Effect of antibiotic-free, low-protein diets with specific amino acid compositions on growth and intestinal flora in weaned pigs†
Junyan
Zhou
 ab,
Yuming
Wang
ab,
Xiangzhou
Zeng
ab,
Tao
Zhang
c,
Peili
Li
ab,
Bingqian
Yao
ab,
Lu
Wang
ab,
Yuming
Wang
ab,
Xiangzhou
Zeng
ab,
Tao
Zhang
c,
Peili
Li
ab,
Bingqian
Yao
ab,
Lu
Wang
 ab,
Shiyan
Qiao
ab and
Xiangfang
Zeng
ab,
Shiyan
Qiao
ab and
Xiangfang
Zeng
 *ab
*ab
aState Key Laboratory of Animal Nutrition, Ministry of Agriculture Feed Industry Centre, China Agricultural University, Beijing 100193, PR. China. E-mail: zjycau@163.com; wudixiaoming@163.com; 1207464069@qq.com; vincentzt@126.com; peilil@126.com; ybq@cau.edu.cn; wanglucau@163.com; qiaoshiyan@cau.edu.cn; ziyangzxf@163.com; Fax: +86-10-62733688; Tel: +86-10-62733588
bBeijing Key Laboratory of bio-feed additives, Beijing 100193, PR. China
cEvonik Degussa (China) Co., Ltd., Beijing 100600, PR. China
First published on 2nd December 2019
Abstract
This study investigated the effects of modulation of the amino acid profile on growth performance and gut health in weaned pigs fed an antibiotic-free, low-protein diet. In experiment 1, 5 treatments were included: a control diet with antibiotics; a low-protein diet with antibiotics; a low-protein diet without antibiotics (LP); a LP diet with 10% more dietary essential amino acids (LP110); and an LP110 diet with 12% more dietary Met + Cys, Thr and Trp. The intestinal digestive enzyme activity and morphology were improved with the increase in dietary essential amino acid levels, while the growth performance was decreased, indicating that the dietary amino acid level was too high. In experiment 2, all 5 treatments of experiment 1 were included, plus a LP diet with 5% more dietary essential amino acids (LP105) and an LP105 diet with 6% more dietary Met + Cys, Thr and Trp. The LP105 treatment showed optimal feed efficiency, a reduced plasma endotoxin concentration, and an increased fecal lactate concentration and increased abundances of Prevotellaceae and Roseburia bacteria. Our results demonstrate that the optimal amino acid profile in an antibiotic-free, low-protein diet can efficiently improve growth performance and gut health and modulate the fecal microbial structure in weaned pigs.
1. Introduction
The routine use of antibiotics in farm animals has promoted animal growth and prevented disease since the 1950s.1 However, antibiotic resistance is causing global concerns and a public health crisis.2 The European Union and the United States banned antibiotics for disease prevention and as growth promoters in animal diets.3,4 Therefore, exploring new strategies for antibiotic alternatives is urgent and necessary. In addition, studying the nutrient requirement of pigs under antibiotic-free conditions is of great importance. A low-protein diet can decrease the cost of feed while effectively relieving the nutritional burden of excess dietary protein, decreasing hindgut microbial protein fermentation and alleviating gastrointestinal tract disorders in pigs and humans.5–7 The development of low-protein diets is probably an efficient antibiotic alternative to relieve gut diseases. However, the removal of antibiotics from the diet may also compromise growth performance and disrupt the intestinal microbial structure.8,9 Furthermore, reducing the dietary protein content and removing antibiotics influence small intestine nutrient absorption10 and mRNA expression of crucial amino acid transporters and receptors,11 besides, removing antibiotics from the diet will inevitably lead to an increase in the number of microorganisms, important amino acid consumers, which may lead to an increased amino acid consumption and a lack of limiting amino acids in the low-protein diet. In summary, additional key amino acids may need to be added to antibiotic-free low-protein diets to meet the nutritional requirement of weaned piglets for optimal growth performance.Essential amino acids play important roles in growth promotion and gut health, and they are precursors of many bioactive substances.12 For example, lysine (Lys), the primary limiting amino acid in pigs, seriously affects protein synthesis and catabolism throughout the whole body.12,13 Appropriate levels of sulfur-containing amino acids can markedly improve intestinal development and contribute to gut maturation.14 Threonine (Thr) has a significant impact on the synthesis of intestinal mucins and maintaining intestinal barrier function.15 Tryptophan (Trp) catabolism through the indoleamine 2,3-dioxygenase pathway is probably involved in the regulation of T cell proliferation and the production of antioxidant molecules, which relieves intestinal immune inflammation.16 Branched-chain amino acids, including leucine (Leu), isoleucine (Ile) and valine (Val), play vital roles in regulating the expression of intestinal amino acid and peptide transporters (especially rBAT and PepT-1) in the small intestine.17 In addition, amino acids can be widely metabolized by gut microorganisms and the dietary amino acid profile, which is altered by altering the amount of crystal amino acid added, has a profound impact on gut flora structure and gut health of the host.18
Many of our previous studies have focused on the impacts of individual amino acids on growth performance and gut health of pigs fed a low-protein diet with antibiotic supplementation. However, the influence of systemic dietary amino acid profiles without antibiotic supplementation has not been investigated. The objective of this study was to investigate the optimal essential amino acid profile for pig growth performance and gut health considering an antibiotic-free, low-protein diet.
2. Materials and methods
2.1 Experimental design
This study was approved by the China Agricultural University Animal Care and Use Committee (Beijing, CAU20150925-2). All pigs were supplied by the Fengning Swine Research Unit of China Agricultural University (Academician Workstation in Chengdejiuyun Agricultural and Livestock Co., Ltd). The pigs had free access to feed and water. Dietary energy was formulated based on the net energy system. Other dietary nutrients were formulated according to the National Research Council (NRC) (2012)19 and our previous studies,20 with slight modifications (Table 1). The details of the experimental diets are shown in Tables S1 and S2.†| Nutrients | Ctr + AGP | LP + AGP | LP | LP105 | LP105 + AA | LP110 | LP110 + AA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Ctr + AGP = normal protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP + AGP = amino acid balanced low protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP = amino acid balanced low protein diet without antibiotics; LP105 = the LP diet with 5% more dietary standardized ileal digestible (SID) essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP105 + AA = the LP105 diet with 6% more dietary SID Met + Cys, Thr and Trp content; LP110 = the LP diet with 10% more dietary SID essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP110 + AA = the LP110 diet with 12% more dietary SID Met + Cys, Thr and Trp content. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Calculated composition, % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Net energy, kcal kg−1 | 2560 | 2560 | 2560 | 2560 | 2560 | 2560 | 2560 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crude protein, % | 21.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID lysine, % | 1.29 | 1.30 | 1.30 | 1.37 | 1.37 | 1.43 | 1.43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID methionine, % | 0.50 | 0.56 | 0.56 | 0.60 | 0.66 | 0.65 | 0.77 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID methionine + cysteine, % | 0.77 | 0.78 | 0.78 | 0.82 | 0.87 | 0.86 | 0.97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID threonine, % | 0.79 | 0.81 | 0.81 | 0.85 | 0.90 | 0.89 | 1.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID tryptophan, % | 0.24 | 0.27 | 0.27 | 0.28 | 0.30 | 0.30 | 0.34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID valine, % | 0.84 | 0.83 | 0.83 | 0.87 | 0.87 | 0.91 | 0.91 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID leucine, % | 1.55 | 1.30 | 1.30 | 1.37 | 1.37 | 1.43 | 1.43 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID isoleucine, % | 0.77 | 0.69 | 0.69 | 0.72 | 0.72 | 0.76 | 0.76 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID arginine, % | 1.11 | 0.80 | 0.80 | 0.78 | 0.77 | 0.76 | 0.75 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID phenylalanine, % | 0.82 | 0.62 | 0.62 | 0.61 | 0.60 | 0.59 | 0.58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID histidine, % | 0.46 | 0.35 | 0.35 | 0.35 | 0.34 | 0.34 | 0.33 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID AA/lysine | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID methionine/lysine | 0.39 | 0.43 | 0.43 | 0.44 | 0.48 | 0.45 | 0.54 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID methionine + cysteine/lysine | 0.60 | 0.60 | 0.60 | 0.60 | 0.64 | 0.60 | 0.68 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID threonine/lysine | 0.61 | 0.62 | 0.62 | 0.62 | 0.66 | 0.62 | 0.70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID tryptophan/lysine | 0.19 | 0.21 | 0.21 | 0.20 | 0.22 | 0.21 | 0.24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID valine/lysine | 0.65 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | 0.64 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID leucine/lysine | 1.20 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID isoleucine/lysine | 0.60 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 | 0.53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID arginine/lysine | 0.86 | 0.62 | 0.62 | 0.57 | 0.56 | 0.53 | 0.52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SID phenylalanine/lysine | 0.64 | 0.48 | 0.48 | 0.45 | 0.44 | 0.41 | 0.41 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In experiment 1, 180 cross-bred (Duroc × Landrace × Yorkshire) weaned pigs (21 d of age; with an average initial body weight of 8.15 ± 1.10 kg) were assigned randomly into one of five dietary treatments with six pigs per pen and six pens per treatment in a randomized complete block design. The treatments are as follows: (1) a control diet (21% crude protein, 21% CP) with chlortetracycline (Ctr + AGP); (2) an amino acid-balanced low-protein diet (17% CP) with chlortetracycline (LP + AGP); (3) an amino acid-balanced low-protein diet (17% CP) without chlortetracycline (LP); (4) a LP diet with 10% of standardized ileal digestible (SID) essential amino acid content in the LP diet, including Lys, Trp, Thr, Leu, Ile, Val and methionine (Met) + cysteine (Cys) (LP110); and (5) an LP110 diet with 12% of dietary SID Met + Cys, Thr and Trp content in the LP105 diet (LP110 + AA). Each treatment was replicated 6 times, with three males and three females in each replicate. The experiment lasted for 4 weeks. During the last week of the trial, 0.25% chromium oxide was added to the diets as an exogenous indicator to measure apparent total tract nutrient digestibility.
The body weight and feed intake of the pigs were recorded at d 1, 14 and 28, and their diarrheal condition was scored by uninformed subjects.21 At d 14, one pig from each replicate was euthanized by electrocution. The middle intestinal segments (approximately 5 cm in length) of the duodenum, jejunum and ileum were flushed with ice-cold physiological saline and stored in 4% paraformaldehyde for histological analysis. The jejunal mucosa was harvested, immediately flash-frozen in liquid nitrogen and stored at −80 °C for analysis of digestive enzyme activity. From d 25 to 27, 3 pigs per pen were randomly selected for the collection of representative fecal samples. Fecal samples from each pen were mixed, and representative aliquots (200 g) were dried at 65 °C for 72 h and stored at −20 °C for subsequent nutrient digestibility analysis. At d 28, one pig from each replicate was selected randomly for collection of blood samples in anticoagulant tubes (Becton, Dickinson & Co., NJ, USA) from the jugular vein after overnight fasting.
In experiment 2, a total of 210 cross-bred (Duroc × Landrace × Yorkshire) weaned pigs (weaned at 21 d of age, with an average initial body weight of 7.21 ± 0.97 kg) were assigned randomly as stated before to one of the following seven treatments for 5 weeks: the same 5 treatments were used as described in experiment 1, plus two additional treatments which included a LP diet with 5% of dietary SID essential amino acid content in the LP diet (LP105) and an LP105 diet with 6% of dietary SID Met + Cys, Thr and Trp content in the LP105 diet (LP105 + AA). Each treatment was replicated 5 times, with three males and three females in each replicate. Thereafter, all pigs were continuously fed the same commercial diet which satisfied the NRC (2012) nutrient recommendation for 57 d. In the 5th, 7th and 9th weeks of the experiment, apparent total tract nutrient digestibility was measured as described above.
The body weight and feed intake of the pigs were recorded at d 1, 14, 35, 64 and 92. The diarrheal condition was scored as stated above. At d 35, one pig from each replicate was selected randomly for the collection of blood samples as stated above; the internal portion of the feces from each replicate was mixed thoroughly and placed into two 10 ml bacteria-free centrifuge tubes; one sample was allocated for the analysis of lactate and short-chain fatty acids (SCFAs) and the other was allocated for microbiota structure determination. All fresh fecal samples were immediately flash-frozen in liquid nitrogen and stored at −80 °C until analysis. From d 33 to 35, 47 to 49 and 68 to 70, fecal samples were collected for nutrient digestibility analysis as described above.
2.2 Chemical analysis of feed and fecal samples
The analysis of feed and fecal dry matter (DM), CP, gross energy (GE) and organic matter (OM) as well as feed calcium and total phosphorus was conducted according to the method of the Association of Official Analytical Chemists (2007).22 Chromium oxide was determined using an atomic absorption spectrophotometer (Hitachi Z-2000 Automatic Absorption Spectrophotometer, Tokyo, Japan) according to the method of Williams.232.3 Plasma chemical analysis
Concentrations of plasma amino acids were determined using an S-433D Amino Acid Analyzer (Sykam, Munich, Germany), as described previously.24 Plasma urea nitrogen concentration was determined using a biochemical analyzer (Bayer, Manufactured Bayer Diagnostics Manufacturing) with a blood urea nitrogen kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).25 The determination of the plasma levels of D-lactate and diamine oxidase was conducted with commercial ELISA kits (Beijing Winter Song Boye Biotechnology Co. Ltd, Beijing, China) according to the manufacturer's protocol. The concentration of plasma endotoxin was determined with a quantitative chromogenic end-point Tachypleus amebocyte lysate endotoxin detection kit (catalog number KTE 20; Xiamen TAL Experimental Plant Co., Ltd, Anhui, China) following the manufacturer's instructions.2.4 Small intestinal morphology
Fixed intestinal samples were embedded in paraffin. Using a rotary microtome, the samples were sectioned at a thickness of approximately 5 μm and stained with hematoxylin and eosin. Villus height and crypt depth were measured using 40× magnification with a light microscope (CK40, Olympus, Tokyo, Japan). A minimum of 10 well-oriented intact villi and their associated crypts from each segment were measured.2.5 Jejunal disaccharidase activity
A substrate (Sigma-Aldrich, Darmstadt, Germany) was used to determine the activities of jejunal sucrase, lactase and maltase according to our previous study.26 The concentration of jejunal mucosal protein was measured using a bicinchoninic acid protein assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the methods of a previous study.27 All the reactions were run in duplicate, and the results are expressed as total activity per milligram of protein.2.6 Fecal concentrations of lactate and SCFAs
Fecal concentrations of lactate and SCFAs were determined as previously described,28 with slight modifications. In brief, approximately 0.6 g of fecal samples was placed into 10 ml centrifuge tubes, diluted with 8 ml ultrapure water and homogenized. Then, the samples were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to obtain the supernatant. The concentrations of SCFAs and lactate in the supernatant were determined using a Dionex ICS-3000 Ion Chromatography System (Dionex, Sunnyvale, CA, USA) and gas chromatography, as described in a previous study.29
000 rpm for 15 min to obtain the supernatant. The concentrations of SCFAs and lactate in the supernatant were determined using a Dionex ICS-3000 Ion Chromatography System (Dionex, Sunnyvale, CA, USA) and gas chromatography, as described in a previous study.29
2.7 Analysis of the fecal microbiota by 16S RNA sequencing
An E.Z.N.A.® soil DNA kit (Omega Bio-Tek, Norcross, GA, USA) was used to extract bacterial DNA according to the manufacturer's instructions, with the addition of a bead-beating step. The bacterial 16S rRNA genes in the V3–V4 region were amplified with a thermocycler polymerase chain reaction system (GeneAmp 9700, ABI, USA). Demultiplexing and quality-filtering of the raw Illumina fastq files were conducted using QIIME software. Operational taxonomic units (OTUs) were defined as those with a similarity threshold of 97% using UPARSE. Then, UCHIME was applied to identify and delete the nonnormal gene sequences. The Ribosomal Database Project classifier (http://rdp.cme.msu.edu/) was also referenced to perform a taxon-dependent analysis of OTUs at a 90% confidence level.2.8 Statistical analysis
Data were obtained with each pen as one experimental unit. GLM models of SAS and Turkey's tests were used to analyze the experimental data. A P-value ≤0.05 was considered significant and a tendency for differences was declared at P < 0.10. The abundance-based coverage estimator (ACE), Chao and observed number of species (Sobs) indices for community richness and the Shannon index for community diversity were calculated from the normalized OTU reads. Weighted UniFrac distance matrices were constructed using the OTU abundance table and visualized with a principal coordinate analysis (PCoA). The abundance of the differential bacteria was classified using the linear discriminant analysis (LDA) effect size algorithm if the logarithmic LDA values of bacteria exceeded 2.0.3. Results
3.1 The amino acid profile modulated growth performance
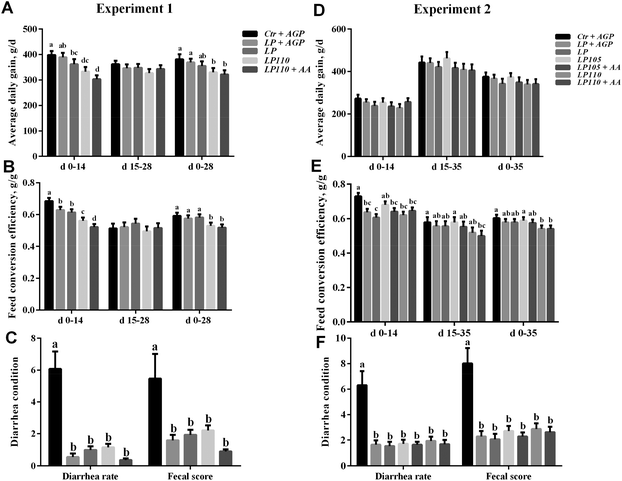
Growth performance data from experiment 1 are shown in Fig. 1 and Table S3.† In experiment 1, the average daily gain (ADG) of pigs fed the LP + AGP diet was not significantly different from that of pigs fed the Ctr + AGP diet. The ADG was markedly decreased in the LP110 and LP110 + AA treatment groups compared with the LP + AGP treatment group during d 1–14 and d 1–28 (P < 0.05) but not during d 15–28. The feed conversion efficiency (FCE) in the LP + AGP and LP treatment groups was higher than that in the Ctr + AGP treatment group during d 1–14 (P < 0.05) but not during d 15–28 or d 1–28. In comparison with the LP and LP + AGP treatment groups, the LP110 and LP110 + AA treatment groups showed a decreased FCE during d 1–14 and d 1–28 (P < 0.05) but not during d 15–28. Compared with the Ctr + AGP treatment group, the diarrhea rate and fecal score were dramatically decreased in the LP + AGP, LP, LP110 and LP110 + AA treatment groups (P < 0.05).The results of experiment 1 showed that a 10% increase in dietary essential amino acids did not improve the growth performance of the pigs fed the antibiotic-free, low-protein diet, although it reduced the diarrhea incidence. We speculated that a 10% increase in dietary essential amino acids might be too high to benefit growth performance or that the experimental time was too short. Therefore, in experiment 2, we included 2 more treatments with a 5% increase in dietary essential amino acids in addition to the 5 treatments used in experiment 1 and extended the experimental period to 5 weeks. Growth performance data from experiment 2 are shown in Fig. 1 and Tables S4 and S5.† Compared with the LP treatment group, the LP + AGP and LP105 groups increased ADG, numerically during d 1–35. Compared with the Ctr + AGP treatment group, the FCE in the LP, LP + AGP, LP105 + AA, LP110 and LP110 + AA treatment groups decreased during d 1–14 (P < 0.05) but not during d 14–35 or d 1–35. The FCE was not different between the Ctr + AGP and LP105 treatment groups during the first five weeks. Compared with the LP105 treatment group, the LP110 and LP110 + AA treatment groups showed a decreased FCE (P < 0.05). The diarrhea rate and fecal score were significantly lower in the LP + AGP, LP, LP105, LP105 + AA, LP110 and LP110 + AA treatment groups than in the Ctr + AGP treatment group during the first five weeks (P < 0.05). To investigate whether gut health promotion induced by a high level of dietary essential amino acids could enhance the growth performance later in life, all the pigs in experiment 2 were fed another formulated diet from d 36 to d 92. During d 36–92, the ADG, average daily feed intake, and FCE were not different among all seven treatments in experiment 2 (Table S5†).
3.2 The amino acid profile affected the small intestine morphology
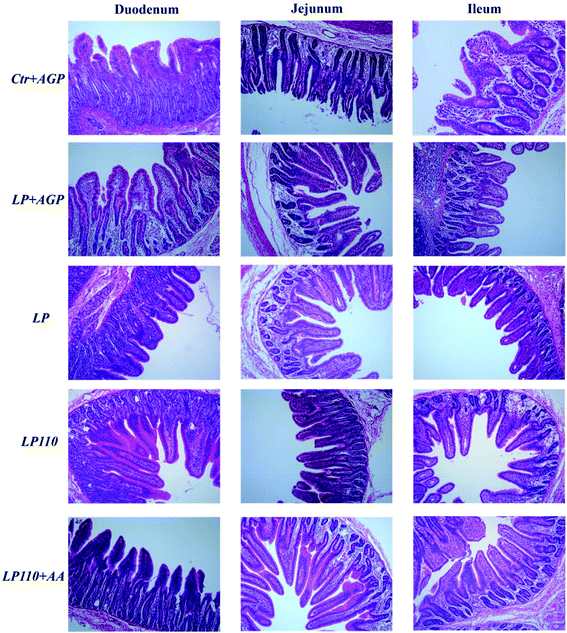
Compared to the Ctr + AGP treatment group, the LP + AGP treatment group presented a notably increased duodenal villus height and jejunal villus height to crypt depth ratio and decreased jejunal crypt depth (P < 0.05). Compared with the LP + AGP treatment group, the LP treatment group showed a decreasing tendency in the duodenal and ileal villus height and an increased jejunal crypt depth. Compared with the LP treatment group, the LP110 + AA treatment group presented a significantly increased ileal villus height (Table 2 and Fig. 2).| Traits | Ctr + AGP | LP + AGP | LP | LP110 | LP110 + AA | SEMb | P-Value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a–cMeans in the same row with different superscripts differ (P ≤ 0.05).a Ctr + AGP = normal protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP + AGP = amino acid balanced low protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP = amino acid balanced low protein diet without antibiotics; LP110 = the LP diet with 10% more dietary standardized ileal digestible (SID) essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP110 + AA = the LP110 diet with 12% more dietary SID Met + Cys, Thr and Trp content.b n = 6. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Villus height, μm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duodenum | 350b | 445a | 382ab | 385ab | 404ab | 14 | 0.05 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jejunum | 369 | 339 | 377 | 357 | 429 | 26 | 0.18 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ileum | 280b | 299ab | 255b | 311ab | 349a | 14 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crypt depth, μm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duodenum | 304 | 321 | 286 | 303 | 286 | 13 | 0.35 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jejunum | 279ab | 221c | 249abc | 234bc | 287a | 15 | 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ileum | 219 | 215 | 223 | 224 | 236 | 10 | 0.70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Villus height![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) crypt depth crypt depth |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Duodenum | 1.15 | 1.38 | 1.35 | 1.28 | 1.42 | 0.06 | 0.06 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Jejunum | 1.32b | 1.54a | 1.53a | 1.53a | 1.49a | 0.04 | 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ileum | 1.31 | 1.42 | 1.30 | 1.39 | 1.50 | 0.07 | 0.10 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
3.3 The amino acid profile enhanced jejunal digestive enzyme activity
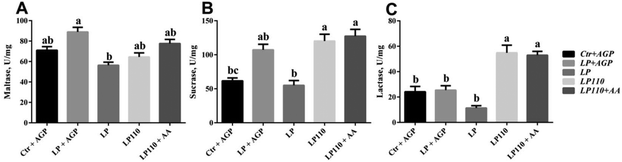
There was no significant difference in the jejunal digestive enzyme activity between the Ctr + AGP and LP + AGP treatment groups (Fig. 3). Compared with the LP + AGP treatment group, the activity of maltase in the LP treatment group was dramatically decreased (Fig. 3A, P < 0.05). Compared with the LP treatment group, the LP110 and LP110 + AA treatment groups presented significantly enhanced jejunal lactase and sucrase activities (Fig. 3B and C, P < 0.05).3.4 The amino acid profile influenced nutrient digestibility
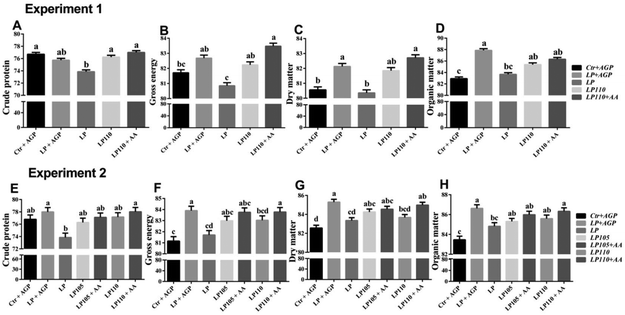
In experiment 1, the LP + AGP treatment group showed higher apparent total tract digestibility of DM and OM than the Ctr + AGP treatment group (Fig. 4C and D, P < 0.05). Compared with the LP + AGP treatment group, the apparent total tract digestibility of GE, DM and OM in the LP treatment group was decreased dramatically (Fig. 4B–D, P < 0.05). Compared with the LP treatment group, the LP110 and LP110 + AA treatment groups showed significantly increased apparent total tract digestibility of CP and GE (Fig. 4A and B, P < 0.05).In experiment 2, during the nursery stage (d 1–35), the LP + AGP treatment group showed significantly higher apparent total tract digestibility of GE, DM and OM than the Ctr + AGP treatment group (Fig. 4F–H, P < 0.05). Compared with the LP + AGP treatment group, the apparent total tract digestibility of CP, GE, DM and OM in the LP treatment group was markedly decreased (Fig. 4E–H, P < 0.05). The apparent total tract digestibility of CP, GE, DM and OM in the LP110 + AA treatment group was significantly higher than that in the LP treatment group (Fig. 4E–H, P < 0.05). During the 7th week, the apparent total tract digestibility of GE in the LP110 and LP110 + AA treatment groups showed an increasing tendency compared with the LP group (Table S6†).
3.5 The amino acid profile affected nitrogen metabolism and intestinal permeability
The plasma data from experiment 1 are shown in Table 3. Compared with those in the Ctr + AGP treatment group, the plasma urea nitrogen and endotoxin concentrations in all four low-protein dietary treatment groups were markedly decreased (P < 0.05). Plasma concentrations of diamine oxidase and D-lactate showed no difference among the different treatment groups. Compared with the Ctr + AGP treatment group, the LP + AGP treatment group showed increased plasma concentrations of Lys, Met, Thr, asparagine, and serine (P < 0.05) and decreased concentrations of histidine and ornithine (P < 0.05). Compared with the LP110 treatment group, the LP110 + AA treatment group showed increased concentrations of Met and Thr and decreased concentrations of glutamate, ornithine and aspartate (P < 0.05).| Traits | Ctr + AGP | LP + AGP | LP | LP110 | LP110 + AA | SEMb | P-Value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a–dMeans in the same row with different superscripts differ (P ≤ 0.05).a Ctr + AGP = normal protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP + AGP = amino acid balanced low protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP = amino acid balanced low protein diet without antibiotics; LP110 = the LP diet with 10% more dietary standardized ileal digestible (SID) essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP110 + AA = the LP110 diet with 12% more dietary SID Met + Cys, Thr and Trp content.b n = 6.c UN = urea nitrogen; EU = endotoxin unit; DAO = diamine oxidase; TAA = total amino acids. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
UN, mmol L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
2.78a | 1.59b | 1.76b | 1.99b | 1.11b | 0.12 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Endotoxins, EU L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
1.89a | 1.43b | 1.32b | 1.24b | 1.43b | 0.05 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DAO, pg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
14.3 | 14.9 | 13.8 | 13.2 | 15.0 | 0.98 | 0.53 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D-Lactate, μmol mL−1 | 4.66 | 5.23 | 4.88 | 4.92 | 4.69 | 0.54 | 0.44 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Essential amino acids, mmol mL−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lysine | 148c | 296b | 247b | 410a | 422a | 24.2 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methionine | 42.2b | 58.1b | 50.0b | 48.7b | 186a | 43.2 | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tryptophan | 25.6c | 38.3b | 30.8bc | 43.5a | 39.6a | 2.03 | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Threonine | 149c | 171bc | 152c | 228b | 302a | 15.1 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Valine | 212 | 230 | 221 | 227 | 207 | 10.6 | 0.97 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenylalanine | 86.0a | 68.2ab | 77.2ab | 66.8ab | 58.4b | 3.11 | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isoleucine | 116 | 109 | 95.9 | 96.3 | 80.7 | 14.6 | 0.12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leucine | 165 | 161 | 169 | 156 | 116 | 26.8 | 0.38 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Histidine | 24.1a | 15.2b | 15.0b | 9.18b | 10.7b | 1.35 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arginine | 180a | 153ab | 136b | 123b | 129b | 5.53 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-essential amino acids, mmol mL−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Citrulline | 66.9 | 66.8 | 58.2 | 63.0 | 59.3 | 4.81 | 0.42 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taurine | 245 | 253 | 313 | 272 | 256 | 33.6 | 0.28 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alanine | 542 | 735 | 619 | 645 | 662 | 52.6 | 0.17 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tyrosine | 71.8 | 76.3 | 59.8 | 59.9 | 70.7 | 6.32 | 0.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glutamate | 360a | 354a | 300ab | 325a | 236b | 14.2 | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asparagine | 41.0b | 77.7a | 50.4b | 74.1a | 83.3a | 4.39 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Serine | 174b | 248a | 171b | 210ab | 215ab | 8.61 | 0.04 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glutamine | 370 | 525 | 376 | 492 | 458 | 25.2 | 0.20 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glycine | 1109 | 1631 | 1210 | 1499 | 1648 | 125 | 0.15 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ornithine | 91.1dc | 78.3d | 115ab | 126a | 101bc | 4.36 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Aspartate | 36.8a | 37.4a | 24.4b | 36.9a | 24.9b | 1.38 | 0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TAA, mmol mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
4043 | 4852 | 4389 | 5154 | 5328 | 179 | 0.13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The plasma data from experiment 2 are shown in Table 4. The plasma concentrations of endotoxin were lower in all six low-protein dietary treatment groups than in the Ctr + AGP treatment group (P < 0.05). The LP105 and LP110 treatment groups, but not the LP105 + AA and LP110 + AA treatment groups, showed decreased plasma endotoxin levels in comparison with the LP treatment group (P < 0.05). The plasma concentrations of diamine oxidase and D-lactate were not different among the different treatment groups. Compared with the Ctr + AGP treatment group, the LP + AGP treatment group showed increased concentrations of Lys and glutamate (P < 0.05) and decreased plasma concentrations of histidine (P < 0.05). Compared with the Ctr + AGP treatment group, the six low-protein dietary treatment groups showed notably increased plasma concentrations of total amino acids (P < 0.05).
| Traits | Ctr + AGP | LP + AGP | LP | LP105 | LP105 + AA | LP110 | LP110 + AA | SEMb | P-Value | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a–dMeans in the same row with different superscripts differ (P ≤ 0.05).a Ctr + AGP = normal protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP + AGP = amino acid balanced low protein diet with antibiotics (75 mg kg−1 chlortetracycline); LP = amino acid balanced low protein diet without antibiotics; LP105 = the LP diet with 5% more dietary standardized ileal digestible (SID) essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP105 + AA = the LP105 diet with 6% more dietary SID Met + Cys, Thr and Trp content; LP110 = the LP diet with 10% more dietary SID essential amino acid content including Lys, Trp, Thr, Leu, Ile, Val and Met + Cys; LP110 + AA = the LP110 diet with 12% more dietary SID Met + Cys, Thr and Trp content.b n = 5.c UN = urea nitrogen; EU = endotoxin unit; DAO = diamine oxidase; TAA = total amino acids. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
UN, mmol L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
2.57a | 1.30bc | 2.08ab | 1.62bc | 1.75bc | 1.61bc | 1.22c | 0.25 | 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Endotoxins, EU L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
1.77a | 1.40bc | 1.46b | 1.16d | 1.34bcd | 1.24cd | 1.40bc | 0.06 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DAO, pg mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
12.0 | 11.1 | 10.7 | 12.7 | 11.9 | 13.2 | 12.0 | 0.86 | 0.45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| D-Lactate, μmol mL−1 | 4.51 | 5.04 | 4.68 | 5.82 | 4.58 | 5.65 | 5.44 | 0.49 | 0.36 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Essential amino acids, mmol mL−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lysine | 342b | 578a | 446ab | 602a | 501ab | 631a | 625a | 0.51 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Methionine | 68.6 | 96.5 | 83.3 | 84.1 | 93.8 | 108 | 92.9 | 15.1 | 0.30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Tryptophan | 44.6 | 40.1 | 48.2 | 50.2 | 45.2 | 47.7 | 46.5 | 4.65 | 0.32 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Threonine | 208c | 334c | 356c | 307c | 427bc | 691a | 632ab | 56.3 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Valine | 321 | 523 | 461 | 493 | 471 | 565 | 533 | 91.2 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phenylalanine | 179 | 167 | 164 | 178 | 191 | 177 | 184 | 11.7 | 0.66 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isoleucine | 146 | 105 | 97.7 | 105 | 116 | 101 | 109 | 19.1 | 0.11 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Leucine | 274 | 283 | 257 | 269 | 275 | 285 | 268 | 17.9 | 0.95 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Histidine | 95.6a | 61.6b | 60.1b | 62.2b | 57.9b | 54.0b | 54.0b | 7.45 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Arginine | 387 | 371 | 369 | 341 | 367 | 374 | 340 | 25.3 | 0.86 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Non-essential amino acids, mmol mL−1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taurine | 379b | 455ab | 447ab | 531a | 433ab | 420ab | 406ab | 34.2 | 0.03 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alanine | 471 | 639 | 630 | 492 | 505 | 541 | 569 | 73.3 | 0.02 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glutamate | 528b | 703a | 637ab | 546b | 557b | 500b | 565ab | 34.1 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Asparagine | 137 | 230 | 175 | 213 | 197 | 238 | 231 | 45.4 | 0.24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Serine | 147 | 174 | 165 | 163 | 158 | 166 | 172 | 19.0 | 0.21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glutamine | 641 | 792 | 660 | 673 | 628 | 644 | 614 | 64.3 | 0.09 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Glycine | 740 | 798 | 818 | 790 | 662 | 698 | 825 | 87.2 | 0.46 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cysteine | 34.2 | 38.6 | 22.8 | 40.0 | 21.7 | 18.4 | 41.5 | 8.81 | 0.21 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Proline | 258 | 300 | 281 | 269 | 251 | 275 | 281 | 21.6 | 0.19 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
TAA, mmol mL−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
5086c | 6493a | 5946b | 5665b | 6018ab | 6090ab | 6372a | 282 | <0.01 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
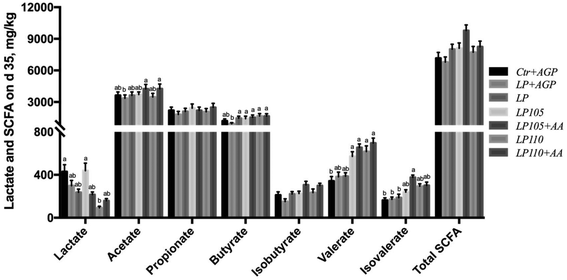
3.6 The amino acid profile affected the fecal concentrations of lactate and SCFAs
Fecal lactate concentrations in the Ctr + AGP and LP105 treatment groups did not differ but were notably higher than that in the LP110 treatment group (Fig. 5, P < 0.05), and tended to be higher than those in the LP + AGP, LP, LP105 + AA and LP110 + AA treatment groups. The concentrations of fecal acetate in the LP105 + AA and LP110 + AA treatment groups were higher than those in the LP + AGP and LP110 treatment groups (Fig. 5, P < 0.05). The concentrations of fecal butyrate in the LP, LP105, LP105 + AA, LP110 and LP110 + AA treatment groups were dramatically higher than that in the LP + AGP treatment group (Fig. 5, P < 0.05), and fecal butyrate in the Ctr + AGP treatment group tended to be more enriched than that in the LP + AGP treatment group. Compared with the LP group, the LP105, LP105 + AA, LP110 and LP110 + AA treatment groups tended to show increased concentrations of valerate and isovalerate (Fig. 5).3.7 The amino acid profile modulated the fecal bacterial community
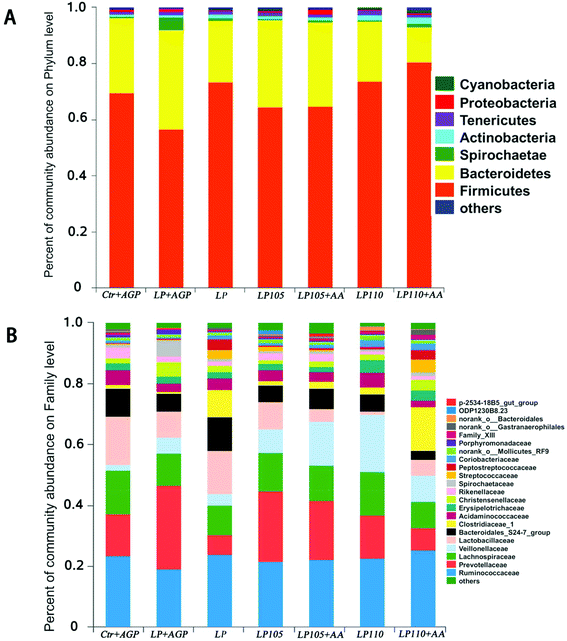
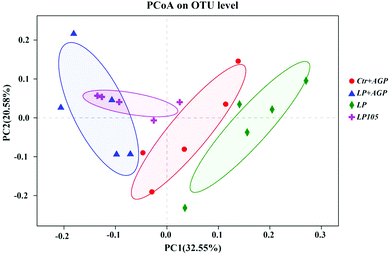
The ACE, Chao and Sobs indices and Shannon index indicated that there was no significant difference in bacterial community richness and diversity among the different treatment groups (Table S7†). A phylum-level analysis revealed that the gut microbiota composition of piglets was consistently dominated by Firmicutes and Bacteroidetes (69.05% and 25.64% of the sequences, respectively); Spirochaetae (1.30%), Actinobacteria (1.26%) and Tenericutes (1.22%) were the minor phyla (Fig. 6A). At the family level, Ruminococcaceae (21.63%), Prevotellaceae (15.63%), Lachnospiraceae (11.67%), Lactobacillaceae (10.86%) and Veillonellaceae (8.76%) were the dominant bacteria (Fig. 6B).To further analyze the membership and structure of the microbiota, a weighted UniFrac distance matrix was constructed based on the OTUs in each sample. The PCoA revealed that antibiotic supplementation, the CP content and the amino acid profile in antibiotic-free, low-protein diets changed the taxonomic and functional structures of the fecal microbial communities, respectively (P < 0.01; Fig. 7).
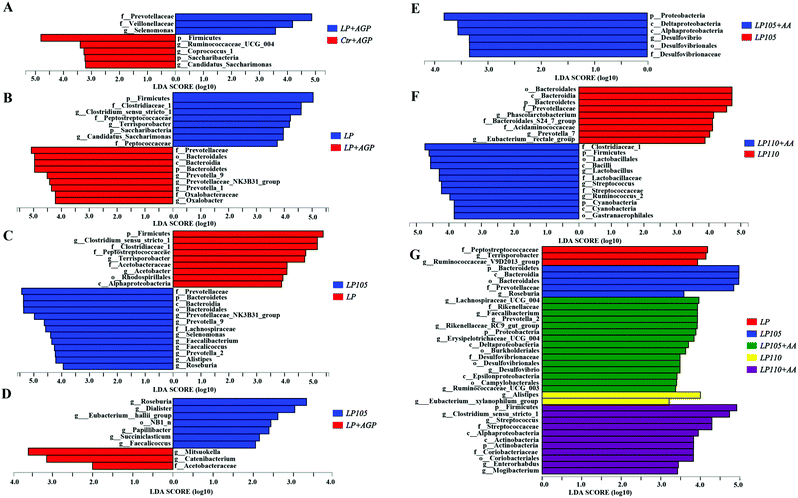
Significant differences in the microbial community among the different treatment groups are shown in Fig. 8A. In the LP + AGP treatment group, Prevotellaceae and Veillonellaceae had higher scores than those in the Ctr + AGP treatment group, whereas Ruminococcaceae_UCG_004 and Coprococcus_1 were enriched in the Ctr + AGP treatment group. Compared with the LP treatment group, the LP + AGP treatment group had a decreased abundance of Clostridiaceae_1 (Fig. 8B). Compared with the LP treatment group, the LP105 treatment group had increased abundances of Prevotellaceae and Roseburia (Fig. 8C). Butyrate-producing bacteria, including Roseburia and Eubacterium_hallii_group, were in greater abundance in the LP105 treatment group than in the LP + AGP treatment group (Fig. 8D). The LP105 + AA diet enriched the abundance of Desulfovibrio compared with the LP105 diet, which may be due to the effect of sulfur-containing amino acids (Fig. 8E). In comparison with the LP110 treatment group, Streptococcaceae and Ruminococcus_2 were enriched in the LP110 + AA treatment group (Fig. 8F). As shown in Fig. 8G, the LP105 treatment group showed significantly increased abundances of Prevotellaceae and Roseburia compared with the other antibiotic-free, low-protein diet treatment groups.
4. Discussion
Since the ban on dietary antibiotic supplementation for growth promotion and disease prevention, numerous studies have explored the feasibility of various types of alternatives to antibiotics. However, few studies analyzed the nutritional requirement of animals under antibiotic free conditions. From the perspective of satisfying the nutritional requirement, we innovatively explored the amino acid profile of weaned piglets without antibiotics, in order to optimize the growth performance of piglets. Our data indicated that the removal of antibiotics from the low-protein diet decreased the growth performance and intestinal development and changed the gut microbiota structure in weaned pigs, which was reversed by appropriately increasing the dietary essential amino acid content.Antibiotics promote growth performance by increasing muscular protein deposition and bactericidal capacity to prevent the establishment of harmful gut microbes.30,31 Herein, our data indicated that the removal of antibiotics from the low-protein diet decreased the growth performance, which was reversed by a moderate increase in dietary essential amino acids, indicating that elevated dietary essential amino acids were important to promote growth in weaned pigs under antibiotic-free dietary conditions. However, an excessive increase in the dietary amino acid level was not associated with a significant increase in growth promotion. Although Met, Thr and Trp play important roles in the maintenance of gut function,32,33 excessive dietary supplementation of these three amino acids did not exert a positive impact on growth performance in this research. Dietary crystalline amino acids can be absorbed quickly, which may cause the energy supply to lag behind the amino acid supply. Glucose and amino acids must be available in appropriately balanced quantities at protein synthesis sites for efficient growth performance.34 Therefore, an excessive proportion of dietary crystalline amino acids in the LP110 and LP110 + AA treatments may have reduced the protein synthesis efficiency due to the lack of a synergistic supply of energy and nitrogen, which can also compromise growth performance in pigs. In addition, given that accelerated oxidation occurs in rapidly absorbed amino acids, an excessive proportion of dietary crystalline amino acids in the two LP110 treatment groups might have induced considerable intestinal first pass amino acid catabolism,35 resulting in inefficient nitrogen utilization. Notably, diarrhea incidence was significantly reduced in all the low-protein diet treatment groups compared with the Ctr + AGP treatment group. One reason was that a low-protein diet relieved a portion of intestinal stress in the pigs during weaning. Additionally, decreased plasma endotoxin levels in pigs fed low-protein diets may explain the lower accidence of diarrhea. A previous study demonstrated that a high level of plasma endotoxin indicated impaired intestinal barrier function and increased intestinal permeability36,37 and diarrhea incidence.
Amino acids play important roles in intestinal development. For instance, a suitable content of Met15,38 and Thr39–41 in diets could contribute to improvements in small intestinal morphology. Dietary Trp supplementation benefited the intestinal mucosa integrity and enhanced the expression of the tight junction protein ZO-1.42 Leu in low-protein diets promoted intestinal mucosal protein synthesis and improved the intestinal health status in piglets.43 In addition, increasing the proportion of synthetic amino acids in diets improved the synthesis of intestinal mucosa proteins.44 A previous study demonstrated that villus height and crypt depth were key indicators of intestinal development.45 In this study, the data from experiment 1 indicated that the LP110 and LP110 + AA treatment groups showed more developed intestinal morphology than the LP treatment group, which may be due to elevated contents of essential amino acids. Additionally, the activities of disaccharidases are vital indicators of intestinal development of piglets.46 In the present study, the LP110 and LP110 + AA treatment groups showed significantly increased jejunal lactase and sucrase activities and an increasing tendency in maltase activity compared with the LP treatment group. This may be related to the effect of functional amino acids, such as Thr and sulfur-containing amino acids, which increase digestive enzyme activities, promote intestinal development, and improve intestinal health.15,47,48
Consistent with intestinal development, increased apparent total tract digestibility of CP was observed in weaned pigs fed the LP110 diet or the LP110 + AA diet compared with those fed the LP diet. This was possibly because synthetic amino acids were more easily absorbed by animals than intact proteins. Similarly, increasing the content of essential amino acids in the low-protein diets also improved apparent total tract digestibility of GE, DM and OM in weaned pigs through improved intestinal development. In addition, promotion of intestinal development induced by increased levels of essential amino acids may exert positive effects on nutrient utilization later in life in pigs. This is partly confirmed by the fact that the GE digestibility in the LP110 and LP110 + AA treatment groups showed increasing tendencies compared with the LP treatment group when the pigs were fed the same commercial diet in the 7th week.
The concentration of plasma urea nitrogen has been recognized as an appropriate indicator for evaluating protein and amino acid utilization.49 Plasma urea nitrogen usually appears low when the nutritional value of protein is high or the dietary amino acid profile meets the demands of the animals.50 In both experiments, all the low-protein treatment groups had lower plasma urea nitrogen levels than the Ctr + AGP treatment group, indicating that nitrogen utilization efficiency in the low-protein treatment groups was much higher than that in the Ctr + AGP treatment group. Consistently, plasma concentrations of total amino acids in all the low-protein diet groups were higher than those in the Ctr + AGP treatment groups in both experiments, which further proves that a low-protein diet can improve nitrogen utilization efficiency and decrease nitrogen excretion. Since we formulated the low-protein diets based on the requirements of the top seven limiting amino acids in pigs and excluded arginine, phenylalanine and histidine, plasma concentrations of these three amino acids in the low-protein treatment groups were obviously lower than those in the Ctr + AGP treatment group, which may have contributed to the lack of essential amino acid nutrition and consequently compromised growth performance. Additionally, the concentrations of plasma endotoxin in all the low-protein treatment groups were lower than that in the Ctr + AGP treatment group, indicating decreased intestinal permeability in pigs fed the low-protein diets. Most notably, the LP105 treatment group had the lowest level of plasma endotoxin. However, the other two intestinal permeability indicators, diamine oxidase and D-lactate, were not different among the treatment groups.
Another interesting finding in our research was that the gut microbiota structure and microbial metabolites were significantly modulated by the dietary CP content and amino acid profile under antibiotic-free conditions. Compared with the Ctr + AGP treatment group, the LP + AGP treatment group showed increased abundances of Prevotellaceae and Veillonellaceae. Most of the members of the Prevotellaceae family can degrade a wide range of complex oligosaccharides and polysaccharides51 and benefit host starch metabolism, while the Veillonellaceae family is often associated with the fermentation of complex carbohydrates. Furthermore, the cross-feeding of primary fermentation metabolites occurs among Veillonellaceae and members of Prevotellaceae.52 Therefore, increased abundances of Prevotellaceae and Veillonellaceae in low-protein diets may promote carbohydrate digestion. Nutrient digestibility data also inferred the same assumptions. In addition, a higher abundance of Coprococcus_1 and Ruminococcus_UCG_004 was observed in the Ctr + AGP treatment group than in the LP + AGP treatment group. Some Coprococcus spp. are able to generate butyrate through the butyrate kinase pathway,53 and some Ruminococcus spp. can produce acetate and butyrate efficiently.54,55 Consistently, fecal acetate and butyrate concentrations in the Ctr + AGP treatment group were higher than those in the LP + AGP treatment group. Considering the differences in the microbial structures and metabolites, we speculate that decreased dietary protein levels decrease the richness of butyrate- and acetate-producing bacteria and ultimately fecal butyrate and acetate concentrations. In comparison with the LP + AGP treatment group, the LP treatment group showed a substantially increased abundance of Clostridiaceae, specifically the Clostridium_sensu_stricto_1 genus. Most members of the Clostridiaceae family can consume mucus- and plant-derived saccharides to produce butyrate in the gut.56 Moreover, Clostridium_sensu_stricto_1 can protect against colonization by bacterial pathogens and degrade amylose starch to generate SCFAs.57 Above all, the higher fecal butyrate concentration in the LP treatment group than that in the LP + AGP treatment group may be attributed to the difference in flora structure.
Among all five antibiotic-free, low-protein treatment groups, Prevotellaceae was enriched in the LP105 treatment group, and Roseburia genus, which belongs to the Lachnospiraceae family, was also particularly prevalent in the LP105 treatment group. Prevotellaceae is a key factor in regulating inflammation and caspase-8-mediated IL-1β maturity58 and is associated with mucin degradation, resulting in a reduced layer of intestinal mucin.59Roseburia inhibits proinflammatory factor signaling pathways in the gut and benefits the maintenance of the intestinal epithelium.60 Therefore, under antibiotic-free conditions, an appropriate amino acid profile may reduce intestinal permeability by optimizing the intestinal flora structure. The lowest plasma endotoxin concentration was observed in the LP105 treatment group in this study, which also inferred the same hypothesis.
Compared with the LP + AGP treatment group, the richness of Roseburia and Eubacterium_hallii_group in the LP105 treatment group was markedly increased. These two genera play dominant roles in butyrate synthesis.61 Therefore, the higher butyrate concentration in the LP105 treatment group than in the LP + AGP treatment group could be at least partly explained by the significant difference in these two bacterial abundances. When compared with the LP105 treatment group, the LP105 + AA treatment group showed a remarkably increased abundance of the Desulfovibrio genus, which may be due to supplementation of DL-Met because previous studies proved that Desulfovibrio could effectively convert sulfur and sulfated amino acids into hydrogen sulfide.62 Given that Streptococcus and Ruminococcus spp. are involved in acetate yield,61 the higher concentration of fecal acetate in the LP110 + AA treatment group than in the LP110 treatment group may be because of the notably higher abundance of Streptococcus and Ruminococcus_2.
5. Conclusion
The optimal dietary essential amino acid profile in antibiotic-free, low-protein diets efficiently improved growth performance, intestinal development and nutrient utilization and modulated the fecal microbial structure in weaned pigs. This study has important implications, as essential amino acids are very important in maintaining the growth performance and gut health of animals in the post-antibiotic era.Abbreviations
| ADG | Average daily gain |
| AGP | Antibiotic growth promotor |
| CP | Crude protein |
| Cys | Cysteine |
| DM | Dry matter |
| FCE | Feed conversion efficiency |
| GE | Gross energy |
| Ile | Isoleucine |
| LDA | Linear discriminant analysis |
| Leu | Leucine |
| Lys | Lysine |
| Met | Methionine |
| NRC | National Research Council |
| OM | Organic matter |
| OTUs | Operational taxonomic units |
| PCoA | Principal coordinate analysis |
| SCFAs | Short-chain fatty acids |
| SID | Standardized ileal digestible |
| Thr | Threonine |
| Trp | Tryptophan |
| Val | Valine |
Authors contributions
The authors’ responsibilities were as follows: JYZ, BQY and XZZ conducted the research; JYZ, YMW, SYQ, TZ and XFZ designed the research; JYZ, XZZ, LW and PLL performed the statistical analysis; JYZ and XFZ wrote and revised the manuscript; XFZ conceived and oversaw the whole study; and all authors approved the final manuscript.Conflicts of interest
The authors declare no competing financial interest.Funding
This work was supported by the National Basic Research Program of China (973 Program) (No. 2017YFD0500506) and Evonik Degussa (China) Co., Ltd.References
- R. H. Gustafson and R. E. Bowen, Antibiotic use in animal agriculture - A Review, J. Appl. Microbiol., 1997, 83, 531–541 CrossRef CAS PubMed.
- M. D. Barton, Antibiotic use in animal feed and its impact on human health, Nutr. Res. Rev., 2000, 13, 279–299 CrossRef CAS PubMed.
- S. Levy, Reduced antibiotic use in livestock: how Denmark tackled resistance, Environ. Health Perspect., 2014, 122, 160–165 Search PubMed.
- M. J. Martin, S. E. Thottathil and T. B. Newman, Antibiotics overuse in animal agriculture: a call to action for health care providers, Am. J. Public Health, 2015, 105, 2409–2410 CrossRef PubMed.
- V. T. S. Rist, E. Weiss, M. Eklund and R. Mosenthin, Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review, Animal, 2013, 7, 1067–1078 CrossRef CAS PubMed.
- W. R. Russell, S. W. Gratz, S. H. Duncan, G. Holtrop, J. Ince, L. Scobbie and G. G. Duthie, High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health, Am. J. Clin. Nutr., 2011, 93, 1062–1072 CrossRef CAS PubMed.
- F. O. Opapeju, D. O. Krause, R. L. Payne, M. Rademacher and C. M. Nyachoti, Effect of dietary protein level on growth performance, indicators of enteric health, and gastrointestinal microbial ecology of weaned pigs induced with postweaning colibacillosis, Anim. Sci., 2009, 87, 2635–2643 CrossRef CAS PubMed.
- E. Rettedal, S. Vilain, S. Lindblom, K. Lehnert, C. Scofield, S. George and V. S. Brözel, Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline, Appl. Environ. Microbiol., 2009, 75, 5489–5495 CrossRef CAS PubMed.
- M. C. Walsh, D. M. Sholly, R. B. Hinson, K. L. Saddoris, A. L. Sutton, J. S. Radcliffe and B. T. Richert, Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding, J. Anim. Sci., 2007, 85, 1799–1808 CrossRef CAS PubMed.
- Y. Pi, K. Gao, Y. Peng, C. Mu and W. Zhu, Antibiotic-induced alterations of the gut microbiota and microbial fermentation in protein parallel the changes in host nitrogen metabolism of growing pigs, Animal, 2019, 13, 262–272 CrossRef CAS PubMed.
- M. Yu, C. Mu, Y. Yang, C. Zhang, Y. Su, Z. Huang and W. Zhu, Increases in circulating amino acids with in-feed antibiotics correlated with gene expression of intestinal amino acid transporters in piglets, Amino Acids, 2017, 49, 1587–1599 CrossRef CAS PubMed.
- G. Wu, Amino acids: metabolism, functions, and nutrition, Amino Acids, 2009, 37, 1–17 CrossRef CAS PubMed.
- B. Stoll, J. Henry, P. J. Reeds, H. Yu, F. Jahoor and D. G. Burrin, Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets, J. Nutr., 1998, 128, 606–614 CrossRef CAS PubMed.
- E. Zong, P. Huang, W. Zhang, J. Li, Y. Li, X. Ding and H. Yang, The effects of dietary sulfur amino acids on growth performance, intestinal morphology, enzyme activity, and nutrient transporters in weaning piglets, J. Anim. Sci., 2018, 96, 1130–1139 CrossRef PubMed.
- R. F. P. Bertolo, C. Z. L. Chen, G. Law, P. B. Pencharz and R. O. Ball, Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerably lower than that of piglets receiving an identical diet intragastrically, J. Nutr., 1998, 128, 1752–1759 CrossRef CAS PubMed.
- L. K. Jasperson, C. Bucher, A. Panoskaltsismortari, A. L. Mellor, D. H. Munn and B. R. Blazar, Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (ido), for suppression of graft-versus-host disease (gvhd) lethality, Blood, 2009, 114, 5062–5070 CrossRef PubMed.
- S. Zhang, S. Qiao, M. Ren, X. Zeng, X. Ma, Z. Wu and G. Wu, Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs, Amino Acids, 2013, 45, 1191–1205 CrossRef CAS PubMed.
- Z. Dai, G. Wu and W. Zhu, Amino acid metabolism in intestinal bacteria: links between gut ecology and host health, Front. Biosci., 2011, 16, 1768–1786 CrossRef CAS PubMed.
- United States National Research Council, Nutrient requirements of swine, National Academy Press, Washington, 11th edn, 2012 Search PubMed.
- Y. Wang, J. Zhou, G. Wang, S. Cai, X. Zeng and S. Qiao, Advances in low-protein diets for swine, J. Anim. Sci. Biotechnol., 2018, 9, 60 CrossRef CAS PubMed.
- W. Feng, Y. Wu, G. Chen, S. Fu, B. Li, B. Huang and J. Liu, Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner, Cell. Physiol. Biochem., 2018, 47, 1617–1629 CrossRef CAS PubMed.
- W. Hortwitz and G. W. Latimer, Official methods of analysis of AOAC international, AOAC Int., Gaithersburg, 18th edn Rev. 2nd edn, 2007 Search PubMed.
- C. H. Williams, D. J. David and O. Iismaa, The determination of chromic oxide in faeces samples by atomic absorption spectrophotometry, J. Agric. Sci., 1962, 59, 381–385 CrossRef CAS.
- X. Wang, S. Qiao, Y. Yin, L. Yue, Z. Wang and G. Wu, A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs, J. Nutr., 2007, 137, 1442–1446 CrossRef CAS PubMed.
- C. Xie, S. Zhang, G. Zhang, F. Zhang, L. Chu and S. Qiao, Estimation of the optimal ratio of standardized ileal digestible threonine to lysine for finishing barrows fed low crude protein diets, Asian-Australas. J. Anim. Sci., 2013, 26, 1172–1180 CrossRef CAS PubMed.
- L. Yue and S. Qiao, Effects of low-protein diets supplemented with crystalline amino acids on performance and intestinal development in piglets over the first 2 weeks after weaning, Livest. Sci., 2008, 115, 144–152 CrossRef.
- M. M. Bradford, A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- Z. Wang, S. Qiao, W. Lu and D. Li, Effects of enzyme supplementation on performance, nutrient digestibility, gastrointestinal morphology, and volatile fatty acid profiles in the hindgut of broilers fed wheat-based diets, Poult. Sci., 2005, 84, 875–881 CrossRef CAS PubMed.
- H. Liu, C. Hou, N. Li, X. Zhang, G. Zhang, F. Yang and S. Qiao, Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation, FASEB J., 2019, 33, 4490–4501 CrossRef CAS PubMed.
- M. S. Edmonds, O. A. Izquierdo and D. H. Baker, Feed additive studies with newly weaned pigs: efficacy of supplemental copper, antibiotics and organic acids, J. Anim. Sci., 1985, 60, 462–469 CrossRef CAS PubMed.
- A. Pérez-Bosque, J. Polo and D. Torrallardona, Spray dried plasma as an alternative to antibiotics in piglet feeds, mode of action and biosafety, Porc. Health Manag., 2016, 2, 16 CrossRef PubMed.
- F. Blachier, F. Mariotti, J. F. Huneau and D. Tomé, Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences, Amino Acids, 2007, 33, 547–562 CrossRef CAS PubMed.
- M. R. Ruth and C. J. Field, The immune modifying effects of amino acids on gut-associated lymphoid tissue, J. Anim. Sci. Biotechnol., 2013, 4, 27 CrossRef PubMed.
- S. Liu and P. H. Selle, A consideration of starch and protein digestive dynamics in chicken-meat production, World's Poult. Sci. J., 2015, 71, 297–310 CrossRef.
- M. L. Sawadogo, A. Piva, A. Panciroli, E. Meola, A. Mordenti and B. Seve, Marginal efficiency of free or protected crystalline L-tryptophan for tryptophan and protein accretion in early-weaned pigs, J. Anim. Sci., 1997, 75, 1561–1568 CrossRef CAS PubMed.
- Y. Guo, M. Liu, X. He, C. Jiang and R. Liu, Functional changes of intestinal mucosal barrier in surgically critical patients, World J. Emerg. Med., 2010, 1, 205–208 Search PubMed.
- Y. Zhao, G. Qin, Z. Sun, D. Che, N. Bao and X. Zhang, Effects of soybean agglutinin on intestinal barrier permeability and tight junction protein expression in weaned piglets, Int. J. Mol. Sci., 2011, 12, 8502–8512 CrossRef CAS PubMed.
- W. Wang, S. Qiao and D. Li, Amino acids and gut function, Amino Acids, 2009, 37, 105–110 CrossRef CAS PubMed.
- M. A. Abbasi, A. H. Mahdavi, A. H. Samie and R. Jahanian, Effects of different levels of dietary crude protein and threonine on performance, humoral immune responses and intestinal morphology of broiler chicks, Braz. J. Poultry Sci., 2014, 1, 35–44 CrossRef.
- X. Mao, X. Zeng, S. Qiao, G. Wu and D. Li, Specific roles of threonine in intestinal mucosal integrity and barrier function, Front. Biosci., Elite Ed., 2011, 3, 1192–1200 Search PubMed.
- W. Wang, X. Zeng, X. Mao, G. Wu and S. Qiao, Optimal dietary true ileal digestible threonine for supporting the mucosal barrier in small intestine of weanling pigs, J. Nutr., 2010, 140, 981–986 CrossRef CAS PubMed.
- W. Liu, S. Mi, Z. Ruan, J. Li, X. Shu, K. Yao and Z. Deng, Dietary tryptophan enhanced the expression of tight junction protein ZO-1 in intestine, J. Food Sci., 2017, 82, 562–567 CrossRef CAS PubMed.
- Y. Yin, K. Yao, Z. Liu, M. Gong, Z. Ruan, D. Deng and G. Wu, Supplementing I-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs, Amino Acids, 2010, 39, 1477–1486 CrossRef CAS PubMed.
- P. S. Worsoe, P. T. Sangild, J. B. van Goudoever, B. Koletzko, E. M. van der Beek, M. Abrahamse-Berkeveld and T. Thymann, Growth and clinical variables in nitrogen-restricted piglets fed an adjusted essential amino acid mix: effects of partially intact protein-based diets, J. Nutr., 2018, 148, 1118–1125 Search PubMed.
- D. Kelly, J. A. Smyth and K. J. McCracken, Digestive development of the early-weaned pig, Br. J. Nutr., 1991, 65, 169–180 CrossRef CAS PubMed.
- S. J. Henning, Ontogeny of enzymes in the small intestine, Annu. Rev. Physiol., 1985, 47, 231–245 CrossRef CAS PubMed.
- T. Tsukahara, R. Inoue, M. Nakatani, K. Fukuta, E. Kishino, T. Ito and K. Ushida, Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine, Anim. Sci. J., 2016, 87, 67–75 CrossRef CAS PubMed.
- M. M. M. Azzam, C. Yuan, G. H. Liu and X. T. Zou, Effect of excess dietary threonine on laying performance, egg quality, serum free amino acids, and digestive enzymes activities of laying hens during the post-peak period, J. Appl. Poult. Res., 2014, 23, 605–613 CrossRef CAS.
- B. O. Eggum, Blood urea measurement as a technique for assessing protein quality, Br. J. Nutr., 1970, 24, 983 CrossRef CAS PubMed.
- K. Yagami and R. Takada, Dietary rice improves growth performance, mucosal enzyme activities and plasma urea nitrogen in weaning piglets, Anim. Sci. J., 2017, 88, 2010–2015 CrossRef CAS PubMed.
- S. Dou, P. Gadonna-Widehem, V. Rome, D. Hamoudi, L. Rhazi, L. Lakhal and I. L. Huerou-Luron, Characterisation of early-life fecal microbiota in susceptible and healthy pigs to post-weaning diarrhoea, PLoS One, 2017, 12, e0169851 CrossRef PubMed.
- L. Zhang, W. Wu, Y. K. Lee, J. Xie and H. Zhang, Spatial heterogeneity and co-occurrence of mucosal and luminal microbiome across swine intestinal tract, Front. Microbiol., 2018, 9, 48 CrossRef PubMed.
- H. J. Flint, S. H. Duncan, K. P. Scott and P. Louis, Links between diet, gut microbiota composition and gut metabolism, Proc. Nutr. Soc., 2015, 74, 13–22 CrossRef CAS PubMed.
- H. Tran, C. L. Anderson, J. W. Bundy, S. C. Fernando and T. E. Burkey, Effects of spray-dried porcine plasma on fecal microbiota in nursery pigs, J. Anim. Sci., 2018, 96, 2017–2031 CrossRef PubMed.
- A. L. Demain, M. Newcomb and J. Wu, Cellulase, clostridia, and ethanol, Microbiol. Mol. Biol. Rev., 2005, 69, 124–154 CrossRef CAS.
- P. K. Wuest, M. A. Horn and H. L. Drake, Clostridiaceae and enterobacteriaceae as active fermenters in earthworm gut content, ISME J., 2011, 5, 92–106 CrossRef CAS PubMed.
- X. Chen, P. Song, P. Fan, T. He, D. Jacobs, C. L. Levesque and J. Zhang, Moderate dietary protein restriction optimized gut microbiota and mucosal barrier in growing pig model, Front. Cell. Infect. Microbiol., 2018, 8, 246 CrossRef PubMed.
- J. R. Lukens, P. Gurung, P. Vogel, G. R. Johnson, R. A. Carter, D. J. McGoldrick and T. D. Kanneganti, Dietary modulation of the microbiome affects autoinflammatory disease, Nature, 2014, 516, 246–249 CrossRef CAS PubMed.
- B. M. Brinkman, F. Hildebrand, M. Kubica, D. Goosens, J. Del Favero, W. Declercq and P. Vandenabeele, Caspase deficiency alters the murine gut microbiome, Cell Death Dis., 2011, 2, e20 Search PubMed.
- K. Hiippala, H. Jouhten, A. Ronkainen, A. Hartikainen, V. Kainulainen, J. Jalanka and R. Satokari, The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation, Nutrients, 2018, 10, 988 CrossRef PubMed.
- A. Koh, F. De Vadder, P. Kovatcheva-Datchary and F. BäCkhed, From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites, Cell, 2016, 165, 1332–1345 CrossRef CAS.
- K. P. Scott, S. W. Gratz, P. O. Sheridan, H. J. Flint and S. H. Duncan, The influence of diet on the gut microbiota, Pharmacol. Res., 2013, 69, 52–60 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9fo02724f |
| This journal is © The Royal Society of Chemistry 2020 |