A new category-specific nutrient rich food (NRF9f.3) score adds flavonoids to assess nutrient density of fruit†
Adam
Drewnowski
*a and
Britt
Burton-Freeman
 b
b
aCenter for Public Health Nutrition, University of Washington, Seattle, Washington, USA. E-mail: adamdrew@u.washington.edu; Fax: +1 (206) 685-1696; Tel: +1 (206) 543-8016
bDepartment of Food Science and Nutrition, Center for Nutrition Research, Illinois Institute of Technology, Chicago, Illinois, USA
First published on 15th January 2020
Abstract
Nutrient profiling (NP) models, intended to capture the full nutritional value of plant-based foods, ought to incorporate bioactive phytochemicals, including flavonoids, in addition to standard nutrients. The well-established Nutrient Rich Food (NRF9.3) score is based on 9 nutrients to encourage (protein, fiber, vitamins A, C, D and calcium, iron, potassium, magnesium) and 3 nutrients to limit (saturated fat, added sugar, sodium). The new category-specific NRF9f.3 score kept the same algorithm based on sums of percent daily values (%DVs) but swapped vitamin D for total flavonoids from the USDA database. NRF9f.3 was applied to the USDA fruit group categories, comparing nutrient density of citrus fruit, citrus juice, dried fruit, raw and cooked fruit, berries, fruit mixtures, fruit salads, non-citrus fruit juice, and fruit nectars. Adding total flavonoids to NRF9f.3 allowed for a recalibration of fruit total nutritional value. Citrus fruits and juices had significantly higher flavanones, berries had significantly higher anthocyanidins, and dried fruit and berries had significantly higher flavan-3-ols, than other fruits (all p < 0.05). Citrus fruit, citrus juice and berries had significantly higher NRFf9.3 scores than all other fruit subcategories (p < 0,05), but were not different from each other. The more innovative NP models are both category specific and make effective use of new nutrient composition databases. NRF9.3 when applied to the fruit group discriminates primarily on fiber, vitamin C, and added sugar content. Incorporating flavonoid and polyphenol data modernizes NP models to better capture nutrient density of plant foods that can aid in dietary guidance and policy development to improve diversity and nutritional value of the diet.
Introduction
Nutrient profiling (NP) models are intended to capture nutrient density of foods.1,2 One NP goal is to help consumers distinguish between foods that are nutrient-rich and those that are energy-dense and relatively nutrient poor.3 When combined with metrics of food cost, consumer preferences, and environmental impact, NP models can identify those foods or food groups that are nutrient-rich, affordable, and appealing and also have a low impact on the environment.4,5Capturing nutrient density of foods has presented a number of challenges, both conceptual and methodological.1,2 Decisions regarding the type of NP model, the selection of index nutrients and reference standards, and the basis of calculation have to be made.2,6 Multiple NP algorithms have been generated and tested in previously published research7 and the resulting models were validated in different ways.3,8 The major steps in developing NP models have been published before.2
The NP methodology, first developed in 2004,1 has benefited from a number of innovations. The first is a shift from across-the-board NP models to category-specific NP models,2 that were designed specifically for dairy products,9 vegetables,10 beverages,11 or ultra-processed foods.12 The Unilever Choices model,13 and the Nestle Nutrient Profiling system14 are both category specific.
An important feature of NP models is flexibility. As new recommendations and new datasets become available, the classic algorithm structure is maintained but some index nutrients can be easily swapped, added or removed. For example, the 2015–2020 Dietary Guidelines for Americans listed vitamin D as a shortfall nutrient. Newer versions of the Nutrient Rich Food 9.3 (NRF9.3) model have replaced vitamin E with vitamin D; given that data for vitamin D were not available in 2004.
When it comes to the USDA fruit group (which includes fresh, frozen, cooked and processed fruit), most existing NP models distinguish among different fruit largely on the basis of vitamin C, fiber and added sugar content.10 The release of the USDA flavonoid nutrient composition database is therefore highly important, since it can lead to an improved NP modeling of nutrient density of vegetables and fruit.10
Research has supported the importance of flavonoids in health and disease risk reduction.15–22 Flavonoids are polyphenol compounds found in plant foods and are the major source of polyphenols in the diet accounting for about 2/3 of polyphenol intakes. Although fruits vary in flavonoid content and composition; current recommendations for fruit only distinguish between whole fruit and 100% fruit juices, which is mainly based on free sugars and fiber. By contrast, vegetables are separated by color (dark green versus red/orange) that is to say by phytochemical content. Using flavonoids in NP modeling of fruit (and vegetable) nutrient density could provide a degree of precision for potential use in updating the Dietary Guidelines for Americans (DGA).
The goal of this project was to develop and test a new category-specific NRF model for fruit. Accordingly, the US Department of Agriculture (USDA) What We Eat in America nutrient composition database23 was merged with the USDA expanded flavonoid database data.
Methods
The FNDDS database
The NRF models were developed using the open-access Food and Nutrient Database for Dietary Studies (FNDDS) available from the USDA.23 The FNDDS provided energy and nutrient content for 60 nutrients per 100 g of food, edible portion, for 6940 foods from all food groups. Additional data for added sugar and vitamin D were obtained from the USDA.24 Reference Amounts Customarily Consumed (RACC) were custom-added to the FNDDS database. Food labeling in the US is based on RACC serving sizes mandated by the Food and Drug Administration (FDA).25The fruit category in the FNDDS database was identified by first digit code ID 6. Based on the USDA classification the fruit category was subdivided into citrus fruit (ID code 611) and citrus juice (ID 612); dried fruit (ID 621), fruit, raw or cooked (ID 631); berries (ID 632), fruit mixtures (ID 633), fruit salads (ID 634), fruit juices (ID 641) and fruit nectars (ID 642). Dried cranberries were assigned to the dried fruit category and cranberry juices to fruit juices category, following the USDA classification scheme. Raw, canned/cooked cranberries and cranberry salads are in the berry category, also following USDA. Some rarely consumed outliers (e.g. acerola juice) were removed. A total of 332 fruits and fruit-derived products in 6 categories were available for NP analysis. As usual, the FNDDS database contained fruits that were fresh, frozen, canned, or cooked, and fruit prepared in dishes or food mixtures, often with the addition of other ingredients (e.g. sugar, cream). One advantage of using the What We Eat in America database is that the nutrient composition data are for foods as commonly consumed as opposed to as purchased in the supermarket.
The USDA expanded flavonoid database
The Expanded Flavonoid Database for the Assessment of Dietary Intakes (FDB-EXP) was the primary database used for the analysis. It is comprised of analytical values for 26 flavonoid compounds for about 500 food items from the Flavonoid Database (FDB) 3.1 and three isoflavones for over 550 food items found in the Isoflavone Database (IDB) 2.0. That gives a total of 29 flavonoid compounds plus calculated totals of each major class.26 Subclasses of flavonoids included flavonols, flavones, flavan-3-ols (e.g. catechins), flavanones, isoflavones, and anthocyanidins.The unit of measure for the flavonoid compounds is mg per 100 g edible portion on fresh weight basis. The database contains values for: (1) flavonols (quercetin, kaempferol, myricetin, isorhamnetin); (2) flavones (luteolin, apigenin) (3) flavanones (hesperetin, naringenin, eriodictyol); (4) flavan-3-ols ((+)-catechin, (+)-gallocatechin, (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin 3-gallate, (−)-epigallocatechin 3-gallate, theaflavin, theaflavin 3-gallate, theaflavin 3′-gallate, theaflavin 3,3′ digallate, thearubigins); (5) anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin), (6) isoflavones (daidzein, genistein, glycitein).
Nutrient composition data from What We Eat in America database was merged with the Expanded Flavonoid database. Of 332 fruit items, 31 did not have flavonoid data after the merge. All missing data were juices and all but three (unsweetened prune, ambrosia and acerola juices) were mixed fruit juices. Sweetened prune juice flavonoid values were used for the unsweetened juice. No data were available for ambrosia and acerola juice items. For the remaining 28 mixed juice items, values for flavonoids were calculated based on equal fruit juice representation (i.e., if three fruit juices, then each assigned 1/3 from respective juice). Flavonoid data from individual juices were used to calculate values for the mixed juices based on aforementioned assumptions. All merged and calculated values had a quality control check against the most recent versions of the individual flavonoids and isoflavone databases (FDB 3.3 and IDB 2.0).
Category-specific NRF score for fruit
The selection of nutrients for the previously described NRF9.3 score had been guided by Dietary Guidelines for Americans 2005–2010 and followed nutrient standards developed by the US Food and Drug Administration. The early NR9 subscore was based on protein, fiber, vitamins A and C, calcium, and iron (the 6 FDA nutrients) plus vitamin E, magnesium, and potassium. The current NR9 subscore draws from current regulations using protein, fiber, vitamins A, C, and D, and calcium, iron, potassium, and magnesium.27 Those nutrients are either part of the FDA definition of “healthy” or have been identified by the DGA as nutrients consumed in insufficient amounts by the US population. Flavonoids are not currently part of regulatory guidance, however growing interest and health benefits evidence suggest inclusion in NP algorithms may be appropriate for some categories, such as the fruit category. The three nutrients to limit in the LIM subscore of the NRF9.3 model were saturated fat, added sugar and sodium2,3 same as in the French SAIN, LIM model.2,6,28Selection of nutrient standards
Nutrient standards are typically based on local reference dietary amounts. The U.S. Reference Daily Values29,30 used on nutrition labels and published by the FDA are summarized in Table 1. Maximum recommended values (MRVs) for disqualifying nutrients were 20 g for saturated fat, 125 g for total sugar, 50 g for added sugar, and 2400 mg for sodium, all based on a 2000 kcal d−1 diet.2,6| Nutrient | RDV |
|---|---|
| Abbreviation: RDV, reference daily values. | |
| Protein | 50 g |
| Fiber | 25 g |
| Vit A | 5000 IU |
| Vit C | 60 mg |
| Vit D | 400 IU (10 mcg) |
| Calcium | 1000 mg |
| Iron | 18 mg |
| Potassium | 3500 mg |
| Magnesium | 400 mg |
The NRF approach was to convert nutrient amounts per 100 kcal of food to percent daily values (% DV) per 100 kcal. Percent DVs were capped at 100% so that foods containing very large amounts of a single nutrient would not have a disproportionately high index score.
No daily values are available for total flavonoids. The present approach used an estimate of median intake of total flavonoids at 150 mg d−1 to include in NRF9f.3 calculations. The estimated intake value was based on NHANES data and other published work31–33 taken together with associations between healthy eating index and flavonoid intake34 and relative contribution of tea in estimates of flavonoid intake in tea and non-tea consumers. A value of 150 mg d−1 represents ∼75% percentile flavonoid intake in non-tea consumers.32
Basis of calculation
The NRF9.3 score was based on 100 kcal. In past studies, additional analyses calculated NRF9.3 values per RACC. Using RACC values has the advantage of linking nutrient profiles to nutrient values as they appear on food labels. RACC values are the rough inverse of energy density, being set lower for energy-dense sugar (4 g), fats and oils (15 g), and cheeses (30 g) than for meats (85 g), vegetables and fruit (120 g), or milk, juices, and other beverages (240 g).The final NRF9.3 algorithm was based on the unweighted sum of capped percent DVs for the 9 qualifying nutrients (NR9) and the sum of capped percent MRV for the 3 disqualifying nutrients (LIM). The composite NRF9.3 scores were then calculated by subtracting LIM from NR9 scores, both expressed per 100 kcal. The decision was to use the sum rather than the mean: NRF algorithms based on subtraction (NR9–LIM) yielded a better distribution of values than did those based on ratios (NR9/LIM). The final product was described as NRF9.3.
The present NRF9f.3 score was based on the sum of percent daily values for protein, fiber, vitamins A and C, calcium, iron, potassium and magnesium. Vitamin D was replaced with an estimated %DV for total flavonoids, based on current reading of the literature. LIM calculations were unchanged.
Plan of analysis
Differences in NRF9f.3 scores by fruit category were tested using one-way anovas followed by post hoc tests between means with Duncan's test for multiple comparisons using SPSS.Results
Selected nutrients and flavonoids of fruit categories
Table 2 shows mean and standard deviations (SD) for fiber, vitamin C, and selected flavonoid content per 100 g fruit for 9 fruit categories within the fruit group, as defined by the USDA. As expected, dried fruit were highest in fiber, whereas citrus fruit and citrus juices were highest in vitamin C (p < 0.05 compared to other fruits). Berries were the highest in total flavonoids and anthocyanidins. One-way anova followed by post hoc Duncan's test showed that berries were significantly higher in total flavonoids and anthocyanidins than every other fruit category (p < 0.01). Citrus fruit and citrus juice were higher in flavanone content than any other category (p < 0.01) but were not different from each other. Dried fruit and berries were highest in flavan-3-ols (p < 0.05 compared to other fruits).| USDA fruit | N | Fiber (g) | Vitamin C (mg) | Total flavonoids (mg) | Total anthocyanidins (mg) | Total flavanones (mg) | Total flavan-3-ols (mg) |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| a Data are means and standard deviations (SD). Abbreviations: Ckd, cooked. ** significantly different from all other items; * significantly different from all except *. Statistical significance determined at p value < 0.05. | |||||||
| Citrus fruit | 22 | 2.2 (2.4) | 37 (23) | 30 (24) | 0 (0) | 28 (21)** | 0 (0) |
| Citrus juice | 34 | 0.3 (0.2) | 32 (21) | 18 (17) | 0.2 (1) | 17 (17)** | 0.1 (0) |
| Fruit, dried | 38 | 5.8 (2.8) | 11 (32) | 35 (67) | 16 (60) | 0 (0) | 13 (15)* |
| Fruit, raw & ckd | 115 | 1.9 (1.3) | 15 (28) | 10 (18) | 5 (17) | 0 (0) | 4 (3) |
| Berries | 31 | 3.6 (1.8) | 18 (16) | 78 (52)** | 62 (42)** | 0 (0) | 12 (15)* |
| Fruit mixtures | 12 | 1.8 (0.8) | 10 (9) | 16 (17) | 7 (13) | 4 (6) | 3 (1) |
| Fruit salads | 35 | 1.7 (1.1) | 15 (12) | 9 (8) | 2 (5) | 3 (6) | 3 (3) |
| Fruit juice | 25 | 0.4 (0.3) | 13 (13) | 16 (26) | 10 (20) | 0 (1) | 4 (6) |
| Fruit nectars | 10 | 0.6 (0.2) | 7 (7) | 2 (2) | 0.3 (1) | 0 (0) | 1 (1) |
| Total | 322 | 2.2 (2.2) | 17 (25) | 22 (38) | 11 (32) | 4 (12) | 5 (9) |
Nutrient density of selected nutrients and flavonoids of fruit categories
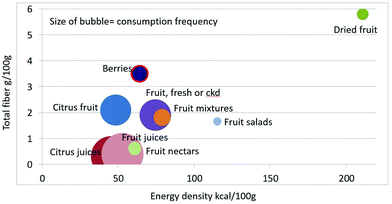
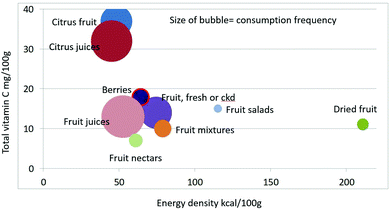
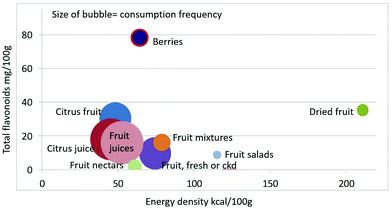
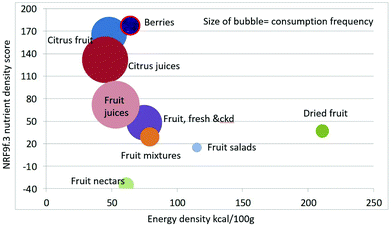
As shown in Fig. 1 calculating mean fiber content (g per 100 g) of fruit categories plotted against energy density (kcal per 100 g), citrus juices and non-citrus containing fruit juices had the least fiber and lowest energy density. Dried fruit had the most fiber and the highest energy density.Berries and whole fruit were in the middle of the scale. The size of the bubble denotes the frequency of consumption based on NHANES 2009–2010 for each category (for a total n = 322). Fig. 2 shows mean vitamin C content (mg per 100 g) of fruit categories plotted against energy density. Citrus fruit and citrus juices had the most vitamin C and a mean energy density of 0.45 kcal g−1. Dried fruit, fruit mixtures and fruit nectars had the least vitamin C. Dried fruit had the highest energy density. Fig. 3 shows mean flavonoid content (mg per 100 g) of fruit categories plotted against energy density. Berries had the highest flavonoid content and a mean energy density of only 0.6 kcal g−1.
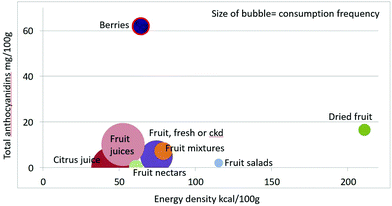
Mean anthocyanidin content (mg per 100 g) of fruit categories plotted against energy density are shown in Fig. 4. Berries are the only fruit category with substantial amounts of anthocyanidins.
Nutrient profiling scores
Mean NR9f, LIM, and NRF9.3f scores for 9 fruit categories within the fruit group, as defined by the USDA are shown in Table 3. Also shown are values for total and added sugar (g per 100 g). Dried fruit were highest in total sugar, whereas fruit nectars were highest in added sugar. For other fruit categories, added sugar came from prepared dishes that contained fruit and were listed in USDA FNDDS.| N | NRF9f | LIM | NRF9f.3 | Added sugar (g) | Total sugar (g) | |
|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| a Data are means and standard deviations (SD). Abbreviations: Ckd, cooked; NRF9f, nutrient rich food 9 nutrients + flavonoids; NRF9f.3, nutrient rich food 9 nutrients + flavonoids – LIM; LIM, nutrients to limit (see Methods for explanation). ** significantly different from all other items; * significantly different from all except *. Statistical significance, p value <0.05. | ||||||
| Citrus fruit | 22 | 176.1 (80.5) | 10.0 (16.9) | 166.1 (91.9)* | 3.3 (6.4) | 9.8 (4.3) |
| Citrus juice | 34 | 135.5 (43.7) | 3.8 (8.7) | 131.6 (48.0) | 0.8 (2.2) | 9.5 (5.9) |
| Fruit, dried | 38 | 48.1 (22.4) | 11.11 (17.4) | 37.0 (31.0) | 9.1 (20.2) | 42.5 (17.4) |
| Fruit, raw & ckd | 115 | 70.4 (52.2) | 22.0 (28.5) | 48.2 (69.4) | 10.0 (15.2) | 14.6 (8.0) |
| Berries | 31 | 197.2 (111.3)** | 19.6 (30.1) | 178.0 (135.0)* | 10.3 (18.4) | 11.6 (9.5) |
| Fruit mixtures | 12 | 56.6 (26.2) | 28.0 (30.1) | 28.6 (50.9) | 15.22 (23.6) | 15.8 (9.6) |
| Fruit salads | 35 | 46.6 (26.7) | 31.2 (21.8) | 15.5 (35.3) | 13.2 (13.3) | 14.7 (7.3) |
| Fruit juice (non-citrus) | 22 | 77.8 (54.5) | 5.0 (15.0) | 72.4 (60.4) | 1.3 (4.1) | 12.1 (2.7) |
| Fruit nectars | 10 | 31.5 (13.5) | 66.6 (25.4) | -35.1 (25.0) | 20.3 (8.0) | 14.8 (1.2) |
| Total | 319 | 90.6 (75.6) | 19.0 (26.4) | 71.3 (89.9) | 8.7 (15.1) | 16.6 (13.2) |
The NRF9.3f was calculated as NR9f–LIM. Data show that berries scored highest and were closely followed by citrus fruit and citrus juice. Berries and citrus fruit and citrus juices had significantly higher NRF9f.3 scores than all other fruit groups (p < 0.05) but were not different from each other. Lowest scores were given to fruit nectars (added sugar) and fruit salads (added sugar and cream).
Mean NRF9.3f nutrient density scores for fruit categories plotted against energy density are shown in Fig. 5. Berries had the highest mean score and energy density comparable to whole fruit, raw or cooked. Fruit nectars had low energy density but also low nutrient density scores.
Discussion
The major food groups as defined by the USDA for the What We Eat in America database are milk and milk products; meat, poultry, and fish; eggs; legumes, nuts, and seeds; grain products; fruits; vegetables; fats and oils; and sugars, sweets, and beverages. As documented in previously published research,2,3 the standard across-the-board NRF9.3 nutrient density scores fall along a continuum that range roughly from sugar to spinach. Foods with low energy density and a high content of nutrients to encourage have higher NRF scores, whereas more energy dense foods, some containing saturated fat, added sugars, and sodium, have lower scores. Vegetables and fruit score higher than grains, fats, and sweets. Focusing on only the fruit category as in the present research, further distinctions among fruit sub-categories are illustrated and aided by including flavonoids in the model.Dietary guidelines are increasingly food based. DGA emphasize eating a variety of fruits and vegetables.35 For vegetables, variety is emphasized by providing specific guidance on type (dark-green, beans and peas, red and orange, starchy, and other) and recommended intake amounts per sub-category as part of the USDA Food Patterns. For fruit, the only distinctions are that at least half the fruit consumed be whole and no more than half should be juice. The limited guidance fails to consider the differing phytochemical content of fruits and their corresponding potential health benefits. In a time when fruit and vegetable consumption continues to be well below recommended intakes for health, strategies to improve guidance and its translation to behavior is paramount.
Consistent with modernizing regulated food labels, the FDA also has begun assessing the use of key labeling terms. The FDA is now permitting the term “healthy” to be applied to foods with healthy components, as in the case of avocados or nuts.36 The FDA indicates the use of the term “healthy” can vary for different food categories (e.g., fruits and vegetables, or seafood and game meat) (see 21 CFR 101.65(d)(2)). Taking this under advice and considering the health value of flavonoid compounds, we have investigated exchanging vitamin D (which fruits do not contain naturally) for flavonoids in the NRF9.3 scoring algorithm to more accurately reflect nutrient density of fruits. USDA database for fruit includes a variety of fresh, canned and frozen fruit as well as 100% and fortified fruit juices. Also included are cooked and prepared fruit and foods with fruit that may contain added sugar, saturated fat or both. NRF9.3 distinguishes fruits primarily on fiber, added sugar and vitamin C, which range from sugar-sweetened fruit nectars to citrus fruit and citrus juice. Dried fruit mostly contain inherent (vs. added) sugar but have high energy density due to low moisture content. Consequently, the nutrient-to-calorie ratio and therefore the nutrient density score of dried fruits is lowered. Including flavonoids in the NRF9.3 NP model to create a category-specific NRF9f.3 score for fruit highlighted some fruits that were otherwise not well recognized for their nutritional value. Specifically, the berry category separated out when applying NRF9f.3. Berries have a unique combination of flavonoid compounds and are particularly rich in anthocyanins and flavan-3-ols. Anthocyanins are water soluble pigment compounds that give them their distinctive red, blue and purple color. Epidemiological research provides evidence for an association between dietary anthocyanins intake and reduced risk of cardiovascular disease (CVD) and diabetes.37–41 Clinical trials provide further evidence demonstrating biologically relevant effects of consuming berries as whole fruits and juices and anthocyanin extracts on risk factors of CVD and diabetes.21,42–44 Similar beneficial effects have been published on berries, anthocyanins and improved cognitive function.45,46 A recent meta-analysis has also highlighted the important role of flavan-3-ols in cardio-metabolic risk protection.47 Incorporating flavonoids in the new NRF9f.3 has allowed us to discriminate better among different sub-categories of fruits. The most nutrient-dense fruit could potentially join the list of healthy dietary ingredients, a designation of the FDA. Likewise, discriminating better among sub-categories can aid specific guidance for fruits in DGA and support consumer educational efforts encouraging a varied fruit diet.
The NRF approach utilizes % DV of nutrients to encourage and caps them at 100% so that foods containing very large amounts of a single nutrient, such as with fortified products, would not have a disproportionately high index score. However, one limitation with including phytochemicals in NRF is the lack of established DVs, with the exception of fiber, which has a DV. To overcome this issue with flavonoids in the present research, intake of total flavonoids was set at 150 mg d−1. Reviewing published intake literature revealed that flavonoid intake was generally skewed, where few people consume a lot of flavonoids and many people consume much lower amounts.48 This is in large part reflective of low fruit and vegetable intake in majority of the USA population.18 Tea intake increases flavonoid values markedly due to its high flavan-3-ol content. Therefore, in establishing a flavonoid intake value for the NRF9f.3 model, we considered flavonoid intake from both tea and non-tea consumers,32,34 variance in assessment methods48 and the association with another measure of a healthy dietary pattern, i.e., the Healthy Eating Index. A value of 150 mg d−1 was an appropriate value to incorporate in the model, as it also represents ∼75% percentile flavonoid intake in non-tea consumers.32 Another limitation is that flavonoid databases are still in development, such that data for some fruit juices had to be calculated (∼8%) as indicated in the Methods section. A major strength, however, is that these analytical databases are frequently updated with new foods and their availability to the public allows for analyses such as in the present research. Future research may apply NRF9f.3 to other food categories and consider the value of expanding beyond flavonoids.
Current research on diets and health has shifted away from individual nutrients to focus more on composite food patterns. Most NP models continue to be based on nutrients alone. This is also about to change.5 There are proposals for hybrid NP models that incorporate selected food groups and dietary ingredients along with nutrients of public health concern.5 This strategy taking into account foods, food groups, and sub-groups that are not nutritionally interchangeable based on the nutrients and phytochemicals they contribute to the diet can offer even better precision in healthy dietary planning. Consumers understand numbers and colors when communicated simply. As NP models are flexible and able to evolve with the science, these models will be important for updating policy and developing targeted education and communication modules helping consumers choose the most nutrient dense foods, in this case within the fruit group to maximize health.
Conclusions
NP models need to innovate and incorporate the latest information from databases of interest. These initiatives are consistent with the call by the World Health Organization for greater industry engagement in improving the nutrient density and quality of the global food supply.Conflicts of interest
Adam Drewnowski has received contracts, consulting fees, and honoraria from entities both public and private with an interest in nutrient profiling of individual foods and composite food patterns.Acknowledgements
Supported by the National Berry Crops Initiative (nationalberrycrops.org).References
- A. Drewnowski, Concept of a nutritious food: toward a nutrient density score, Am. J. Clin. Nutr., 2005, 82, 721–732 CrossRef CAS PubMed.
- A. Drewnowski and V. Fulgoni, Nutrient profiling of foods: Creating a nutrient-rich food index, Nutr. Rev., 2008, 66, 23–39 CrossRef PubMed.
- A. Drewnowski and V. L. Fulgoni, Nutrient density: principles and evaluation tools, Am. J. Clin. Nutr., 2014, 99, 1223S–1228S CrossRef CAS PubMed.
- Knorr and WWF, Future 50 Foods, 2019.
- A. Drewnowski, J. Dwyer, J. C. King and C. M. Weaver, A proposed nutrient density score that includes food groups and nutrients to better align with dietary guidance, Nutr. Rev., 2019, 77, 404–416 CrossRef PubMed.
- A. Drewnowski, M. Maillot and N. Darmon, Testing nutrient profile models in relation to energy density and energy cost, Eur. J. Clin. Nutr., 2009, 63, 674–683 CrossRef CAS PubMed.
- M. Maillot, N. Darmon, M. Darmon, L. Lafay and A. Drewnowski, Nutrient-Dense Food Groups Have High Energy Costs: An Econometric Approach to Nutrient Profiling, J. Nutr., 2007, 137, 1815–1820 CrossRef CAS PubMed.
- V. L. Fulgoni, D. R. Keast and A. Drewnowski, Development and Validation of the Nutrient-Rich Foods Index: A Tool to Measure Nutritional Quality of Foods, J. Nutr., 2009, 139, 1549–1554 CrossRef PubMed.
- A. Drewnowski, W. Tang and R. Brazeilles, Calcium requirements from dairy foods in France can be met at low energy and monetary cost, Br. J. Nutr., 2015, 114, 1920–1928 CrossRef CAS PubMed.
- N. Darmon, M. Darmon, M. Maillot and A. Drewnowski, A nutrient density standard for vegetables and fruits: Nutrients per calorie and nutrients per unit cost, J. Am. Diet. Assoc., 2005, 105, 1881–1887 CrossRef PubMed.
- B. M. Popkin, L. E. Armstrong, G. M. Bray, B. Caballero, B. Frei and W. C. Willett, A new proposed guidance system for beverage consumption in the United States, Am. J. Clin. Nutr., 2006, 83, 529–542 CrossRef CAS PubMed.
- S. Gupta, T. Hawk, A. Aggarwal and A. Drewnowski, Characterizing ultra-processed foods by energy density, nutrient density, and cost, Front. Nutr., 2019, 6, 1–9 CrossRef PubMed.
- Informed Choices, https://www.unilever.com/sustainable-living/improving-health-and-well-being/improving-nutrition/nutritious-diets/informed-choices/, (accessed September 2019).
- Nestlé Nutritional Foundation, The Nestlé Nutritional Profiling System, Its Product Categories and Sets of Criteria, 2014 Search PubMed.
- P. Knekt, J. Kumpulainen, R. Järvinen, H. Rissanen, M. Heliövaara, A. Reunanen, T. Hakulinen and A. Aromaa, Flavonoid intake and risk of chronic diseases, Am. J. Clin. Nutr., 2002, 76, 560–568 CrossRef CAS PubMed.
- P. J. Mink, C. G. Scrafford, L. M. Barraj, L. Harnack, C. P. Hong, J. A. Nettleton and D. R. Jacobs, Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women, Am. J. Clin. Nutr., 2007, 85, 895–909 CrossRef CAS PubMed.
- Y. Kim and Y. Je, Flavonoid intake and mortality from cardiovascular disease and all causes: A meta-analysis of prospective cohort studies, Clin. Nutr. ESPEN, 2017, 20, 68–77 CrossRef PubMed.
- M. M. Murphy, L. M. Barraj, D. Herman, X. Bi, R. Cheatham and R. K. Randolph, Phytonutrient intake by adults in the United States in relation to fruit and vegetable consumption, J. Acad. Nutr. Diet., 2012, 112, 222–229 CrossRef CAS PubMed.
- B. Burton-Freeman, Postprandial metabolic events and fruit-derived phenolics: a review of the science, Br. J. Nutr., 2010, 104, S1–S14 CrossRef CAS PubMed.
- D. Del Rio, A. Rodriguez-Mateos, J. P. E. Spencer, M. Tognolini, G. Borges and A. Crozier, Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases, Antioxid. Redox Signaling, 2013, 18, 1818–1892 CrossRef CAS PubMed.
- B. Burton-Freeman, M. Brzeziński, E. Park, A. Sandhu, D. Xiao and I. Edirisinghe, A Selective Role of Dietary Anthocyanins and Flavan-3-ols in Reducing the Risk of Type 2 Diabetes Mellitus: A Review of Recent Evidence, Nutrients, 2019, 11, 841 CrossRef CAS PubMed.
- S. Joseph, I. Edirisinghe and B. Burton-Freeman, Fruit Polyphenols: A Review of Anti-Inflammatory Effects in Humans, Crit. Rev. Food Sci. Nutr., 2016, 56, 419–444 CrossRef CAS PubMed.
- USDA Food and Nutrient Database for Dietary Studies 2015-2016, U.S. Department of Agriculture, Agricultural Research Service, Beltsville, MD, 2019 Search PubMed.
- S. Bowman, J. Friday and A. Moshfegh, MyPyramid Equivalents Database, 2.0 for USDA Survey Foods, 2003-2004, 2008. https://www.ars.usda.gov/research/publications/publication/?seqNo115=229527%0D Search PubMed.
- Code of Federal Regulations, Title 21–Food and Drugs, 2018.
- S. Bhagwat, D. B. Haytowitz and S. Wasswa-Kintu, USDA's Expanded Flavonoid Database for the assessment of Dietary Intakes, Beltsville, MD, 2014 Search PubMed.
- A. Drewnowski, C. Rehm and F. Vieux, Breakfast in the United States: Food and Nutrient Intakes in Relation to Diet Quality in National Health and Examination Survey 2011–2014. A Study from the International Breakfast Research Initiative, Nutrients, 2018, 10, 1200 CrossRef PubMed.
- N. Darmon, F. Vieux, M. Maillot, J. L. Volatier and A. Martin, Nutrient profiles discriminate between foods according to their contribution to nutritionally adequate diets: A validation study using linear programming and the SAIN,LIM system, Am. J. Clin. Nutr., 2009, 89, 1227–1236 CrossRef CAS PubMed.
- P. Trumbo, A. A. Yates, S. Schlicker and M. Poos, Dietary reference intakes : Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc, J. Am. Diet. Assoc., 2001, 101, 294–301 CrossRef CAS PubMed.
- P. Trumbo, S. Schlicker, A. Yates and M. Poos, Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids, J. Am. Diet. Assoc., 2002, 102, 1621–1630 CrossRef PubMed.
- K. Kim, T. M. Vance and O. K. Chun, Estimated intake and major food sources of flavonoids among US adults: changes between 1999–2002 and 2007–2010 in NHANES, Eur. J. Nutr., 2016, 55, 833–843 CrossRef CAS PubMed.
- W. O. Song and O. K. Chun, Tea Is the Major Source of Flavan-3-ol and Flavonol in the U.S. Diet, J. Nutr., 2008, 138, 1543S–1547S CrossRef CAS PubMed.
- K. J. Murphy, K. M. Walker, K. A. Dyer and J. Bryan, Estimation of daily intake of flavonoids and major food sources in middle-aged Australian men and women, Nutr. Res., 2019, 61, 64–81 CrossRef CAS PubMed.
- R. S. Sebastian, C. Wilkinson Enns, J. D. Goldman, C. L. Martin, L. C. Steinfeldt, T. Murayi and A. J. Moshfegh, A New Database Facilitates Characterization of Flavonoid Intake, Sources, and Positive Associations with Diet Quality among US Adults, J. Nutr., 2015, 145, 1239–1248 CrossRef CAS PubMed.
- U.S. Department of Health and, Human Services and U.S. Department of Agriculture, Dietary Guidelines for Americans 2015-2020, Washington, D.C., 2015 Search PubMed.
- Center for Food Safety and Applied Nutrition, Use of the term ‘Healthy’ in the labeling of human food products: Guidance for Industry, 2016, vol. 20740 Search PubMed.
- A. Jennings, A. A. Welch, S. J. Fairweather-Tait, C. Kay, A. M. Minihane, P. Chowienczyk, B. Jiang, M. Cecelja, T. Spector, A. Macgregor and A. Cassidy, Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women, Am. J. Clin. Nutr., 2012, 96, 781–788 CrossRef CAS PubMed.
- N. M. Wedick, A. Pan, A. Cassidy, E. B. Rimm, L. Sampson, B. Rosner, W. Willett, F. B. Hu, Q. Sun and R. M. Van Dam, Dietary flavonoid intakes and risk of type 2 diabetes in US men and women, Am. J. Clin. Nutr., 2012, 95, 925–933 CrossRef CAS PubMed.
- X. Guo, B. Yang, J. Tan, J. Jiang and D. Li, Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies, Eur. J. Clin. Nutr., 2016, 70, 1360–1367 CrossRef CAS PubMed.
- J. Mursu, J. K. Virtanen, T.-P. Tuomainen, T. Nurmi and S. Voutilainen, Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study, Am. J. Clin. Nutr., 2014, 99, 328–333 CrossRef CAS PubMed.
- H. D. Sesso, J. M. Gaziano, D. J. A. Jenkins and J. E. Buring, Strawberry intake, lipids, c-reactive protein, and the risk of cardiovascular disease in women, J. Am. Coll. Nutr., 2007, 26, 303–310 CrossRef CAS PubMed.
- A. Basu, Role of Berry Bioactive Compounds on Lipids and Lipoproteins in Diabetes and Metabolic Syndrome, Nutrients, 2019, 11, 1983 CrossRef PubMed.
- I. Edirisinghe and B. Burton-Freeman, Anti-diabetic actions of Berry polyphenols - Review on proposed mechanisms of action, J. Berry Res., 2016, 6, 237–250 Search PubMed.
- E. Turrini, L. Ferruzzi and C. Fimognari, Possible Effects of Dietary Anthocyanins on Diabetes and Insulin Resistance, Curr. Drug Targets, 2017, 18, 629–640 CrossRef CAS PubMed.
- E. L. Boespflug, J. C. Eliassen, J. A. Dudley, M. D. Shidler, W. Kalt, S. S. Summer, A. L. Stein, A. N. Stover and R. Krikorian, Enhanced neural activation with blueberry supplementation in mild cognitive impairment, Nutr. Neurosci., 2018, 21, 297–305 CrossRef CAS PubMed.
- N. Travica, N. M. D'Cunha, N. Naumovski, K. Kent, D. D. Mellor, J. Firth, E. N. Georgousopoulou, O. M. Dean, A. Loughman, F. Jacka and W. Marx, The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials, Brain, Behav., Immun. DOI:10.1016/j.bbi.2019.04.001 , in press.
- G. Raman, E. E. Avendano, S. Chen, J. Wang, J. Matson, B. Gayer, J. A. Novotny and A. Cassidy, Dietary intakes of flavan-3-ols and cardiometabolic health: systematic review and meta-analysis of randomized trials and prospective cohort studies, Am. J. Clin. Nutr., 2019, 110, 1067–1078 CrossRef PubMed.
- J. J. Peterson, J. T. Dwyer, P. F. Jacques and M. L. McCullough, Improving the estimation of flavonoid intake for study of health outcomes, Nutr. Rev., 2015, 73, 553–576 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02344e |
| This journal is © The Royal Society of Chemistry 2020 |