Construction of multi-scale vascular chips and modelling of the interaction between tumours and blood vessels†
Jing
Nie‡
ab,
Qing
Gao‡
ab,
Chaoqi
Xie‡
ab,
Shang
Lv
ab,
Jingjiang
Qiu
c,
Yande
Liu
d,
Mengzi
Guo
e,
Rui
Guo
 f,
Jianzhong
Fu
ab and
Yong
He
f,
Jianzhong
Fu
ab and
Yong
He
 *ab
*ab
aState Key Laboratory of Fluid Power and Mechatronic Systems, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China. E-mail: yongqin@zju.edu.cn
bKey Laboratory of 3D Printing Process and Equipment of Zhejiang Province, School of Mechanical Engineering, Zhejiang University, Hangzhou 310027, China
cSchool of Mechanics and Safety Engineering, Zhengzhou University, Zhengzhou 450001, China
dSchool of Mechatronics & Vehicle Engineering, East China Jiaotong University, Nanchang 330013, China
eMcCormick School of Engineering, Northwestern University, USA
fDepartment of Biomedical Engineering, Jinan University, Guangzhou 510632, China
First published on 28th August 2019
Abstract
Despite the substantial progress in the construction of vascular structures in the past few years, building a whole blood circulatory system model containing both large vessels and capillaries inside cell-laden hydrogels remains a big challenge. Here, we present a flexible and novel process to construct a hydrogel-based microfluidic chip with such a multi-scale network by combining high-resolution three-dimensional (3D) printing and a twice-crosslinking strategy. The whole process includes: (1) vascular system design (from arteries and capillaries to veins), (2) template printing (ultrafine fiber network), (3) hydrogel material casting (formation of partially-crosslinked hydrogel sheets), (4) template peeling off (creation of microgrooves on the surfaces of the hydrogel sheets), (5) hydrogel sheet bonding (formation of a closed channel network) and (6) cell loading (specific cells seeded onto specific positions mimicking in vivo conditions). We demonstrated that it is easy to fabricate the ubiquitous structures of biological vascular systems, highly-branched networks, spiral vessels, stenosis, etc. The endothelial cell (EC) channels exhibit representative vascular functions. As a proof of concept, a bulk breast tumor tissue with a functional vascular network was built. Additionally, a vascular–tumour co-culture concept has been proposed and constructed through the process to investigate the interaction between tumours and blood vessels. The proposed strategy can also be applied to help engineer diverse meaningful in vitro models for extensive biomedical applications, from physiology and disease study to therapy evaluation.
New conceptsA new concept, multi-scale vascular chips, has been proposed for the first time. We pioneer the application of a combination of multi-scale 3D printing technology and a twice-cross-linking strategy to construct this model. Whereas most of the previous studies about the construction of vascular models are limited to a certain scale range, we aim to realize the construction of the whole vascular system containing both vessels and capillaries. This is achieved by a shift of the printing mode between FDM printing and EHD printing. Additionally, ubiquitous structures mimicking biological vascular morphologies are established through the proposed method. Our study further proposed a vascular–tumour co-culture system for modelling the interaction between tumours and blood vessels and we envision that it can pave a new way for cancer study and anti-tumour drug screening. The significance of the built-in vessel has been proven through an initial study of the migration behaviour of the tumour cells towards the endothelial barrier and the introduction of drug candidates through an endothelial barrier. |
Introduction
The ultimate goal of tissue engineering is to construct organ substitutes for surgical transplants and pharmaceutical testing.1–3 To achieve this ambitious goal,4,5 large-scale cell-laden artificial tissues should be established and functionalized. A basic prerequisite for this is an efficient substance exchange system due to the limitation of the penetration depth of the extracellular matrix (ECM).6–10 In nature, vascular networks have evolved to deal with this issue by integrating multi-scale and highly-branched space-filling architectures. Thus, the development of biomimetic vasculatures would be of vital importance.2,6,9 Despite the significant improvements, demands still exist for a feasible protocol to construct large-scale tissues with the complete vascular network containing both large blood vessels and capillaries.11,12Numerous approaches have been proposed for building large blood vessels, including the deformation of cell sheets into tubular structures,13–16 preparation of fibres with cell-embedded hydrogels,17,18 thin wire-based moulding,19–21 use of sacrificial templates,22–26 3D bio-printing,27–33 light-assisted processes,34–36 and layer-by-layer assembly.37–42 However, the major difficulty lies in the construction of a bifurcation structure and capillaries at the end.43 Few studies have been reported about the engineering of capillaries, which are essential components of a vascular system. To remodel organized and stable capillaries, a self-development strategy is applied by extending new capillaries from pre-existing vessels toward angiogenic signals.44–47 However, the use of growth factors on a large scale is cost-prohibitive and inefficient.48,49 An alternative approach, referred to as a physical structure-based strategy, is to engineer ultrafine channels before EC seeding.50 This approach makes it convenient to control the distribution of the capillaries, from direction to range. In addition, the in vivo vascular morphology is more diverse than a single linear structure,25 which is closely related to physiological and pathological conditions. For example, spiral arteries reside in the uterine endometrium,51,52 while stenosis can lead to inflammation and dysfunction.53,54 This complicated organization motivates the development of new techniques featuring excellent flexibility, cost-effectiveness and versatility.
Tumour development is a complicated multi-step process, involving the origin, growth, and metastasis. In particular, the vascular network is the crucial condition for tumour development,63,64 and the interaction between tumours and the vascular network plays an important role in the process. Angiogenic growth factors secreted by tumour cells prompt angiogenesis, while newly-induced capillaries supply nutrition for tumour growth and the necessary pathway for tumour metastasis.55,56 A series of tumour cell-laden constructs have been created.57,58 Multi-cellular tumour sphere models consisting of aggregated cells emerged, showing an excellent structure to mimic solid tumours.59,60 In addition, a set of microfluidic systems have been introduced as vascularized models for the study of tumour development.12,61,62,65,66 However, a microfluidic device is far from a real soft tissue.67 Accordingly, there is an urgent demand for an effective model to study the interaction between tumour tissues and blood vessels, as well as the key processes during metastasis.68–70
This study pioneers the combination of multi-scale 3D printing and a twice-crosslinking strategy to construct multi-scale structures within hydrogel materials. This method has the ability to construct the whole vascular hierarchy (from arteries to veins, as shown in Fig. S1, ESI†) and to emulate the ubiquitous structures of biological vascular systems. As a proof of concept, a vascularized breast tumour model is established. In addition, the strategy can also be applied to cell-laden hydrogel materials for engineering ubiquitous in vitro biological structures for a wide range of biomedical applications. Based on this method, a demonstrative vascular–tumour co-culture model with a parallel-channel structure is designed and constructed as a feasible system for comprehensive and systematic investigation of tumour-related physiological phenomena, including tumour development and anti-tumour drug screening. A meaningful vascular–tumour co-culture model has been proposed, showing great potential in future applications through a series of preliminary phenomena and basic data. The migration ability of the tumour cells and the barrier function of the vessels to anti-cancer drugs are verified. Furthermore, a simple model mimicking tumour development is engineered, showing the processes of primary tumours, angiogenesis, and invasion, which can be further used as an experimental platform to investigate metastasis. The initial feasibility and potential application scenario are displayed through the preliminary phenomena and basic data, ensuring the matching of the materials, structures, and processes with the potential application.
In comparison with other reported in vitro tumour models,66,71–73 this model displays several innovative features and outstanding merits, including: (i) development of a multi-scale vascular network, (ii) 3D culture conditions mimicking the in vivo environment, (iii) convenience to construct varied tumour tissues with diverse vascular networks, (iv) layer-by-layer assembly for the production of heterogeneous tumour tissues, (v) real-time loading of functional materials at reserved vacancies, (vi) diversity in bio-materials for customized models with individual and personal designs and (vii) the endothelial channel which offers a tool to study the invasion process of tumour cells and provides a barrier to drug candidates, mimicking in vivo drug delivery.74,75
Results
Construction of multi-scale structures within a hydrogel bulk
The whole process for engineering hydrogel constructs equipped with multi-scale inner structures is shown in Fig. 1, including model designing, template printing, casting, stripping, bonding and cell loading. First of all, a multi-scale template was designed and printed through a multi-scale direct writing system which consists of fused deposition modeling (FDM) printing and electro-hydrodynamic (EHD) printing. The detailed printing principle of the multi-scale template is presented in Section S2 in the ESI.† The following steps are based on a twice-cross-linking strategy, which has been proposed in our previous work.42 Section S1 in the ESI† discusses in detail the process and mechanism of the approach. The whole process was performed under mild conditions, without causing damage to cells, which allows its application to cell-laden hydrogels.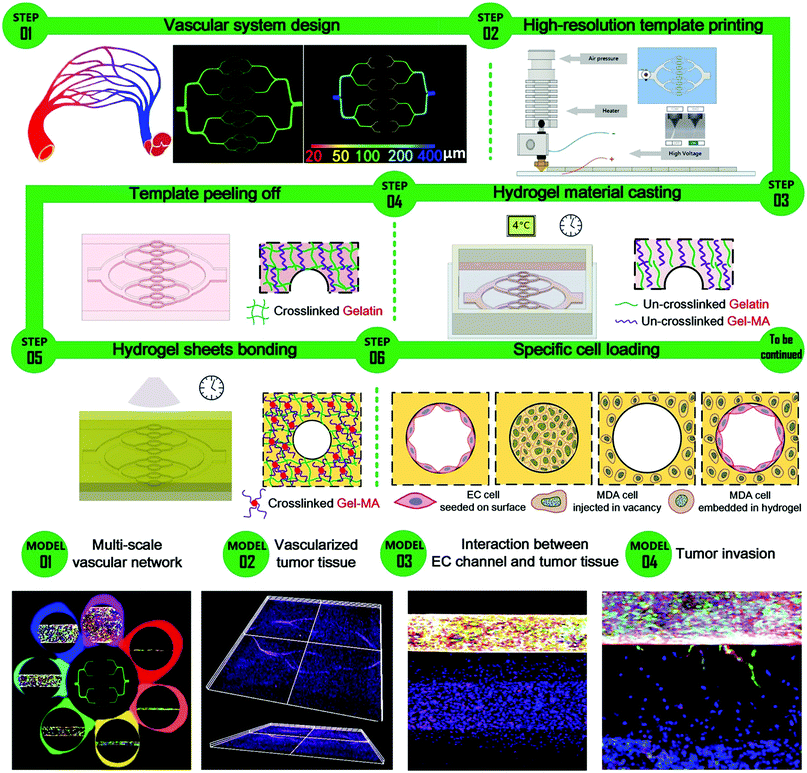 | ||
| Fig. 1 Schematic for the construction of a multi-scale vascular chip and display of the representative models through confocal images. | ||
Moreover, it is simple to reserve a vacancy of a specific shape at a designated location for post injection and deposition on demand, bringing flexibility to the construction of various heterogeneous structures. Different cell loading conditions are displayed in Fig. 1, including EC cells seeded onto the surface of the channel, tumor cells injected into the vacancy and tumor cells embedded in the surrounding hydrogel bulk.
Several models were constructed to display the versatility of this strategy, including a multi-scale vascular network model, a parallel-channel model, a vascularized tumour model and a tumor invasion model, as shown in Fig. 1.
Processing parameters and channel structures
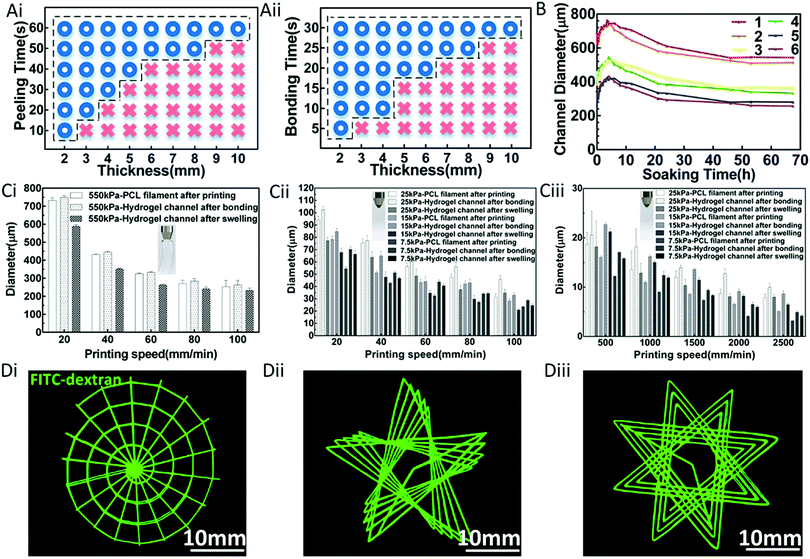
According to the above process, it is feasible to fabricate a hydrogel construct with multi-scale inner channels. In order to establish the optimal production program and achieve the precise regulation of the channel scales, a set of relevant experiments were conducted.By adopting the minimal dichotomy, the feasible domains for peeling off and bonding were determined,42 as shown in Fig. 2A. This revealed that the minimal time for peeling off and bonding increased with the increasing thickness of the hydrogel constructs.
Notably, the cross-linking of the hydrogel compositions during the peeling off and bonding processes brought about, to a certain extent, the increase of the diameter of the channel compared with the diameter of the template, showing good repeatability. Also, the subsequent swelling process during the soaking of the hydrogel constructs in medium or buffer caused a certain decrease of the channel diameter, which could reach a stable balance point after 53 hours. This was verified through experiments, as shown in Fig. 2B.
The effects of the printing parameters on the diameters of the filaments were systematically investigated for the FDM and EHD printing, respectively. During the printing process, the diameters of the printed filaments could be adjusted in the corresponding scale range and were affected by several parameters, including extrusion pressure and printing speed, as shown in Fig. 2C. The results indicated that the diameter of the filaments increased with increasing extrusion pressure, whereas it decreased with increasing printing speed. This occurred because a higher extrusion pressure directly increased the extrusion quantity, eventually leading to an increase of the diameter of the filament. Additionally, the filament was stretched and became thinner with the increase of the printing speed. In this study, both the printing speed and extrusion pressure were used to adjust the diameter of the filament. Instructively, by adjusting the printing parameters (i.e., printing speed and extrusion pressure), macro-scale filaments ranging from 253 to 732 μm and micro-scale filaments ranging from 3.16 to 94.19 μm could be obtained during the FDM printing process and EHD printing process, respectively, as shown in Fig. 2C. In conclusion, a final channel network containing macro-scale channels ranging from 233 to 589 μm and micro-scale channels ranging from 4.17 to 102.36 μm could be obtained.
Based on these results, interesting channel patterns could be successfully constructed through the special design of printing paths, such as a snail-like structure, a pentagram-like structure and a flower-like structure, as shown in Fig. 2D. It is conceivable that this process can be used to obtain various intricate and detailed internal channels over a wide scale range within hydrogel materials, such as a multi-scale structure, a superfine patterned structure, and a variable-scale structure, which can be further used as vascular system, capillary network, and vascular disease models.
An in vivo vascular network contains arteries, veins and capillaries with different scales and functions. A new concept, multi-scale vascular chips, has been demonstrated for the first time. Existing research studies about the construction of vascular models are limited to a certain scale range (Section S2, ESI†), while the multi-scale printing technology in this paper overcomes the limitation and can be applied to realizing the construction of the whole vascular system containing both big vessels and capillaries.
Various EC channels simulating real blood vessels
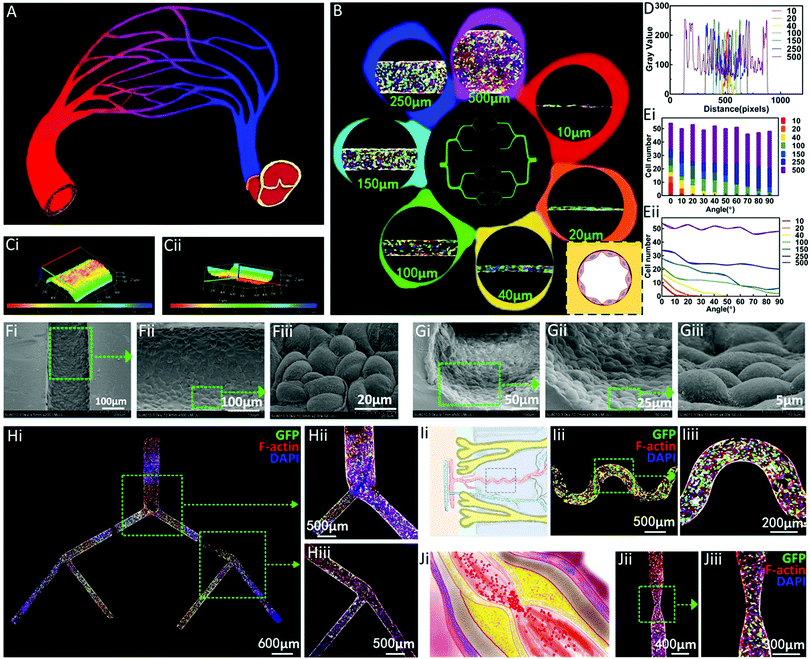
Inspired by the human blood vessel network in vivo (Fig. 3A), a multi-stage network mimicking the whole blood vessel hierarchy was designed and constructed through the above method (Fig. 3B). The results demonstrated that this method had the ability to construct the whole vascular system through the construction of EC channels with diameters of 10, 20, 40, 100, 150, 250, and 500 μm. The microscopy morphologies of the ECs seeded onto the channels with different diameters are shown in Fig. 3B. The cells on the inner wall were observed to attach and spread well on the inner wall of the channel. The 3D stacking confocal images showed the complete distribution of ECs along the whole channels (Fig. 3C). The transformed grey level data along the radial direction of the endothelial channels in different diameters were collected and displayed (Fig. 3D), showing the feasibility of the proposed method to construct the multi-scale vascular model containing both big vessels and capillaries. Furthermore, the directions of the EC stretching on the channels with different diameters were measured and statistically analyzed in both discrete and continuous ways, as shown in Fig. 3E. The results indicated that the direction of EC stretching tended to gradually become isotropic with the increase of the diameter of the channel. The cells were oriented to a certain extent in the thinner channels, and the directivity was strengthened as the diameter of the channel decreased. In the 10 and 20 μm channels, almost all the ECs stretched along the axial direction of the channels, while in the 250 and 500 μm channels, the numbers of cells stretching in all directions were almost equal. The different stretching situations on the channels of different scales indicate the directional effect of the capillary channel.Furthermore, Fig. 3F and G display the microscopy morphologies of the ECs seeded onto the channel from both the longitudinal and axial directions through high magnification images, showing a healthy state, indicating the excellent bio-compatibility of the proposed system.
A three-level EC network was visualized through confocal microscopy, showing substantial spreading of ECs (Fig. 3H). Emulating the ubiquitous structures of biological vascular systems in hydrogels is significant for the construction of in vitro models. However, in spite of the lot of progress, the existing methods are restricted by the complicated microfabrication process, introduction of toxic materials, and consumption of disposable molds. In contrast, in this method, a trace amount of PCL materials was printed according to the specific demand for every single unique structure. For example, a spiral EC channel inspired by the spiral artery residing in the uterine endometrium was constructed by this method, as shown in Fig. 3I. As a main cause and risk factor of cardiovascular diseases, stenosis can bring about the reduction of blood flow and ischemia. A stenosis-mimicking EC channel was also constructed as a disease model for further investigation of relevant drugs, as shown in Fig. 3J.
A model of vascularized tumour tissue
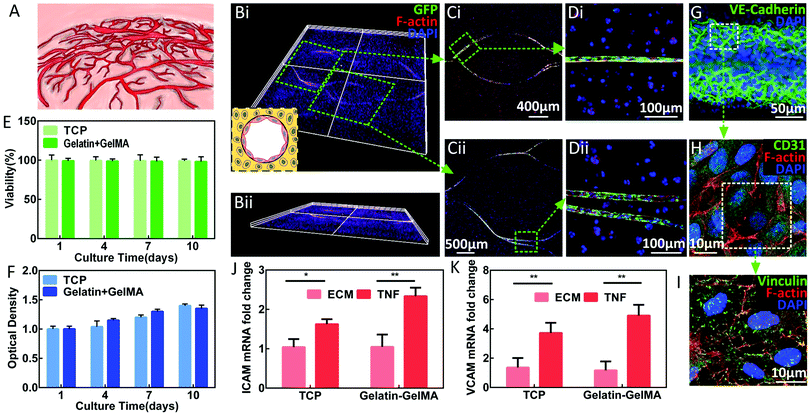
Cell-laden artificial tissue models are attracting more attention. Among them, hydrogel-based microfluidics shows its surpassing advantages in mimicking the in vivo environment. The first and most important factor is an efficient vascular network,76,77 as shown in Fig. 4A. For tumour cells, 3D culture conditions can better simulate the in vivo growth environment than 2D culture conditions. As a result, tumour cells cultured under 3D conditions show behaviours closer to those of in vivo cells in terms of phenotype and genotype.78–82 However, the simulation of the tumour microenvironment is very complicated,61,83–85 and the development of a tumour model equipped with the vascular system is an important objective.In this paper, a breast cancer model containing tumour stroma (Gel-Gel-MA hydrogel), a functional vascular system (EC channels), and tumour cells (MDA-MB-231) was developed, as shown in Fig. 4B–D. The cytoskeleton staining of the construct confirmed the conceived distribution of specific cell types. Data revealed that the cells maintained high viability and a good proliferation ability, as shown in Fig. 4E and F. The expression of VE-cadherin protein indicated the formation of cell–cell junctions (Fig. 4G), which is significant for the barrier function of the EC channels. The expression of CD31 protein demonstrated the achievement of key endothelial function (Fig. 4H). The expression of vinculin protein further supported the tight adherence between cells and channels (Fig. 4I).42
In order to reconstruct the vascular inflammation model, a similar protocol from previously published work42 was applied to this model and the obtained results confirmed the realization of the simulation of the pathological conditions based on our model (Fig. 4J and K).
Together the above results proved that the engineered in vitro EC network could model endothelial functionality under different physiological conditions to mimic the native vascular system.
A model of interaction between tumour tissues and blood vessels
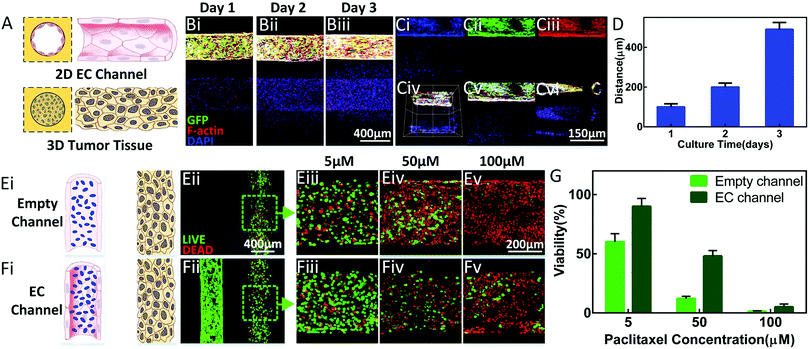
In order to study the interaction between tumour cells and blood vessels, a parallel-channel model with better unity was designed and constructed to avoid the random variable quantity caused by sample inconsistency. The MDA-laden Gel-MA pre-polymer solution was injected into one of the channels before being properly cured to simulate the tumour tissue. Medium containing ECs was injected into another channel and flipped for 3 h before culturing for 48 h to allow the formation of a confluent EC monolayer. This model is schematically displayed in Fig. 5A. Our work is focused on the extending the vascular–tumour co-culture model to the study of the interaction between tumours and blood vessels.It was observed that the MDAs gradually migrated towards the EC channel spontaneously during the culture period, as shown in Fig. 5B. Eventually, the tumour cells reached the EC channels, migrating a total distance of 500 μm (Fig. 5C and D).
Although this model could not be completely generalized to the complex in vivo tumour microenvironment, it greatly shortened the gap between 2D monolayer culture and in vivo conditions.
This model can additionally be used as a preclinical tool for drug screening. We conducted a set of studies to apply the established model in the initial drug dose-reaction to mimic the in vivo endothelial barrier to drug candidates. Paclitaxel solutions of different concentrations were separately introduced into both the empty channels and the EC channels and then, through diffusion, affected the tumor cells in the neighboring parallel channels, as shown in Fig. 5E and F. The data revealed that the viability of the tumor cells decreased with the increase of the drug concentration, which was the same for both the empty channel conditions and the EC channel conditions. Furthermore, the presence of ECs blocked the effect of the drug to a certain degree, indicating a good barrier function, as shown in Fig. 5E–G.
A model of multi-step tumor development
Based on the parallel-channel model, we aimed to further simulate the tumour development process. The single endothelial channel was applied in the construction of a tumour development model for better biomimetic performance and for serving as a comparison between physical structure-induced and chemically-induced capillaries. Furthermore, the uniform vessel helps in better investigation of the exact interaction between tumours and blood vessels without random changes due to the structures.The development of a tumour occurs through a multi-step mechanism, and several critical processes (as shown in Fig. 6A) were reproduced based on the above parallel-channel model containing tumour stroma (Gel-Gel-MA hydrogel), chemical environment (endothelial growth factor), functional vascular system (EC channels) and tumour cells (MDA-MB-231).
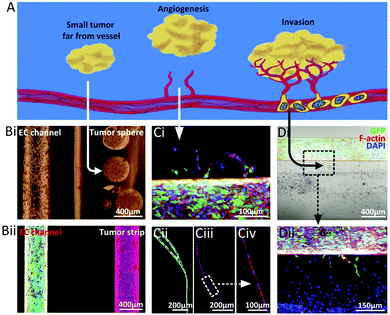
The first step is the formation of a primary tumour. This small tumour tissue is far from blood vessels and the nutrients required for tumour cell growth are supplied through penetration from the microenvironment of the tissue and organ. Tumour spheres and tumour strips located far from the EC channels were constructed to mimic the small primary tumour at an early stage, as shown in Fig. 6B.
The second step is angiogenesis and vascularization, which is the crucial requirement for tumor growth, invasion and metastasis. When the diameter of the tumor tissue reaches 1–2 mm, the nutrients provided by penetration cannot satisfy the growth of the tumor tissue. Tumour cells start to secrete angiogenesis factors to stimulate the growth and migration of vascular ECs, and capillaries gradually sprout from the original blood vessels, providing nourishment for tumour growth. Tumor cell-induced capillaries were observed on the established model, as shown in Fig. 6Ci. However, the degree of angiogenesis was limited by the hydrogel matrix properties, which could be well solved through the pre-construction of superfine channel structures, as shown in Fig. 6Cii. Compared with the chemically-induced capillaries, the physical structure-induced ones showed better integrity, continuity and customization. Subsequently, the tumor tissue gradually grew with the steady supply of nutrients from blood vessels.
The third step is tumour invasion. During the growth process, the tumour cells continuously secrete factors to promote the formation of new capillaries. At the same time, these abundant capillaries provide a basic way for tumour cells to enter the circulatory system. Malignant tumour cells are qualified for invasion into the blood vessel as long as they reach a newly-formed capillary. The arrival of the migrated tumour cells to the capillaries was observed in the above model, as shown in Fig. 6D, which is the prerequisite for the realization of the invasion process.
After that, tumour cells spread to a secondary tissue or organ through the vascular system and infiltration occurs before a secondary tumour forms.
During the whole process, the vascular system and the tumour tissue show a mutual improvement. On the one hand, the vascular system provides the necessary conditions for tumour growth and further metastasis. On the other hand, the tumour cells continuously secrete factors to promote the development of new capillaries. The proposed model for simulating tumour development is initially verified through a set of basic data which indicate the realization of the prerequisites for tumour-related studies, including angiogenesis, migration, and endothelial barrier function.
Discussion
The construction of a multi-scale functional vascular system is a fundamental prerequisite for the construction of in vitro models, especially reliable platforms for disease study and drug evaluation. In recent years, multiple studies have focused on the construction of vascular networks. In the present study, we have proposed a novel process for the construction of a multi-scale vascular model within a cell-laden hydrogel construct. By combining the multi-scale 3D printing process and twice-crosslinking strategy, we have demonstrated the possibility of engineering vascular models in various structures.In order to develop an effective tumor therapy, reliable tumor models for preclinical testing and screening are urgently demanded. However, challenges exist in the clinical translation of potential anti-cancer drugs and treatments due to the discrepancy between the existing models and in vivo conditions.86 2D monolayer tumour models87 have difficulty in recapitulating the native microenvironment, and cells are unable to mimic their natural behaviors and responses to anti-cancer drugs.76,88,89 On the other hand, animal experiments sometimes show false effects.90,91 To overcome these obstacles, a promising strategy is to develop preclinical in vitro 3D tumor models based on human tumour cells,79,80,88 which are supposed to be able to physically and chemically recapitulate the tumor physiological environment,92,93 and further be used to develop effective cancer therapy.94 However, the existing 3D in vitro tumor models are far from being able to mimic the complex tumor microenvironment. In the present study, we have demonstrated the versatility of this method through the construction of a series of vascularized tumour models, and they greatly narrow the gap between the 2D monolayer culture and the in vivo environment.88,95–97 We envision that the proposed new strategy and the established in vitro models can pave a new way and provide a new idea for the study of the interaction between tumours and blood vessels.
This vascular–tumour co-culture platform can also be adapted to other biological systems and will be used as a valuable tool to model the interaction between different cells/tissues and to control the microenvironment.
In addition, more types of cells, such as stroma cells, immune cells and stem cells, can further be introduced into the models,84 to conduct research on the interaction between different cells. These models have shown the potential to provide valuable insights into tumour development.
Conclusions
In summary, we have presented a universal strategy for the construction of a series of in vitro models with vascular structures in a flexible way, including a multi-scale hydrogel channel network to mimic the vascular system (from arteries to veins), a tumour tissue model with an inner vascular network, and a parallel-channel model containing a tumor tissue strip and an endothelial channel.For the multi-scale vascular construction, a set of experimental data showing the feasibility of the proposed method to construct a multi-scale vascular model containing both big blood vessels and capillaries, the directional effect of the capillary channel, and the excellent bio-compatibility of the proposed system have been displayed. Ubiquitous structures of biological vascular systems have been fabricated to demonstrate the versatility of this method.
Based on this method, a vascularized tumour tissue model exhibiting representative vascular functions under both physiological and pathological conditions has been constructed.
In our work, a parallel-channel model has been proposed for better quantitative analysis of the interaction between tumours and blood vessels, as well as the drug effects, which can further be used as a platform to investigate the metastasis process. Based on these data, we anticipate that this method can further be used as a significant preclinical tool for evaluating and screening novel cancer therapies.
Funding
This work was sponsored by the National Key Research and Development Program of China (2018YFA0703000), the National Natural Science Foundation of China (No. 51805474, 51622510, U1609207, 51605429), and the Science Fund for Creative Research Groups of National Natural Science Foundation of China (No. 51821093).Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge the Testing Center in Suzhou Intelligent Manufacturing Research Institute for SEM and confocal microscopy.Notes and references
- V. Mironov, T. Boland, T. Trusk, G. Forgacs and R. R. Markwald, Trends Biotechnol., 2003, 21, 157–161 CrossRef CAS.
- R. Lanza, R. Langer and J. P. Vacanti, Principles of tissue engineering, Academic Press, 2011 Search PubMed.
- H. Wang, Z. Zhao, Y. Liu, C. Shao, F. Bian and Y. Zhao, Sci. Adv., 2018, 4, eaat2816 CrossRef.
- B. Derby, Science, 2012, 338, 921–926 CrossRef CAS.
- E. S. Lippmann, S. M. Azarin, J. E. Kay, R. A. Nessler, H. K. Wilson, A. Al-Ahmad, S. P. Palecek and E. V. Shusta, Nat. Biotechnol., 2012, 30, 783 CrossRef CAS PubMed.
- R. K. Jain, P. Au, J. Tam, D. G. Duda and D. Fukumura, Nat. Biotechnol., 2005, 23, 821 CrossRef CAS.
- N. Asakawa, T. Shimizu, Y. Tsuda, S. Sekiya, T. Sasagawa, M. Yamato, F. Fukai and T. Okano, Biomaterials, 2010, 31, 3903–3909 CrossRef CAS.
- D. B. Kolesky, R. L. Truby, A. S. Gladman, T. A. Busbee, K. A. Homan and J. A. Lewis, Adv. Mater., 2014, 26, 3124–3130 CrossRef CAS PubMed.
- T. Kaully, K. Kaufman-Francis, A. Lesman and S. Levenberg, Tissue Eng., Part B, 2009, 15, 159–169 CrossRef CAS.
- H. Bae, A. S. Puranik, R. Gauvin, F. Edalat, B. Carrillo-Conde, N. A. Peppas and A. Khademhosseini, Sci. Transl. Med., 2012, 4, 160ps123 Search PubMed.
- A. Khademhosseini, J. P. Vacanti and R. Langer, Sci. Am., 2009, 300, 64–71 CrossRef CAS.
- J. Wang, L. Sun, M. Zou, W. Gao, C. Liu, L. Shang, Z. Gu and Y. Zhao, Sci. Adv., 2017, 3, e1700004 CrossRef.
- J. Yang, M. Yamato, T. Shimizu, H. Sekine, K. Ohashi, M. Kanzaki, T. Ohki, K. Nishida and T. Okano, Biomaterials, 2007, 28, 5033–5043 CrossRef CAS.
- B. Yuan, Y. Jin, Y. Sun, D. Wang, J. Sun, Z. Wang, W. Zhang and X. Jiang, Adv. Mater., 2012, 24, 890–896 CrossRef CAS.
- Y. Yamagishi, T. Masuda, M. Matsusaki, M. Akashi, U. Yokoyama and F. Arai, Biomicrofluidics, 2014, 8, 064113 CrossRef.
- H.-H. G. Song, R. T. Rumma, C. K. Ozaki, E. R. Edelman and C. S. Chen, Cell Stem Cell, 2018, 22, 340–354 CrossRef CAS.
- M. I. Santos, K. Tuzlakoglu, S. Fuchs, M. E. Gomes, K. Peters, R. E. Unger, E. Piskin, R. L. Reis and C. J. Kirkpatrick, Biomaterials, 2008, 29, 4306–4313 CrossRef CAS.
- Y. Yu, L. Shang, J. Guo, J. Wang and Y. Zhao, Nat. Protoc., 2018, 1 Search PubMed.
- N. Sadr, M. Zhu, T. Osaki, T. Kakegawa, Y. Yang, M. Moretti, J. Fukuda and A. Khademhosseini, Biomaterials, 2011, 32, 7479–7490 CrossRef CAS.
- B. Trappmann, B. M. Baker, W. J. Polacheck, C. K. Choi, J. A. Burdick and C. S. Chen, Nat. Commun., 2017, 8, 371 CrossRef PubMed.
- J. W. Nichol, S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar and A. Khademhosseini, Biomaterials, 2010, 31, 5536–5544 CrossRef CAS.
- T. J. Hinton, Q. Jallerat, R. N. Palchesko, J. H. Park, M. S. Grodzicki, H.-J. Shue, M. H. Ramadan, A. R. Hudson and A. W. Feinberg, Sci. Adv., 2015, 1, e1500758 CrossRef.
- W. Wu, A. DeConinck and J. A. Lewis, Adv. Mater., 2011, 23, H178–H183 CrossRef CAS.
- V. K. Lee, D. Y. Kim, H. Ngo, Y. Lee, L. Seo, S.-S. Yoo, P. A. Vincent and G. Dai, Biomaterials, 2014, 35, 8092–8102 CrossRef CAS PubMed.
- K. H. Song, C. B. Highley, A. Rouff and J. A. Burdick, Adv. Funct. Mater., 2018, 28, 1801331 CrossRef.
- B. S. Kim, G. Gao, J. Y. Kim and D. W. Cho, Adv. Healthcare Mater., 2019, 8, 1801019 CrossRef.
- E. A. Roth, T. Xu, M. Das, C. Gregory, J. Hickman and T. Boland, Biomaterials, 2004, 25, 3707–3715 CrossRef CAS.
- L. Zhao, V. K. Lee, S.-S. Yoo, G. Dai and X. Intes, Biomaterials, 2012, 33, 5325–5332 CrossRef CAS.
- A. B. Dababneh and I. T. Ozbolat, J. Manuf. Sci. Eng., 2014, 136, 061016 CrossRef.
- Q. Gao, Y. He, J.-Z. Fu, A. Liu and L. Ma, Biomaterials, 2015, 61, 203–215 CrossRef CAS.
- J. M. Grolman, D. Zhang, A. M. Smith, J. S. Moore and K. A. Kilian, Adv. Mater., 2015, 27, 5512–5517 CrossRef CAS PubMed.
- L. Ouyang, C. B. Highley, W. Sun and J. A. Burdick, Adv. Mater., 2017, 29, 1604983 CrossRef PubMed.
- G. Gao, J. H. Lee, J. Jang, D. H. Lee, J. S. Kong, B. S. Kim, Y. J. Choi, W. B. Jang, Y. J. Hong and S. M. Kwon, Adv. Funct. Mater., 2017, 27, 1700798 CrossRef.
- S. Suri, L.-H. Han, W. Zhang, A. Singh, S. Chen and C. E. Schmidt, Biomed. Microdevices, 2011, 13, 983–993 CrossRef CAS.
- R. Raman, B. Bhaduri, M. Mir, A. Shkumatov, M. K. Lee, G. Popescu, H. Kong and R. Bashir, Adv. Healthcare Mater., 2016, 5, 610–619 CrossRef CAS.
- R. Zhang and N. B. Larsen, Lab Chip, 2017, 17, 4273–4282 RSC.
- N. W. Choi, M. Cabodi, B. Held, J. P. Gleghorn, L. J. Bonassar and A. D. Stroock, Nat. Mater., 2007, 6, 908 CrossRef CAS.
- M. P. Cuchiara, A. C. Allen, T. M. Chen, J. S. Miller and J. L. West, Biomaterials, 2010, 31, 5491–5497 CrossRef CAS PubMed.
- Y. Zheng, P. W. Henderson, N. W. Choi, L. J. Bonassar, J. A. Spector and A. D. Stroock, Biomaterials, 2011, 32, 5391–5401 CrossRef CAS PubMed.
- L. S. Wray, K. Tsioris, E. S. Gi, F. G. Omenetto and D. L. Kaplan, Adv. Funct. Mater., 2013, 23, 3404 CrossRef CAS.
- J. He, R. Chen, Y. Lu, L. Zhan, Y. Liu, D. Li and Z. Jin, Mater. Sci. Eng., C, 2016, 59, 53–60 CrossRef CAS PubMed.
- J. Nie, Q. Gao, Y. Wang, J. Zeng, H. Zhao, Y. Sun, J. Shen, H. Ramezani, Z. Fu and Z. Liu, Small, 2018, 14, 1802368 CrossRef.
- S. Paulsen and J. Miller, Dev. Dyn., 2015, 244, 629–640 CrossRef CAS.
- H. Sekine, T. Shimizu, K. Sakaguchi, I. Dobashi, M. Wada, M. Yamato, E. Kobayashi, M. Umezu and T. Okano, Nat. Commun., 2013, 4, 1399 CrossRef.
- J. M. Isner and T. Asahara, J. Clin. Invest., 1999, 103, 1231–1236 CrossRef CAS PubMed.
- K. Y. Lee, M. C. Peters, K. W. Anderson and D. J. Mooney, Nature, 2000, 408, 998 CrossRef CAS PubMed.
- T. P. Richardson, M. C. Peters, A. B. Ennett and D. J. Mooney, Nat. Biotechnol., 2001, 19, 1029 CrossRef CAS.
- X. Chen, A. S. Aledia, C. M. Ghajar, C. K. Griffith, A. J. Putnam, C. C. Hughes and S. C. George, Tissue Eng., Part A, 2008, 15, 1363–1371 CrossRef PubMed.
- S. Rafii and D. Lyden, Nat. Med., 2003, 9, 702 CrossRef CAS.
- D. Xue, Y. Wang, J. Zhang, D. Mei, Y. Wang and S. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 19428–19435 CrossRef CAS.
- G. Burton, A. Woods, E. Jauniaux and J. Kingdom, Placenta, 2009, 30, 473–482 CrossRef CAS.
- R. Pijnenborg, L. Vercruysse and M. Hanssens, Placenta, 2006, 27, 939–958 CrossRef CAS.
- K. J. Woollard and F. Geissmann, Nat. Rev. Cardiol., 2010, 7, 77 CrossRef PubMed.
- F. A. Jaffer, P. Libby and R. Weissleder, Arterioscler., Thromb., Vasc. Biol., 2009, 29, 1017–1024 CrossRef CAS PubMed.
- S. Knowlton, S. Onal, C. H. Yu, J. J. Zhao and S. Tasoglu, Trends Biotechnol., 2015, 33, 504–513 CrossRef CAS.
- C. Fischbach and D. J. Mooney, Biomaterials, 2007, 28, 2069–2076 CrossRef CAS.
- D. W. McMillin, J. Delmore, E. Weisberg, J. M. Negri, D. C. Geer, S. Klippel, N. Mitsiades, R. L. Schlossman, N. C. Munshi and A. L. Kung, Nat. Med., 2010, 16, 483 CrossRef CAS.
- X. Dai, C. Ma, Q. Lan and T. Xu, Biofabrication, 2016, 8, 045005 CrossRef.
- Y. Chen, D. Gao, H. Liu, S. Lin and Y. Jiang, Anal. Chim. Acta, 2015, 898, 85–92 CrossRef CAS.
- C. L. Chaffer and R. A. Weinberg, Science, 2011, 331, 1559–1564 CrossRef CAS PubMed.
- N. Reymond, B. B. d'Agua and A. J. Ridley, Nat. Rev. Cancer, 2013, 13, 858 CrossRef CAS.
- J. S. Jeon, I. K. Zervantonakis, S. Chung, R. D. Kamm and J. L. Charest, PLoS One, 2013, 8, e56910 CrossRef CAS.
- D. Richards, J. Jia, M. Yost, R. Markwald and Y. Mei, Ann. Biomed. Eng., 2017, 45, 132–147 CrossRef PubMed.
- P. Bao, A. Kodra, M. Tomic-Canic, M. S. Golinko, H. P. Ehrlich and H. Brem, J. Surg. Res., 2009, 153, 347–358 CrossRef CAS PubMed.
- Q. Zhang, T. Liu and J. Qin, Lab Chip, 2012, 12, 2837–2842 RSC.
- M. B. Chen, J. A. Whisler, J. Fröse, C. Yu, Y. Shin and R. D. Kamm, Nat. Protoc., 2017, 12, 865 CrossRef CAS PubMed.
- J. H. Jeong, V. Chan, C. Cha, P. Zorlutuna, C. Dyck, K. J. Hsia, R. Bashir and H. Kong, Adv. Mater., 2012, 24, 58–63 CrossRef CAS.
- D. Hanahan and R. A. Weinberg, Cell, 2000, 100, 57–70 CrossRef CAS.
- G. P. Gupta and J. Massagué, Cell, 2006, 127, 679–695 CrossRef CAS PubMed.
- E. T. Roussos, J. S. Condeelis and A. Patsialou, Nat. Rev. Cancer, 2011, 11, 573 CrossRef CAS.
- J. S. Jeon, S. Bersini, M. Gilardi, G. Dubini, J. L. Charest, M. Moretti and R. D. Kamm, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, E818 CrossRef.
- I. K. Zervantonakis, S. K. Hughes-Alford, J. L. Charest, J. S. Condeelis, F. B. Gertler and R. D. Kamm, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 13515–13520 CrossRef.
- Z. Du, S. Mi, X. Yi, Y. Xu and W. Sun, Biofabrication, 2018, 10, 034102 CrossRef.
- B. R. Seo, P. DelNero and C. Fischbach, Adv. Drug Delivery Rev., 2014, 69, 205–216 CrossRef PubMed.
- Y. Zhao, Z. Xie, H. Gu, C. Zhu and Z. Gu, Chem. Soc. Rev., 2012, 41, 3297–3317 RSC.
- C. K. Griffith, C. Miller, R. C. Sainson, J. W. Calvert, N. L. Jeon, C. C. Hughes and S. C. George, Tissue Eng., 2005, 11, 257–266 CrossRef CAS.
- H. Lodish, J. E. Darnell, A. Berk, C. A. Kaiser, M. Krieger, M. P. Scott, A. Bretscher, H. Ploegh and P. Matsudaira, Molecular cell biology, Macmillan, 2008 Search PubMed.
- C. Fischbach, R. Chen, T. Matsumoto, T. Schmelzle, J. S. Brugge, P. J. Polverini and D. J. Mooney, Nat. Methods, 2007, 4, 855 CrossRef CAS PubMed.
- G. Rijal and W. Li, Sci. Adv., 2017, 3, e1700764 CrossRef.
- X. Tian, M. E. Werner, K. C. Roche, A. D. Hanson, H. P. Foote, K. Y. Stephanie, S. B. Warner, J. A. Copp, H. Lara and E. L. Wauthier, Nat. Biomed. Eng., 2018, 2, 443 CrossRef CAS.
- G. Y. Lee, P. A. Kenny, E. H. Lee and M. J. Bissell, Nat. Methods, 2007, 4, 359 CrossRef CAS.
- G. Ying, N. Jiang, C. Yu and Y. S. Zhang, Bio-des. Manuf., 2018, 1, 215–224 CrossRef CAS.
- R. Kalluri and M. Zeisberg, Nat. Rev. Cancer, 2006, 6, 392 CrossRef CAS PubMed.
- J. A. Joyce and J. W. Pollard, Nat. Rev. Cancer, 2009, 9, 239 CrossRef CAS.
- Y. Zhao, H. Gu, Z. Xie, H. C. Shum, B. Wang and Z. Gu, J. Am. Chem. Soc., 2012, 135, 54–57 CrossRef.
- J. Vanderburgh, J. A. Sterling and S. A. Guelcher, Ann. Biomed. Eng., 2017, 45, 164–179 CrossRef.
- K. K. Van Rompay, Antiviral Res., 2010, 85, 159–175 CrossRef CAS.
- F. Pampaloni, E. G. Reynaud and E. H. Stelzer, Nat. Rev. Mol. Cell Biol., 2007, 8, 839 CrossRef CAS PubMed.
- L. G. Griffith and M. A. Swartz, Nat. Rev. Mol. Cell Biol., 2006, 7, 211 CrossRef CAS PubMed.
- I. Levinger, Y. Ventura and R. Vago, Advances in cancer research, Elsevier, 2014, vol. 121, pp. 383–414 Search PubMed.
- D. Huh, G. A. Hamilton and D. E. Ingber, Trends Cell Biol., 2011, 21, 745–754 CrossRef CAS PubMed.
- D. Huh, B. D. Matthews, A. Mammoto, M. Montoya-Zavala, H. Y. Hsin and D. E. Ingber, Science, 2010, 328, 1662–1668 CrossRef CAS.
- Š. Selimović, M. R. Dokmeci and A. Khademhosseini, Curr. Opin. Pharmacol., 2013, 13, 829–833 CrossRef.
- C. Wang, Z. Tang, Y. Zhao, R. Yao, L. Li and W. Sun, Biofabrication, 2014, 6, 022001 CrossRef.
- S. Bian, M. Repic, Z. Guo, A. Kavirayani, T. Burkard, J. A. Bagley, C. Krauditsch and J. A. Knoblich, Nat. Methods, 2018, 15, 631 CrossRef CAS PubMed.
- L. Hutchinson and R. Kirk, Nat. Rev. Clin. Oncol., 2011, 8, 189–190 CrossRef PubMed.
- Q. Gao, P. Zhao, R. Zhou, P. Wang, J. Fu and Y. He, Bio-des. Manuf., 2019, 2, 1–9 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9mh01283d |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2020 |