Dimension conversion: from a 1D metal–organic gel into a 3D metal–organic porous network with high-efficiency multiple enzyme-like activities for cascade reactions†
Zhong Wei
Jiang‡
a,
Ting Ting
Zhao‡
a,
Yuan Fang
Li
 *a and
Cheng Zhi
Huang
*a and
Cheng Zhi
Huang
 *ab
*ab
aKey Laboratory of Luminescent and Real-Time Analytical Chemistry, Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Beibei, Chongqing 400715, China. E-mail: chengzhi@swu.edu.cn; liyf@swu.edu.cn
bChongqing Key Laboratory of Biomedical Analysis, Chongqing Science and Technology Commission, College of Pharmaceutical Sciences, Southwest University, Beibei, Chongqing 400716, China
First published on 14th August 2019
Abstract
Rational design and synthesis of high-efficiency artificial enzymes with multiple enzyme-like activities in one unit and high selectivity for specific substrates remain great challenges. In this work, for the first time, a stable three-dimensional (3D) Cu-based metal–organic porous network (Cu-MOPN) with intrinsic oxidase- and peroxidase-mimicking activities was successfully fabricated from a one-dimensional (1D) Cu-based metal–organic gel (Cu-MOG) under microwave irradiation. Owing to its high BET surface area, hierarchically porous structure and inherent mass transfer channels, Cu-MOPN shows higher catalytic activity than Cu-MOG. Furthermore, on the basis of the excellent cysteine (Cys) oxidase- and peroxidase-mimicking activities of Cu-MOPN, the highly selective detection of Cys in serum was well realized by a cascade reaction. This study may provide a new strategy for constructing high-efficiency artificial enzymes with versatile functions, and will be highly beneficial for a broad range of applications including biocatalysis, bioassays and biomedicine.
New conceptsArtificial enzymes, as alternatives to natural enzymes, are currently attracting immense attention. Up to now, although various functional materials with enzyme-like characteristics have been developed, their efficacy is still unsatisfactory, including single enzyme activity, insufficient catalytic activity and lack of selectivity towards given substrates. Besides, current research efforts mostly focus on integrated artificial enzyme cascade systems by integrating different materials together, which are usually limited by the uncertainty of assembling individual enzymes into organized functional systems and the incompatibility of different components and catalytic reactions. In this study, a stable 3D Cu-based metal–organic porous network (Cu-MOPN) with intrinsic oxidase- and peroxidase-mimicking activities was successfully synthesized from a 1D Cu-based metal–organic gel (Cu-MOG) via a microwave-assisted in situ transformation strategy. The high BET surface area, hierarchically porous structure and inherent mass transfer channels of Cu-MOPN endow it with high-efficiency catalytic activity. In particular, Cu-MOPN exhibited a high selectivity towards cysteine. And by coupling the cysteine oxidase-like catalytic ability and peroxidase-mimicking activity, a self-organized cascade reaction system was successfully constructed for highly selective detection of cysteine. This contribution not only provides a promising candidate for artificial enzymes, but also sheds light on a new perspective for constructing high-efficiency artificial enzymes with versatile functions. |
In recent years, artificial enzymes, as alternative candidates of natural enzymes, have received great attention due to their attractive merits of ease of storage, low cost, and excellent stability against harsh environments, as well as adjustable catalytic activity.1,2 Since the discovery of inert Fe3O4 nanoparticles that exhibit intrinsic horseradish peroxidase (HRP)-like activity,3 numerous exciting nanomaterials such as carbon-based nanomaterials,4–6 metallic oxide/noble metal nanoparticles,7–13 and metal–organic frameworks (MOFs)14–19 have been demonstrated to exhibit enzyme-mimicking activity and have shown promising potential in environmental detection and biomedical sensing applications. Recently, metal–organic gels (MOGs), a newly developed metal organic hybrid material formed by self-assembly of metal ions and organic linkers, have attracted extensive attention in the field of enzyme mimics owing to their easy preparation, porous structure and inherently present open metal sites.20–23 As is well-known, their sufficient pore size, high stability, firm mass transfer channels and hierarchical porosity are four prerequisites for high catalytic activity. However, MOGs as enzyme mimics developed to date still suffer from insufficient activity because their slow diffusion rates inside the micropores of one-dimensional (1D) nanofibers limit the accessibility of substrate molecules to the active sites. Besides, the mass transfer channels within the pore and network of MOGs occupied by large quantities of solvent molecules may collapse during the drying and reaction processes; thus the catalytic activity can also be inhibited. Therefore, it is a great challenge to obtain high catalytic activity by constructing hierarchical porosity and firm mass transfer channels.
Generally, artificial enzymes, like traditional natural enzymes, only exhibit a single enzyme activity. In order to simulate the complexity and multi-functionality of natural systems for cascade reactions, a common method is to prepare hybrid nanomaterials by integrating different materials together.24–27 Achieving this goal, however, is a significant challenge, not only because of the uncertainty of assembling individual nano-enzymes into organized functional systems, but also because of the incompatibility of different components and catalytic reactions. Until now, only AuNP-based composites, such as EMSN–AuNPs,24 AuNPs/Cu-TCPP,25 PtAu@SiO2,28 cyclodextrin–AuNPs,29 Fe3O4–Au@mesoporous SiO2,30 V2O5–AuNPs,31 and Au@BSA–GO,32 have been developed as multifunctional nanozymes with cascade glucose oxidase–peroxidase catalytic properties. Thus, it is necessary to develop a single component artificial enzyme with multiple enzyme activities for cascade reactions.33 Besides, compared with natural enzymes, artificial enzymes have limited selectivity.2,34 Therefore, designing and developing artificial enzymes with high selectivity for specific substrates is also one of the biggest challenges to be tackled.
Herein, a stable 3D Cu-based metal–organic porous network (Cu-MOPN) with high BET surface area, hierarchically porous structure and firm mass transfer channels have been successfully synthesized from a 1D Cu-based metal–organic gel (Cu-MOG) via a microwave-assisted in situ transformation strategy (Fig. 1A), and it shows intrinsic oxidase- and peroxidase-mimicking activities. The high BET surface area, hierarchically porous structure and inherent mass transfer channels improve its diffusion rate and mass transfer efficiency, thus boosting its catalytic activity. Furthermore, by coupling the Cys oxidase-like catalytic ability and peroxidase-mimicking activity, a self-organized cascade reaction system has been successfully constructed for highly selective detection of Cys (Fig. 1B).
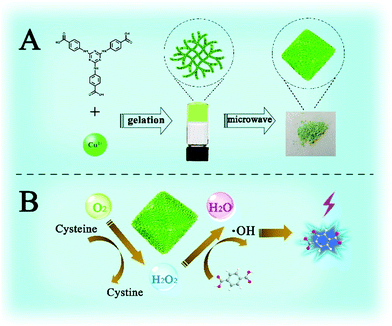 | ||
| Fig. 1 Schematic illustration of the synthesis (A) and cascade Cys oxidase- and peroxidase-mimicking activities of Cu-MOPN (B). | ||
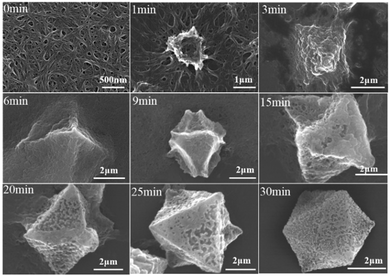
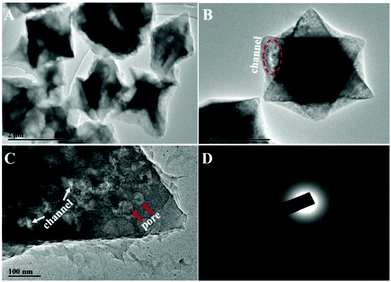
For the preparation of Cu-MOPN, Cu-MOG was first synthesized according to our previous work, which displays a network structure constructed from 1D nanofibers (Fig. S1, ESI†).22 Unexpectedly, an interesting evolutionary process of Cu-MOG to Cu-MOPN was observed by scanning electron microscopy (SEM) under continuous microwave irradiation (Fig. 2 and Fig. S2, ESI†). First, under microwave irradiation, in order to minimize the total surface energy, nanofibers gradually aggregated and formed an initial nucleus. With the prolongation of the reaction time, the nanofibers continually accumulated to the initial nucleus and cross-linked with each other, and finally formed a 3D octahedron sponge-like morphology. However, other physical stimulations, such as ultrasound and heat, did not change the morphology of Cu-MOG significantly. Moreover, a large number of pores and mass transfer channels were formed due to insufficient crosslinking during the reaction process. The transmission electron microscopy (TEM) images in Fig. 3 clearly showed the formation of channels and pores. In the selected area electron diffraction (SAED) pattern, there is no obvious diffraction spot except for the diffraction ring, which indicates that Cu-MOPN is an amorphous structure (Fig. 3D). With the increase of reaction time, in the powder X-ray diffraction (PXRD) patterns, the diffraction peaks at about 25° gradually disappear, and two new peaks can be observed at about 9° and 20°, indicating that the phase structure has changed (Fig. S3, ESI†). Meanwhile, the PXRD patterns show broad peaks, also proving its non-crystalline structure. The Brunauer–Emmett–Teller (BET) surface area of Cu-MOG is calculated to be 7.3 m2 g−1. However, as the reaction time increased, the BET surface area of Cu-MOPN significantly increased to 312.6 m2 g−1 (Fig. 4). Besides, the pore size and porosity also increased gradually as the reaction time increased. The high BET surface area and the hierarchically porous structure (including micropores and mesopores) of Cu-MOPN may play an important role in improving its catalytic activity.
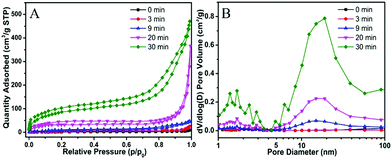 | ||
| Fig. 4 The time-dependent N2 adsorption–desorption isotherms (A) and the corresponding pore size distribution curves (B) of Cu-MOG and Cu-MOPN. | ||
In the Fourier transform infrared (FT-IR) spectra, the absorption peaks at 1689 cm−1, 1290 cm−1 and 990 cm−1 are assigned to the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, νC–O and γO–H vibrations in the carboxyl group of free H3TATAB, respectively (Fig. S4, ESI†). Obviously, the absorption band of the νC
O, νC–O and γO–H vibrations in the carboxyl group of free H3TATAB, respectively (Fig. S4, ESI†). Obviously, the absorption band of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations shifted to 1581 cm−1, and the νC–O and γO–H vibrations almost disappeared as expected, indicating that copper ions coordinated with the carboxyl groups in H3TATAB. The thermogravimetric analysis (TGA) shows that the weight loss ratio of Cu-MOPN is lower than that of Cu-MOG, revealing its higher thermostability, up to 300 °C (Fig. S5, ESI†), even higher than that of Cu-MOF (mesoMOF-1) prepared with Cu2+ and H3TATAB.35
O vibrations shifted to 1581 cm−1, and the νC–O and γO–H vibrations almost disappeared as expected, indicating that copper ions coordinated with the carboxyl groups in H3TATAB. The thermogravimetric analysis (TGA) shows that the weight loss ratio of Cu-MOPN is lower than that of Cu-MOG, revealing its higher thermostability, up to 300 °C (Fig. S5, ESI†), even higher than that of Cu-MOF (mesoMOF-1) prepared with Cu2+ and H3TATAB.35
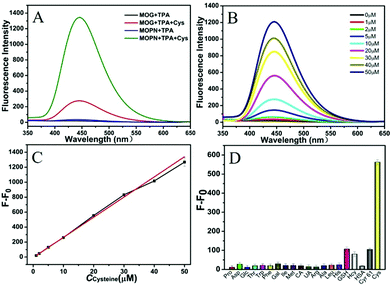
For investigating its multiple enzyme-like activities, the oxidase-mimicking activity of Cu-MOPN was evaluated first. It was found that Cu-MOPN could catalyze the oxidation of o-phenylenediamine (OPD) to 2,3-diaminophenazine (2,3-DPA) with fluorescence emission in the absence of H2O2, and showed a higher fluorescence intensity than Cu-MOG (Fig. 5A), proving that Cu-MOPN exhibited high oxidase-mimicking activity. Furthermore, the peroxidase-mimetic activity of Cu-MOPN was also investigated by using terephthalic acid (TPA) as a typical fluorescence indicator of hydroxyl radicals (˙OH). As expected, Cu-MOPN can catalyze the decomposition of H2O2 to produce ˙OH, thus promoting the reaction of ˙OH with TPA to generate 2-hydroxyterephthalic acid (TAOH) with blue fluorescence (Fig. 5B). Although Cu-MOG can also catalyze the oxidation of TPA in the presence of H2O2, the catalytic activity is much lower than that of Cu-MOPN. All these results indicate Cu-MOPN shows intrinsic oxidase- and peroxidase-mimetic activities, higher than those of Cu-MOG, which may be attributed to the high BET surface area, hierarchically porous structure and inherent mass transfer channels of Cu-MOPN.
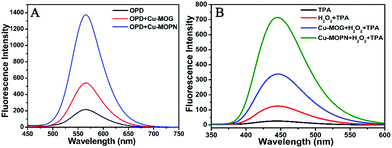 | ||
| Fig. 5 The oxidase- (A) and peroxidase-mimetic activities (B) of Cu-MOG and Cu-MOPN towards catalytic oxidation of OPD and TPA. | ||
Interestingly, the fluorescence of TAOH could be observed when Cys was present in the Cu-MOG + TPA and Cu-MOPN + TPA reaction systems without H2O2 (Fig. 6A). To clarify the reaction mechanism, the Raman spectra of Cys before and after the reaction were explored (Fig. S6, ESI†). The intense peak at 2553 cm−1 assigned to the stretching vibration of S–H nearly disappeared and an obvious peak at 499 cm−1 attributed to the stretching vibration of S–S could be observed after the reaction, indicating that Cys was oxidized to cystine.36 The XPS measurements show that the Auger peak of copper in Cu-MOPN shifted from 570.6 eV to 571.8 eV after Cys treatment, confirming the formation of Cu(I) after reacting with Cys (Fig. S7, ESI†).37,38
To verify the generation of H2O2 in the catalytic oxidation of Cys by Cu-MOPN, the catalase (CAT) was introduced into the TPA–Cys–Cu-MOPN system. Distinctly, the fluorescence of TAOH in the presence of catalase was much weaker than that in its absence, revealing the generation of H2O2 (Fig. S8, ESI†). Noteworthily, the O2-saturated TPA–Cys–Cu-MOPN/Cu-MOG system showed a higher fluorescence signal of TAOH than the N2-saturated reaction system. Besides, when cytochrome c (Cyt c), an electron capture agent, was present in the reaction system, the fluorescence signal also greatly decreased. According to the above results, the catalytic mechanism can be described in the following reactions:
| Cu(II) + Cys + O2 → Cu(I) + cystine + H2O2 | (1) |
| Cu(I) + H2O2 → Cu(II) + ˙OH + OH− | (2) |
| TPA + ˙OH → TAOH | (3) |
Furthermore, to verify the generation of ˙OH during the Cys-induced cascade reaction, isopropanol, acting as an ˙OH scavenger, was added into the reaction systems. Obviously, the fluorescence intensity of TAOH decreased significantly with increasing isopropanol concentration in the TPA + H2O2 + Cu-MOPN system, and the same phenomenon could be observed in the Cys + TPA + Cu-MOPN system, proving the production of ˙OH in the cascade reaction (Fig. S9A, ESI†). As direct evidence, in the Cys + Cu-MOPN system, a typical quartet characteristic peak in the electron spin resonance (ESR) spectrum with relative intensities 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed, further demonstrating the generation of ˙OH (Fig. S9B, ESI†).
1 was observed, further demonstrating the generation of ˙OH (Fig. S9B, ESI†).
In order to investigate the cascade enzyme activity of Cu-MOPN and Cu-MOG, the time-dependent fluorescence changes of TAOH (Fig. S10A and B, ESI†) and apparent steady-state kinetic curves (Fig. S10C and D, ESI†) were measured in the presence of various concentrations of Cys. As results, with the increase of Cys concentration, the formation rate of TAOH accelerated. The data were analyzed by using the Lineweaver–Burk double reciprocal plot to calculate the catalytic parameters Km and Vmax (inset in Fig. S10 and Table S1, ESI†). Obviously, compared to Cu-MOG, Cu-MOPN shows lower apparent Km and higher Vmax values, suggesting its higher cascade enzyme activity than that of Cu-MOG.
For the stability study of Cu-MOPN, after treatment with HCl (pH = 2) and NaOH (pH = 12) for 6 hours, respectively, the morphology, XRD pattern, BET surface area and pore size distribution of Cu-MOPN did not show any significant change, demonstrating its exceptionally high chemical stability (Fig. S11–S13, ESI†). However, Cu-MOG disintegrated immediately when it was placed in solutions of HCl (pH = 2) and NaOH (pH = 12), indicating the poor chemical stability of Cu-MOG (Fig. S14, ESI†). The catalytic activity of Cu-MOPN was enhanced after HCl treatment and decreased after NaOH treatment (Fig. S15, ESI†). This may be due to the fact that H+ can activate the Cu(II) active site, while OH− passivates the Cu(II) active site. Besides, there was no significant loss of catalytic activity in the five successive catalytic cycles, suggesting that Cu-MOPN exhibits long-term catalytic activity (Fig. S16, ESI†).
Based on the cascade reaction system that combined the Cys oxidase- and peroxidase-mimicking activities of Cu-MOPN, a fluorescence approach to detect Cys in serum samples was established. As shown in Fig. 6B, the fluorescence signal of TAOH increased with the increase of Cys concentration. To achieve a highly sensitive determination, the reaction conditions including pH, temperature and the concentrations of Cu-MOPN and TPA were optimized (Fig. S17, ESI†). Under the optimal reaction conditions, the standard curve for Cys was constructed over the range from 1 μM to 50 μM and with a detection limit of 93 nM (Fig. 6C). In addition, the selectivity of the developed method was investigated by considering other amino acids, biomolecules and peptides/proteins (such as glutathione (GSH), human serum albumin (HSA) and human Cyr61) that exist in serum (Fig. 6D). Although the concentrations of the interfering substances were much higher than that of Cys, only Cys provided a significant fluorescence response, indicating that the cascade reaction detection platform has very high specificity toward Cys. Besides, the standard addition method was used to approve the precision and reliability of this method. The acceptable recovery rates of 95.5–104.0% were obtained, demonstrating that the method can be used for the detection of Cys in serum samples (Table S2, ESI†). Compared with some systems recently reported for Cys detection, the cascade reaction system based on Cu-MOPN presented a high selectivity and a low detection limit (Table S3, ESI†).
Conclusions
To sum up, we, for the first time, successfully developed a 3D Cu-based metal–organic porous network derivatized from a 1D Cu-based metal–organic gel via a microwave-assist conversion strategy, which shows high-efficiency oxidase- and peroxidase-mimicking activities due to its high BET surface area, hierarchically porous structure and inherent mass transfer channels. On the basis of the cascade Cys oxidase- and peroxidase-mimicking activities, a highly sensitive and high selectivity fluorometric method for the determination of Cys was developed. This contribution not only provides a promising candidate for artificial enzymes for a wide range of applications including biocatalysis, bioassays, and nano-biomedicine, but also brings light to a new opportunity for design and synthesis of multifunctional nanozymes with enhanced catalytic activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, No. 21575117).Notes and references
- Y. Lin, J. Ren and X. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang and N. Gu, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- M. Shamsipur, A. Safavi and Z. Mohammadpour, Sens. Actuators, B, 2014, 199, 463–469 CrossRef CAS.
- Y. Song, X. Wang, C. Zhao, K. Qu, J. Ren and X. Qu, Chem. – Eur. J., 2010, 16, 3617–3621 CrossRef CAS PubMed.
- W. He, X. Wu, J. Liu, X. Hu, K. Zhang, S. Hou, W. Zhou and S. Xie, Chem. Mater., 2010, 22, 2988–2994 CrossRef CAS.
- Y. Lin, J. Ren and X. Qu, Adv. Mater., 2014, 26, 4200–4217 CrossRef CAS.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS.
- Y. Jv, B. Li and R. Cao, Chem. Commun., 2010, 46, 8017–8019 RSC.
- J. S. Mu, Y. Wang, M. Zhao and L. Zhang, Chem. Commun., 2012, 48, 2540–2542 RSC.
- L. Zhan, C. M. Li, W. B. Wu and C. Z. Huang, Chem. Commun., 2014, 50, 11526–11528 RSC.
- Y. J. Long, Y. F. Li, Y. Liu, J. J. Zheng, J. Tang and C. Z. Huang, Chem. Commun., 2011, 47, 11939–11941 RSC.
- L. Ai, L. Li, C. Zhang, J. Fu and J. Jiang, Chem. – Eur. J., 2013, 19, 15105–15108 CrossRef CAS.
- Z. Jiang, P. Gao, L. Yang, C. Huang and Y. Li, Anal. Chem., 2015, 87, 12177–12182 CrossRef CAS.
- I. Nath, J. Chakraborty and F. Verpoort, Chem. Soc. Rev., 2016, 45, 4127–4170 RSC.
- Y. Xiong, S. Chen, F. Ye, L. Su, C. Zhang, S. Shen and S. Zhao, Chem. Commun., 2015, 51, 4635–4638 RSC.
- Y. L. Liu, X. J. Zhao, X. X. Yang and Y. F. Li, Analyst, 2013, 138, 4526–4531 RSC.
- W. H. Chen, M. Vazquez-Gonzalez, A. Kozell, A. Cecconello and I. Willner, Small, 2018, 14, 1703149 CrossRef.
- L. He, Z. W. Jiang, W. Li, C. M. Li, C. Z. Huang and Y. F. Li, ACS Appl. Mater. Interfaces, 2018, 10, 28868–28876 CrossRef CAS.
- L. He, Z. W. Peng, Z. W. Jiang, X. Q. Tang, C. Z. Huang and Y. F. Li, ACS Appl. Mater. Interfaces, 2017, 9, 31834–31840 CrossRef CAS.
- T. T. Zhao, Z. W. Jiang, S. J. Zhen, C. Z. Huang and Y. F. Li, Microchim. Acta, 2019, 186, 168 CrossRef.
- M. X. Guo and Y. F. Li, Spectrochim. Acta, Part A, 2019, 207, 236–241 CrossRef CAS.
- Y. Lin, Z. Li, Z. Chen, J. Ren and X. Qu, Biomaterials, 2013, 34, 2600–2610 CrossRef CAS.
- Y. Huang, M. Zhao, S. Han, Z. Lai, J. Yang, C. Tan, Q. Ma, Q. Lu, J. Chen, X. Zhang, Z. Zhang, B. Li, B. Chen, Y. Zong and H. Zhang, Adv. Mater., 2017, 29, 1700102 CrossRef.
- Y. Lin, L. Wu, Y. Huang, J. Ren and X. Qu, Chem. Sci., 2015, 6, 1272–1276 RSC.
- Y. Liu, Y. Zheng, Z. Chen, Y. Qin and R. Guo, Small, 2019, 15, 1804987 CrossRef.
- Z. Ma, L. Wu, K. Han and H. Han, Nanoscale Horiz., 2019, 4, 1124–1131 RSC.
- Y. Zhao, Y. Huang, H. Zhu, Q. Zhu and Y. Xia, J. Am. Chem. Soc., 2016, 138, 16645–16654 CrossRef CAS.
- X. He, L. Tan, D. Chen, X. Wu, X. Ren, Y. Zhang, X. Meng and F. Tang, Chem. Commun., 2013, 49, 4643–4645 RSC.
- K. Qu, P. Shi, J. Ren and X. Qu, Chem. – Eur. J., 2014, 20, 7501–7506 CrossRef CAS.
- H. Zhang, X. Liang, L. Han and F. Li, Small, 2018, 14, e1803256 CrossRef.
- J. Wu, S. Li and H. Wei, Nanoscale Horiz., 2018, 3, 367–382 RSC.
- X. Wang, Y. Hu and H. Wei, Inorg. Chem. Front., 2016, 3, 41–60 RSC.
- X. S. Wang, S. Q. Ma, D. F. Sun, S. Parkin and H. C. Zhou, J. Am. Chem. Soc., 2006, 128, 16474–16475 CrossRef CAS.
- H. Lee, M. S. Kim and S. W. Suh, J. Raman Spectrosc., 1991, 22, 91–96 CrossRef CAS.
- S. Poulston, P. M. Parlett, P. Stone and M. Bowker, Surf. Interface Anal., 1996, 24, 811–820 CrossRef CAS.
- P. D. Kirsch and J. G. Ekerdt, J. Appl. Phys., 2001, 90, 4256–4264 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00293f |
| ‡ Zhong Wei Jiang and Ting Ting Zhao contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |