more compact floc at higher pH was attributed to sweep flocculation. Yu36 applied breakage tests as an indicator of floc strength, where floc were exposed to a high ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value (184 s−1) and changes in size measured. It was observed that floc with low Df produced smaller particle sizes (∼38 to 58 μm, 41 to 60 μm) than floc with higher Df (∼76 to 102 μm, 130 to 133 μm), suggesting that Df has a positive correlation with strength. Feng41 and Dong43 also reported rapid breakage for floc formed at pH 4.0 when compared to those formed at alkaline pH suggesting lower strength. Results indicate that sweep flocculation produces floc with higher Df than charge neutralization, and that the Df of floc formed by monomeric coagulants is higher at acidic pH and lower at alkaline pH when compared to polymeric coagulants.
value (184 s−1) and changes in size measured. It was observed that floc with low Df produced smaller particle sizes (∼38 to 58 μm, 41 to 60 μm) than floc with higher Df (∼76 to 102 μm, 130 to 133 μm), suggesting that Df has a positive correlation with strength. Feng41 and Dong43 also reported rapid breakage for floc formed at pH 4.0 when compared to those formed at alkaline pH suggesting lower strength. Results indicate that sweep flocculation produces floc with higher Df than charge neutralization, and that the Df of floc formed by monomeric coagulants is higher at acidic pH and lower at alkaline pH when compared to polymeric coagulants.
Previous studies have examined the impact of ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value and contact time on floc size and Df. A positive correlation between contact time and floc size has been reported in multiple studies.28,37 At a
value and contact time on floc size and Df. A positive correlation between contact time and floc size has been reported in multiple studies.28,37 At a ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) value of 45 s−1, Cho28 observed that floc size increased (90 ± 9 to 447 ± 23 μm) as contact time increased from 20 min to 8 h, while Df decreased (2.29 ± 0.04 to 1.89 ± 0.01). The observation of a decrease in Df with contact time is unexpected, and while an explanation was not provided it may be related to the dramatic increase in floc size. Lower
value of 45 s−1, Cho28 observed that floc size increased (90 ± 9 to 447 ± 23 μm) as contact time increased from 20 min to 8 h, while Df decreased (2.29 ± 0.04 to 1.89 ± 0.01). The observation of a decrease in Df with contact time is unexpected, and while an explanation was not provided it may be related to the dramatic increase in floc size. Lower ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) values are expected to result in larger floc size,35,64 while higher
values are expected to result in larger floc size,35,64 while higher ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) values result in greater Df than floc formed by Type 1.22,35,37 Floc formed at higher
values result in greater Df than floc formed by Type 1.22,35,37 Floc formed at higher ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) values are expected to be more compact due to increased particle collisions, floc breakup, and restructuring. As floc grow, cluster–cluster interactions become more important and smaller compact clusters have the chance to penetrate the pores of larger flocs.
values are expected to be more compact due to increased particle collisions, floc breakup, and restructuring. As floc grow, cluster–cluster interactions become more important and smaller compact clusters have the chance to penetrate the pores of larger flocs.
3.4. Type 3: conventional coagulation
Only one study has considered the impact of configuration Type 3 on floc properties,22 investigating the effect of hydrodynamic conditions on floc size and Df (Table 2). After flocculation, floc sizes (∼1 to 303 μm) are of similar magnitude as those of Type 2 conditions; however, neither floc size nor Df were reported after sedimentation. Therefore, the reported floc sizes are expected to be larger than those that would have been present during subsequent membrane filtration, as larger floc would have been removed by settling. Amjad22 reported that floc with higher Df values were not preferentially removed by settling, and that the Df values of floc that were settled and those that were not settled were not statistically different. While high Df values may indicate more compact floc as mentioned in section 3, it is not necessarily an indication of higher density or floc that are more readily settled.4. Impact of configuration types on membrane fouling
4.1. Overview of membrane fouling theory
As discussed in section 3, coagulation/flocculation configuration types dictate floc characteristics, which combined with membrane operating conditions, including flux, transmembrane pressure (TMP), and dead-end vs. crossflow modes, influence membrane fouling.33,65,66 Membrane fouling depends on the mass flux of floc to the membrane surface as well as particle adsorption on the membrane surface and in membrane pores. Particle-membrane interactions initially dictate cake layer formation, followed by particle–particle interactions that may become more prominent once a cake layer has formed. Total membrane resistance, R(t), which increases with time, can be quantified using the relationships in eqn (1) and (2):17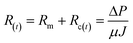 | (1) |
| Rc(t) = Rrev(t) + Rirr(t) | (2) |
| Rc(t) = αM = αJCb | (3) |
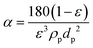 | (4) |
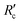 , which is the resistance per unit depth of foulant as expressed in eqn (5).68
, which is the resistance per unit depth of foulant as expressed in eqn (5).68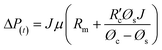 | (5) |
4.2. Summary of results
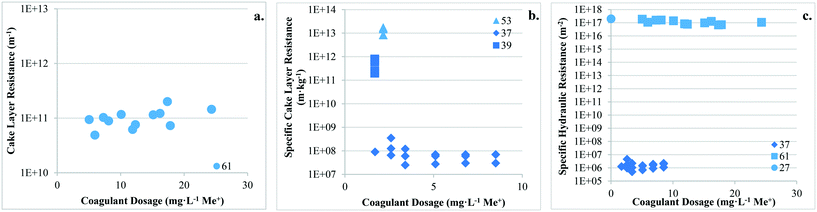
This section discusses the impact of coagulation/flocculation configuration types on membrane fouling in terms of resistance (i.e. resistance to flow), fouling rate, as well as hydraulically and chemically reversible/irreversible fouling. For comparison with configuration types, results for membrane filtration with no coagulant addition have also been included. Published results on the impact of coagulation/flocculation configuration types on membrane resistance (i.e. total resistance, cake layer resistance, specific cake layer resistance, specific hydraulic resistance) for MF and UF are summarized in Tables 3 and 4, respectively. Results for membrane resistance are presented graphically in Fig. 3–5 and discussed for each configuration type in sections 4.3 to 4.5. As results are limited and experimental conditions (e.g. water matrix, coagulant dose, pH, hydrodynamic conditions) vary across studies, it is difficult to observe trends when comparing the configuration types. Amjad22 is the only known study to compare the impact of all three configuration types on membrane fouling with similar experimental conditions, highlighting the need for further research to address current knowledge gaps. Potential relationships between coagulant dose and membrane resistance for MF and UF have been highlighted for configuration Types 1, 2, and 3 with results compiled from several studies (Fig. 9–11).| Coagulant/dosage | Feedwater | Membrane type | Coagulation | Flocculation | Membrane resistance | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
R (t) (m−1) | R c(t) (m−1) | α (m kg−1) | (m−2) | ||||
| a N/A = not available. b Determined over a TMP range of 20–80 kPa. | |||||||||||||
| Type 1: coagulation + no/incidental flocculation | |||||||||||||
| No coagulant | Surface water | MF GVHP 0.22 μm (nominal) | N/Aa | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 69 | |
| 3 mg L−1 DOC | |||||||||||||
| pH 7.4 | |||||||||||||
| Hydrophobic acids | 3.20 × 1011 | ||||||||||||
| Transphilic acids | 2.00 × 1011 | ||||||||||||
| Hydrophilic charged | 1.80 × 1011 | ||||||||||||
| Hydrophilic neutral | 5.50 × 1011 | ||||||||||||
| 3.18 mg L−1 (Al2O3) | River | MF GVWP 0.22 μm (nominal) | 150 | 20 min | N/A | N/A | N/A | N/A | N/A | N/A | 4.11 × 1012 | N/A | 28 |
| 2–3 mg L−1 DOC | 1 h | 3.65 × 1012 | |||||||||||
| pH N/A | 2 h | 3.10 × 1012 | |||||||||||
| 4 h | 2.50 × 1012 | ||||||||||||
| 8 h | 1.86 × 1012 | ||||||||||||
| No coagulant | Reservoir | MF PES 0.1 μm (mean) | 230 rpm | 180 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 2.0 × 1017 | 27 |
| Fe-Based/0.027 mM (Fe) | 2.4 mg L−1 TOC | 1.5 × 1017 | |||||||||||
| 0.036 mM (Fe) | pH ∼5.4 | 6.0 × 1016 | |||||||||||
| 0.045 mM (Fe) | 4.0 × 1016 | ||||||||||||
| 0.054 mM (Fe) | 3.0 × 1016 | ||||||||||||
| 0.072 mM (Fe) | 5.0 × 1015 | ||||||||||||
| PACl/2 mg L−1 (Al) | River | MF PVDF 0.1 μm | 100 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 70 | |
| 1 mg L−1 DOC | 14.1 × 1011 | ||||||||||||
| 0.5 mg L−1 DOC | 14.1 × 1011 | ||||||||||||
| 2.0 mg L−1 DOC | 16.0 × 1011 | ||||||||||||
| 1.6 mg L−1 DOC | 18.5 × 1011 | ||||||||||||
| 1.0 mg L−1 DOC | 16.2 × 1011 | ||||||||||||
| No coagulant | Latex particles | N/A | 140 | 10–220 min | N/A | N/A | N/A | N/A | N/A | N/A | 0.49–5.07 × 1012b | N/A | 63 |
| 1–16 mg L−1 | Cellulose | ||||||||||||
| pH 3–12 | 0.45 μm | ||||||||||||
| Type 2: coagulation + flocculation | |||||||||||||
| PACla/0.08 mM (Al) | Synthetic (HA) | MF PVDF 0.22 μm (mean) | 200 rpm | 1 min | N/A | 40 rpm | 15 min | N/A | 1.9 × 1011 | N/A | 8.4 × 1012 | N/A | 34 |
| PAClb/0.08 mM (Al) | 3.0–3.13 mg L−1 DOC | 3.4 × 1011 | 1.7 × 1013 | ||||||||||
| PAClp/0.08 mM (Al) | pH 7.8–7.9 | 3.0 × 1011 | 1.5 × 1013 | ||||||||||
| Alum/1.7 mg L−1 (Al) | Synthetic (HA) 7.5 mg L−1 pH 8.3 | MF GVWP 0.22 μm (nominal) | 100 | 60 | 6000 | 25 | 1200 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
N/A | N/A | 9.00 × 107 | 1.25 × 106 | 42 |
| 2.6 mg L−1 (Al) | 6.50 × 107 | 0.95 × 106 | |||||||||||
| 3.4 mg L−1 (Al) | 2.50 × 107 | 0.50 × 106 | |||||||||||
| 5.1 mg L−1 (Al) | 2.75 × 107 | 0.70 × 106 | |||||||||||
| 6.8 mg L−1 (Al) | 3.00 × 107 | 0.90 × 106 | |||||||||||
| 8.5 mg L−1 (Al) | 3.00 × 107 | 1.10 × 106 | |||||||||||
| PACl/2.6 mg L−1 (Al) | 12.5 × 107 | 1.70 × 106 | |||||||||||
| 3.4 mg L−1 (Al) | 6.00 × 107 | 1.05 × 106 | |||||||||||
| 5.1 mg L−1 (Al) | 6.00 × 107 | 1.40 × 106 | |||||||||||
| 6.8 mg L−1 (Al) | 6.00 × 107 | 1.60 × 106 | |||||||||||
| 8.5 mg L−1 (Al) | 7.00 × 107 | 2.00 × 106 | |||||||||||
| ACH/2.6 mg L−1 (Al) | 35.0 × 107 | 4.50 × 106 | |||||||||||
| 3.4 mg L−1 (Al) | 12.0 × 107 | 2.20 × 106 | |||||||||||
| 5.1 mg L−1 (Al) | 7.00 × 107 | 1.50 × 106 | |||||||||||
| 6.8 mg L−1 (Al) | 7.00 × 107 | 1.90 × 106 | |||||||||||
| 8.5 mg L−1 (Al) | 7.00 × 107 | 2.20 × 106 | |||||||||||
| PACl/3.18 mg L−1 (Al2O3) | River 2–3 mg L−1 DOC pH N/A | MF GVWP 0.22 μm (nominal) | 150 | 3 min | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
45 | 20 min | N/A | N/A | N/A | 0.80 × 1012 | N/A | 28 |
| 1 h | 0.70 × 1012 | ||||||||||||
| 2 h | 0.60 × 1012 | ||||||||||||
| 4 h | 0.25 × 1012 | ||||||||||||
| 8 h | 0.20 × 1012 | ||||||||||||
| Al2SO4/0.11 mM | Natural 6.30–7.81 mg L−1 DOC pH 6.9–7.1 | MF Cellulose acetate 0.22 μm | 300 rpm | 60 | N/A | 30 rpm | 30–90 | N/A | N/A | 0.49 × 1011 | N/A | 1.10 × 1017 | 38 |
| 0.22 mM | 0.62 × 1011 | 0.84 × 1017 | |||||||||||
| 0.33 mM | 0.73 × 1011 | 0.71 × 1017 | |||||||||||
| PACl/0.15 mM | 0.89 × 1011 | 1.62 × 1017 | |||||||||||
| 0.30 mM | 1.22 × 1011 | 1.29 × 1017 | |||||||||||
| 0.45 mM | 1.45 × 1011 | 1.08 × 1017 | |||||||||||
| FeSO4/0.13 mM | 1.03 × 1011 | 1.58 × 1017 | |||||||||||
| 0.22 mM | 0.76 × 1011 | 0.78 × 1017 | |||||||||||
| 0.31 mM | 2.01 × 1011 | 0.69 × 1017 | |||||||||||
| FeCl3/0.09 mM | 0.94 × 1011 | 1.89 × 1017 | |||||||||||
| 0.18 mM | 1.17 × 1011 | 1.41 × 1017 | |||||||||||
| 0.27 mM | 1.15 × 1011 | 0.98 × 1017 | |||||||||||
| Type 3: conventional coagulation | |||||||||||||
| Alum/10 mg L−1 | River 2.0–2.5 mg L−1 TOC pH 7–8 | MF PVDF 0.22 μm (nominal) | 100 rpm | 3 min | N/A | 30 rpm | 20 min | N/A | N/A | N/A | 3.5 × 1011 | N/A | 44 |
| 4.0 × 1011 | |||||||||||||
| 4.5 × 1011 | |||||||||||||
| 4.5 × 1011 | |||||||||||||
| 100 mg L−1 | 2.5 × 1012 | ||||||||||||
| 3.0 × 1012 | |||||||||||||
| 3.4 × 1012 | |||||||||||||
| 4.0 × 1012 | |||||||||||||
| Coagulant/dosage | Feedwater | Membrane type | Coagulation | Flocculation | Membrane resistance | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) (s−1) (s−1) |
Time (s) |
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t ·t |
R (t) (m−1) | R c(t) (m−1) | α (m kg−1) | (m−2) | ||||
| a N/A = not available. b 2 ppm FeCl3 dosed during first cycle and 1 ppm in subsequent cycles over first 30 min of a 60 min permeation cycle. c Lower values in range typical of Type 2, upper values Type 1; not distinguished in study, therefore data only presented for Type 2. | |||||||||||||
| Type 1: coagulation + no/incidental flocculation | |||||||||||||
| No coagulant | Lake (biopolymers) pH N/A | UF PES 150 kDa MWCO | N/Aa | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 12 | |
| 0.22 mg L−1 | 2.4 × 1012 | ||||||||||||
| 0.24 mg L−1 | 2.0 × 1012 | ||||||||||||
| 0.47 mg L−1 | 3.6 × 1012 | ||||||||||||
| 0.50 mg L−1 | 2.5 × 1012 | ||||||||||||
| 0.51 mg L−1 | 3.1 × 1012 | ||||||||||||
| 0.54 mg L−1 | 4.0 × 1012 | ||||||||||||
| 0.57 mg L−1 | 3.0 × 1012 | ||||||||||||
| 0.62 mg L−1 | 3.9 × 1012 | ||||||||||||
| 0.80 mg L−1 | 5.3 × 1012 | ||||||||||||
| 0.89 mg L−1 | 3.8 × 1012 | ||||||||||||
| 0.90 mg L−1 | 5.7 × 1012 | ||||||||||||
| No coagulant | Synthetic (HA/SA) 10 mg L−1 TOC pH 7.8 | UF PES 150 kDa MWCO | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 71 | |
| HA | 1.50 × 1014 | ||||||||||||
| + 1 mM Ca2+ | 3.00–3.50 × 1014 | ||||||||||||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HA/SA) 1 (HA/SA) |
4.20 × 1014 | ||||||||||||
| + 1 mM Ca2+ | 2.20–2.25 × 1015 | ||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HA/SA) 1 (HA/SA) |
5.70 × 1015 | ||||||||||||
| + 1 mM Ca2+ | 3.10–3.90 × 1015 | ||||||||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (HA/SA) 3 (HA/SA) |
8.50 × 1014 | ||||||||||||
| + 1 mM Ca2+ | 3.90–4.00 × 1015 | ||||||||||||
| SA | 1.80 × 1015 | ||||||||||||
| + 1 mM Ca2+ | 4.00–4.60 × 1015 | ||||||||||||
| No coagulant | Synthetic (HA) DOC N/A pH 4.81–8.73 | UF PES 100 kDa MWCO | 60 rpm | 3 min | N/A | N/A | N/A | N/A | 12 × 1010 | N/A | N/A | N/A | 32 |
| Alum/0.59 mg L−1 Al | 110 × 1010 | ||||||||||||
| 0.59 mg L−1 Al | 35 × 1010 | ||||||||||||
| 1.17 mg L−1 Al | 6 × 1010 | ||||||||||||
| 1.76 mg L−1 Al | 7 × 1010 | ||||||||||||
| 2.34 mg L−1 Al | 8.5 × 1010 | ||||||||||||
| 2.93 mg L−1 Al | 10 × 1010 | ||||||||||||
| No coagulant | Lake 3909 ppb TOC pH N/A | UF PES 100 kDa MWCO | N/A | N/A | N/A | N/A | N/A | N/A | 9.7–15.0 × 1011 | N/A | N/A | N/A | 9 |
| FeCl3/1.0 ppm | 7.4–8.2 × 1011 | ||||||||||||
| 2.0 ppm | 7.4–8.9 × 1011 | ||||||||||||
| 5.0 ppm | 7.4–10.2 × 1011 | ||||||||||||
| 2.0/1.0 ppmb | 5.3–10.4 × 1011 | ||||||||||||
| Alum/70 mg L−1 | Synthetic (HA) 10 mg L−1 TOC pH 4.8–5.5 | UF PES 50 kDa MWCO | N/A | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5.25 | 360 | 1890 | N/A | N/A | 3.17–5.93 × 1013 | N/A | 22 |
| No coagulant | River 2.3–2.9 ppm DOC pH 7.2–7.8 | UF PS 0.01 μm (nominal) | N/A | N/A | N/A | 0 | 720 | N/A | N/A | 2.1–3.7 × 1010 | 0.1–0.5 × 1013 | N/A | 29 |
| PACl/4.1 ppm (Al2O3) | 7.0–9.0 × 1013 | ||||||||||||
| Type 2: coagulation + flocculation | |||||||||||||
| Alum/70 mg L−1 | Synthetic (HA) 10 mg L−1 TOC pH 4.8–5.5 | UF PES 50 kDa MWC O | N/A | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
14.85 | 1200 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
N/A | N/A | 3.17–4.16 × 1013 | N/A | 22 |
| 42.00 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
3.10–5.10 × 1013 | |||||||||||
| No coagulant | River 2.3–2.9 ppm DOC pH 7.2–7.8 | UF PS 0.01 μm (nominal) | N/A | N/A | N/A | N/A | N/A | 0.1–0.5 × 1013 | N/A | 29 | |||
| PACl/4.1 ppm (Al2O3) | 58–350c | 1800–3600 | 1.8–5.4 × 109 | 6.0 × 1012–1.1 × 1013 | |||||||||
| Type 3: conventional coagulation | |||||||||||||
| Alum/70 mg L−1 | Synthetic (HA) 10 mg L−1 TOC pH 4.8–5.5 | UF PES 50 kDa MWCO | N/A | N/A | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
5.25 | 360 | 1890 | N/A | N/A | 3.37–6.79 × 1013 | N/A | 22 |
| 14.85 | 1200 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 820 |
7.96–9.80 × 1013 | ||||||||||
| 42.00 | 1200 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
9.29–11.91 × 1013 | ||||||||||
| No coagulant | Lake 2.13 ± 0.08 mg L−1 TOC pH 8.18 ± 0.2 | UF PVDF 0.04 μm (nominal) | N/A | N/A | N/A | N/A | N/A | N/A | 4.00–8.25 × 1011 | N/A | N/A | N/A | 8 |
| Alum/0.5 mg L−1 | 4.30–7.30 × 1011 | ||||||||||||
| 15 mg L−1 | 5.65–10.80 × 1011 | ||||||||||||
| No coagulant | Lake 4.25 ± 0.06 mg L−1 TOC pH 8.05 ± 0.12 | 8.60–13.40 × 1011 | |||||||||||
| Alum/0.5 mg L−1 | 4.05–7.80 × 1011 | ||||||||||||
| 15 mg L−1 | 2.05–7.80 × 1011 | ||||||||||||
| No coagulant | River 5.99 mg L−1 TOC pH 8.1 ± 0.37 | 14.65–17.00 × 1011 | |||||||||||
| Alum/0.5 mg L−1 | 5.65–11.25 × 1011 | ||||||||||||
| 15 mg L−1 | 8.25–11.75 × 1011 | ||||||||||||
4.3. Type 1: coagulation + no/incidental flocculation
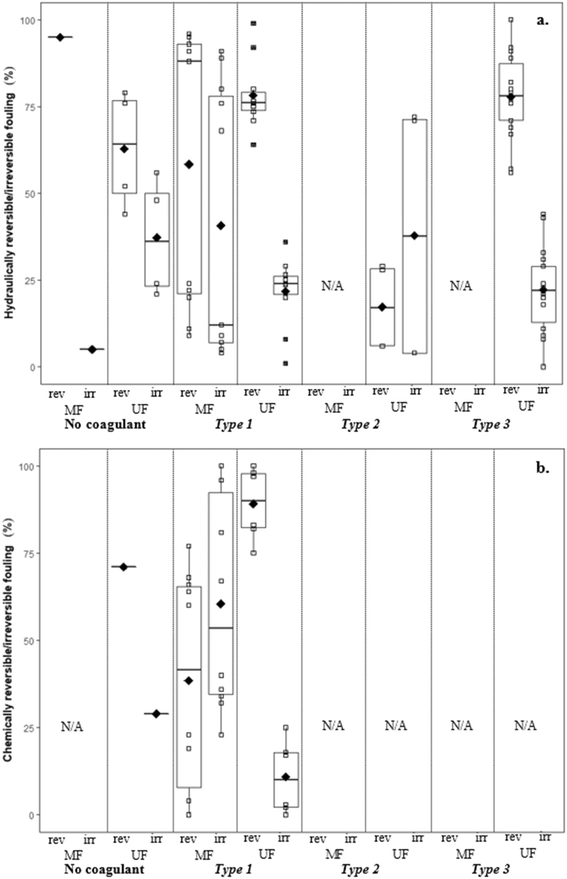
There are no apparent trends in the results for total membrane resistance (Fig. 3a); however, both Pronk9 and Choi and Dempsey32 reported lower fouling for configuration Type 1 when compared to no coagulant addition. The limited results presented in Fig. 3b suggest that cake layer resistance for UF may be lower for configuration Type 1 when compared to no coagulant and Type 3, but higher when compared to Type 2. It was suggested that greater specific cake layer resistance for Type 1 could be due to the formation of smaller floc, which may form a less permeable cake layer when compared to the larger floc formed by configuration Type 2 (ref. 22 and 29) (Fig. 3c). As noted by Amjad,22 specific cake layer resistance for Type 1 is likely to be lower when compared to that of Type 3 because of smaller floc that remain following settling (Fig. 3c). Specific cake layer resistance has been reported to decrease as rapid mixing time increased (20 min to 8 h) due to a decrease in the fractal dimension of floc.28 Lower fractal values may not reduce resistance where compression occurs, although this may be negligible when operating under low or moderate pressures (e.g. ≤40 kPa). Amjad22 observed that while cake layer thickness and resistance increased over the duration of a permeation cycle, porosity also increased.
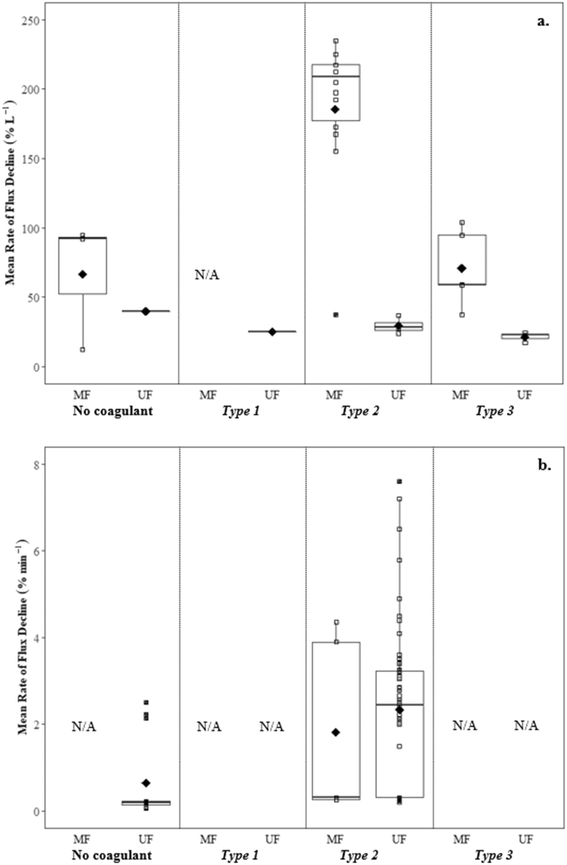
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t when compared to flux declines for medium (∼36.7% L−1) and high
t when compared to flux declines for medium (∼36.7% L−1) and high ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t (∼36.7% L−1, ∼30.0% L−1) (Table 6). Low flux decline with low
t (∼36.7% L−1, ∼30.0% L−1) (Table 6). Low flux decline with low ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t coincided with the smallest floc size and lowest solids removal (60.3%).
t coincided with the smallest floc size and lowest solids removal (60.3%).
| Coagulant/dosage | Feedwater | Membrane type | Backwash frequency | Permeation duration | Initial TMP | Mean rate of TMP increase | Ref. |
|---|---|---|---|---|---|---|---|
| a N/A = not available. b Crossflow mode. c Dead-end mode. | |||||||
| Type 1: coagulation + no/incidental flocculation | |||||||
| No coagulant | Reservoir | MF PES 0.1 μm (mean) | 1/10 min | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
N/Aa | 0.05 kPa min−1 | 27 |
| Fe-based/0.018 mM (Fe) | 8000 s | 0.11 kPa min−1 | |||||
| 0.027 mM (Fe) | 2.4 mg L−1 TOC | 5000 s | 0.11 kPa min−1 | ||||
| 0.036 mM (Fe) | 8000 s | 0.05 kPa min−1 | |||||
| 0.045 mM (Fe) | pH ∼5.4 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 s 500 s |
0.03 kPa min−1 | ||||
| 0.054 mM (Fe) | 5000 s | 0.03 kPa min−1 | |||||
| 0.072 mM (Fe) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
0.01 kPa min−1 | |||||
| No coagulant | River | UF PS 0.01 μm (nominal) | 1/60 min | 9 hb | ∼45–50 kPa | 0.037 kPa min−1 | 29 |
| PACl/4.1 ppm (Al2O3) | 2.3–2.9 ppm DOC | 26 h | 0.013 kPa min−1 | ||||
| No coagulant | pH 7.2–7.8 | 1/20 min | 2 hb | 0.167 kPa min−1 | |||
| 4.1 ppm (Al2O3) | 37 h | 0.009 kPa min−1 | |||||
| Type 2: coagulation + flocculation | |||||||
| 4.1 ppm (Al2O3) | River | UF PS 0.01 μm (nominal) | 1/60 min | 30 hc | ∼45–50 kPa | 0.011 kPa min−1 | 29 |
| 2.3–2.9 ppm DOC | |||||||
| 4.1 ppm (Al2O3) | 1/20 min | 50 hc | 0.007 kPa min−1 | ||||
| pH 7.2–7.8 | |||||||
| Coagulant/dosage | Feedwater | Membrane type | Coagulation/flocculation conditions | Volume filtered per cycle | Permeation time per cycle | Mean rate of flux decline | Ref. |
|---|---|---|---|---|---|---|---|
| a Not available. | |||||||
| Type 1: coagulation + no/incidental flocculation | |||||||
| Alum/70 mg L−1 | Synthetic (HA) | UF PES 50 kDa MWCO | Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (5.25 s−1) 600 + 1200 s slow mix (5.25 s−1) |
∼1.5 L | N/Aa | 25% L−1 | 22 |
| 10 mg L−1 TOC | |||||||
| pH 4.8–5.5 | |||||||
| No coagulant | Synthetic (HA/SA) | UF | N/A | N/A | 71 | ||
| 10 mg L−1 TOC | |||||||
| pH 7.8 | |||||||
| HA | 6.5 h | 0.14% min−1 | |||||
| +1 mM Ca2+ | 7 h | 0.19% min−1 | |||||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HA/SA) 1 (HA/SA) |
7 h | 0.07% min−1 | |||||
| +1 mM Ca2+ | 6.75 h | 0.20% min−1 | |||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (HA/SA) 1 (HA/SA) |
7 h | 0.08% min−1 | |||||
| +1 mM Ca2+ | PE | 6.75 h | 0.21% min−1 | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (HA/SA) 3 (HA/SA) |
7 h | 0.10% min−1 | |||||
| +1 mM Ca2+ | 150 kDa MWCO | 7 h | 0.21% min−1 | ||||
| SA | 6.5 h | 0.17% min−1 | |||||
| +1 mM Ca2+ | 6.5 h | 0.22% min−1 | |||||
| Type 2: coagulation + flocculation | |||||||
| No coagulant | River | MF | Stirred | 900 mL | N/A | 92.2% L−1 | 24 |
| Alum/3.2 mg L−1 (Al) | 9.0 mg L−1 DOC | PP | |||||
| 58.9% L−1 | |||||||
| pH 6.0 | 0.2 μm (nominal) | ||||||
| No coagulant | Synthetic (HA) | MF | 60 s rapid mix (100 s−1) + 1200 s slow mix (25 s−1) | 400 mL | N/A | <12.5% L−1 | 42 |
| Alum/1.7 mg L−1 (Al) | 212.5% L−1 | ||||||
| 2.6 mg L−1 (Al) | 172.5% L−1 | ||||||
| 3.4 mg L−1 (Al) | 155% L−1 | ||||||
| 5.1 mg L−1 (Al) | 167.5% L−1 | ||||||
| 6.8 mg L−1 (Al) | 197.5% L−1 | ||||||
| 8.5 mg L−1 (Al) | 197.5% L−1 | ||||||
| PACl/1.7 mg L−1 (Al) | 37.5% L−1 | ||||||
| 2.6 mg L−1 (Al) | 7.5 mg L−1 | GVWP | 217.5% L−1 | ||||
| 3.4 mg L−1 (Al) | 192.5% L−1 | ||||||
| 5.1 mg L−1 (Al) | 205% L−1 | ||||||
| 6.8 mg L−1 (Al) | 212.5% L−1 | ||||||
| 8.5 mg L−1 (Al) | 217.5% L−1 | ||||||
| ACH/1.7 mg L−1 (Al) | pH 8.3 | 0.22 μm (nominal) | 37.5% L−1 | ||||
| 2.6 mg L−1 (Al) | 235% L−1 | ||||||
| 3.4 mg L−1 (Al) | 225% L−1 | ||||||
| 5.1 mg L−1 (Al) | 212.5% L−1 | ||||||
| 6.8 mg L−1 (Al) | 217.5% L−1 | ||||||
| 8.5 mg L−1 (Al) | 225% L−1 | ||||||
| PACl/3.18 mg L−1 (Al2O3) | River | MF | 3 min–8 h rapid mix (150 s−1) | N/A | 15 min | 3.8–4.9% min−1 | 28 |
| 2–3 mg L−1 DOC | GVWP | ||||||
| pH N/Aa | 0.22 μm (nominal) | 3 min rapid mix (150 s−1) + 3 min–8 h slow mix (45 s−1) | 2.9–4.9% min−1 | ||||
| AC/8 mg L−1 (Al) | Synthetic (HA) | UF | 90 s rapid mix (200 rpm) + 900 s slow mix (40 rpm) | N/A | 300 min | 0.21–0.26% min−1 | 41 |
| PACb/8 mg L−1 (Al) | 5.35 mg L−1 DOC | PES | 300 min | 0.23–0.24% min−1 | |||
| PACc/8 mg L−1 (Al) | pH 4–8 | 100 kDa MWCO | 275–300 min | 0.20–0.26% min−1 | |||
| No coagulant | Synthetic (HA) | UF | 90 s rapid mix (175 s−1) + 900 s slow mix (20 s−1) | 1000 mL | N/A | 40% L−1 | 33 |
| PACl/0.025 mM (Al) | 5 mg L−1 | PES | 27% L−1 | ||||
| PACl/0.1 mM (Al) | pH 7.5 | 100 kDa MWCO | 24% L−1 | ||||
| FeCl3/22 mg L−1 (Fe) | Synthetic (HA) | UF | 90 s rapid mix (200 rpm) + 900 s slow mix (40 rpm) | N/A | ∼14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s 000 s |
0.21–0.30% min−1 | 43 |
| PFC10/22 mg L−1 (Fe) | 4.67 ± 0.25 mg L−1 DOC | PES | 0.21–0.31% min−1 | ||||
| PFC22/22 mg L−1 (Fe) | pH 4–9 | 100 kDa MWCO | 0.31% min−1 | ||||
| synthetic (HA/BSA) pH 6 | UF PES 50 kDa MWCO | 60 s rapid mix (250 rpm) + 740 s slow mix (100 rpm) | N/A | 600–700 s | 26 | ||
| AlCl3/0.25–50 μM (Al) | 10 mg L−1 HA | 0.07–1.54% min−1 | |||||
| AlCl3/0.25–50 μM (Al) | 5 mg L−1 HA + 5 mg L−1 BSA | 3.20–3.90% min−1 | |||||
| AlCl3/1–50 μM (Al) | 10 mg L−1 BSA | 2.00–7.60% min−1 | |||||
| pH 7 | |||||||
| No coagulant | 10 mg L−1 HA | 2.23% min−1 | |||||
| AlCl3/2.5–30 μM (Al) | 2.23–3.00% min−1 | ||||||
| No coagulant | 5 mg L−1 HA + | 2.30% min−1 | |||||
| AlCl3/2.5–20 μM (Al) | 5 mg L−1 BSA | 2.49–3.23% min−1 | |||||
| No coagulant | 10 mg L−1 BSA | 2.50% min−1 | |||||
| AlCl3/1–20 μM (Al) | 2.60–6.40% min−1 | ||||||
| pH 8 | |||||||
| AlCl3/0.025–30 μM (Al) | 10 mg L−1 HA | 2.12–3.05% min−1 | |||||
| AlCl3/0.25–100 μM (Al) | 5 mg L−1 HA + 5 mg L−1 BSA | 2.03–3.23% min−1 | |||||
| AlCl3/0.25–30 μM (Al) | 10 mg L−1 BSA | 2.14–2.83% min−1 | |||||
| Alum/70 mg L−1 | Synthetic (HA) | UF | Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (14.85 s−1) 600 + 1200 s slow mix (14.85 s−1) |
∼1.5 L | N/A | 36.7% L−1 | 22 |
| PES | |||||||
| 50 kDa MWCO | |||||||
| 10 mg L−1 TOC | |||||||
Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (42.00 s−1) 600 + 1200 s slow mix (42.00 s−1) |
30.0% L−1 | ||||||
| pH 4.8–5.5 | |||||||
| Type 3: conventional coagulation | |||||||
| No coagulant | River | MF | Settling | 900 mL | N/A | 92.2% L−1 | 24 |
| 9.0 mg L−1 DOC | PP | ||||||
| Alum/3.2 mg L−1 (Al) | pH 6.0 | 0.2 μm (nominal) | 58.9% L−1 | ||||
| No coagulant | River | MF | Rapid mix + 30 min slow mix (60 s−1) | 400–900 mL | N/A | ∼94.7% L−1 | 23 |
| Alum/4 mg L−1 | ∼104% L−1 | ||||||
| 12 mg L−1 | 3.1 mg L−1 DOC | PP | ∼94.7% L−1 | ||||
| 25 mg L−1 | ∼58.7% L−1 | ||||||
| 50 mg L−1 | pH 6.9–7.3 | 0.2 μm (nominal) | ∼37.3% L−1 | ||||
| Alum/70 mg L−1 | Synthetic (HA) | UF | Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (5.25 s−1) 600 + 1200 s slow mix (5.25 s−1) |
∼1.5 L | N/A | 22.7% L−1 | 22 |
| 10 mg L−1 TOC | PES | Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (14.85 s−1) 600 + 1200 s slow mix (14.85 s−1) |
24.0% L−1 | ||||
| pH 4.8–5.5 | 50 kDa MWCO | Rapid mix ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) ·t 12 ·t 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 + 1200 s slow mix (42.00 s−1) 600 + 1200 s slow mix (42.00 s−1) |
17.3% L−1 | ||||
| Coagulant/dosage | Feedwater | Membrane type | Hydraulic/chemical cleaning conditions | Reversible/irreversible fouling (%) | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Hydraulically reversible | Hydraulically irreversible | Chemically reversible | Chemically irreversible | |||||
| a Determined after physical wiping. b N/A = not available. c 2 ppm FeCl3 dosed during first cycle and 1 ppm in subsequent cycles over first 30 min of a 60 min permeation cycle. d Reported as normalized fouling. | ||||||||
| Type 1: coagulation + no/incidental flocculation | ||||||||
| PACl/2 mg L−1 (Al) | River | MF | 1 per 30 min for 30 s at 94 LMH/NaClO (24 h) | 70 | ||||
| 1 mg L−1 DOC | 22a | 78 | 23–60 | 40–67 | ||||
| 0.5 mg L−1 DOC | NaOH (24 h) | 11 | 89 | 0–64 | 36–100 | |||
| 2.0 mg L−1 DOC | PVDF | HCl (24 h) | 9 | 91 | 4–66 | 34–96 | ||
| 1.6 mg L−1 DOC | 0.1 μm | 24 | 76 | 4–68 | 32–96 | |||
| 1.0 mg L−1 DOC | 20 | 80 | 19–77 | 23–81 | ||||
| No coagulant | Reservoir | MF | 1 per 10 min at 200 LMH and 2 bar | 95 | 5 | N/Ab | N/A | 27 |
| Fe-based/0.018 mM (Fe) | 95 | 5 | ||||||
| 0.027 mM (Fe) | 2.4 mg L−1 TOC | PES | 96 | 4 | ||||
| 0.036 mM (Fe) | 91 | 9 | ||||||
| 0.045 mM (Fe) | pH ∼5.4 | 0.1 μm (mean) | 93 | 7 | ||||
| 0.054 mM (Fe) | 88 | 12 | ||||||
| 0.072 mM (Fe) | 93 | 7 | ||||||
| No coagulant | Lake | UF | 1 per 90 min for 60 s at 0.65 bar and forward flush for 60 s at 0.3 L min−1 | 75–77 | 23–25 | N/A | N/A | 9 |
| FeCl3/ | 3909 ppb TOC | 64–83 | 17–36 | |||||
| 1.0 ppm | pH N/A | PES | ||||||
| 2.0 ppm | 68–84 | 16–32 | ||||||
| 5.0 ppm | 100 kDa MWCO | 70–83 | 17–30 | |||||
| 2.0/1.0 ppmc | 69–73 | 27–31 | ||||||
| No coagulant | Synthetic (HA) | UF | N/A/NaOH then DI water permeation for 30 min each at 150 LMH | 79 | 21 | 71 | 29 | 32 |
| Alum/0.59 mg L−1 Al | 75 | 25 | 98 | 2 | ||||
| 0.59 mg L−1 Al | PES | 99 | 1 | 97 | 3 | |||
| 1.17 mg L−1 Al | DOC N/A | 92 | 8 | 83 | 17 | |||
| 1.76 mg L−1 Al | 100 kDa MWCO | 64 | 36 | 100 | 0 | |||
| 2.34 mg L−1 Al | pH 4.81–8.73 | 76 | 24 | 82 | 18 | |||
| 2.93 mg L−1 Al | 80 | 20 | 75 | 25 | ||||
| Type 2: coagulation + flocculation | ||||||||
| No coagulant | Synthetic | UF | 1 per 350 mL with 100 mL ultrapure water | 44–52d | 48–56 | N/A | N/A | 33 |
| PACl/0.025 mM (Al) | 5 mg L−1 HA | PES | 28–29 | 71–72 | ||||
| PACl/0.1 mM (Al) | pH 7.5 | 100 kDa MWCO | 6 | 4 | ||||
| Type 3: conventional coagulation | ||||||||
| No coagulant | Lake | UF | 1 per 30 min for 10 min at 30 LMH | 76–100 | 0–24 | N/A | N/A | 8 |
| Alum/0.5 mg L−1 | 2.13 ± 0.08 mg L−1 TOC | 57–79 | 21–43 | |||||
| 15 mg L−1 | pH 8.18 ± 0.2 | 56–79 | 21–44 | |||||
| No coagulant | Lake | 76–89 | 11–24 | |||||
| Alum/0.5 mg L−1 | 4.25 ± 0.06 mg L−1 TOC | PVDF | 71–89 | 11–29 | ||||
| 15 mg L−1 | pH 8.05 ± 0.12 | 71–80 | 20–29 | |||||
| No coagulant | River | 0.04 μm (nominal) | 77–91 | 9–23 | ||||
| Alum/0.5 mg L−1 | 5.99 mg L−1 TOC | 69–92 | 8–31 | |||||
| 15 mg L−1 | pH 8.1 ± 0.37 | 67–82 | 18–33 | |||||
Several studies investigated the impact of coagulant dose on reversible/irreversible fouling. Applying a range of coagulant dosages at various pH levels, Choi and Dempsey32 observed comparatively high hydraulically and chemically reversible fouling for a low coagulant dose (0.59 mg L−1) (99% and 97%). This may have been due to partial charge neutralization, and formation of floc with a slightly negative charge that are more readily removed from the negatively charged membrane surface. Depending on coagulation conditions, results suggest that there may be a trade-off between greater membrane fouling and more frequent cleaning, as well as the recovery of permeability (i.e. increased fouling but higher permeability recovery). While specific hydraulic resistance decreased with increasing coagulant dose, Judd and Hillis27 reported that the ratio of hydraulically reversible/irreversible resistance remained approximately the same at all coagulant dosages.
4.4. Type 2: coagulation + flocculation
Operating MF membranes in dead-end mode, Lee44 reported that relative specific cake layer resistance (αcoagulation/αraw) was lower when charge neutralization was the destabilization mechanism (<0.7) when compared to sweep flocculation (>1.0). Floc size distributions were similar for both mechanisms, thus it was suggested that the difference was due to floc compressibility, where floc formed under sweep flocculation were three times more compressible than floc formed by charge neutralization. That floc formed by sweep flocculation are more compressible appears counter-intuitive given that several studies have reported that floc formed by sweep flocculation have higher Df than those formed by charge neutralization. However, the higher compressibility of floc formed by sweep flocculation may be due to their higher water content, while at the same time they are gelated, more compact, and less porous because of being predominantly made up of aluminum hydroxide precipitates.44 Lee44 suggested that floc formed by charge neutralization consist of aluminum cation and inorganic/organic complexes that are less compressible.
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t followed by direct filtration Amjad22 observed lower specific cake layer resistances for Type 2, but a greater rate of flux decline. This was attributed to a greater mass flux towards the membrane surface without settling.
t followed by direct filtration Amjad22 observed lower specific cake layer resistances for Type 2, but a greater rate of flux decline. This was attributed to a greater mass flux towards the membrane surface without settling.
Again, it was observed that a threshold coagulant dose exists below which fouling rate may increase. However, it may also be the case that low coagulant doses result in lower flux decline due to insufficient floc development and fewer particles being retained on the membrane.42 Ma26 observed critical doses of Al that resulted in dramatic flux reduction for water matrices containing humic acid (HA), bovine serum albumin (BSA), and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mass ratio of HA/BSA, which were mainly induced by particle size. Flux decline varied slightly with pH for HA and significantly for BSA solutions, indicating that NOM type plays an important role in fouling. Dong43 reported that at pH ranging from 7.0 to 9.0 flux declines were more dramatic than at pH ranging from 4.0 to 6.0. It was suggested that lower fouling rates at lower pH were due to either greater floc size or lower Df. In addition, the better performance of FeCl3 and polyferric chloride with basicity of 1.0 (PFC10) at pH 6.0 and 7.0 was attributed to the predominance of monomeric and polymeric species.43
1 mass ratio of HA/BSA, which were mainly induced by particle size. Flux decline varied slightly with pH for HA and significantly for BSA solutions, indicating that NOM type plays an important role in fouling. Dong43 reported that at pH ranging from 7.0 to 9.0 flux declines were more dramatic than at pH ranging from 4.0 to 6.0. It was suggested that lower fouling rates at lower pH were due to either greater floc size or lower Df. In addition, the better performance of FeCl3 and polyferric chloride with basicity of 1.0 (PFC10) at pH 6.0 and 7.0 was attributed to the predominance of monomeric and polymeric species.43
4.5. Type 3: conventional coagulation
![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t conditions followed by settling, Amjad22 reported lower rates of flux decline with settling (ranging from 17.3 to 24.0% L−1) than without settling (ranging from 25.0 to 36.7% L−1), likely due to the reduction of solids in the feedwater when measured gravimetrically. Multiple studies have reported similar flux declines when evaluating raw water and water following coagulation with an optimum coagulant dosage for turbidity removal,23,24 suggesting NOM as being the main contributor to flux decline. At higher coagulant dosages more suitable for NOM removal (3.2 to 4.0 mg L−1 Al3+), reported flux declines were much lower.
t conditions followed by settling, Amjad22 reported lower rates of flux decline with settling (ranging from 17.3 to 24.0% L−1) than without settling (ranging from 25.0 to 36.7% L−1), likely due to the reduction of solids in the feedwater when measured gravimetrically. Multiple studies have reported similar flux declines when evaluating raw water and water following coagulation with an optimum coagulant dosage for turbidity removal,23,24 suggesting NOM as being the main contributor to flux decline. At higher coagulant dosages more suitable for NOM removal (3.2 to 4.0 mg L−1 Al3+), reported flux declines were much lower.
Wray and Andrews8 reported different results for the impact of coagulant dose on hydraulically reversible/irreversible fouling for various source waters. It was reported that for Lake Ontario, the addition of 15 mg L−1 alum increased reversible fouling, and greater variability in the hydraulic reversibility of fouling was observed at this higher coagulant dose. In contrast, for Lake Simcoe and Otonabee River waters the addition of alum reduced reversible fouling at dosages of both 0.5 and 15 mg L−1. As mentioned in section 2.2.3., this emphasizes the importance of differences in water quality when considering coagulant dosages for membrane pretreatment.
5. Performance of configuration types related to removal of organic matter
5.1. Summary of results
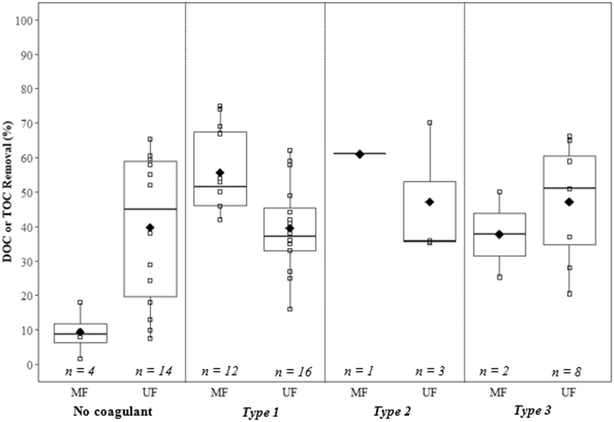
Removals of dissolved and total organic carbon (DOC/TOC) for coagulation/flocculation configuration types are summarized in Fig. 6 (Table 9). For comparison with configuration types, results for membrane filtration with no coagulant addition have also been included. While less results are available for configuration Type 1 regarding floc properties, when compared to Types 2 and 3 there are more results for Type 1 regarding DOC/TOC removal. This may be because the analysis of floc properties for configuration Type 1 is more difficult due to smaller floc size.5.2. Type 1: coagulation + no/incidental flocculation
A number of studies have investigated the impact of coagulant dosage, coagulant type, and hydrodynamic conditions on the removal of organics and particulates for configuration Type 1. Reported DOC/TOC removal ranges from 42% to 75%, with a mean of 56% for MF, and 16% to 62%, with a mean of 40% for UF (Fig. 6). The mean removal for MF is greater than that reported for no coagulant (9%), below the mean value for configuration Type 2 (61%), and above the mean for configuration Type 3 (38%). The mean removal for UF is approximately the same as that reported for no coagulant (40%), and below the mean values for both configuration Types 2 (47%) and 3 (47%). With no coagulant, results indicate that UF achieves greater DOC/TOC removal when compared to MF, but with the addition of coagulant performance is similar for all configuration types. In general, results suggest that the removal of DOC/TOC increases with increasing coagulant doses.25,27 There is evidence that charge neutralization, sweep flocculation, and low dose conditions at pH <5.0 are favorable for the removal of DOC/TOC, while low dose conditions at pH 7.0 to 9.0 lead to poor removal.30,32 Fe-Based coagulants may perform better than Al-based coagulants for biopolymer removal, possibly due to faster generation rates of hydrolysis products and precipitates.75 Guigui25 noted that at approximately neutral pH (5.5 to 7.5), there was a greater variance in DOC removal for high (16 to 42%) compared to low (27 to 33%) coagulant doses.Amjad22 reported poor solids removal (60.3%, as measured gravimetrically) at low ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t (1890) for configuration Type 1 when compared to higher
t (1890) for configuration Type 1 when compared to higher ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t for Types 2 and 3, likely because of incomplete coagulation and dissolved solids not being retained on the membrane surface. Similarly, Howe and Clark23 reported turbidity reductions after MF of 57% for rapid mixing alone compared to ≥89% for configuration Type 3. Again, this may be expected due to the formation of smaller floc, resulting in lower retention on the membrane surface.
t for Types 2 and 3, likely because of incomplete coagulation and dissolved solids not being retained on the membrane surface. Similarly, Howe and Clark23 reported turbidity reductions after MF of 57% for rapid mixing alone compared to ≥89% for configuration Type 3. Again, this may be expected due to the formation of smaller floc, resulting in lower retention on the membrane surface.
5.3. Type 2: coagulation + flocculation
As for configuration Type 1, studies for configuration Type 2 have examined the impact of coagulant dosage, type, and hydrodynamic conditions on the removal of DOC/TOC and particulates. The reported DOC/TOC removal is 61% for MF, and ranges from 35% to 70%, with a mean of 47% for UF (Fig. 6). The reported removal for MF is greater than that reported for no coagulant, as well as configuration Types 1 and 3, while the me removal reported for UF is greater than that reported for both no coagulant and configuration Type 1, and approximately the same as Type 3. Comparing the removal of TOC by MF for no coagulant addition with that obtained with charge neutralization (10 mg L−1, pH 5.0) and sweep flocculation (30 mg L−1, pH 7.5) mechanisms, Lee44 observed the greatest reduction in TOC for charge neutralization. The greater TOC removal may be attributed to the charge of soluble organics being less electronegative at pH 5.0, thus improving particle agglomeration. At pH 8.3 with sweep flocculation as the predominant coagulation mechanism, Wang42 reported UV254 removals of ≥90% for alum, PACl, and ACH doses of ≥1.7, 2.6, and 3.4 mg L−1 Al, respectively. Yao33 observed greater reductions in both DOC and UV254 using an alum dosage optimized for turbidity removal compared to a low dosage. With a coagulant dosage optimized for DOC and UV254 removal, Zhang40 reported greater removals of both DOC and UV254 for coagulation–UF compared to UF with no coagulant addition.Considering medium and high ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t (17
t (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 and 50
820 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) for configuration Type 2, Amjad22 observed nearly 100% solids removal following UF. This solids removal was greater than the removal reported for configuration Type 1, and indicated effective aggregation of humic acid. Howe and Clark23 observed similar turbidity reductions (57%) after rapid mixing with 4 min flocculation (65 rpm) followed by MF or UF. Despite the addition of flocculation, turbidity removal was the same as that for 30 s rapid mixing only.
400) for configuration Type 2, Amjad22 observed nearly 100% solids removal following UF. This solids removal was greater than the removal reported for configuration Type 1, and indicated effective aggregation of humic acid. Howe and Clark23 observed similar turbidity reductions (57%) after rapid mixing with 4 min flocculation (65 rpm) followed by MF or UF. Despite the addition of flocculation, turbidity removal was the same as that for 30 s rapid mixing only.
5.4. Type 3: conventional coagulation
Several studies have considered DOC/TOC removal following configuration Type 3; however, the majority of results reported in the literature are for removal following sedimentation only. DOC/TOC removal after sedimentation ranged from 22% to 51% with a mean of approximately 37% (Table 9). Reported DOC/TOC removal ranges from 25.3% to 50% for MF, with a mean of 38%, while reported DOC/TOC removal ranges from 20.5% to 66.2% for UF, with a mean of 47% (Fig. 6). The mean removal for MF is greater than that reported for no coagulant addition, and lower than that reported for configuration Types 1 and 2, while the mean removal reported for UF is greater than that reported for both no coagulant addition and configuration Type 1, and approximately the same as Type 2. Using alum and PAC, Kabsch-Korbutowicz46 observed TOC removals to increase by 24.1% and 14.8% for configuration Type 3-UF compared to conventional coagulation with settling alone. Dixon47 used Type 3-UF to treat river water with PACl dosages optimized for UV254 removal and enhanced coagulation, and reported similar DOC and UV254 removals after conventional coagulation with settling and Type 3-UF for both coagulant doses. If the removal of organic matter following settling does not increase it could be attributed to dissolved organics and colloids passing through the membrane.24Amjad22 observed that for hydrodynamic conditions of low (1790), medium (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820), and high (50
820), and high (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400)
400) ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t, solids removals following settling were approximately 19%, 76%, and 82%, respectively. Subsequent removals by UF were 41.0%, 22.2%, and 15.5% totaling approximately 60% (similar to low
t, solids removals following settling were approximately 19%, 76%, and 82%, respectively. Subsequent removals by UF were 41.0%, 22.2%, and 15.5% totaling approximately 60% (similar to low ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t without settling), and nearly 100% for the latter two conditions. Solids removals indicate that medium and high
t without settling), and nearly 100% for the latter two conditions. Solids removals indicate that medium and high ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t result in the formation of larger, more settlable floc than low
t result in the formation of larger, more settlable floc than low ![[G with combining overline]](https://www.rsc.org/images/entities/i_char_0047_0305.gif) t that are more readily removed by UF.
t that are more readily removed by UF.
6. Discussion and conclusion
Results indicate that configuration Type 1 (coagulation + no/incidental flocculation) leads to the formation of small floc with low Df. There is evidence that this causes the formation of less porous cake layers with greater resistance than those formed by configuration Type 2 (coagulation + flocculation) (Fig. 3), and results in lower removal of solids and turbidity (section 5.2.). As a result, configuration Type 1 may not be optimal for fouling control and DOC/TOC removal when compared to Types 2 and 3; however, more evidence is required. When considering fouling rate, reversibility, and performance in terms of DOC/TOC removal, floc size has been identified as an important factor (sections 4.2. and 5.2.). While a wide range of coagulant doses have been examined for configuration Type 1, it may be beneficial to achieve a balance between thresholds where internal fouling is minimized and DOC/TOC removal targets are achieved, while limiting additional fouling from the accumulation of hydrolytic coagulant products on the membrane surface (section 2.2.1.). Results of several studies indicate that such a balance may be realized at low dose conditions. While characteristically short contact times (≤2 min) appear adequate for developing floc sizes greater than those of membrane pores, the effects of hydrodynamic conditions on floc properties are not clear, perhaps because of difficulty in accurately simulating these conditions at bench-scale. In addition, more information is needed comparing the impact of NOM type on floc properties and subsequent membrane fouling. It is expected that small floc with low Df would form a cake layer with higher resistance. However, there is also evidence that floc formed at low dosages and acidic pH conditions can result in permeable cake layers with high hydraulic and chemical recoveries (section 3.3), though it is not clear if this would occur for configuration Type 1 without flocculation. Phased coagulation, whereby coagulant is applied during only a portion of the permeation cycle (e.g. 50%), is an operational variation that should be investigated further, as it has been reported that fouling and coagulant requirements may be reduced simultaneously (section 4.2.1.).Application of configuration Type 2 may provide greater control of floc properties. As for configuration Type 1, a range of coagulation doses and destabilization mechanisms have been examined, and as expected for increased particle collisions and contact time, reported floc sizes were generally larger and Df higher (section 3.3.). Floc formed at acidic pH (≤5.0) have a slower growth rate, but reach larger steady-state size, which could lead to the formation of a more porous cake layer. Results also suggest that floc formed at acidic pH have lower Df. During mixing floc with lower Df experienced greater breakage than those formed by the sweep flocculation mechanism with higher Df. The apparent correlation between Df and the degree of floc breakage indicates that a correlation also exists between Df and floc strength. However, it has been reported that floc formed by sweep flocculation are more compressible, which can reduce cake layer permeability due to hydraulic pressure during membrane operation. Future research efforts should be directed towards more clearly distinguishing shear strength and compressibility of floc. While some studies reported that larger floc resulted in lower cake layer resistance, others reported similar resistances to those observed without flocculation (section 4.3.1.). Amjad22 explained that despite forming cake with higher intra-particle permeability, the higher Df of floc could compensate for this with lower inter-particle permeability. Finally, without settling, mass flux of floc towards the membrane surface may be comparatively high. While the larger floc may form a more porous and easily removed cake layer, rapid accumulation of material could increase cake layer thickness causing rapid flux decline (sections 4.4.2. and 4.5.2.). A trade-off may exist between cleaning efficiency and frequency. There is evidence that Type 2 conditions result in greater NOM removal when compared to Type 1 (section 5.3.).
For configuration Type 3 (conventional coagulation), settling prior to membrane filtration results in lower mass accumulation on the membrane surface, thus lower fouling rate, but higher specific cake layer resistance (section 4.5.). While the summarized results for DOC/TOC removal (Fig. 6) do not suggest a clear trend when comparing configuration types, Amjad22 reported greater solids removal for configuration Type 3 when compared to Type 1 and similar solids removal when compared to Type 2. In some cases, it was reported that the rate of flux decline was similar with and without settling, which was attributed to poor removal of NOM despite effective reduction of turbidity (section 4.5.2.). In order to reduce membrane fouling using configuration Type 3, the application of enhanced coagulation for increased NOM removal may be required. Limited information exists regarding the cleaning efficiency of membranes incorporating conventional coagulation (section 4.5.3.). While cake layers are anticipated to be thin, they may also be more compact. In addition, the accumulated foulants may include a lower concentration of hydrolytic coagulant products, which could result in greater irreversible fouling.
This review compiled the results of 36 studies on the impact of coagulation/flocculation pretreatment on floc properties and membrane performance. Despite the significant number of published studies, no clear guidance can yet be obtained to optimally design coagulation/flocculation pretreatment for membrane filtration.
Appendix
| Coagulant/dosage | Feedwater | Membrane type | DOC/TOC removal (%) | Ref. |
|---|---|---|---|---|
| a N/A = not available. | ||||
| No coagulant | ||||
| No coagulant | River | MF | 1.66 (DOC) | 45 |
| 1.53 ± 0.41 mg L−1 DOC | PVDF | 9.66 (DOC) | ||
| pH N/Aa | 0.1 μm (nominal) | |||
| No coagulant | Reservoir | MF | 8 (TOC) | 27 |
| 2.4 mg L−1 TOC | Hydrophilic PES | |||
| pH ∼5.4 | 0.1 μm (mean) | |||
| No coagulant | River | MF | 18 (DOC) | 39 |
| 10.8 ± 0.8 mg L−1 DOC | PVDF | |||
| pH 8.14 ± 0.09 | 0.1 μm (nominal) | |||
| No coagulant | Synthetic (HA,SA,BSA) | UF | 52.1 (TOC) | 76 |
| N/A | 60.5 (TOC) | |||
| 4 mg L−1 TOC | 150 kDa MWCO | 65.3 (TOC) | ||
| pH 7.0±0.3 | UF | 57.9 (TOC) | ||
| PES | 59.4 (TOC) | |||
| 100 kDa MWCO | 65.4 (TOC) | |||
| No coagulant | River | UF | 10 (DOC) | 31 |
| CA | ||||
| 2–6 mg L−1 DOC | 150 kDa MWCO | |||
| UF | 18 (DOC) | |||
| pH 7 | ||||
| PAN | ||||
| 200 kDa MWCO | ||||
| No coagulant | River | UF | 7.5 (DOC) | 48 |
| 5.341–6.29 mg L−1 DOC | PVDF | |||
| pH 7.1–7.5 | 150 kDa MWCO | |||
| No coagulant | Synthetic | UF | 38.0 (TOC) | 46 |
| 9.43 mg L−1 TOC | PES | |||
| 37.0 (TOC) | ||||
| pH 5–10 | 30 kDa MWCO | |||
| No coagulant | Canal | UF | 13 (DOC) | 25 |
| 5.4 ppm DOC | Cellulose | |||
| pH 5.5–7.5 | N/A | |||
| No coagulant | Natural | UF | 24.3 (DOC) | 40 |
| 3.521 ± 1.423 mg L−1 DOC | PVDF | |||
| pH 7.1–7.3 | 0.01 μm (nominal) | |||
| No coagulant | River | UF | 55.1 (TOC) | 30 |
| 6.53 mg L−1 TOC | PES | |||
| pH 7.8–8.0 | N/A | |||
| Type 1: coagulation + no/incidental flocculation | ||||
| Fe-based/0.018 mM (Fe) | Reservoir | MF | 50 (TOC) | 27 |
| 0.027 mM (Fe) | 46 (TOC) | |||
| 0.036 mM (Fe) | 2.4 mg L−1 TOC | Hydrophilic PES | 69 (TOC) | |
| 0.045 mM (Fe) | 42 (TOC) | |||
| 0.054 mM (Fe) | pH ∼5.4 | 0.1 μm (mean) | 54 (TOC) | |
| 0.072 mM (Fe) | 75 (TOC) | |||
| FeCl3/5 ppm Fe | Canal | UF | 33 (DOC) | 25 |
| 5 ppm Fe | 36 (DOC) | |||
| 5 ppm Fe | 5.4 ppm DOC | Cellulose | 27 (DOC) | |
| 10 ppm Fe | 42 (DOC) | |||
| 10 ppm Fe | pH 5.5–7.5 | N/A | 33 (DOC) | |
| 10 ppm Fe | 16 (DOC) | |||
| Alum/0.59–2.93 mg L−1 Al | Synthetic | UF | 35 (TOC) | 32 |
| DOC N/A | PES | |||
| pH 4.81–8.73 | 100 kDa MWCO | |||
| FeCl3/2.8 mg L−1 Fe | River | UF | 44.2 (TOC) | 30 |
| Fe2(SO4)3/2.8 mg L−1 Fe | 6.53 mg L−1 TOC | PES | 59.1 (TOC) | |
| Al2(SO4)3/3.6 mg L−1 Al | pH 7.8–8.0 | N/A | 57.9 (TOC) | |
| 1 mg L−1 Al | River | UF | 25 (DOC) | 31 |
| 5 mg L−1 Al | CA | 35 (DOC) | ||
| 7 mg L−1 Al | 2–6 mg L−1 DOC | 150 kDa MWCO | 38 (DOC) | |
| 5 mg L−1 Al | UF | 62 (DOC) | ||
| 5 mg L−1 Al | pH 5.5–7.5 | PAN | 49 (DOC) | |
| 5 mg L−1 Al | 200 kDa MWCO | 41 (DOC) | ||
| Type 2: coagulation + flocculation | ||||
| PACl/15 mg L−1 | River | MF | 61 (DOC) | 39 |
| 10.8 ± 0.8 mg L−1 DOC | PVDF | |||
| pH 8.14 ± 0.09 | 0.1 μm (nominal) | |||
| PACl/0.025 mM (Al) | Synthetic | UF | 35.32 (DOC) | 33 |
| 5 mg L−1 HA | PES | |||
| 0.1 mM (Al) | 70.2 (DOC) | |||
| pH 7.5 | 100 kDa MWCO | |||
| Alum/0.06 mM Al | Natural | UF | 35.8 (DOC) | 40 |
| 3.521 ± 1.423 mg L−1 DOC | PVDF | |||
| pH 7.1–7.3 | 0.01 μm (nominal) | |||
| Sedimentation | ||||
| Al2(SO4)3/3.59 mg L−1 Al | Synthetic | UF | 42.1 (TOC) | 46 |
| PAC10WA/3.59 mg L−1 Al | 9.43 mg L−1 TOC | PES | 44.1 (TOC) | |
| NaAlO2/3.59 mg L−1 Al | pH 5–10 | 30 kDa MWCO | 22.0 (TOC) | |
| PACl/14 and 28 mg L−1 | River | UF | 38 (DOC) | 47 |
| 4.5 mg L−1 DOC | PVDF | |||
| 51 (DOC) | ||||
| pH N/A | 0.02 μm (nominal) | |||
| PACl/6 mg L−1 | Synthetic | UF | 36 (DOC) | 77 |
| 31 (DOC) | ||||
| 4.104 ± 0.043 mg L−1 DOC | PES | 32 (DOC) | ||
| 41 (DOC) | ||||
| pH 8.12 | 100 kDa MWCO | 35 (DOC) | ||
| 37 (DOC) | ||||
| Type 3: conventional coagulation | ||||
| Al-based/20 mg L−1 | River | MF | 25.3 (DOC) | 45 |
| 1.53 ± 0.41 mg L−1 DOC | PVDF | |||
| pH N/A | 0.1 μm (nominal) | |||
| PACl/15 mg L−1 | River | MF | 50 (DOC) | 39 |
| 10.8 ± 0.8 mg L−1 DOC | PVDF | |||
| pH 8.14 ± 0.09 | 0.1 μm (nominal) | |||
| Al2(SO4)3/3.59 mg L−1 Al | Synthetic | UF | 66.2 (TOC) | 46 |
| PAC10WA/3.59 mg L−1 Al | PES | 58.9 (TOC) | ||
| NaAlO2/3.59 mg L−1 Al | 9.43 mg L−1 TOC | 30 kDa MWCO | 37.0 (TOC) | |
| Al2(SO4)3/3.59 mg L−1 Al | UF | 65.0 (TOC) | ||
| PAC10WA/3.59 mg L−1 Al | pH 5–10 | Cellulose | 51.0 (TOC) | |
| NaAlO2/3.59 mg L−1 Al | 30 kDa MWCO | 28.0 (TOC) | ||
| PACl/14 and 28 mg L−1 | River | UF | 51 (DOC) | 47 |
| 4.5 mg L−1 DOC | PVDF | |||
| pH N/A | 0.02 μm (nominal) | |||
| Alum/4 mg L−1 | River | UF | 20.5 (DOC) | 48 |
| 5.341–6.29 mg L−1 DOC | PVDF | |||
| pH 7.1–7.5 | 150 kDa MWCO | |||
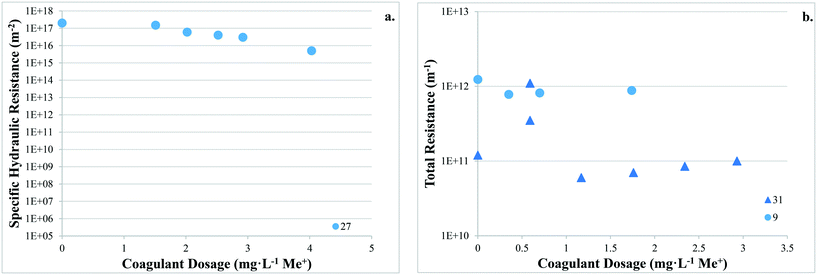 | ||
| Fig. 9 Type 1: coagulation + no/incidental flocculation a. specific hydraulic resistance vs. coagulant dosage (MF), and b. total resistance vs. coagulant dosage (UF). | ||
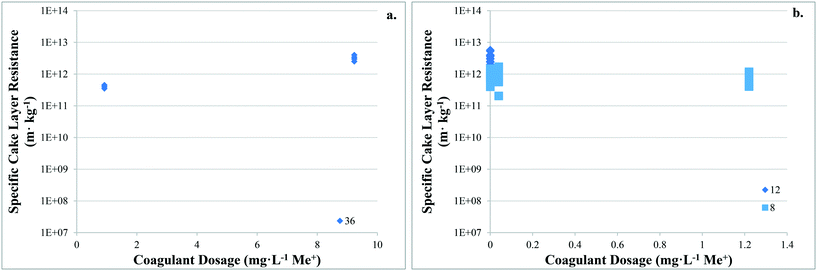 | ||
| Fig. 11 Type 3: conventional coagulation a. specific cake layer resistance vs. coagulant dosage (MF), b. specific cake layer resistance vs. coagulant dosage (UF). | ||
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors acknowledge the Natural Sciences and Engineering Research Council of Canada (NSERC) Industrial Chair in Drinking Water Research at the University of Toronto for funding this work.References
- W. Gao, H. Liang, J. Ma, M. Han, Z. Chen and Z. Han, et al. Membrane fouling control in ultrafiltration technology for drinking water production: A review, Desalination, 2011, 272(1–3), 1–8 Search PubMed.
- C. M. Chew, M. K. Aroua, M. A. Hussain and W. M. Z. W. Ismail, Evaluation of ultrafiltration and conventional water treatment systems for sustainable development: an industrial scale case study, J. Cleaner Prod., 2016, 112, 3152–3163 Search PubMed.
- S. Robinson, S. Abdullah, P. Berube and P. Le-Clech, Ageing of Membranes For Water Treatment: Linking Changes to Performance, J. Membr. Sci., 2015, 503 Search PubMed.
- G. Amy, Fundamental understanding of organic matter fouling of membranes, Desalination, 2008, 231(1–3), 44–51 Search PubMed.
- D. M. McKnight and G. R. Aiken, Sources and Age of Aquatic Humus, in Aquatic Humic Substances: Ecology and Biogeochemistry, ed. D. O. Hessen and L. J. Tranvik, Springer, Berlin, Heidelberg, 1998, pp. 9–39, [cited 2020 Apr 22], (Ecological Studies). Available from: DOI:10.1007/978-3-662-03736-2_2.
- E. Aoustin, A. I. Schäfer, A. G. Fane and T. D. Waite, Ultrafiltration of natural organic matter, Sep. Purif. Technol., 2001, 22–23, 63–78 Search PubMed.
- C. Jucker and M. M. Clark, Adsorption of aquatic humic substances on hydrophobic ultrafiltration membranes, J. Membr. Sci., 1994, 97, 37–52 Search PubMed.
- H. E. Wray and R. C. Andrews, Optimization of coagulant dose for biopolymer removal: Impact on ultrafiltration fouling and retention of organic micropollutants, J. Water Process. Eng., 2014, 1, 74–83 Search PubMed.
- W. Pronk, J. Traber and G. Kaminska, in Optimization of process parameters of the coagulation/ultrafiltration process for the reduction of membrane fouling, The International Water Association, Oslo, Norway, 2016, pp. 198–205 Search PubMed.
- N. M. Peleato, R. L. Legge and R. C. Andrews, Characterization of UF foulants and fouling mechanisms when applying low in-line coagulant pre-treatment, Water Res., 2017, 126, 1–11 Search PubMed.
- Z. Su, T. Liu, W. Yu, X. Li and N. J. D. Graham, Coagulation of surface water: Observations on the significance of biopolymers, Water Res., 2017, 126, 144–152 Search PubMed.
- J. Tian, M. Ernst, F. Cui and M. Jekel, Correlations of relevant membrane foulants with UF membrane fouling in different waters, Water Res., 2013, 47(3), 1218–1228 Search PubMed.
- E. Barbot, S. Moustier, J. Y. Bottero and P. Moulin, Coagulation and ultrafiltration: Understanding of the key parameters of the hybrid process, J. Membr. Sci., 2008, 325(2), 520–527 Search PubMed.
- H. Huang, K. Schwab and J. G. Jacangelo, Pretreatment for Low Pressure Membranes in Water Treatment: A Review, Environ. Sci. Technol., 2009, 43(9), 3011–3019 Search PubMed.
- X. Shi, G. Tal, N. P. Hankins and V. Gitis, Fouling and cleaning of ultrafiltration membranes: A review, J. Water Process. Eng., 2014, 1, 121–138 Search PubMed.
- T. Leiknes, The effect of coupling coagulation and flocculation with membrane filtration in water treatment: A review, J. Environ. Sci., 2009, 21(1), 8–12 Search PubMed.
- J. C. Crittenden, R. R. Trussell, D. W. Hand, K. J. Howe and G. Tchobanoglous, Water Treatment: Principles and Design, John Wiley & Sons, Ltd, 3rd edn, 2012, pp. 541–639 Search PubMed.
- A. P. Black, F. B. Birkner and J. J. Morgan, The effect of polymer adsorption on the electrokinetic stability of dilute clay suspensions, J. Colloid Interface Sci., 1966, 21(6), 626–648 Search PubMed.
- H. H. Hahn and W. Stumm, Kinetics of coagulation with hydrolyzed AI (III): The rate-determining step, J. Colloid Interface Sci., 1968, 28(1), 134–144 Search PubMed.
- R. F. Packham, Some studies of the coagulation of dispersed clays with hydrolyzing salts, J. Colloid Sci., 1965, 20(1), 81–92 Search PubMed.
- S. Youn and D. F. Lawler, The (relative) insignificance of G revisited to include nanoparticles, AWWA Water Sci., 2019, 1(3), e1138 Search PubMed.
- H. Amjad, Z. Khan and V. V. Tarabara, Fractal structure and permeability of membrane cake layers: Effect of coagulation–flocculation and settling as pretreatment steps, Sep. Purif. Technol., 2015, 143, 40–51 Search PubMed.
- K. J. Howe and M. M. Clark, Effect of coagulation pretreatment on membrane filtration performance, J. – Am. Water Works Assoc., 2006, 98(4), 133–146 Search PubMed.
- T. Carroll, S. King, S. R. Gray, B. A. Bolto and N. A. Booker, The fouling of microfiltration membranes by NOM after coagulation treatment, Water Res., 2000, 34(11), 2861–2868 Search PubMed.
- C. Guigui, J. C. Rouch, L. Durand-Bourlier, V. Bonnelye and P. Aptel, Impact of coagulation conditions on the in-line coagulation/UF process for drinking water production, Desalination, 2002, 147(1–3), 95–100 Search PubMed.
- B. Ma, W. Yu, H. Liu and J. Qu, Effect of low dosage of coagulant on the ultrafiltration membrane performance in feedwater treatment, Water Res., 2014, 51, 277–283 Search PubMed.
- S. J. Judd and P. Hillis, Optimisation of combined coagulation and microfiltration for water treatment, Water Res., 2001, 35(12), 2895–2904 Search PubMed.
- M. H. Cho, C. H. Lee and S. Lee, Effect of flocculation conditions on membrane permeability in coagulation-microfiltration, Desalination, 2006, 191(1–3), 386–396 Search PubMed.
- P. Park, C. Lee, S.-J. Choi, K.-H. Choo, S.-H. Kim and C.-H. Yoon, Effect of the removal of DOMs on the performance of a coagulation-UF membrane system for drinking water production, Desalination, 2002, 145(1–3), 237–245 Search PubMed.
- K. Konieczny, D. Sąkol, J. Płonka, M. Rajca and M. Bodzek, Coagulation—ultrafiltration system for river water treatment, Desalination, 2009, 240(1–3), 151–159 Search PubMed.
- R. Bian, Y. Watanabe, N. Tambo and G. Ozawa, Removal of humic substances by UF and NF membrane systems, Water Sci. Technol., 1999, 40(9), 121–129 Search PubMed.
- K. Y. Choi and B. A. Dempsey, In-line coagulation with low-pressure membrane filtration, Water Res., 2004, 38(19), 4271–4281 Search PubMed.
- M. Yao, J. Nan, Q. Li, D. Zhan, T. Chen and Z. Wang, et al. Effect of under-dosing coagulant on coagulation–ultrafiltration process for treatment of humic-rich water with divalent calcium ion, J. Membr. Sci., 2015, 495(Complete), 37–47 Search PubMed.
- B. Zhao, D. Wang, T. Li, C. W. K. Chow and C. Huang, Influence of floc structure on coagulation–microfiltration performance: Effect of Al speciation characteristics of PACls, Sep. Purif. Technol., 2010, 72(1), 22–27 Search PubMed.
- D. Wang, R. Wu, Y. Jiang and C. W. K. Chow, Characterization of floc structure and strength: Role of changing shear rates under various coagulation mechanisms, Colloids Surf., A, 2011, 379(1), 36–42 Search PubMed.
- W. Yu, J. Gregory, H. Liu and J. Qu, Investigation of the property of kaolin–alum flocs at acidic pH, Colloids Surf., A, 2014, 443(Complete), 177–181 Search PubMed.
- R. K. Chakraborti, K. H. Gardner, J. F. Atkinson and J. E. Van Benschoten, Changes in fractal dimension during aggregation, Water Res., 2003, 37(4), 873–883 Search PubMed.
- A. T. Pikkarainen, S. J. Judd, J. Jokela and L. Gillberg, Pre-coagulation for microfiltration of an upland surface water, Water Res., 2004, 38(2), 455–465 Search PubMed.
- S. Wang, C. Liu and Q. Li, Impact of polymer flocculants on coagulation-microfiltration of surface water, Water Res., 2013, 47(13), 4538–4546 Search PubMed.
- Y. Zhang, X. Zhao, X. Zhang and S. Peng, The change of NOM in a submerged UF membrane with three different pretreatment processes compared to an individual UF membrane, Desalination, 2015, 360(Complete), 118–129 Search PubMed.
- R. Feng, Q. Yue, B. Gao, L. Feng, W. Wang and H. Dong, et al. Effect of pH on floc properties and membrane fouling in coagulation–ultrafiltration hybrid process with different Al-based coagulants, Desalin. Water Treat., 2016, 57(54), 26041–26049 Search PubMed.
- J. Wang, J. Guan, S. R. Santiwong and T. D. Waite, Characterization of floc size and structure under different monomer and polymer coagulants on microfiltration membrane fouling, J. Membr. Sci., 2008, 321(2), 132–138 Search PubMed.
- H. Dong, B. Gao, Q. Yue, Y. Wang and Q. Li, Effect of pH on floc properties and membrane fouling in coagulation – Ultrafiltration process with ferric chloride and polyferric chloride, Chemosphere, 2015, 130, 90–97 Search PubMed.
- J.-D. Lee, S.-H. Lee, M.-H. Jo, P.-K. Park, C.-H. Lee and J.-W. Kwak, Effect of Coagulation Conditions on Membrane Filtration Characteristics in Coagulation−Microfiltration Process for Water Treatment, Environ. Sci. Technol., 2000, 34(17), 3780–3788 Search PubMed.
- J. Moon, M.-S. Kang, J.-L. Lim, C.-H. Kim and H.-D. Park, Evaluation of a low-pressure membrane filtration for drinking water treatment: pretreatment by coagulation/sedimentation for the MF membrane, Desalination, 2009, 247(1–3), 271–284 Search PubMed.
- M. Kabsch-Korbutowicz, Effect of Al coagulant type on natural organic matter removal efficiency in coagulation/ultrafiltration process, Desalination, 2005, 185(1–3), 327–333 Search PubMed.
- M. Dixon, C. Staaks, R. Fabris, V. Vimonses, C. W. K. Chow and S. Panglisch, et al. The impact of optimised coagulation on membrane fouling for coagulation/ultrafiltration process, Desalin. Water Treat., 2013, 51(13–15), 2718–2725 Search PubMed.
- Y. Chen, B. Z. Dong, N. Y. Gao and J. C. Fan, Effect of coagulation pretreatment on fouling of an ultrafiltration membrane, Desalination, 2007, 204(1–3), 181–188 Search PubMed.
- Q. Ding, H. Yamamura, N. Murata, N. Aoki, H. Yonekawa and A. Hafuka, et al. Characteristics of meso-particles formed in coagulation process causing irreversible membrane fouling in the coagulation-microfiltration water treatment, Water Res., 2016, 101, 127–136 Search PubMed.
- Q. Ding, H. Yamamura, H. Yonekawa, N. Aoki, N. Murata and A. Hafuka, et al. Differences in behaviour of three biopolymer constituents in coagulation with polyaluminium chloride: Implications for the optimisation of a coagulation–membrane filtration process, Water Res., 2018, 133, 255–263 Search PubMed.
- K. Kimura and N. Ando, Maximizing biopolymer removal by coagulation for mitigation of fouling in the following membrane process, Sep. Purif. Technol., 2016, 163, 8–14 Search PubMed.
- W. Yu, H. Liu, L. Xu, J. Qu and N. Graham, The pre-treatment of submerged ultrafiltration membrane by coagulation—Effect of polyacrylamide as a coagulant aid, J. Membr. Sci., 2013, 446(Complete), 50–58 Search PubMed.
- J. Xu, Y. Zhao, B. Gao, S. Han, Q. Zhao and X. Liu, The influence of algal organic matter produced by Microcystis aeruginosa on coagulation-ultrafiltration treatment of natural organic matter, Chemosphere, 2018, 196, 418–428 Search PubMed.
- R. Fabris, C. W. K. Chow and M. Drikas, Evaluation of chitosan as a natural coagulant for drinking water treatment, Water Sci. Technol., 2010, 61(8), 2119–2128 Search PubMed.
- W. Wang, S. Zhao, Q. Yue, B. Gao, W. Song and L. Feng, Purification, characterization and application of dual coagulants containing chitosan and different Al species in coagulation and ultrafiltration process, J. Environ. Sci., 2017, 51, 214–221 Search PubMed.
- B. Garcia-Fayos, J. M. Arnal, M. Sancho and I. Rodrigo, Moringa oleifera for drinking water treatment: influence of the solvent and method used in oil-extraction on the coagulant efficiency of the seed extract, Desalin. Water Treat., 2016, 57(48–49), 23397–23404 Search PubMed.
- S. Y. Choy, K. N. Prasad, T. Y. Wu, M. E. Raghunandan and R. N. Ramanan, Performance of conventional starches as natural coagulants for turbidity removal, Ecol. Eng., 2016, 94, 352–364 Search PubMed.
- G. Galjaard, J. van Paassen, P. Buijs and F. Schoonenberg, Enhanced pre-coat engineering (EPCE) for micro- and ultrafiltration: the solution for fouling?, Water Sci. Technol.: Water Supply, 2001, 1(5–6), 151–156 Search PubMed.
- J. Wang, J. Guan, S. R. Santiwong and T. D. Waite, Effect of aggregate characteristics under different coagulation mechanisms on microfiltration membrane fouling, Desalination, 2010, 258(1–3), 19–27 Search PubMed.
- D. W. Schaefer, Polymers, Fractals, and Ceramic Materials, Science, 1989, 243(4894), 1023–1027 Search PubMed.
- J. Gregory, Particles in water: properties and processes, CRC Press, Boca Raton, Florida, 2006 Search PubMed.
- P. Jarvis, B. Jefferson, J. Gregory and S. A. Parsons, A review of floc strength and breakage, Water Res., 2005, 39(14), 3121–3137 Search PubMed.
- P.-K. Park, C.-H. Lee and S. Lee, Permeability of Collapsed Cakes Formed by Deposition of Fractal Aggregates upon Membrane Filtration, Environ. Sci. Technol., 2006, 40(8), 2699–2705 Search PubMed.
- Y. Wang, B.-Y. Gao, X.-M. Xu, W.-Y. Xu and G.-Y. Xu, Characterization of floc size, strength and structure in various aluminum coagulants treatment, J. Colloid Interface Sci., 2009, 332(2), 354–359 Search PubMed.
- T. D. Waite, A. I. Schäfer, A. G. Fane and A. Heuer, Colloidal Fouling of Ultrafiltration Membranes: Impact of Aggregate Structure and Size, J. Colloid Interface Sci., 1999, 212(2), 264–274 Search PubMed.
- Y.-N. Wang and C. Y. Tang, Protein fouling of nanofiltration, reverse osmosis, and ultrafiltration membranes—The role of hydrodynamic conditions, solution chemistry, and membrane properties, J. Membr. Sci., 2011, 376(1–2), 275–282 Search PubMed.
- R. J. Baker, A. G. Fane, C. J. D. Fell and B. H. Yoo, Factors affecting flux in crossflow filtration, Desalination, 1985, 53(1), 81–93 Search PubMed.
- R. H. Davis and D. C. Grant, Theory for dead end microfiltration, ed. W. S. Ho and K. K. Sirkar, Van Norstrand, Reinhold, N.Y., 1996 Search PubMed.
- L. Fan, J. L. Harris, F. A. Roddick and N. A. Booker, Influence of the characteristics of natural organic matter on the fouling of microfiltration membranes, Water Res., 2001, 35(18), 4455–4463 Search PubMed.
- K. Kimura, K. Tanaka and Y. Watanabe, Microfiltration of different surface waters with/without coagulation: Clear correlations between membrane fouling and hydrophilic biopolymers, Water Res., 2014, 49(Complete), 434–443 Search PubMed.
- K. S. Katsoufidou, D. C. Sioutopoulos, S. G. Yiantsios and A. J. Karabelas, UF membrane fouling by mixtures of humic acids and sodium alginate: Fouling mechanisms and reversibility, Desalination, 2010, 264(3), 220–227 Search PubMed.
- S. A. Lee, A. G. Fane, R. Amal and T. D. Waite, The Effect of Floc Size and Structure on Specific Cake Resistance and Compressibility in Dead-End Microfiltration, Sep. Sci. Technol., 2003, 38(4), 869–887 Search PubMed.
- D. Antelmi, B. Cabane, M. Meireles and P. Aimar, Cake Collapse in Pressure Filtration, Langmuir, 2001, 17(22), 7137–7144 Search PubMed.
- B. Cabane, M. Meireles and P. Aimar, Cake collapse in frontal filtration of colloidal aggregates: mechanisms and consequences, Desalination, 2002, 146(1–3), 155–161 Search PubMed.
- H. A. Bustamante, S. Raj Shanker, R. M. Pashley and M. E. Karaman, Interaction between cryptosporidium oocysts and water treatment coagulants, Water Res., 2001, 35(13), 3179–3189 Search PubMed.
- M. Schulz, A. Soltani, X. Zheng and M. Ernst, Effect of inorganic colloidal water constituents on combined low-pressure membrane fouling with natural organic matter (NOM), J. Membr. Sci., 2016, 507(Complete), 154–164 Search PubMed.
- W. Wang, Q. Yue, K. Guo, F. Bu, X. Shen and B. Gao, Application of Al species in coagulation/ultrafiltration process: Influence of cake layer on membrane fouling, J. Membr. Sci., 2019, 572, 161–170 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |