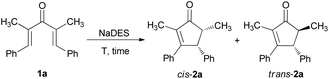
Natural deep eutectic solvents as an efficient and reusable active system for the Nazarov cyclization†
Stefano
Nejrotti
 ,
Marta
Iannicelli
,
Salwa Simona
Jamil
,
Davide
Arnodo
,
Marta
Iannicelli
,
Salwa Simona
Jamil
,
Davide
Arnodo
 ,
Marco
Blangetti
,
Marco
Blangetti
 and
Cristina
Prandi
and
Cristina
Prandi
 *
*
Dipartimento di Chimica, Università di Torino, via P. Giuria 7, I-10125 Torino, Italy. E-mail: cristina.prandi@unito.it
First published on 19th November 2019
Abstract
Natural deep eutectic solvents have emerged as alternative non-toxic, non-aqueous solvents for an increasing number of synthetic transformations. Remarkably, in some cases one (or more) components of the NaDES plays an active role in the reaction mechanism and directly participates as either a catalyst or a reagent in the reaction. In this paper, we tested several NaDESs in which one of the components is a carboxylic acid as a medium to perform the Nazarov cyclization of divinyl ketones to obtain cyclopentenones, a widespread motif in natural compounds. The reaction conditions were optimized and the scope was investigated on C-, O- and N-derived compounds. To assess the full sustainability of the proposed approach, the recyclability and scalability of the process were investigated, thus proving that multi-gram preparations are possible with complete recycling of the medium.
1. Introduction
The Nazarov reaction or Nazarov cyclization (NC) was first reported in 1942 and consists of the 4π conrotatory electrocyclization of divinyl ketones to cyclopentenones in the presence of Brønsted or Lewis acids (Scheme 1).1 The reaction is tolerant to substituents on the dienone skeleton (Scheme 1, A) and thus allows for the facile synthesis of functionalized cyclopentenones B, including relatively simple non-annulated structures as well as complex polynuclear cyclopentenoid skeletons annulated with one or more non-aromatic, aromatic or heteroaromatic rings.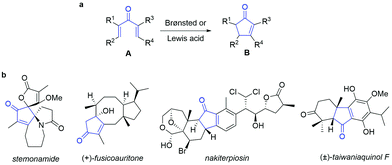 | ||
| Scheme 1 (a) The Nazarov reaction. (b) Examples of the occurrence of the cyclopentenone motif in natural compounds. | ||
Over the years, the Nazarov reaction has found wide application in synthesis and has become one of the most useful tools for the preparation of various cyclopentenone motifs.1b,2 The study of the torquoselectivity of the reaction allowed for stereochemical control of the substituted cyclopentenones obtained,3 and enantioselective versions of the NC were reported.4 Modern protocols for the facile generation of the pentadienyl cation intermediate include a wide number of precursors beyond the traditional divinyl ketones A,5 including oxiranes,6 unsaturated carbinols,7 allene ethers8 and propargylic esters.9 Besides strong Brønsted acids, the NC can be catalysed by Lewis acids, such as main-group Lewis acids and transition metals: successful data are for example reported for BF3·Et2O,10 Pd(0),11 Ag,12 Au(I),13 Au(III)9b,14 and halides.15 Remarkably, organocatalytic versions of the NC were also reported in which chiral phosphoric acid derivatives,16 diamines,17 thioureas,18 and cinchona alkaloids19 were also used.20
Despite its importance as a synthetic tool for the preparation of natural and pharmaceutically active compounds and its undeniable potential as a synthetic tool which fulfills green chemistry principles in terms of atom economy, the NC still presents some drawbacks related to sustainability. Indeed, strong Brønsted acids (e.g. TFA, TfOH, and MsOH), which are toxic and corrosive, are usually employed and are often required in a stoichiometric or super-stoichiometric amount.21 On the other hand, the use of Lewis acids is affected by the cost and air-sensitivity of the metal catalyst. Furthermore, volatile organic solvents (VOCs) and in particular chlorinated solvents are by far the most common solvent system for the NC.2e Up to now, only a few references report on more sustainable versions of the NC, in which non-toxic and recyclable homogeneous22 and heterogeneous23 catalysts were used. A remarkable example in this sense is represented by the cellulose–SO3H catalyst reported by Rostami.24 Finally, a Nazarov cyclization performed in water in the presence of surfactants (and Lewis acids) was reported.25
Deep eutectic solvents (DESs) have emerged as a new promising class of green solvents, since they are non-flammable, non-toxic, biodegradable and very easily prepared from cheap and readily accessible components.26 An appropriate choice of the constituents paves the way for the design of different matrices with specific physicochemical properties.27 When the compounds that constitute the DESs are primary metabolites and in general natural compounds, namely amino acids, organic acids, sugars or choline derivatives, the DESs are called natural deep eutectic solvents (NaDESs). NaDESs totally fulfill green chemistry principles and are now foreseen as potential contributors to more sustainable industrial processes.28
Since the first report by Abott,29 DESs have been successfully employed in several fields of application,26c,27 including organic synthesis.30 It has been shown that, apart from constituting a more sustainable alternative to traditional solvents, DESs often behave as a non-innocent reaction medium and can play an active role in promoting organic reactions, by improving the reaction rate and the yield and allowing for milder conditions. This has been attributed to the supramolecular structure of these mixtures, characterized by an extensive hydrogen bonding pattern.30c–e
In this work, we wish to report on our study on the Nazarov cyclization in deep eutectic solvents, in open-air and under mild conditions. Naturally occurring carboxylic acids are employed as components of the DES, allowing for mild conditions in a renewable active solvent system, which combines the lower toxicity and good biodegradability31 with its low cost. As to our knowledge, this is the first example of a NC promoted by weak carboxylic acids. Remarkably, the medium can be recycled up to 4 times without considerable loss in its activity.
2. Results and discussion
To start our project and investigate the feasibility of the NC in NaDESs, we chose the symmetric tetra-substituted dienone 1a as a model substrate.32 Various choline chloride (ChCl)-carboxylic acid based NaDESs were tested on the basis of their ability to convert 1a into the product cyclopentenone 2a (Table 1). First, ChCl/L-(+)-lactic acid in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio was tested and was ineffective in promoting the reaction at room temperature, even after 24 h (Table 1, entry 1). On the other hand, ChCl/oxalic acid 1
2 molar ratio was tested and was ineffective in promoting the reaction at room temperature, even after 24 h (Table 1, entry 1). On the other hand, ChCl/oxalic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 partially converted the substrate at the same temperature (entry 2). Raising the temperature up to 60 °C resulted in an improvement of the reaction yield to 91% after 16 h (entry 3). NaDESs based on L-(−)-malic acid (entry 4), citric acid (entry 5) and L-(+)-tartaric acid (entry 6) were less effective, as lower yields were obtained with these NaDESs under the same experimental conditions. Conversely, malonic acid (entry 7) and maleic acid (entry 8) based NaDESs performed well, affording 2a with a yield of 92% and 87% respectively. ChCl/p-toluenesulfonic acid (TsOH) in 1
1 partially converted the substrate at the same temperature (entry 2). Raising the temperature up to 60 °C resulted in an improvement of the reaction yield to 91% after 16 h (entry 3). NaDESs based on L-(−)-malic acid (entry 4), citric acid (entry 5) and L-(+)-tartaric acid (entry 6) were less effective, as lower yields were obtained with these NaDESs under the same experimental conditions. Conversely, malonic acid (entry 7) and maleic acid (entry 8) based NaDESs performed well, affording 2a with a yield of 92% and 87% respectively. ChCl/p-toluenesulfonic acid (TsOH) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio was also tested, and in this case a yield up to 99% was obtained (entry 9).21,31 NaDESs based on betaine (entry 11) and L-proline (entry 12), instead of ChCl, were found to be ineffective in promoting the reaction. We then performed some control experiments, which are summarized in Table 1, entries 13–16. A DES not containing an acid, such as ChCl/H2O 1
2 molar ratio was also tested, and in this case a yield up to 99% was obtained (entry 9).21,31 NaDESs based on betaine (entry 11) and L-proline (entry 12), instead of ChCl, were found to be ineffective in promoting the reaction. We then performed some control experiments, which are summarized in Table 1, entries 13–16. A DES not containing an acid, such as ChCl/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (entry 13), and a polar organic solvent, such as ethanol (entry 14), were ineffective in promoting the reaction, indicating that an acid-containing DES is required.
2 (entry 13), and a polar organic solvent, such as ethanol (entry 14), were ineffective in promoting the reaction, indicating that an acid-containing DES is required.
| Entry | Solvent | T (°C) | Time | 2a (%) | cis/transb |
|---|---|---|---|---|---|
| Reaction conditions: 1a (0.2 mmol), in 1 g of the appropriate DES.a Yield of the isolated product.b Determined by 1H-NMR analysis of purified compounds.c Higher temperature was required due to the viscosity of the medium.d 0% enantiomeric excess. | |||||
| 1 | ChCl/L-(+)-lactic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
rt | 24 h | Traces | — |
| 2 | ChCl/oxalic acid dihydrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
rt | 16 h | 30% | 57/43 |
| 3 | ChCl/oxalic acid dihydrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 16 h | 91% | 14/86 |
| 4 | ChCl/L-(−)-malic acid/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 | 16 h | 22% | 26/74d |
| 5 | ChCl/citric acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% H2O 1 + 2% H2O |
60 | 16 h | 36% | 67/33 |
| 6 | ChCl/L-(+)-tartaric acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% H2O 1 + 2% H2O |
80c | 16 h | 67% | 17/83d |
| 7 | ChCl/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 16 h | 92% | 17/83 |
| 8 | ChCl/maleic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 16 h | 87% | 16/84 |
| 9 | ChCl/TsOH monohydrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 | 16 h | 99% | 15/85 |
| 10 | ChCl/R-(−)-mandelic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 16 h | 59% | 20/80d |
| 11 | Betaine/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 | 16 h | — | — |
| 12 |
L-Proline/oxalic acid dihydrate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 | 16 h | — | — |
| 13 | ChCl/H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
60 | 16 h | — | — |
| 14 | EtOH | 60 | 16 h | — | — |
| 15 | EtOH (10 eq. malonic acid) | 60 | 16 h | 33% | 24/76 |
| 16 | DCE (10 eq. malonic acid) | 60 | 16 h | Traces | — |
| 17 | H2O (10 eq. malonic acid) | 60 | 16 h | 23% | 30/70 |
The acidic component of the eutectic mixture, malonic acid, was tested in polar and apolar solvents such as ethanol (entry 15), 1,2-dichloroethane (entry 16) and water (entry 17), but very low yields were obtained in these cases, suggesting that the activity is a peculiar property of the eutectic mixture and does not derive from its acidic component per se.33 Considering that in general the acidity of the DES is governed by the acidity of the HBD, we then tried to rationalize these results, taking into consideration the pKa of the acidic component of the NaDES. The NaDESs containing malic acid (pKa 3.40), citric acid (2.92, 4.28 and 5.21) and tartaric acid (2.99) were almost ineffective, while the best results were obtained with oxalic (1.23), malonic (2.83) and maleic acids (1.93),34 with malonic acid better performing (see entry 7, Table 1) than maleic acid (entry 8, Table 1). Taken together, these data do not account for a direct correlation between performance in the NC and pKa of the acidic component of the DES. According to a recently published study,35 the acidic properties of DES mixtures can differ from those of their components alone, and moreover they can be significantly affected by the temperature. This behaviour suggests the crucial role played by the supramolecular interactions in the eutectic mixture and is particularly relevant in the cases of ChCl/oxalic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ChCl/malonic acid 1
1 and ChCl/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where a drop in the pH values is observed as the temperature increases.36 These observations are in line with our results, which showed how in ChCl/oxalic acid 1
1, where a drop in the pH values is observed as the temperature increases.36 These observations are in line with our results, which showed how in ChCl/oxalic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ChCl/malonic acid 1
1 and ChCl/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entries 3 and 7 of Table 1) at 60 °C 2a was obtained in both cases in 92% yield despite the different pKa of the HBD component (1.23 for oxalic acid and 2.83 for malonic acid). The diastereoselectivity of the reaction was also evaluated in terms of the cis/trans ratio. At room temperature (entry 2) the cyclization is not diastereoselective. At 60 °C the selectivity improves in favour of trans-2a to a 14/86 ratio in the case of ChCl/oxalic acid (entry 3).
1 (entries 3 and 7 of Table 1) at 60 °C 2a was obtained in both cases in 92% yield despite the different pKa of the HBD component (1.23 for oxalic acid and 2.83 for malonic acid). The diastereoselectivity of the reaction was also evaluated in terms of the cis/trans ratio. At room temperature (entry 2) the cyclization is not diastereoselective. At 60 °C the selectivity improves in favour of trans-2a to a 14/86 ratio in the case of ChCl/oxalic acid (entry 3).
Similar results have been obtained with ChCl/malonic acid and ChCl/maleic acid (entries 7 and 8) thus proving that the most efficient NaDESs are also those inducing the best diastereoselectivity. It should be noted that in the case of ChCl/citric acid (entry 5) the diastereoselectivity is in favour of cis-2a. A proposed mechanism to account for the diastereoselectivity either in favour of the cis or the trans cyclopentenone and based on the acidic character of the melt might involve multiple key activation roles of the solvent (Brønsted acid and hydrogen bond formation) as well as a plausible structure of the eutectic mixture. Further computational and physicochemical investigations are needed to elucidate these aspects. Regarding the enantioselectivity of the reaction, when we employed NaDESs composed of a chiral carboxylic acid (entries 4, 6 and 10) we did not observe any induction of chirality in the product.
Based on the optimization study, we extended the scope of the reaction to different substrates, choosing ChCl/malonic acid, ChCl/oxalic acid or ChCl/TsOH as a possible reaction medium (entries 7, 3 and 9 of Table 1). The results reported in Scheme 2 refer to the best score we obtained for each of the substrates.
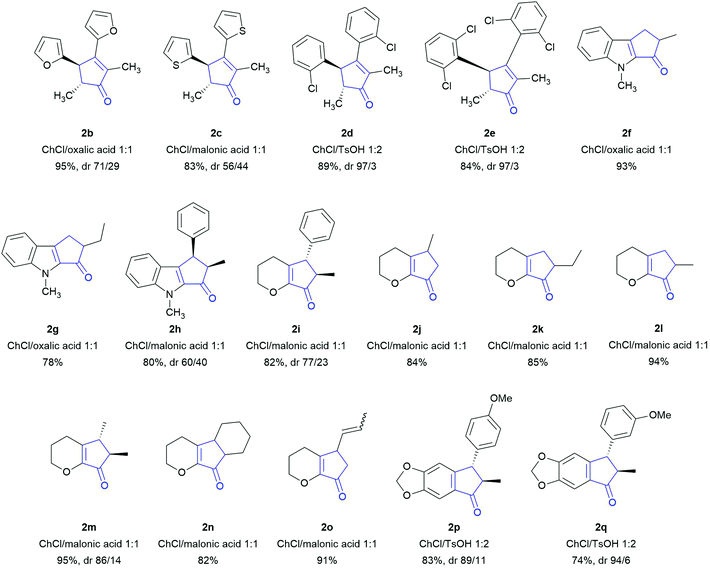 | ||
| Scheme 2 Scope of the Nazarov cyclization in NaDESs. All reactions were performed at 60 °C for 16 h and extracted with cyclopentyl methyl ether (CPME), as described in the ESI.† | ||
Symmetrical dienones bearing electron-rich or electron-poor aryl substituents were successfully cyclized to the corresponding cyclopentenones 2b–e with very good yields (Scheme 2). Sterically encumbered substrates leading to 2d and 2e afforded the corresponding products with almost complete diastereoselectivity for the trans isomer.
Since one of the most synthetically useful aspects of the NC is represented by the possibility to access fused (hetero)polycyclic molecular scaffolds and due to our own research interest in the synthesis of heterocyclic natural compounds,37 we investigated the reactivity of several dienones whose structure is embedded in an indole or dihydropyran moiety. To our delight, for all the substrates the reaction proceeded smoothly to afford cyclopentenones 2f–o in 78% to 95% yields. Finally, we showed that also aryl vinyl ketones, which are less prone to undergo the cyclization due to the interruption of the aromatic system as a consequence of the cyclization,38 are converted into the corresponding Nazarov products 2p and 2q in good yield with diastereoselectivity.
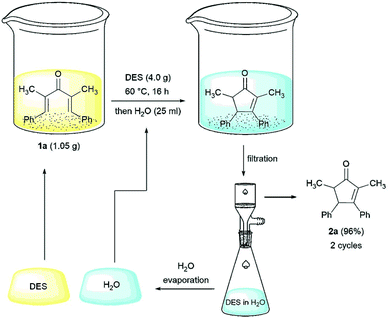
One of the most attractive features of a catalytic system is undoubtedly the recyclability. We tested the possibility to recycle the ChCl/malonic acid eutectic mixture for the cyclization of dienone 1a, at a concentration of 0.2 mmol g−1 (i.e. mmol of substrate per g of NaDES). The procedure for the recovery of the NaDES, shown in Fig. 3, is highly feasible and involves the addition of water to the reaction mixture upon completion of the reaction, causing the precipitation of the product, which can be collected by filtration. Water is then evaporated to restore the eutectic mixture, which is used without further manipulations for another run. As shown in Fig. 1, the activity of the NaDES remains essentially unchanged for the first two cycles and is acceptable up to the fourth one, while in the fifth run a drop in the yield of product 2a is observed.
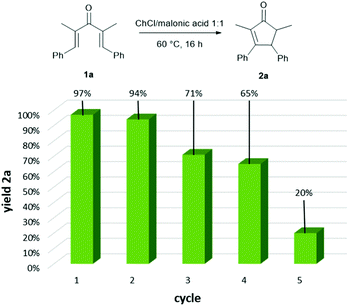 | ||
| Fig. 1 Recyclability of the ChCl/malonic acid DES for the Nazarov cyclization of 1a. Yields were determined by quantitative 1H NMR analysis using CH3NO2 as the internal standard. | ||
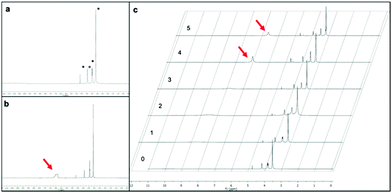
The activity of the NaDES strongly depends on the dynamic behaviour of its molecules and possibly in this case on the intermolecular interactions in which the COOH of the NaDES is involved. It seems then reasonable that the protic nature of the carboxylic acid component becomes stronger or weaker either in a more restricted or more disordered supramolecular network. To investigate how the activity of the NaDES changes in each recycling cycle we decided to evaluate the structure of the NaDES ChCl/malonic acid by 1H and 13C NMR (see the ESI† for full size spectra).39
In Fig. 2 the 1H NMR spectrum of pure NaDES ChCl/malonic acid is reported. The 1H NMR signals at 3.1 (9H), 3.4 (2H) and 4.1 ppm (2H) are attributable to ChCl (*), while at 3.5 ppm the CH2 of malonic acid is detectable (○). The COOH proton of malonic acid is entrapped in the tight network of intermolecular H bonds and is detectable in the 1H NMR spectrum as a very broad signal between 10 and 11 ppm. In Fig. 2b, the 1H NMR spectrum of ChCl/malonic acid in which a 10% of water has been added on purpose is reported. A signal at around 7 ppm is detectable and assigned to water entrapped in the NaDES, while free water is detectable at 4.7 ppm. In Fig. 2c, the 1H NMR spectra of the NaDES recovered after each cycle are reported. It is evident that the structure of the NaDES remains substantially unchanged after the first cycle (Fig. 2c, 1), compared to the freshly prepared one (Fig. 2c, 0). In these cases yields of 2a of 94% and 97% respectively were obtained (see Fig. 1). Starting from the NaDES recovered from the second cycle, a broad signal around 8 ppm starts to appear and becomes progressively more evident after the fourth and fifth cycles, shifting towards 7 ppm. The presence of some other minor peaks might indicate that the structure of the DES is partially decomposed.40 To rationalize these results, it should be worthwhile to recall that the activity of the NaDES ChCl/malonic acid is higher than that of malonic acid alone (see entries 13–15 in Table 1), as the supramolecular structure of the DES might enhance the polarizability of COOH groups and facilitate the reactivity. The increase in the amount of water contained in the DES (and not removable by simple evaporation) is apparently correlated with the observed drop in yield. Further investigations are needed to assess how the supramolecular structure of the NaDES is modified in each cycle.41
The successful recycling procedure prompted us to further increase the efficiency of the process, with a view to improving its scalability. To this aim, we tested various substrate concentrations in NaDESs, in order to find the highest concentration at which the product yield remains unaltered, which was found to be 1 mmol g−1, provided that the substrate was well dispersed in the medium and the stirring of the mixture was effective. Therefore, we set up a gram-scale reaction starting from 1.05 g (4.0 mmol) of substrate 1a and 4.0 g of ChCl/malonic acid (Fig. 3). The NaDES was recovered and used again for a second run through the above described procedure. To minimize waste in this scale preparation, the water recovered at the rotavapor after the first cycle was used again for the second cycle.
The overall process afforded 2.01 g of cyclopentenone 2a by using only 4.0 g of ChCl/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The E-factor parameter for the process is 19.5, a value that confirms the green chemistry credentials of our protocol (see the ESI† for the calculations).42
1. The E-factor parameter for the process is 19.5, a value that confirms the green chemistry credentials of our protocol (see the ESI† for the calculations).42
3. Conclusions
In this paper, for the first time we have disclosed a conceptually new, flexible, versatile, and high-yielding Nazarov cyclization of divinyl ketones performed in deep eutectic solvents, in which the reaction medium plays a dual role both as a solvent and as a promoter of the cyclization. The combination of divinyl ketones and NaDESs composed of carboxylic acids as an environmentally benign reaction medium enables access to the targeted compounds with excellent yields and good stereocontrol, at moderate temperature. The scope of the reaction is wide and several substituents on the divinyl ketone framework are well tolerated.Furthermore, the potential of this reaction in terms of coherence with green chemistry principles was addressed and several sustainable features of this transformation have been developed. In particular, we demonstrated that the process is scalable and multi-gram preparations are possible. A practical and easy work-up allows for the recovery of both the NaDES and water. We demonstrated that 4 cycles of reaction is tolerated with an overall acceptable yield. The beneficial effect of the DES is further showcased by its ability to obtain, in some cases, pure compounds, thus avoiding purification by column chromatography.
4. Experimental section
4.1 Materials
Flasks and all equipment used for the generation and reaction of moisture-sensitive compounds were dried by using an electric heat gun under nitrogen. Unless specified, all reagents were used as received without further purification. Anhydrous THF was obtained by distillation over LiAlH4, followed by distillation over Na-benzophenone; benzaldehyde was distilled under vacuum. Flash column chromatography was performed over silica gel (40–63 μm, 230–400 mesh); Rf values refer to TLC carried out on silica gel plates. 1H NMR and 13C NMR spectra were recorded on a Jeol ECZR600, in CDCl3, using a residual solvent peak as an internal standard (CHCl3, 1H: 7.26 ppm, 13C: 77.16 ppm). NMR spectra of DESs were recorded in a capillary tube, using D2O as the locking solvent. Multiplicities are reported as follows: s (singlet), d (doublet), t (triplet), q (quartet), quin (quintet), sext (sextet), m (multiplet), and br (broad). GC-MS spectra were recorded at an ionizing voltage of 70 eV. Deep eutectic solvents were prepared by methods reported in the literature (see the ESI† for details). Divinyl ketone substrates 1a–q were synthesized according to the literature (see the ESI† for details).4.2 General procedure for the Nazarov cyclization in DESs
A vial was charged with the dienone substrate (0.2–0.5 mmol), then the DES (5 g per mmol of substrate) was added and the mixture was stirred at 60 °C for 16 h. Water was added and the mixture was extracted three times with cyclopentyl methyl ether (CPME); the combined organic layers were dried over anhydrous Na2SO4 and filtered and the solvent was evaporated under reduced pressure. When required, the crude product was purified by flash column chromatography.4.3 General procedure for recycling cycles
A vial was charged with the dienone 1a (1.0–4.0 mmol), then ChCl/malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (5 g or 1 g per mmol of substrate) was added and the mixture was stirred at 60 °C for 16 h. Water was added, causing the precipitation of the product. The solid product was recovered by filtration, while water was evaporated under reduced pressure to afford the eutectic mixture, which was used for the next reaction cycle.
1 (5 g or 1 g per mmol of substrate) was added and the mixture was stirred at 60 °C for 16 h. Water was added, causing the precipitation of the product. The solid product was recovered by filtration, while water was evaporated under reduced pressure to afford the eutectic mixture, which was used for the next reaction cycle.
Synthesized according to the general procedure with ChCl/malonic acid 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. White solid, 92% yield, trans/cis ratio 83
1. White solid, 92% yield, trans/cis ratio 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17. Rf 0.25 (PE/Et2O 9
17. Rf 0.25 (PE/Et2O 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). trans-2a1H NMR (600 MHz) δ (ppm): 7.34–7.24 (m, 5H), 7.23–7.19 (m, 2H), 7.15–7.11 (m, 1H), 7.09–7.06 (m, 2H), 3.98 (quin, 1H, J = 2.2 Hz), 2.41 (qd, 1H, J = 7.3, 2.8 Hz), 2.03 (d, 3H, J = 2.0 Hz), 1.36 (d, 3H, J = 7.5 Hz). 13C NMR (150 MHz) δ (ppm): 211.1 (Cq), 167.2 (Cq), 142.2 (Cq), 136.9 (Cq), 135.3 (Cq), 129.1 (CH), 128.9 (CH), 128.5 (CH), 128.4 (CH), 127.7 (CH), 126.8 (CH), 56.5 (CH), 51.4 (CH), 15.4 (CH3), 10.3 (CH3). GC-MSm/z (%): 262 [M]+ (100), 247 (88), 219 (38), 115 (36). cis-2a1H NMR (600 MHz) δ (ppm): 7.40–7.37 (m, 2H), 7.34–7.24 (m, 4H), 7.24–7.19 (m, 2H), 7.18–7.16 (m, 1H), 7.00 (br s, 1H), 4.63–4.60 (m, 1H), 2.92 (quin, 1H, J = 7.4 Hz), 2.09 (d, 3H, J = 1.7 Hz), 0.76 (d, 3H, J = 7.5 Hz). 13C NMR (150 MHz) δ (ppm): 211.6 (Cq), 166.4 (Cq), 139.3 (Cq), 137.1 (Cq), 135.9 (Cq), 129.1 (CH), 128.5 (CH), 128.5 (CH), 128.4 (CH), 127.7 (CH), 127.0 (CH), 52.7 (CH), 45.6 (CH), 12.4 (CH3), 10.4 (CH3). GC-MSm/z (%): 262 (100), 247 (92), 219 (40), 115 (40).
1). trans-2a1H NMR (600 MHz) δ (ppm): 7.34–7.24 (m, 5H), 7.23–7.19 (m, 2H), 7.15–7.11 (m, 1H), 7.09–7.06 (m, 2H), 3.98 (quin, 1H, J = 2.2 Hz), 2.41 (qd, 1H, J = 7.3, 2.8 Hz), 2.03 (d, 3H, J = 2.0 Hz), 1.36 (d, 3H, J = 7.5 Hz). 13C NMR (150 MHz) δ (ppm): 211.1 (Cq), 167.2 (Cq), 142.2 (Cq), 136.9 (Cq), 135.3 (Cq), 129.1 (CH), 128.9 (CH), 128.5 (CH), 128.4 (CH), 127.7 (CH), 126.8 (CH), 56.5 (CH), 51.4 (CH), 15.4 (CH3), 10.3 (CH3). GC-MSm/z (%): 262 [M]+ (100), 247 (88), 219 (38), 115 (36). cis-2a1H NMR (600 MHz) δ (ppm): 7.40–7.37 (m, 2H), 7.34–7.24 (m, 4H), 7.24–7.19 (m, 2H), 7.18–7.16 (m, 1H), 7.00 (br s, 1H), 4.63–4.60 (m, 1H), 2.92 (quin, 1H, J = 7.4 Hz), 2.09 (d, 3H, J = 1.7 Hz), 0.76 (d, 3H, J = 7.5 Hz). 13C NMR (150 MHz) δ (ppm): 211.6 (Cq), 166.4 (Cq), 139.3 (Cq), 137.1 (Cq), 135.9 (Cq), 129.1 (CH), 128.5 (CH), 128.5 (CH), 128.4 (CH), 127.7 (CH), 127.0 (CH), 52.7 (CH), 45.6 (CH), 12.4 (CH3), 10.4 (CH3). GC-MSm/z (%): 262 (100), 247 (92), 219 (40), 115 (40).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Prof. Vito Capriati for helpful discussions and advice, Dr Matteo Tiecco for providing the betaine/malonic acid DES, and Huvepharma srl, CRT (Cassa di Risparmio di Torino), Regione Piemonte and the Italian Ministry of Research for financial support.References
- (a) K. L. Habermas, S. E. Denmark and T. K. Jones, Org. React., 2004, 45, 1–158 Search PubMed; (b) M. G. Vinogradov, O. V. Turova and S. G. Zlotin, Org. Biomol. Chem., 2017, 15, 8245–8269 RSC.
- (a) M. A. Tius, Eur. J. Org. Chem., 2005, 2193–2206 CrossRef CAS; (b) D. R. Wenz and J. Read de Alaniz, Eur. J. Org. Chem., 2015, 23–37 CrossRef CAS; (c) H. Pellissier, Tetrahedron, 2005, 27, 6479–6517 CrossRef; (d) A. J. Frontier and C. Collison, Tetrahedron, 2005, 61, 7577–7606 CrossRef CAS; (e) T. Vaidya, R. Eisenberg and A. J. Frontier, ChemCatChem, 2011, 3, 1531–1548 CrossRef CAS.
- (a) E. G. Occhiato, C. Prandi, A. Ferrali, A. Guarna and P. Venturello, J. Org. Chem., 2003, 68, 9728–9741 CrossRef CAS PubMed; (b) C. Prandi, A. Ferrali, A. Guarna, P. Venturello and E. G. Occhiato, J. Org. Chem., 2004, 69, 7705–7709 CrossRef CAS; (c) A. Cavalli, M. Masetti, M. Recanatini, C. Prandi, A. Guarna and E. G. Occhiato, Chem. – Eur. J., 2006, 12, 2836–2845 CrossRef CAS; (d) A. Cavalli, A. Pacetti, M. Recanatini, C. Prandi, D. Scarpi and E. G. Occhiato, Chem. – Eur. J., 2008, 14, 9292–9304 CrossRef CAS; (e) B. L. Flynn, N. Manchala and E. H. Krenske, J. Am. Chem. Soc., 2013, 135, 9156–9163 CrossRef CAS; (f) T. D. R. Morgan, L. M. LeBlanc, G. H. Ardagh, R. J. Boyd and D. J. Burnell, J. Org. Chem., 2015, 80, 1042–1051 CrossRef CAS PubMed.
- (a) N. Shimada, C. Stewart and M. A. Tius, Tetrahedron, 2011, 67, 5851 CrossRef CAS PubMed; (b) C. E. Sleet, U. K. Tambar and P. Maity, Tetrahedron, 2017, 73, 4023–4038 CrossRef CAS; (c) M. Rueping, A. Kuenkel and I. Atodiresei, Chem. Soc. Rev., 2011, 40, 4539–4549 RSC; (d) S. P. Simeonov, J. P. Nunes, K. Guerra, V. B. Kurteva and C. A. Afonso, Chem. Rev., 2016, 116, 5744–5893 CrossRef CAS.
- W. T. Spencer III, T. Vaidya and A. J. Frontier, Eur. J. Org. Chem., 2013, 3621–3633 CrossRef.
- (a) G. Sudhakar and K. Satish, Chem. – Eur. J., 2015, 21, 6475–6480 CrossRef CAS PubMed; (b) J. A. Malona, K. Cariou and A. J. Frontier, J. Am. Chem. Soc., 2009, 131, 7560–7561 CrossRef CAS PubMed.
- (a) X. Liu, X. Xu, L. Pan, Q. Zhang and Q. Liu, Org. Biomol. Chem., 2013, 11, 6703–6706 RSC; (b) B. N. Kakde, P. Kumari and A. Bisai, J. Org. Chem., 2015, 80, 9889–9899 CrossRef CAS PubMed; (c) Z. Wang, X. Xu, Z. Gu, W. Feng, H. Qian, Z. Li, X. Sun and O. Kwon, Chem. Commun., 2016, 52, 2811–2814 RSC; (d) B. N. Kakde, N. Kumar, P. K. Mondal and A. Bisai, Org. Lett., 2016, 18, 1752–1755 CrossRef CAS.
- (a) M. A. Tius, Chem. Soc. Rev., 2014, 43, 2979–3002 RSC; (b) R. J. Fradette, M. Kang and F. West, Angew. Chem., Int. Ed., 2017, 56, 6335–6338 CrossRef CAS; (c) V. Nair, S. Bindu, V. Sreekumar and A. Chiaroni, Org. Lett., 2002, 4, 2821–2823 CrossRef CAS.
- (a) M. Petrovic, D. Scarpi, B. Fiser, E. Gomez-Bengoa and E. G. Occhiato, Eur. J. Org. Chem., 2015, 3943–3956 CrossRef CAS; (b) M. Hoffmann, J.-M. Weibebl, P. de Fremont, P. Pale and A. Blanc, Org. Lett., 2014, 16, 908–911 CrossRef CAS PubMed; (c) L. Zhang and S. Wang, J. Am. Chem. Soc., 2006, 128, 1442–1443 CrossRef CAS.
- V. M. Marx and D. J. Burnell, J. Am. Chem. Soc., 2010, 132, 1685–1689 CrossRef CAS PubMed.
- N. Shimada, C. Stewart, W. F. Bow, A. Jolit, K. Wong, Z. Zhou and M. A. Tius, Angew. Chem., Int. Ed., 2012, 51, 5727–5729 CrossRef CAS.
- (a) P. Cordier, C. Aubert, M. Malacria, E. Lacote and V. Gandon, Angew. Chem., Int. Ed., 2009, 48, 8757–8760 CrossRef CAS PubMed; (b) S. S. Subramanium, S. Handa, A. J. Miranda and L. M. Slaughter, ACS Catal., 2011, 1, 1371–1374 CrossRef CAS.
- (a) A. Rinaldi, M. Petrović, S. Magnolfi, D. Scarpi and E. G. Occhiato, Org. Lett., 2018, 20, 4713–4717 CrossRef CAS PubMed; (b) G.-Y. Lin, C.-W. Li, S.-H. Hung and R.-S. Liu, Org. Lett., 2008, 10, 5059–5062 CrossRef CAS PubMed.
- (a) T. Jin and Y. Yamamoto, Org. Lett., 2008, 10, 3137–3139 CrossRef CAS PubMed; (b) M. E. Krafft, D. V. Vidhani, J. W. Cran and M. Manoharan, Chem. Commun., 2011, 47, 6707–6709 RSC.
- (a) J. J. Koenig, T. Arndt, N. Gildemeister, J. M. Neudorfl and M. Breugst, J. Org. Chem., 2019, 84, 7587–7605 CrossRef CAS PubMed; (b) A. Dreger, P. Wonner, E. Engelage, S. M. Walter, R. Stoll and S. M. Huber, Chem. Commun., 2019, 55, 8262–8265 RSC.
- (a) M. Rueping, W. Ieawsuwan, A. P. Antonchick and B. J. Nachtsheim, Angew. Chem., Int. Ed., 2007, 46, 2097–2100 CrossRef CAS; (b) A. Jolit, P. M. Walleser, G. P. Yap and M. A. Tius, Angew. Chem., Int. Ed., 2014, 53, 6180–6183 CrossRef CAS PubMed; (c) A. Jolit, C. F. Dickinson, K. Kitamura, P. M. Walleser, G. P. Yap and M. A. Tius, Eur. J. Org. Chem., 2017, 6067–6076 CrossRef CAS.
- W. F. Bow, A. K. Basak, A. Jolit, D. A. Vicic and M. A. Tius, Org. Lett., 2010, 12, 440–443 CrossRef CAS.
- A. K. Basak, N. Shimada, W. F. Bow, D. A. Vicic and M. A. Tius, J. Am. Chem. Soc., 2010, 132, 8266–8267 CrossRef CAS PubMed.
- Y.-W. Huang and A. J. Frontier, Tetrahedron Lett., 2015, 56, 3523–3526 CrossRef CAS PubMed.
- (a) J. Ouyang, J. L. Kennemur, C. K. De, C. Farès and B. List, J. Am. Chem. Soc., 2019, 141, 3414–3418 CrossRef CAS PubMed; (b) M. Vogler, L. Süsse, J. H. LaFortune, D. W. Stephan and M. Oestreich, Organometallics, 2018, 37, 3303–3313 CrossRef CAS.
- M. Amere, J. Blanchet, M.-C. Lasne and J. Rouden, Tetrahedron Lett., 2008, 49, 2541–2545 CrossRef CAS.
- M. Barbero, S. Cadamuro, A. Deagostino, S. Dughera, P. Larini, E. G. Occhiato, C. Prandi, S. Tabasso, R. Vulcano and P. Venturello, Synthesis, 2009, 2260–2266 CrossRef CAS.
- (a) K. Murugan, S. Srimurugan and C. Chen, Chem. Commun., 2010, 46, 1127–1129 RSC; (b) Y. Shimoda and H. Yamamoto, Tetrahedron Lett., 2015, 56, 3090–3092 CrossRef CAS; (c) W. Cao and B. Yu, Adv. Synth. Catal., 2011, 353, 1903–1907 CrossRef CAS.
- Z. Daneshfar and A. Rostami, RSC Adv., 2015, 5, 104695–104707 RSC.
- S. Wang, R. William, K. K. G. E. Seah and X.-W. Liu, Green Chem., 2013, 15, 3180–3183 RSC.
- (a) A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS; (b) M. Francisco, A. van den Bruinhorst and M. C. Kroon, Angew. Chem., Int. Ed., 2013, 52, 3074–3085 CrossRef CAS; (c) E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jerome, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- (a) H. Vanda, Y. Dai, E. G. Wilson, R. Verpoorte and Y. H. Choi, C. R. Chim., 2018, 21, 628–638 CrossRef CAS; (b) Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS; (c) Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- A. P. Abott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- (a) C. Ruß and B. König, Green Chem., 2012, 14, 2969–2982 RSC; (b) J. García-Álvarez, Eur. J. Inorg. Chem., 2015, 2015, 5147–5157 CrossRef; (c) D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org. Chem., 2016, 612–632 CrossRef CAS; (d) P. Liu, J.-W. Hao, L.-P. Mo and Z.-H. Zhang, RSC Adv., 2015, 5, 48675–48704 RSC; (e) S. Khandelwal, Y. K. Tailor and M. Kumar, J. Mol. Liq., 2016, 215, 345–386 CrossRef CAS; (f) S. Handy, in Ionic Liquids-Current State of the Art, InTech, 2015 CrossRef.
- B.-Y. Zhao, P. Xu, F.-X. Yang, H. Wu, M.-H. Zong and W.-Y. Lou, ACS Sustainable Chem. Eng., 2015, 3, 2746–2755 CrossRef CAS . Since TsOH is not a bio-derived compound, formally ChCl/TsOH is not a NaDES, however its toxicity and biodegradability were found to be comparable to those of the other acid-based NaDESs.
- P. Yates, N. Yoda, W. Brown and B. Mann, J. Am. Chem. Soc., 1958, 80, 202–205 CrossRef CAS.
- F. Douelle, L. Tal and M. F. Greaney, Chem. Commun., 2005, 660–662 RSC.
- R. Williams, W. Jencks and F. Westheimer, Williams, 2004. Available online: https://www.chem.wisc.edu/areas/reich/pkatable/pKa_compilation-1-Williams.
- A. Skulcova, A. Russ, M. Jablonsky and J. Sima, BioResources, 2018, 13, 5042–5051 CAS.
- (a) M. A. Kareem, F. S. Mjalli, M. A. Hashim and I. M. AlNashef, J. Chem. Eng. Data, 2010, 55, 4632–4637 CrossRef CAS; (b) C. D'Agostino, R. C. Harris, A. P. Abbott, L. F. Gladden and M. D. Mantle, Phys. Chem. Chem. Phys., 2011, 13, 21383–21391 RSC.
- (a) C. Prandi, E. G. Occhiato, S. Tabasso, P. Bonfante, M. Novero, D. Scarpi, M. E. Bova and I. Miletto, Eur. J. Org. Chem., 2011, 3781–3793 CrossRef CAS; (b) C. Prandi, H. Rosso, B. Lace, E. G. Occhiato, A. Oppedisano, S. Tabasso, G. Alberto and M. Blangetti, Mol. Plant, 2013, 6, 113–127 CrossRef CAS PubMed; (c) C. Prandi, G. Ghigo, E. G. Occhiato, D. Scarpi, S. Begliomini, B. Lace, G. Alberto, E. Artuso and M. Blangetti, Org. Biomol. Chem., 2014, 12, 2960–2968 RSC; (d) E. Mayzlish-Gati, D. Laufer, C. F. Grivas, J. Shaknof, A. Sananes, A. Bier, S. Ben-Harosh, E. Belausov, M. D. Johnson, E. Artuso, O. Levi, O. Genin, C. Prandi, I. Khalaila, M. Pines, R. I. Yarden, Y. Kapulnik and H. Koltai, Cancer Biol. Ther., 2015, 16, 1682–1688 CrossRef CAS PubMed; (e) C. Lombardi, E. Artuso, E. Grandi, M. Lolli, F. Spirakys, E. Priola and C. Prandi, Org. Biomol. Chem., 2017, 15, 8218–8231 RSC; (f) S. Parisotto, B. Lace, E. Artuso, C. Lombardi, A. Deagostino, R. Scudu, C. Garino, C. Medana and C. Prandi, Org. Biomol. Chem., 2017, 15, 884–893 RSC.
- X. Zhou, Y. Zhao, Y. Cao and L. He, Adv. Synth. Catal., 2017, 359, 3325–3331 CrossRef CAS.
- (a) I. Delso, C. Lafuente, J. Muñoz-Embid and M. Artal, J. Mol. Liq., 2019, 111236 CrossRef; (b) C. Florindo, F. S. Oliveira, L. P. N. Rebelo, A. M. Fernandes and I. M. Marrucho, ACS Sustainable Chem. Eng., 2014, 2, 2416–2425 CrossRef CAS; (c) O. S. Hammond, D. T. Bowron and K. J. Edler, Angew. Chem., Int. Ed., 2017, 56, 9782–9785 CrossRef CAS PubMed; (d) N. López-Salas, J. M. Vicent-Luna, S. Imberti, E. Posada, M. J. Roldán, J. A. Anta, S. R. G. Balestra, R. M. Madero Castro, S. Calero, R. J. Jiménez-Riobóo, M. C. Gutiérrez, M. L. Ferrer and F. del Monte, ACS Sustainable Chem. Eng., 2019, 21, 17565–17573 CrossRef.
- N. Rodriguez Rodriguez, A. van den Bruinhorst, L. J. B. M. Kollau, M. C. Kroon and K. Binnemans, ACS Sustainable Chem. Eng., 2019, 7, 11521–11528 CrossRef CAS.
- F. Gabriele, M. Chiarini, R. Germani, M. Tiecco and N. Spreti, J. Mol. Liq., 2019, 291, 111301 CrossRef.
- R. A. Sheldon, Pure Appl. Chem., 2000, 72, 1233–1246 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc03465j |
| This journal is © The Royal Society of Chemistry 2020 |