A highly asymmetric interfacial superstructure in WC: expanding the classic grain boundary segregation and new complexion theories†
Zhishan
Luo‡
a,
Chongze
Hu‡
bc,
Lin
Xie
d,
Hongbo
Nie
e,
Congying
Xiang
a,
Xinfu
Gu
f,
Jiaqing
He
d,
Wenqing
Zhang
*d,
Zhiyang
Yu
*ad and
Jian
Luo
 *bc
*bc
aState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou, Fujian 350002, P. R. China. E-mail: yuzyemlab@fzu.edu.cn
bProgram of Materials Science and Engineering, University of California San Diego, La Jolla, California 92093, USA. E-mail: jluo@alum.mit.edu
cDepartment of NanoEngineering, University of California San Diego, La Jolla, California 92093, USA
dDepartment of Physics, Southern University of Science and Technology, Shenzhen, Guangdong 518055, P. R. China. E-mail: zhangwq@sustc.edu.cn
eXiamen Tungsten Co., LTD, Xiamen, Fujian 361126, P. R. China
fSchool of Materials Science and Engineering, University of Science and technology Beijing, Beijing 100083, P. R. China
First published on 12th August 2019
Abstract
Solute segregation (adsorption) at grain boundaries (GBs), which is ubiquitous in polycrystalline materials and can remarkably alter various properties, is one of the classic materials science problems. Despite decades of research, the understanding of the atomic level GB segregation structures is still limited, mostly to symmetric GBs. Here, we combine aberration-corrected electron microscopy and first-principles calculations to reveal a highly asymmetric interfacial superstructure in WC. Several striking features are observed concurrently at this GB. First, the segregations of Ti and Co are highly asymmetric. Second, the maxima of the Ti and Co adsorption profiles are both off the center in the opposite sides, separated by an intermediate W-rich atomic layer with much less adsorbates. Third, accompanying asymmetric interfacial structural transitions occur to form a cubic-TiC-like interfacial layer on the one side and a partially disordered Co-rich segregation layer on the other side. Such a highly asymmetric interfacial superstructure knowingly differs from all prior experimental observations and is beyond the predictions of any existing models. Thus, this observation extends both the classic GB segregation models and the new complexion theory. First-principles calculations further verified all observed phenomena and provided new insights based on the analysis of differential charge transfer and bond ordering. A new descriptor, sum of bond ordering, is introduced to predict the segregation trend in complicated interfacial structures.
New conceptsIn this communication, we reveal a highly asymmetric interfacial superstructure at a mixed twist and tilt grain boundary (GB) of WC that significantly broadens our understanding of GB segregation structures. Specifically, this interfacial superstructure exhibits the following features that differ strikingly from all prior experimental observations and are beyond the predictions of any existing theoretical models: (i) the segregation profiles of Ti and Co are highly asymmetric; (ii) the maxima of the Ti and Co segregation both occur at the off-the-center atomic planes in the opposite sides, separated by a W-rich atomic layer in between; and (iii) solute segregations further induce asymmetric interfacial structural transitions, i.e., Ti segregation drives an interfacial symmetry change from the hexagonal WC grain to a cubic-TiC-like interfacial layer on the one side, while Co induces a partially-disordered segregation layer on the other side. Such a highly asymmetric interfacial superstructure is not anticipated from the classic Langmuir–Mclean type GB segregation model, as well as the newer diffuse-interface and multilayer lattice-adsorption models; moreover, it is beyond (differs significantly from) the Dillon-Harmer complexions and recent observations of several other interfacial superstructures. Yet, we expect that similar highly asymmetric interfacial superstructures may not be uncommon (despite being unrecognized in prior studies), and we propose factors favoring their formation. Thus, this study extends our fundamental knowledge of atomic level GB segregation structures, which can have broad technological implications. |
Introduction
The enrichment and redistribution of solute atoms at the boundaries between two crystalline grains, known as “grain boundary (GB) segregation (a.k.a. adsorption),”1 could drastically alter the microstructural evolution and various material properties of virtually all engineered polycrystalline materials.2,3 Hence, understanding GB segregation is a key avenue to demystifying the complicated structure–processing–property relationship, thereby being a key interest of materials scientists. Classic thermodynamic adsorption models, including the well-known Langmuir–Mclean model and its various extensions,4,5 were adopted to describe the equilibrium segregation behaviors. Those models typically treated GBs as thermodynamic entities without much structural detail. More recently, diffuse-interface6,7 and multilayer adsorption8–10 models were developed to describe spatially-varying GB segregation, but they are limited in predicting GB structural transitions (other than GB disordering in the diffuse-interface models6,7), e.g., interfacial symmetry change and/or reconstruction. Furthermore, atomistic simulations using density functional theory (DFT) or hybrid Monte Carlo and molecular dynamics (hybrid MC/MD) methods enable more realistic modeling of GB segregation. However, most prior atomistic simulations are limited to the symmetric tilt or twist GBs (albeit a recent report suggested that segregation can induce symmetry breaking at an otherwise symmetric GB11). Yet, asymmetric GBs of mixed twist and tilt character, which are ubiquitous in real polycrystalline materials and can often be the weaker link chemically and mechanically, have not been studied thoroughly.With the rapid development of advanced electron microscopy, interesting solute segregation patterns have been revealed at the atomic scale. Apart from the well-known monolayer and submonolayer segregation at symmetric tilt GBs,12 nanoscale, equilibrium-thickness, intergranular films (IGFs) with compositional and structural gradients were widely observed at GBs in Si3N4 and various other ceramic materials,13–15 as well as some metallic alloys.16,17 Notably, Dillon et al. further discovered a series of six discrete “complexions” (a.k.a. interfacial phases that are thermodynamically 2-D), including: intrinsic (“clean”) GB, (Langmuir–McLean type) monolayer, bilayer, trilayer, nanolayer (equilibrium-thickness IGF), and wetting layer.18,19 They can be understood as segregated adsorbates with nominal thickness of 0, 1, 2, 3, x (nanoscale), and +∞ (arbitrary) of atomic layers, respectively.18,19 More recently, several so-called interfacial “superstructures” have also been revealed, showing further complexity of the possible GB segregation structures. These GB superstructures include an ordered defect superstructure at the Σ5 symmetric tilt GB of MgO,20 the symmetric Y/Zr/Y adsorption structure at the Σ3 tilt GB of yttria-stabilized zirconia (YSZ),21 the “sub-surface” segregation of La and Ta at perovskite GBs,22 and interfacial reconstruction within the adsorbed Bi bilayers at Ni general GBs.23
In this study, we have discovered, characterized, and modeled a highly asymmetric GB superstructure in Ti doped WC–Co cermet; it combines features of asymmetric and off-the-center (subsurface) segregation that induces asymmetric GB structural transitions of interfacial symmetry alteration (reconstruction) and disordering in the opposite sides. This GB superstructure is not only significantly more complex than the Dillon-Harmer complexions and/or other interfacial superstructures reported previously, but also beyond the predictions of all existing interfacial thermodynamic models.
Results and discussion
The two phases in the Ti-doped WC + 10 wt% Co cermet are the primary hexagonal WC and a secondary Co binder phase, where WC GBs are the dominant interfaces in this composite (Fig. S1A and S3, ESI†). Via aberration-corrected scanning transmission electron microscopy (AC STEM) high-angle annular dark-field (HAADF) imaging, we identify a (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and [2
0) and [2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]//[2
0]//[2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3] WC GB near a WC–Co interface and align this GB to an edge-on condition (Fig. S1B, ESI†). A crystallography analysis based on Kikuchi patterns shows that this is a “near Σ28” GB (Fig. S1C, ESI†). Here, the Σ value is not exact, but based on a near-coincident cell theory of Bonnet et al.24 for hexagonal crystals (where approximate even Σ values are allowed; see Fig. S13, ESI† for detail). We catalog this GB as an asymmetric “mixed twist and tilt GB” following Rohrer et al.,23,25 which represents the most general group of GBs (vs. the more special symmetric twist, symmetric tilt, and asymmetric tilt GBs).
3] WC GB near a WC–Co interface and align this GB to an edge-on condition (Fig. S1B, ESI†). A crystallography analysis based on Kikuchi patterns shows that this is a “near Σ28” GB (Fig. S1C, ESI†). Here, the Σ value is not exact, but based on a near-coincident cell theory of Bonnet et al.24 for hexagonal crystals (where approximate even Σ values are allowed; see Fig. S13, ESI† for detail). We catalog this GB as an asymmetric “mixed twist and tilt GB” following Rohrer et al.,23,25 which represents the most general group of GBs (vs. the more special symmetric twist, symmetric tilt, and asymmetric tilt GBs).
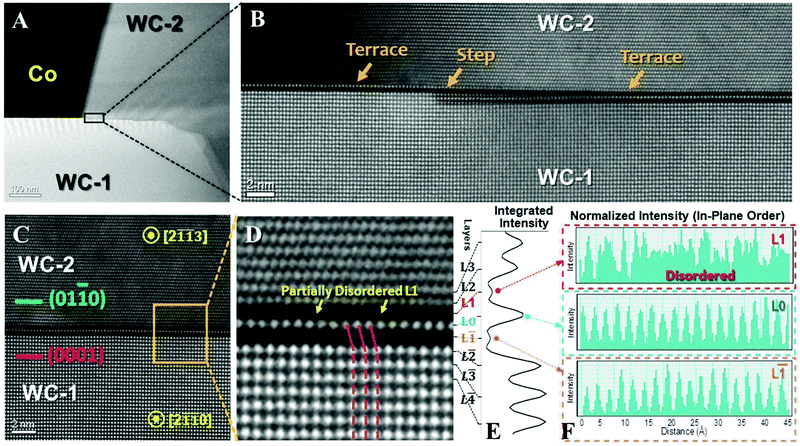
Interestingly, this GB is interrupted by multiple steps or disconnections (Fig. 1A). Similar dark/bright/dark fringes persist at multiple, disconnected, straight terraces (that can be >30 nm long, see Fig. S2, ESI†). Thus, the GB structure (Fig. 1) likely represents the stable configuration because it re-appears in several independent segments (Fig. S2, ESI†). An analysis of EBSD mapping suggests that this specific type of (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) and [2
0) and [2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]//[2
0]//[2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3] GB represents about 2–3% of ∼1000 GBs analyzed (Fig. S3A, ESI†). Its frequency is on a par with the top three most frequently-observed GBs in a WC–Co specimen reported by a prior EBSD study.25
3] GB represents about 2–3% of ∼1000 GBs analyzed (Fig. S3A, ESI†). Its frequency is on a par with the top three most frequently-observed GBs in a WC–Co specimen reported by a prior EBSD study.25
Atomic-resolution HAADF images (Fig. 1C and D) show a unique segregation superstructure with dark/bright/dark fringes that have never been reported before. The enlarged intensity profile in Fig. 1E verifies this dark/bright/dark intensity variation. For convenience, we define the center atomic plane with the brighter fringe as the layer L0. The layers in the WC-2 grain with the (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) terminal orientation are labeled as L1, L2, etc., and the layers in the WC-1 grain with the (0001) terminal orientation are labeled as L
0) terminal orientation are labeled as L1, L2, etc., and the layers in the WC-1 grain with the (0001) terminal orientation are labeled as L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , L
, L![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) , etc. Since W is the heaviest element in the system, the dark/bright/dark fringes from L
, etc. Since W is the heaviest element in the system, the dark/bright/dark fringes from L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) to L1 suggest that the segregation of lighter elements is stronger in the two off-the-center atomic planes (L
to L1 suggest that the segregation of lighter elements is stronger in the two off-the-center atomic planes (L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and L1), which is sometimes called “sub-surface segregation.” This off-the-center or sub-surface segregation is further confirmed by an atomic-resolution compositional analysis (Fig. 2A and B)
and L1), which is sometimes called “sub-surface segregation.” This off-the-center or sub-surface segregation is further confirmed by an atomic-resolution compositional analysis (Fig. 2A and B)
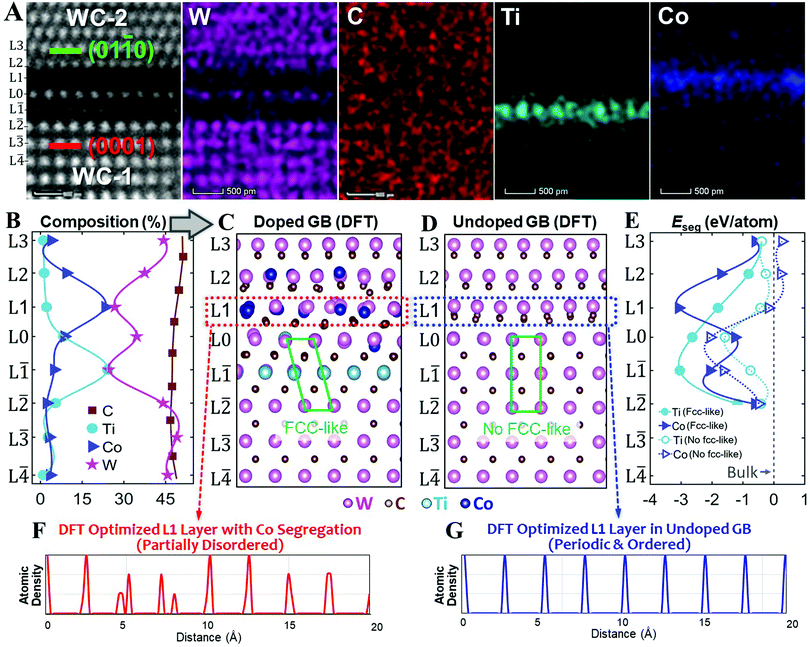 | ||
Fig. 2 (A) Experimental HAADF and corresponding EDS elemental maps of W, C, Ti, and Co. (B) Measured compositional profiles. (C) DFT optimized doped WC GB structure based on the experimentally measured compositional profiles shown in panel (B), where the green parallelogram shows the formation of an FCC-like interfacial layer (from L![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) to L0) at the (0001) side. (D) DFT optimized undoped WC GB structure (for comparison) without the formation of an FCC-like interfacial layer. (E) DFT calculated segregation energies (Eseg) of Ti and Co at different layers in the dilute limit; the dashed lines represent the segregation energies without the formation of the FCC-like interfacial layer. The atomic density profiles of the L1 layer (F) in the doped GB, which became partially disordered, and (G) in the undoped GB, which remained periodic and ordered, after relaxations. Both structures were ordered before the DFT relaxations. This comparison suggests that Co segregation induces disordering in the L1 layer, which supports the experimental observation shown in Fig. 1F. to L0) at the (0001) side. (D) DFT optimized undoped WC GB structure (for comparison) without the formation of an FCC-like interfacial layer. (E) DFT calculated segregation energies (Eseg) of Ti and Co at different layers in the dilute limit; the dashed lines represent the segregation energies without the formation of the FCC-like interfacial layer. The atomic density profiles of the L1 layer (F) in the doped GB, which became partially disordered, and (G) in the undoped GB, which remained periodic and ordered, after relaxations. Both structures were ordered before the DFT relaxations. This comparison suggests that Co segregation induces disordering in the L1 layer, which supports the experimental observation shown in Fig. 1F. | ||
Atomic-resolution STEM-based EDS elemental maps of W, C, Ti, and Co are shown in Fig. 2A. The center L0 layer consists of mostly W, while the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer is enriched in Ti and the L1 layer is enriched in Co, respectively. A quantitative analysis (Fig. 2B) shows that the segregation peak compositions of Co and Ti, respectively, both occur off the center asymmetrically in the opposite directions. Specifically, the maximum segregation occurs at the layer L1 with 23 at% Co and the layer L
layer is enriched in Ti and the L1 layer is enriched in Co, respectively. A quantitative analysis (Fig. 2B) shows that the segregation peak compositions of Co and Ti, respectively, both occur off the center asymmetrically in the opposite directions. Specifically, the maximum segregation occurs at the layer L1 with 23 at% Co and the layer L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with 24 at% Ti, respectively. The center atomic plane L0 is still largely WC with 35 at% W and ∼49 at% C, but only 9 at% Co and 8 at% Ti. See Table S1 in the ESI† for more detail. Since the HAADF signal intensity is approximately proportional to the square of the atomic number Z,26 where ZTi = 22, ZCo = 27, and ZW = 74, this explains the brighter HAADF intensity at the center L0 layer with two darker layers at L1 and L
with 24 at% Ti, respectively. The center atomic plane L0 is still largely WC with 35 at% W and ∼49 at% C, but only 9 at% Co and 8 at% Ti. See Table S1 in the ESI† for more detail. Since the HAADF signal intensity is approximately proportional to the square of the atomic number Z,26 where ZTi = 22, ZCo = 27, and ZW = 74, this explains the brighter HAADF intensity at the center L0 layer with two darker layers at L1 and L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) on both sides (Fig. 1 and Fig. 2A). A prior atom probe tomography (APT) experiment revealed the co-segregation of Co and Ti at WC GBs,27 but the asymmetric distribution of Co and Ti solutes had not been recognized.
on both sides (Fig. 1 and Fig. 2A). A prior atom probe tomography (APT) experiment revealed the co-segregation of Co and Ti at WC GBs,27 but the asymmetric distribution of Co and Ti solutes had not been recognized.
Thus, we can conclude that the L1 layer at the (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) side is enriched in Co and the L
0) side is enriched in Co and the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer at the (0001) side is enriched in Ti, while the center L0 layer in between is W rich. Such a chemically asymmetric and off-the-center segregation profile has never been observed before, nor predicted by any existing model or theory.
layer at the (0001) side is enriched in Ti, while the center L0 layer in between is W rich. Such a chemically asymmetric and off-the-center segregation profile has never been observed before, nor predicted by any existing model or theory.
The asymmetric segregation further suggests that the solute segregation should be dictated by the terminal boundary plane orientation (instead of the commonly believed misorientation). Somewhat similarly, Swiatnicki et al. showed that Ti preferred to segregate on a rhombohedral plane while Si was favorable to stay on a basal plane of the Al2O3 GBs.28 However, Swiatnicki et al.'s observation was on two different GBs in Al2O3, while the current study showed a highly asymmetric and off-the-center segregation at opposite sides of the same GB.
More interestingly, the asymmetric segregation further induces asymmetric interfacial structural transitions at both sides of this GB. On the one hand, the Ti segregation induced a symmetry change on the (0001) side of the WC-1 grain from the hexagonal WC to a FCC-like interfacial layer that spans from the layer L![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) to L0 (Fig. 1D); i.e., this interfacial layer is represented by one unit cell of the rocksalt TiC-like structure (centered at the L
to L0 (Fig. 1D); i.e., this interfacial layer is represented by one unit cell of the rocksalt TiC-like structure (centered at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer) that accounts for two lattice spacings in its apparent thickness. On the other hand, the Co-rich segregation layer on the (01
layer) that accounts for two lattice spacings in its apparent thickness. On the other hand, the Co-rich segregation layer on the (01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) side or WC-2 grain is partially disordered, as indicated by the yellow arrows in Fig. 1D and further analysis in Fig. 1F. The existence of structural disorder in this Co-enriched segregation layer at L1 is further confirmed by our theoretical calculations (e.g., Fig. 2F) that will be discussed below.
0) side or WC-2 grain is partially disordered, as indicated by the yellow arrows in Fig. 1D and further analysis in Fig. 1F. The existence of structural disorder in this Co-enriched segregation layer at L1 is further confirmed by our theoretical calculations (e.g., Fig. 2F) that will be discussed below.
While the possible existence of a structurally-ordered, but chemically-disordered, complexion has been proposed in Cantwell et al.’ overview,18 several new features, including the highly asymmetric and off-the-center segregation, accompanying an interfacial symmetry change and disordering in the opposite sides, have been revealed by the detailed AC-STEM and atomic-resolution EDS analysis for the current case. However, the formation mechanism of this complex GB superstructure is hitherto unclear. Hence, we carry out first-principles DFT calculations to achieve a deeper understanding. The Vienna Ab initio simulation package (VASP)29,30 is used. The input WC GB structure (Fig. S4, ESI†; using a large cell of 592 atoms) is constructed based on HAADF images and compositional analysis. We adopt the nonlocal optB86b-vdW31 functional after testing a gamut of DFT methods (Supplementary discussion section 1 and Table S2, ESI†).
First, based on the DFT optimized undoped GB structures with and without the FCC-like interfacial layer, we have calculated segregation energies of Ti and Co solutes in different layers in the dilute limit (Fig. 2E; see Method section 5 in ESI† for the definition). The GB with an FCC-like interfacial layer exhibits the most negative segregation energy Eseg of −3.08 eV per atom for Co at the L1 layer and the most negative Eseg of −3.04 eV per atom for Ti at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer, as shown by the solid lines in Fig. 2E. These results agree well with our HAADF and EDS observations (Fig. 2A). In comparison, without the formation of the FCC-like interfacial layer, the strongest segregation (most negative Eseg) occurs at the center L0 layer for both Co and Ti, as shown by the dashed lines in Fig. 2E. Thus, the formation of the FCC-like interfacial layer is essential to induce the asymmetric and off-the-center segregation in this GB.
layer, as shown by the solid lines in Fig. 2E. These results agree well with our HAADF and EDS observations (Fig. 2A). In comparison, without the formation of the FCC-like interfacial layer, the strongest segregation (most negative Eseg) occurs at the center L0 layer for both Co and Ti, as shown by the dashed lines in Fig. 2E. Thus, the formation of the FCC-like interfacial layer is essential to induce the asymmetric and off-the-center segregation in this GB.
Second, we performed detailed structural analysis for two DFT optimized (relaxed) interfacial structures: the doped GB (Fig. 2C) based on the experimental composition profiles (Table S1, ESI†) with the FCC-like interfacial layer (Fig. 1C), as well as the undoped WC GB without the FCC-like interfacial layer (Fig. 2D) for comparison. On the one hand, Fig. 2D shows that undoped WC GB exhibits highly ordered W layers from L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) to L1 layers although some C atoms near the L1 layer are slightly disordered. Specifically, the periodic and ordered L1 layer in the undoped GB is shown in Fig. 1G. On the other hand, in the optimized structure of the doped GB, the Ti-rich L
to L1 layers although some C atoms near the L1 layer are slightly disordered. Specifically, the periodic and ordered L1 layer in the undoped GB is shown in Fig. 1G. On the other hand, in the optimized structure of the doped GB, the Ti-rich L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer maintains a highly ordered structure, but the Co-rich L1 layer becomes partially disordered (Fig. 2E), although the starting doped GB structure before the DFT relaxation was ordered. Moreover, Co segregation can induce structural disorder in not only the surrounding C atoms, but also the W atoms at the adjacent L1 layer (Fig. 2C). This predicted Co segregation induced partial disorder further validates our experimental observation (Fig. 1F).
layer maintains a highly ordered structure, but the Co-rich L1 layer becomes partially disordered (Fig. 2E), although the starting doped GB structure before the DFT relaxation was ordered. Moreover, Co segregation can induce structural disorder in not only the surrounding C atoms, but also the W atoms at the adjacent L1 layer (Fig. 2C). This predicted Co segregation induced partial disorder further validates our experimental observation (Fig. 1F).
Third, we further calculated a stability diagram as a function of Ti and Co segregation amounts by comparing relative energies of the GB structures with and without the formation of the FCC-like interfacial layer. This stability diagram (Fig. S5, ESI†) indicates that the FCC-like interfacial layer is stable when the Ti fraction at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer is above a threshold (virtually independent of the Co fraction at the L1 layer). Hence, we conclude that the Ti segregation induces an interfacial symmetry change to form the FCC-like interfacial layer. It is worth noting that a segregation induced FCC-like interfacial structure was also found at the WC/Co phase boundary by both experimental32 and theoretical33,34 studies.
layer is above a threshold (virtually independent of the Co fraction at the L1 layer). Hence, we conclude that the Ti segregation induces an interfacial symmetry change to form the FCC-like interfacial layer. It is worth noting that a segregation induced FCC-like interfacial structure was also found at the WC/Co phase boundary by both experimental32 and theoretical33,34 studies.
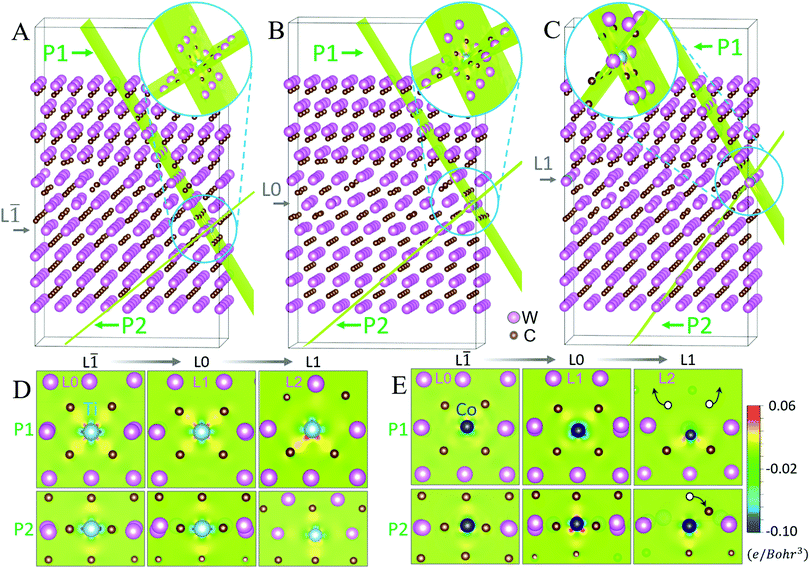
Fourth, we examined the changes of coordination environments at each layer in the dilute segregation structures to investigate the causes for the Co and Ti segregation. To illustrate bonding environments clearly, the GB structures are cut along the P1 and P2 planes for three cases if the segregation occurs at the layer L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Fig. 3A), layer L0 (Fig. 3B), and layer L1 (Fig. 3C), respectively; the detailed coordination environments are shown in Fig. 3D for Ti and Fig. 3E for Co, respectively.
(Fig. 3A), layer L0 (Fig. 3B), and layer L1 (Fig. 3C), respectively; the detailed coordination environments are shown in Fig. 3D for Ti and Fig. 3E for Co, respectively.
On the one hand, the Ti atom is mostly octahedrally coordinated with six carbon atoms at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer (Fig. 3D), suggesting a TiC-based FCC-like rocksalt structure35,36 (Fig. S6A and B, ESI†). A similar coordination environment of Ti is also found in the L0 layer (Fig. 3D). On the other hand, if Ti segregated to the L1 layer (Fig. 3D), the W atoms (in the L2 layer), which are not octahedrally coordinated with carbon atoms, would distort the preferred carbon coordination environment of Ti. For example, DFT optimization shows that the upper two carbon atoms would move away from the Ti atom, thereby distorting the Ti octahedron at the L1 layer and reducing the corresponding coordination number. Since octahedral coordination with six Ti–C bonds is the most stable for Ti,37,38 this destabilizes the segregation of Ti at the L1 layer. This analysis explains the formation of an FCC-like interfacial layer with the preferred segregation of Ti in the L
layer (Fig. 3D), suggesting a TiC-based FCC-like rocksalt structure35,36 (Fig. S6A and B, ESI†). A similar coordination environment of Ti is also found in the L0 layer (Fig. 3D). On the other hand, if Ti segregated to the L1 layer (Fig. 3D), the W atoms (in the L2 layer), which are not octahedrally coordinated with carbon atoms, would distort the preferred carbon coordination environment of Ti. For example, DFT optimization shows that the upper two carbon atoms would move away from the Ti atom, thereby distorting the Ti octahedron at the L1 layer and reducing the corresponding coordination number. Since octahedral coordination with six Ti–C bonds is the most stable for Ti,37,38 this destabilizes the segregation of Ti at the L1 layer. This analysis explains the formation of an FCC-like interfacial layer with the preferred segregation of Ti in the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer.
layer.
Furthermore, we find that Co also exhibits an FCC-like sublattice with six surrounding carbon atoms at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and L0 layers (Fig. 3E). However, the adjacent carbon atoms can be easily repelled away from the Co atom segregated at the L1 layer, because the loosely-packed W atoms at the L2 layer provide a large space for them to relax; see, e.g., the white circles with arrows in the right panel of Fig. 3E. Since a tetrahedral coordination with three carbon atoms is more stable for Co,38 the Co segregation at the L1 layer is favorable.
and L0 layers (Fig. 3E). However, the adjacent carbon atoms can be easily repelled away from the Co atom segregated at the L1 layer, because the loosely-packed W atoms at the L2 layer provide a large space for them to relax; see, e.g., the white circles with arrows in the right panel of Fig. 3E. Since a tetrahedral coordination with three carbon atoms is more stable for Co,38 the Co segregation at the L1 layer is favorable.
Thus, DFT calculations discussed above suggest that the observed asymmetric segregation stems from the different coordination environments: the octahedral coordination with six carbons for Ti at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer vs. the tetrahedral coordination with only approximately three carbons for Co at the L1 layer. Furthermore, a strong distortion in the carbon coordination at the L1 layer can lead to interfacial disordering (Fig. 2F), which explains the experimental observation of the partially disordered Co segregation layer (Fig. 1F).
layer vs. the tetrahedral coordination with only approximately three carbons for Co at the L1 layer. Furthermore, a strong distortion in the carbon coordination at the L1 layer can lead to interfacial disordering (Fig. 2F), which explains the experimental observation of the partially disordered Co segregation layer (Fig. 1F).
Fifth, we further calculated the differential charge density (DCD) to reveal the correlation between segregation and charge transfer. The projected DCD maps (Fig. 3D) show that strong charge accumulation occurred between Ti and six coordinated C atoms in the FCC-like interfacial layer (at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and L0 layers), while less accumulation is found at the L1 layer. Since large charge transfer can prompt the segregation of the solute atom,39,40 the formation of an FCC-like interfacial layer should favor the Ti segregation. A quantitative analysis of the excess charge transfer, Δqex, further confirms this suggestion (Fig. S7, ESI†). In comparison, the strong charge transfer near Co at the L1 layer (Fig. 3E) favors strong Co segregation at this L1 layer, whereas the much weaker charge transfer between Co and six individual C atoms at the L
and L0 layers), while less accumulation is found at the L1 layer. Since large charge transfer can prompt the segregation of the solute atom,39,40 the formation of an FCC-like interfacial layer should favor the Ti segregation. A quantitative analysis of the excess charge transfer, Δqex, further confirms this suggestion (Fig. S7, ESI†). In comparison, the strong charge transfer near Co at the L1 layer (Fig. 3E) favors strong Co segregation at this L1 layer, whereas the much weaker charge transfer between Co and six individual C atoms at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and L0 layers (Fig. 3E) accounts for weak segregation there. These predictions from charge transfers again agree well with experimental observations (Fig. 1).
and L0 layers (Fig. 3E) accounts for weak segregation there. These predictions from charge transfers again agree well with experimental observations (Fig. 1).
The above discussions illustrate that both coordination environment and charge transfer are useful to understand solute segregation at the GB. However, neither of them can illustrate the true bonding environment and local chemical structure. Thus, we propose a new quantity, sum of bond ordering (SBO),41 to uncover the relationship between the solute segregation and the bonding environment.
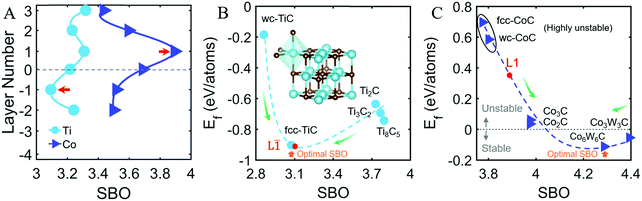
On the one hand, Fig. 4A shows calculated SBO of Ti and Co segregated at each GB layer, in comparison with all possible Ti- and Co-based carbides (Fig. 4B and C). Notably, the Ti SBO of 3.09 at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer (represented by the red dot in Fig. 4B) is very close to that of the FCC TiC, the titanium carbide with the lowest formation energy Ef (Fig. 4B). The similarity of the SBO values also suggests the favorable formation of a Ti-based FCC-like interfacial layer at the L
layer (represented by the red dot in Fig. 4B) is very close to that of the FCC TiC, the titanium carbide with the lowest formation energy Ef (Fig. 4B). The similarity of the SBO values also suggests the favorable formation of a Ti-based FCC-like interfacial layer at the L![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) layer, in excellent agreement with our experiments and calculations.
layer, in excellent agreement with our experiments and calculations.
On the other hand, Fig. 4C shows a Co SBO value of 3.90 at the L1 layer (represented by the red dot) towards relatively stable Co2C and Co3C phases (with coordination of 3 and 2), whereas the segregated Co at all other layers has smaller SBO values (of ∼3.4–3.6), towards highly unstable FCC- and WC-structured CoC phases (both with 6 coordination). Consequently, the most favorable SBO of the segregated Co occurs at the L1 layer and corresponds to a loosely bonding environment between Co and C, which promotes interfacial disordering.
Furthermore, there are two stable ternary phases (Co6W6C and Co3W3C with Ef < 0) in Fig. 4C, where Co and C atoms do not form direct bonds but are separated by W atoms. This also implies that a direct Co–C bond is less stable (Fig. S8C and D). Similarly, the intermediate W-rich L0 layer of the observed asymmetric segregation superstructure (Fig. 1 and 2A) can also allow an intermediate layer for a gradual transition from a highly stable, six-coordinated Ti–C to a relatively unstable and less-coordinated Co–C bonding environment. Thus, this SBO analysis explains the formation of the off-the-center, asymmetric segregation structure (Fig. 1 and 2A).
The success of the proposed SBO theory to explain segregation behavior implies that SBO can be used as a new descriptor to predict the segregation trend in complex interfacial structures.
Finally, we also find two additional (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) GBs in our experiments, which are not orientated to [2
0) GBs in our experiments, which are not orientated to [2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0]//[2
0]//[2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 3] as the example (Fig. 1 and 2) discussed above, that exhibit similar interfacial superstructures. One such (0001)//(01
3] as the example (Fig. 1 and 2) discussed above, that exhibit similar interfacial superstructures. One such (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) GB exhibits a long terrace with similar dark/bright/dark fringes in HAADF imaging, where the corresponding EDS mapping has revealed a similar Ti/W/Co asymmetric segregation pattern (Fig. S11, ESI†). In another general (0001)//(01
0) GB exhibits a long terrace with similar dark/bright/dark fringes in HAADF imaging, where the corresponding EDS mapping has revealed a similar Ti/W/Co asymmetric segregation pattern (Fig. S11, ESI†). In another general (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) GB, discrete terraces with dark/bright/dark contrast are separated by atomic-level steps (Fig. S12, ESI†). Altogether, all three (different) (0001)//(01
0) GB, discrete terraces with dark/bright/dark contrast are separated by atomic-level steps (Fig. S12, ESI†). Altogether, all three (different) (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) GBs examined by STEM in this study exhibit similar interfacial superstructures, thereby suggesting its generality. Since all (0001)//(01
0) GBs examined by STEM in this study exhibit similar interfacial superstructures, thereby suggesting its generality. Since all (0001)//(01![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) GBs, regardless of their in-plane rotation angles, account for about 8–9% of all GBs in this Ti-doped WC–Co specimen (Fig. S10, ESI†), such interfacial superstructures are not uncommon.
0) GBs, regardless of their in-plane rotation angles, account for about 8–9% of all GBs in this Ti-doped WC–Co specimen (Fig. S10, ESI†), such interfacial superstructures are not uncommon.
The combination of all the three cases also further supports that the orientations of the terminal grain surfaces, instead of the misorientation between the two abutting grains, dictate the segregation and interfacial structure. The interaction between the two asymmetric segregation profiles can lead to even more complex asymmetric interfacial structural transitions, thereby promoting unexpected features like off-the-center segregation.
In general, we expect that highly asymmetric segregation may also occur in other materials, particularly at mixed GBs with two low-index grain surface terminal planes, but with little (or less) lattice matching. Different bonding natures of the hosting and two co-dopants may also favor the formation of highly asymmetric interfacial superstructures, which are further discussed in Supplementary discussion section 3 in the ESI.†
In a broader context, our experiments and theoretical calculations demonstrate that interfacial superstructures can be much more complicated than what has been previously observed and believed. This highly asymmetric interfacial superstructure with highly asymmetric and off-the-center segregation, occurring concurrently with two different interfacial structural transitions on two sides, is significantly more complex than Dillon et al.'s complexions18,19 as well as the interfacial superstructures observed at symmetric GBs of MgO20 and YSZ21 and general GBs in a perovskite22 and Ni–Bi.23
Specifically, multiple dopants such as Ti, V and Cr, etc. are often used to inhibit grain growth in WC–Co cermets, and they can alter the microstructures and mechanical properties substantially. Thus, understanding and controlling the formation of complex interfacial superstructures are of fundamental importance to tailor the material processing and properties.
Conclusions
We observed highly asymmetric and off-the-center segregation of Ti and Co in mixed GBs in WC, along with different interfacial structural transitions of symmetry change (reconstruction) and interfacial disordering in the two sides, via detailed AC-STEM characterization and atomic resolution EDS mapping. Large-scale first-principles calculations further verified the energetic stability of this highly asymmetric interfacial superstructure and further reveal the important roles of the coordination and bonding environments of the solute atoms in determining the segregation pattern and interfacial structure.Our results not only shed light on the complex GB segregation structures in Ti-doped WC–Co, but also demonstrate the possible existence of asymmetric complex interfacial superstructures that differ significantly from all prior experimental observations and are beyond the predictions of any existing models. Thus, this discovery greatly expands our knowledge of atomic-level segregation structures in real polycrystalline materials, with potentially broad impacts.
Author contributions
Z. Y., J. L., and H. N. conceived the idea and supervised the experiments and calculations. H. N. provided the WC–Co sample. Z. L., Z. Y., and L. X. carried out STEM-HAADF characterization and C. X. analyzed the EDS mapping data. W. Z., L. X., and J. H. provided the AC-STEM facility and assistance. C. H. performed theoretical calculations. X. G. analyzed crystallography. Z. Y., C. H., Z. L., and J. L. drafted the manuscript. All authors reviewed the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
Z. Yu would like to acknowledge the National Natural Science Foundation of China (Grant No. 51871058 and 51701170), and project of Science and Technology Plan of Fujian Province (Grant No. 2018J01520). C. H. and J. L.'s work on WC at UCSD is unfunded.References
- L. Priester, Springer Series in Materials Science, 2013, vol. 172 Search PubMed.
- J. Luo, H. Cheng, K. M. Asl, C. J. Kiely and M. P. Harmer, Science, 2011, 333, 1730–1733 CrossRef CAS.
- R. F. Klie, J. P. Buban, M. Varela, A. Franceschetti, C. Jooss, Y. Zhu, N. D. Browning, S. T. Pantelides and S. J. Pennycook, Nature, 2005, 435, 475–478 CrossRef CAS.
- D. McLean, Grain Boundaries in Metals, Clarendon Press, Oxford, 1957 Search PubMed.
- E. D. Hondros and M. P. Seah, Metall. Trans., 1977, 8A, 1363–1371 CrossRef CAS.
- M. Tang, W. C. Carter and R. M. Cannon, Phys. Rev. Lett., 2006, 97, 075502 CrossRef.
- Y. Mishin, W. J. Boettinger, J. A. Warren and G. B. McFadden, Acta Mater., 2009, 57, 3771–3785 CrossRef CAS.
- P. Wynblatt and D. Chatain, Mater. Sci. Eng., A, 2008, 495, 119–125 CrossRef.
- J. Luo, Appl. Phys. Lett., 2009, 95, 071911 CrossRef.
- J. M. Rickman and J. Luo, Curr. Opin. Solid State Mater. Sci., 2016, 20, 225–230 CrossRef CAS.
- S. Yang, N. Zhou, H. Zheng, S. Y. Ong and J. Luo, Phys. Rev. Lett., 2018, 120, 085702 CrossRef CAS.
- G. Duscher, M. F. Chisholm, U. Alber and M. Rühle, Nat. Mater., 2004, 3, 621–626 CrossRef CAS.
- N. Shibata, S. J. Pennycook, T. R. Gosnell, G. S. Painter, W. A. Shelton and P. F. Becher, Nature, 2004, 428, 730–733 CrossRef CAS.
- J. Luo, Crit. Rev. Solid State Mater. Sci., 2007, 32, 67–109 CrossRef CAS.
- D. R. Clarke, J. Am. Ceram. Soc., 1987, 70, 15–22 CrossRef CAS.
- T. Hu, S. Yang, N. Zhou, Y. Zhang and J. Luo, Nat. Commun., 2018, 9, 2764 CrossRef.
- J. Luo, V. K. Gupta, D. H. Yoon and H. M. Meyer, Appl. Phys. Lett., 2005, 87, 231902 CrossRef.
- P. R. Cantwell, M. Tang, S. J. Dillon, J. Luo, G. S. Rohrer and M. P. Harmer, Acta Mater., 2014, 62, 1–48 CrossRef CAS.
- S. J. Dillon, M. Tang, W. C. Carter and M. P. Harmer, Acta Mater., 2007, 55, 6208–6218 CrossRef CAS.
- Z. Wang, M. Saito, K. P. McKenna, L. Gu, S. Tsukimoto, A. L. Shluger and Y. Ikuhara, Nature, 2011, 479, 380–383 CrossRef CAS.
- B. Feng, T. Yokoi, A. Kumamoto, M. Yoshiya, Y. Ikuhara and N. Shibata, Nat. Commun., 2016, 7, 11079 CrossRef CAS.
- H.-I. Yoon, D.-K. Lee, H. B. Bae, G.-Y. Jo, H.-S. Chung, J.-G. Kim, S.-J. L. Kang and S.-Y. Chung, Nat. Commun., 2017, 8, 1417 CrossRef PubMed.
- Z. Yu, P. R. Cantwell, Q. Gao, D. Yin, Y. Zhang, N. Zhou, G. S. Rohrer, M. Widom, J. Luo and M. P. Harmer, Science, 2017, 358, 97–101 CrossRef CAS PubMed.
- R. Bonnet, E. Cousineau and D. H. Warrington, Acta Crystallogr., 1981, A37, 184–189 CrossRef CAS.
- C.-S. Kim and G. S. Rohrer, Interface Sci., 2004, 12, 19–27 CrossRef CAS.
- Z. Yu, J. Luo, B. Shi, J. Zhao, M. P. Harmer and J. Zhu, Sci. Rep., 2015, 5, 16960 CrossRef CAS.
- J. Weidow and H.-O. Andrén, Int. J. Refract. Met. Hard Mater., 2011, 29, 38–43 CrossRef CAS.
- W. Swiatnicki, S. Lartigue-Korinek and J. Y. Laval, Acta Mater., 1995, 43, 795–805 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS PubMed.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169 CrossRef CAS.
- J. Klimeš, D. R. Bowler and A. Michaelides, J. Phys.: Condens. Matter, 2009, 22, 022201 CrossRef PubMed.
- X. Liu, X. Song, H. Wang, X. Liu, F. Tang and H. Lu, Acta Mater., 2018, 149, 164–178 CrossRef CAS.
- S. A. E. Johansson and G. Wahnstrom, Philos. Mag. Lett., 2010, 90, 599–609 CrossRef CAS.
- S. A. E. Johansson and G. Wahnström, Acta Mater., 2011, 59, 171–181 CrossRef CAS.
- C. Hu, J. Huang, B. G. Sumpter, E. Meletis and T. Dumitrică, J. Phys. Chem. C, 2017, 121, 26007–26018 CrossRef CAS.
- C. Hu, J. Huang, B. G. Sumpter, E. Meletis and T. Dumitrică, ACS Appl. Nano. Mater., 2018, 1, 2029–2035 CrossRef CAS.
- D. Conntable, Mater. Res. Express, 2016, 3, 126502 CrossRef.
- A. Jain, S. P. Ong, G. Hautier, W. Chen, W. D. Richards, S. Dacek, S. Cholia, D. Gunter, D. Skinner, G. Ceder and K. A. Persson, APL Mater., 2013, 1, 011002 CrossRef.
- C. Hu and J. Luo, Scr. Mater., 2019, 158, 11–15 CrossRef CAS.
- H. Zheng, R. Tran, X.-G. Li, B. Radhakrishnan and S. P. Ong, Acta Mater., 2018, 145, 470–476 CrossRef CAS.
- N. G. Limas and T. A. Manz, RSC Adv., 2018, 8, 2678–2707 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mh00969h |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |