Open Access Article
Open Access ArticleGastrointestinal digestion of dietary advanced glycation endproducts using an in vitro model of the gastrointestinal tract (TIM-1)†
Timme
van der Lugt
 *a,
Koen
Venema
b,
Stefan
van Leeuwen
c,
Misha F.
Vrolijk
d,
Antoon
Opperhuizen
ae and
Aalt
Bast
ad
*a,
Koen
Venema
b,
Stefan
van Leeuwen
c,
Misha F.
Vrolijk
d,
Antoon
Opperhuizen
ae and
Aalt
Bast
ad
aDepartment of Pharmacology and Toxicology, Maastricht University, Maastricht, The Netherlands. E-mail: t.vanderlugt@maastrichtuniversity.nl
bCentre for Healthy Eating & Food Innovation (HEFI), Maastricht University - campus Venlo, Venlo, The Netherlands
cWageningen Food Safety Research, Wageningen University and Research, Wageningen, The Netherlands
dCampus Venlo, Maastricht University, Venlo, The Netherlands
eOffice for Risk Assessment and Research, Netherlands Food and Consumer Product Safety Authority (NVWA), Utrecht, The Netherlands
First published on 30th June 2020
Abstract
Protein- and sugar-rich food products processed at high temperatures contain large amounts of dietary advanced glycation endproducts (dAGEs). Our earlier studies have shown that specifically protein-bound dAGEs induce a pro-inflammatory reaction in human macrophage-like cells. To what extent these protein-bound dAGEs survive the human gastrointestinal (GI) tract is still unclear. In this study we analysed gastric and small intestinal digestion of dAGEs using the validated, standardised TNO in vitro gastroIntestinal digestion model (TIM-1), a dynamic in vitro model which mimics the upper human GI tract. This model takes multiple parameters into account, such as: dynamic pH curves, peristaltic mixing, addition of bile and pancreatic digestive enzymes, and passive absorption. Samples of different digested food products were collected at different time points after (i) only gastric digestion and (ii) after both gastric plus small intestinal digestion. Samples were analysed for dAGEs using UPLC-MS/MS for the lysine derived Nε-carboxymethyllysine (CML) and Nε-carboxyethyllysine (CEL), and the arginine derived methylglyoxal-derived hydroimidazolone-1 (MG-H1), and glyoxal-derived hydroimidazolone-1 (G-H1). All AGEs were quantified in their protein-bound and free form. The results of this in vitro study show that protein-bound dAGEs survive gastrointestinal digestion and are additionally formed during small intestinal digestion. In ginger biscuits, the presence MG-H1 in the GI tract increased with more than 400%. This also indicates that dAGEs enter the human GI tract with potential pro-inflammatory characteristics.
1. Introduction
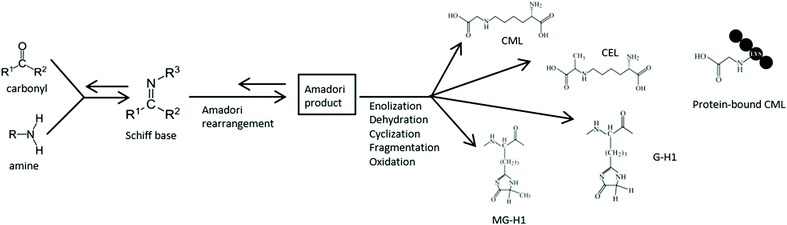
A typical characteristic of the Western diet is the abundance of fried and otherwise processed and heat-treated foods. During thermal processing of food, large amounts of dietary advanced glycation endproducts (dAGEs) are formed.1 Especially in food products containing ample amounts of carbohydrates and proteins, dAGEs are highly present. This formation of dAGEs occurs via the Maillard reaction (Fig. 1) in which the carbonyl group of a reducing sugar reacts with an amino moiety of an amino acid under the influence of heat.2 dAGEs are formed at the final stage of this reaction and are largely responsible for the brown colour and palatability of heat-processed food. Although in some studies dAGES are considered to play an important role in the negative health effects attributed to the Western diet, the health consequences of these compounds are not yet well understood. In a recent study, we demonstrated pro-inflammatory effects of dAGEs on human macrophage-like cells through binding to the receptor for advanced glycation endproducts (RAGE).3 Only protein-bound dAGEs were found to have this pro-inflammatory effect, which was measured as TNF-α secretion, whereas free dAGEs, such as Nε-carboxymethyllysine (CML) and methylglyoxalderived-hydroimidazolone-1 (MG-H1), did not induce any TNF-α secretion.Since the dAGE-protein binding may be vulnerable to acid hydrolysis and proteolysis that occur during gastrointestinal (Gl) digestion, it is to be expected that GI digestion may influence the pro-inflammatory effects. After the ingestion of protein-rich food products, several mechanical and enzymatic processes break down the ingested proteins during GI digestion. In the stomach, acid hydrolysis occurs and the proteolytic enzyme pepsin cleaves proteins into smaller polypeptides. In the small intestine the pH is increased to a neutral level and different proteolytic enzymes from the pancreatic juice cleave the polypeptides further to dipeptides and peptides, which become then available for absorption. The possible release of the AGEs from proteins into their free form may result in a reduced pro-inflammatory effect of dAGEs. Thus far, the influence of GI digestion on the pro-inflammatory potency of dAGEs has not been investigated.
The existing evidence on the effects of digestion in dAGEs is conflicting. While some studies found breakdown of protein binding during digestion,4,5 most studies found a survival of protein bound AGEs in the GI tract.6–8 Moreover, it has previously been suggested that especially high molecular weight dAGES, which include the protein-bound dAGEs, were able to inhibit intestinal protease activity. Already in 1993, Pitotti et al. found an inhibiting effect of Maillard reaction products on protease activity to glycated proteins.9 However, one of the issues that arise when assessing these studies for human relevance, is the use of static GI digestion in vitro models. Often, the stomach phase of these models consists of a mixture of digestion enzymes with a constant pH of 2–2.5 where the food products are exposed to for two hours. After the stomach phase the digests are transferred to a new static environment consisting of different buffers and digestion enzymes with a constant pH of 6.5–7.5 for 4–6 hours.6–8
In contrast to these static GI digestion in vitro models, the human GI tract is a dynamic system that includes mixing via peristaltic movement and alternating pH levels. Additionally, digestive fluids and meal components are removed from the GI tract by both passive and active absorption.10 The TNO gastroIntestinal model (TIM-1) is a dynamic in vitro model that is able to mimic much of the human GI tract. This model takes multiple parameters into account, such as: dynamic pH curves, peristaltic mixing, addition of bile and pancreatic enzymes, and passive absorption.11 The TIM-1 model accurately mimics the in vivo food and drug behaviour with a high prediction capability compared to other models.12
The studies that have been performed on the effect of GI digestion on dAGEs thus far, not only poorly reflect the in vivo situation, they also did not use accurate methods to measure dAGEs. These methods include fluorescence and/or competitive ELISA of CML and ‘methylglyoxal – derived AGEs’.13 Additionally, most studies only assessed meal-resembling systems, and most of these studies focussed on the effect of glycation on protein digestion instead of the digestion of the dAGEs themselves.
Therefore, in this study we aimed to investigate to what extent the dAGE-protein binding is affected by human GI digestion. This is done by digesting a food-based matrix and actual food products (ginger biscuits and apple juice) using the TIM-1 GI model and analysing the digests for different free-dAGEs and protein-bound dAGEs by ultrahigh pressure liquid chromatography coupled to triple quadrupole mass spectrometry (UPLC-MS/MS).
2. Materials and methods
2.1 Chemicals and reagents
Casein from bovine milk, α-lactose monohydrate, NaOH, sodium-phosphate, and 2-mercaptoethanol were obtained from Sigma-Aldrich (Saint Louis, MO USA). Pancreatin (Pancrex V) was obtained from Paines and Birne (Greenford, UK). Porcine bile extract was obtained from Merck (Darmstadt, Germany). Analytical standards of CML (>99%), CEL (>98%), GH-1 (98.2%) and MG-H1 (>98.2%), as well as the deuterium labelled internal standards CML-D4, CEL-D4, and MG-H1-D3 and G-H1-13C2, were obtained from Iris Biotech (Marktredwitz, Germany). Boric acid (99.5%), chloroform (99.5%), nonafluoropentanoic acid (NFPA; 99%), sodium hydroxide (98%), sodium borohydride (96%), and trifluoroacetic acid (TFA; 99%), were obtained from Sigma (Zwijndrecht, Netherlands). HPLC-grade acetonitrile and methanol were obtained from Actu-all Chemicals (Oss, Netherlands).2.2 Preparation of samples
Glycated casein (GC) for use as food-based matrix was prepared by combining casein, glucose, and lactose, in the double proportions of milk powder (22 mM glucose, 0.4 M lactose, 20 g L−1 casein from bovine milk), and diluted in 50 mM phosphate buffer, pH 7.4. One part of the mixture was aliquoted and stored at −80 °C for use as control sample (unglycated casein (UC)). The remaining mixture was heated in an Erlenmeyer on a heating plate at 100 °C for 1 hour. After 1 hour samples were taken and immediately cooled in ice water. Samples were aliquoted and stored at −80 °C.The food products ginger biscuits and apple juice from the same respective batches were bought at a local Dutch supermarket.
2.3 TNO in vitro model of the stomach and small intestine (TIM-1)
Fig. S1† shows a schematic of the in vitro model, which has been described extensively before.14 Briefly, the model comprises four connected glass compartments, representing the stomach, duodenum, jejunum and ileum, respectively. Inside each glass compartment is a flexible silicone inner wall. The space between the inner and outer walls is filled with water of 37 °C. By periodically applying pressure on the water, the flexible inner walls are squeezed, simulating peristalsis. In each individual compartment the pH is measured continuously and regulated by ‘secretion’ of hydrochloric acid (gastric compartment) or sodium bicarbonate (intestinal compartments). The set-points of pH, gastric emptying and intestinal transit time are controlled by a computer. The current experiments were performed in duplicate under the average physiological conditions as found in the human gastrointestinal tract for adults (Fig. S2†). The gastric emptying, intestinal residence time and gastric and intestinal pH-curves mimicked the situation as found in humans for intake after a meal (Fig. S2†). Additional to Fig. S2,† intestinal pH were set at 6.8 in the duodenum, 6.8 in the jejunum, and 7.2 in the ileum. The concentrations of secretion fluids (electrolytes, enzymes, bile, and pancreatic juice) were adjusted to the average concentrations as described for adults after ingestion of a meal. Pancreatic output was simulated by secreting 10% pancreatin (protease, lipase, and amylase) in small intestinal electrolyte solution. Biliary output was simulated by secreting a 2% bile (porcine bile extract) solution at 0.5 ml min−1. Prior to the experiment the compartments were filled with start residues as described before,15 except for the gastric residue, which was mixed with the ‘meal’. Hollow fibre membrane systems continuously dialyzed the digested and dissolved low-molecular weight compounds from the jejunum (Fig. S1-MN† left) and ileum compartments (Fig. S1-MN† right), which simulated absorption of nutrients by the body, and which maintained physiological concentrations of bile and electrolytesThe enzyme activity of pepsin was 2500 units per mg. These units are defined as a change in the absorbance at λ = 280 nm of 0.001 per minute at pH 2.0 at 37 °C, measured as trichloroacetic acid (TCA)-soluble products using haemoglobin as substrate. The enzyme activities of the pancreatin was expressed in international units and were: amylase 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 IU g−1, protease 1000 IU g−1, and lipase 15
000 IU g−1, protease 1000 IU g−1, and lipase 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 IU g−1.
000 IU g−1.
Experiments were done both in the gastric compartment only, as well as in the complete model. In the experiments in which only the gastric compartment was used, the duodenal compartment was only used for neutralization of the gastric efflux, without secretion of bile and pancreatin. This neutralization occurred before sampling.
In the gastric experiments, during 3 hours approximately 95% of the gastric contents were gradually delivered into the small intestine for neutralization (Fig. S2†) through the ‘pyloric sphincter’ (Fig. S1-B†). Experiments in the complete model lasted 6 hours, after which approximately 90% of the small-intestinal contents were gradually delivered (Fig. S2†) into the ‘large intestine’ (sampling bottle) through the ‘ileo-caecal sphincter’ (Fig. S1-H†). Samples were taken every hour from the jejunal and ileal diaysates (Fig. S1-MN,† left and right respectively), and the ileal efflux (Fig. S1-H†). After termination of the experiments, the residues remaining in the system were collected as well, to allow a mass-balance to be made. In case of the liquid food products GC and applejuice, the starting product contained 300 grams of food and for the solid ginger biscuits 30 grams of biscuits were run through the model.
All results that were later obtained with the UPLC MS/MS were corrected for the amount of AGEs present in the gastro-intestinal fluids and enzymes of the TIM-1 model by running a control experiment with only 50 mM phosphate buffer.
2.4 Quantification of dAGEs in digests by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)
In this study, every sample was analysed in both the hydrolysed (total dAGEs, i.e. free + protein-bound dAGEs) and unhydrolysed form (free dAGEs). The sample preparation is based on earlier studies1,16 and similar to our previous study.3 The technical procedure is described in van der Lugt et al. (2018).3 The details on the sample preparation and UPLC-MS/MS settings are mentioned in the supplements. The free dAGE content in the samples was analysed by omitting the hydrolysis step in the sample preparation procedure, thereby circumventing the release of protein-bound dAGEs.Quantification was performed using the precursor-product ion multiple reaction monitoring (MRM) transitions reported in Table S1.† The accuracy of the analysis was monitored by spiking each sample with a specific dAGE standard for each analysed dAGE. The average accuracies ranged from 75–133% (see Table S2†), demonstrating that no severe losses occurred during sample preparation, and no signal enhancements or suppression occurred during UPLC-MS/MS analysis. Ion ratios between the quantifier ion and qualifier ion were monitored. In case ion ratios deviated more than 20% from the ratio observed in the standard, the identity of the peak could not be confirmed. In the case of MG-H1 and G-H1, ion-ratio deviations were observed in GC, ginger cookies and apple juice. A brief investigation into this issue revealed that isomers of MG-H1 (i.e. MG-H2 and MG-H3) and G-H1 (i.e. G-H2 and G-H3) may co-exist in the samples. Additional explorative experiments showed that these may co-elute and alter the ion-ratios (data not shown). Future work is needed to explore this work further, but with the isomers issue in consideration, deviating ion ratios were accepted for MG-H1 and G-H1. It is expected that this also played a role in the slightly elevated (or lower) accuracies for MG-H1 and G-H1 (Table S2†). The LOQ for the protein-bound dAGEs was 125 μg L−1 and 10 μg L−1 for the free dAGEs (Table S3†). This 12.5 fold difference can be explained by omitting the acid hydrolysis step, which introduces a dilution of the sample. By omitting this step, the sample is not diluted, resulting in lower LOQs.
2.5 Analysis of protein size by SDS-page and Coomassie Blue staining
A sample of 10 μg of digested and undigested GC and UG with reducing laemmli buffer was loaded onto an Any kD™ Mini-PROTEAN® TGX™ Precast Protein gel (Bio-rad, Hercules, CA, USA) and run in a Bio-rad cell. Due to volume constraints, the protein amount for small intestinal digested UG was adjusted to 7 μg. For assessment of protein size, the gels were stained by Coomassie Brilliant Blue R250 (Bio-Rad) and imaged with Amersham Imager 600 (GE Healthcare, Chicago, IL, USA). For casein assessment, the samples were treated as previously mentioned. After running, the protein was transferred to a Nitrocellulose membrane (Bio-Rad). Primary antibodies used: Anti-Casein (ab166596, Abcam, Cambridge, UK) Secondary antibody used: Anti-rabbit IgG, HRP-linked Antibody (Cell Signaling Technology, Danvers, MA, USA). The membrane was then incubated with Clarity Western ECL Substrate (Bio-rad) for colour development. Staining of the membranes was analysed using Amersham Imager 600.2.5 Data assessment
Every sample was analysed in both the hydrolysed (total dAGEs) and unhydrolysed form (free dAGEs), as discussed above. To obtain the concentration of protein-bound dAGEs in the samples, the results from the free dAGE measurement were subtracted from the total dAGE measurement of each sample and each dAGE. To correct for any dAGEs present in the TIM-1 solutions and enzymes, a control run of TIM-1 with 50 mM phosphate buffer was conducted and analysed using the UPLC-MS/MS. The small quantities found in the digests from phosphate buffer were subtracted from all dAGE results in the samples. The UPLC-MS/MS results (in μg ml−1) were then multiplied by the volume of the effluents to obtain the absolute amount of dAGEs.3. Results
3.1 Digestion of dietary AGEs – protein binding
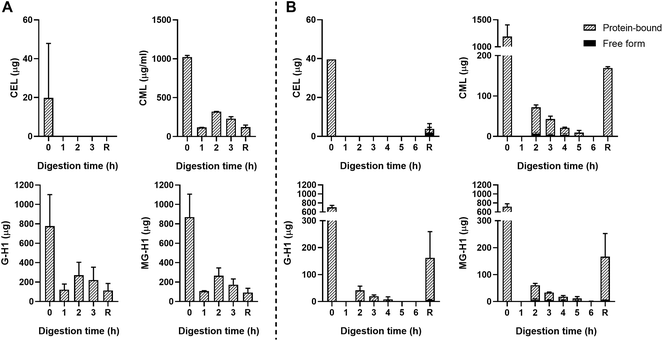
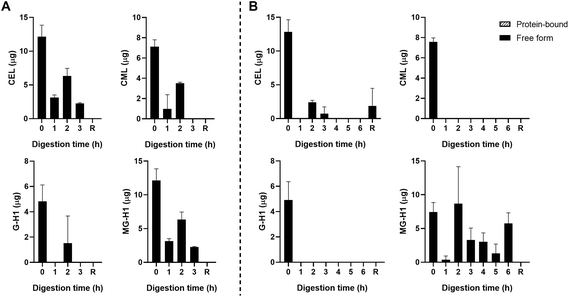
The starting product (undigested GC, t = 0 h) did not contain any free dAGEs. After gastric digestion, none of dAGEs measured were present in free form (Fig. 2A). Fig. 2B shows the results of consecutive gastric and small intestinal digestion. Free CEL was only found in the residue, which is everything left in the TIM-1 system after 6 hours that did not reach the effluent but would naturally enter the colon. Free CML was recovered in the effluent after 2 hours of digestion (9.2% ± 3.4% of total CML) (mean ± SD). Similar percentages of free-form CML were also found after 3 and 4 hours of digestion. Only marginal amounts of free G-H1 was found in the samples in general (6.7% ± 0.9% of total G-H1 after 3 hours of digestion). The amount of free MG-H1 ranged from 10.9% ± 3.6% of total MG-H1 after 2 hours of digestion to 25.8% ± 10.5% of total MG-H1 after 5 hours. After 6 hours of digestion all MG-H1 found was present in the free form (1.4 ± 0.3 μg).
In general, concentrations of protein-bound CML, G-H1, and MG-H1 were highest after 2–3 hours of gastric digestion (Fig. 2A) and combined gastric and small intestinal digestion (Fig. 2B), while gradually decreasing afterwards. The residue contained relatively high amounts of protein-bound CML, G-H1, and MG-H1. Passive absorption of nutrients from the jejunum and ileum was assessed by continuously dialyzing the digested and dissolved low-molecular weight compounds from the jejunum and ileum compartments through hollow fiber membrane systems. No free or protein-bound dAGEs were found in the dialysate of either jejunum or ileum (data not shown).
As a control, unglycated casein (UC) was used which consisted of the same components as GC but it did not undergo thermal treatment. No dAGEs were found in the UC (data not shown).
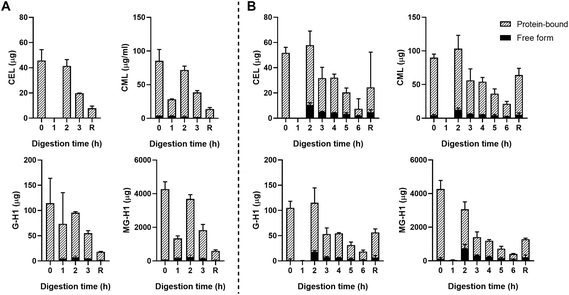
Fig. 3B shows the results for the combination of gastric and small intestinal digestion. Overall, MG-H1 concentrations (both the free and protein-bound form) in the ginger biscuit are higher than the other three dAGEs. After 2 hours of digestion, the amounts of all free dAGEs peaked, containing 22.1% ± 1.2% of total CEL, 12.0% ± 0.1% of total CML, 15.7% ± 2.1% of total G-H1 and 24.1% ± 2.3% of total MG-H1. After 2 hours the amounts of both protein-bound and free dAGEs gradually decreased.
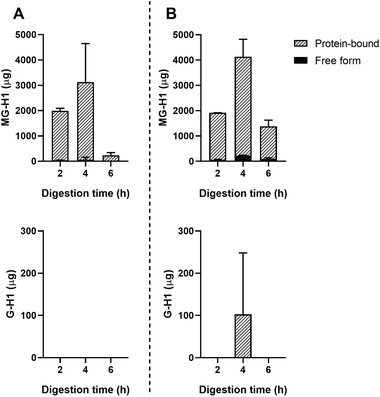
Passive absorption of protein-bound and free MG-H1 occurred in both the jejunum and ileum (Fig. 4), largely in protein-bound form. In absolute amounts, a total of 8.1 ± 1.6 mg MG-H1 (free and protein-bound) left the small intestine in the effluent, while 12.8 ± 0.4 mg of MG-H1 (which is 61% of the total detected protein-bound MG-H1) was absorbed via passive diffusion. In contrast, passively absorbed G-H1 was only found in the ileum after 4 hours and in only 1 sample.
3.2 Digestion of glycated casein – protein digestion
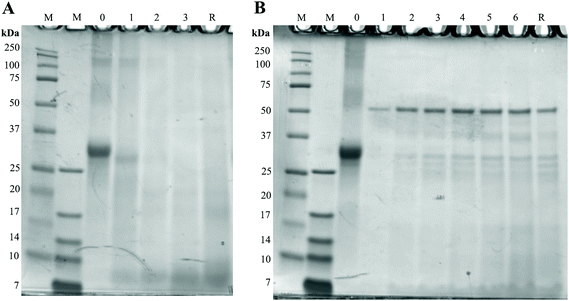
To assess the possible effects of glycation on protein digestion, the digests of GC were first separated with SDS-page and stained with the Coomassie stain. Results can be seen in Fig. 6. In Fig. 6A, the large band just above the 25 kDa before digestion (0 h) reduces during digestion, indicating a decrease in protein size. During the combination of gastric and small intestinal digestion (Fig. 6B) the larger proteins disappear and more smaller size proteins are present in the samples. The persistent band seen at 50 kDa is very likely to be pancreatic lipase.17 The difference between digestion of GC and UC was assessed by immunostaining the SDS-page membranes for bovine casein. Fig. 7 shows the results for digested GC versus UC in the gastric experiments. During the gastric digestion of UC, casein is only detectable in very low levels in the digests of the stomach after 2 hours of digestion, whereas casein is still present in the digests of GC after 3 hours of digestion. Immunostaining the small intestinal digests for casein did not show any differences (data not shown).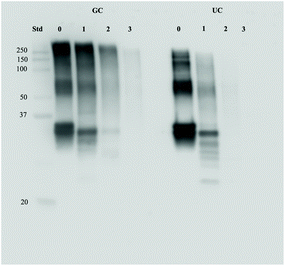 | ||
| Fig. 7 Protein levels of casein in GC and UC digests after 0, 1, 2, and 3 hours of gastric digestion. | ||
3.3 Digestion of AGEs – recovery
In order to calculate the absolute amounts of the different dAGEs (free and protein-bound) leaving the stomach and small intestine compartments, every effluent of the TIM-1 model was weighed to allow for a mass-balance to be made. These absolute amounts were then expressed as percentages of the total amounts of the starting products that were ingested in order to assess the recovery of dAGEs in the TIM-1 model. The results can be seen in Table 1 for both total (free and protein-bound) dAGEs and only protein-bound dAGEs. For GC, the recoveries after gastric digestion for all dAGEs was <100%, and further decreased during small intestinal digestion. Recoveries for the ginger biscuit were much higher. After gastric digestion the recoveries for protein-bound dAGEs ranged from 152 ± 13% for CEL to 231 ± 156% for G-H1. After additional small intestinal digestion, the recoveries for protein-bound dAGEs increased even further and ranged from 276 ± 96% for CEL to 454 ± 73% for MG-H1. Recoveries for the free-form dAGEs in apple juice were all lower than 100% after gastric digestion. Remarkably, no CML and G-H1 and 37 ± 25% for CEL were recovered after small intestinal digestion, whereas free MG-H1 was recovered for 296 ± 63% after the small intestinal digestion.| Gastric | Small Intestinal | ||||
|---|---|---|---|---|---|
| Total (%) | Protein-bound (%) | Total (%) | Protein-bound (%) | ||
| GC | CEL | 0 ± 0 | 0 ± 0.0 | 5 ± 7 | 5 ± 7 |
| CML | 77 ± 4 | 77 ± 4 | 27 ± 5 | 26 ± 5 | |
| G-H1 | 91 ± 13 | 91 ± 13 | 33 ± 13 | 32 ± 13 | |
| MG-H1 | 73.5 ± 0.4 | 73.5 ± 0.4 | 42 ± 15 | 38 ± 15 | |
| Ginger biscuit | CEL | 152 ± 13 | 152 ± 13 | 332 ± 104 | 276 ± 96 |
| CML | 182 ± 27 | 180 ± 30 | 372 ± 54 | 350 ± 53 | |
| G-H1 | 247 ± 159 | 231 ± 156 | 406 ± 221 | 371 ± 218 | |
| MG-H1 | 175 ± 3 | 163 ± 2 | 494 ± 76 | 454 ± 73 | |
| Apple juice | CEL | 96.8 ± 0.5 | — | 37 ± 25 | — |
| CML | 62 ± 15 | — | 0.0 ± 0.0 | — | |
| G-H1 | 27 ± 38 | — | 0.0 ± 0.0 | — | |
| MG-H1 | 23 ± 33 | — | 296 ± 63 | — | |
4. Discussion
In this study, the digestion of the dAGE-protein-binding was assessed in a sophisticated human gastrointestinal in vitro model using a food-based matrix and actual food products. dAGEs are largely present in thermally treated food products. Earlier findings have shown that specifically protein-bound dAGEs induce a pro-inflammatory reaction in human macrophage-like cells. To what extent these protein-bound dAGEs survive the GI tract was unclear until now.Our results show that the protein binding of dAGEs is able to survive gastric and small intestinal digestion and consequently stays intact throughout the whole GI tract (Fig. 2, 3, and 4). These findings underline findings from earlier studies that used static digestion models, where the protein binding survived digestion. These studies were mostly focused on assessing the effect of glycation on protein digestibility. Among others, Zhao et al. (2017) saw a decrease in digestibility of β-casein after glycation with glyoxal.6 In addition, Pinto et al. (2014) found larger aggregates of casein when casein was heated combined with glucose than when casein was heated alone. Moreover, the heated glucose-casein combination was less digestible than heated native casein,18 indicating that glycation interferes and complicates protein digestion. These results correspond to the results that were observed in the current study when comparing the digestion of GC with UC (Fig. 7), but only in the gastric compartment. The digestion of the casein protein was assessed with immunostaining and hampered gastric digestion of casein was seen as protein levels of casein in UC were almost completely diminished after 2 hours, whereas casein was still detectable in the digests of GC after 3 hours of digestion. In the small intestines, no more differences were present between protein levels of casein in GC and UC. This was also seen by Joubran et al. (2017), who found that most peptides were liberated in the duodenum in an in vitro infant gastrointestinal tract.7 In addition Corzo-Martinez et al. (2012) saw differences in proteolytic breakdown between different glycated proteins, so the type of protein may play a role in digestion efficiency.5 Despite the fact that the casein protein was broken down in the small intestines, our results show that the dAGE-protein binding stays intact. As Coomassie staining and immunoblotting for casein (Fig. 6) showed a decrease in protein size during small intestinal digestion and break down of casein, it is possible that the dAGEs are bound to these smaller proteins or peptides after digestion.
There are several ways through which glycation can interfere with protein digestion in the small intestines. Inhibition occurs via steric hindrance of the proteolytic cleavage site by an AGE and via the direct binding of an AGE to the proteolytic cleavage site.7,19 Lysine and arginine contain tryptic binding sites because the ε-amino groups of lysine and the guanido groups of arginine are charged positively and will therefore be attracted to the negatively charged active centre of trypsin.20 However, the same ε-amino group of lysine and the guanido group of arginine are major reactive sites in the Maillard reaction, which possibly explains why glycation of proteins may disrupt protein digestion. Within the group of amino acids, lysine is the most reactive in the Maillard reaction.21–23 CML and CEL are lysine-derived dAGEs and MG-H and G-H are arginine-derived dAGEs. By blocking the residues on these amino acids, dAGEs inhibit proteolytic digestion.20 Interestingly, in this study a distinct effect of glycation on gastric digestion of casein rather than small intestinal digestion was observed (Fig. 7). The mechanism through which glycation may interfere with gastric digestion is less well known. Hydrolysis and pepsin proteolysis are the primarily responsible mechanisms for protein digestion in the stomach. The gastric pH plays an essential role in the activity of gastric enzymes and thus gastric digestion. A major advantage of the TIM-1 model is the dynamic pH range of the stomach compartment (Fig. S2†). In the previously used static in vitro models, the pH is set to 2–2.5 for two hours, which poorly reflects the physiological situation as in the human stomach the pH is more dynamic. The fasted gastric pH varies between 1–1.5, but after ingestion, the pH increases to 5–7, depending on the buffer capacity of the meal. After approximately 3 hours of digestion, the pH has returned to basal levels.24 Human gastric emptying time is of large influence on the gastric pH, but this is also highly variable and dependent on the type of meal eaten. Sams et al. (2016) found that with liquid meals, the gastric pH ranges between 4 to 7 after 50% of gastric emptying, while the gastric pH is still 4.6 after ingestion of a solid meal and 50% gastric emptying. Pepsin exerts maximal activity at a pH of 2, is still 70% active at a pH of 4, but then rapidly declines in activity with increasing pH.25 Denaturation of the proteins by gastric acid leads to increased accessibility of pepsin to the protein.26,27 The high pH of the stomach in the first hours of digestion might explain the exit of undigested casein from the stomach into the duodenum in the UC samples; the pH of the stomach was not low enough for pepsin to exert its activity. The largest difference in casein digestion between GC and UC was seen in the stomach (Fig. 7). Opposite to lysine and arginine as tryptic cleavage sites, peptic cleavage sites include tyrosine, tryptophan, phenylalanine, and leucine. Even though these amino acids do not contain the primarily reactive residues for the Maillard reaction, all N-terminal groups of amino acids can react with 1,2-dicarbonyls. For instance, tryptophan has an indole side chain that can be glycated.28 But since the Maillard reaction prefers lysine, the reduced digestibility that is seen in the GC vs. UC gastric digestion is probably to be largely attributed to other mechanisms happening during the Maillard reaction, such as the previously discussed steric hindrance.6
In the current study a large portion of protein bound MG-H1 in ginger biscuits was absorbed by the TIM-1 model by passive absorption through the dialysis filters (Fig. 4). This effect was not seen in the results of the other food products or for the other dAGEs, except one G-H1 outlier. An explanation for this can be the large concentrations of MG-H1 in the starting ginger biscuit compared to the other dAGEs. GC and apple juice also contain smaller concentrations of MG-H1. The large dilution occurring in the dialysate may have led the other dAGEs to be present in concentrations below the level of quantification of the UPLC-MS/MS. It is however remarkable that protein-bound MG-H1 is passively absorbed to such a large extent. The physiological relevance of this finding is unclear, firstly because the method by which the passive absorption occurs is not very close to the real-life situation. Secondly, because previous in vitro absorption studies by Hellwig et al. (2011) showed uptake of MG-H1 in Caco-2 cells, but strong retainment of MG-H1 in the intestinal cell.29 It will therefore most probably not enter the systemic circulation via passive absorption.
A very interesting result of the present study was that all food products showed endogenous formation of AGEs in the small intestinal tract, with ginger biscuits showing the highest amount of endogenously formed MG-H1 represented by the recovery of more than 400% (Table 1). This endogenous formation was seen in earlier studies using the static in vitro models by Martinez-Saez et al. (2019) and Bains et al. (2017).13,30 Additionally, Helou et al. (2015) used the TIM-1 model to assess the digestion of fluorescent AGEs from bread crust, or soluble melanoidins as they called them, and found an increase in fluorescent properties of the soluble melanoidins.31 The authors concluded that, since the TIM-1 model only has a temperature of 37 °C, the increase was related to “the enzymatic and chemical release of fluorescent and soluble melanoidins from larger insoluble melanoidin-skeletons”. This would mean that proteins conceal glycated parts that are released during glycation. However, our method of assessing dAGE concentrations includes hydrolysing the dAGEs from their protein-binding, circumventing this problem. We show that new AGEs are being formed and not released from their structures. Moreover, UC did not lead to any endogenous formation of AGEs, indicating that the thermal treatment that food products undergo are vital for the formation of endogenous AGEs. In this study AGEs are only formed endogenously after digestion of products that also contained AGEs, which might indicate an underlying need for the presence of AGE precursors in food, such as glyoxal and methylglyoxal. As previously stated, ginger biscuits displayed a much larger formation of food-derived endogenous AGEs than GC and apple juice. A second mechanism for the formation of endogenous AGEs in the GI tract could therefore be the presence of fructose in the ginger biscuit, which is highly reactive in the Maillard reaction due to its reduced stability of the ring structure compared to glucose.32 Although conflicting evidence exists on whether there is a correlation between dAGE uptake and AGE serum and urine levels, a perspective by DeChristopher published in 2017 proposed that a source for increased serum AGE levels may be the formation of AGEs in the GI tract.33 Fructose is actively absorbed in the human GI tract together with glucose in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Many processed food products contain high fructose corn syrup (HFCS), making the ratio fructose
1 ratio. Many processed food products contain high fructose corn syrup (HFCS), making the ratio fructose![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose < 1, resulting in fructose malabsorption. This would leave the fructose in the GI tract to react with proteins present. In this study a small proportion of free AGEs were seen after digestion. However, due to this additional new formation of AGEs, it is not possible to conclude whether these AGEs are liberated from their protein-binding during digestion or were newly formed.
glucose < 1, resulting in fructose malabsorption. This would leave the fructose in the GI tract to react with proteins present. In this study a small proportion of free AGEs were seen after digestion. However, due to this additional new formation of AGEs, it is not possible to conclude whether these AGEs are liberated from their protein-binding during digestion or were newly formed.
This formation of dAGEs in the intestinal tract was not seen with the food-based matrix GC, from which dAGEs were digested during gastric and small intestinal digestion. This was contrary to the study conducted by Martinez-Saez using different meal resembling systems that did show an increase in dAGEs during digestion.13 These contradicting results can be explained by the difference in constituents of the meal resembling systems between this study and the one from Martinez-Saez. The nutrients used in the different studies are important for the progress of the Maillard reaction, something that is underlined in the aforementioned study.
This indicates that meal resembling systems are actually poor models for food products. However, many digestion studies on dAGEs and the Maillard reaction make use of meal resembling systems. Our results show that the outcomes of these studies only using meal resembling systems should be interpreted with caution.
Gastric digestion led in both GC and apple juice to a loss of dAGEs (Table 1), this implies a breakdown of dAGEs during digestion. In the case of protein-bound dAGEs in GC, the hydrolysing of the sample before UPLC-MS/MS measurement may have led to a loss of dAGEs bound to peptides, since some small peptides may not have precipitated. This is not the case for the loss seen in free dAGEs in apple juice, since these samples are not hydrolysed. Another problem that arose during the UPLC-MS/MS measurement is the presence of different isomers of G-H (G-H1, G-H2, and G-H3), the isomerization may have interfered with the measurement, causing the large standard deviations seen in the absolute results of G-H1. Another aspect that has to be taken into account is that in the current UPLC-MS/MS method a higher LOQ is obtained for the hydrolysed samples versus unhydrolysed samples. This is due to omitting the hydrolysis step in the latter approach (thereby omitting a dilution step) (Table S3†). This may have resulted in a possible underestimation of the amount of protein-bound dAGEs versus free dAGEs.
The results of this study show that protein-bound dAGEs in food products stay bound to proteins during mimicked human GI digestion in both the meal resembling system and the food product. This implies for human health that dAGEs enter the human GI tract in a pro-inflammatory state where they might cause or aggravate inflammation. Earlier studies have shown that RAGE is upregulated in inflamed areas of the colon in Crohn's Disease patients, indicating a possible role for dAGEs in inflammatory bowel diseases.34 Glycation can also interfere with the digestion of proteins, leading to a loss of amino acids, both essential and non-essential.6,13 A human study compared the protein digestibility in adolescents of a low dAGE diet and a high dAGE diet and found only a small, but significant difference. The proteins in the high dAGE diet were 6% less digested than the low dAGE diets.35 A more recent study assessed the effects of different glycation levels on post-prandial lysine availability in humans and saw a decrease in lysine bioavailability with increasing glycation levels.36 This result was only seen for lysine and not for other amino acids.
In addition to having negative effects on amino acid bioavailability, some indications also exist of newly formed bioactive peptides during the Maillard reaction in foods.7 A consequence of reduced protein and amino acid digestibility is the presence of proteins in the colon. Protein fermentation in the colon leads to the formation of detrimental compounds such as ammonia, amines, phenols and sulfides.37 Excessive protein fermentation in the colon has been linked to colon cancer and Ulcerative Colitis.38 There is no consensus on the effect of dAGEs in the colon and on the colonic microbiota as the effects depend on both the type of food product and the thermal treatment.39,40
As stated before, the pH of the stomach is crucial for protein digestion, as acid hydrolysis of the protein liberates proteolytic binding sites. The higher the pH, the less access for proteolytic enzymes. People taking anti-acid medication pharmacologically elevate their stomach pH, making acid hydrolysis less effective and thereby interfering with the digestion of protein-bound dAGEs. Additionally, age-related differences exist in stomach pH, where the infant stomach is less acidic than the adult stomach and the GI tract has different transit times.41,42 Infant formulas are extensively processed and contain protein-bound dAGEs that might have a detrimental effect on the developing infant GI tract.43,44
5. Conclusion
In this study we have shown in a validated, sophisticated in vitro GI model that dAGEs enter the small intestinal and large intestinal tract in predominantly protein-bound and thereby pro-inflammatory state. Furthermore, it can be concluded that glycation hampered casein digestion. Additionally, we have shown that endogenous AGEs are formed in the GI tract with the quantities depending on the type of food product and thermal treatment. Our results show that the effect of digestion on food-based matrices, or meal-resembling systems, do not correspond to the effects on actual food products. This is important for future research into dAGEs.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Jessica Verhoeven and Sanne Verbruggen at HEFI for their valuable help with the TIM-1 model, Stanislava Vonsovic, Anouk Lentjes and Henk Gerritsen at WFSR for performing the UPLC-MS/MS measurements, and Casper Schalkwijk and Jean Scheijen for their feedback and suggestions during the data analysis.This research is supported by the Dutch Food and Consumer Safety Authority (NVWA) (BAS project code WOT-02-002-004). The research was also partly funded by the Dutch Province of Limburg, with a grant to HEFI.
References
- J. Scheijen, E. Clevers, L. Engelen, P. C. Dagnelie, F. Brouns, C. D. A. Stehouwer and C. G. Schalkwijk, Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary AGE database, Food Chem., 2016, 190, 1145–1150 CrossRef CAS PubMed.
- M. W. Poulsen, R. V. Hedegaard, J. M. Andersen, B. de Courten, S. Bugel, J. Nielsen, L. H. Skibsted and L. O. Dragsted, Advanced glycation endproducts in food and their effects on health, Food Chem. Toxicol., 2013, 60, 10–37 CrossRef CAS PubMed.
- T. van der Lugt, A. Weseler, W. Gebbink, M. Vrolijk, A. Opperhuizen and A. Bast, Dietary Advanced Glycation Endproducts Induce an Inflammatory Response in Human Macrophages in Vitro, Nutrients, 2018, 10, 1868 CrossRef PubMed.
- I. Alamir, C. Niquet-Leridon, P. Jacolot, C. Rodriguez, M. Orosco, P. M. Anton and F. J. Tessier, Digestibility of extruded proteins and metabolic transit of N-epsilon-carboxymethyllysine in rats, Amino Acids, 2013, 44, 1441–1449 CrossRef CAS PubMed.
- M. Corzo-Martinez, M. Avila, F. J. Moreno, T. Requena and M. Villamiel, Effect of milk protein glycation and gastrointestinal digestion on the growth of bifidobacteria and lactic acid bacteria, Int. J. Food Microbiol., 2012, 153, 420–427 CrossRef CAS PubMed.
- D. Zhao, L. Li, T. T. Le, L. B. Larsen, G. Su, Y. Liang and B. Li, Digestibility of Glyoxal-Glycated beta-Casein and beta-Lactoglobulin and Distribution of Peptide-Bound Advanced Glycation End Products in Gastrointestinal Digests, J. Agric. Food Chem., 2017, 65, 5778–5788 CrossRef CAS PubMed.
- Y. Joubran, A. Moscovici, R. Portmann and U. Lesmes, Implications of the Maillard reaction on bovine alpha-lactalbumin and its proteolysis during in vitro infant digestion, Food Funct., 2017, 8, 2295–2308 RSC.
- A. M. Moscovici, Y. Joubran, V. Briard-Bion, A. Mackie, D. Dupont and U. Lesmes, The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants, Food Funct., 2014, 5, 1898–1908 RSC.
- A. Pitotti, A. Dalbo and M. Stecchini, Effect of Maillard Reaction-Products on Proteases Activity in vitro, J. Food Qual., 1994, 17, 211–220 CrossRef CAS.
- A. Guerra, L. Etienne-Mesmin, V. Livrelli, S. Denis, S. Blanquet-Diot and M. Alric, Relevance and challenges in modeling human gastric and small intestinal digestion, Trends Biotechnol., 2012, 30, 591–600 CrossRef CAS PubMed.
- P. A. Dickinson, R. Abu Rmaileh, L. Ashworth, R. A. Barker, W. M. Burke, C. M. Patterson, N. Stainforth and M. Yasin, An investigation into the utility of a multi-compartmental, dynamic, system of the upper gastrointestinal tract to support formulation development and establish bioequivalence of poorly soluble drugs, AAPS J., 2012, 14, 196–205 CrossRef CAS PubMed.
- S. Souliman, S. Blanquet, E. Beyssac and J. M. Cardot, A level A in vitro/in vivo correlation in fasted and fed states using different methods: applied to solid immediate release oral dosage form, Eur. J. Pharm. Sci., 2006, 27, 72–79 CrossRef CAS PubMed.
- N. Martinez-Saez, B. Fernandez-Gomez, W. Cai, J. Uribarri and M. D. Del Castillo, In vitro formation of Maillard reaction products during simulated digestion of meal-resembling systems, Food Res. Int., 2019, 118, 72–80 CrossRef CAS PubMed.
- I. Surono, J. Verhoeven, S. Verbruggen and K. Venema, Microencapsulation increases survival of the probiotic Lactobacillus plantarum IS-10506, but not Enterococcus faecium IS-27526 in a dynamic, computer-controlled in vitro model of the upper gastrointestinal tract, J. Appl. Microbiol., 2018, 124, 1604–1609 CrossRef CAS PubMed.
- M. Minekus, P. Marteau, R. Havenaar and J. H. J. Huis, In't Veld, A multicompartmental dynamic computer-controlled model simulating the stomach and small intestine, Altern. Lab. Anim., 1995, 23, 197–209 Search PubMed.
- S. H. Assar, C. Moloney, M. Lima, R. Magee and J. M. Ames, Determination of Nepsilon-(carboxymethyl)lysine in food systems by ultra performance liquid chromatography-mass spectrometry, Amino Acids, 2009, 36, 317–326 CrossRef CAS PubMed.
- K. Iizuka, H. Higurashi, J. Fujimoto, Y. Hayashi, K. Yamamoto and H. Hiura, Purification of human pancreatic lipase and the influence of bicarbonate on lipase activity, Ann. Clin. Biochem., 1991, 28(Pt 4), 373–378 CrossRef CAS PubMed.
- M. S. Pinto, J. Leonil, G. Henry, C. Cauty, A. F. Carvalho and S. Bouhallab, Heating and glycation of beta-lactoglobulin and beta-casein: Aggregation and in vitro digestion, Food Res. Int., 2014, 55, 70–76 CrossRef CAS.
- G. A. A. van Lieshout, T. T. Lambers, M. C. E. Bragt and K. A. Hettinga, How processing may affect milk protein digestion and overall physiological outcomes: A systematic review, Crit. Rev. Food Sci. Nutr., 2019, 1–24, DOI:10.1080/10408398.2019.1646703.
- Y. Wada and B. Lonnerdal, Effects of Different Industrial Heating Processes of Milk on Site-Specific Protein Modifications and Their Relationship to in Vitro and in Vivo Digestibility, J. Agric. Food Chem., 2014, 62, 4175–4185 CrossRef CAS PubMed.
- E. H. Ajandouz and A. Puigserver, Nonenzymatic browning reaction of essential amino acids: effect of pH on caramelization and Maillard reaction kinetics, J. Agric. Food Chem., 1999, 47, 1786–1793 CrossRef CAS PubMed.
- E. J. Kwak and S. I. Lim, The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control, Amino Acids, 2004, 27, 85–90 CrossRef CAS PubMed.
- S. H. Ashoor and J. B. Zent, Maillard Browning of Common Amino Acids and Sugars, J. Food Sci., 1984, 49, 1206–1207 CrossRef CAS.
- L. Sams, J. Paume, J. Giallo and F. Carriere, Relevant pH and lipase for in vitro models of gastric digestion, Food Funct., 2016, 7, 30–45 RSC.
- D. W. Piper and B. H. Fenton, pH stability and activity curves of pepsin with special reference to their clinical importance, Gut, 1965, 6, 506–508 CrossRef CAS PubMed.
- J. M. Berg, J. L. Tymoczko and L. Stryer, Proteins Are Degraded to Amino Acids in Biochemistry, W H Freeman, New York, 5th edn, 2002 Search PubMed.
- M. Schlamowitz and L. U. Peterson, Studies on the optimum pH for the action of pepsin on “native” and denatured bovine serum albumin and bovine hemoglobin, J. Biol. Chem., 1959, 234, 3137–3145 CAS.
- G. Munch, D. Schicktanz, A. Behme, M. Gerlach, P. Riederer, D. Palm and R. Schinzel, Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library, Nat. Biotechnol., 1999, 17, 1006–1010 CrossRef CAS PubMed.
- M. Hellwig, S. Geissler, R. Matthes, A. Peto, C. Silow, M. Brandsch and T. Henle, Transport of free and peptide-bound glycated amino acids: synthesis, transepithelial flux at Caco-2 cell monolayers, and interaction with apical membrane transport proteins, ChemBioChem, 2011, 12, 1270–1279 CrossRef CAS PubMed.
- Y. Bains, A. Gugliucci and R. Caccavello, Advanced glycation endproducts form during ovalbumin digestion in the presence of fructose: Inhibition by chlorogenic acid, Fitoterapia, 2017, 120, 1–5 CrossRef CAS PubMed.
- C. Helou, S. Denis, M. Spatz, D. Marier, V. Rame, M. Alric, F. J. Tessier and P. Gadonna-Widehem, Insights into bread melanoidins: fate in the upper digestive tract and impact on the gut microbiota using in vitro systems, Food Funct., 2015, 6, 3737–3745 RSC.
- D. Hemmler, C. Roullier-Gall, J. W. Marshall, M. Rychlik, A. J. Taylor and P. Schmitt-Kopplin, Insights into the Chemistry of Non-Enzymatic Browning Reactions in Different Ribose-Amino Acid Model Systems, Sci. Rep., 2018, 8, 16879 CrossRef PubMed.
- L. R. DeChristopher, Perspective: The Paradox in Dietary Advanced Glycation End Products Research-The Source of the Serum and Urinary Advanced Glycation End Products Is the Intestines, Not the Food, Adv. Nutr., 2017, 8, 679–683 CrossRef CAS PubMed.
- R. Ciccocioppo, A. Vanoli, C. Klersy, V. Imbesi, V. Boccaccio, R. Manca, E. Betti, G. C. Cangemi, E. Strada, R. Besio, A. Rossi, C. Falcone, S. Ardizzone, P. Fociani, P. Danelli and G. R. Corazza, Role of the advanced glycation end products receptor in Crohn's disease inflammation, World J. Gastroenterol., 2013, 19, 8269–8281 CrossRef CAS PubMed.
- I. Seiquer, J. Diaz-Alguacil, C. Delgado-Andrade, M. Lopez-Frias, A. Muñoz Hoyos, G. Galdo and M. P. Navarro, Diets rich in Maillard reaction products affect protein digestibility in adolescent males aged 11-14 y, Am. J. Clin. Nutr., 2006, 83, 1082–1088 CrossRef CAS PubMed.
- J. Nyakayiru, G. A. A. van Lieshout, J. Trommelen, J. van Kranenburg, L. B. Verdijk, M. C. E. Bragt and L. J. C. van Loon, The glycation level of milk protein strongly modulates post-prandial lysine availability in humans, Br. J. Nutr., 2019, 1–22, DOI:10.1017/S0007114519002927.
- M. S. Gilbert, N. Ijssennagger, A. K. Kies and S. W. C. van Mil, Protein fermentation in the gut; implications for intestinal dysfunction in humans, pigs, and poultry, Am. J. Physiol.: Gastrointest. Liver Physiol., 2018, 315, G159–G170 CrossRef CAS PubMed.
- K. M. Tuohy, D. J. Hinton, S. J. Davies, M. J. Crabbe, G. R. Gibson and J. M. Ames, Metabolism of Maillard reaction products by the human gut microbiota–implications for health, Mol. Nutr. Food Res., 2006, 50, 847–857 CrossRef CAS PubMed.
- M. Snelson and M. T. Coughlan, Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology, Nutrients, 2019, 11(2) CrossRef PubMed , pii: E215.
- S. Perez-Burillo, S. Pastoriza, N. Jimenez-Hernandez, G. D'Auria, M. P. Francino and J. A. Rufian-Henares, Effect of Food Thermal Processing on the Composition of the Gut Microbiota, J. Agric. Food Chem., 2018, 66, 11500–11509 CrossRef CAS PubMed.
- G. Yu, Q. S. Zheng and G. F. Li, Similarities and differences in gastrointestinal physiology between neonates and adults: a physiologically based pharmacokinetic modeling perspective, AAPS J., 2014, 16, 1162–1166 CrossRef CAS PubMed.
- D. Dupont, G. Mandalari, D. Molle, J. Jardin, J. Leonil, R. M. Faulks, M. S. Wickham, E. N. Mills and A. R. Mackie, Comparative resistance of food proteins to adult and infant in vitro digestion models, Mol. Nutr. Food Res., 2010, 54, 767–780 CrossRef CAS PubMed.
- I. Penndorf, D. Biedermann, S. V. Maurer and T. Henle, Studies on N-terminal glycation of peptides in hypoallergenic infant formulas: quantification of alpha-N-(2-furoylmethyl) amino acids, J. Agric. Food Chem., 2007, 55, 723–727 CrossRef CAS PubMed.
- D. Keller, R. Van Dinter, H. Cash, S. Farmer and K. Venema, Bacillus coagulans GBI-30, 6086 increases plant protein digestion in a dynamic, computer-controlled in vitro model of the small intestine (TIM-1), Benefic. Microbes, 2017, 8, 491–496 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0fo00450b |
| This journal is © The Royal Society of Chemistry 2020 |