Baicalein reduces hepatic fat accumulation by activating AMPK in oleic acid-induced HepG2 cells and high-fat diet-induced non-insulin-resistant mice
Wenlong
Sun
 *a,
Panpan
Liu
a,
Tianqi
Wang
b,
Xudong
Wang
c,
Weilong
Zheng
d and
Jingda
Li
*b
*a,
Panpan
Liu
a,
Tianqi
Wang
b,
Xudong
Wang
c,
Weilong
Zheng
d and
Jingda
Li
*b
aInstitute of Biomedical Research, School of Life Sciences, Shandong University of Technology, Zibo, Shandong, People's Republic of China. E-mail: 512649113@qq.com
bCollege of Life Science, Yangtze University, Jingzhou, Hubei, People's Republic of China. E-mail: jingdali_shenqi@163.com
cCollege of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou, Zhejiang, People's Republic of China
dInstitute of Biomass Resources, Taizhou University, Taizhou, Zhejiang, People's Republic of China
First published on 19th December 2019
Abstract
Nonalcoholic fatty liver disease (NAFLD) has become the most common liver disease worldwide; thus, a dietary supplement that can restrict hepatic fat accumulation is needed. Baicalein, a major component of Scutellaria baicalensis, is used as a dietary supplement in Eastern and Western cultures and can reduce hepatic fat accumulation. However, the detailed mechanism by which baicalein exerts this effect has yet to be elucidated in vivo and in vitro. In this study, we characterized the hepatic fat-lowering activity of baicalein and found that baicalein reduced hepatic fat accumulation by activating AMPK and suppressing SREBP1 cleavage, thus consequently inhibiting the transcriptional activity of SREBP1 and the synthesis of hepatic fat in oleic acid-induced HepG2 cells and high-fat diet-induced non-insulin-resistant mice. Moreover, baicalein improved NAFLD by decreasing TC, increasing HDLC, decreasing LDLC, affecting antioxidant activity, and exerting other effects. Therefore, the mechanism of baicalein with regard to NAFLD prevention and treatment might involve effects on multiple targets and pathways. Our study supports the use of baicalein as a dietary supplement due to its ability to reduce hepatic fat accumulation and to ameliorate NAFLD-related biochemical abnormalities.
1. Introduction
Nonalcoholic fatty liver diseases (NAFLDs) include nonalcoholic simple fatty liver, nonalcoholic steatohepatitis and hepatocellular carcinoma.1 Recent studies revealed that approximately 20% to 30% of adults have NAFLD in Western countries. NAFLD has become the most common liver disease worldwide.2 Additionally, patients with NAFLD have a higher incidence of liver cancer, which is seriously harmful to human health and affects quality of life.3Although the underlying mechanism of the development and progression of NAFLD is complex and multifactorial, which is known to be closely related to obesity, diabetes, genetics, malnutrition, the environment, etc.4 NAFLD is characterized by fat accumulation, ranging from simple accumulation to steatosis combined with varying degrees of inflammation and fibrosis.5 Therefore, directly limiting the accumulation of fats, especially fatty acids (the elementary components of fat) and triglycerides (TGs) in the liver, is a fundamental approach for NAFLD prevention and treatment. Fatty acids in the liver are mainly derived from the diet and de novo synthesis.6 The diet is the leading source of liver fatty acids, and dietary restriction is beneficial for NAFLD.7 However, for many severe NAFLD patients, diet restriction alone or in combination with lifestyle intervention, did not achieve favorable therapeutic benefits.8,9 A dietary supplement or drug intervention that could restrict the synthesis of fatty acids is also needed.
Flavonoids, a class of polyphenol secondary metabolites, are found in large quantities in fruits, vegetables, grains, cola, tea, coffee, cocoa, beer, and red wine.10 Baicalein (5,6,7-trihydroxyflavone, PubChem CID: 5281605) is a flavonoid found in Scutellaria baicalensis (also known as “Huangqin”). Decoctions and tinctures prepared from the root of S. baicalensis have been widely used for the prevention and treatment of fever, viral infections, bacterial infections, inflammation, and cancer.11–13 In addition to traditional formulations, baicalein is given as a dietary food supplement in Eastern and Western cultures.14 Baicalein is also considered a natural compound with low toxicity. Single oral doses of 100–2800 mg of baicalein were safe and well tolerated by healthy subjects.15 Despite a relatively high tolerated dose, the excessive uptake of baicalein is not advisable. The appropriate dose of baicalein depends on several factors, such as the user's age, health, and several other conditions. In our and other previous studies, baicalein was also reported to reduce hepatic fat in diabetic mice.16,17 However, the detailed mechanism through which baicalein reduces hepatic fat in vivo and in vitro and improves NAFLD has yet to be elucidated.
In this study, we investigated the mechanism through which baicalein improved fat accumulation in vitro and in vivo and evaluated the preventive effects of baicalein on NAFLD in mice without diabetes and insulin resistance (IR) induced by a high-fat diet (HFD).
2. Materials and methods
2.1. Materials
Baicalein (purity, more than 98%; HPLC), isolated from a water–ethanol extract of S. baicalensis, was purchased from Nantong Feiyu Biological Technology Co., Ltd (Shanghai, China). The Western Bright ECL kit, Dulbecco's modified Eagle's medium (DMEM), hematoxylin, eosin, oil red O, antibiotics, Lipofectamine 3000, fetal bovine serum, and trypsin were purchased from Solarbio Co., Ltd (Beijing, China). Hygromycin B was purchased from Roche Co, Ltd (Basel, Switzerland). The luciferase assay kit and the luciferase reporter vector were purchased from Promega Co., Ltd (Beijing, China). Sodium carboxymethylcellulose (CMC-Na) and other chemical reagents were purchased from Damao Co., Ltd (Tianjin, China). The insulin kit was purchased from Cusabio (Wuhan, China). The membrane protein extraction kit, the BCA protein assay kit and the kits used to measure the level of glucose (glucose oxidase method), glycogen, aspartate transaminase (AST), alanine transaminase (ALT), superoxide dismutase (SOD), total antioxidative capacity (T-AOC), glutathione peroxidase (GPx), TG, total cholesterol (TC), high-density lipoprotein cholesterol (HDLC), and low-density lipoprotein cholesterol (LDLC) were purchased from Jiancheng Institute of Biotechnology (Nanjing, China). The polyvinylidene fluoride (PVDF) membrane was purchased from Millipore Co., Ltd (Massachusetts, USA). HepG2 (human hepatocellular carcinoma) cells were obtained from the American Type Culture Collection (Maryland, USA).Primary antibodies against adenosine 5′-monophosphate (AMP)-activated protein kinase (AMPK; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, PA555883), phospho-AMPK (T183/172) (pAMPK; 1
1000, PA555883), phospho-AMPK (T183/172) (pAMPK; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, PA247163), precursor sterol regulatory element-binding protein 1 (pSREBP1; 1
1000, PA247163), precursor sterol regulatory element-binding protein 1 (pSREBP1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, PA005992), and phospho-SREBP1 (1;1000, PA050140) were purchased from Cusabio Technology Co., Ltd (Wuhan, China). Primary antibodies against acetyl coenzyme a carboxylase (ACC; 1
1000, PA005992), and phospho-SREBP1 (1;1000, PA050140) were purchased from Cusabio Technology Co., Ltd (Wuhan, China). Primary antibodies against acetyl coenzyme a carboxylase (ACC; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs2745R), phospho-ACC (Ser79) (pACC; 1
1000, bs2745R), phospho-ACC (Ser79) (pACC; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs3039R), fatty acid synthase (FASN; 1
1000, bs3039R), fatty acid synthase (FASN; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs1498R), and mature SREBP1 (mSREBP1; 1
1000, bs1498R), and mature SREBP1 (mSREBP1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, bs1402R) were purchased from Bioss Technology Co., Ltd (Beijing, China). Primary antibodies against β-actin (1
1000, bs1402R) were purchased from Bioss Technology Co., Ltd (Beijing, China). Primary antibodies against β-actin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, K200058 M) and secondary antibodies were purchased from Solarbio Co., Ltd (Beijing, China).
1000, K200058 M) and secondary antibodies were purchased from Solarbio Co., Ltd (Beijing, China).
Male Kunming mice (16–20 g) were purchased from Shandong Laboratory Animal Center (Jinan, China) with the permission number SCXK 2014-0007. The animal husbandry system was maintained under standard laboratory conditions. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shandong University of Technology and approved by the Animal Ethics Committee of Shandong University of Technology.
2.2. In vitro studies
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 in TBST). The peroxidase activity was visualized using an ECL kit. The densitometric analysis of the immunoblots was performed using a gel documentation system. For the detection of pSREBP1, phospho-SREBP, ACC, pACC, and FASN, the protein samples were separated by 8% SDS-PAGE.
1000 in TBST). The peroxidase activity was visualized using an ECL kit. The densitometric analysis of the immunoblots was performed using a gel documentation system. For the detection of pSREBP1, phospho-SREBP, ACC, pACC, and FASN, the protein samples were separated by 8% SDS-PAGE.
2.3. In vivo studies
2.4. Statistical analysis
Statistical analysis was performed using SPSS 17.0, and the results are expressed as the mean ± standard deviation for in vitro experiments and the mean ± standard error for in vivo experiments. Significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001) were analyzed using one-way ANOVA followed by the least significant difference (LSD) test for multiple comparisons.3. Results
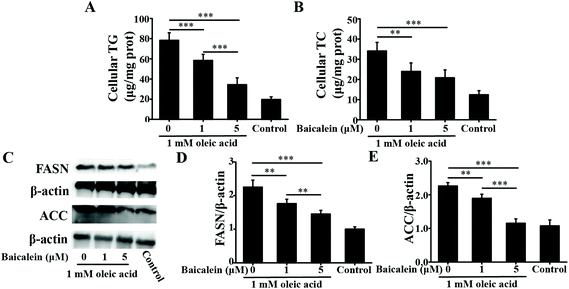
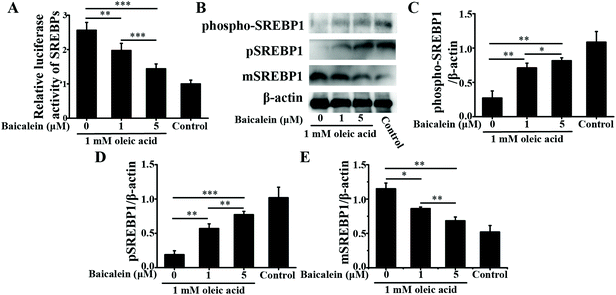
3.1. In vitro studies
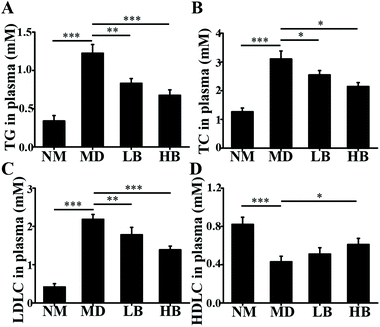
3.2. In vivo studies
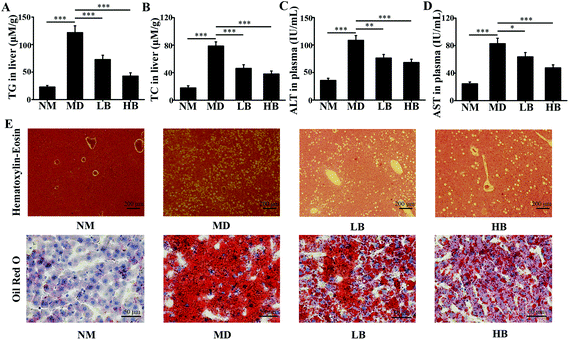
| NM | MD | LB | HB | |
|---|---|---|---|---|
| NM, Normal mice administered 0.5% CMC-Na; MD, NAFLD mice administered 0.5% CMC-Na; LB, NAFLD mice administered 50 mg kg−1 d−1 baicalein in 0.5% CMC-Na; HB, NAFLD mice administered 200 mg kg−1 d−1 baicalein in 0.5% CMC-Na. Values are shown as the mean ± standard error, n = 8 for each group. Significant differences (*P < 0.05, **P < 0.01 and ***P < 0.001 compared with NM group) were analyzed using one-way ANOVA followed by LSD test for multiple comparisons. | ||||
| Bodyweiht (g) | 43.58 ± 1.80 | 52.68 ± 5.59* | 51.68 ± 5.53* | 53.46 ± 8.26* |
| Food intake (g d−1) | 5.41 ± 0.18 | 4.69 ± 0.34* | 4.83 ± 0.25* | 4.60 ± 0.20* |
| FPG (mM) | 3.95 ± 0.25 | 4.82 ± 0.48 | 4.97 ± 0.21 | 4.76 ± 0.38 |
| 2h-PG (mM) | 4.80 ± 0.30 | 5.52 ± 0.52 | 5.67 ± 0.57 | 5.85 ± 0.52 |
| FINS (mIU L−1) | 348.60 ± 24.66 | 368.47 ± 38.08 | 373.62 ± 57.93 | 365.42 ± 56.69 |
| HOMA-IR | 62.06 ± 8.42 | 79.16 ± 12.22 | 81.84 ± 12.60 | 76.01 ± 13.34 |
4. Discussion
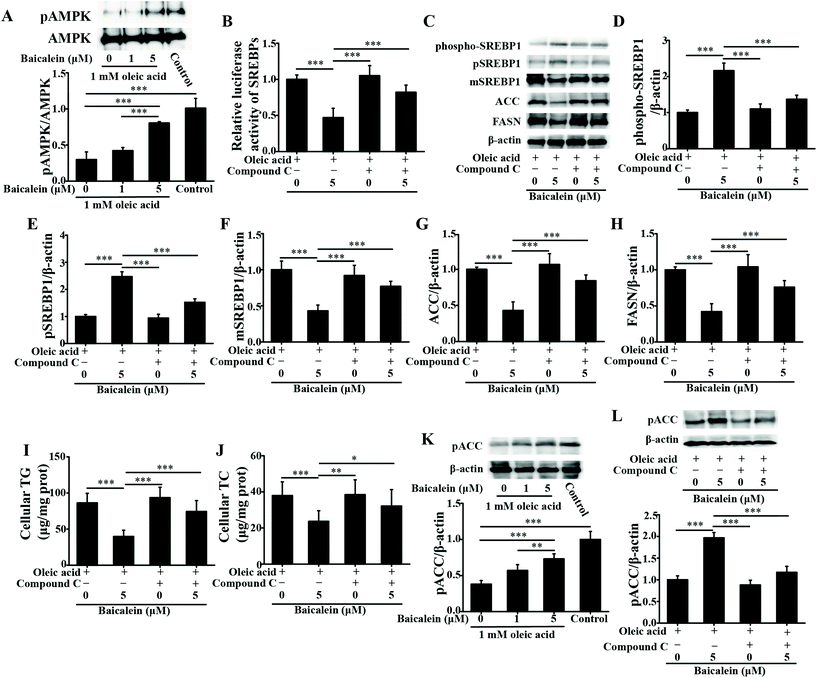
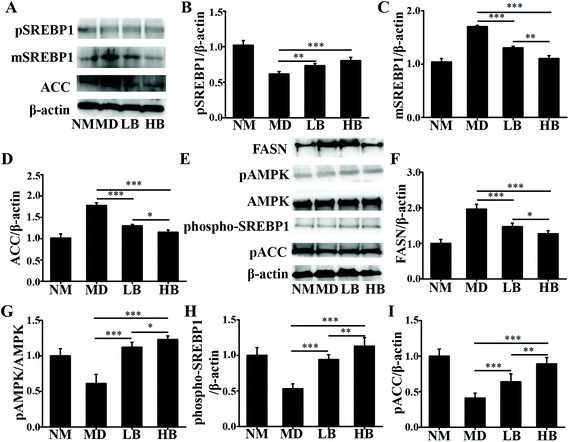
As a dietary food supplement in Eastern and Western cultures, baicalein has been widely used for many diseases, including hepatic disease. We and other researchers have found that baicalein can improve lipid metabolism in diabetic animals. However, IR plays an important role in the occurrence and development of NAFLD, and baicalein can ameliorate IR.20 Therefore, the hepatic fat-lowering effect and the anti-NAFLD effect of baicalein on a non-IR animal needed to be directly evaluated. Moreover, the mechanism through which baicalein reduced fat accumulation also needed to be elucidated in detail. The results of the present study demonstrated that baicalein increased the phosphorylation of AMPK, thus consequently inhibiting the transcriptional activity of SREBP1 and inhibiting the synthesis of hepatic fat to exert hepatic fat-lowering effects in oleic acid-induced fatty HepG2 cells and in HFD-induced non-IR NAFLD mice.NAFLD is characterized by fat or TG accumulation, and directly limiting fat accumulation in the liver is a fundamental approach for NAFLD prevention and treatment. Fatty acids are the primary components of fat, and FASN and ACC are the rate-limiting enzymes of fatty acid synthesis.21 Reducing the abundance of FASN and ACC is a main approach to reducing fat accumulation. SREBP1 is a nuclear transcription factor that has been reported to widely regulate key enzymes that synthesize fatty acids, including FASN and ACC.22 Moreover, the phosphorylation of AMPK suppressed the cleavage and nuclear translocation of SREBP1 and then further repressed the expression of the SREBP1-mediated target genes in hepatic cells when the cells were treated with oleic acid, leading to a reduction in lipid accumulation.23 Additionally, the phosphorylation state of AMPK was found to be influenced by fluctuations in the levels of peripheral blood glucose and lipids.24 Therefore, we used mice with similar blood glucose and lipid profiles in immunoblot analysis to avoid false positive results caused by fluctuations in peripheral blood glucose and lipids. Our data suggested that baicalein increased the phosphorylation of AMPK and SREBP1, further suppressed the cleavage of SREBP1, and thus consequently inhibited the transcriptional activity of SREBP1 and the synthesis of hepatic fat both in vitro and in vivo. Notably, we used a reporter gene to assay the transcriptional activity of SREBP1, which a more direct measurement.25 These results were further confirmed by blocking the phosphorylation of AMPK with compound C. According to Fig. 3, compared with baicalein alone, the combination of baicalein and compound C increased the transcriptional activity of SREBP1, the abundance of ACC and FASN, and the levels of intracellular TG, suggesting that the AMPK phosphorylation inhibitor inhibited the effect of baicalein on SREBP1. Furthermore, many researchers have found that AMPK can inhibit the activity of ACC by increasing its phosphorylation level.26 In our study, baicalein also increased the phosphorylation of ACC, and this increase was blocked by the AMPK phosphorylation inhibitor. Therefore, baicalein reduced hepatic lipid accumulation by activating AMPK. Furthermore, since plasma-adipose-liver tissue exchange free fatty acids (FFA), baicalein could also reduce the plasma TG.27 Taking these results into consideration, increasing the phosphorylation of AMPK and inhibiting the transcriptional activity of SREBP1 might be one of the main mechanisms through which baicalein reduces hepatic fat accumulation and plasma lipid profiles, especially TG levels.
NAFLD is also characterized by the accumulation of TC or free cholesterol, and reducing TC accumulation is important for NAFLD prevention and treatment.28 Our results suggested that baicalein could reduce hepatic and plasma TC. The key enzymes in cholesterol biosynthesis are 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), and HMGCR could be regulated by the AMPK-SREBP2 signaling pathway.29 Our results indicated that baicalein increased the phosphorylation of AMPK and that this might activate the phosphorylation of SREBP2, suppress the cleavage and nuclear translocation of SREBP2, and then further repress the expression of SREBP2-mediated target genes, for example HMGCR. Thus, the regulation of cholesterol biosynthesis by baicalein via AMPK/SREBP2 might be a mechanism to reduce hepatic TC, and because of the exchange of cholesterol between plasma-adipose-liver tissue, this might also be the mechanism to reduce plasma TC.30
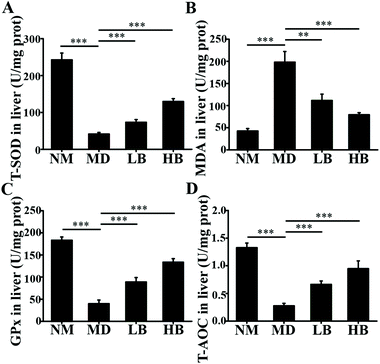
The liver damage caused by lipid peroxidation and inflammation leads to hepatic dysfunction, hepatitis, fibrosis, cirrhosis and even carcinoma.31,32 In the present study and our previous studies, baicalein protected the liver from oxidative and inflammatory injury.33 These therapeutic effects were also beneficial for NAFLD prevention and treatment. For antioxidative activity, nuclear factor (erythroid-derived 2)-like 2 (Nrf2) is a master regulator of cellular detoxification responses and redox status that induces the expression of multiple antioxidant genes.34 Recent studies demonstrated that the AMPK/Akt2/Nrf2 pathway is an important regulator of antioxidative activity in the liver.35 Our data indicated that increased AMPK phosphorylation might activate the nuclear transcriptional activity of Nrf2 and enhance the antioxidant activity of the liver. Baicalein and associated glycosides were also reported to upregulate the gene expression of antioxidant enzymes.36 In addition, our previous antioxidant capacity study demonstrated that baicalein was a natural antioxidant and could eliminate free radicals directly in vivo.37 For the anti-inflammatory effect, many researchers have shown that baicalein exhibited anti-inflammatory activity through the suppression of NF-κB transactivation by inhibiting the Trx system.38 Moreover, our previous research indicated that baicalein could increase fecal SCFA content and improve gut barrier function by improving gut microbiota to reduce systemic inflammation.33 Based on the above results, baicalein has antioxidant activity and anti-inflammatory activity, and these activities are beneficial for NAFLD prevention and treatment.
Our data also indicated that baicalein could increase the level of HDLC and reduce the level of LDLC. Additionally, our previous studies and the previous work of many others also confirmed these therapeutic effects.16,17,33 These therapeutic effects are also beneficial for NAFLD prevention and treatment. However, the mechanism of these therapeutic effects is not clear and needs to be elucidated. We are trying to elucidate these mechanisms in vivo and in vitro.
5. Conclusion
In summary, baicalein reduced hepatic fat accumulation in vitro and in vivo by increasing the activation of AMPK and suppressing the cleavage of SREBP1, thus consequently inhibiting the transcriptional activity of SREBP1 and the synthesis of hepatic fat. Moreover, baicalein also showed TC-lowering, HDLC-raising, LDLC-lowering, antioxidant, and anti-inflammatory activities. Therefore, acting on multiple targets and pathways might be the mechanism through which baicalein impacts NAFLD prevention and treatment, and these results further support the use of baicalein as a dietary supplement in Eastern and Western cultures. In addition, this study will also be beneficial for understanding the anti-NAFLD mechanism of many other flavonoids.Abbreviations
| 2h-PG | 2 h-postprandial glucose |
| ACC | Acetyl coenzyme a carboxylase |
| ALT | Alanine transaminase |
| AMPK | 5′-Adenosine monophosphate (AMP)-activated protein kinase |
| AST | Aspartate transaminase |
| CMC-Na | Sodium carboxymethylcellulose |
| DMEM | Dulbecco's modified Eagle's medium |
| FASN | Fatty acid synthase |
| FFA | Free fatty acid |
| FINS | Fasting insulin |
| FPG | Fasting plasma glucose |
| GPx | Glutathione peroxidase |
| HDLC | High-density lipoprotein cholesterol |
| HFD | High-fat diet |
| HMGCR | 3-Hydroxy-3-methylglutaryl-CoA reductase |
| HOMA-IR | Homeostasis model assessment-IR |
| IR | Insulin resistance |
| LDLC | Low-density lipoprotein cholesterol |
| LSD | Least significant difference |
| mSREBP1 | Mature SREBP1 |
| NAFLD | Nonalcoholic fatty liver diseases |
| Nrf2 | Nuclear factor (erythroid-derived 2)-like 2 |
| pSREBP1 | Precursor SREBP1 |
| PVDF | Polyvinylidene fluoride |
| SDS-PAGE | SDS–polyacrylamide gel electrophoresis |
| SOD | Superoxide dismutase |
| SREBP1 | Sterol regulatory element-binding protein 1 |
| T-AOC | Total antioxidative capacity |
| TBST | Tris-buffered saline with Tween-20 |
| TC | Total cholesterol |
| TG | Total triglyceride |
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The research was supported by the fund for the National Natural Science Foundation of China (Grant No. 81903878) and the Natural Science Foundation of Shandong Province, China (Grant No. ZR2019BH049).References
- M. Ekstedt, P. Nasr and S. Kechagias, Natural History of NAFLD/NASH, Curr. Hepatol. Rep., 2017, 16(4), 391–397 CrossRef PubMed.
- Z. Younossi, Q. M. Anstee, M. Mariett, T. Hardy, L. Henry, M. Eslam, J. George and E. Bugianesi, Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention, Nat. Rev. Gastroenterol. Hepatol., 2018, 15(1), 11–20 CrossRef PubMed.
- G. A. Michelotti, M. V. Machado and A. M. Diehl, NAFLD, NASH and liver cancer, Nat. Rev. Gastroenterol. Hepatol, 2013, 10(11), 656–665 CrossRef CAS PubMed.
- E. Buzzetti, M. Pinzani and E. A. Tsochatzis, The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD), Metabolism, 2016, 65(8), 1038–1048 CrossRef CAS PubMed.
- Q. Liu, S. Bengmark and S. Qu, The role of hepatic fat accumulation in pathogenesis of non-alcoholic fatty liver disease (NAFLD), Lipids Health Dis., 2010, 9, 42 CrossRef PubMed.
- K. L. Donnelly, C. I. Smith, S. J. Schwarzenberg, J. Jessurun, M. D. Boldt and E. J. Parks, Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease, J. Clin. Invest., 2005, 115(5), 1343–1351 CrossRef CAS PubMed.
- A. L. Menezes, M. P. Pereira, S. L. Buzelle, M. P. Dos Santos, S. A. de França, A. M. Baviera, C. M. Andrade, M. A. Garófalo, I. C. Kettelhut, V. E. Chaves and N. H. Kawashita, A low-protein, high-carbohydrate diet increases de novo fatty acid synthesis from glycerol and glycerokinase content in the liver of growing rats, Nutr. Res., 2013, 33(6), 494–502 CrossRef CAS PubMed.
- D. M. Torres and S. A. Harrison, Diagnosis and therapy of nonalcoholic steatohepatitis, Gastroenterology, 2008, 134(6), 1682–1698 CrossRef CAS PubMed.
- E. Reynoso and J. E. Lavine, NAFLD: The role of exercise in treating NAFLD, Nat. Rev. Gastroenterol. Hepatol., 2012, 9(7), 368–370 CrossRef PubMed.
- C. Faggio, A. Sureda, S. Morabito, A. Sanches-Silva, A. Mocan, S. F. Nabavi and S. M. Nabavih, Flavonoids and platelet aggregation: A brief review, Eur. J. Pharmacol., 2017, 807, 91–101 CrossRef CAS PubMed.
- H. Trinh, Y. Yoo, K. H. Won, H. T. T. Ngo, J. E. Yang, J. G. Cho, S. W. Lee, K. Y. Kim and T. H. Yi, Evaluation of in vitro antimicrobial activity of Artemisia & apiacea H. and Scutellaria baicalensis G. extracts, J. Med. Microbiol., 2018, 67(4), 489–495 CrossRef CAS PubMed.
- C. S. Cheng, J. Chen, H. Y. Tan, N. Wang, Z. Chen and Y. Feng, Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies, Am. J. Chin. Med., 2018, 46(1), 25–54 CrossRef CAS PubMed.
- S. Salini, T. Chubicka, N. Sasidharan, E. R. Sindhu and T. D. Babu, Cytotoxic and antioxidant properties of selected Scutellaria species from the Western Ghats of Peninsular India, Pharm. Biol., 2013, 51(2), 152–159 CrossRef CAS PubMed.
- S. Havermann, R. Rohrig, Y. Chovolou, H. U. Humpf and W. Watjen, Molecular effects of baicalein in Hct116 cells and Caenorhabditis elegans : activation of the Nrf2 signaling pathway and prolongation of lifespan, J. Agric. Food Chem., 2013, 61(9), 2158–2164 CrossRef CAS PubMed.
- M. Li, A. Shi, H. Pang, W. Xue, Y. Li, G. Cao, B. Yan, F. Dong, K. Li, W. Xiao, G. He, G. Du and X. Hu, Safety, tolerability, and pharmacokinetics of a single ascending dose of baicalein chewable tablets in healthy subjects, J. Ethnopharmacol., 2014, 156, 210–215 CrossRef CAS PubMed.
- P. Pu, X. A. Wang, M. Salim, L. H. Zhu, L. Wang, K. J. Chen, J. F. Xiao, W. Deng, H. W. Shi, H. Jiang and H. L. Li, Baicalein, a natural product, selectively activating AMPKα2 and ameliorates metabolic disorder in diet-induced mice, Mol. Cell. Endocrinol., 2012, 362(1–2), 128–138 CrossRef CAS PubMed.
- W. Sun, J. Sun, B. Zhang, Y. Xing, X. Yu, X. Li, Z. Xiu and Y. Dong, Baicalein improves insulin resistance via regulating SOCS3 and enhances the effect of acarbose on diabetes prevention, J. Funct. Foods, 2017, 37, 339–353 CrossRef CAS.
- Y. Mi, D. Tan, Y. He, X. Zhou, Q. Zhou and S. Ji, Melatonin Modulates lipid Metabolism in HepG2 Cells Cultured in High Concentrations of Oleic Acid: AMPK Pathway Activation may Play an Important Role, Cell Biochem. Biophys., 2018, 76(4), 463–470 CrossRef CAS PubMed.
- Z. G. Zheng, Y. P. Zhou, X. Zhang, P. M. Thu, Z. S. Xie, C. Lu, T. Pang, B. Xue, D. Q. Xu, Y. Chen, X. W. Chen, H. J. Li and X. Xu, Anhydroicaritin improves diet-induced obesity and hyperlipidemia and alleviates insulin resistance by suppressing SREBPs activation, Biochem. Pharmacol., 2016, 122, 42–61 CrossRef CAS PubMed.
- F. Angelico, M. D. Ben, R. Conti, S. Francioso, K. Feole, S. Fiorello, M. Burattin, M. Mohanna, B. Zalunardo and F. Lirussi, Insulin resistance (IR) but not reduced insulin secretion (IS) is associated to non alcoholic fatty liver disease (NAFLD), J. Hepatol., 2003, 38(2), 190 CrossRef.
- J. D. Horton, J. L. Goldstein and M. S. Brown, SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver, J. Clin. Invest., 2002, 109(9), 1125–1131 CrossRef CAS PubMed.
- L. F. Wang, X. N. Wang, C. C. Huang, L. Hu, Y. F. Xiao, X. H. Guan, Y. S. Qian, K. Y. Deng and H. B. Xin, Inhibition of NAMPT aggravates high fat diet-induced hepatic steatosis in mice through regulating Sirt1/AMPKalpha/SREBP1 signaling pathway, Lipids Health Dis., 2017, 16(1), 82 CrossRef PubMed.
- Y. Li, S. Xu, M. M. Mihaylova, B. Zheng, X. Hou, B. Jiang, O. Park, Z. Luo, E. Lefai, J. Y. Shyy, B. Gao, M. Wierzbicki, T. J. Verbeuren, R. J. Shaw, R. A. Cohen and M. Zang, AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice, Cell Metab., 2011, 13(4), 376–388 CrossRef CAS PubMed.
- Z. Wan, C. Durrer, D. Mah, S. Simtchouk, E. Robinson and J. P. Little, Reduction of AMPK activity and altered MAPKs signalling in peripheral blood mononuclear cells in response to acute glucose ingestion following a short-term high fat diet in young healthy men, Metabolism, 2014, 63(9), 1209–1216 CrossRef CAS PubMed.
- G. Welch, R. Damoiseaux and L. Miraglia, Making It All Work: Functional Genomics and Reporter Gene Assays, Methods Mol. Biol., 2018, 1755, 89–105 CrossRef CAS PubMed.
- Z. Zheng, Y. Zhou, X. Zhang, P. M. Thu, Z. Xie, C. Lu, T. Pang, B. Xue, D. Xu, Y. Chen, X. Chen, H. Li and X. Xu, Anhydroicaritin improves diet-induced obesity and hyperlipidemia and alleviates insulin resistance by suppressing SREBPs activation, Biochem. Pharmacol., 2016, 122, 42–61 CrossRef CAS PubMed.
- N. D. Oakes, P. G. Thale'n, S. M. Jacinto and B. Ljung, Thiazolidinediones Increase Plasma-Adipose Tissue FFA Exchange Capacity and Enhance Insulin-Mediated Control of Systemic FFA Availability, Diabetes, 2001, 50(5), 1158 CrossRef CAS PubMed.
- K. Ray, NAFLD-HCC: target cholesterol, Nat. Rev. Gastroenterol. Hepatol., 2018, 15(7), 390 CrossRef PubMed.
- H. Tang, R. Yu, S. Liu, B. Huwatibieke, Z. Li and W. Zhang, Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling, EBioMedicine, 2016, 6, 139–148 CrossRef PubMed.
- K. Ren, T. Jiang and G. J. Zhao, Quercetin induces the selective uptake of HDL-cholesterol via promoting SR-BI expression and the activation of the PPARγ/LXRα pathway, Food Funct., 2018, 9(1), 624–635 RSC.
- M. M. Rahman, A. Y. Muse, D. Khan, I. H. Ahmed, N. Subhan, H. M. Reza, M. A. Alam, L. Nahar and S. D. Sarker, Apocynin prevented inflammation and oxidative stress in carbon tetra chloride induced hepatic dysfunction in rats, Biomed. Pharmacother., 2017, 92, 421–428 CrossRef CAS PubMed.
- G. C. Farrell, F. Haczeyni and S. Chitturi, Pathogenesis of NASH: How Metabolic Complications of Overnutrition Favour Lipotoxicity and Pro-Inflammatory Fatty Liver Disease, Adv. Exp. Med. Biol., 2018, 1061, 19–44 CrossRef CAS PubMed.
- B. Zhang, W. Sun, N. Yu, J. Sun, X. Yu, X. Li, Y. Xing, D. Yan, Q. Ding, Z. Xiu, B. Ma, L. Yu and Y. Dong, Anti-diabetic effect of baicalein is associated with the modulation of gut microbiota in streptozotocin and high-fat-diet induced diabetic rats, J. Funct. Foods, 2018, 46, 256–267 CrossRef CAS.
- I. Bellezza, I. Giambanco, A. Minelli and R. Donato, Nrf2-Keap1 signaling in oxidative and reductive stress, Biochim. Biophys. Acta, Mol. Cell Res., 2018, 1865(5), 721–733 CrossRef CAS PubMed.
- L. Wang, S. Zhang, H. Cheng, H. Lv, G. Cheng and X. Ci, Nrf2-mediated liver protection by esculentoside A against acetaminophen toxicity through the AMPK/Akt/GSK3beta pathway, Free Radicals Biol. Med., 2016, 101, 401–412 CrossRef CAS PubMed.
- V. Y. Waisundara, S. Y. Siu, A. Hsu, D. Huang and B. K. Tan, Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats, Life Sci., 2011, 88(23–24), 1016–1025 CrossRef CAS PubMed.
- W. Sun, Y. Sang, B. Zhang, X. Yu, Q. Xu, Z. Xiu and Y. Dong, Synergistic effects of acarbose and an Oroxylum indicum seed extract in streptozotocin and high-fat-diet induced prediabetic mice, Biomed. Pharmacother., 2017, 87, 160–170 CrossRef CAS PubMed.
- R. S. Patwardhan, D. Sharma, M. Thoh, R. Checker and S. K. Sandur, Baicalein exhibits anti-inflammatory effects via inhibition of NF-kappaB transactivation, Biochem. Pharmacol., 2016, 108, 75–89 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |