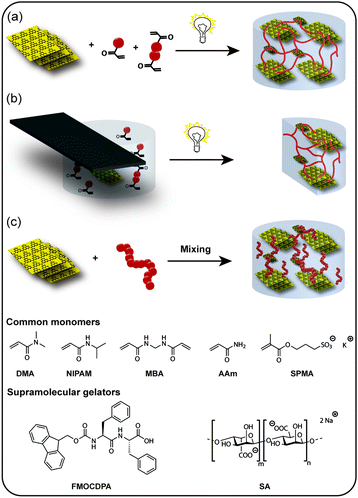
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Graphitic carbon nitride and polymers: a mutual combination for advanced properties
Qian
Cao
a,
Baris
Kumru
a,
Markus
Antonietti
 a and
Bernhard V. K. J.
Schmidt
a and
Bernhard V. K. J.
Schmidt
 *ab
*ab
aMax Planck Institute of Colloids and Interfaces, Am Mühlenberg 1, 14476 Potsdam, Germany
bUniversity of Glasgow, School of Chemistry, G12 8QQ, Glasgow, UK. E-mail: bernhard.schmidt@glasgow.ac.uk; Tel: +44 (0)141 330 8469
First published on 19th November 2019
Abstract
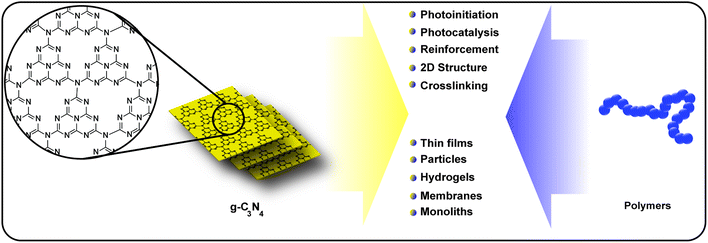
The sheet-like material graphitic carbon-nitride (g-C3N4) is one of the most promising metal-free photocatalysts and utilized for various purposes, e.g. energy conversion, waste water remediation or organic synthesis. g-C3N4 features a suitable band gap in the visible light range and outstanding physicochemical stability. However, g-C3N4 features drawbacks such as structural disorder, low conductivity, poor dispersibility and in turn low processability. Amongst the strategies to improve g-C3N4 properties, combination with polymers is a promising avenue toward advanced materials. The present critical review highlights the development and investigation of g-C3N4/polymer combination, including (1) g-C3N4 as photoinitiator for polymer snythesis, (2) polymer modified g-C3N4 for improved dispersibility, (3) g-C3N4/polymer hybrid materials fabricated via physical or covalent attachment and (4) g-C3N4 based hydrogels. The fabrication methods and application of these areas will be critically reviewed and the advantage of g-C3N4/polymer combination comprehensively presented. Moreover, the broad range of applications is highlighted, e.g. photocatalysis, batteries, biosensors, H2 evolution and films. Finally, the review will conclude with a summary and perspective on future directions as well as current challenges of this research area in order to stimulate new research regarding the design and construction of g-C3N4/polymer materials.
1. Introduction
Sustainability has an increasing impact both on academia and industry, orienting many research fields such as biomass conversion, batteries, polymers and photochemistry.1–5 A significant direction is the integration of free and abundant sunlight into current technology, such as energy harvesting via photovoltaic devices or photo-mediated material synthesis. Semiconductors (such as Si, TiO2, CdS, hybrid perovskites, polythiophenes) absorb light which results in the formation of excited electrons, and are promising candidates for photovoltaic devices, transistors and for photoinduced reaction catalysis.6–8 Many factors should be considered for the efficiency of a semiconductor materials, such as suitable absorption and bandgap values, lifetime of excited electrons, and charge migration which delays recombination. Even though excessive research has been conducted on these materials, their metal content, possible toxicity and synthesis from non-abundant sources restrict applications. Therefore, suitable candidates which have at least similar photoactivity but are formed via sustainable synthetic conditions are highly sought.9Graphitic carbon-nitride (g-C3N4) is a sheet like material that is traditionally formed from a regular arrangement of tri-s-triazine units (Scheme 1).10,11 It features a variety of modifications, which have direct influence on the band gap resulting in photoactivity in the visible and UV range of light.12–15 Thus, g-C3N4 is utilized frequently as visible light induced heterogeneous photocatalyst, for example for organic transformations,16,17 hydrogen evolution,18–20 pollution degradation21 or CO2 reduction.22,23 Moreover, g-C3N4 is utilized in ion transport membranes,24,25 photoelectrochemistry26 or in organic photovoltaics27–29 as well as for emulsion stabilization.30 The synthesis of g-C3N4 is usually performed from metal-free, oxygen-free, abundant and nitrogen-rich precursors, for example cyanamide,31,32 guanidine hydrochloride,33,34 melamine35–37 or cyanuric acid.38 The process can be realized via several methods. Amongst them, thermal condensation is the most common method for fabrication of bulk g-C3N4, which proceeds under inert atmosphere between 400 and 600 °C. Recently, other methods like chemical vapor deposition or electrochemical deposition were introduced for fabricating film or membrane g-C3N4.39 The microwave method was utilized as well, e.g. to produce fluorescent g-C3N4.40,41 However, the type of the precursors and treatment can significantly influence the physicochemical properties of as-prepared g-C3N4. Compared to traditional semiconductors which have defined formulas, g-C3N4 represents a large family of materials with a variety of properties. For example, one way to obtain well-defined g-C3N4 is the utilization of a supramolecular precursor complex of cyanuric acid and melamine that already resembles the final g-C3N4 structure.42,43 As such, porosity,44–46 surface charge,47 light absorption, photoluminescence, and band gap48 can be tailored according to the needs. Recently, also the overall shape of g-C3N4 could be tuned via various synthetic methodologies, e.g. a control over precursor crystal structures/shapes.49–52 As summary, g-C3N4 seems to satisfy sustainability requirement as semiconductor by being metal free and synthesized from benign precursors, in addition it possesses tunable properties, however it has some major drawbacks such as structural disorder, as well as being non-processable in bulk.
In that sense, a combination of carbon nitride with polymers seems to be a promising avenue for advanced materials (Scheme 1). Polymers can introduce processability (e.g. film formation) into materials as well as enhancing dispersibility. Moreover, a plethora of conducting polymers allows a fine tuning of electron transport process in materials. Therefore, polymers can be utilized to introduce new properties to g-C3N4 or to enhance existing properties (e.g. photocatalysis or conductivity). Likewise, incorporation of g-C3N4 into polymer materials is an avenue to tailor mechanical properties of the polymer, e.g. in the bulk or in hydrogels. Another promising property of g-C3N4 that is of significant interest for polymer science is its inherent property to form radicals under irradiation with visible light. As such it acts as photoinitiator and can be used for polymer synthesis (e.g. polymer particles) in a convenient way. Polymers and g-C3N4 have various points of contact, and by hybridization improved or novel materials properties can be synthesized.
In the following review, this combination is discussed. The research in recent years is divided into four parts. At first the photoinitiator properties of g-C3N4 and its application in various polymerization systems is discussed. Next, the dispersibility of g-C3N4 and polymer- or functionalization-based routes towards improved dispersibility are highlighted. As the main part, g-C3N4/polymer hybrid and composite materials are presented with emphasis on H2 evolution, photocatalysis, biosensors, electrochemical energy storage and solar cells, films, nano particles and polymer properties. Finally, the area of g-C3N4-based hydrogels is introduced with a focus on mechanical properties as well as photocatalytic properties. The review is closed with a summary and discussion of future aspects.
2. Carbon nitride as polymerization initiator
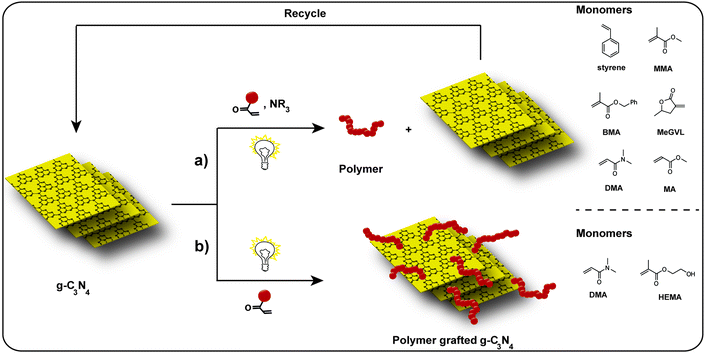
Commonly, g-C3N4 has been employed as a highly active photocatalyst. As g-C3N4 features a suitable bandgap to absorb visible light, it generates electrons and holes under visible light irradiation. In these cases, the formation of reactive species (e.g., ˙OH, O2−˙, and HO2˙, or also CN-centered species) takes place, which is not only of interest for photocatalysis but for polymerization processes as well (Scheme 2). Therefore, two ways are depicted in Scheme 2 where g-C3N4 acts as initiator for photopolymerization, which is accompanied with various advantages. First of all, g-C3N4 polymerizations can be operated in the visible light region, which is a benign and abundant trigger. Several monomers were investigated for polymerization via g-C3N4 as photoinitiator, such as styrene,53 methyl methacrylate (MMA),54 α-methylene-γ-valerolactone (MeGVL),55 methyl acrylate (MA).56 On top, photopolymerizations have various useful features, like spatial and temporal control. Moreover, g-C3N4 is a heterogenous catalyst, which allows easy separation and recycling. Albeit, the conditions can be altered in a way that the growing polymer chains are grafted from g-C3N4 and as such recycling or separation is not straight-forward. In this case grafting polymers from g-C3N4 opens up various opportunities for hybrid material formation, which also broadens the utilization in bioapplications due to the inherent photoluminescent properties of g-C3N4.As a proof of concept, Yagci and coworkers utilized g-C3N4 as visible light photoinitiator for free radical polymerization.57 Mesoporous g-C3N4 (mpg-C3N4) was employed as heterogeneous visible light photoinitiator in the presence of tertiary amine as reactive co-initiator, conducting free radical polymerization with vinyl monomers such as methyl methacrylate (MMA). Based on the photoredox chemistry of mpg-C3N4, the photopolymerization process was realized by exposing the monomer mixture to visible light in the absence of O2. The initiation mechanism is presumably the transfer of the CN-based hole and the amine. The photochemically formed holes oxidize amines to the corresponding radical cations which abstract hydrogen from another amine leading to the formation of initiating radicals. Moreover, mpg-C3N4 demonstrated enhanced activity in the polymerization process due to a larger external surface compared to non-porous bulk g-C3N4.
Recently, our group employed g-C3N4 as radical photoinitiator for emulsion photopolymerization.58 Emulsions of aromatic monomers (styrene and benzyl methacrylate) and MMA in water were formed via g-C3N4 (cyanuric acid-melamine-derived g-C3N4 (CM) or 1-decene modified CM) as stabilizer. Subsequently, the mixture was exposed to visible light to conduct polymerization. Radicals were formed on the surface of g-C3N4. Kinetic studies and in-depth studies of the polymer particles indicated that the g-C3N4 surface acted as the reaction locus for polymer chain growth and particle formation. The polymerization mechanism was investigated by addition of hole or electron scavenger to the reaction system, respectively, and it was demonstrated that the emulsion photopolymerization was mainly initiated by the hole. Overall, very well-defined latexes were obtained, which was attributed to the fast nucleation of the latex particles. Moreover, compared to previous works, radicals were formed via holes on the g-C3N4 surface directly without co-initiator addition. The direct radical formation leads to crosslinked latex particles directly due to the multifunctionality of g-C3N4. Furthermore, our team has employed g-C3N4 as heterogeneous photocatalyst for free radical polymerization of MeGVL.55 At first MeGVL is efficiently synthesized from renewable resources via continuous flow reaction from γ-valerolactone over hierarchical basic zeolite. MeGVL is structurally similar to methacrylic monomers, and further valorization of this compound via efficient polymerization demonstrates the ability to make novel biomass-derived polymer with significant industrial interest.
As a semiconductor, g-C3N4 possesses a quite high negative position of the conduction band, thus contributing to a high activity of oxygen capture and reduction. Meanwhile, the moderate oxidation potential of g-C3N4 efficiently prevents the decomposition of the prepared polymer by the photogenerated holes. Both effects play a crucial role in the photopolymerization process.59,60 Thus researchers introduced g-C3N4 as a promoter for electron transfer in reversible deactivation radical polymerization (RDRP). For example, Yagci and coworkers56 utilized mpg-C3N4 as photoactivator for reduction of initially loaded copper(II) species, for the in situ formation of copper(I) species, which act as catalytic species in atom transfer radical polymerization (ATRP). Subsequently, polymerization was successfully conducted under sunlight or UV-light irradiation. It was shown that the light-induced electron transfer from g-C3N4 is due to the large reduction potential (ECB) of −1.2 eV. Vinyl monomers such as, methyl acrylate (MA), MMA and styrene were successfully polymerized during the reaction with precise control on molecular dispersity (Đ). A work reported by Qiao and coworkers introduced g-C3N4/amine cocatalysts to a photoinduced electron/energy transfer (PET) oxygen-tolerant reversible addition–fragmentation chain transfer (RAFT) polymerization (Fig. 1).61 g-C3N4 was employed directly without prior deoxygenation of the reaction mixture to enable electron transfer from added tertiary amines (i.e. triethanolamine TEOA) to dissolved molecular oxygen. Thus, the trithiocarbonate (TTC) chain transfer agents were activated via oxygen reduction, followed by polymerization of acrylic species such as MA, n-butyl acrylate (BA) and water-soluble monomer N,N-dimethylacrylamide (DMA) (Fig. 1a). In the mechanism, molecular oxygen takes a prominent part in the photoredox cycle. Thus, benign reaction conditions under visible light, without deoxygenation of monomer solution, are achieved that are time consuming in the common case. Polymers with different polymerization degree could be achieved easily with this process as shown via size exclusion chromatography (SEC) (Fig. 1b). Moreover, block copolymers could be synthesized, e.g. PMA-b-PBA (Fig. 1c). Such PET-RAFT approach had merits of low toxicity, organic solvent tolerance and facile post-polymerization removal of the catalyst.62
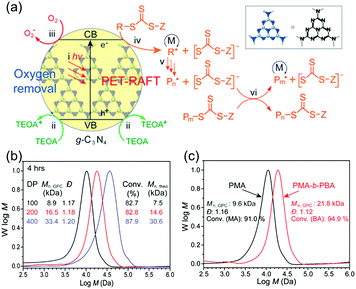 | ||
| Fig. 1 (a) Schematic overview over the mechanism of deoxygenation and photoinduced electron/energy transfer-reversible addition–fragmentation chain transfer (PET-RAFT) polymerization employing g-C3N4: (i) photo-induced activation of g-C3N4, (ii–iv) electron transfers from amine to g-C3N4, from g-C3N4 to O2, and from g-C3N4 to the trithiocarbonate (TTC), respectively; (v) initiation and chain propagation of monomers (M); and (vi) reversible degenerative chain transfer (RAFT process). (b) size exclusion chromatography (SEC) traces of PET-RAFT polymerization derived poly(methyl acrylate) (PMA) with different degree of polymerization (DP) (DP = 100, 200, and 400). (c) SEC traces of PMA-b-poly(n-butyl acrylate) (PBA) diblock copolymer product and precursor (Reprinted with permission.61 Copyright 2017 American Chemical Society). | ||
Very recently, our group conducted dithiol–ene click reactions between lignocellulosic biomass-derived 4-pentenoic acid (4-PEA) and different dithiols using g-C3N4 as photocatalyst.63 Visible light induced click chemistry was utilized for the reactions between 4-PEA and 1,2-ethanedithiol (EDT), 2,2-(ethylenedioxy)-diethanethiol (EDDT), and 1,4-benzenedimethanethiol (BDT) leading to polymers in high yields and purities in order to reduce the dependence on petroleum-derived monomers. Moreover, g-C3N4 has been utilized as photoinitiator to fabricate g-C3N4-based polymer composites under light irradiation via a “grafting from” method, which is defined as chain growth via monomer propagation starting from a surface, i.e. here a g-C3N4 surface. As radicals are produced by g-C3N4 under visible light irradiation, the grafting occurs presumably via the presence of uncondensed –NH2 or –NH groups at g-C3N4. These groups act as active sites to initiate polymer chain growth from g-C3N4 surface. In such a way, g-C3N4-based polymer hybrid materials are fabricated, with the firmly attachment of polymer on to g-C3N4 surface, which facilitates further applications.
Another area where g-C3N4 photoinitiation is widely utilized is hydrogel formation. For hydrogel formation the same initiation mechanisms are exploited, i.e. radicals are formed on the surface of g-C3N4 with phototreatment and g-C3N4 directly participates in the hydrogelation without electron transfer or co-initiator addition. The formation of hydrogels will be covered in detail in Section 5. Most notably, hydrogels could be formed without addition of external crosslinker, which indicates initiation from the g-C3N4 surface and covalent incorporation of g-C3N4 into the gel network.64 As such, these findings support the mechanism found for g-C3N4 initiated polymerization without addition of radical transfer agent.
3. Carbon nitride dispersibility
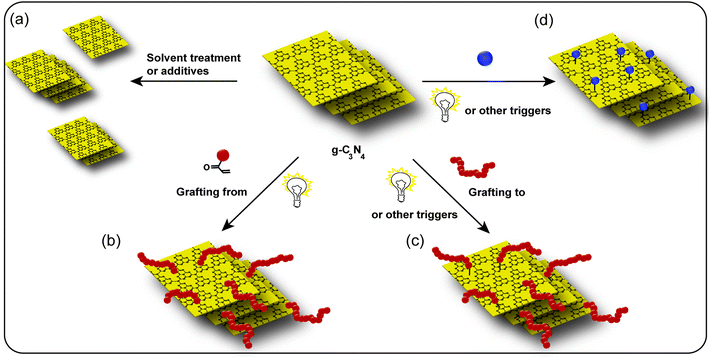
A major issue for the application of g-C3N4 is its poor dispersibility of the pure material. As the strong van der Waals interactions (π–π stacking) of g-C3N4 sheets causes the agglomeration in the liquid phase. Thus, dispersibility in organic as well as aqueous environment is rather low,65 and results in restricted applicability as well as activity.65,66 Especially the formation of thin (2–3 nm) semiconductor films are of great interest for optoelectronics and photovoltaics. However, to form such g-C3N4 films rather elaborate methods like chemical vapor deposition have to be utilized.67,68 A solution based process would be simpler and suitable for many real life applications, and therefore, attention has been addressed to enhance dispersibility of g-C3N4 powder. Several researchers focused on exfoliation of the bulk g-C3N4 into thin sheets, in which the electrostatic repulsion between g-C3N4 sheets can enable the formation of well-dispersed g-C3N4 colloids. Hence, stable dispersions of g-C3N4 can be prepared, yet the achievable concentration of dispersed g-C3N4 is rather low. A route to disperse g-C3N4 is via additives or physical treatment, e.g. via addition solvents in the dispersion step,69,70 hydrothermal treatment,71 chemical oxidation,72 thermal oxidation73 or ultrasonication.74 The other option is chemical modification via attachment of molecular moieties. On one hand, with the attachment of charged groups to the g-C3N4 surface, the stability of dispersed g-C3N4 nanosheets can be increased in aqueous environment via electrostatic repulsion. On the other hand, attachment of hydrophilic polymers can stabilize g-C3N4 in dispersion via steric repulsion in aqueous dispersion. In organic solvent, the dispersibility of g-C3N4 can be increased via the attachment of organo soluble small molecules or polymers, where steric repulsion leads to enhanced stability of the dispersion.A reported option is the complex formation with organic modified montmorillonite to facilitate enhanced stability in organic environment, which was utilized to form poly(styrene) (PS)/g-C3N4 composites.77 Exfoliating g-C3N4 in 1,3-butanediol results in graphene-like g-C3N4 with around 2–6 layers of g-C3N4 with a thickness of circa 1–2 nm. However, ultrasonication for 24 hours and possibility to exfoliate only low amount of g-C3N4 is the drawback of the approach.31
Another functionalization route to enhance dispersibility of g-C3N4 is by pre-condensation or post-condensation. In the case of pre-condensation a specific monomer mixture is used, for example additional phenyl moieties are introduced into the system.78 The utilization of a phenyl-functional precursor for g-C3N4 synthesis prevents growth of larger g-C3N4 sheets which results in quantum dot structure that possess enhanced dispersibility compared to traditional g-C3N4 sheets. Another approach is post-condensation functionalization that allows the introduction of various functional groups via chemical treatment, e.g. oligo(ethylene glycol) (oligoEG) (Fig. 2).75 Well dispersed colloids (Fig. 2a) with high biocompatibility and bioimaging properties could be achieved in that way (Fig. 2b). As such Kim and coworkers firstly oxidized g-C3N4via KMnO4 and exfoliated it via ultrasonication, followed by covalent modification with monomesylated oligoEG. Attachment of oligoEG resulted in oligoEG-modified g-C3N4 sheets, which exhibited water dispersibility to be utilized for bioimaging. Oxygen plasma can be employed for introducing protonated hydroxylamine functional groups to g-C3N4 surface, which provides extreme hydrophilic character.79 Other functional groups such as sulfonic acid,80 hydroxyl81 or aromatic groups48 can also be introduced for enhancing dispersibility of g-C3N4 sheets. However, alternative facile and less stringent routes to enhance dispersibility with high g-C3N4 solid content yields would be beneficial.
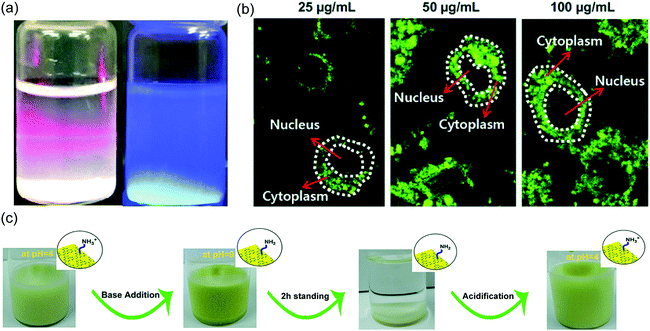 | ||
| Fig. 2 (a) A photo of suspensions of oligo(ethylene glycol) (oligoEG)-grafted g-C3N4 in water showing the Tyndall effect (left) and fluorescence (right). (b) Confocal fluorescence microscopy image of RAW264.7 cells after incubation at different concentrations with the oligoEG-g-C3N4 nanodots (25, 50, and 100 μg mL−1) for 24 h at 37 °C (Reprinted with permission.75 Copyright 2018 John Wiley and Sons). (c) Allylamine-modified g-C3N4 in water (1 wt%) at acidic pH (pH = 4), observation of immediate precipitation after base addition (pH = 9), complete sedimentation after standing for 2 h, and redispersion after reacidification (pH = 4) (Reprinted with permission.76 Copyright 2017 American Chemical Society). | ||
To improve dispersibility, we investigated solvent mixtures (Scheme 3a). The mixture of water and EG in equal volume enabled dispersion with significant g-C3N4 weight contents of up to 4 wt%, compared to a maximum of 0.6 wt% in pure water. g-C3N4 is a photoactive compound that is a semiconductor and thus forms electron/hole pairs via light irradiation. This feature was exploited to perform photo-initiated polymerization reactions (Scheme 3b). Thus, to improve dispersibility of g-C3N4 further, a polymerization approach was introduced via g-C3N4 photoinitiation. DMA was added to a g-C3N4 dispersion in EG/water and treated with visible light, which led to a weakly associated gel. The viscous gel was formed from PDMA grafted g-C3N4 and could be easily dispersed in water. In addition, such dispersions possess high stability (up to 2 months) due to steric stabilization of g-C3N4 colloids.82
Furthermore, pre-formed ene end functionalized polymers could be grafted onto g-C3N4 as well (Scheme 3c, refer to Section 4 as well). A way to obtain dispersible-CN via a more exact approach that does not rely on polymerization was also studied. For that, the parent g-C3N4 is dispersed in aqueous or organic solution, an ene-compound is added, and the mixture subjected to visible light (Scheme 3d).76 Due to the radical formation at the g-C3N4 surface, addition of the ene-compound takes place. To prevent polymerization of the added small molecule, allylic compounds were utilized as they possess no propagation tendency. Hence, the molecules are grafted on g-C3N4 directly. Notable, the modification of the g-C3N4 surface chemistry significantly influences the dispersion properties of g-C3N4. For example, 3-allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt (AHPA) was grafted on g-C3N4 to improve water dispersibility with up to 10 wt% while stable dispersions were obtained, e.g. for 48 hours. Grafting of 11-decene led to organo dispersibility of up to 2 wt% g-C3N4 in solvents like THF, DCM or toluene. In addition, pH-sensitive dispersibility could be introduced via allylamine that leads to dispersibility in acidic solution and precipitation at basic pH (Fig. 2c). Recently, methyl vinyl thiazole was grafted onto g-C3N4, which led to a significant improvement of organo dispersibility via an intrinsic electrostatic stabilization mechanism. The two components create an autonomous donor–acceptor type structure and enhance excited electron–hole separation.83 Moreover, surface functionality has a profound effect on photocatalytic activity as well.
4. Carbon nitride/polymer hybrid materials
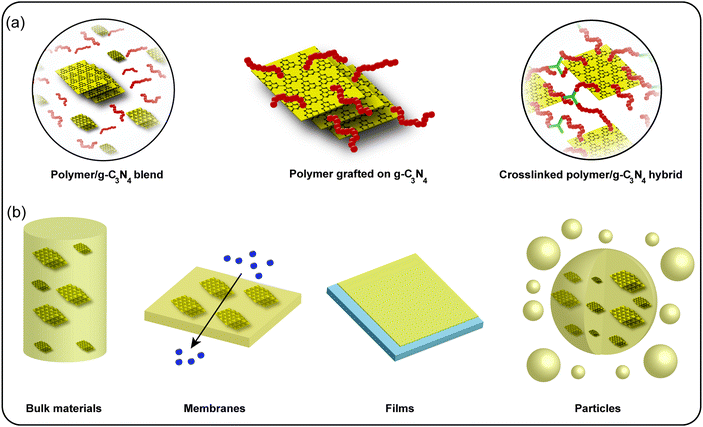
Polymers are playing a significant role in industry as well as daily life and are widely investigated. Especially, polymer materials with functional properties are of focal interest today, such as self-healing,84 stimuli response,85 biodegradability86 or electrical conductivity.87 A combination of traditional polymers with the novel metal-free semiconductor g-C3N4 materials is highly worthwhile (Scheme 4). g-C3N4 is widely investigated as a metal-free semiconductor, but poor processing inhibits a scale-up of utilization, which is an issue for applicability.88,89 A combination of g-C3N4 with polymers appears to be a useful solution,90,91 as properties of both material classes can be coupled via combination of the individual parts.92 Due to g-C3N4, photocatalytic, photoluminescence, as well as enhanced physical and mechanical properties are obtained, while the polymers bring improved processability or conductivity. There are several ways to fabricate g-C3N4/polymer hybrid materials (Scheme 4a). (1) Physical absorption or deposition, mostly prepared in the liquid phase. In the same way, one can also list blending of both materials. (2) “Grafting from” method, in which monomers are polymerized from the g-C3N4 surface. (3) “Grafting to” method that is based on chemical bond formation between reactive sites on g-C3N4 and active polymer end groups.Such g-C3N4/polymer hybrid materials can be utilized for various applications as discussed in Section 4.1, e.g. photocatalytic H2 evolution, photocatalysis, biosensors, electrochemical energy storage according to different type of the hybrids (Scheme 4b). As film and membrane materials are commonly applied to electrochemical area and particles are mostly used in biosensors, Table 1 shows the summary of specific g-C3N4/polymer compositions and their application area as well as the specific enhanced properties and performance compared to the single component g-C3N4. In addition, polymers can be utilized to include g-C3N4 into specific material morphologies, e.g. spherical particles or thin films as presented in Section 4.2. Moreover, g-C3N4/hybridization enables the improvement of polymer properties, especially mechanical properties as shown in Section 4.3. As such, polymers and g-C3N4 can be utilized for mutual benefit. On one hand, polymers improve processing of g-C3N4 amongst other properties. On the other hand, g-C3N4 improves the properties of polymer materials, e.g. the mechanical properties.
| Application area | Composition | Synthesis methods | Enhanced properties | Ref. |
|---|---|---|---|---|
| Hydrogen evolution | P3HT/Pt/g-C3N4/Au | Self-assembly | Efficient charge transfer, improved H2 evolution rate (320 μmol h−1) | 93 |
| Hydrogen evolution | PPyg-C3N4 | Ultrasonication | Efficient charge transfer, 50 times over g-C3N4 | 94 |
| Hydrogen evolution | PAN/g-C3N4 | Thermal condensation | Efficient charge transfer, 37 μmol h−1 (H2 evolution rate), 3.8 times over g-C3N4 | 95 |
| Hydrogen evolution | P3HT/g-C3N4 | Physical absorption | Efficient charge transfer, 300 times over g-C3N4 | 96 |
| Degradation of MB | PANI/g-C3N4/ZnO | In situ polymerization | Efficient charge transfer, 3.6 and 3.3 times over pristine g-C3N4 | 99 |
| Degradation of MB | PANI/g-C3N4 | In situ polymerization | Efficient charge transfer, 5.1 times over g-C3N4 | 107 |
| Degradation of MB | PANI/SP-g-C3N4(sulfur and phosphorous doped g-C3N4) | In situ polymerization | Efficient charge transfer, synergistic effect, maximum degradation (99.9%) | 108 |
| Degradation of RhB | PAN nanofibers/g-C3N4 | Electrospinning | Prevent agglomeration of powder g-C3N4 | 110 |
| Oil and organic solvent capture | Acrylic resin/g-C3N4 | In situ polymerization | Improved capillary and penetration interaction for oil absorption, ∼33.5 g g−1 (toluene), ∼20.6 g g−1 (DMF), 15 wt% (MCNFs) | 111 |
| CO2 reduction | 1H,1H,2H,2H-perfluorodecanethiol/PGMA/g-C3N4 | Photoinduced chemical reaction | Improved mass transfer of CO2 by 34 times | 97 |
| CO2 reduction | Polymeric cobalt phthalocyanine/mpg-C3N4 | In situ polymerization | Efficient charge transfer | 98 |
| Eliminating antibiotics | Poly(ethylene terephthalate) nanofibers/g-C3N4 | Electrospinning | Increased active sites, improved mass transfer and light absorption | 113 |
| Eliminating hexavalent chromium and antibiotics | Cellulose aerogel/polyester fibers/g-C3N4 | Blending | Improved mechanical strength and higher impact resistance | 114 |
| Eliminating antibiotics | Poly(ethylene terephthalate)/g-C3N4 | Electrospinning | Improved photocatalytic stability and reusability | 115 |
| Degradation of tetracycline (TC) hydrochloride | Polyester fiber/g-C3N4 | Hot-melt adhesive | Improved contact to contaminants, recycling performance and structural stability | 116 |
| Electrochemiluminescence biosensor | DexP/g-C3N4 | Blending | Wider linear response range (0.05 ng mL−1 to 100 ng mL−1), lower detection limit (17 pg mL−1) | 122 |
| Electrochemiluminescence biosensor | AuNF@PANI/g-C3N4 | In situ polymerization | lower limit of detection (1.7 × 10−9 M) | 123 |
| Electrochemiluminescence biosensor | PPy/g-C3N4 | In situ polymerization | Improved electro-conductive network, high sensitivity (645.7 μA mM−1 cm−2) and lower detection limit (8.0 μM) | 124 |
| Fuel cell | Poly(arylene ether ketone)s/g-C3N4 | Blending | Improved anion exchange, ionic conductivity, alkaline resistance and methanol permeability | 161 |
| Proton exchange membrane for fuel cells | PVP-phosphonated poly(2,6-dimethyl-1,4-phenylene oxide)/g-C3N4 | Solution casting | Improved proton conductivity (74.4 mS cm−1) and mechanical properties | 133 |
| Proton exchange membrane for fuel cells | PES-PVP/g-C3N4 | Blending | Improved proton conductivity (0.104 S cm−1) and power density (512 mW cm−2) | 134 |
| Direct methanol fuel cells | Nafion/g-C3N4 | Blending | Enhances movement of hydronium ions | 132 |
| Direct methanol fuel cells | SPEEK/g-C3N4 | Solution-casting | Enhanced movement of hydronium ions | 135 |
| Vanadium redox flow battery | SPEEK/g-C3N4 | Solution-casting | Higher coulombic efficiency and improved cycling stability | 139 |
| Vanadium redox flow battery | SPEEK/g-C3N4 | Solution-casting | Higher coulombic efficiency and improved structure stability over 300 charge–discharge cycles | 141 |
| Vanadium redox flow battery | Nafion/g-C3N4 | Blending | Higher coulombic efficiency and energy efficiency | 142 |
| Lithium metal batteries | Bis(trifluoromethanesulfonimide) lithium salt/di(ethylene glycol) dimethyl ether/g-C3N4 | Blending | Improved mechanical strength, interfacial resistance and battery stability | 143 |
| Supercapacitors | Camphor sulfonic acid/polycarbazole/g-C3N4 | Chemical oxidative polymerization | Improved charge transfer and structure stability (over 1000 cycles) | 144 |
| Supercapacitors | Cellulose/PPy/tubular/g-C3N4 | Self-assembly | Improved specific capacitance (158 F g−1) | 145 |
| Supercapacitors | PANI/g-C3N4 | In situ polymerization | Improved specific capacitance (797.8 F g−1) and capacitance retention (84.4%) | 146 |
| Supercapacitors | PEDOT:PSS/g-C3N4 | Self-assembly | Improved specific capacitance, 137 F g−1 (H2SO4) and 200 F g−1 (Na2SO4), improved cycling stability | 147 |
| Solar cells | Bulk-heterojunction polymer/g-C3N4 | Blending | Enhanced power conversion efficiencies, improved active layer conductivity and charge transfer | 149 |
A significant issue with polymer hybrids and composites is the compatibility of polymer and the mixed-in material. In the case of g-C3N4, favorable interactions are present in many cases. For example, g-C3N4 is prone to establish π–π interactions with polymers like PS or poly(benzyl methacrylate) (PBMA). On the other hand, g-C3N4 contains polar groups at the edges, which allows interactions (mostly hydrogen bonding) with polar polymers like PDMA or poly(2-hydroxyethyl methacrylate) (PHEMA).
4.1 Applications of g-C3N4/polymer hybrid materials
For example, Yan and coworkers combined P3HT and g-C3N4 by impregnating g-C3N4 with a chloroform solution of P3HT overnight, followed by evaporation of the solvent, which resulted in physical attachment of P3HT to g-C3N4 surface (Fig. 3a).96 With the increased deposition of P3HT, a remarkable increase of H2 evolution of 300 times was achieved utilizing Na2S and Na2SO3 as electron donors. The improved catalytic activity was attributed to the enhanced electron conductivity after P3HT incorporation. Later, the same group established a g-C3N4/Au/P3HT/Pt layer structure using a self-assembly method.93 A tight g-C3N4/Au conjunction was formed by photodeposition, then P3HT/Pt was combined with g-C3N4/Au due to the formation of Au–sulfur association between Au on g-C3N4 and sulfur in the P3HT structure. Thus, chemical bonds were used instead of physical adsorption to ensure a tight junction between the individual g-C3N4 and P3HT layers. Such layered structures were demonstrated to be efficient for the separation of photoinduced electron–hole pairs and for H2 evolution.
 | ||
| Fig. 3 (a) A proposed mechanism of visible light-induced H2 evolution on g-C3N4–poly(3-hexylthiophene) (P3HT) polymer composite photocatalysts (Reprinted with permission.96 Copyright 2011 Royal Society of Chemistry). (b) The mechanism to understand the role of the dispersed poly(pyrrole) (PPy) nanoparticles in enhancing the photocatalytic activity of PPy-g-C3N4 for H2 evolution (Reprinted with permission.94 Copyright 2013 Royal Society of Chemistry.) (c) Illustration for the enhanced photogenerated charge carriers separation and transfer in graphitized-poly(acrylonitrile) (g-PAN)/g-C3N4 composites under visible light irradiation (λ > 400 nm) (Reprinted with permission.95 Copyright 2014 American Chemical Society). | ||
Nevertheless, P3HT has the drawback of a limited processability in aqueous media, which is the preferred choice for g-C3N4 so far. An option to circumvent that problem is to switch to PPy, which possesses high stability in the oxidized state as well as high conductivity. For example, Chen and coworkers reported the loading of highly dispersed PPy nanoparticles onto the g-C3N4 surface via sonochemical approach in a physical attachment (Fig. 3b).94 PPy-g-C3N4 suspension was treated with ultrasonication for 12 hours and drying at 80 °C. The addition of PPy nanoparticles showed no effects on the absorption edge of g-C3N4 but influenced the intensity of the g-C3N4 emission peak, indicating the more effective separation of photogenerated electrons and holes in PPy-g-C3N4 compared to pristine g-C3N4. The activity of H2 evolution was dramatically improved with the increasing loading amount of PPy. Furthermore, graphitized-poly(acrylonitrile) (g-PAN) nanosheets were deposited on g-C3N4via one step thermal condensation method as reported by Hao and coworkers (Fig. 3c).95 Simple mixing of g-C3N4 precursors with PAN and thermal treatment under 650 °C led to graphitization of PAN. Thus, a layered structure of g-PAN/g-C3N4 was obtained. Compared to aggregated polymer morphologies, the g-PAN with aromatic conjugated structure possesses more reactive sites and short diffusion distance, which decreases the recombination rate of photogenerated charge carriers. Hence g-PAN acts as an effective electron transfer channel in the g-PAN/g-C3N4 composites and obviously enhanced the photocatalytic performance for H2 evolution.
Very recently, hydrophobic polymer grafted g-C3N4 was employed as a three-phase photocatalyst for enhanced selectivity and activity of CO2 reduction.97 Hydrophobic 1H,1H,2H,2H-perfluorodecanethiol was utilized to modify poly(glycidyl methacrylate) (PGMA) grafted on CM (pDFe-PGMA/CM) via thiol-epoxy addition reaction. Subsequently, an in situ photoloading method was applied for loading Pt on the pDFe-PGMA/CM surface. A three-phase contact photocatalyst of CO2 (gas), H2O (liquid) and catalyst (solid) was fabricated to enable a high concentration of CO2 molecules on the catalyst surface directly. Moreover, the mass transfer limitation of CO2 was overcome due to the hydrophobic catalytic surface, which contributed to an enhanced CO2 reduction reaction and suppressed hydrogen evolution reaction. The observed efficiency was about 34 times higher than commonly achieved by hydrophilic catalysts. Reisner and Roy showed an avenue to CO2 reduction as well.98 In their experiments, mpg-C3N4 was combined with a polymeric cobalt phthalocyanine catalyst. To obtain a good interfacial contact, 1,2,4,5-tetracyanobenzene together with Co2+ was polymerized directly in the presence of mpg-C3N4. A synergistic effect of CN porosity, solar energy harvesting and photosensitization facilitated remarkable activity of the phthalocyanine catalyst in CO2 reduction under visible light.
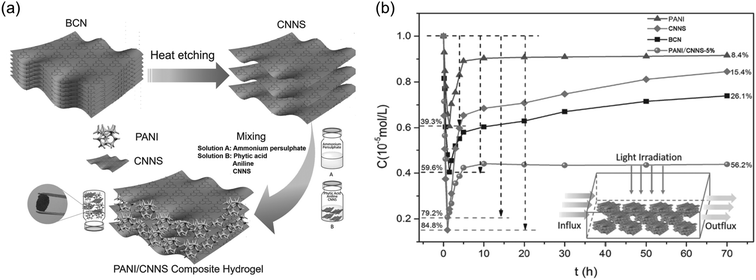 | ||
| Fig. 4 (a) Schematic plot of the preparation of poly(aniline)/g-C3N4 nano sheet (PANI/CNNS) composite hydrogel from bulk g-C3N4 (BCN). (b) Photocatalytic degradation of Methylene Blue (MB) in flow system (1.0 L s−1 flow rate, 200 mg catalyst, 1 × 10−5 mol L−1 MB) (Reprinted with permission.106 Copyright 2016 John Wiley and Sons). | ||
A good way to improve efficiency of g-C3N4-based waste water remediation by improved transport engineering is the incorporation into fiber structures. The group of Chen fabricated visible light responsive nanofibers based on PAN dispersed g-C3N4via electrospinning, to disperse g-C3N4 and immobilized by PAN fiber structure.110 The g-C3N4/PAN hybrids demonstrated efficient photocatalytic properties of Rhodamine B (RhB) degradation over a wide pH range and were recycled in a simple way. Othman and coworkers incorporated g-C3N4 into PAN nanofibers using electrospinning as well.111 A liquid-permeable self-supporting photocatalytic nanofiber was fabricated, demonstrating 85% degradation capability for purification of oil contaminated water under visible light irradiation. Liu and coworkers utilized electrospun PAN nanofibers to immobilize a g-C3N4/BiOI heterojunction via a facile in site synthesis strategy.112 The efficient separation of the electron–hole pairs and strong absorption in the visible region of PAN/g-C3N4/BiOI hybrids resulted in superior photocatalytic activity in the degradation of RhB and toxic Cr(VI) ions under visible-light. Moreover, the film-like and self-supporting nanostructure enabled the hybrids for floating photocatalyst application.
An issue of growing interest is the removal of antibiotic contaminations in water, which can be tackled via g-C3N4 photocatalysis as well. For example, g-C3N4@poly(ethylene terephthalate) nanofibers were fabricated using poly(ethylene terephthalate) as a support and polyethylene glycol (PEG) as a porogen via electrospinning,113 which is beneficial for catalyst–substrate contact. This work demonstrated high photocatalytic activity for the degradation of antibiotics such as sulfaquinoxaline and sulfadiazine under solar irradiation. Polyester fibers (from poly(ethylene terephthalate)) were utilized to support cellulose (CA) containing nanosheet g-C3N4 in another work as well,114 aerogel g-C3N4@CA/poly(ethylene terephthalate) for enhanced photocatalytic activity towards the removal of hexavalent chromium and antibiotics, simultaneously. Chen and coworkers used poly(ethylene terephthalate) as support for g-C3N4via electrospinning and subsequently hydrothermal treatment, which enabled the exposure of g-C3N4 on the poly(ethylene terephthalate) surface, avoided aggregation and improved recyclability.115 Low melting sheath-core composite polyester fibers were immobilized with g-C3N4 to induce recyclability as well as enhanced photocatalytic degradation capability.116
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N of 305 kJ mol−1 and 615 kJ mol−1, respectively.133 Another reason is that the amino (–NH2) and imino (–NH) groups of g-C3N4 can interact with acid groups in the polymer matrix, which leads to enhancement of the Grotthuss-mechanism proton transfer. For instance, Zhou and coworkers blended PVP–phosphonated-poly(2,6-dimethyl-1,4-phenylene oxide) (PVP/pPPO) with g-C3N4 nanosheets via a solution casting method,133 by mixing a certain amount of g-C3N4 in 70% PVP/pPPO solution, then the solution was casted onto a glass plate to obtain the nanocomposite membranes. Improved proton conductivity and mechanical properties were realized due to the proton accepting sites provided by NH2 and the interaction of g-C3N4 with polymer chains, thus leading to higher proton conductivity (74.4 mS cm−1) and power density (294 mW cm−2) at 180 °C with a content of g-C3N4 nanosheets of 5 wt%.
N of 305 kJ mol−1 and 615 kJ mol−1, respectively.133 Another reason is that the amino (–NH2) and imino (–NH) groups of g-C3N4 can interact with acid groups in the polymer matrix, which leads to enhancement of the Grotthuss-mechanism proton transfer. For instance, Zhou and coworkers blended PVP–phosphonated-poly(2,6-dimethyl-1,4-phenylene oxide) (PVP/pPPO) with g-C3N4 nanosheets via a solution casting method,133 by mixing a certain amount of g-C3N4 in 70% PVP/pPPO solution, then the solution was casted onto a glass plate to obtain the nanocomposite membranes. Improved proton conductivity and mechanical properties were realized due to the proton accepting sites provided by NH2 and the interaction of g-C3N4 with polymer chains, thus leading to higher proton conductivity (74.4 mS cm−1) and power density (294 mW cm−2) at 180 °C with a content of g-C3N4 nanosheets of 5 wt%.
To improve the proton conductivity of PA doped PES-PVP membrane material, Lu and coworkers introduced g-C3N4 nanosheets to the polymer composite matrix through a blending method (Fig. 5).134 The as-prepared nanocomposites have shown a significantly improved proton conductivity of 0.104 S cm−1 and power density of 512 mW cm−2 with 0.5% content of g-C3N4. Meanwhile, due to the physical reinforcement effect of 2D g-C3N4 nanosheets, the mechanical properties of the composite membranes were enhanced compared to PES-PVP without g-C3N4. Jiang and coworkers introduced g-C3N4 nanosheets to sulfonated poly(ether ether ketone) composites (SPEEK),135 exhibiting a 68% increase of tensile strength of nanocomposite membranes with g-C3N4 content of 0.5 wt% due to the intrinsic mechanical stability of g-C3N4 nanosheets and favorable interfacial interactions of g-C3N4 nanosheets with the SPEEK matrix. The g-C3N4/SPEEK composites were applied to PEMFCs demonstrating a 39% increase in maximum power density at a g-C3N4 content of 0.5 wt%.
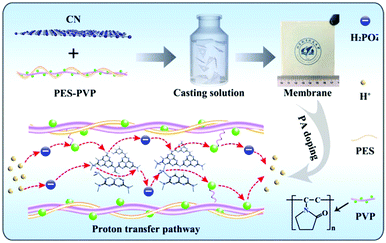 | ||
| Fig. 5 The preparation and proton conductivity mechanism of the g-C3N4 incorporated PES-PVP composite membranes. (PVP: poly(vinyl pyrrolidone); PES: poly(ether sulfone)) (Reprinted with permission.134 Copyright 2019 Elsevier.) | ||
Other energy storage devices such as vanadium redox flow battery (VRB), lithium metal batteries and supercapacitors were also combined with g-C3N4 for improved stability and battery efficiency. Particularly, for VRB, sulfonated aromatic polymers such as SPEEK,136 sulfonated poly(sulfone) (SPSF)137 or sulfonated polyimide (SPI)138 are widely used for fabrication of membranes due to excellent proton conductivity and mechanical properties. However, improved proton conductivity and ion selectivity are still required. Incorporation of g-C3N4 regulates the interfacial interaction of the membrane materials, thus the ion selectivity, and vanadium ion permeation and structure stability could be effectively controlled.139–141 Xiang and coworkers introduced g-C3N4 nanosheets into a Nafion matrix membrane to reduce vanadium ion crossover (Fig. 6a).142 Crosslinking interaction between Nafion matrix and g-C3N4 nanosheets efficiently induced the shrinkage of the Nafion membrane (Fig. 6b and c), resulting in a lamellar structure, thus the vanadium ion crossover is significantly reduced. An improved coulombic efficiency of 97% and energy efficiency of 85% at a current density of 80 mA cm−2 was achieved (Fig. 6d). Moreover, Li and coworkers proposed a lightweight polymer-reinforced electrolyte based on g-C3N4 mesoporous microspheres as electrolyte filler in lithium metal batteries.143 Due to the high mechanical strength and nanosheet-built hierarchical structure of g-C3N4, this electrolyte can effectively suppress lithium dendrite growth during cycling. The Li/Li symmetrical cell based on this electrolyte exhibited a long-term cycling of at least 120 cycles with a high capacity of 6 mA h cm−2. Additionally, g-C3N4 was embedded onto conductive polymers as an efficient electrode material for supercapacitors to improve electrochemical and mechanical stability.144–146 Yang and coworkers prepared a novel electrode material for supercapacitors composed of poly(3,4-ethylenedioxythiophene) (PEDOT):poly(styrenesulfonate) (PSS) and g-C3N4 by the layer-by-layer assembly method.147 Compared with pure PEDOT, the PEDOT/g-C3N4 composite demonstrated excellent electrochemical stability in neutral electrolyte and enhanced electrochemical performance of capacitance of 137 F g−1 in H2SO4 and 200 F g−1 in Na2SO4, respectively.
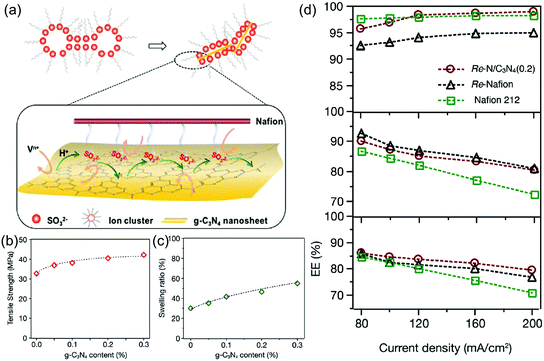 | ||
| Fig. 6 (a) Proton and vanadium ion transport behaviors of the Re-N/CN(x) composite membrane. (b) Tensile strength and (c) swelling ratio of the composite Re-N/CN(x) membranes with various amounts of g-C3N4 nanosheets compared to that of Re-Nafion membranes. (d) Single battery efficiency performance. Coulombic efficiency (CE), voltage efficiency (VE), and energy efficiency (EE) at current densities of 80–200 mA cm−2 of the Vanadium redox flow battery (VRB) with the Re-N/g-C3N4 (0.2) membrane compared to those of the VRB with the Re-Nafion membrane and the commercial Nafion 212 membrane (Reprinted with permission.142 Copyright 2018 Royal Society of Chemistry). | ||
Additionally, few studies reported the combination of g-C3N4 with polymers for solar cell utilization.148 Yang and coworkers for the first time introduced g-C3N4 quantum dots (C3N4QDs) into the active layer of bulk-heterojunction (BHJ) polymer solar cells (PSCs).149 Solution-processable C3N4QDs were prepared by acid treatment of bulk g-C3N4, followed by a solvothermal treatment. Finally, they were doped to the active layers of the PSC with a doping ratio of 0.2 mg mL−1. The different active layers of the C3N4QDs doped BHJ-PSC device demonstrated an obvious enhancement of power conversion efficiencies from 17.5% to 11.6%, depending on the utilized active layer compared to the reference device in the absence of C3N4QDs. The effect of C3N4QDs on surface morphology, optical absorption, PL properties as well as charge transfer properties was specifically studied, hence the mechanism for such efficiency enhancement was proposed. Very recently, following our recent discovery of modifying g-C3N4 with 4-methyl-5-vinylthiazole (vTA), in which the grafting of vTA led to spontaneous polarization and migration of negative charges on thiazole rims whereas g-C3N4 remained positive. Thus significantly influencing the electron transport process,83 which was exploited in another work of our group that applied vTA-grafted g-C3N4 nanosheets (g-C3N4-vTA) as interfacial transporting layers (ETLs) for inverted perovskite solar cells (PVSCs).29 Homogenous films of g-C3N4-vTA were formed via facile spin-coating on two different layers to study the influence of g-C3N4-vTA on the electronic properties of methylammonium lead iodide based PVSCs. The implementation of g-C3N4-vTA enabled interface enhancement via suppression of charge recombination, achieving 1.09 V in Voc and a rise to 20.17 mA cm−2 in short circuit current. Besides, enhanced carrier collection due to the extra light absorption at short wavelengths was observed. Moreover, the introduction of g-C3N4-vTA as alternative interface layer featured metal-free, cheap and benign photophysical properties with high tenability.
4.2 g-C3N4/polymer hybrids for designed material morphologies
For example, Hu and coworkers fabricated a series of sodium alginate (SA) nanocomposite films with different g-C3N4 loading levels via casting technology.156 A physical adsorption method was utilized here to fabricate SA/g-C3N4 composites, SA and g-C3N4 mixture were ultrasonicated at 40 °C to form hydrogen bonds between –OH on SA and uncondensed –NH2 on g-C3N4. Subsequently, the mixture was poured onto a transparent flat dish and left undisturbed for 36 h at 40 °C. The hydrogen bonding behaviour, thermal stability and mechanical performance of the g-C3N4/SA film were studied. Recently, our group investigated a “grafting to” method to graft allyl-end functionalized polymers onto g-C3N4 under visible light irradiation (Fig. 7). Decene end functionalized PMMA, poly(isobornyl methacrylate) (PIBA) and PGMA were firstly synthesized via ATRP.155 In the next step the polymers were mixed with g-C3N4 dispersed in THF and irradiated with visible light to form a covalent bond. The as-prepared polymer/g-C3N4 with different grafting density could be easily processed for fabrication of polymer/g-C3N4 film by dispersing the composites in THF and spin coating on glass slides (Fig. 7b). As such, smooth thin polymer/g-C3N4 films with thicknesses in the range of 60 nm were obtained (Fig. 7c). In addition, the PGMA-based materials could be further modified via nucleophilic ring-opening of the epoxides with thiol compounds (Fig. 7d). In such a way, sodium 2-mercaptoethanesulfonate or 1H,1H,2H,2H-perfuorodecanethiol were further grafted on the g-C3N4-brushes, which led to significant changes in surface hydrophilicity as shown via contact angle measurements after film formation.
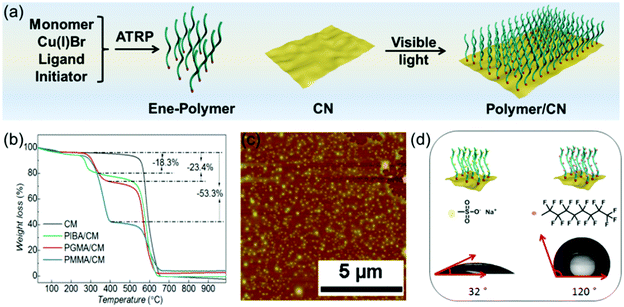 | ||
| Fig. 7 (a) Overview for atom transfer radical polymerization and grafting of polymer brushes onto g-C3N4 under visible light irradiation, (b) thermogravimetric analysis of cyanuric acid-melamine-derived g-C3N4 (CM) precursor and ene-polymer grafted CM, (c) atomic force microscopy profile of spin coated poly(isobornyl acrylate)(PIBA)/CM film, (d) modification of poly(glycidyl methacrylate)(PGMA)/CM via thiol-addition mediated epoxy ring-opening, with sodium 2-mercaptoethanesulfonate and 1H,1H,2H,2H-perfluorodecanethiol, respectively (Licensed under CC-BY).155 | ||
Another work from our group described a g-C3N4-based polymer thermoset coating, and thicker films were obtained via a prepolymer route, which is similar to already discussed g-C3N4-PDMA prepolymers.157 In this case, a prepolymer of PHEMA on g-C3N4 was formed in EG/water mixture that led to the formation of a viscous precursor material composed of PHEMA grafted g-C3N4, EG and water. The viscosity of the precursor was tuned in way that injectable material was obtained. As such, the precursor could be applied to various surfaces, including PS, wood and copper, with spatial control. After addition of citric acid and film formation on a glass slide, crosslinking was performed via heating. The so-formed thermosets formed smooth hydrophobic coatings that could be used for further processing. As the g-C3N4 in the coating retains its photoactivity, PDMA or PS could be modified to the surface via the “grafting from” method to tailor the surface polarity, as shown via contact angle measurements. Moreover, the photoactive surfaces could be used in dye degradation experiments as well as photoelectrochemistry.
Additionally, as discussed in Section 2, g-C3N4 can effectively produce radical species under visible light irradiation, thus polymerization can be conducted on g-C3N4 and g-C3N4/polymer nanocomposites can be obtained. For example, Weber and coworkers reported the formation of g-C3N4-based PBA composites via an aerosol polymerization process (Fig. 8a).160 Spherical mesoporous g-C3N4 (SMCN) was initially prepared with mesoporous silica nanoparticles as template (Fig. 8b), then monomer was continuously added via the gas phase and polymerized in proximity of g-C3N4via photoinitiation under UV light irradiation. Later, spherical g-C3N4/polymer composite particles (Fig. 8c) were obtained without solvent or surfactant. Such a strategy is suitable for fabrication of spherical nanocomposites with hydrophobic polymers. The as-prepared mesoporous CN acts not only as photoinitiator but also as filler and template.
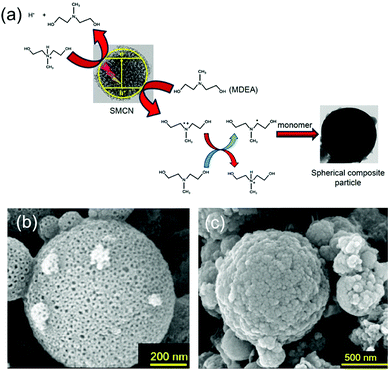 | ||
| Fig. 8 (a) Initiation mechanism of the photopolymerization using spherical mesoporous g-C3N4 (SMCN) in the presence of the methyl diethanolamine (MDEA) as co-initiator. (b) SEM image of SMCN replicas. (c) SEM image of PBA-SMCN composites produced by aerosol-photopolymerization (Reprinted with permission.160 Copyright 2016 American Chemical Society). | ||
Very recently, our team attempted to utilize g-C3N4 as emulsifier and photoinitiator at the same time to conduct emulsion photopolymerization.58 Herein, the emulsion photopolymerization of styrene was studied with non-functionalized g-C3N4, which led to PS latexes with particle diameters around 170 nm. Nevertheless, no satisfying MMA latexes could be obtained that way albeit BMA formed latexes with narrowly distributed particle sizes. Apparently, the monomer structure has a significant impact on the polymerization process, i.e. the interactions of monomer and g-C3N4 seem to play a significant role to create the particle nucleation site. It is very likely that styrene and BMA feature enhanced interactions with g-C3N4 due to π–π interactions, while MMA interacts to a lesser extent. Notably, the utilization of decene-functionalized g-C3N4 enabled the formation of PMMA latexes, probably due to the improved interaction of the initiating stabilizer with the monomer. The formed latexes feature polymer particles that incorporated g-C3N4 and were crosslinked directly. The specific location of g-C3N4 was investigated, demonstrating that g-C3N4 nanosheets ranging from 50–100 nm appeared to be inside of the latex with STEM tilt observation, and small pieces attached outside of latexes with a negative surface charge that grants a stable emulsion latex/g-C3N4 composite. The combination of the traditional PS latex with the outstanding features of environmentally friendly g-C3N4 provides novel polymer composites with multifunctional modern applications, e.g. in bioimaging or 3D printing.
4.3 Improved properties of polymer materials via combination with g-C3N4
It is reported that hybridizing g-C3N4 and polymers on one side significantly improves surface and photocatalytic properties of g-C3N4, on the other side also enhances the thermal and mechanical properties of polymers as well as surface properties.162–164 Few studies reported that the introduction of g-C3N4 to polymers also lead to the improvement of mechanical properties of polymers.165 Myllymaa and coworkers deposited Si-doped CN (CN-Si) on poly(propylene) (PP) disc samples via pulsed laser deposition.166 The coating of CN-Si can significantly convert the PP surface from being hydrophobic to hydrophilic, leading to enhanced adherence of Saos-cells on PP. Hu and coworkers reported a mix of PP-grafted maleic anhydride (PP-g-MA) with g-C3N4 by refluxing the mixture for 4 hours in xylene.167 It was reported that the incorporation of g-C3N4 with PP-g-MA significantly improved the storage modulus, i.e. 2445 MPa for neat PP-g-MA and 2784 MPa for pp-g-MA/g-C3N4, respectively. Moreover, optical results showed that the hybrid materials possessed fascinating UV absorption. Cai and coworker introduced g-C3N4 as reinforcing filler for wood plastic compositions (WPCs), pp-g-MA was added as a coupling agent to increase the interaction between different components.168 The tensile modulus indicating an increase of 143% with g-C3N4 contents of 5 wt%, and the thermal tests demonstrated that the degradation temperature shifted to higher values after g-C3N4 addition. Lin and coworker fabricated g-C3N4/poly(vinyl alcohol) (PVA) nanocomposites by solution casting using water as solvent, demonstrating that the g-C3N4 could be well dispersed in PVA matrix.169 The introduction of g-C3N4 nanosheets increased the glass transition temperature and crystallinity of the nanocomposites, leading to improved mechanical performance with ∼71% enhancement of tensile strength.169 Very recently, Guo and coworkers synthesized g-C3N4 on carbon fiber surfaces in situ in order to enhance interfacial properties of carbon fiber reinforced epoxy resin composites.170 The g-C3N4 on the carbon fiber surface greatly increased the roughness, the content of polar functional groups and wettability of carbon fibers. Thus, significant enhancement of interfacial properties of the composites was obtained.5. Carbon nitride-based hydrogels
Hydrogels constitute an important class of polymeric materials due to their unique features such as swelling properties or soft character while being shape persistent.171 Consisting of a crosslinked hydrophilic network, hydrogels contain significant amounts of water. Their similarity to natural tissues is another important point for the frequent investigation of hydrogels, especially when biomedical applications like tissue-engineering or drug-delivery are targeted.172,173 Other discussed applications comprise actuators,174 self-healing,175 absorption of contaminants176 or shape-memory materials.177 Commonly, hydrogels possess rather weak mechanical properties but research has introduced reinforced hydrogels with partly extraordinary properties. For that, several methods are utilized, e.g. double network hydrogels,178,179 topological (slide-ring) gels,180,181 nanofiber reinforced hydrogels182 or the introduction of charged supports.183–185 Especially, the introduction of particles as reinforcer provides increased stress dissipation, in some cases charge–charge repulsion between the single filler particles or sheets leads to an additional stabilization especially against compression.20 One common example are clay nanosheets that combine ionic interaction and hydrogen bonding with polymeric chains acting as additional crosslinking points.186–188As mentioned before, g-C3N4 can be utilized as photoinitiator for polymerization reactions. As such g-C3N4 can be utilized as well for the formation of hydrogels under visible light. Most notably, these hydrogels incorporate g-C3N4, which might have various effects on materials properties.189 For example, g-C3N4-based hydrogels feature remarkable mechanical properties like compressibility and high storage moduli as discussed in Section 5.1. In addition, g-C3N4-based hydrogels retain the photocatalytic properties of g-C3N4, which allows utilization in contaminant degradation or H2 evolution (Section 5.2). Besides the formation of hydrogels via covalent bonds, g-C3N4 can be utilized in supramolecular hydrogels as well as blended into hydrogel scaffolds to improved mechanical properties for instance, which is discussed in Section 5.3.
5.1 Hydrogels with tailored mechanical properties
It was already stated that the photoactive properties of g-C3N4 enable the formation of radicals in aqueous dispersion.190 Thus, after addition of monomer and crosslinker, hydrogels can be formed with greatest ease via visible light irradiation (Scheme 5a and Table 2). For example, water-soluble acrylamide-derivatives can be utilized, which feature fast gelation rates and a broad spectrum of functionalities. g-C3N4 then indeed does not only act as photoinitiator but also as reinforcer. The reinforcement effect is due to the lateral extension of the g-C3N4 sheets that dissipate mechanical stress throughout the network and thus improve mechanical properties, very similar to the previously mentioned clay nano sheets.| Synthesis method | Monomers/gelators | Properties/applications | Ref. |
|---|---|---|---|
| Photopolymerization | DMA/MBA | Photocatalytic dye degradation and H2 evolution | 191 |
| Photopolymerization | DMA/MBA | High G′ (up to 8.3 kPa), salt and pH response | 64 |
| Photopolymerization | NIPAM/MBA | Thermoresponse | 190 |
| Photopolymerization | AAm/DMA/MBA | High compressibility | 193 |
| Photopolymerization in water/EG | DMA/MBA | High G′ (up to 729 kPa) | 196 |
| Photopolymerization with g-C3N4 prepolymer | DMA/SPMA/MBA | Low friction | 197 |
| Photopolymerization | AAm/MBA | Tetracycline degradation | 200 |
| Photopolymerization | AAm/MBA | Photooxidation of Cr(VI) | 201 |
| Redox polymerization | AAm/MBA | Gel electrophoresis | 192 |
| Thermal polymerization | AAm/acrylic acid/MBA | Ag+ sensing and pH sensitivity | 203 |
| Blending | Graphene/PPy | Photooxidation of Cr(VI) and degradation of phenol | 202 |
| Blending | FMOCDPA | Photo- and enzymatic catalysis | 204 |
| Blending | SA | 3D printing and photocatalysis | 205 |
| Blending | IL | H2S gas sensor | 206 |
| Blending | Agar | Photocatalytic dye degradation | 208 and 209 |
| Supramolecular | Partially hydrolyzed g-C3N4 | Selective dye adsorption | 207 |
As a first example our group employed DMA and N,N′-methylene bisacrylamide (MBA) with a dispersion of 0.6 wt% CM in water for hydrogel formation.191 Hydrogels were obtained after several hours of visible light irradiation. Notably, the hydrogels retained the photocatalytic properties of g-C3N4. In addition, remarkable mechanical properties were observed, such as super-stretchability or being very tough and flexible at the same time. Such behavior opens up a completely new field of applications. In the next step the mechanical properties and origin of gel formation were studied in more detail (Fig. 9).64 To investigate the role of g-C3N4 in the network formation, control reactions were performed, i.e. hydrogel formation with a common photoinitiator/without g-C3N4 (Fig. 9a), which lead to very weak hydrogels. Furthermore, hydrogelation employing g-C3N4 without external crosslinker and non-nitrogen containing monomers was investigated as well. Indeed, hydrogelation took place even without addition of external crosslinker, which indicates the incorporation of g-C3N4 into the network. Moreover, hydrogels could be formed from non-nitrogen containing monomers and crosslinkers, which indicates that the reaction is not dependent on radical transfer from g-C3N4 to amines as instrumentalized in various other reactions.56,57 Moreover, remarkable mechanical properties were obtained (G′ up to 8.3 kPa at solid contents of 11 wt%) (Fig. 9c). One reason is the particular structure of g-C3N4 that acts as colloidal filler via formation of a secondary network of inorganic sheets inside of the hydrogel providing additional strength to the structure. In addition, g-C3N4 introduces additional crosslinking points, which strengthens the hydrogel further. This effect could be analyzed via a control experiment of hydrogel formation in g-C3N4 dispersion but with redox initiation in the absence of light (Fig. 9b). Compared to a reference sample without g-C3N4, improved mechanical properties were found albeit the mechanical properties from visible light g-C3N4 mediated hydrogels were not reached. Thus, both reinforcement via inorganic secondary network as well as via additional crosslinking points are important. The fabricated hydrogels showed a significant shear thinning effect. Such an effect is common to reinforced hydrogels as shear leads to alignment of the polymer network as well as g-C3N4 particles, and such ordering is weakening the g-C3N4-g-C3N4 interactions.
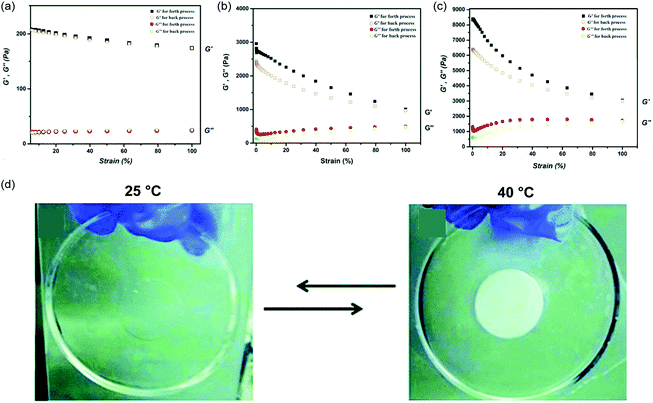 | ||
| Fig. 9 Comparison of storage (G′, black and orange) and loss modulus (G′′, red and green) values of g-C3N4 free N,N-dimethylacrylamide (DMA) hydrogel (a), g-C3N4 embedded DMA hydrogel without covalent bonding (b), and g-C3N4 derived DMA hydrogels with nanosheet integration (c) (Reprinted with permission.64 Copyright 2017 American Chemical Society). (d) Pictures of a monolithic NIPAM/g-C3N4 hydrogel at ambient temperature and elevated temperature, respectively (Licensed under CC-BY).190 | ||
Liu and coworkers showed the formation of a g-C3N4/NIPAM hydrogel.190 A hydrogel was formed via visible light mediated photopolymerization albeit no external crosslinker was added. The NIPAM-based hydrogels showed thermoresponsive properties. For example, the viscosity, storage and loss modulus of the obtained hydrogels decreased until the lower critical solution temperature (LCST) of PNIPAM and increased again above the LCST. Moreover, the authors could form the hydrogels in specific patterns that changed transparency reversibly according to temperature (Fig. 9d). Farzaneh and coworkers physically incorporated g-C3N4 into acrylamide hydrogels for gel electrophoresis via a redox polymerization of AAm and MBA.192 Due to the thermal conductivity of g-C3N4, Joule heating in the hydrogels was reduced and band broadening in the electrophoresis was lowered. Moreover, the presence of g-C3N4 allowed to refrain from utilization of tetramethyl ethylenediamine as polymerization catalyst, which can be a disadvantage for some analytes.
As g-C3N4 provides the opportunity to modify the surface charge (zeta potential), surface area and light absorption via variation in the precursor composition,43,194,195 effects of the precursor composition on mechanical properties were investigated as well. Notably, the zeta potential had a profound effect on storage modulus. It was found that stronger negative zeta potential g-C3N4 compounds led to stronger hydrogels.64 Such an effect can be explained by g-C3N4 sheet repulsion that increases with more negative zeta potentials. A similar effect was already described in the literature for other reinforcing particles.185,188 The effect of surface charge was further investigated by our group employing AHPA-modified g-C3N4, which features significantly lower zeta potentials and high dispersibilities due to the sulfonic acid group.193 A mixture of AAm, DMA and MBA were used as monomers together with CM-AHPA as initiator. The hydrogels obtained were rather soft with G′ in the range of 100–200 Pa and contained solid contents below 10 wt%. However, the hydrogels featured remarkable compression properties, e.g. withstand loads above 12 MPa (Fig. 10) and resisted multiple hits with a hammer. Probably, the extreme compressibility is due to the fact that the gels contained highly negatively charged g-C3N4, which shows significant repulsion of the g-C3N4 sheets in compression. In addition, the gels were soft and could dissipate the compressive force over the whole structure by electrostatic coupling. Compression led to a complete flattening of the structure and a return to the initial shape after release of the force. In order to get further insights into the origin of the remarkable compression properties, hydrogels were formed from the individual monomers with MBA. It was found that the DMA-based hydrogels were stronger but less compression resistant, while the AAm-based hydrogels were weaker but more resistant to compression. Swelling the AAm/DMA hydrogels with salt solution (NaCl or CaCl2) showed increased strength but less compressibility, which might be due to the screening of the negative charges on the g-C3N4 surface. Hence, the surface charge of g-C3N4 is certainly the main reason for the enhanced compressibility, while the monomer mixture supports compressibility due to enhanced elasticity, which leads to improved distribution of the stress in the network.
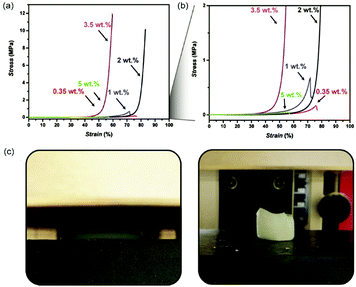 | ||
| Fig. 10 (a) Compression test results of g-C3N4-AHPA (g-C3N4-3-allyloxy-2-hydroxy-1-propanesulfonic acid sodium salt)-based hydrogels (red: 0.35 wt%; blue: 1 wt%; black: 2 wt%; violet: 3.5 wt%; green: 5 wt%), (b) magnification of compression test results of g-C3N4-AHPA hydrogels, (c) images of 2 wt% g-C3N4-AHPA hydrogel during (left) and after (right) compression (Reproduced with permission.193 Copyright 2019 John Wiley and Sons). | ||
Regarding the mechanical properties of the hydrogels, not only the charge on the g-C3N4 matters but also the amount of incorporated g-C3N4. Therefore, EG/water mixtures were used to increase the amount of non-functionalized g-C3N4 during hydrogel formation.196 Incorporating g-C3N4 contents up to 4 wt% led to hydrogels with G′ of 88 kPa for 2 wt%, 430 kPa for 3 wt% and 729 kPa for 4 wt% of g-C3N4 at 0.1% strain, which is a remarkable increase of two orders of magnitude compared to the first hydrogels with 0.6 wt% g-C3N4. In addition to enhanced mechanical performance, hydrogelation was much faster with higher g-C3N4 content which proves g-C3N4 acts as photoinitiator. As the hydrogel formation is photoinitiated, patterning was investigated as well. For that, parts of the reaction mixture were covered with a photomask to obtain an inversely shaped hydrogel (Scheme 5b and Fig. 11). The success of such experiments also proves that there is no significant radical transfer to dark area (solution) and the polymerization takes place only in the illuminated area via g-C3N4 initiation. In order to enable the fabrication of hydrogels with a broader range of monomers, e.g. charged monomers, our group utilized g-C3N4-PDMA prepolymer formed in EG/water mixture without crosslinker.82 With prepolymer, hydrogels from 3-sulfopropyl methacrylate potassium salt (SPMA) and DMA were obtained. The SPMA monomer introduces negative charges into the hydrogel structure, which is a useful feature for low friction surfaces.197 Hence, hydrogels with both very low friction coefficients (around 0.03) and remarkable compression properties due to g-C3N4 incorporation were obtained. This combination of properties is rather uncommon and challenging to achieve but of particular interest for applications, e.g. in artificial cartilage.198
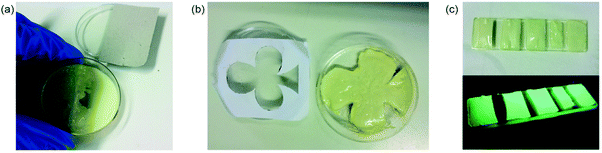 | ||
| Fig. 11 Spatially controlled photopolymerization for gel formation: (a) formation of a self-standing half-circle, (b) self-standing club shape and (c) photopatterning of stripes on a glass slide (Licensed under CC-BY).196 | ||
5.2 Utilization of photophysical properties
The incorporation of g-C3N4 into hydrogels occurs under retention of its photophysical properties in the dispersed state inside of the network (Table 2). As such, various properties are accessible, e.g. photocatalysis or water contaminant degradation. Dong and coworkers obtained acrylamide-based hydrogels for “light filtering” applications.199 AAm was utilized as monomer in a visible light induced polymerization. Due to the hydrogen bonding interactions between g-C3N4 and PAAm in the hydrogel self-healing was observed after cutting the hydrogel. Similar mechanical properties (compression and tensile) were observed before and after healing. The hydrogels featured high photostability and could be utilized for UV shielding due to the broad absorption of g-C3N4 in the gel. For example, for UVA, UVB and UVC a transmittance of 0 to 28% was observed, while at 550 nm a transmittance of 89% was measured. Thus, these gels might be used as sun-light blockers.Shalom and coworkers formed g-C3N4-based hydrogels for photocatalytic applications (Fig. 12a).191 For example dye degradation reactions could be performed easily. Several dyes were probed as reference compounds for other impurities in waste water. Depending on the type, absorption in the gel proceed with different efficiency, which was mainly attributed to ionic interactions. In addition, hydrogen evolution could be performed after addition of platinum cocatalyst (Fig. 12b) and the catalytic hydrogel could be recycled easily (Fig. 12c). Another hydrogel for waste water treatment was described by Yao and coworkers.200 In this case, bentonite, g-C3N4, AAm and MBA were combined to form hydrogel monoliths via thermal polymerization. Finally, the monoliths were cut into small cubes, and adsorption of tetracycline was tested. The incorporation of bentonite led to enhanced adsorption of the organic contamination. In the next step, tetracycline was degraded via visible light irradiation. Furthermore, adsorption and degradation were studied in the flow process that showed a high removal efficiency and cycling stability.
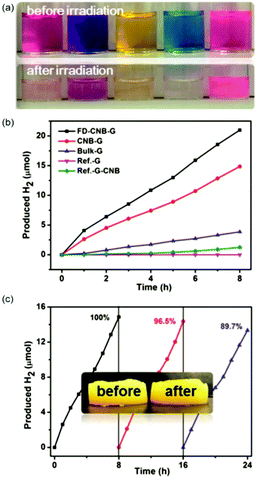 | ||
| Fig. 12 (a) Photodegradation of various dyes catalyzed by a g-C3N4 hydrogel (congo red, crystal violet, methyl orange, MB, and RhB, from left to right). (b) Kinetics of H2 production from water with g-C3N4 hydrogels (FD-CNB-G: freeze dried g-C3N4-based hydrogel and CNB-G: g-C3N4-based hydrogel) and references (Bulk-G: hydrogel from melamine-derived g-C3N4; ref.-G: hydrogel without g-C3N4 incorporation; ref.-G-CNB: reference gel after adsorption of g-C3N4 suspension) under white LED irradiation. (c) Cycling measurements of H2 generation via wet g-C3N4 hydrogel (the inset shows the g-C3N4 hydrogel before and after H2 production) (Reprinted with permission.191 Copyright 2017 American Chemical Society). | ||
Another photocatalytic process employing g-C3N4-based hydrogels was presented by Lamkaho and Randorn.201 A PAAm hydrogel was formed via photopolymerization of AAm and MBA under UV light. Finally, the hydrogel was utilized for the photocatalytic reduction of Cr(VI) to Cr(III). Cui and coworkers described a hydrogel formed from g-C3N4, graphene and PPy.202 Improvements of the photocatalytic properties were obtained via graphene as electron transporter and PPy as hole transporter. The obtained hydrogels were utilized for photodegradation of phenol and reduction/adsorption of Cr(VI). Tu and coworkers introduced another application of g-C3N4 based hydrogels. An AAm/acrylic acid-based hydrogel was formed via thermal initiation that could be utilized as sensor for Ag+ ions.203 Due to the acrylic acid incorporation, the hydrogels were pH sensitive, i.e. the swelling state changed according to pH. Moreover, the hydrogels showed fluorescent behavior because of the g-C3N4 incorporation. The fluorescence could in turn be utilized for sensing. The change in fluorescence after addition of various metal ions was tested, and in the case of Ag+ a significant quenching effect was observed down to concentrations of 6.31 μM. Notably, the detection of Ag+ was still possible when other contaminant ions were present.
5.3 Blending and supramolecular hydrogels
In addition to covalent hydrogels formed via polymerization, g-C3N4 was incorporated into hydrogels via blending or supramolecular interactions as well (Scheme 5c and Table 2). For example, Park and coworkers presented the combination of g-C3N4 and peptide materials.204 In those experiments, Fmoc-diphenylalanine (FMOCDPA) was utilized to undergo self-assembly in the presence of g-C3N4 yielding a hydrogel. The formed hydrogel was utilized to reduce NAD+ to NADH. Moreover, the incorporation of an enzyme facilitated the combination of photo and enzymatic catalysis. A remarkable hydrogel was described by Fan and coworkers, who combined g-C3N4 with the Ca2+/alginate supramolecular hydrogel and 3D printing (Fig. 13a).205 Hence, 3D hydrogel scaffolds were obtained that could be utilized for photocatalytic tasks. A carbon nitride nanosheet/sodium alginate (CNNS-SA)-based ink was printed and crosslinked in CaCl2/glycerol solution (Fig. 13b and c). Inclusion of gold nanopyramids in such 3D scaffolds boosted the photocatalytic activity for solar wastewater remediation. Thus, the gel architecture could be utilized to tailor the catalysis efficiency via enhanced substrate transport. Ayajan and coworkers presented a gel from g-C3N4 and ionic liquids (IL) forming an amphiphilic network that was utilized as H2S gas sensor at ambient temperature.206 ILs served as exfoliating agent for g-C3N4 sheets at high temperatures, and gel formation was observed at 200 °C after 24 h, where exfoliated g-C3N4 nanosheets assembled in the IL matrix.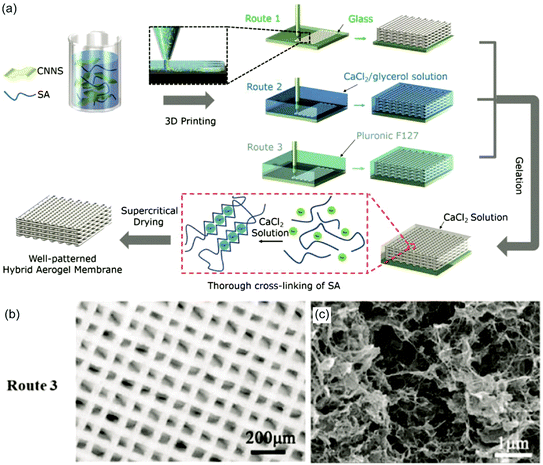 | ||
Fig. 13 (a) Schematic illustration of the 3D fabrication process: a highly concentrated homogeneous CNNS-SA (g-C3N4 nano sheet-sodium alginate) ink was extruded and directly printed onto a glass substrate covered with a layer of Vaseline (Route 1), into a reservoir composed of a CaCl2/glycerol solution (Route 2) or Pluronic F127 (Route 3). Subsequently, the printed lattices were submerged in a CaCl2 aqueous solution overnight to crosslink the SA. (b) Optical image of a woodpile structure (mass ratio CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SA = 1 SA = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) printed via Pluronic F127 (Route 3) and (c) a cross-sectional SEM image (Reprinted with permission.205 Copyright 2018 John Wiley and Sons). 2) printed via Pluronic F127 (Route 3) and (c) a cross-sectional SEM image (Reprinted with permission.205 Copyright 2018 John Wiley and Sons). | ||
A hydrogel solely formed from g-C3N4 was investigated by Zhang and coworkers.207 Here, g-C3N4 was partially hydrolyzed in sodium hydroxide solution. The obtained material formed reversible hydrogel structures via bubbling with CO2 or N2 that could be utilized for selective dye absorption. In the presence of agar, heating–cooling polymerization yielded hydrogels including g-C3N4.208 The agar-based hydrogels were further utilized for effective photocatalytic degradation due to enhanced adsorption capacity. In a similar way, Zhu and coworkers utilized a g-C3N4/agar hydrogel for photocatalytic tasks.209 Therefore, g-C3N4 and agar were dispersed in water and heated. After cooling a hydrogel was obtained that was used for the photodegradation of MB and phenol. A significant activity was obtained due to the adsorptive behaviour of the hydrogel.
6. Conclusion and outlook
Combining exfoliated g-C3N4 nanosheets with polymers is a recent hotspot of materials research, thus enabling many advanced functionalities and hybrid materials with unusual properties extending the previous range of the possible. The development is mainly driven by typical g-C3N4 properties, such as photoinitiation, photocatalysis, or photoluminescence. However, it turned out that these properties are significantly improved or optimized in the polymer hybrids, which is due to improved delamination, but also due to synergies of the two sides. Introducing for instance conducting polymers to g-C3N4 enables overcoming the drawback of low electron conductivity of g-C3N4, and hence the photocatalytic or H2 evolution properties of g-C3N4 are promoted. In biosensor systems, polymer functionalized g-C3N4 has shown an improved sensitivity and selectivity, which we attribute to improved dispersion and access to the sensing sites. Polymer modified g-C3N4 showed enhanced dispersibility and processability, and for instance can be employed in film formation.On the other hand, g-C3N4 with its multiple properties brings advantages to polymer materials as well. For example, doping the polymer matrix with g-C3N4 enhances the performance of electrode materials in energy storage and improves stability as well as efficiency compared to the pure polymer matrix. Photoinitiation with g-C3N4 facilitates polymerization in solution and on g-C3N4 to obtain g-C3N4/polymer nanocomposites with inherent photoluminescence that can be applied for various applications. In addition, combination of g-C3N4 with polymers enhances the thermal and mechanical properties of polymer materials. In the realm of bulk soft materials, g-C3N4 is particularly useful as reinforcer in hydrogels and g-C3N4/hydrogel hybrids can be obtained. Overall, the plethora of monomer combinations and g-C3N4 types allows the fabrication of tailored hydrogel materials with very unusual and most useful properties, e.g. ultralow friction, being thermoresponsive, or tough but compressible. Indeed, the storage modulus of hydrogels could be varied over a broad range (approximately 7 kPa to 700 kPa) via g-C3N4 content and type, only, while the toughness of the material could be adjusted by monomer mixtures, e.g. AAm and DMA. By inherent photopolymerization, reinforced hydrogels can be obtained also in a spatially controlled way and directions of additive manufacturing are certainly a promising approach a toolbox is needed for.210,211
Polymers might pave the way for novel applications for g-C3N4, however some main issues still need to be addressed. Although promising results were reported so far, the research for g-C3N4/polymer hybrids is still in the infant stage, and further investigations and developments are still required. To date, there are a number of issues, which need to be resolved for improved combination of g-C3N4 and polymer. For example, most of the research reported utilizes the simple blending method of fabrication, thus connection between polymer and g-C3N4 is enabled by physical interaction, only, which may cause restrictions for the future commercial application. Moreover, the synthesis process mainly depends on organic solvents, which is not environment friendly when expanded to commercial scale, thus solvent-free, mechanochemical and green routes should be developed in the future research. In oxygen evolution and organic synthesis, g-C3N4 is also of great significance as a photocatalyst. Nevertheless, g-C3N4/polymer hybrids are rarely applied in these areas so far. As such, one can expect significant outcomes in the future in organic synthesis and oxygen evolution. Overall, g-C3N4 has a promising future especially in energy conversion and storage, and enhancing surface area of g-C3N4 by polymers might become key for utilization of g-C3N4 in batteries, supercapacitors and other high-efficiency energy conversion devices.
Polymers have opened up new doors for g-C3N4 which could not be imagined seven years ago. Previously, dispersibility and processability issues were the main problems for integration of g-C3N4 materials, however with the current ideas in mind dispersibility becomes less of an issue. Ultimately, g-C3N4 is a sustainable and cheap alternative for other semiconductors, and we believe that with precise tailoring of both g-C3N4 properties and design of a surrounding polymer, g-C3N4 will play an increasingly important role both in academia and in the industry of the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for funding from Max-Planck society and the University of Glasgow as well as funding for Open Access from the University of Glasgow.Notes and references
- D. Larcher and J. M. Tarascon, Nat. Chem., 2014, 7, 19 CrossRef.
- Y. Zhu, C. Romain and C. K. Williams, Nature, 2016, 540, 354 CrossRef CAS.
- Y. Zheng, L. Lin, B. Wang and X. Wang, Angew. Chem., Int. Ed., 2015, 54, 12868–12884 CrossRef CAS.
- D. Esposito and M. Antonietti, Chem. Soc. Rev., 2015, 44, 5821–5835 RSC.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- M. Chhowalla, D. Jena and H. Zhang, Nat. Rev. Mater., 2016, 1, 16052 CrossRef CAS.
- T. M. Brenner, D. A. Egger, L. Kronik, G. Hodes and D. Cahen, Nat. Rev. Mater., 2016, 1, 15007 CrossRef CAS.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC.
- X. Wang, S. Blechert and M. Antonietti, ACS Catal., 2012, 2, 1596–1606 CrossRef CAS.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2008, 8, 76 CrossRef.
- J. Liu, H. Wang and M. Antonietti, Chem. Soc. Rev., 2016, 45, 2308–2326 RSC.
- G. Algara-Siller, N. Severin, S. Y. Chong, T. Björkman, R. G. Palgrave, A. Laybourn, M. Antonietti, Y. Z. Khimyak, A. V. Krasheninnikov, J. P. Rabe, U. Kaiser, A. I. Cooper, A. Thomas and M. J. Bojdys, Angew. Chem., Int. Ed., 2014, 53, 7450–7455 CrossRef CAS PubMed.
- W. J. Ong, L. L. Tan, Y. H. Ng, S. T. Yong and S. P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS.
- J. Ran, T. Y. Ma, G. Gao, X.-W. Du and S. Z. Qiao, Energy Environ. Sci., 2015, 8, 3708–3717 RSC.
- S. Hu, F. Li, Z. Fan, F. Wang, Y. Zhao and Z. Lv, Dalton Trans., 2015, 44, 1084–1092 RSC.
- K. K. R. Datta, B. V. S. Reddy, K. Ariga and A. Vinu, Angew. Chem., Int. Ed., 2010, 49, 5961–5965 CrossRef CAS.
- B. Kurpil, B. Kumru, T. Heil, M. Antonietti and A. Savateev, Green Chem., 2018, 20, 838–842 RSC.
- K. Schwinghammer, B. Tuffy, M. B. Mesch, E. Wirnhier, C. Martineau, F. Taulelle, W. Schnick, J. Senker and B. V. Lotsch, Angew. Chem., Int. Ed., 2013, 52, 2435–2439 CrossRef CAS.
- G. Zhang, G. Li, Z. A. Lan, L. Lin, A. Savateev, T. Heil, S. Zafeiratos, X. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2017, 56, 13445–13449 CrossRef CAS.
- J. Yang, C.-R. Han, X.-M. Zhang, F. Xu and R.-C. Sun, Macromolecules, 2014, 47, 4077–4086 CrossRef CAS.
- N. Cheng, J. Tian, Q. Liu, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6815–6819 CrossRef CAS.
- R. Kuriki, K. Sekizawa, O. Ishitani and K. Maeda, Angew. Chem., Int. Ed., 2015, 54, 2406–2409 CrossRef CAS.
- J. Lin, Z. Pan and X. Wang, ACS Sustainable Chem. Eng., 2014, 2, 353–358 CrossRef CAS.
- K. Xiao, L. Chen, R. Chen, T. Heil, S. D. C. Lemus, F. Fan, L. Wen, L. Jiang and M. Antonietti, Nat. Commun., 2019, 10, 74 CrossRef.
- K. Xiao, B. Kumru, L. Chen, L. Jiang, B. V. K. J. Schmidt and M. Antonietti, Beilstein J. Nanotechnol., 2019, 10, 1316–1323 CrossRef CAS.
- G. Peng, M. Volokh, J. Tzadikov, J. Sun and M. Shalom, Adv. Energy Mater., 2018, 8, 1800566 CrossRef.
- X. Chen, Q. Liu, Q. Wu, P. Du, J. Zhu, S. Dai and S. Yang, Adv. Funct. Mater., 2016, 26, 1719–1728 CrossRef CAS.
- T. R. Chetia, M. S. Ansari and M. Qureshi, J. Mater. Chem. A, 2016, 4, 5528–5541 RSC.
- D. Cruz, J. Garcia Cerrillo, B. Kumru, N. Li, J. Dario Perea, B. Schmidt, I. Lauermann, C. J. Brabec and M. Antonietti, J. Am. Chem. Soc., 2019, 140, 17532–17537 Search PubMed.
- J. Xu and M. Antonietti, J. Am. Chem. Soc., 2017, 139, 6026–6029 CrossRef CAS PubMed.
- Y. Shiraishi, S. Kanazawa, Y. Sugano, D. Tsukamoto, H. Sakamoto, S. Ichikawa and T. Hirai, ACS Catal., 2014, 4, 774–780 CrossRef CAS.
- Y. Chen, W. Huang, D. He, Y. Situ and H. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 14405–14414 CrossRef CAS.
- P. Gibot, F. Schnell and D. Spitzer, Microporous Mesoporous Mater., 2016, 219, 42–47 CrossRef CAS.
- D. A. Erdogan, M. Sevim, E. Kısa, D. B. Emiroglu, M. Karatok, E. I. Vovk, M. Bjerring, Ü. Akbey, Ö. Metin and E. Ozensoy, Top. Catal., 2016, 59, 1305–1318 CrossRef CAS.
- M. Xie, W. Wei, Z. Jiang, Y. Xu and J. Xie, Ceram. Int., 2016, 42, 4158–4170 CrossRef CAS.
- G. Li, Z. Lian, W. Wang, D. Zhang and H. Li, Nano Energy, 2016, 19, 446–454 CrossRef CAS.
- J. Fang, H. Fan, M. Li and C. Long, J. Mater. Chem. A, 2015, 3, 13819–13826 RSC.
- Q. Cui, J. Xu, X. Wang, L. Li, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2016, 55, 3672–3676 CrossRef CAS.
- K. Xiao, B. Tu, L. Chen, T. Heil, L. Wen, L. Jiang and M. Antonietti, Angew. Chem., Int. Ed., 2019, 58, 12574–12579 CrossRef CAS.
- D. Xiao, D. Yuan, H. He and J. Lu, Luminescence, 2013, 28, 612–615 CrossRef CAS.
- J. Bian, Q. Li, C. Huang, J. Li, Y. Guo, M. Zaw and R.-Q. Zhang, Nano Energy, 2015, 15, 353–361 CrossRef CAS.
- M. Shalom, S. Inal, C. Fettkenhauer, D. Neher and M. Antonietti, J. Am. Chem. Soc., 2013, 135, 7118–7121 CrossRef CAS.
- J. Barrio and M. Shalom, ChemCatChem, 2018, 10, 5573–5586 CrossRef CAS.
- K. S. Lakhi, W. S. Cha, S. Joseph, B. J. Wood, S. S. Aldeyab, G. Lawrence, J.-H. Choy and A. Vinu, Catal. Today, 2015, 243, 209–217 CrossRef CAS.
- M. Groenewolt and M. Antonietti, Adv. Mater., 2005, 17, 1789–1792 CrossRef CAS.
- Y. Wang, X. Wang, M. Antonietti and Y. Zhang, ChemSusChem, 2010, 3, 435–439 CrossRef CAS.
- J. Zhou, Y. Yang and C.-y. Zhang, Chem. Commun., 2013, 49, 8605–8607 RSC.
- J. Zhang, G. Zhang, X. Chen, S. Lin, L. Möhlmann, G. Dołęga, G. Lipner, M. Antonietti, S. Blechert and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 3183–3187 CrossRef CAS.
- Y. Chen, F. Ding, A. Khaing, D. Yang and Z. Jiang, Appl. Surf. Sci., 2019, 757–764 CrossRef.
- J. Barrio, L. Lin, P. Amo-Ochoa, J. Tzadikov, G. Peng, J. Sun, F. Zamora, X. Wang and M. Shalom, Small, 2018, 14, 1800633 CrossRef.
- J. Barrio and M. Shalom, ACS Appl. Mater. Interfaces, 2018, 10, 39688–39694 CrossRef CAS.
- T. Jordan, N. Fechler, J. Xu, T. J. K. Brenner, M. Antonietti and M. Shalom, ChemCatChem, 2015, 7, 2826–2830 CrossRef CAS.
- Q. Cao, Q. Cui, Y. Yang, J. Xu, C. Han and L. Li, Chem. – Eur. J., 2018, 24, 2286–2291 CrossRef CAS.
- B. Kiskan, J. Zhang, X. Wang, M. Antonietti and Y. Yagci, ACS Macro Lett., 2012, 1, 546–549 CrossRef CAS.
- M. Al-Naji, B. Puertolas, B. Kumru, D. Cruz, M. Baumel, B. V. K. J. Schmidt, N. V. Tarakina and J. Perez-Ramirez, ChemSusChem, 2019, 12, 2628–2636 CrossRef CAS.
- S. Dadashi-Silab, M. A. Tasdelen, B. Kiskan, X. Wang, M. Antonietti and Y. Yagci, Macromol. Chem. Phys., 2014, 215, 675–681 CrossRef CAS.
- B. Kiskan, J. Zhang, X. Wang, M. Antonietti and Y. Yagci, ACS Macro Lett., 2012, 1, 546–549 CrossRef CAS.
- Q. Cao, T. Heil, B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Polym. Chem., 2019, 10, 5315–5323 RSC.
- D. J. Martin, K. Qiu, S. A. Shevlin, A. D. Handoko, X. Chen, Z. Guo and J. Tang, Angew. Chem., Int. Ed., 2014, 53, 9240–9245 CrossRef CAS.
- D. J. Martin, P. J. Reardon, S. J. Moniz and J. Tang, J. Am. Chem. Soc., 2014, 136, 12568–12571 CrossRef CAS.
- Q. Fu, Q. Ruan, T. G. McKenzie, A. Reyhani, J. Tang and G. G. Qiao, Macromolecules, 2017, 50, 7509–7516 CrossRef CAS.
- L. Zhang, G. Ye, X. Huo, S. Xu, J. Chen and K. Matyjaszewski, ACS Omega, 2019, 4, 16247–16255 CrossRef CAS.
- B. Kumru, J. Mendoza Mesa, M. Antonietti and M. Al-Naji, ACS Sustainable Chem. Eng., 2019, 7, 17574–17579 CrossRef CAS.
- B. Kumru, M. Shalom, M. Antonietti and B. V. K. J. Schmidt, Macromolecules, 2017, 50, 1862–1869 CrossRef CAS.
- E. G. Gillan, Chem. Mater., 2000, 12, 3906–3912 CrossRef CAS.
- J. Sun, R. Phatake, A. Azoulay, G. Peng, C. Han, J. Barrio, J. Xu, X. Wang and M. Shalom, Chem. – Eur. J., 2018, 24, 14921–14927 CrossRef CAS.
- H. Arazoe, D. Miyajima, K. Akaike, F. Araoka, E. Sato, T. Hikima, M. Kawamoto and T. Aida, Nat. Mater., 2016, 15, 1084 CrossRef CAS.
- N. Karjule, R. Phatake, M. Volokh, I. Hod and M. Shalom, Small Methods, 2019, 1900401 CrossRef CAS.
- G. Peng, L. Xing, J. Barrio, M. Volokh and M. Shalom, Angew. Chem., Int. Ed., 2018, 57, 1186–1192 CrossRef CAS.
- X. She, H. Xu, Y. Xu, J. Yan, J. Xia, L. Xu, Y. Song, Y. Jiang, Q. Zhang and H. Li, J. Mater. Chem. A, 2014, 2, 2563–2570 RSC.
- Q. Han, B. Wang, Y. Zhao, C. Hu and L. Qu, Angew. Chem., Int. Ed., 2015, 54, 11433–11437 CrossRef CAS.
- L. Chen, D. Huang, S. Ren, T. Dong, Y. Chi and G. Chen, Nanoscale, 2013, 5, 225–230 RSC.
- P. Niu, L. Zhang, G. Liu and H.-M. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS.
- J. K. Kim, S. Park, R. J. Yoo, H. J. Jeong, J. Oh, Y. J. Lee, S. Park and D. W. Kim, Chem. – Eur. J., 2018, 24, 3506–3511 CrossRef CAS.
- B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Langmuir, 2017, 33, 9897–9906 CrossRef CAS.
- Y. Shi, B. Wang, L. Duan, Y. Zhu, Z. Gui, R. K. K. Yuen and Y. Hu, Ind. Eng. Chem. Res., 2016, 55, 7646–7654 CrossRef CAS.
- Q. Cui, J. Xu, X. Wang, L. Li, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2016, 55, 3672–3676 CrossRef CAS.
- X. Bu, J. Li, S. Yang, J. Sun, Y. Deng, Y. Yang, G. Wang, Z. Peng, P. He, X. Wang, G. Ding, J. Yang and X. Xie, ACS Appl. Mater. Interfaces, 2016, 8, 31419–31425 CrossRef CAS.
- R. B. N. Baig, S. Verma, M. N. Nadagouda and R. S. Varma, Sci. Rep., 2016, 6, 39387 CrossRef CAS.
- Y. Zheng, Z. Zhang, C. Li and S. Proulx, Mater. Res. Bull., 2016, 84, 46–56 CrossRef CAS.
- B. Kumru, V. Molinari, M. Hilgart, F. Rummel, M. Schäffler and B. V. K. J. Schmidt, Polym. Chem., 2019, 10, 3647–3656 RSC.
- B. Kumru, D. Cruz, T. Heil, B. V. K. J. Schmidt and M. Antonietti, J. Am. Chem. Soc., 2018, 140, 17532–17537 CrossRef CAS.
- B. J. Blaiszik, S. L. B. Kramer, S. C. Olugebefola, J. S. Moore, N. R. Sottos and S. R. White, Annu. Rev. Mater. Res., 2010, 40, 179–211 CrossRef CAS.
- M. A. Stuart, W. T. Huck, J. Genzer, M. Muller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- L. S. Nair and C. T. Laurencin, Prog. Polym. Sci., 2007, 32, 762–798 CrossRef CAS.
- Y. Wang and X. Jing, Polym. Adv. Technol., 2005, 16, 344–351 CrossRef CAS.
- J. Zhang, M. Zhang, L. Lin and X. Wang, Angew. Chem., Int. Ed., 2015, 54, 6297–6301 CrossRef CAS.
- Z. Zhao, Y. Sun and F. Dong, Nanoscale, 2015, 7, 15–37 RSC.
- A. C. Balazs, T. Emrick and T. P. Russell, Science, 2006, 314, 1107 CrossRef CAS.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350–1375 CrossRef CAS.
- S. Patnaik, D. P. Sahoo and K. Parida, in Nanocomposites for Visible Light-induced Photocatalysis, ed. M. M. Khan, D. Pradhan and Y. Sohn, Springer International Publishing, Cham, 2017, pp. 251–294 DOI:10.1007/978-3-319-62446-4_9.
- Y. Zhang, F. Mao, H. Yan, K. Liu, H. Cao, J. Wu and D. Xiao, J. Mater. Chem. A, 2015, 3, 109–115 RSC.
- Y. Sui, J. Liu, Y. Zhang, X. Tian and W. Chen, Nanoscale, 2013, 5, 9150–9155 RSC.
- F. He, G. Chen, Y. Yu, S. Hao, Y. Zhou and Y. Zheng, ACS Appl. Mater. Interfaces, 2014, 6, 7171–7179 CrossRef CAS.
- H. Yan and Y. Huang, Chem. Commun., 2011, 47, 4168–4170 RSC.
- A. Li, Q. Cao, G. Zhou, B. V. K. J. Schmidt, W. Zhu, X. Yuan, H. Huo, J. Gong and M. Antonietti, Angew. Chem., Int. Ed., 2019, 58, 14549–14555 CrossRef CAS.
- S. Roy and E. Reisner, Angew. Chem., Int. Ed., 2019, 58, 12180–12184 CrossRef CAS.
- K. Pandiselvi, H. Fang, X. Huang, J. Wang, X. Xu and T. Li, J. Hazard. Mater., 2016, 314, 67–77 CrossRef CAS.
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- S. Xiao, Z. Wang, H. Ma, H. Yang and W. Xu, Adv. Powder Technol., 2014, 25, 574–581 CrossRef CAS.
- M. Panizza and G. Cerisola, Chem. Rev., 2009, 109, 6541–6569 CrossRef CAS.
- C. P. Athanasekou, N. G. Moustakas, S. Morales-Torres, L. M. Pastrana-Martínez, J. L. Figueiredo, J. L. Faria, A. M. T. Silva, J. M. Dona-Rodriguez, G. E. Romanos and P. Falaras, Appl. Catal., B, 2015, 178, 12–19 CrossRef CAS.
- Y. Zhao, Y. Zhang, A. Liu, Z. Wei and S. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 4006–4014 CrossRef CAS PubMed.
- Y. Huang, H. Li, M. S. Balogun, W. Liu, Y. Tong, X. Lu and H. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 22920–22927 CrossRef CAS.
- W. Jiang, W. Luo, R. Zong, W. Yao, Z. Li and Y. Zhu, Small, 2016, 12, 4370–4378 CrossRef CAS PubMed.
- L. Ge, C. Han and J. Liu, J. Mater. Chem., 2012, 22, 11843–11850 RSC.
- B. Vellaichamy and P. Periakaruppan, Ind. Eng. Chem. Res., 2018, 57, 6684–6695 CrossRef CAS.
- X. Bu, Y. Lu, S. Chen, D. Li, Z. Zhang and P. Qian, Chem. Eng. Sci., 2019, 355, 299–308 CrossRef CAS.
- T. Xu, F. Wu, Y. Gu, Y. Chen, J. Cai, W. Lu, H. Hu, Z. Zhu and W. Chen, RSC Adv., 2015, 5, 86505–86512 RSC.
- N. H. Alias, J. Jaafar, S. Samitsu, N. Yusof, M. H. D. Othman, M. A. Rahman, A. F. Ismail, F. Aziz, W. N. W. Salleh and N. H. Othman, Chemosphere, 2018, 204, 79–86 CrossRef CAS.
- X. Zhou, C. Shao, S. Yang, X. Li, X. Guo, X. Wang, X. Li and Y. Liu, ACS Sustainable Chem. Eng., 2018, 6, 2316–2323 CrossRef CAS.
- D. Qin, W. Lu, Z. Zhu, N. Li, T. Xu, G. Wang and W. Chen, Ind. Eng. Chem. Res., 2017, 56, 11151–11160 CrossRef CAS.
- S. Chen, W. Lu, J. Han, H. Zhong, T. Xu, G. Wang and W. Chen, Chem. Eng. J., 2019, 359, 119–129 CrossRef CAS.
- D. Qin, W. Lu, X. Wang, N. Li, X. Chen, Z. Zhu and W. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 25962–25970 CrossRef CAS PubMed.
- Y. Chen, W. Lu, X. Wang and W. Chen, Appl. Surf. Sci., 2018, 453, 110–119 CrossRef CAS.
- C. Zhang, Y. Li, D. Shuai, Y. Shen, W. Xiong and L. Wang, Chemosphere, 2019, 214, 462–479 CrossRef CAS.
- R. Li, Y. Ren, P. Zhao, J. Wang, J. Liu and Y. Zhang, J. Hazard. Mater., 2019, 365, 606–614 CrossRef CAS.
- L. Ghalamchi, S. Aber, V. Vatanpour and M. Kian, Chem. Eng. Res. Des., 2019, 147, 443–457 CrossRef CAS.
- J. Tian, Q. Liu, C. Ge, Z. Xing, A. M. Asiri, A. O. Al-Youbi and X. Sun, Nanoscale, 2013, 5, 8921–8924 RSC.
- Q. Cui, J. Xu, X. Wang, L. Li, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2016, 55, 3672–3676 CrossRef CAS.
- X. Ou, X. Tan, X. Liu, Q. Lu, S. Chen and S. Wei, Biosens. Bioelectron., 2015, 70, 89–97 CrossRef CAS PubMed.
- Q. Lu, J. Zhang, X. Liu, Y. Wu, R. Yuan and S. Chen, Analyst, 2014, 139, 6556–6562 RSC.
- B. K. Shrestha, R. Ahmad, S. Shrestha, C. H. Park and C. S. Kim, Biosens. Bioelectron., 2017, 94, 686–693 CrossRef CAS PubMed.
- Y. Liu, H. Ma, Y. Zhang, X. Pang, D. Fan, D. Wu and Q. Wei, Biosens. Bioelectron., 2016, 86, 439–445 CrossRef CAS.
- H. Pu, Q. Liu, L. Qiao and Z. Yang, Polym. Eng. Sci., 2005, 45, 1395–1400 CrossRef CAS.
- A. Bozkurt and W. H. Meyer, J. Polym. Sci., Part B: Polym. Phys., 2001, 39, 1987–1994 CrossRef CAS.
- Y. Huang, L. Chuang, A. M. Kannan and C. Lin, J. Power Sources, 2009, 186, 22–28 CrossRef CAS.
- S. Lu, R. Xiu, X. Xu, D. Liang, H. Wang and Y. Xiang, J. Membr. Sci., 2014, 464, 1–7 CrossRef CAS.
- N. G. Sahoo, Y. Pan, L. Li and S. H. Chan, Adv. Mater., 2012, 24, 4203–4210 CrossRef CAS.
- D. He, H. Tang, Z. Kou, M. Pan, X. Sun, J. Zhang and S. Mu, Adv. Mater., 2017, 29 Search PubMed.
- P. Velayutham and A. K. Sahu, J. Phys. Chem. C, 2018, 122, 21735–21744 CrossRef CAS.
- G. Zou, W. Wu, C. Cong, X. Meng, K. Zhao and Q. Zhou, RSC Adv., 2016, 6, 106237–106247 RSC.
- H. Bai, H. Wang, J. Zhang, C. Wu, J. Zhang, Y. Xiang and S. Lu, J. Membr. Sci., 2018, 558, 26–33 CrossRef CAS.
- M. Gang, G. He, Z. Li, K. Cao, Z. Li, Y. Yin, H. Wu and Z. Jiang, J. Membr. Sci., 2016, 507, 1–11 CrossRef CAS.
- W. Dai, Y. Shen, Z. Li, L. Yu, J. Xi and X. Qiu, J. Mater. Chem. A, 2014, 2, 12423–12432 RSC.
- L. Semiz, N. Demirci Sankir and M. Sankir, J. Membr. Sci., 2014, 468, 209–215 CrossRef CAS.
- L. Cao, Q. Sun, Y. Gao, L. Liu and H. Shi, Electrochim. Acta, 2015, 158, 24–34 CrossRef CAS.
- F. Wang, G. Wang, J. Zhang, B. Li, J. Zhang, J. Deng, J. Chen and R. Wang, J. Electroanal. Chem., 2017, 797, 107–112 CrossRef CAS.
- Z. Mai, H. Zhang, X. Li, S. Xiao and H. Zhang, J. Power Sources, 2011, 196, 5737–5741 CrossRef CAS.
- R. Niu, L. Kong, L. Zheng, H. Wang and H. Shi, J. Membr. Sci., 2017, 525, 220–228 CrossRef CAS.
- C. Wu, S. Lu, J. Zhang and Y. Xiang, Phys. Chem. Chem. Phys., 2018, 20, 7694–7700 RSC.
- J. Hu, J. Tian and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 11615–11625 CrossRef CAS PubMed.
- P. Praveena, M. Sheril Ann, S. Dhanavel, D. Kalpana, T. Maiyalagan, V. Narayanan and A. Stephen, J. Mater. Sci.: Mater. Electron., 2019, 30, 8736–8750 CrossRef CAS.
- F. Li, Y. Dong, Q. Dai, T. T. Nguyen and M. Guo, Vacuum, 2019, 161, 283–290 CrossRef CAS.
- J. Ma, X.-Y. Tao, S.-X. Zhou, X.-Z. Song, Y.-B. Zhu, L.-T. Guo, Z.-S. Liu, H.-L. Fan and X.-Y. Wei, J. Electroanal. Chem., 2019, 835, 346–353 CrossRef CAS.
- X. Chen, X. Zhu, Y. Xiao and X. Yang, J. Electroanal. Chem., 2015, 743, 99–104 CrossRef CAS.
- R. A. Senthil, J. Theerthagiri, J. Madhavan, K. Murugan, P. Arunachalam and A. K. Arof, J. Solid State Chem., 2016, 242, 199–206 CrossRef CAS.
- X. Chen, Q. Liu, Q. Wu, P. Du, J. Zhu, S. Dai and S. Yang, Adv. Funct. Mater., 2016, 26, 1719–1728 CrossRef CAS.
- J. Xu, T. J. Brenner, L. Chabanne, D. Neher, M. Antonietti and M. Shalom, J. Am. Chem. Soc., 2014, 136, 13486–13489 CrossRef CAS.
- S. Barman and M. Sadhukhan, J. Mater. Chem. A, 2012, 22, 21832–21837 RSC.
- K. Xiao, P. Giusto, L. Wen, L. Jiang and M. Antonietti, Angew. Chem., Int. Ed., 2018, 57, 10123–10126 CrossRef CAS.
- C. Tang, A. Tracz, M. Kruk, R. Zhang, D. M. Smilgies, K. Matyjaszewski and T. Kowalewski, J. Am. Chem. Soc., 2005, 127, 6918–6919 CrossRef CAS.
- X. Zhang, X. Xie, H. Wang, J. Zhang, B. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 18–21 CrossRef CAS.
- Q. Cao, B. Kumru, M. Antonietti and B. V. K. J. Schmidt, Macromolecules, 2019, 52, 4989–4996 CrossRef CAS.
- Y. Shi, S. Jiang, K. Zhou, C. Bao, B. Yu, X. Qian, B. Wang, N. Hong, P. Wen, Z. Gui, Y. Hu and R. K. Yuen, ACS Appl. Mater. Interfaces, 2014, 6, 429–437 CrossRef CAS.
- B. Kumru, J. Barrio, J. Zhang, M. Antonietti, M. Shalom and B. V. K. J. Schmidt, ACS Appl. Mater. Interfaces, 2019, 11, 9462–9469 CrossRef CAS PubMed.
- C. Han, Q. Cui, P. Meng, E. R. Waclawik, H. Yang and J. Xu, Langmuir, 2018, 34, 10135–10143 CrossRef CAS PubMed.
- Y. Luo, Y. Yang, Q. Cui, R. Peng, R. Liu, Q. Cao and L. Li, ACS Appl. Bio Mater., 2019, 2, 5127–5135 CrossRef CAS.
- J. Poostforooshan, A. Badiei, M. Kolahdouz and A. P. Weber, ACS Appl. Mater. Interfaces, 2016, 8, 21731–21741 CrossRef CAS.
- Y. Lu, X. Pan, N. Li, Z. Hu and S. Chen, Appl. Surf. Sci., 2019, 144071 Search PubMed.
- C. Duan, D. Yuan, Z. Yang, S. Li, L. Tao, Q. Wang and T. Wang, Composites, Part A, 2018, 113, 200–208 CrossRef CAS.
- M. Khalifa, A. Mahendran and S. Anandhan, J. Polym. Res., 2019, 26, 73 CrossRef.
- Y. Shi, B. Wang, L. Duan, Y. Zhu, Z. Gui, R. K. K. Yuen and Y. Hu, Ind. Eng. Chem. Res., 2016, 55, 7646–7654 CrossRef CAS.
- Y. Shi, L. Wang, L. Fu, C. Liu, B. Yu, F. Yang and Y. Hu, J. Colloid Interface Sci., 2019, 539, 1–10 CrossRef CAS.
- K. Myllymaa, S. Myllymaa, H. Korhonen, M. J. Lammi, H. Saarenpaa, M. Suvanto, T. A. Pakkanen, V. Tiitu and R. Lappalainen, J. Mater. Sci.: Mater. Med., 2009, 20, 2337–2347 CrossRef CAS.
- Y. Shi, Z. Gui, B. Yu, R. K. K. Yuen, B. Wang and Y. Hu, Composites, Part B, 2015, 79, 277–284 CrossRef CAS.
- B. Lei, Y. Zhang, Y. He, Y. Xie, B. Xu, Z. Lin, L. Huang, S. Tan, M. Wang and X. Cai, Mater. Des., 2015, 66, 103–109 CrossRef CAS.
- S. He, J. Wang, M. Yu, Y. Xue, J. Hu and J. Lin, Polymers, 2019, 11 Search PubMed.
- B. Song, T. Wang, H. Sun, H. Liu, X. Mai, X. Wang, L. Wang, N. Wang, Y. Huang and Z. Guo, Compos. Sci. Technol., 2018, 167, 515–521 CrossRef CAS.
- E. A. Appel, X. J. Loh, S. T. Jones, F. Biedermann, C. A. Dreiss and O. A. Scherman, J. Am. Chem. Soc., 2012, 134, 11767–11773 CrossRef CAS.
- J. Zhu and R. E. Marchant, Expert Rev. Med. Devices, 2011, 8, 607–626 CrossRef CAS PubMed.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- H. Yuk, S. Lin, C. Ma, M. Takaffoli, N. X. Fang and X. Zhao, Nat. Commun., 2017, 8, 14230 CrossRef CAS.
- X. Dai, Y. Zhang, L. Gao, T. Bai, W. Wang, Y. Cui and W. Liu, Adv. Mater., 2015, 27, 3566–3571 CrossRef CAS.
- P. Samaddar, S. Kumar and K.-H. Kim, Polym. Rev., 2019, 59, 418–464 CrossRef CAS.
- W. Guo, C. H. Lu, R. Orbach, F. Wang, X. J. Qi, A. Cecconello, D. Seliktar and I. Willner, Adv. Mater., 2015, 27, 73–78 CrossRef CAS.
- A. Nakayama, A. Kakugo, J. P. Gong, Y. Osada, M. Takai, T. Erata and S. Kawano, Adv. Funct. Mater., 2004, 14, 1124–1128 CrossRef CAS.
- B. G. Cooper, R. C. Stewart, D. Burstein, B. D. Snyder and M. W. Grinstaff, Angew. Chem., Int. Ed., 2016, 55, 4226–4230 CrossRef CAS.
- T. Murakami, B. V. K. J. Schmidt, H. R. Brown and C. J. Hawker, Macromolecules, 2015, 48, 7774–7781 CrossRef CAS.
- A. Bin Imran, K. Esaki, H. Gotoh, T. Seki, K. Ito, Y. Sakai and Y. Takeoka, Nat. Commun., 2014, 5, 5124 CrossRef.
- A. Agrawal, N. Rahbar and P. D. Calvert, Acta Biomater., 2013, 9, 5313–5318 CrossRef CAS PubMed.
- M. Fukasawa, T. Sakai, U.-i. Chung and K. Haraguchi, Macromolecules, 2010, 43, 4370–4378 CrossRef CAS.
- O. Okay and W. Oppermann, Macromolecules, 2007, 40, 3378–3387 CrossRef CAS.
- S. Tamesue, M. Ohtani, K. Yamada, Y. Ishida, J. M. Spruell, N. A. Lynd, C. J. Hawker and T. Aida, J. Am. Chem. Soc., 2013, 135, 15650–15655 CrossRef CAS PubMed.
- J. Djonlagic, A. Lancuski, M. S. Nikolic, J. Rogan, S. Ostojic and Z. Petrovic, J. Appl. Polym. Sci., 2017, 134, 44535 CrossRef.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef CAS PubMed.
- M. Liu, Y. Ishida, Y. Ebina, T. Sasaki, T. Hikima, M. Takata and T. Aida, Nature, 2015, 517, 68 CrossRef CAS PubMed.
- C. Hu, Y.-R. Lin and H.-C. Yang, ChemSusChem, 2019, 12, 1794–1806 CrossRef CAS PubMed.
- J. Liu, T. An, Z. Chen, Z. Wang, H. Zhou, T. Fan, D. Zhang and M. Antonietti, J. Mater. Chem. A, 2017, 5, 8933–8938 RSC.
- J. Sun, B. V. K. J. Schmidt, X. Wang and M. Shalom, ACS Appl. Mater. Interfaces, 2017, 9, 2029–2034 CrossRef CAS PubMed.
- M. Zarei, H. Ahmadzadeh, E. K. Goharshadi and A. Farzaneh, Anal. Chim. Acta, 2015, 887, 245–252 CrossRef CAS PubMed.
- B. Kumru, V. Molinari, R. Dünnebacke, K. G. Blank and B. V. K. J. Schmidt, Macromol. Rapid Commun., 2018, 40, 1800712 CrossRef.
- H. Li, L. Wang, Y. Liu, J. Lei and J. Zhang, Res. Chem. Intermed., 2016, 42, 3979–3998 CrossRef CAS.
- Y. Zheng, Y. Jiao, J. Chen, J. Liu, J. Liang, A. Du, W. Zhang, Z. Zhu, S. C. Smith, M. Jaroniec, G. Q. Lu and S. Z. Qiao, J. Am. Chem. Soc., 2011, 133, 20116–20119 CrossRef CAS PubMed.
- B. Kumru, V. Molinari, M. Shalom, M. Antonietti and B. V. K. J. Schmidt, Soft Matter, 2018, 14, 2655–2664 RSC.
- J. P. Gong, T. Kurokawa, T. Narita, G. Kagata, Y. Osada, G. Nishimura and M. Kinjo, J. Am. Chem. Soc., 2001, 123, 5582–5583 CrossRef CAS PubMed.
- M. M. Blum and T. C. Ovaert, Mater. Sci. Eng., C, 2013, 33, 4377–4383 CrossRef CAS PubMed.
- B. Ye, C. Yao, M. Yan, H. Zhang, F. Xi, J. Liu, B. Li and X. Dong, Macromol. Mater. Eng., 2018, 1800500 Search PubMed.
- Q. Hao, T. Chen, R. Wang, J. Feng, D. Chen and W. Yao, J. Cleaner Prod., 2018, 197, 1222–1230 CrossRef CAS.
- S. Lamkhao and C. Randorn, J. Photopolym. Sci. Technol., 2017, 30, 425–429 CrossRef CAS.
- Y. Liang, X. Wang, W. An, Y. Li, J. Hu and W. Cui, Appl. Surf. Sci., 2019, 466, 666–672 CrossRef CAS.
- M. Li, H. Liao, Q. Deng, Y. Wu, F. Xiao, X. Wei and D. Tu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2018, 55, 408–413 CrossRef CAS.
- J. W. Ko, W. S. Choi, J. Kim, S. K. Kuk, S. H. Lee and C. B. Park, Biomacromolecules, 2017, 18, 3551–3556 CrossRef CAS PubMed.
- P. He, X. Tang, L. Chen, P. Xie, L. He, H. Zhou, D. Zhang and T. Fan, Adv. Funct. Mater., 2018, 28, 1801121 CrossRef.
- J. Yan, M.-T. F. Rodrigues, Z. Song, H. Li, H. Xu, H. Liu, J. Wu, Y. Xu, Y. Song, Y. Liu, P. Yu, W. Yang, R. Vajtai, H. Li, S. Yuan and P. M. Ajayan, Adv. Funct. Mater., 2017, 27, 1700653 CrossRef.
- Y. Zhang, Z. Zhou, Y. Shen, Q. Zhou, J. Wang, A. Liu, S. Liu and Y. Zhang, ACS Nano, 2016, 10, 9036–9043 CrossRef CAS.
- L. Tan, C. Yu, M. Wang, S. Zhang, J. Sun, S. Dong and J. Sun, Appl. Surf. Sci., 2019, 467–468, 286–292 CrossRef CAS.
- M. Zhang, W. Jiang, D. Liu, J. Wang, Y. Liu, Y. Zhu and Y. Zhu, Appl. Catal., B, 2016, 183, 263–268 CrossRef CAS.
- D. Gräfe, A. Wickberg, M. M. Zieger, M. Wegener, E. Blasco and C. Barner-Kowollik, Nat. Commun., 2018, 9, 2788 CrossRef.
- S. C. Ligon, R. Liska, J. r. Stampfl, M. Gurr and R. Mülhaupt, Chem. Rev., 2017, 117, 10212–10290 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |