Activation of Nrf2 by lead sulfide nanoparticles induces impairment of learning and memory†
Yanhua
Cao
abc,
Dong
Wang
d,
Qingzhao
Li
b,
Huajie
Liu
 e,
Cuihong
Jin
ac,
Jinghua
Yang
ac,
Shengwen
Wu
ac,
Xiaobo
Lu
ac and
Yuan
Cai
*ac
e,
Cuihong
Jin
ac,
Jinghua
Yang
ac,
Shengwen
Wu
ac,
Xiaobo
Lu
ac and
Yuan
Cai
*ac
aDepartment of Toxicology, School of Public Health, China Medical University, Shenyang 110001, China. E-mail: cmuycai@163.com; Fax: +86-315-3725312
bDepartment of Toxicology, School of Public Health, Hebei United University, Tangshan 063000, China
cDepartment of Occupational and Environmental Health, School of Public Health, China Medical University, Shenyang 110001, China
dDepartment of Nutrition, Beidaihe Sanatorium of Beijing Military Region, Qinhuangdao 066000, China
eSchool of Chemical Science and Engineering, Tongji University, Shanghai 201800, China
First published on 26th October 2019
Abstract
Lead sulfide nanoparticles (PbS NPs) are semiconductor materials that have been widely applied to light-emitting diodes (LEDs), biological fluorescent probes, infrared detection, solar receivers, ion-selective electrodes, and ion-sensitive materials. However, the effects of PbS NPs on the central nervous system are still unclear. Thus, this study aimed to determine, using rats, the mechanism of action of PbS NPs, exposure to which results in persistent alterations in nervous system function. The results of the Morris water maze test showed that PbS NPs significantly impaired learning and memory. Compared with that in the control group, the lead content in the hippocampal tissue was significantly elevated after PbS NP exposure. Exposure to PbS NPs led to increased oxidative damage in blood and hippocampal tissues, and significantly inhibited the activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) while increasing the serum malondialdehyde (MDA) content. In addition, reactive oxygen species triggered the activation of Nrf2 and the antioxidant system, including HO-1, r-GCS, and GSH-Px. Moreover, we observed significant apoptosis in the hippocampi of the rats using the TUNEL assay and transmission electron microscopy. The MOD values from the TUNEL assay of the hippocampi were all significantly higher than those of the control group, which increased as the concentration of the PbS NPs increased. There were also changes in the ultrastructure of the hippocampal neurons and synapses in the PbS-treated rats, including a shorter synaptic active zone, smaller curvature of the synaptic interface, and thicker postsynaptic density. Therefore, PbS NP exposure could lead to increased brain lead content, oxidative damage, and apoptosis.
Significance to metallomicsLead sulfide nanoparticles (PbS NPs) are a semiconductor material which has been widely applied in light emitting diodes (LEDs), biological fluorescent probes, infrared detection, solar receivers, ion selective electrodes and ion sensitive materials. However, the effects of PbS NPs on the central nervous system are still unclear. Thus, this study aimed to determine the mechanism of how PbS NP exposure results in persistent alterations in nervous system function. Our results showed that PbS NPs significantly impair the behaviors of learning and memory. Exposure to PbS NPs led to an increased oxidative damage index in both blood and hippocampus tissues. In addition, ROS triggered the activation of Nrf2 and its mediated antioxidant system, including HO-1, r-GCS and GSH-Px. Generally, PbS NPs exposure could alter the brain lead content, the oxidative damage index and the apoptosis index. |
Introduction
Lead sulfide nanoparticles (PbS NPs) are important semiconductor materials, and they have been widely used in light emitting diodes (LEDs), biological fluorescence probes, in vivo imaging, infrared detection, solar energy collectors, and other fields1–3 due to their unique physicochemical properties. With the wide application and production of nanomaterials, there is a chance that people will be increasingly exposed to PbS NPs. Thus, the health safety evaluation of nanomaterials is becoming an important topic of research.4,5 Because of the small size of nanoparticles, they can enter tissues that conventional particles cannot; therefore, the biological effects of the former are often stronger than those of the latter.6–8Lead is a kind of nerve poison that can damage the central nervous system (CNS) and impair learning and memory. The cerebral cortex and hippocampus are two tissues of target organs of lead, which play key roles in learning and memory.9–11 Long-term potentiation (LTP) in the hippocampus is one of the important mechanisms related to the development of learning and memory.12 Studies have shown that lead can affect the levels of Ca2+ in nerve cells and interfere with LTP, which can lead to dysfunction of the CNS and impairment of learning and memory. The hippocampus is reported to be the main area where brain damage occurs. However, reports on the effects of nanoscale lead on brain tissue are limited, and the mechanisms are not clear.
Even trace amounts of foreign chemicals can cause brain damage because the CNS is very sensitive to changes in the microenvironment and there is a lack of effective defense mechanisms. Therefore, the CNS is likely to be affected by nanoparticles. Our previous study found that exposure to PbS NPs could lead to an increase in the number of errors in the Y-maze test and a decrease in learning and memory. However, the mechanism of learning and memory impairment by nanoscale lead remains unclear. Previous studies have shown that the toxic effects of nano lead may be related to oxidative damage, imbalance in calcium homeostasis, abnormal metabolism of neurotransmitters, and abnormal opening of ion channels.13,14 It is necessary to carry out research on the toxicities of nano lead to clarify its effect and mechanism of action and to provide a scientific basis for the evaluation of the risk of exposure to nano lead.
Results and discussion
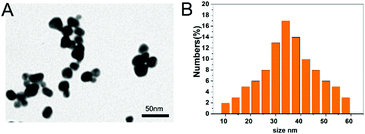
The morphology of the PbS NP sample was examined by TEM. Fig. 1A illustrates uniform and spherical nanoparticles with an average diameter of 36.8 nm (Fig. 1B) calculated using a Zetasizer. We used a Morris water maze (MWZ) test to detect the learning and memory abilities of rats that were treated with PbS NPs for 6 weeks. The results of the MWZ test are shown in Fig. 2. Compared with those in the control group, the escape latency and time to reach the platform for the rats that were exposed to PbS NPs significantly increased, while the objective quadrant time and number of rats that crossed the platform significantly decreased. In addition, as the dose of PbS NPs increased, the escape latency and the time to reach the platform consistently increased (P < 0.05); yet, the objective quadrant time and the number of rats that crossed the platform consistently decreased (P < 0.05). These signs of learning and memory inhibition in the rats that received intragastric administration of PbS NPs indicated that PbS NPs may accumulate in the hippocampus, cause hippocampal injury, and alter the activities of antioxidant enzymes. These suppositions were confirmed by follow-up assays that were used to determine the lead content, antioxidant enzyme activity, hippocampal apoptosis, and morphology. There were no significant differences observed in the average body weights of the five groups. The coefficients of the brains in the 100 mg kg−1 PbS NP-treated group significantly increased in comparison with those in the control group (P < 0.05) (Table S1, ESI†), suggesting that 100 mg kg−1 of PbS NPs caused brain damage in the treated rats.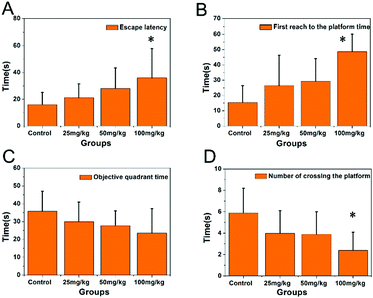 | ||
| Fig. 2 Escape latency (A), time of first reaching the platform (B), objective quadrant time (C), and number of rats crossing the platform (D). | ||
In the present study, 25, 50, and 100 mg kg−1 of PbS NPs (30 nm) were given to the rats by intragastric administration for 30 days. In comparison with the control group, higher coefficients of the brains were observed in the 100 mg kg−1 PbS NP group (P < 0.05). Our previous study indicated14 that higher brain coefficients were observed in the low- and high-dose groups, which were administered 15 and 30 mg kg−1 of PbS NPs (30 nm), respectively, to the lungs by tracheal injections once every 7 days for 3 consecutive months. Li et al. reported that a fixed dose of 30 mg kg−1 of PbS NPs (30 nm and 60 nm) administrated via the trachea once every 7 days for 3 consecutive months significantly increased the lung coefficients compared with the untreated group (P < 0.05).13 Some evidence suggested that the increase in brain coefficients may be due to edema or hypertrophy.15 Consistent with previous reports, the studies demonstrated that PbS NPs at 100 mg kg−1 were toxic to the brains of rats.
An MWZ test was utilized to assess the effect of PbS NPs on the learning and memory of rats. MWZ is recognized as a model for measuring learning and memory in experimental animals by observing their spatial discrimination16 abilities. The results indicated that PbS NPs impaired rat learning and memory. These signs of learning and memory inhibition in the rats that received intragastric administration of PbS NPs indicated that PbS NPs may accumulate in the hippocampus, cause hippocampal injury, and alter the activities of antioxidant enzymes. These suppositions were confirmed by follow-up assays that were used to determine the contents of the lead, antioxidant enzyme activity, hippocampal apoptosis, and morphology.
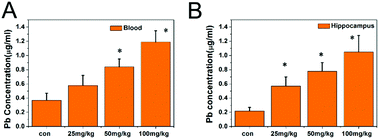
The lead content in the whole blood and hippocampal tissues is shown in Fig. 3. As the exposure dose increased, the lead levels in the whole blood and hippocampi were significantly elevated (P < 0.05), indicating the accumulation of lead. For instance, the lead levels in the whole blood and hippocampal tissues of the 100 mg kg−1 PbS NP group were significantly higher than those in the 50 mg kg−1 PbS NP group, which were significantly higher than those in the 25 mg kg−1 PbS NP group (P < 0.05).
The activities of the antioxidative enzymes are shown in Fig. 4. The activities of SOD (Fig. 4A), GSH-Px (Fig. 4B), and CAT (Fig. 4C) in the hippocampal tissue of the 100 mg kg−1 PbS NP-treated group were significantly lower than those in the control group (P < 0.05), while the MDA level (Fig. 4D) was significantly higher than that in the control group (P < 0.05).
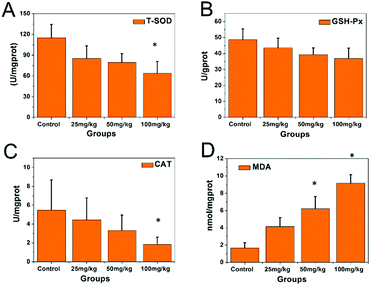 | ||
| Fig. 4 Concentration results of the SOD, GSH-Px, CAT, and MDA analyses from the hippocampi of the mice that received PbS NPs (* compared with control group P < 0.05). | ||
The GR and γ-GCS activities and GSH levels in the hippocampi are shown in Fig. 5. Compared with those in the control group, the GR and γ-GCS activities in the 50 and 100 mg kg−1 PbS NP-treated groups were significantly lower (P < 0.05). Similarly, the GSH levels of the 50 and 100 mg kg−1 PbS NP-treated groups were lower than those in the control group (P < 0.05). As shown in Fig. 5D and 6, compared with that in the control group, the apoptosis rate of hippocampal cells exposed to PbS NPs significantly increased. With the increase in the PbS NP dose, the apoptosis rate of the hippocampal cells increased, which was the highest in the 100 mg kg−1 PbS NP-treated group, followed by those in the 50 and 25 mg kg−1 PbS NP-treated groups.
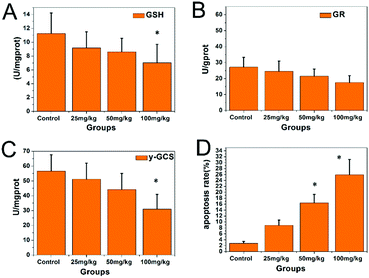 | ||
| Fig. 5 Concentrations of GSH (A), GR (B), and GCS (C) in the hippocampi of the mice that received PbS NPs, and the apoptosis rates of the groups (D) (* compared with control group P < 0.05). | ||
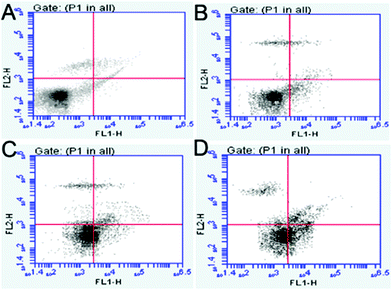 | ||
| Fig. 6 Results of the FCM flow cytometry of the different groups. Control (A), 25 (B), 50 (C), and 100 mg kg−1 (D). | ||
To further study the brain damage caused by PbS NPs, we carried out lead content measurements and hippocampal ultrastructure characterization. As expected, with increases in the PbS NP doses used, the accumulation of lead in the whole blood and hippocampi was elevated. The lead contents in the whole blood and hippocampi of the 100 mg kg−1 PbS NP group were significantly higher than those of the 100 mg kg−1 PbS micron particle group. This implied that PbS NPs could enter the hippocampus via the oral tract and accumulate in the whole blood and hippocampus, which led to learning and memory damage. This is consistent with reports that showed that lead exposure caused impairment in LTP17 and suggests that it may have been mediated through oxidative damage.18
The TUNEL-positive cells of the hippocampi were dark red under the optical microscope, as illustrated in Fig. 7. The hippocampal nuclei at the corresponding region in the control group were not stained or were pale in color. The MOD value of the apoptotic cells was determined in 10 areas of the hippocampus for each rat. A dose–response relationship was observed; that is, with an increasing dose of PbS NPs, the number of TUNEL-positive hippocampal cells gradually increased, and so did the MOD value (Table S2, ESI†).
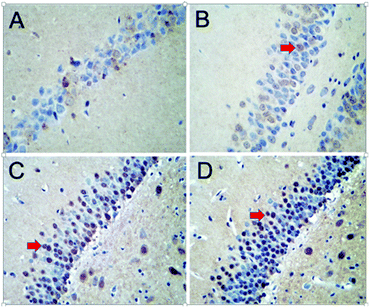 | ||
| Fig. 7 Images of the TUNEL stains. The red arrows show the positive cells. Control (A), 25 (B), 50 (C), and 100 mg kg−1 (D). | ||
Transmission electron microscopy (TEM) pictures of the hippocampal neurons are shown in Fig. 8. For the control group not exposed to PbS NPs, the nuclear membranes of the hippocampal neurons were clear and complete (Fig. 8A) and a uniform distribution of nuclear chromatin and abundant organelles, such as mitochondria and the endoplasmic reticulum, could be seen in the cytoplasm. Although the nuclear membranes were complete and the nuclear chromatin was evenly distributed in the low-dose-treated group (25 mg kg−1 PbS NPs), the size of the hippocampal neurons and nucleolus decreased and some of the cytoplasm dissolved (Fig. 8B). Furthermore, a portion of the mitochondria in the cytoplasm showed swelling and vacuolar changes. For the medium-dose group treated with 50 mg kg−1 of PbS NPs, the boundaries of the hippocampal neuronal nuclei and cell membranes were fuzzy. The structures of the organelles in the cytoplasm were unclear, and the mitochondria showed swelling and vacuolar changes (Fig. 8C). Small compensatory mitochondria were also observed. For the high-dose group treated with 100 mg kg−1 of PbS NPs, the nuclei of the hippocampal neurons showed pyknosis, and the chromatin was massive. Compared with those in the control group, the number of organelles in the cytoplasm decreased significantly (Fig. 8D).
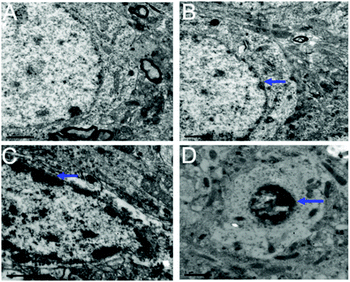 | ||
| Fig. 8 TEM images of the hippocampal cells. Control (A), 25 (B), 50 (C), and 100 mg kg−1 (D). The blue arrow shows the damage. | ||
Changes in the ultrastructure of the synapses of the hippocampi are shown in Fig. 9. A long active zone, smooth synaptic curvature, and thick post-synaptic density were observed in the control group (Fig. 9A). Ultrastructural changes were observed in the synapses of the hippocampi of the rats treated with PbS NPs. These ultrastructural changes included a short active zone, uneven synaptic curvature, and low post-synaptic density as shown by red arrows (Fig. 9B–D).
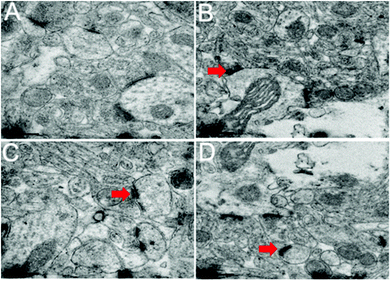 | ||
| Fig. 9 TEM images of the synapses of the hippocampus. Control (A), 25 (B), 50 (C), and 100 mg kg−1 (D). | ||
Compared with the control group, the expression levels of the mRNAs of Nrf2, HO-1γ-GCS, and GPx-1 in the hippocampi of the PbS NP-exposed groups significantly increased (P < 0.05) (Fig. 10). With an increase in exposure dose, the transcription level of the mRNAs of Nrf2, HO-1γ-GCS, and GPx-1 increased, which meant that the transcription level was significantly higher in the 100 mg kg−1 PbS NP group than in the 50 mg kg−1 PbS NP group, and the transcription level was significantly higher in the 50 mg kg−1 PbS NP group than in the 25 mg kg−1 PbS NP group.
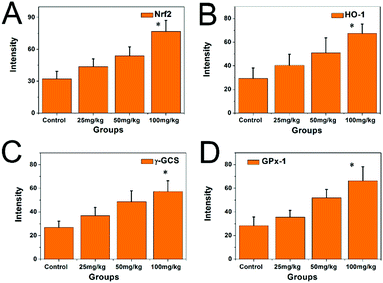 | ||
| Fig. 10 Expression levels of the mRNAs of Nrf2, HO-1γ-GCS, and GPx-1 in the hippocampi of the different groups. | ||
As shown in Fig. 11, the protein expression levels of Nrf2, HO-1, γ-GCS, and GPx-1 in the hippocampi of the PbS NP groups were significantly higher than those of the control group. The protein expression levels of Nrf2, HO-1, γ-GCS, and GPx-1 in the hippocampi increased with the increase of the PbS NP exposure dose, and these protein expression levels were the highest in the 100 mg kg−1 PbS NP group, followed by the 50 and 25 mg kg−1 PbS NP groups, respectively (P < 0.05).
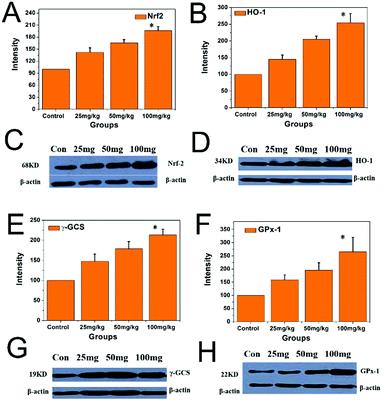 | ||
| Fig. 11 Expression levels of the proteins of Nrf2, HO-1γ-GCS, and GPx-1 in the hippocampi of the different groups. | ||
We also observed significant ultrastructural changes in the hippocampi of the rats, such as membrane shrinkage, a short active zone, uneven synaptic curvature, and thin post-synaptic density. These observations indicated that PbS NPs can injure rat hippocampi. The postsynaptic density gradually increased with the increase in the PbS NP dose, which may be related to the upregulation of some synaptic proteins.
Park reported that the ethanol extract of Inula helenium L(EIH) reduced inflammatory effects via the induction of the p38 MAPK/Nrf2/HO-1 signaling pathway.19 Other research showed that gymnasterkoreayne B (GKB) could induce Nrf2 translocation and expression by the differential regulation of the ERK and PKC pathways in HCT116 cells.20 In addition, caffeoyl serotonin (CaS) could protect against oxidative stress-induced keratinocyte cell death, in part, through the activation of Nrf2-mediated HO-1 induction via the PI3K/Akt and/or PKC pathways.21 All of these results indicated that the expression of ERK, PI3K, and PKC could activate the expression of Nrf2 and its downstream antioxidant enzymes. In this study, Nrf2 was upregulated in the PbS NP exposure groups, suggesting that the thickness of the postsynaptic density may be related to the activation of synaptic proteins via Nrf2. Changes in the ultrastructure of the hippocampus synapse would have a negative effect on the learning and memory of rats.22
To study the mechanism of PbS NP-induced neurotoxicity, we measured the activity of antioxidant enzymes in the hippocampus. Previous studies have shown that the formation of ROS and oxidative stress are the main mechanisms of the biological toxicity caused by nanomaterials.23,24 It was suggested that C60 can induce acute oxidative stress in rat lung tissues.25 Wang et al. showed that after lead exposure, the ROS and MDA content increased compared with that in the control group.26 Our data showed a significant increase in the ROS and MDA content in the hippocampi from the 25, 50, and 100 mg kg−1 PbS NP-treated groups and the 100 mg kg−1 PbS micron particle-treated group, indicating that severe oxidative stress occurred in the PbS NP-treated rat hippocampi. The overproduction of ROS would destroy the balance of the oxidative/antioxidative system in the hippocampus, leading to lipid peroxidation. MDA is the end product of peroxide decomposition of unsaturated fatty acids. The content of MDA indirectly reflects the severity of the damage to cells attacked by free radicals, and the activities of antioxidative enzymes like SOD, GSH-Px, and CAT, as well as nonenzymatic antioxidants like GSH, directly reflect the ability to eliminate oxygen free radicals.
In our study, the activities of SOD, GSH-Px, CAT, γ-GCS, and GR were significantly inhibited, and the content of GSH in the rat hippocampus was significantly reduced at higher doses of PbS NPs. With an increase in the PbS NP dose, the capacity of eliminating free radicals decreased. When the cell membrane, mitochondria, and cell nucleus are attacked by ROS, tissue injury and cellular disfunction could occur in addition to the modification of functional molecules and structural integrity by activating signal transduction pathways.26 These injury events eventually promote inflammation, fibrosis, apoptosis, necrosis, and other functional disorders. Excessive lead can induce the production of high amounts of ROS and induce apoptosis in the body.27 Its main feature is nuclear condensation, chromatin condensation, cell shrinkage, and apoptotic bodies. In this study, the apoptosis rates of the hippocampal neurons were evaluated by flow cytometry. We also observed DNA breakage by the TUNEL method. The results of the TUNEL method indicated that the number of positively stained cells increased with the increase in the dose of PbS NPs, and a dose–effect relationship was observed. Compared with the 100 mg kg−1 PbS micron particle group, the number of positively stained cells significantly increased. The results of the DNA breakage analysis by the TUNEL assay and the apoptosis rate by flow cytometry were consistent.
There are many mechanisms of tissue injury caused by nanoparticles, but oxidative stress is the critical mechanism of injury induced by NPs.28,29 One of the defense mechanisms of cells in response to oxidative stress is the antioxidant response element (ARE), which is a cis-acting element responsible for the expression of antioxidation enzymes/phase II enzymes regulated by the nuclear transcription factor Nrf2 (NF-E2-related factor 2). Nrf2, which is a member of the cap ‘n’ collar family of bZIP proteins, is an important regulator of inducible ARE-related gene expression and the innate immune response. Under oxidative stress, Nrf2 is translocated to the nucleus where it forms a heterodimer protein with Maf and other proteins. It then identifies and combines with the GCTGAGTCA sequence on the ARE30 and transactivates a series of ARE-regulated genes including phase II detoxifying enzymes (i.e., NQO1 and HO-1) and endogenous antioxidants (SOD, GSH-Px, and CAT).
Experimental
Materials and chemicals
Globular PbS NPs (30 nm) were prepared by the College of Environmental Science and Engineering at Nankai University. They were synthesized by the reported colloidal chemistry method 13, and the details of the method are as follows.31 6 ml of a lead nitrate [Pb(NO3)2] solution (1 mmol l−1) was added to a conical flask that contained 2 ml of trisodium citrate (1 mmol l−1), and the solution sat for 15 h at room temperature. Then, 0.1 mol of a Na2S solution was added to the flask. The mixture was magnetically stirred at 1000 rpm for 5 min, followed by centrifugation at 2000 rpm for 5 min. Lastly, the precipitate was washed several times with water and ethanol. The average size and surface charge of the PbS NPs in pure water were measured using a Zetasizer 3000HSA (Malvern Company, UK) at 25 °C. The morphology of the precipitate was characterized by transmission electron microscopy (JEOL-2010, JEOL Company, Japan).Animals and treatment
Specific pathogen free (SPF) Sprague–Dawley (SD) rats (100 males that weighed 220–240 g) were purchased from the Laboratory Animal Center of Hebei United University (Tangshan, China). The rats were housed in a ventilated room with controlled temperature and light, and they had free access to water and food. All procedures used on the laboratory animals were in accordance with NIH guidelines and the local ethics committee. Then, the SD rats were randomly divided into four groups based on their body weight after 1 week of adaptation to the new environment. Each group was composed of 20 rats. The control group was exposed to ultrapure water, and the low exposure group (25 mg kg−1 PbS NPs), medium exposure group (50 mg kg−1 PbS NPs) and high exposure group (100 mg kg−1 PbS NPs) were exposed to the PbS NPs. All of the animals were exposed to a specific substance by intragastric administration once per day, 5 days per week, which continued for 6 weeks. After 6 weeks, all of the rats were weighed and recorded. Then, each rat was sacrificed, and the blood and brain tissues were collected. The brains were washed with 95% saline and weighed, and the hippocampal tissues were subsequently isolated. The specimens were either stored at −80 °C or the hippocampi were fixed in glutaraldehyde fluid.Morris water maze experiment
After 6 weeks, the spatial learning and memory in all the rats were examined by the Morris water maze (MWM) method. The maze was a circular pool (120 cm in diameter) filled with water (23 ± 1 °C) to a height of 50 cm. The objective for the rat was to find the platform, which was 10 cm in diameter and made of black metal. The platform was hidden 2 cm below the water surface in the middle of one quadrant of the pool, 40 cm away from the wall. The testing included a place navigation test and a spatial probe test. For the place navigation test, the rats were trained once a day for 3 days. The testing phase was conducted on the fourth day. Each trial started by gently placing the rat into the water with its head towards the pool wall in any of the quadrants. If an animal found the platform within 120 s, it was left to remain on the platform for 10 s. If an animal did not find the platform, it was gently guided to the platform by the experimenter and remained on the platform for 10 s. The escape latency of the rats was defined by the time it took the rats to find the hidden underwater platform. On the fifth day, there was a spatial probe test in which the platform was removed, the rats were placed into the water with their head towards the pool wall, and the number of animals that crossed the platform within 120 s, the time to the first quadrant, and the time to the platform were recorded.Between the trials, all mice were placed back in their home cages using a spoon net in order to avoid direct contact with the experimenter. All trials were tracked automatically by a digital tracking system that assessed the path length, swimming speed, and latency to escape from the water.
Brain coefficients
After the behavioral tests, the rats and the whole brains were weighed. The brain coefficient was calculated according to the formula brain coefficient (%) = [brain quality (g)/body mass (g)] × 100%.Measurement of the lead content in the whole blood and hippocampus
About 0.1 g hippocampus or whole blood was weighed, digested, and analyzed for the lead content. Then, 5 ml of mixed acid was added to the 0.1 g tissue sample (whole blood or hippocampus) in a PTFE beaker, and the tissues in the mixed acid were digested overnight. The samples were then heated at 180 °C until they were completely digested. Next, the solutions were heated at 120 °C to dry. Finally, 10 ml of 1% nitric acid was added. The lead content was determined with an ICP-MS 7500A (Agilent Company, USA). The working conditions of the ICP-MS were: emission power, 1420 w; frequency, 27.12 MHz; atomization pressure, 32 Ibf/in2; auxiliary gas flow, 1.08 l min−1; injection speed, 1.85 ml min−1; dilute nitric acid (2%) flushing time, 1 min; ultrapure water flushing time, 1 min.Biochemical parameter assay
The hippocampal tissue (about 1.0 g) was homogenized in 10 ml of cold saline solution (0.9%) by a homogenizer (FA25, FLUKO equipment, Shanghai). The homogenate was centrifuged at 5000 rpm for 10 min at 4 °C, and the supernatant was used for analysis. The SOD, GSH-Px, CAT, GR, MDA and GSH dynamics of the serum and homogenate of the hippocampal tissue were measured using SOD (19160), GSH-Px (CGP1), CAT (100), GR(GRSA), and MDA (085) and GSH (CS0260) assay kits (Sigma-Aldrich, China) according to the manufacturer's manual. The γ-GCS level was measured by γ-GCS (E3800) assay kits as well (TSZ, USA).Apoptosis index measurement
The apoptosis index of the left hemispheres of the rat brains was determined by the TUNEL method. First the specimens were fixed in 10% formol and then the hemispheres were placed in an oven for 20 min at 60 °C followed by xylene treatment for 20 min. Next, the hemispheres were dehydrated through graded ethanol (70, 80, 90%, 95%, 100%). Finally, the hemispheres were embedded in paraffin blocks and were cut into 5 μm sections. These sections were processed for the TUNEL assay using POD kits (Roche, Penzberg, Germany) to reveal the degree of apoptosis of the hippocampal cells treated with different doses of PbS NPs.The apoptosis rate was determined with the annexin V-FITC/PI dual stain kit (Abcam, U.S.) according to the instructions. The hippocampal tissues were obtained immediately after the rats were sacrificed, and hippocampal single cell suspensions were prepared. Then, the cells were resuspended in 200 μl of cold binding buffer. Next, 10 μl of annexin V-FITC and 5 μl of PI were added in turn. Finally, the samples were kept in the dark for 15 min at room temperature. The cells (1 × 106 cells per sample) were analyzed using flow cytometry within 1 h.
Hippocampal histopathology
The hippocampus was conventionally fixed in a 2.5% glutaraldehyde solution, dehydrated, penetrated, sliced ultrathin, and electron stained. The ultrastructural changes were observed with a transmission electron microscope H-7650 (Hitachi Company, Japan).Expression assay of Nrf2, HO-1, γ-GCS, and GSH-Px1
The levels of mRNA expression of Nrf2, heme oxygenase (HO-1), γ-GCS, and GSH-Px1 in the rat hippocampal tissues were measured by real-time quantitative RT polymerase chain reaction (RT-PCR). The PCR primers were designed by Primer Express Software, and their sequences are available upon request. The primers of the PCR sequences are shown in Table S3 (ESI†). We determined the protein expression levels of Nrf2 and HO-1 by Western blot analysis. Densitometric analysis of the Western blots was performed using Image Lab™ software (Quantity ONE7.0). The antibodies were all obtained from Abcam (USA).Statistical analysis
All data were analyzed using a one-way univariate analysis of variance (ANOVA), Tukey test, or Dunnett's T3 test between groups using the Statistical Package for Social Sciences (SPSS version 16.0; Chicago, IL, USA). The results are represented as mean ± SD. All the tests were two sided, and P < 0.05 was considered statistically significant.Conclusions
In summary, Nrf2 is an important transcription factor that was induced by PbS NPs during cellular defense. There was a correlation between the activation of the Nrf2 pathway by PbS NP exposure, the transcription and protein expression levels of its downstream target genes, such as HO-1, γ-GCS, and GPx-1, and oxidative damage of the hippocampus, toxic damage, and learning and memory impairment. However, further study is required to elucidate the specific mechanisms.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Basic Research Program of China (973 Program 2013CB932800), National Natural Science Foundation (21103219), Shanghai Pujiang Program (11PJ1412000) and SRF for ROCS, SEM.Notes and references
- R. Hu, W. C. Law, G. Lin, L. Ye, J. Liu, J. Liu, J. L. Reynolds and K. T. Yong, PEGylated Phospholipid Micelle-Encapsulated Near-Infrared PbS Quantum Dots for in vitro and in vivo Bioimaging, Theranostics, 2012, 2, 723–733 CrossRef CAS PubMed.
- J. Cao, H. Zhu, D. Deng, B. Xue, L. Tang, D. Mahounga, Z. Qian and Y. Gu, In vivo NIR imaging with PbS quantum dots entrapped in biodegradable micelles, J. Biomed. Mater. Res., Part A, 2012, 100, 958–968 CrossRef PubMed.
- D. Wang, J. Qian, F. Cai, S. He, S. Han and Y. Mu, ‘Green’-synthesized near-infrared PbS quantum dots with silica-PEG dual-layer coating: ultrastable and biocompatible optical probes for in vivo animal imaging, Nanotechnology, 2012, 23, 245701 CrossRef CAS PubMed.
- G. Oberdorster, E. Oberdorster and J. Oberdorster, Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles, Environ. Health Perspect., 2005, 113, 823–839 CrossRef CAS PubMed.
- A. Nel, T. Xia, L. Madler and N. Li, Toxic potential of materials at the nanolevel, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- Q. Zhang, Y. Kusaka, X. Zhu, K. Sato, Y. Mo, T. Kluz and K. Donaldson, Comparative toxicity of standard nickel and ultrafine nickel in lung after intratracheal instillation, J. Occup. Health, 2003, 45, 23–30 CrossRef CAS PubMed.
- S. Bakand, A. Hayes and F. Dechsakulthorn, Nanoparticles: a review of particle toxicology following inhalation exposure, Inhalation Toxicol., 2012, 24, 125–135 CrossRef CAS PubMed.
- M. A. Gatoo, S. Naseem, M. Y. Arfat, A. M. Dar, K. Qasim and S. Zubair, Physicochemical properties of nanomaterials: implication in associated toxic manifestations, BioMed Res. Int., 2014, 2014, 498420 Search PubMed.
- D. W. Anderson, W. A. Mettil and J. S. Schneider, Rearing environment, sex and developmental lead exposure modify gene expression in the hippocampus of behaviorally naive animals, Neurochem. Int., 2013, 62, 510–520 CrossRef CAS PubMed.
- J. Lebedová, Z. Nováková and Z. Večeřa, et al., Impact of acute and subchronic inhalation exposure to PbO nanoparticles on mice, Nanotoxicology, 2018, 12(4), 290–304 CrossRef PubMed.
- G. Oszlánczi, A. Papp, A. Szabó and L. Nagymajtényi, et al., Nervous system effects in rats on subacute exposure by lead-containing nanoparticles via the airways, Inhal. Toxicol., 2011, 4, 173–181 CrossRef PubMed.
- E. R. Kandel, The molecular biology of memory storage: a dialogue between genes and synapses, Science, 2001, 294, 1030–1038 CrossRef CAS PubMed.
- Q. Li, X. Hu, Y. Bai, M. Alattar, D. Ma, Y. Cao, Y. Hao, L. Wang and C. Jiang, The oxidative damage and inflammatory response induced by lead sulfide nanoparticles in rat lung, Food Chem. Toxicol., 2013, 60, 213–217 CrossRef CAS.
- Y. Cao, H. Liu, Q. Li, Q. Wang, W. Zhang, Y. Chen, D. Wang and Y. Cai, Effect of lead sulfide nanoparticles exposure on calcium homeostasis in rat hippocampus neurons, J. Inorg. Biochem., 2013, 126, 70–75 CrossRef CAS.
- L. Ma, J. Liu, N. Li, J. Wang, Y. Duan, J. Yan, H. Liu, H. Wang and F. Hong, Oxidative stress in the brain of mice caused by translocated nanoparticulate TiO2 delivered to the abdominal cavity, Biomaterials, 2010, 31, 99–105 CrossRef CAS.
- Y. Deng-Bryant, L. Y. Leung, K. Caudle, F. Tortella and D. Shear, Cognitive Evaluation Using Morris Water Maze in Neurotrauma, Methods Mol. Biol., 2016, 1462, 539–551 CrossRef CAS.
- Y. Z. Xu, D. Y. Ruan, Y. Wu, Y. B. Jiang, S. Y. Chen, J. Chen and P. Shi, Nitric oxide affects LTP in area CA1 and CA3 of hippocampus in low-level lead-exposed rat, Neurotoxicol. Teratol., 1998, 20, 69–73 CrossRef CAS.
- R. Karamian, A. Komaki, I. Salehi, L. Tahmasebi, H. Komaki, S. Shahidi and A. Sarihi, Vitamin C reverses lead-induced deficits in hippocampal synaptic plasticity in rats, Brain Res. Bull., 2015, 116, 7–15 CrossRef CAS.
- E. J. Park, Y. M. Kim, S. W. Park, H. J. Kim, J. H. Lee, D. U. Lee and K. C. Chang, Induction of HO-1 through p38 MAPK/Nrf2 signaling pathway by ethanol extract of Inula helenium L. reduces inflammation in LPS-activated RAW 264.7 cells and CLP-induced septic mice, Food Chem. Toxicol., 2013, 55, 386–395 CrossRef CAS.
- K. M. Lee, K. Kang, S. B. Lee and C. W. Nho, Nuclear factor-E2 (Nrf2) is regulated through the differential activation of ERK1/2 and PKC alpha/betaII by Gymnasterkoreayne B, Cancer Lett., 2013, 330, 225–232 CrossRef CAS PubMed.
- C. N. Nguyen, H. E. Kim and S. G. Lee, Caffeoylserotonin protects human keratinocyte HaCaT cells against H2O2-induced oxidative stress and apoptosis through upregulation of HO-1 expression via activation of the PI3K/Akt/Nrf2 pathway, Phytother. Res., 2013, 27, 1810–1818 CrossRef CAS.
- Y. Y. Luo, D. M. Zhu and D. Y. Ruan, Galantamine rescues lead-impaired synaptic plasticity in rat dentate gyrus, Toxicology, 2011, 289, 45–51 CrossRef CAS.
- P. H. Danielsen, Y. Cao, M. Roursgaard, P. Moller and S. Loft, Endothelial cell activation, oxidative stress and inflammation induced by a panel of metal-based nanomaterials, Nanotoxicology, 2015, 9, 813–824 CrossRef.
- A. Rama Narsimha Reddy, Y. Narsimha Reddy, V. Himabindu and D. Rama Krishna, Induction of oxidative stress and cytotoxicity by carbon nanomaterials is dependent on physical properties, Toxicol. Ind. Health, 2011, 27, 3–10 CrossRef CAS.
- C. M. Sayes, A. A. Marchione, K. L. Reed and D. B. Warheit, Comparative pulmonary toxicity assessments of C60 water suspensions in rats: few differences in fullerene toxicity in vivo in contrast to in vitro profiles, Nano Lett., 2007, 7, 2399–2406 CrossRef CAS PubMed.
- Z. Wang, Y. Yan, X. Yu, W. Li, B. Li and C. Qin, Protective effects of chitosan and its water-soluble derivatives against lead-induced oxidative stress in mice, Int. J. Biol. Macromol., 2016, 83, 442–449 CrossRef CAS.
- S. Dewanjee, T. K. Dua, R. Khanra, S. Das, S. Barma, S. Joardar, N. Bhattacharjee, M. Zia-Ul-Haq and H. Z. Jaafar, Water Spinach, Ipomoea aquatic (Convolvulaceae), Ameliorates Lead Toxicity by Inhibiting Oxidative Stress and Apoptosis, PLoS One, 2015, 10, e0139831 CrossRef PubMed.
- P. V. Prabhakar, U. A. Reddy, S. P. Singh, A. Balasubramanyam, M. F. Rahman, S. Indu Kumari, S. B. Agawane, U. S. Murty, P. Grover and M. Mahboob, Oxidative stress induced by aluminum oxide nanomaterials after acute oral treatment in Wistar rats, J. Appl. Toxicol., 2012, 32, 436–445 CrossRef CAS.
- M. I. Setyawati, C. Y. Tay and D. T. Leong, Effect of zinc oxide nanomaterials-induced oxidative stress on the p53 pathway, Biomaterials, 2013, 34, 10133–10142 CrossRef CAS.
- H. Motohashi, T. O'Connor, F. Katsuoka, J. D. Engel and M. Yamamoto, Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors, Gene, 2002, 294, 1–12 CrossRef CAS.
- P. S. Khiew, S. Radiman and N. M. Huang, et al., Studies on the growth and characterization of CdS and PbS nanoparticles using sugar-ester nonionic water-in-oilmicroemulsion, J. Cryst. Growth, 2003, 254, 235–243 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1 Gene primer sequence. Table S2 Brain coefficient values. See DOI: 10.1039/c9mt00151d |
| This journal is © The Royal Society of Chemistry 2020 |