Open Access Article
Open Access ArticleNanocrystalline microstructure in Sm3+ and Gd3+ doped K2O–MgO–Al2O3–SiO2–F glass-ceramic sealant (SOFC)
Mrinmoy
Garai
 *ab,
C. Hari Venkateswara
Rao
ac and
Basudeb
Karmakar
b
*ab,
C. Hari Venkateswara
Rao
ac and
Basudeb
Karmakar
b
aOffice of the Additional Director General, Aeronautical Quality Assurance (AQA), Koraput, India. E-mail: mrinmoygarai@yahoo.in
bGlass Science and Technology Section, CSIR-Central Glass & Ceramic Research Institute (CGCRI), Kolkata, India
cDepartment of Mechanical Engineering, Osmania University, Hyderabad, India
First published on 11th May 2020
Abstract
In order to demonstrate the effects of Sm3+ and Gd3+ ions on the crystalline microstructures of the magnesium-boro-alumino-silicate (MBAS) system, the K2O–MgO–B2O3–Al2O3–SiO2–F glass doped with 0–5 mol% Sm2O3 and Gd2O3 were synthesized by melt-quenching (1550 °C). The addition of Sm3+ and Gd3+ content was found to increase the density (2.74–2.91 g cm−3) of the base glass. By controlled heat-treatment at 950 °C, the MBAS glasses were converted into opaque glass-ceramics with crystalline phases (XRD), containing fluorophlogopite mica [KMg3(AlSi3O10)F2], norbergite [Mg2SiO4·MgF2] and enstatite [MgSiO3]. The FESEM study revealed the development of rock-like and plate-like crystallite particles (average size 2–4 μm) randomly dispersed in the heat-treated MBAS microstructure, which on the addition of Sm3+ and Gd3+ ions is restructured into nanocrystalline (size = ∼50–400 nm) morphology. The substantial change in the microstructure influenced the corresponding density and thermal expansion properties. The coefficient of thermal expansion for MBAS was estimated to be 10.47(±0.10) × 10−6 K−1 (50–800 °C), which increased to 11.11–11.29 × 10−6 K−1 when doped with Sm3+ and Gd3+. Such large thermal expansion makes the Sm2O3- and Gd2O3-doped K2O–MgO–B2O3–Al2O3–SiO2–F glasses suitable for high temperature sealing applications (like SOFC).
Introduction
Fluorophlogopite mica-based glass-ceramics are technologically important materials because of their well-grained microstructure, resulting in a wide range of thermal expansion value suitable for high temperature sealing applications.1–3 K2O–MgO–B2O3–Al2O3–SiO2–F is a type of magnesium-boro-alumino-silicate (MBAS) system that can be easily crystallized into mica glass-ceramics containing fluorophlogopite [KMg3(AlSi3O10)F2] phase.3,4 For this, the most studied technique is the controlled in situ crystallization of glass over a temperature 800 °C.4 In addition to the flexibility to develop an improved fine-grained microstructure with desired thermal properties, these glass-ceramics exhibit consistent reproducibility of properties due to the homogeneity of the as-cast melt glass.4–6 They typically contain a finite quantity of crystalline ceramic phase produced by the controlled nucleation of highly viscous glass forming melts.5Mica glass-ceramics with dense nanocrystalline-grained microstructure and improved thermal properties are interesting materials for high temperature sealing applications such as solid oxide fuel cells (SOFCs).7 This is typically due to their compatible thermal expansion with other components used (viz. metal electrode, solid electrolyte, interconnect material, etc.) in SOFC cell.6,7 Moreover, they possess layered crystalline structure that can avert the generation and growth of micro-crack during thermal recycling operation performed at high temperature.6–8 However, the large thermal shock resistivity for those mica glass-ceramics arises due to the wide thermal expansion value.9–11 Moreover, the wide thermal expansion in the K2O–MgO–B2O3–Al2O3–SiO2–F glasses is obtained due to the layered structure of the mica crystals, which permit structural relaxation along the planes of the flat structural network.5,10 In general, the thermal properties of the mica glass-ceramic body are affected by the particle size, amount of precipitated mica crystals and their strength.12 In the production of glass-ceramics, two major factors, namely (i) nucleating agents, and (ii) temperature and time of the heat-treatment influence the size and number of the crystals.4,5 In magnesium-boro-alumino-silicate based glass-ceramic, the tuning of crystallization has been studied with the doping of nucleating agent such as rare-earth (RE) ions having high ionic field strength.9,10 Compared to the mono- and di-valent modifier ions, the trivalent RE ions (viz., Nd3+, Sm3+, and Gd3+) play different structural roles both over short and intermediate-range in the alumino-silicate glass.9–11 During the process of crystallization the RE ions (RE3+) tend to ‘cluster’ that makes a minority of oxygen ions (O2−) involved in the RE–O–RE linkages, and isolated from the aluminosilicate glass.10,13 Nicoleau et al.13 studied the impact of rare-earth silicate crystallization on the borosilicate glass structural configuration and argued that the crystallization leads to the reorganization of the cation distribution around the rare-earth elements. Due to low solubility, the lanthanides lead to crystalline phases in the Si–O–B based glass during cooling.13,14 This means that the RE ions influence the crystallization behavior, which is essential for preparing glass-ceramics with desired thermal properties required for high-temperature sealing applications (SOFC).10–13 Salinigopal et al.14 studied the effects of Nd2O3 and Gd2O3 on the BaO–Al2O3–B2O3–SiO2 glass and observed that the coefficient of thermal expansion for glass-ceramics lie within the range 12.32–12.81 × 10−6 °C−1, which is suitable for the solid oxide fuel cell (SOFC) applications.
In this study, based on the previous experiences,9–11 40SiO2–12MgO–16Al2O3–10B2O3–10K2O–12MgF2 (mol%) glass doped with Sm2O3/Gd2O3 (5 mol%) was considered as the starting composition in order to achieve a fluorophlogopite (phase) dominated glass-ceramics possessing a high thermal expansion value (>11 × 10−6 K−1 at 50–800 °C) suitable for high temperature sealant applications. In this regard, the role of Sm2O3 or Gd2O3 to improve the glass-ceramic morphology and thermal expansion was also investigated.
Experimental
Three different glasses of (i) base composition (mol%) 40SiO2–12MgO–16Al2O3–10B2O3–10K2O–12MgF2 (G-1) and doped (5 mol%) with (ii) Sm2O3 (G-2) and (iii) Gd2O3 (G-3) were synthesized via a conventional melt-quench technique using highly pure reagent grade fine chemicals. Most of the chemicals used were in the form of oxides, hydroxides and carbonates as precursor materials: SiO2 (Quartz Powder, LobaChemie, Mumbai, India), Mg(OH)2 (97%, LobaChemie, Mumbai, India), Al(OH)3 (97%, LobaChemie, Mumbai, India), H3BO3 (99.5%, LobaChemie, Mumbai, India), K2CO3 (98%, LobaChemie, Mumbai, India), MgF2 (99.9%, LobaChemie, Mumbai, India), Sm2O3 (99.99%, Indian Rare Earths Ltd, Udyogamandal, India) and Gd2O3 (99.99%, Indian Rare Earths Ltd, Udyogamandal, India). Homogeneously mixed batches were allowed to melt at ∼1550 °C (2 h) using an electric furnace (Kanthal), followed by stirring for 0.5 min with a silica glass rod in an open platinum (Pt) crucible. Molten glasses were then allowed to cast into a pre-heated carbon plate in open-air atmosphere. The as-synthesized glasses were then heat-treated at ∼950 °C (2 h) for controlled crystallization.The density (d) of the investigated glass and glass-ceramic bulk samples was determined (with an accuracy of ±0.7%) by the Archimedes principle using distilled water as the immersion liquid (density = 1 g cc−1) in a digital balance (MettlerToledo), which enables weighing the bulk solid in air (Wair) as well as in the solvent (Wwater):
| d = [Wair/(Wair − Wwater)]dwater |
The microstructural morphology of the studied glass-ceramics (heat-treated at 950 °C/2 h) were examined via field emission scanning electron microscopy (FESEM model S430i, LEO, CEA, USA) using polished glass-ceramic samples (chemically etched by immersion in 5 vol% aqueous HF solution for 5 min).
In order to investigate the thermal properties, the coefficient of thermal expansion (CTE) of the studied glass-ceramics were evaluated using a cylinder-shaped sample with a length of ∼25 mm and diameter of ∼6 mm using a horizontal dilatometer, NETZSCH DIL 402 PC (NETZSCH-Gerätebau GmbH, Germany) at a heating rate of 5 °C min−1 under ±1% accuracy after calibration with a standard Al2O3 cylinder.
Results and discussion
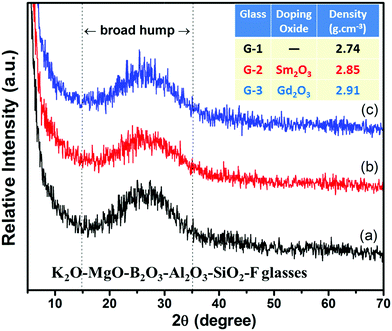
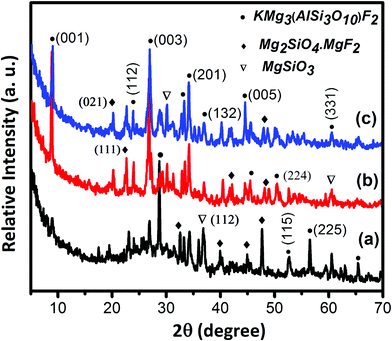
The melt-quenched monoliths of 40SiO2–12MgO–16Al2O3–10B2O3–10K2O–12MgF2–Sm2O3/Gd2O3 (0–5 mol%) composition were opaque in nature. Base glass G-1 contained no rare-earth composition; it possessed density value 2.74 ± 0.02 g cm−3. On adding Sm2O3 and Gd2O3 in the G-1 glass, the density increased to 2.85 ± 0.02 and 2.91 ± 0.02 g cm−3, respectively.10 The as-synthesized glasses were heat-treated at 950 °C for 2 h over, which they were converted into the glass-ceramics. The crystalline nature of the glasses and corresponding glass-ceramics were accounted from the XRD pattern taken in 5–70° (2θ). It is evident from Fig. 1 that the broad hump appearing at (2θ) 15–35° for all the glasses signifies their amorphous nature.10,12 On heating over 950 °C, those glasses converted into glass-ceramic signifying their crystalline pattern (XRD), as exhibited in Fig. 2. The crystalline peaks shown in Fig. 2 at the (2θ) positions of 8.92, 23.82, 26.92, 33.43, 34.18, 37.03, 44.54, 46.05, 50.42, 52.79, 56.48, 60.57 and 65.26°, corresponding to the (001), (112), (003), (200), (201), (132), (005), (222), (224), (115), (225), (331) and (007) planes are developed due to the formation of the phase fluorophlogopite mica, KMg3AlSi3O10F2 (molecular weight = 421, monoclinic end-centered system, JCPDS-PDF number 10-0494 & 71-1542; lattice parameter a = 5.299, b = 9.188, c = 10.13).3,5 All the peaks with corresponding planes are summarized in Table 1.| Peak position (2θ) (degree) | Corresponding planes | Crystalline phase | JCPDS file |
|---|---|---|---|
| 8.92, 23.82, 26.92, 33.43, 34.18, 37.03, 44.54, 46.05, 50.42, 52.79, 56.48, 60.57, 65.26 | (001), (112), (003), (200), (201), (132), (005), (222), (224), (115), (225), (331), (007) | Fluorophlogopite mica [KMg3AlSi3O10F2] | 10-0494 and 71-1542 |
| 19.93, 22.67, 32.42, 39.90, 41.75, 45.40, 48.94 | (021), (111), (131), (210), (004), (142), (151) | Norbergite (Mg2SiO4·MgF2) | 71-2401 |
| 30.20, 36.78, 60.45 | (321), (112), (650) | Enstatite (MgSiO3) | 83-2057 |
The characteristic peaks appearing at (2θ) 19.93, 22.67, 32.42, 39.90, 41.75, 45.40 and 48.94° diffracted from the (021), (111), (131), (210), (004), (142) and (151) crystalline planes, correspond to the formation of magnesium fluoride silicate, ‘Norbergite’ (Mg2SiO4·MgF2), molecular weight = 203, JCPDS file number 71-2401 (orthorhombic, primitive system; lattice parameter a = 4.710, b = 10.27, c = 8.747). Norbergite is formed in all the glass-ceramics; however, the development of fluorophlogopite mica is supported by rare-earth ions.10,13,14 A different crystalline phase enriched by the Mg and Si contents, magnesium silicate, ‘enstatite’ (MgSiO3), molecular weight = 100.39, was also found to appear with peaks (2θ) 30.20, 36.78 and 60.45° due to the crystalline planes (321), (112) and (650); JCPDS file number 83-2057 (orthorhombic, primitive system; lattice parameter a = 18.21, b = 8.813, c = 5.179).15 During the heat-treatment, Mg2+ and F− ions tend to form MgF2 precipitates at ∼600–650 °C.16 The tiny particles of MgF2 then mix with magnesium silicates to form the additive compound i.e., norbergite crystallites.10,16 In the base glass-ceramic, the XRD pattern manifests the appearance of norbergite and enstatite phases predominantly. The trivalent ions Sm3+ or Gd3+ strongly support the heterogeneous phase separation in the SiO2–MgO–Al2O3–B2O3–K2O–MgF2 composition.10,14 Due to the large ionic field strength (charge/radius) and small critical nuclei, they tend to form ‘clusters’ during initial heating, and hence are isolated from the silicate glass network (Si–O–Si).10,11 Thus, the maximum XRD peaks corresponding to the fluorophlogopite phase are observed in the case of G-2 (i.e., Sm2O3 content) and G-3 (i.e., Gd2O3 content) glass-ceramics.13,14 Under the same heat-treatment, the slight shift of the diffraction peaks in G-2 and G-3 (Fig. 2) is associated with the crystallite size and high concentration of the fluorophlogopite phase.15,17
Mica belongs to the monoclinic system with the co-existence of several types of atoms, where the volume fraction, crystallinity and grain size of the crystalline phase is controlled by the heat-treatment as well as the doping agents.5,17 In the present report, the controlled nucleation in the SiO2–MgO–Al2O3–B2O3–K2O–MgF2 glass is performed under two selected parameters: (i) heat-treatment at 950 °C for 2 h and (ii) the addition of 5 mol% Sm2O3 and Gd2O3. Thus, it is of interest to compare the microstructures of the crystallized glasses of same composition with different RE ions. Volume crystallization with a rather fine morphology was observed for all the glass-ceramics studied under same heat-treatment.18–20 The examples of the FESEM micrographs (taken using etched G-1 to G-3 specimens) are given in Fig. 3. The XRD analysis of these samples indicated that the base glass-ceramic microstructure predominantly contained norbergite and enstatite constituents, and the rare-earth doped samples (Sm2O3 and Gd2O3) largely comprised fluorophlogopite mica particles.10 As seen from Fig. 3a, the G-1 glass-ceramic microstructure is composed of rock-like and plate-like crystallite particles (average size 2 to 4 μm), which are randomly dispersed throughout the matrix. In a closer look it seems that the plenty of rock-shaped (100–500 nm sized) particles are in-homogeneously positioned to fill the gaps amongst the plate-shape crystallites of dimension 1–4 μm. The bulk density of the G-1 glass-ceramic was calculated as 2.77 ± 0.02 g cm−3. In the presence of Sm2O3 (i.e., Sm3+), the glass-ceramic structure is largely changed into the nanocrystalline morphology,10–14 where 50–200 nm sized spherical droplets such as crystallite particles are homogeneously distributed to form a fine-grained microstructure (Fig. 3b). Such a compact morphology is ascribed for its high density value (3.02 ± 0.02 g cm−3).6,20 During the heat-treatment at 950 °C, the nucleating agents such as Sm3+ or Gd3+ ions can either support the heterogeneous phase separation or these can cause the accumulation in a specific microphase or nanophase of the phase separated glass.3,10 Those trivalent ions Sm3+ or Gd3+ having large ionic field strength (charge/radius) and smaller critical nuclei tend to form ‘cluster’, which makes a minority of oxide ions involved in the Sm–O–Sm or Gd–O–Gd linkages, and hence isolated from the silicate glass network (Si–O–Si).10,11,13 When Gd2O3 (i.e., Gd3+) is present in the base composition G-1, the spherical granules are agglomerated in the surrounds of Gd3+ to form ‘cluster’. The clusters then shaped into bigger spherical crystals mostly of size, 200–400 nm (Fig. 3c).10,14 From samarium (Sm) to gadolinium (Gd) the ionic radii decreases and hence the ionic field strength increases by virtue of “lanthanide contraction”. Therefore, it can be guessed that Gd3+ have occupied the interstitial places in the glass network governed by Si–O–Si/B–O–B/Si–O–B and their increase in field strength as well as atomic weight have made the glass-ceramic network more compact, resulting in an increase in the molecular weight without increasing the volume.10 Thus, the maximum density is estimated for G-3 as 3.06 ± 0.02 g cm−3.6,20
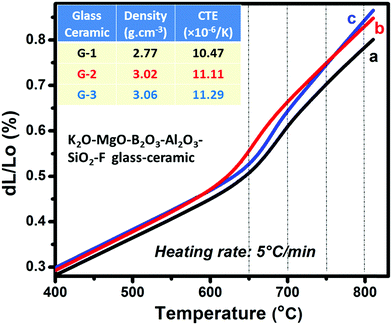
The dilatometry of the heat-treated samples (i.e., glass-ceramics) was carried out in order to investigate the thermal expansion-sealant (SOFC) behavior.21,22 A plot of the CTE of the glass-ceramics between 400 °C and 800 °C is presented in Fig. 4. Linear increase in CTE up to 800 °C was obtained for all the studied glass-ceramics; however, the linear trend is quite different for the Sm2O3- and Gd2O3-doped samples. For the G-1 glass-ceramic, the CTE at 50–500 °C was 8.00(±0.08) × 10−6 K−1 and increased to 8.20, 9.37 and 10.54 × 10−6 K−1 at 50–600, 50–700 and 50–800 °C, respectively. For G-2, where 5 mol% Sm2O3 was added, the CTE was evaluated as 8.44(±0.08) and 8.56(±0.09) × 10−6 K−1 at 50–500 °C and 50–600 °C, respectively. The obtained CTE values are summarized in Table 2. For fluorophlogopite mica-based glass-ceramics, the CTE strongly depends on the size, shape of crystallite particles as well as crystalline fraction in the microstructure.23,24 The dopant ions Sm3+ or Gd3+ have tendency to participate in the overall bonding of the MBAS glass to tailor the thermal expansion since their structural roles in the Si–O–Si matrix are related to their size and coordination number.9–11 Nanocrystalline morphology advocates structural relaxation in the G-2 glass-ceramic in comparison to the G-1 system.10,13,23 At 50–700 and 50–800 °C, large CTE values were thus estimated for the G-2 sample with the values being 10.22(±0.10) and 11.11(±0.11) × 10−6 K−1, respectively. Gd3+ having a smaller size gets surrounded by the [AlO4]− tetrahedra during heat-treatment. In the present composition, 5 mol% Gd2O3 was present in the 40SiO2–12MgO–16Al2O3–10B2O3–10K2O–12MgF2 system and the microstructure of that became less homogeneous.10,11 As is evident from Fig. 3c, the dimension of the spherical crystallite particles increased, whereas the compactness of the morphology decreased. Because of the combining effects, the CTE value of G-3 is comparatively larger at 50–800 °C (Table 2).9,10
| Sample identity | Coefficient of thermal expansion (× 10−6 K−1) | |||
|---|---|---|---|---|
| 50–500 °C | 50–600 °C | 50–700 °C | 50–800 °C | |
| G-1 | 8.00(±0.08) | 8.20(±0.08) | 9.37(±0.09) | 10.47(±0.10) |
| G-2 | 8.44(±0.08) | 8.56(±0.09) | 10.22(±0.10) | 11.11(±0.11) |
| G-3 | 8.56(±0.09) | 8.53(±0.09) | 9.89(±0.10) | 11.29(±0.11) |
For intermediate temperature (700–800 °C) solid oxide fuel cell (SOFC) designs, the glass sealant must have thermal expansion characteristics that do not contribute to the creation of thermal stress between a variety of ceramic and metallic materials used in the SOFC stack; must be thermochemically compatible with the other materials; must be stable at operational temperatures (700–800 °C) of SOFCs. These all requirements are however controlled by the CTE value.25–29 Thus, a linear thermal expansion up to 800 °C as well as large CTE value at that temperature are prime requisites for SOFC sealant.28 The large CTE (>11 × 10−6 K−1) at 50–800 °C as observed for the samples G-2 and G-3 (Table 2), is well-matched with the SOFC components like electrode (Ni/Fe), solid electrolyte (YSZ), interconnect (Crofer-22APU) etc. in the operating temperature ∼700–800 °C.23,24 Hence, Sm2O3 and Gd2O3 doped SiO2–MgO–Al2O3–B2O3–K2O–MgF2 glasses can act as a potential SOFC sealant material.13,30
Conclusions
This report highlights the effect of the addition of samarium (Sm3+) and gadolinium (Gd3+) on the nucleation behavior, alteration of microstructure, physical and thermal properties of low alkali containing magnesium-boro-alumino-silicate (MBAS) glass. 0–5 mol% Sm2O3 and Gd2O3 doped K2O–MgO–B2O3–Al2O3–SiO2–F glasses were synthesized by melt-quenching at 1550 °C. The major conclusions are summarized below:• Base glass G-1 (no rare-earth composition) possessed density value of 2.74 ± 0.02 g cm−3, which after the addition of Sm3+ and Gd3+ increased to 2.85–2.91 g cm−3.
• The MBAS glasses, by controlled heat-treatment at 950 °C (2 h), were converted into multi-crystalline grass-ceramics with predominantly crystalline phase (XRD) containing fluorophlogopite mica [KMg3(AlSi3O10)F2], norbergite [Mg2SiO4·MgF2] and enstatite [MgSiO3].
• Field emission scanning electron microscopy revealed the development of rock-like and plate-like crystallites (average size ∼2–4 μm) randomly dispersed in the base glass-ceramic matrix. In the presence of Sm3+ and Gd3+, the microstructure restructured into nanocrystalline morphology packed by droplet-like crystallite particles (size ∼50–400 nm).
• The significant variation in microstructure is ascribed to the corresponding density and thermal expansion value. The coefficient of thermal expansion (CTE) for the base glass-ceramic was estimated to be 10.47(±0.10) × 10−6 K−1 at 50–800 °C that increased to 11.11–11.29 × 10−6 K−1 at 50–800 °C for glass-ceramics containing Sm3+ and Gd3+. Such large thermal expansion makes the Sm2O3- and Gd2O3-doped K2O–MgO–B2O3–Al2O3–SiO2–F glasses applicable for high temperature sealing application (like SOFC).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
MG thankfully acknowledges CSIR for the financial support under the CSIR-NMITLI project TLP 0005. The authors are very much thankful to Dr K. Muraleedharan, Director and Dr Ranjan Sen, Head, Glass Division and Dr R. N. Basu, Head, Fuel Cell and Battery Division of CSIR-CGCRI, Kolkata for their encouragement and support to carry out this work. They thankfully acknowledge the technical supports provided by the XRD and SEM Sections of CSIR-CGCRI.References
- W. Holand, V. Rheinberger and M. Schweiger, Adv. Eng. Mater., 2001, 3, 768–774 CrossRef CAS.
- G. H. Beall, Int. J. Appl. Glass Sci., 2014, 5, 93–103 CrossRef.
- M. Garai, N. Sasmal, A. R. Molla, S. P. Singh, A. Tarafder and B. Karmakar, J. Mater. Sci., 2014, 49, 1612–1623 CrossRef CAS.
- J. Deubener and W. Höland, Front. Mater., 2017, 4, 14 CrossRef.
- W. Wolfgang and C. Rüssel, CrystEngComm, 2015, 17(45), 8671–8675 RSC.
- M. Garai and B. Karmakar, Front. Mater., 2020, 7, 57 CrossRef.
- Y. S. Chou, J. W. Stevenson and P. Singh, J. Power Sources, 2005, 152, 168–174 CrossRef CAS.
- H. Liu, X. Du, Z. Yu, D. Tang and T. Zhang, RSC Adv., 2016, 6, 17151 RSC.
- N. Sasmal, M. Garai, A. R. Molla, A. Tarafder, S. P. Singh and B. Karmakar, J. Non-Cryst. Solids, 2014, 387, 62–70 CrossRef CAS.
- M. Garai and B. Karmakar, J. Alloys Compd., 2016, 678, 360–369 CrossRef CAS.
- N. Sasmal, M. Garai and B. Karmakar, J. Asian Ceram. Soc., 2016, 4, 29–38 CrossRef.
- A. Chen, W. Tan, H. He, G. Li, X. Wu, Q. Tao and J. Zhu, Phys. Chem. Miner., 2019, 46(3), 259–270 CrossRef CAS.
- E. Nicoleau, F. Angeli, S. Schuller, T. Charpentier, P. Jollivet and M. Moskura, J. Non-Cryst. Solids, 2016, 438, 37–48 CrossRef CAS.
- M. S. Salinigopal, N. Gopakumar, P. S. Anjana and O. P. Pandey, J. Non-Cryst. Solids, 2020, 535, 119956 CrossRef CAS.
- T. Benitez, A. Veber, K. P. Furlan, L. B. Rebouças, D. de Ligny, D. Hotza, A. P. N. de Oliveira and N. Travitzky, Int. J. Appl. Glass Sci., 2020, 11(1), 155–169 CrossRef CAS.
- M. Garai, N. Sasmal, A. R. Molla, A. Tarafder and B. Karmakar, J. Mater. Sci. Technol., 2015, 31, 110–119 CrossRef CAS.
- T. Behraznia, A. S. Alzahrani, M. Rashwan, A. J. Bushby and R. G. Hill, J. Eur. Ceram. Soc., 2020, 40(3), 887–892 CrossRef CAS.
- H. Yu, W. Li, W. G. Zhu and H. B. Wu, Glass Phys. Chem., 2019, 45(6), 555–564 CrossRef.
- M. Eilaghi, M. Montazerian and B. E. Yekta, Trans. Indian Ceram. Soc., 2016, 75(1), 1–6 CrossRef CAS.
- M. Garai, A. K. Maurya and S. Roy, MRS Adv., 2018, 3, 3525–3533 CrossRef CAS.
- V. Kumar, M. Kaur, G. Kaur, S. K. Arya and G. Pickrell, Stacking designs and sealing principles for IT-solid oxide fuel cell, Intermediate Temperature Solid Oxide Fuel Cells, Elsevier, 2020, pp. 379–410 Search PubMed.
- S. Ghosh, P. Kundu, A. Das Sharma, R. N. Basu and H. S. Maiti, J. Eur. Ceram. Soc., 2008, 28, 69–76 CrossRef CAS.
- M. Garai, N. Sasmal and B. Karmakar, Indian J. Mater. Sci., 2015, 2015, 638341, DOI:10.1155/2015/638341.
- A. A. Reddy, A. Goel, D. U. Tulyaganov, M. Sardo, L. Mafra, M. J. Pascual, V. V. Kharton, E. V. Tsipis, V. A. Kolotygin and J. M. F. Ferreira, J. Mater. Chem. A, 2014, 2(6), 1834–1846 RSC.
- M. Garai, T. S. R. Ch Murthy and B. Karmakar, Ceram. Int., 2018, 44, 22308–22317 CrossRef CAS.
- P. Nandi, A. S. Patil, B. Paul, A. Sarkar, M. Goswami and G. P. Kothiyal, Trans. Indian Ceram. Soc., 2012, 71(4), 235–238 CrossRef CAS.
- M. Garai, N. Sasmal, A. R. Molla and B. Karmakar, Solid State Sci., 2015, 44, 10–21 CrossRef CAS.
- D. A. Krainova, S. T. Zharkinova, N. S. Saetova, A. A. Raskovalov, A. V. Kuz’min, V. A. Eremin, E. A. Sherstobitova, S. V. Pershina, M. V. Dyadenko, X. Zhang and S. Jiang, Russ. J. Appl. Chem., 2017, 90(8), 1278–1284 CrossRef CAS.
- X. V. Nguyen, C. T. Chang, G. B. Jung, S. H. Chan, W. T. Lee, S. W. Chang and I. C. Kao, Int. J. Hydrogen Energy, 2016, 41(46), 21812–21819 CrossRef CAS.
- S. Kurama and G. Saydam, J. Aust. Ceram. Soc., 2017, 53(2), 293–298 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |