Is there such a thing as a molecular organic alloy?
Xinjing
Huang†
a,
Xiao
Liu†
 b,
Kan
Ding
c and
Stephen R.
Forrest
*abcd
b,
Kan
Ding
c and
Stephen R.
Forrest
*abcd
aApplied Physics Program, University of Michigan, Ann Arbor, MI 48109, USA. E-mail: stevefor@umich.edu
bDepartment of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA
cDepartment of Physics, University of Michigan, Ann Arbor, MI 48109, USA
dDepartment of Material Science and Engineering University of Michigan, Ann Arbor, MI 48109, USA
First published on 10th September 2019
Abstract
In inorganic materials, an alloy is a mixture of two or more substances that generally exhibits electronic and/or physical properties that differ from those of its constituents. In organic systems, the formation of a “molecular alloy” comprising mixtures of molecular organic materials has also been proposed. We test the validity of this concept via the study of the optoelectronic properties of a ternary system that has previously been identified to form a molecular acceptor alloy, namely a blend of a poly(3-hexylthiophene) (P3HT) donor, with two acceptors indene-C60 bisadduct (ICBA) and phenyl-C61-butyric acid methyl ester (PC61BM) [R. A. Street, et al., J. Am. Chem. Soc., 2013, 135, 986–989]. Using photoelectron spectroscopy, we find that the ICBA:PC61BM blend shows the same highest occupied molecular orbital and exciton energies as that of ICBA, indicating the absence of a new exciton state in the blend. Furthermore, charge transfer state spectra of ternary blends are found to comprise a simple linear superposition of the corresponding binaries. From these results, no evidence of new, emergent electronic states is found to support the existence of a molecular alloy in this system. To our knowledge there is as yet no clear evidence of the existence of an alloy in any organic semiconductor system. We discuss the criteria that should be met by a molecular organic alloy and procedures needed for their unambiguous identification.
New conceptsFor 30+ years, the concept of molecular alloy has been used to explain a variety of phenomena in organic materials from doping in OLEDs, to charge transfer salts. Recently, the term has been used in the context of charge transfer in ternary mixtures of donor and acceptor molecules in organic solar cells. However, there is no coherent or consistent definition of what, exactly, is a molecular alloy. In this paper we describe the conventional definition of an alloy, and then proceed to set forth criteria that, if met, would clearly define the presence of such behavior in molecular organic solids. We then apply these criteria, as an example, to the analysis of a recently published ternary mixture found in organic solar cells. We find no evidence for alloy formation for this particular example ternary mixture. To broaden our discussion from this archetype substance, we apply our criteria to a variety of molecular systems including charge transfer salts, polymer eutectics, heavily conductivity-doped organic films, and so on. None appear to show clear alloy behavior. Yet we do not rule out the possibility that such will be found in the future, but rather suggest means for identifying these potentially interesting materials. |
Introduction
An alloy is commonly understood to be a physical mixture of two or more constituents with variable proportions that exhibits electronic and/or physical properties different from its components.1 Common examples of metal alloys are steel (C + Fe) or brass (Zn + Cu). Binary semiconductors such as SixGe1−x, ternary semiconductors such as InxGa1−xAs, and other quaternary and quintenary inorganic semiconductors are also commonly referred to as alloys.1 All of these materials have optical and electrical properties that are distinct from the substances of which they are comprised. The physical properties (i.e. Young's moduli, melting points, etc.) are also different in most alloy mixtures than in their constituents.2 Their characteristics derive almost entirely from the strong chemical bonds formed between atoms in the mixtures.In contrast to inorganic alloys comprising atomic constituents, organic materials are bonded by far weaker, electrostatic van der Waals forces. It has been proposed that in some cases these weakly bonded organic mixtures form a new substance which is called a “molecular alloy”. One potential class of molecular alloys are charge transfer (CT) salts and their solid solutions,3–7 another are organic eutectics.8–12 The formation of molecular alloys has also been claimed to be observed in disordered organic solid solutions.13 Recently, ternary blends in the active regions of organic photovoltaic (OPV) cells and so-called organic co-crystals have been identified to form organic alloys.14–23 Although there has been frequent mention of molecular organic alloys over many decades, to our knowledge no uniform framework has been developed to clearly define what is meant by this term, and how an organic alloy differs from chemically bonded mixtures whose electronic, optical and physical properties are not a mere extrapolation of those of the individual constituents.
A chemical bond between organic molecules commonly results in an entirely new compound, not an alloy mixture. Given the absence of chemical bonds between molecules, the correspondence between a molecular organic alloy and alloys found in metals and inorganic semiconductors admittedly cannot be precise. The weak and electrostatic nature of van der Waals forces in molecular alloys as compared with chemical bonds in atomic alloys implies that the term alloy, itself, will have a different meaning for mixtures in these different systems. To clarify these substantially different definitions, we propose that a molecular alloy should meet the following criteria:
1. It should be a physical mixture of two or more species of organic molecules with no chemical reaction occurring during mixture formation. The proportion of constituents can vary over a wide range.
2. It should have electron orbital interactions between different molecular species that result in modifications of electronic states that cannot be ascribed to a simple linear superposition of those inherent to the constituents.
3. The orbital interactions should lead to electronic or physical properties clearly distinguishable from those of its constituents.
Note that electron transfer between molecules needs special attention relative to Criterion 2. For example, the electron transfer in a donor:acceptor blend is driven by the energy offset between the corresponding states in the participating molecules. This does not meet the requirements of Criterion 2 if the inherent properties of the electronic states involved are left unchanged by the transfer process.
The changes in electronic or physical properties mentioned in Criterion 3 are often observed. Specifically, changes in electronic properties have been used to claim the formation of a molecular organic alloy.3,5,6,14–18,22 Also, changes in physical properties (e.g. eutectic formation) may indicate the presence of an alloy.9–11 These changes, too, are often the result of electronic interactions, although prior studies have not provided evidence that the other two criteria are also fulfilled.
In this work, we investigate a ternary mixture that forms the active region of an organic photovoltaic (OPV) cell that has previously been claimed to be an organic alloy.14 The ternary system comprises a donor (D) poly(3-hexylthiophene) (P3HT), blended with two acceptors indene-C60 bisadduct (ICBA, A1), and phenyl-C61-butyric acid methyl ester (PC61BM, A2).14,24 Evidence for the existence of an alloy between the two acceptors was based on the observed dependence of the CT state absorption energy and the open circuit voltage (VOC) of the OPV on the blend ratio. Although these composition-dependent electronic properties may suggest the formation of an organic alloy, investigation of the origins of the observed changes are needed to identify whether or not they fully meet the three criteria discussed above. Here we provide a detailed investigation of the highest occupied molecular orbital (HOMO), the Frenkel exciton, and CT states of this system. The various electronic states of the blends are found to be linear superpositions of those of the constituents, without evidence for the emergence of new electronic properties that are expected for an organic alloy. Based on these findings and those in other such reports, we provide a set of criteria that should be met when characterizing a molecular material mixture as an alloy. To our knowledge, there is as yet no definitive identification of such a substance that unambiguously meet these criteria.
Results
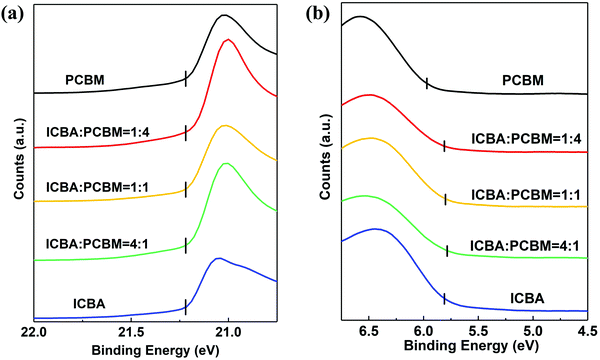
Fig. 1 shows the ultraviolet photoelectron spectra (UPS) of the two acceptors and their blends in the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The short vertical lines in Fig. 1(a) indicate the emission cutoff extracted from the spectra due to the 21.2 eV He–I transition. Fig. 1(b) shows the low-binding-energy region of the spectra. The short vertical lines show that the HOMO energy for ICBA and the blends is at −5.8 ± 0.1 eV (referenced to vacuum), and for PC61BM the HOMO energy is at −6.0 ± 0.1 eV.
1. The short vertical lines in Fig. 1(a) indicate the emission cutoff extracted from the spectra due to the 21.2 eV He–I transition. Fig. 1(b) shows the low-binding-energy region of the spectra. The short vertical lines show that the HOMO energy for ICBA and the blends is at −5.8 ± 0.1 eV (referenced to vacuum), and for PC61BM the HOMO energy is at −6.0 ± 0.1 eV.
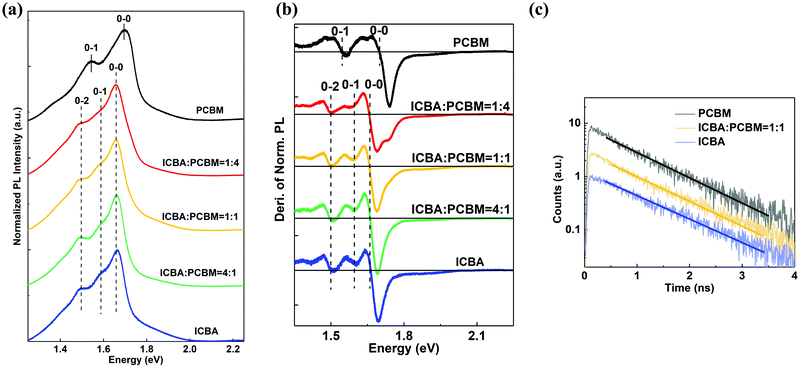
Photoluminescence (PL) was used to study the exciton states of individual acceptors and blends, with the results shown in Fig. 2. The normalized steady-state PL spectra are provided in Fig. 2(a). The dominant 0–0 transition peaks have energies of 1.66 ± 0.01 eV for ICBA, 1.70 ± 0.01 eV for PC61BM, and 1.66 ± 0.01 eV for all of the blends. Also, ICBA and the blends show 0–1 transition emission at 1.60 ± 0.01 eV, and 0–2 transition peaks at 1.51 ± 0.01 eV. PC61BM has its 0–1 transition at 1.55 ± 0.01 eV. Fig. 2(b) presents the first derivatives of the normalized PL spectra to more accurately determine the peak positions. The data indicate that all acceptor blends have the same peak positions as that of ICBA. To further explore whether there exists a new excitonic state, the dynamics of the exciton state recombination were studied using time-resolved PL, with results shown in Fig. 2(c). The transients are fit by a single exponential decay shown by solid lines, yielding exciton lifetimes of τ = 0.98 ± 0.04 ns for ICBA, τ = 0.94 ± 0.04 ns for PC61BM, and τ = 0.95 ± 0.04 ns for the blend. All of these decay rates are equal to within experimental accuracy.
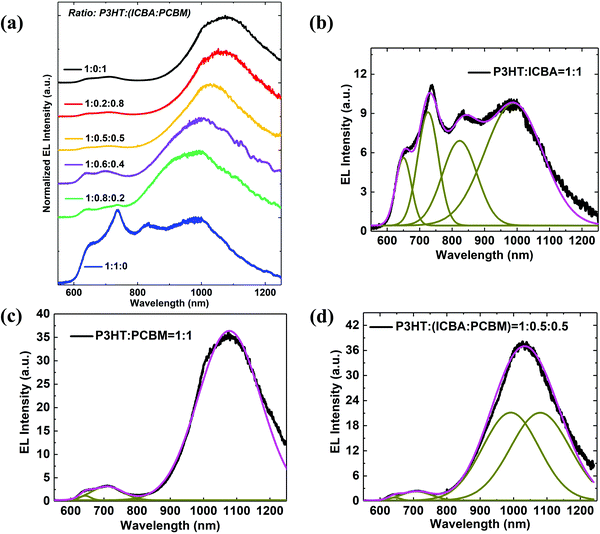
The electroluminescence (EL) spectra of ternary OPVs with different blend ratios are shown Fig. 3(a). The CT state emission peak monotonically blue shifts from 1080 nm to 990 nm as the proportion of ICBA in the two acceptors increases from 0 to 100%. To understand this hypsochromic shift, we used a series of Gaussians to fit the EL spectra. As shown in Fig. 3(b), the EL spectrum of the DA1 binary is fit using a P3HT exciton peak at 646 nm, ICBA exciton peaks at 725 nm and 823 nm, and the DA1 binary CT state at 990 nm with a full width at half maximum of FWHM = 205 nm. Fig. 3(c) is the EL spectrum of the DA2 binary fit assuming a P3HT exciton peak at 645 nm, and of PC61BM at 708 nm. Then the DA2 binary CT state is at 1080 nm with FWHM = 215 nm. Fig. 3(d) is an example of the Gaussian fit to the EL spectrum of the ternary DA1A2 with ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0.5
(0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5). It has the same exciton peak positions of P3HT and PC61BM. The CT emission peak comprising the two binaries is fit by their linear superposition with peaks at 990 nm and 1080 nm, and with FWHM = 205 nm and 215 nm, respectively.
0.5). It has the same exciton peak positions of P3HT and PC61BM. The CT emission peak comprising the two binaries is fit by their linear superposition with peaks at 990 nm and 1080 nm, and with FWHM = 205 nm and 215 nm, respectively.
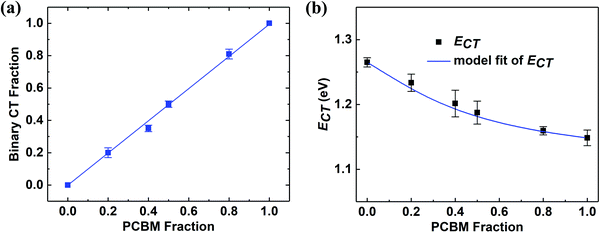
A similar fitting procedure is applied to DA1A2 junctions of the same composition but with different acceptor blend ratios. The intensities of the DA1 and DA2 CT emission are found from the areas under the corresponding Gaussian distributions. In Fig. 4(a), the ordinate is the fraction of DA2 CT state emission intensity, CT(DA2), in the total ternary CT emission, CT(DA1) + CT(DA2). The abscissa is the fraction of PC61BM (A2) in the acceptor mixture (A1 + A2). We find a linear relationship between CT(DA2) and A2 blend fraction with unity slope and an intercept at the origin. Fig. 4(b) shows CT state energy (ECT) vs. blend ratio. The blue solid line represents the simulated peak position found from the sum of two Gaussians centered at ECT1 = 1.15 eV and ECT2 = 1.25 eV, corresponding to the two binary CT state energies. The peak position is plotted as a function of the intensity fraction, which is the same as PC61BM fraction as illustrated in Fig. 4(a).
Discussion
Analysis of the UPS data in Fig. 1 indicates that the HOMO levels of the acceptor blends are the same as that of ICBA, independent of the acceptor blend ratio, with no evidence found for the emergence of new ground state. The steady-state PL data in Fig. 2(a) and (b) show that the exciton energies of the acceptor blends are also the same as for ICBA, nor does the exciton lifetime extracted from time-resolved PL in Fig. 2(c) depend on the blend ratio. Thus, the excited states in the acceptor blends also do not appear to be influenced by the mixture composition.The CT state EL spectrum in Fig. 3 for a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0.5
(0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) DA1A2 blend does not exhibit spectral features independent of those of the individual binaries. In addition, the CT emission of the ternary blends is a linear superposition of the binary spectra whose intensities are proportional to their concentration in the blends, see Fig. 4(a).
0.5) DA1A2 blend does not exhibit spectral features independent of those of the individual binaries. In addition, the CT emission of the ternary blends is a linear superposition of the binary spectra whose intensities are proportional to their concentration in the blends, see Fig. 4(a).
In the less clearly resolved absorption spectral analysis of Street, et al.,14 it was found that the CT peak position of the blend follows Vegard's law via:
| ECT = (1 − x)ECT1 + xECT2 − bx(1 − x) |
Since the totality of our measurements show no evidence for the emergence of new electronic states, we conclude that the properties of the P3HT:ICBA:PC61BM active region are explained by the linear superposition of the characteristics of the two constituent heterojunctions. Hence, we can eliminate, at least in this case, the existence of a molecular alloy.
One exogenous cause for the lack of evidence for alloying in P3HT:ICBA:PC61BM blends based on our three criteria may be phase separation between the two acceptor components. That is, if separate domains of P3HT:ICBA and P3HT:PC61BM are formed in the blend, the opportunity for electronic interactions between the two acceptors would not exist, thus explaining why no alloys are detected. However, similar chemical structures and thin film surface energies of ICBA (24.9 mN m−1) and PC61BM (27.6 mN m−1) suggest the two molecules are highly miscibile.25 Furthermore, detailed nuclear magnetic resonance studies of the acceptor and ternary blends show shifts of the 1H and 13C resonance frequencies of the two acceptors compared to their individual spectra. This is attributed to homogeneous, random contacts between acceptors in the blends, indicating a lack of phase separation.26 Hence, at least in this particular system, the lack of evidence for an alloy cannot be attributed to spatial separation of the two donor:acceptor constituents in the blend.
The forgoing characterization illustrates a general procedure by which to identify the existence of an alloy. Based on the criteria stated in the introduction, the first step is to measure and compare the electronic states of the organic constituents and mixtures. If the electronic states of the mixtures are unchanged from those in the constituents, no alloy has been formed. If a difference is found, other reasons should be considered and if possible excluded, such as a linear superposition of electronic states of the constituents, aggregation of one molecular species when introducing another or other morphology changes,27 and trap-state filling,28etc. The final identification of a molecular alloy can be made after this procedure is complete.
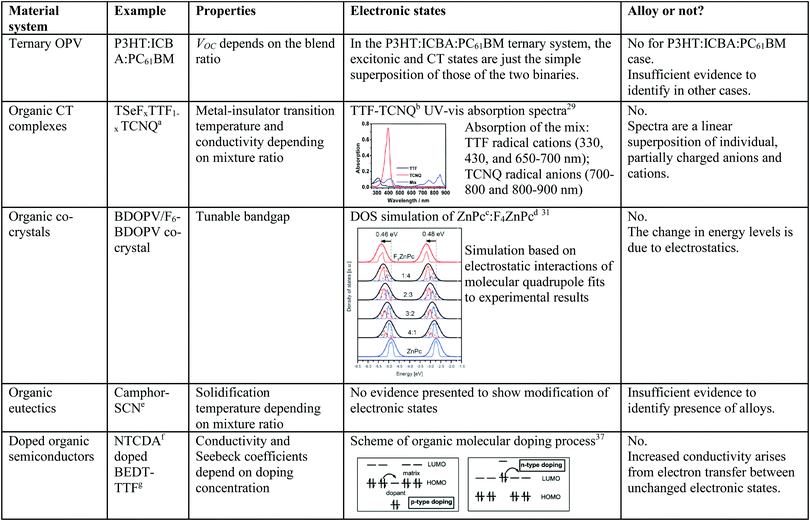
With these criteria in mind, we can attempt to evaluate other possible molecular alloy systems. Table 1 is a summary of some of the organic mixtures that have previously been described as alloys. Organic CT salt complexes and their solid solutions are frequently mentioned as representing a class of organic alloys.3–7 The absorption and Raman spectra of organic CT salts are composed of the superposition of the spectra of the individual anionic and cationic states.4,5,7,29 This suggests that even though there is substantial charge transfer between constituents of the salts, the result is again a simple mixture of anions and cations that do not form alloys whose properties differ from those of the partially reduced or oxidized constituents.
| a Tetraselenafulvalene (TSeF)–tetrathiafulvalene (TTF)–tetracyano-p-quinodimethane (TCNQ). b Tetrathiafulvalene (TTF)–tetracyano-p-quinodimethane (TCNQ). c Zinc phthalocyanine. d Tetrafluoro-zinc phthalocyanine. e Succinonitrile. f Naphthalenetetracarboxylic dianhydride. g Bis-ethylenedithio-tetrathiafulvalene. | ||||
|---|---|---|---|---|
|
Another materials class with potential to form alloys are organic co-crystals, defined as a single phase material comprising two or more molecular organic species.22,23,30 Although some co-crystals appear to have tunable energy levels such as halogenated benzodifurandione-based oligo(p-phenylene vinylene) (BDOPV)-based small molecules,22 the origin of this energy level change is due only to electrostatic interactions between molecular dipoles or higher order multipoles.31,32 Note that in this situation it is important to distinguish electrostatic interactions from intermolecular orbital interactions. For example, simulations based on electrostatic interactions and its consistency with experimental results31 can provide evidence as to whether electrostatic effects or alloying is responsible for the modification in electronic states. Furthermore, organic eutectics are materials that have phase transition temperatures dependent on the relative proportion of their constituents.8–12 This distinct physical property is similar to inorganic alloys and may, indeed, indicate formation of an organic alloy. But the evidence thus far presented has not shown intermolecular electronic coupling. Hence, it is inconclusive that these mixtures are true alloys.
Doped organic semiconductors whose conductivities and Seebeck coefficients depend on doping concentration have also been studied as possible examples of alloys.33–37 Doping with donors or acceptors results in the charge transfer between electronic states from the dopant to the host.36,37 Even at the high molar densities typically used in highly doped organics (∼0.1 molar ratio of dopant to host), new electronic states have not been reported. Other organic mixtures that do not belong to these categories have also been studied,13,38,39 including disordered organic solid solutions and binary helicies, yet once again no evidence has been presented to support the claim of a modification of the parent electronic states. To our understanding, therefore, there is yet no complete evidence to identify electronic coupling that would justify unambiguous identification of a molecular alloy in any of these previously reported systems.
Conclusion
In this work we propose criteria that must be met to unambiguously identify a molecular organic alloy: that is, a mixture of two or more molecular organic substances that have intermolecular electron coupling that results in modification of the electronic states of the constituent molecules. Specifically, we investigated the P3HT:(ICBA:PC61BM) ternary system which has previously been proposed to form an alloy between the two acceptors in the blend. The HOMO levels and the exciton energies of the acceptor blends are the same as for the individual components. Furthermore, the CT states in the ternary blends of varying acceptor ratios are a linear superposition of the two distinct binary CT states. According to our criteria, this ternary system is simply a combination of two binary heterojunctions that do not form an organic molecular alloy. Using our procedure to identify a molecular organic alloy, no clear example has yet been found for the existence of such a material.Methods
The ICBA/PC61BM acceptor blends with ICBA weight fractions of 0, 0.2, 0.5, 0.8, 1 were dissolved in chlorobenzene (CB) at total concentration of 20 mg mL−1 and stirred overnight at 60 °C at 300 rpm. The solutions were then spin-coated at 1000 rpm for 60 s on 150 nm thick indium tin oxide (ITO) on glass substrates to form 90–100 nm thick acceptors films for UPS measurements. The films were thermally annealed at 150 °C for 60 min, 20 min, 50 min, 20 min, and 10 min, respectively. The measurements were done in an ultrahigh vacuum chamber (base pressure < 1 × 10−9 Torr) using the 21.22 eV He–I gas-discharge lamp emission. The spectra were collected by a hemispherical electron energy analyzer (Thermal VG) with a pass function FWHM = 0.16 eV.The acceptor films were similarly prepared for PL measurements on quartz substrates. The samples were excited in low vacuum at wavelength λ = 422 nm using a continuous wave He–Cd laser to obtain steady-state PL. The spectra were collected using a fiber-coupled monochromator (Princeton Instruments SP-2300i) equipped with a Si charge-coupled device (CCD) (PIXIS:400). For time-resolved PL measurements, the samples were excited at λ = 480 nm using 150 fs pulses at a 1 kHz repetition rate from a Ti:sapphire laser (Clark-MXR CPA series) pumped optical parametric amplifier (TOPAS-C). The photon counts were measured using a time-correlated single photon counter (PicoHarp 300) coupled to a Si single photon avalanche detector (PDM series).
The OPV devices were grown on a solvent cleaned, 145 nm thick film of ITO, patterned into 1 mm wide stripes on a glass substrate. The ternary blends comprising P3HT:(ICBA:PC61BM) of different acceptor weight ratios were separately prepared in CB at total concentration 20 mg mL−1 and stirred overnight at 60 °C at 300 rpm. A 45 nm thick layer of poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) (PEDOT:PSS) (CLEVIOS™ P VP AI 4083, filtered with a Whatman™ 0.45 μm nylon filter w/glass micro fiber) was first spin-coated on the ITO and baked at 150 °C for 30 min. Subsequently, the P3HT:(ICBA:PC61BM) active layer was spin-coated in an ultrapure nitrogen environment (O2 < 0.1 ppm, H2O < 0.1 ppm) on top of the PEDOT:PSS at 1000 rpm for 60 s to form a 90–100 nm thick film. Then the samples were transferred into a high vacuum chamber (base pressure ∼10−7 Torr) for Al thermal evaporation at 1 Å s−1 to form a 100 nm thick film. The samples were transferred back to the nitrogen environment for thermal annealing at 150 °C. The thermal annealing time was 10 min for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 20 min for 1
0, 20 min for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 and 1
0.8 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 40 min for 1
0.2, 40 min for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4, 50 min for 1
0.4, 50 min for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 60 min for 1
0.5 and 60 min for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.24 The charge transfer state EL spectra were measured under 4 V forward-bias, collected using a fiber-coupled monochromator equipped with a Si CCD and an InGaAs photoreceiver with sensitivity from λ = 800 to 1700 nm (Newport Model 2153). The EL spectra were fit with Gaussian distributions using OriginPro 2017. The VOC were measured in a glovebox filled with nitrogen (O2 < 0.1 ppm, H2O < 0.1 ppm), using a solar simulator with a 300 W Xe lamp with an AM1.5G filter, whose 1 sun intensity was calibrated with a National Renewable Energy Laboratory-traceable Si reference cell.
1.24 The charge transfer state EL spectra were measured under 4 V forward-bias, collected using a fiber-coupled monochromator equipped with a Si CCD and an InGaAs photoreceiver with sensitivity from λ = 800 to 1700 nm (Newport Model 2153). The EL spectra were fit with Gaussian distributions using OriginPro 2017. The VOC were measured in a glovebox filled with nitrogen (O2 < 0.1 ppm, H2O < 0.1 ppm), using a solar simulator with a 300 W Xe lamp with an AM1.5G filter, whose 1 sun intensity was calibrated with a National Renewable Energy Laboratory-traceable Si reference cell.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Dr Yongxi Li, Dr Quinn Burlingame and Boning Qu for valuable discussions. This work was supported by Office of Naval Research Grant No. N00014-17-1-2211 and the National Science Foundation, Grant No. DMR 1905401.References
- S. Adachi, Properties of Semiconductor Alloys: Group-IV, III–V and II–VI Semiconductors, Wiley, 2009 Search PubMed.
- J. R. Davis and R. Joseph, Alloying: Understanding the Basics, ASM International, 2001 Search PubMed.
- E. M. Engler, B. A. Scott, S. Etemad, T. Penney and V. V. Patel, Organic Alloys: Synthesis and Properties of Solid Solutions of Tetraselenafulvalene-Tetracyano-p-Quinodimethane (TSeF-TCNQ) and Tetrathiafulvalene-Tetracyano-p-Quinodimethane (TTF-TCNQ), J. Am. Chem. Soc., 1977, 99(18), 5909–5916, DOI:10.1021/ja00460a011.
- T. Murata, X. Shao, Y. Nakano, H. Yamochi, M. Uruichi, K. Yakushi, G. Saito and K. Tanaka, Tuning of Multi-Instabilities in Organic Alloy, [(EDO-TTF)1−x(MeEDO-TTF)x]2PF6, Chem. Mater., 2010, 22(10), 3121–3132, DOI:10.1021/cm100051b.
- K. Kubo and M. Yamashita, New BEDT-TTF Radical Cation Salt with Mixed Anions: α’-[BEDT-TTF]2[CuBr2]0.4[CuCl2]0.6, Crystals, 2012, 2(2), 284–293, DOI:10.3390/cryst2020284.
- T. Ishikawa, Y. Urasawa, T. Shindo, Y. Okimoto, S. Koshihara, S. Tanaka, K. Onda, T. Hiramatsu, Y. Nakano and K. Tanaka, et al., Optical Study of Electronic Structure and Photoinduced Dynamics in the Organic Alloy System [(EDO-TTF)0.89(MeEDO-TTF)0.11]2PF6, Appl. Sci., 2019, 9(6), 1174, DOI:10.3390/app9061174.
- T. Murata, X. Shao, Y. Nakano, H. Yamochi, G. Saito, M. Uruichi, K. Yakushi and K. Tanaka, Metal-Insulator Transition of Alloyed Radical Cation Salts, (Me XEDO-TTF)2PF6, Phys. B, 2010, 405(11 SUPPL), 45–48, DOI:10.1016/j.physb.2009.12.042.
- U. S. Rai and S. George, Physicochemical studies on organic eutectics and the 1 : 1 addition compound: benzidine-α-naphthol system, J. Mater. Sci., 1992, 27, 711–718 CrossRef CAS.
- L. Sturz, V. T. Witusiewicz, U. Hecht and S. Rex, Organic Alloy Systems Suitable for the Investigation of Regular Binary and Ternary Eutectic Growth, J. Cryst. Growth, 2004, 270(1–2), 273–282, DOI:10.1016/j.jcrysgro.2004.06.004.
- U. S. Rai and H. Shekhar, Solidification Behaviour of Binary Organic Eutectic Alloys, Cryst. Res. Technol., 1994, 29(4), 533–542, DOI:10.1002/crat.2170290415.
- V. T. Witusiewicz, L. Sturz, U. Hecht and S. Rex, Thermodynamic Description and Unidirectional Solidification of Eutectic Organic Alloys: I. Succinonitrile-(D)Camphor System, Acta Mater., 2004, 52(15), 4561–4571, DOI:10.1016/j.actamat.2004.06.013.
- U. S. Rai and R. N. Rai, Physical Chemistry of Organic Eutectic and Monotectic: Hexamethylbenzene - Succinonitrile System, Chem. Mater., 1999, 11(11), 3031–3036, DOI:10.1021/cm9806937.
- J. C. Bellows and P. N. Prasad, Molecular Motions and Lattice Stability of a Disordered Organic Alloy: Binary Solid Solutions of 1,4-Dihalonaphthalenes, J. Chem. Phys., 1977, 67(12), 5802–5808, DOI:10.1063/1.434787.
- R. A. Street, D. Davies, P. P. Khlyabich, B. Burkhart and B. C. Thompson, Origin of the Tunable Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Organic Solar Cells, J. Am. Chem. Soc., 2013, 135(3), 986–989, DOI:10.1021/ja3112143.
- P. P. Khlyabich, A. E. Rudenko, B. C. Thompson and Y. L. Loo, Structural Origins for Tunable Open-Circuit Voltage in Ternary-Blend Organic Solar Cells, Adv. Funct. Mater., 2015, 25(34), 5557–5563, DOI:10.1002/adfm.201502287.
- A. D. de Zerio and C. Müller, Glass Forming Acceptor Alloys for Highly Efficient and Thermally Stable Ternary Organic Solar Cells, Adv. Energy Mater., 2018, 1702741, 1–18, DOI:10.1002/aenm.201702741.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, Conjugated Polymer-Small Molecule Alloy Leads to High Efficient Ternary Organic Solar Cells, J. Am. Chem. Soc., 2015, 137(25), 8176–8183, DOI:10.1021/jacs.5b03449.
- P. P. Khlyabich, M. Sezen-Edmonds, J. B. Howard, B. C. Thompson and Y. L. Loo, Formation of Organic Alloys in Ternary-Blend Solar Cells with Two Acceptors Having Energy-Level Offsets Exceeding 0.4 eV, ACS Energy Lett., 2017, 2(9), 2149–2156, DOI:10.1021/acsenergylett.7b00620.
- P. Cheng, C. Yan, Y. Wu, J. Wang, M. Qin, Q. An, J. Cao, L. Huo, F. Zhang and L. Ding, et al., Alloy Acceptor: Superior Alternative to PCBM toward Efficient and Stable Organic Solar Cells, Adv. Mater., 2016, 28(36), 8021–8028, DOI:10.1002/adma.201602067.
- T. Ameri, P. Khoram, J. Min and C. J. Brabec, Organic Ternary Solar Cells: A Review, Adv. Mater., 2013, 25(31), 4245–4266, DOI:10.1002/adma.201300623.
- L. Lu, M. A. Kelly, W. You and L. Yu, Status and Prospects for Ternary Organic Photovoltaics, Nat. Photonics, 2015, 9(8), 491–500, DOI:10.1038/nphoton.2015.128.
- J. Dou, Z.-A. Yu, J. Zhang, Y.-Q. Zheng, Z.-F. Yao, Z. Tu, X. Wang, S. Huang, C. Liu and J. Sun, et al., Organic Semiconducting Alloys with Tunable Energy Levels, J. Am. Chem. Soc., 2019, b13471, DOI:10.1021/jacs.8b13471.
- M. Dabros, P. R. Emery and V. R. Thalladi, A Supramolecular Approach to Organic Alloys: Cocrystals and Three- and Four-Component Solid Solutions of 1,4-Diazabicyclo[2.2.2]Octane and 4-X-Phenols (X = Cl, CH3, Br), Angew. Chem., Int. Ed., 2007, 46(22), 4132–4135, DOI:10.1002/anie.200604830.
- P. P. Khlyabich, B. Burkhart and B. C. Thompson, Efficient Ternary Blend Bulk Heterojunction Solar Cells with Tunable Open-Circuit Voltage, J. Am. Chem. Soc., 2011, 133(37), 14534–14537, DOI:10.1021/ja205977z.
- P. P. Khlyabich, A. E. Rudenko, R. A. Street and B. C. Thompson, Influence of Polymer Compatibility on the Open-Circuit Voltage in Ternary Blend Bulk Heterojunction Solar Cells, ACS Appl. Mater. Interfaces, 2014, 6(13), 9913–9919, DOI:10.1021/am502122a.
- D. Angmo, M. Bjerring, N. C. Nielsen, B. C. Thompson and F. C. Krebs, Fullerene Alloy Formation and the Benefits for Efficient Printing of Ternary Blend Organic Solar Cells, J. Mater. Chem. C, 2015, 3(21), 5541–5548, 10.1039/c5tc00781j.
- S. A. Mollinger, K. Vandewal and A. Salleo, Microstructural and Electronic Origins of Open-Circuit Voltage Tuning in Organic Solar Cells Based on Ternary Blends, Adv. Energy Mater., 2015, 5(23), 1–12, DOI:10.1002/aenm.201501335.
- N. Felekidis, A. Melianas and M. Kemerink, Design Rule for Improved Open-Circuit Voltage in Binary and Ternary Organic Solar Cells, ACS Appl. Mater. Interfaces, 2017, 9, 37070–37077, DOI:10.1021/acsami.7b08276.
- J. Xiao, Z. Yin, H. Li, Q. Zheng, F. Boey, H. Zhang and Q. Zhang, Postchemistry of Organic Particles: When TTF Microparticles Meet Tcnq Microstructures in Aqueous Solution, J. Am. Chem. Soc., 2010, 132(20), 6926–6928, DOI:10.1021/ja102154b.
- K. Sada, K. Inoue, T. Tanaka, A. Epergyes, A. Tanaka, N. Tohnai, A. Matsumoto and M. Miyata, Multicomponent Organic Alloys Based on Organic Layered Crystals, Angew. Chem., Int. Ed., 2005, 44(43), 7059–7062, DOI:10.1002/anie.200501678.
- M. Schwarze, W. Tress, B. Beyer, F. Gao, R. Scholz, C. Poelking, K. Ortstein, A. A. Günther, D. Kasemann and D. Andrienko, et al., Band Structure Engineering in Organic Semiconductors, Science, 2016, 352(6292), 1446–1449, DOI:10.1126/science.aaf0590.
- I. Salzmann, S. Duhm, G. Heimel, M. Oehzelt, R. Kniprath, R. L. Johnson, J. P. Rabe and N. Koch, Tuning the Ionization Energy of Organic Semiconductor Films: The Role of Intramolecular Polar Bonds, J. Am. Chem. Soc., 2008, 130(39), 12870–12871, DOI:10.1021/ja804793a.
- M. Pfeiffer, A. Beyer, T. Fritz and K. Leo, Controlled Doping of Phthalocyanine Layers by Cosublimation with Acceptor Molecules: A Systematic Seebeck and Conductivity Study, Appl. Phys. Lett., 1998, 73(22), 3202–3204, DOI:10.1063/1.122718.
- A. Nollau, M. Pfeiffer, T. Fritz and K. Leo, Controlled N-Type Doping of a Molecular Organic Semiconductor: Naphthalenetetracarboxylic Dianhydride (NTCDA) Doped with Bis(Ethylenedithio)-Tetrathiafulvalene (BEDT-TTF), J. Appl. Phys., 2000, 87(9), 4340–4343, DOI:10.1063/1.373413.
- G. H. Kim, L. Shao, K. Zhang and K. P. Pipe, Engineered Doping of Organic Semiconductors for Enhanced Thermoelectric Efficiency, Nat. Mater., 2013, 12(8), 719–723, DOI:10.1038/nmat3635.
- B. Lüssem, M. Riede and K. Leo, Doping of Organic Semiconductors, 2013, vol. 210, DOI:10.1002/pssa.201228310.
- K. Walzer, B. Männig, M. Pfeiffer and K. Leo, Highly Efficient Organic Devices Based on Electrically Doped Transport Layers, Chem. Rev., 2007, 107(4), 1233–1271, DOI:10.1021/cr050156n.
- Y. Lei, Y. Sun, Y. Zhang, H. Zhang, H. Zhang, Z. Meng, W. Y. Wong, J. Yao and H. Fu, Complex Assembly from Planar and Twisted π-Conjugated Molecules towards Alloy Helices and Core-Shell Structures, Nat. Commun., 2018, 9(1), 4358 CrossRef.
- Y. Masumoto and T. Mori, Application of Organic Bathocuproine-Based Alloy Film to Organic Light-Emitting Diodes, Thin Solid Films, 2008, 516(10), 3350–3356, DOI:10.1016/j.tsf.2007.11.082.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |