A “PDMS-in-water” emulsion enables mechanochemically robust superhydrophobic surfaces with self-healing nature†
Mingzheng
Ge
 a,
Chunyan
Cao
b,
Fanghua
Liang
a,
Rong
Liu
a,
Yu
Zhang
a,
Wei
Zhang
*a,
Tianxue
Zhu
c,
Bo
Yi
a,
Chunyan
Cao
b,
Fanghua
Liang
a,
Rong
Liu
a,
Yu
Zhang
a,
Wei
Zhang
*a,
Tianxue
Zhu
c,
Bo
Yi
 b,
Yuxin
Tang
b,
Yuxin
Tang
 *d and
Yuekun
Lai
*d and
Yuekun
Lai
 *c
*c
aSchool of Textile & Clothing, National & Local Joint Engineering Research Center of Technical Fiber Composites for Safety and Health, Nantong University, Nantong 226019, P. R. China. E-mail: zhangwei@ntu.edu.cn
bDepartment of Biomedical Sciences, City University of Hong Kong, Hong Kong 999077, P. R. China
cNational Engineering Research Center of Chemical Fertilizer Catalyst (NERC-CFC), College of Chemical Engineering, Fuzhou University, Fuzhou 350116, P. R. China. E-mail: yklai@fzu.edu.cn
dInstitute of Applied Physics and Materials Engineering, University of Macau, Macau 999078, P. R. China. E-mail: yxtang@um.edu.mo
First published on 13th September 2019
Abstract
It is highly challenging to construct a durable superhydrophobic coating for practical applications since the coating is easily destroyed by mechano-chemical attack. To address this issue, a “PDMS-in-water” emulsion approach is for the first time adopted to design a mechanochemically robust superhydrophobic cotton fabric with intelligent self-healing nature, without using any fluorine-containing components. With this approach, PDMS molecules firstly penetrate into the cotton fiber, and then graft onto the surface of the cotton fabric with a strong binding force, creating hierarchical rough structures and lowering the surface energy simultaneously. Benefitting from this design, the PDMS@cotton fabric exhibits high superhydrophobicity with a water contact angle over 155°, surpassing all the PDMS-in-organic solvent based approaches. Impressively, the surface repairs its superhydrophobicity throughout the whole lifetime though damaged by machine washing or abrasion (>100 cycles), due to the self-diffusion process of PDMS molecules from the inner part to the outer surface of the cotton fibers to minimize surface free energy. Besides, the superhydrophobic coatings display superior chemical stability in strongly acidic and alkaline solution, and maintain similar textile physical properties of the cotton fabric, such as elongation at break, tensile strength, etc. Our environment-friendly “PDMS-in-water” approach can be easily integrated into industrial textile finishing treatment and is promising to apply to various substrates with robust superhydrophobic surfaces.
New conceptsHydrophobic polydimethylsiloxane (PDMS) is a commonly used fluorine-free polymer binder for constructing superhydrophobic coatings due to its excellent chemical stability, good washing/abrasion-resistance, outstanding water-repellent property, and high adhesion with the substrate. Traditionally, the PDMS coating is fabricated by dissolving PDMS in organic solvent or water/organic solvent mixed solutions first, and then the superhydrophobic cotton fabrics are obtained by top-coating of PDMS molecular. In the current work, we for the first time develop a new concept to disperse PDMS polymer in water to form a “PDMS-in-water” emulsion, which allows the construction of intelligent self-healing and robust superhydrophobic coatings on cotton fiber. Benefitting from the intensified inherent hierarchical nanostructures, the PDMS@cotton fabric exhibits high superhydrophobicity with a water contact angle over 155°, surpassing all the PDMS-in-organic solvent based approaches. Impressively, the coating exhibits excellent self-healing properties against intensive mechanical damage. This environment-friendly and cost-effective method can be employed on various materials in the nanoscience field for environment applications, such as anti-icing, self-cleaning surfaces, catalysis, etc. |
Introduction
Inspired by gecko feet, lotus leaves, and other organisms in nature, bio-inspired superhydrophobic interfaces/surfaces have been successfully constructed by rational design,1 which is widely used in oil–water separation,2 water collection,3 droplet manipulation,4 drag reduction,5etc.6 It is widely known that rough micro/nano-structures and low surface energy are the two crucial factors to construct superhydrophobic surfaces, which can be fabricated via solvothermal/hydrothermal methods, chemical vapor deposition, dip/spray-coating techniques, etc. by one or two steps.7 However, their practical applications are limited by some disadvantages, especially poor mechanical and chemical stability, inferior interfacial binding force between the substrate and modified materials, and pollutive fabrication methods by using organic solvents or fluorine-based components.8Recently, although great efforts have been devoted to constructing superhydrophobic coatings on diverse substrates, it is still a major challenge to solve the durability of superhydrophobic coatings for practical applications, which is usually destroyed by mechano-chemical attack.9 For example, a superhydrophobic cotton fabric was obtained by modifying with tea polyphenol and Fe nanoparticles via a facile dip-coating technique;10a however, the binding force between the modified materials and cotton fabric was weak. Therefore, the coating was easily damaged by mechanical stress, and the contact angle decreased from 163° to 153° only after 5 washing cycles. To address this problem, an emerging concept based on superhydrophobic surfaces/interfaces with intelligent self-healing function is proposed, and it is promising for commercial applications. For instance, a self-healing superhydrophobic coating containing fluoroalkylsilane components is successfully designed.10b With this approach, when the primary top fluoroalkylsilane layer was destroyed by an etching process, the preserved self-healing components could migrate towards the surface to restore the damaged surface, converting hydrophilicity to superhydrophobicity again. However, the utility of superhydrophobic materials by this method was still hindered for real application due to the decreased content of low-surface-energy agents and physically degraded structure when exposing to mechanical damage.11 Moreover, most of the reported self-healing processes were achieved via the migration of low-surface-energy fluorine-containing components to the top-surface, which were usually expensive, toxic and harmful to the environment and human health, especially for the cotton fabric.12 Therefore, endowing mechanochemical robust superhydrophobic surfaces with a self-healing property via an environment-friendly technique is promising to enhance the durability of artificial superhydrophobicity.
From an environment-friendly and mechanical perspective, polydimethylsiloxane (PDMS) is a commonly used fluorine-free polymer binder for constructing superhydrophobic coatings due to its excellent chemical stability, good washing/abrasion-resistance, outstanding water-repellent property, and high adhesion with the substrate.13 Traditionally, the PDMS coating is fabricated as followed. The PDMS is firstly dissolved in organic solvent or water/organic solvent mixed solutions, and then the superhydrophobic cotton fabrics are obtained by top-coating of PDMS.14 For example, Xue et al. fabricated superhydrophobic PDMS modified textiles by dissolving PDMS in tetrahydrofuran (THF) solution via a phase-separation technique.15 The self-roughened coatings have superior durability against long laundering and abrasion tests due to the improved interfacial binding force. However, the organic solvent involved in the fabrication of the PDMS coating is not only expensive, but also harmful to the environment. By considering the above disadvantages, it is highly desirable to construct mechanochemically robust superhydrophobic cotton fabric surfaces with self-healing nature by a clean aqueous solution approach from PDMS without using any organic solvents.
Herein, a “PDMS-in-water” emulsion approach is applied to fabricate a mechanochemically robust superhydrophobic cotton fabric with intelligent self-healing nature, without using any fluorine-containing components. With this approach, PDMS was uniformly dispersed in cotton fiber, and grafted on the bulk surface of cotton fabric via a strong interfacial binding force, enhancing inherent hierarchical micro-structures and decreasing the surface energy simultaneously. Benefitting from our method, the PDMS@cotton fabric displayed a water contact angle higher than 155°, which was much higher than that fabricated in organic solvent solutions, such as THF, hexadecane, etc. Interestingly, the superhydrophobic PDMS@cotton fabrics could recover their water-repellency though damage by abrasion or machine washing throughout its lifetime. Moreover, the superhydrophobic coatings displayed superior chemical stability in strongly acidic and alkaline solution. Furthermore, the superhydrophobic PDMS@cotton fabric maintained similar textile physical properties of the cotton fabric, such as elongation at break, tensile strength, and air permeability, etc. In addition, the superhydrophobic coatings not only showed excellent anti-fouling and self-cleaning performance, but also exhibited high efficiency in oil–water separation. This simple fluorine-free and cost-effective method is very promising for large-scale industry application.
Results and discussion
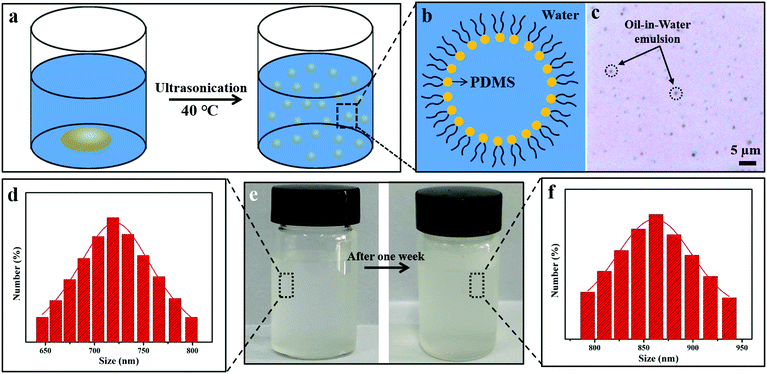
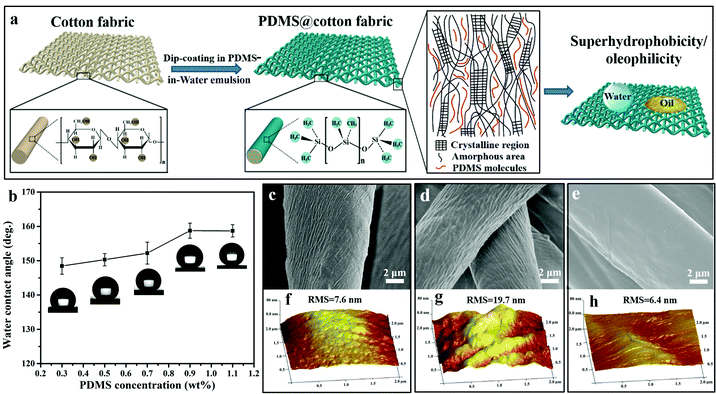
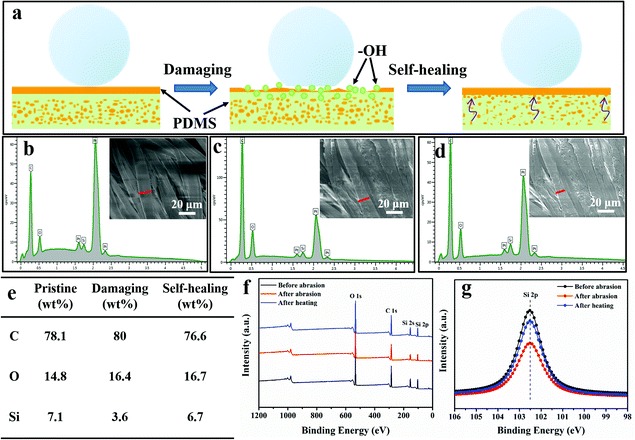
As we know, PDMS is insoluble in water. To facilitate PDMS dispersing in water, it was pretreated by plasma treatment in air, which produced lots of hydrophilic groups on its surface. Due to the existence of both hydrophilic (–OH) and hydrophobic (–CH3) groups on the PDMS molecules, they were encapsulated by water and became a PDMS-in-water emulsion under ultrasonication, resulting in the formation of a homogeneous solution (Fig. 1a–c). After ultrasonication at 40 °C for 30 min, PDMS was uniformly dispersed in deionized water and the solution became translucent (Fig. 1e, left). As confirmed by particle size analysis, the average size of the PDMS polymer dispersion was about 719 nm (Fig. 1d). Even after one-week storage, no precipitation was observed (Fig. 1e, right), and the average particle size only slightly increased to 865 nm (Fig. 1f), indicating that the PDMS-in-water emulsion could remain stable for a long time. Such a stable dispersion system will greatly facilitate the feasibility of the coating onto various substrates with desirable water absorption by the dip-coating technique.The fabrication procedure of superhydrophobic cotton fabrics by one-step dip-coating in PDMS-in-water emulsion is displayed in Fig. 2a. When wetting the cotton fabric with water, water would penetrate into the amorphous areas of cellulose in the cotton fiber, decreasing the binding force among cellulose macromolecules. Therefore, PDMS penetrated into the inner space and occupied the amorphous areas of cotton fiber along with water after a facile dip-coating and drying process, and PDMS molecules were grafted onto the outer bulk surface of cotton fiber via a strong binding force simultaneously after water evaporation. The PDMS with identical terminate groups of methyl on the surface could lower the surface energy and enhance the inherent hierarchical micro-structures of cotton fabric, bringing a superhydrophobic surface. Therefore, PDMS@cotton fabric had a water contact angle (CA) as high as 158.7 ± 2.2° and methyl-blue dyed water can stand on the cotton surface for a long time without any permeation (Fig. S1, ESI†). For comparison, we also dissolved PDMS in THF, hexadecane, dichloromethane and toluene organic solvent solutions (denoted as PDMS-in-THF, hexadecane, dichloromethane, and toluene solution). With the same PDMS concentration, the modified cotton fabrics showed a hydrophobic surface with water CAs around 130°, which is much lower than the counterparts in PDMS-in-water emulsion. With increasing PDMS concentration in organic solvent solutions, the water contact angle kept relatively stable (Fig. S2, ESI†). These results demonstrate that PDMS dissolved in an all-water-based system induces the best superhydrophobicity for modified cotton fabrics, which is attributed to the penetration of water-soluble PDMS molecules into the amorphous areas of the entire hydrophilic cotton fiber (including the uniform coating on outer surface), as well as the intensified inherent hierarchical nanostructures (Fig. 2d and g), which significantly amplifies the hydrophobicity.
In order to unveil the influence of PDMS concentration on the superhydrophobicity of PDMS@cotton fabric fabricated in PDMS-in-water emulsion, the water CA changes with various PDMS concentrations are summarized in Fig. 2b. It was found that the water CA gradually increased from 148.6° ± 2.4° to 158.7° ± 2.2° when increasing the concentration of PDMS from 0.3 wt% to 0.9 wt%. However, when further increasing the PDMS concentration to 1.1 wt%, the water CA kept unchanged. These results suggest that the low content of PDMS cannot fully cover on the surface of cotton fabric, while increasing PDMS concentration induces higher superhydrophobicity due to the better coverage of the low surface energy of PDMS molecules. However, the PDMS is saturated and the excessive PDMS cannot be dispersed uniformly in water when the PDMS concentration was more than 0.9 wt%. Therefore, 0.9 wt% PDMS was the most optimal concentration for endowing cotton fabrics with the best superhydrophobicity. As displayed in Fig. 2c, pristine cotton fabric inherently had many natural cracks and grooves at the microscale. After being immersed in PDMS-in-water emulsion, groove structures were highly intensified and became more distinct (Fig. 2d). In comparison, the surface of the cotton fabrics became smoother with no apparent hierarchal structures after dip-coating in PDMS-in-THF solution (Fig. 2e), which was similar to PDMS-in-hexadecane, dichloromethane and toluene solutions (Fig. S3, ESI†). This further verified that using an all-water-based system can largely increase the surface roughness, thus improving superhydrophobicity. Accordingly, the surface roughness was also confirmed by atomic force microscopy (AFM). The root mean square (RMS) value of the pristine cotton was only 7.6 nm, indicating that the outer surface was comparatively smooth (Fig. 2f). The lower RMS value represented smaller roughness. After dip-coating in PDMS-in-water emulsion, the surface roughness of PDMS@cotton fabric increased with the RMS roughness increasing from 7.6 nm to 19.7 nm (Fig. 2g), while RMS decreased to 6.4 nm after immersing in PDMS-in-THF solution (Fig. 2h), consistent with the SEM observations.
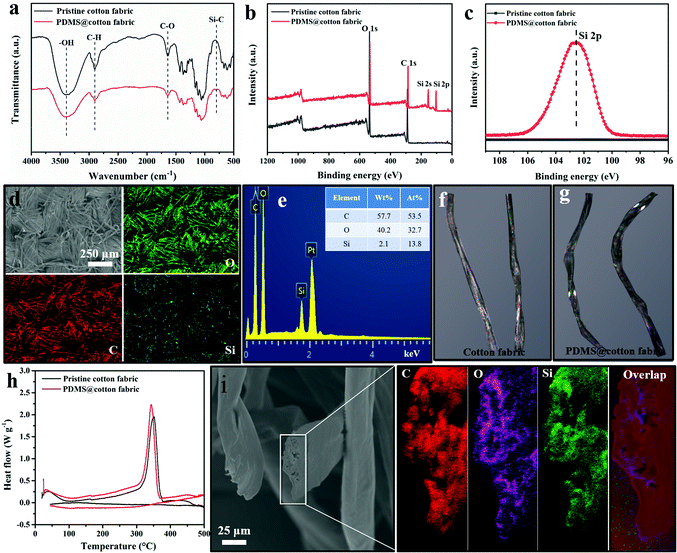
The chemical composition of the pristine cotton fabric and PDMS@cotton fabric was further confirmed by energy disperse spectroscopy (EDS), Fourier transform infrared (FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). As displayed in Fig. 3a, the pristine cotton fabric displayed three strong representative peaks at 3430, 2917 and 1617 cm−1, corresponding to the –OH, C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. After being modified with PDMS, the Si–C bond representative peak appeared at 750 cm−1 (FTIR, Fig. 3a) and in the C1s XPS spectra (Fig. S4, ESI†), indicating that the PDMS was well grafted on the entire cotton fabric. Besides, as depicted in Fig. 3b, the pristine cotton fabric only consisted of carbon and oxygen elements. However, two new Si 2p and 2s representative peaks appeared at 101.0 eV and 152.0 eV after PDMS coating. Furthermore, the high resolution Si 2p peak of PDMS@cotton fabric exhibited a much stronger peak than that of pristine cotton fabric (Fig. 3c), demonstrating that the cotton fabric was well fully encapsulated by PDMS film. Except for XPS, EDS was also used to characterize the element composition distribution on the surface of PDMS@cotton fabric. The elemental mapping images of PDMS@cotton fabric exhibited that the elements of C, O, and Si were uniformly distributed on the surface of the cotton fabric (Fig. 3d and e), demonstrating that PDMS was uniformly grafted on the cotton fabric, consistent with the FTIR and XPS spectra. As displayed in the optical picture of the cotton fabric (Fig. 3f), the light area in a single cotton fiber represents the crystalline region. However, after dip-coating in PDMS-in-water emulsion, the cotton fiber became dark (Fig. 3g), indicating that PDMS successfully penetrated into the cotton fiber. What's more, the melting point of PDMS@cotton fabric was smaller than that of pristine cotton fabric (Fig. 3h) due to the PDMS migration into the amorphous areas of cellulose of the cotton fiber. In addition, the elements of C, O, and Si were also uniformly distributed on the cross section of a single cotton fiber (Fig. 3i), further demonstrating uniform dispersion of PDMS molecules in the cotton fiber.
O groups, respectively. After being modified with PDMS, the Si–C bond representative peak appeared at 750 cm−1 (FTIR, Fig. 3a) and in the C1s XPS spectra (Fig. S4, ESI†), indicating that the PDMS was well grafted on the entire cotton fabric. Besides, as depicted in Fig. 3b, the pristine cotton fabric only consisted of carbon and oxygen elements. However, two new Si 2p and 2s representative peaks appeared at 101.0 eV and 152.0 eV after PDMS coating. Furthermore, the high resolution Si 2p peak of PDMS@cotton fabric exhibited a much stronger peak than that of pristine cotton fabric (Fig. 3c), demonstrating that the cotton fabric was well fully encapsulated by PDMS film. Except for XPS, EDS was also used to characterize the element composition distribution on the surface of PDMS@cotton fabric. The elemental mapping images of PDMS@cotton fabric exhibited that the elements of C, O, and Si were uniformly distributed on the surface of the cotton fabric (Fig. 3d and e), demonstrating that PDMS was uniformly grafted on the cotton fabric, consistent with the FTIR and XPS spectra. As displayed in the optical picture of the cotton fabric (Fig. 3f), the light area in a single cotton fiber represents the crystalline region. However, after dip-coating in PDMS-in-water emulsion, the cotton fiber became dark (Fig. 3g), indicating that PDMS successfully penetrated into the cotton fiber. What's more, the melting point of PDMS@cotton fabric was smaller than that of pristine cotton fabric (Fig. 3h) due to the PDMS migration into the amorphous areas of cellulose of the cotton fiber. In addition, the elements of C, O, and Si were also uniformly distributed on the cross section of a single cotton fiber (Fig. 3i), further demonstrating uniform dispersion of PDMS molecules in the cotton fiber.
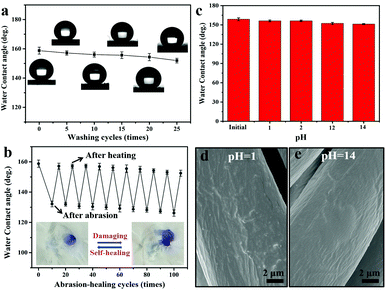
To investigate the feasibility of PDMS@cotton fabric in a real-world application, washing and laundering durability was measured systematically, respectively. Here, the washing durability of PDMS@cotton fabric was examined by an accelerated laundering durability test with the AACC 61-2006 standard method. The CA changes of PDMS@cotton fabric were measured at every 5 cycles. After every 5 cycles, these samples were cleaned by water and then dried. In the original state, PDMS@cotton fabric displayed a non-wetting performance with a high water contact angle nearly 160°. After laundering for 25 cycles, there was no distinct decrease in the CA and the surface still maintained high water CA above 150°. As displayed in Fig. 4a (insert pictures), the water droplets could still retain spherical morphology even after 25 accelerated laundering cycles. The long cycling laundering times only caused a subtle change in the groove structures (Fig. S5a, ESI†), demonstrating the high mechanical stability of the superhydrophobic PDMS coating.
In addition to the laundering durability, PDMS@cotton fabrics also showed good abrasion resistance. The CA variations on the surfaces of PDMS@cotton fabric with different abrasion cycles are shown in Fig. 4b. After 10 times of abrasion, PDMS@cotton fabric lost its superhydrophobicity with the water contact angle decreasing to 132.2°. However, when the damaged cotton fabric was heated at 80 °C for 30 min, it restored superhydrophobicity with a water CA of 157.2°. The water CAs only reduced slightly with increasing the abrasion-heating repeated cycles. Indeed, the mechanical abrasion and laundering also have a great influence on the hysteresis state of PDMS@cotton fabric. For example, before mechano-chemical attack, the PDMS@cotton fabric was highly superhydrophobic with a low sliding angle around 25.5° (Fig. S6a–c, ESI†). However, the sliding angle increased and the droplet could not roll off from the cotton fabric even turning it over (Fig. S6d and e, ESI†), since the mechanical treatment destroyed the hierarchical rough surfaces and reduced the content of the PDMS coating. Herein, we found that the PDMS@cotton fabric wore out after 100 cycles of abrasion and became nearly hydrophilic (right inset picture in Fig. 4b). However, after heating for another 30 min, the superhydrophobicity was restored and the water can stand on the fabric without any penetration (left inset picture in Fig. 4b). It was interesting that the PDMS@cotton fabric maintained its superhydrophobicity even after the fabric had been destroyed. Besides, the PDMS@cotton fabric still retained hierarchical micro-structures even after 100 cycles of abrasion-heating cycles (Fig. S5b, ESI†). The self-healing process of robust superhydrophobic PDMS coating can also take place at room temperature, but the self-healing process needed a long time for almost 1 day. This phenomenon may be because the preserved elastomer PDMS molecules in cotton fibers could release after heating treatment or long-time placement.16
Except for mechanical damage assessment, chemical stability of PDMS@cotton fabric was also evaluated by immersing in highly concentrated acidic and alkali solutions. As exhibited in Fig. 4c, there were no significant changes in the CAs after immersing in the acid solution with the pH value of 1 and 2 for 24 hours. The SEM images of PDMS@cotton fabric in HCl solution (pH = 1) confirmed that no morphological changes occurred after acid treatment (Fig. 4d). Though the Si–O bond was sensitive to alkaline corrosion, the surface still maintained a well-wrapped coating on the cotton fiber (Fig. 4e) and still displayed high water CAs above 150°. Besides, the chemical treatment has little impact on the sliding angle, which only slightly increased to 29.2° (Fig. S6f, ESI†). All these results demonstrated that a mechanochemically robust superhydrophobic cotton fabric with self-healing nature has been successfully synthesized.
The working mechanism of the intelligent self-healing process for PDMS@cotton fabric was demonstrated by EDS line scan and XPS spectra. During the dip-coating process, large quantities of PDMS polymers penetrated into the amorphous areas of cellulose macromolecules along with water, and PDMS preserved in the cotton fiber after water evaporation. Upon damaging the surface mechanically, the primary top PDMS layer was removed, and polar groups (usually –OH groups) were normally introduced, resulting in reduced surface superhydrophobicity and increased surface free energy. However, heating increased the mobility of PDMS molecules, and more superhydrophobic –CH3 groups of preserved PDMS polymer in the cotton fiber moved to the outer surface due to the self-diffusion process, minimizing the surface free energy and leading to superhydrophobicity (Fig. 5a). The molecular rotation and movement could also take place at room temperature because of the low Tg, making cotton fabric superhydrophobic again.17 As displayed in Fig. 5b and e, the content of C, O, and Si elements for PDMS@cotton fabric at a pristine state is 78.1, 14.8, and 7.1 wt%, respectively. However, the content of Si element decreased to 3.6 wt%, and that of O element increased to 16.4 wt%, indicating that the PDMS top coating was destroyed by abrasion (Fig. 5c and e). After heating, the ratio of Si element increased to 6.7 wt%, which was approaching the pristine state, verifying that preserved PDMS polymers in the cotton fiber successfully migrated to the outer surface of the cotton fabric (Fig. 5d and e). For further confirmation, the surface chemical composition of PDMS@cotton fabric before abrasion, and after abrasion and heating treatment was also tested by XPS. As displayed in Fig. 5f and g, the representative peak of Si 2p at 101.0 eV weakened after abrasion. However, the intensity was strengthened after heating treatment, which was consistent with the line scan results.
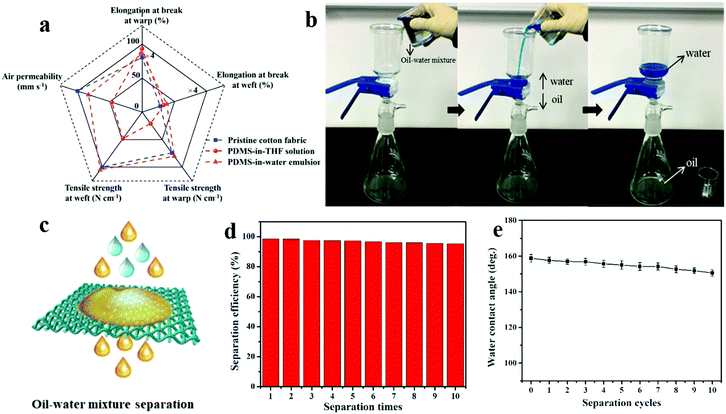
Besides mechanochemical stability, the textile physical properties, self-cleaning and anti-fouling performance were all tested. The PDMS coating has a negligible adverse effect on the important textile physical properties of the cotton fabric, such as tensile strength, air permeability, etc. Due to the excellent mechanical property of PDMS polymer and more regular molecular chains in amorphous areas after PDMS introduction, the strength of PDMS@cotton fabricated in PDMS-in-water emulsion was largely improved from 194 ± 5 N cm−1 to 210 ± 7 N cm−1 (Fig. 6a and Table S1, ESI†). However, the strength of PDMS@cotton fabricated in PDMS-in-THF solution was weakened due to the destroyed structure of cellulose by the corrosive organic solvent. And the elongation also increased after PDMS coating correspondingly (Increased from 20.1% to 21.5%). In addition, compared to PDMS@cotton fabric fabricated in PDMS-in-THF solution, PDMS@cotton fabric fabricated in PDMS-in-water emulsion had a small decrease of air permeability, which was attributed to a large amount of PDMS polymers existing in the cotton fiber, which did not block the interspace between the interwoven yarns. To demonstrate the anti-fouling performance of the pristine fabric and PDMS@cotton fabric, they were immersed in methyl blue-containing water solution. As displayed in Fig. S7a (ESI†), after dipped in methyl blue polluted solution, the liquids wetted the pristine cotton quickly and it was fully contaminated after taking out. However, the superhydrophobic PDMS@cotton fabric still remained clean after being immersed in the contaminated solution (Fig. S7b, ESI†). These results demonstrated that PDMS@cotton fabric had excellent water repellency, which was promising for anti-fouling applications. In addition, self-cleaning performance of PDMS@cotton fabric was also investigated. Water-soluble rhodamine was employed as a model stain and casted on the samples. And then, water was dripped on the stain. When the water contacted the substrates, the pristine cotton fabric was dyed red immediately due to its hydrophilic property (Fig. S8a, ESI†). As for superhydrophobic PDMS@cotton fabric, water was stained red and it showed spherical morphology without penetration (Fig. S8b, ESI†). Furthermore, after removing the water by tilting the fabric, the surface becomes as clean as that of the pristine state. PDMS embedded in cotton fiber and fully covered on the cotton fabric enabled the water droplets to roll off freely, and remove the pollutants simultaneously (Fig. S8c, ESI†).
The as-constructed porous PDMS@cotton fabric has been demonstrated with both superhydrophobic and superoleophilic behavior, which could be used to absorb the light oil floating on water, as shown in Fig. S9 (ESI†). Besides, it could also be used for oil–water separation (Fig. 6b). When 100 mL mixture solution was poured onto the PDMS@cotton fabric, the immiscibility of water and oil made the mixed solution separate rapidly. The heavy oil could pass through the PDMS@cotton fabric via its gravity, while water was blocked and remained above the membrane due to superhydrophobicity (Fig. 6c).18 After 10 separation cycles, the efficiency only slightly decreased from 98.6% to 95.3%, displaying good recyclability (Fig. 6d). In addition, the water CA of the cotton still remained more than 150° after 10 cycles of separation, showing satisfactory superhydrophobicity (Fig. 6e). Thus, PDMS@cotton fabric displayed excellent oil–water separation efficiency and recyclability.
Conclusions
In summary, we have developed a “PDMS-in-water” emulsion approach to design a mechanochemically robust superhydrophobic PDMS@cotton fabric with self-healing nature. The enhanced micro-scaled groove structures combined with low surface energy endow the superhydrophilic cotton fabric with excellent self-cleaning properties, anti-fouling performance, and superhydrophobicity with a water contact angle higher than 155°, which is much higher than that fabricated in PDMS-in-organic solvent solutions. Besides, the as-prepared PDMS@cotton fabric exhibits remarkable laundering and abrasion durability against mechanical stress with numerous repeated cycles. In particular, it was capable of repairing its superhydrophobicity though damaged by abrasion over 100 cycles, due to the intelligent self-healing property driven by the self-diffusion process with minimizing the surface free energy throughout its whole lifetime. Benefitting from our design, the superhydrophobic PDMS@cotton fabric maintained similar textile physical properties of the cotton fabric, such as elongation at break, tensile strength, etc., and the superhydrophobic coatings displayed superior chemical stability in harsh environments. Our invention based on this environment-friendly technique offers a practical solution for industry textile finishing, and it can be adopted for scalable fabrication of multifunctional fabrics for potential applications in anti-icing, oil–water-emulsion separation, etc.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (Grant No. 2016YFB0303101), the National Nature Science Foundation of China (Grant No. 21501127), the Science and Technology Development Fund, Macau SAR (Grant No. 0057/2019/A1), and Nantong Science and Technology Project (No. JC2018105). This work was also supported by the Open Project Program of Key Laboratory of Eco-textiles, Ministry of Education, Jiangnan University (No. KLET1803) and the Large Instruments Open Foundation of Nantong University (No. KBJN1927).Notes and references
- (a) K. Liu, X. Yao and L. Jiang, Chem. Soc. Rev., 2010, 39, 3240–3255 RSC; (b) T. Verho, C. Bower, P. Andrew, S. Franssila, O. Ikkala and R. H. A. Ras, Adv. Mater., 2011, 23, 673–678 CrossRef CAS; (c) N. R. Chiou, C. Lui, J. Guan, L. J. Lee and A. J. Epstein, Nat. Nanotechnol., 2007, 2, 354–357 CrossRef CAS; (d) N. Miljkovic, R. Enright, Y. Nam, K. Lopez, N. Dou, J. Sack and E. N. Wang, Nano Lett., 2013, 13, 179–187 CrossRef CAS; (e) J. Y. Shiu, C. W. Kuo, P. L. Chen and C. Y. Mou, Chem. Mater., 2004, 16, 561–564 CrossRef CAS.
- (a) J. Y. Huang, S. H. Li, M. Z. Ge, L. N. Wang, T. L. Xing, G. Q. Chen, X. F. Liu, S. S. Al-Deyab, K. Q. Zhang, T. Chen and Y. K. Lai, J. Mater. Chem. A, 2015, 3, 2825–2832 RSC; (b) Y. Pan, S. Huang, F. Li, X. Zhao and W. Wang, J. Mater. Chem. A, 2018, 6, 15057–15063 RSC; (c) S. H. Li, J. Y. Huang, Z. Chen, G. Q. Chen and Y. K. Lai, J. Mater. Chem. A, 2017, 5, 31–55 RSC; (d) R. Qu, W. Zhang, X. Li, Y. Liu, T. Shih, Y. Wei and L. Feng, J. Mater. Chem., 2018, 6, 18003–18009 RSC; (e) X. Ge, W. Qin, H. Zhang, G. Wang, Y. Zhang and C. Yu, Nanoscale, 2019, 11, 12161–12168 RSC.
- (a) Y. Zheng, H. Bai, Z. Huang, X. Tian, F. Q. Nie, Y. Zhao, J. Zhai and L. Jiang, Nature, 2010, 463, 640–643 CrossRef CAS; (b) J. Ju, Y. Zheng and L. Jiang, Acc. Chem. Res., 2014, 47, 2342–2352 CrossRef CAS; (c) H. Bai, L. Wang, J. Ju, R. Sun, Y. Zheng and L. Jiang, Adv. Mater., 2014, 26, 5025–5030 CrossRef CAS; (d) D. Li, Z. Wang, D. Wu, G. Han and Z. Guo, Nanoscale, 2019, 11, 11774–11781 RSC; (e) X. Zeng, L. Qian, X. Yuan, C. Zhou, Z. Li, J. Cheng, S. Xu, S. Wang, P. Pi and X. Wen, ACS Nano, 2017, 11, 760–769 CrossRef CAS.
- (a) X. Hong, X. Gao and L. Jiang, J. Am. Chem. Soc., 2007, 129, 1478–1479 CrossRef CAS; (b) X. Tian, H. Jin, J. Sainio, R. H. A. Ras and O. Ikkala, Adv. Funct. Mater., 2014, 24, 6023–6028 CrossRef CAS; (c) J. Jiang, J. Gao, H. Zhang, W. He, J. Zhang, D. Daniel and X. Yao, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 2482–2487 CrossRef CAS; (d) C. Yang, L. Wu and G. Li, ACS Appl. Mater. Interfaces, 2018, 10, 20150–20158 CrossRef CAS; (e) X. Yang, X. Liu, Y. Lu, J. Song, S. Huang, S. Zhou, Z. Jin and W. Xu, J. Phys. Chem. C, 2016, 120, 7233–7240 CrossRef CAS.
- (a) J. Ou, B. Perot and J. P. Rothstein, Phys. Fluids, 2004, 16, 4635–4643 CrossRef CAS; (b) G. Wang, Z. Zeng, H. Wang, L. Zhang, X. Sun, Y. He, L. Li, X. Wu, T. Ren and Q. Xue, ACS Appl. Mater. Interfaces, 2015, 7, 26184–26194 CrossRef CAS; (c) N. J. Shirtcliffe, G. McHale, M. I. Newton and Y. Zhang, ACS Appl. Mater. Interfaces, 2009, 1, 1316–1323 CrossRef CAS; (d) Z. Li, J. Marlena, D. Pranantyo, B. Nguyen and C. Yap, J. Mater. Chem. A, 2019, 7, 16387–16396 RSC; (e) H. Hu, J. Wen, L. Bao, L. Jia, D. Song, B. Song, G. Pan, M. Scaraggi, D. Dini, Q. Xue and F. Zhou, Sci. Adv., 2017, 3, e1603288 CrossRef PubMed; (f) W. Wang, Y. Q. Liu, Y. Liu, B. Han, H. Wang, D. D. Han, J. N. Wang, Y. L. Zhang and H. B. Sun, Adv. Funct. Mater., 2017, 27, 1702946 CrossRef.
- (a) Y. Si, Q. Fu, X. Wang, J. Zhu, J. Yu, G. Sun and B. Ding, ACS Nano, 2015, 9, 3791–3799 CrossRef CAS; (b) M. Zeng, P. Wang, J. Luo, B. Peng, B. Ding, L. Zhang, L. Wang, D. Huang, I. Echols, E. A. Deeb, E. Bordovsky, C. H. Choi, C. Ybanez, P. Meras, E. Situ, M. S. Mannan and Z. Cheng, ACS Appl. Mater. Interfaces, 2018, 10, 22793–22800 CrossRef CAS; (c) G. B. Hwang, K. Page, A. Patir, S. P. Nair, E. Allan and I. P. Parkin, ACS Nano, 2018, 12, 6050–6058 CrossRef CAS; (d) B. Zhang, Q. Zhu, Y. Li and B. Hou, Chem. Eng. J., 2018, 352, 625–633 CrossRef CAS; (e) A. Li, H. X. Sun, D. Z. Tan, W. J. Fan, S. H. Wen, X. J. Qing, G. X. Li, S. Y. Li and W. Q. Deng, Energy Environ. Sci., 2011, 4, 2062–2065 RSC.
- (a) Y. Shen, Y. Xie, J. Tao, H. Chen, C. Zhu, M. Jin and Y. Lu, ACS Sustainable Chem. Eng., 2019, 7, 2702–2708 CrossRef CAS; (b) Y. Xie, H. Chen, Y. Shen, J. Tao, M. Jin, Y. Wu and W. Hou, J. Bionic Eng., 2019, 16, 27–37 CrossRef; (c) H. Zhou, H. Wang, H. Niu, A. Gestos, X. Wang and T. Lin, Adv. Mater., 2012, 24, 2409–2412 CrossRef CAS; (d) B. Wang, W. Liang, Z. Guo and W. Liu, Chem. Soc. Rev., 2015, 44, 336–361 RSC; (e) J. Yong, F. Chen, Q. Yang, J. Huo and X. Hou, Chem. Soc. Rev., 2017, 46, 4168–4217 RSC.
- (a) S. Chen, X. Li, Y. Li and J. Sun, ACS Nano, 2015, 9, 4070–4076 CrossRef CAS; (b) K. Chen, S. Zhou, S. Yang and L. Wu, Adv. Funct. Mater., 2015, 25, 1035–1041 CrossRef CAS; (c) H. Wang, H. Zhou, S. Liu, H. Shao, S. Fu, G. C. Rutledge and T. Lin, RSC Adv., 2017, 7, 33986–33993 RSC; (d) J. Zhao, X. Zhu, X. F. Wang, L. F. Liu, J. Yu and B. Ding, Nanoscale Horiz., 2019, 4, 867–873 RSC; (e) Y. H. Sun and Z. G. Guo, Nanoscale Horiz., 2019, 4, 52–76 RSC.
- (a) S. Huang, G. Liu, K. Zhang, H. Hu, J. Wang, L. Miao and T. Tabrizizadeh, Chem. Eng. J., 2019, 360, 445–451 CrossRef CAS; (b) F. Heale, M. Einhorn, K. Page, I. Parkin and C. Carmalt, RSC Adv., 2019, 9, 20332–20340 RSC; (c) B. Deng, R. Cai, H. Jiang, C. L. Wang, J. Li, L. F. Li, M. Yu, J. Y. Li, L. D. Xie, Q. Huang and C. H. Fan, Adv. Mater., 2010, 22, 5473–5477 CrossRef CAS; (d) Y. Zhao, Y. W. Tang, X. G. Wang and T. Lin, Appl. Surf. Sci., 2010, 256, 6736–6742 CrossRef CAS; (e) W. Liu, P. Fan, M. Cai, X. Luo, C. Chen, R. Pan, H. Zhang and M. Zhong, Nanoscale, 2019, 11, 8940–8949 RSC.
- (a) Q. Zhou, G. Chen and T. Xing, Cellulose, 2018, 25, 1513–1525 CrossRef CAS; (b) Y. Li, S. S. Chen, M. C. Wu and J. Q. Sun, Adv. Mater., 2014, 26, 3344–3348 CrossRef CAS.
- (a) U. Zulfiqar, M. Awais, S. Hussain, I. Hussain, S. Husain and T. Subhani, Mater. Lett., 2017, 192, 56–59 CrossRef CAS; (b) C. Xue, X. Bai and S. Jia, Sci. Rep., 2016, 6, 27262 CrossRef; (c) S. Y. Qiang, K. L. Che, Y. J. Yin and C. X. Wang, Mater. Des., 2017, 116, 395–402 CrossRef CAS; (d) F. Guo, Q. Wen, Y. Peng and Z. Guo, J. Mater. Chem. A, 2017, 5, 21866–21874 RSC; (e) Y. Li, L. Li and J. Sun, Angew. Chem., Int. Ed., 2010, 49, 6129–6133 CrossRef CAS.
- (a) C. H. Xue, Z. D. Zhang, J. Zhang and S. T. Jia, J. Mater. Chem. A, 2014, 2, 15001–15007 RSC; (b) H. Qian, D. Xu, C. Du, D. Zhang, X. Li, L. Huang, L. Deng, Y. Tu, J. M. C. Mol and H. A. Terryn, J. Mater. Chem. A, 2017, 5, 2355–2364 RSC; (c) G. Wu, J. An, X. Z. Tang, Y. Xiang and J. Yang, Adv. Funct. Mater., 2014, 24, 6751–6761 CrossRef CAS; (d) H. Zhang, C. Hou, L. Song, Y. Ma, Z. Ali, J. Gu, B. Zhang, H. Zhang and Q. Zhang, Chem. Eng. J., 2018, 334, 598–610 CrossRef CAS; (e) Z. L. Chu, Y. J. Feng and S. Seeger, Angew. Chem., Int. Ed., 2015, 54, 2328–2338 CrossRef CAS.
- (a) D. Wu, S. Z. Wu, Q. D. Chen, S. Zhao, H. Zhang, J. Jiao, J. A. Piersol, J. N. Wang, H. B. Sun and L. Jiang, Lab Chip, 2011, 11, 3873–3879 RSC; (b) X. Zhao, L. Li, B. Li, J. Zhang and A. Wang, J. Mater. Chem. A, 2014, 2, 18281–18287 RSC; (c) Y. Liu, H. Gu, Y. Jia, J. Liu, H. Zhang, R. Wang, B. Zhang, H. Zhang and Q. Zhang, Chem. Eng. J., 2019, 356, 318–328 CrossRef CAS; (d) H. Chang, K. Tu, X. Wang and J. Liu, RSC Adv., 2015, 5, 30647–30653 RSC; (e) Y. Wu, Y. Shen, J. Tao, Z. He, Y. Xie, H. Chen, M. Jin and W. Hou, New J. Chem., 2018, 42, 18208–18216 RSC.
- (a) S. Gao, X. Dong, J. Huang, J. Dong, Y. Cheng, Z. Chen and Y. Lai, Appl. Surf. Sci., 2018, 459, 512–519 CrossRef CAS; (b) S. Gao, X. Dong, J. Huang, S. Li, Y. Li, Z. Chen and Y. Lai, Chem. Eng. J., 2018, 333, 621–629 CrossRef CAS; (c) J. Yong, F. Chen, J. Huo, Y. Fang, Q. Yang, J. Zhang and X. Hou, Nanoscale, 2018, 10, 3688–3696 RSC; (d) Z. Han, X. Feng, Z. Jiao, Z. Wang, J. Zhang, J. Zhao, S. Niu and L. Ren, RSC Adv., 2018, 8, 26497–26505 RSC; (e) X. Zhou, Y. Y. Lee, K. S. L. Chong and C. He, J. Mater. Chem. B, 2018, 6, 440–448 RSC.
- C. H. Xue, Y. R. Li, J. L. Hou, L. Zhang, J. Z. Ma and S. T. Jia, J. Mater. Chem. A, 2015, 3, 10248 RSC.
- (a) T. Dikic, W. Ming, R. van Benthem, A. Esteves and G. de With, Adv. Mater., 2012, 24, 3701–3704 CrossRef CAS; (b) S. K. Lahiri, P. Zhang, C. Zhang and L. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 10262–10275 CrossRef CAS; (c) Y. Li, Q. Li, C. Zhang, P. Cai, N. Bai and X. Xu, Chem. Eng. J., 2017, 323, 134–142 CrossRef CAS; (d) M. Long, S. Peng, W. Deng, X. Yang, K. Miao, N. Wen, X. Miao and W. Deng, J. Colloid Interface Sci., 2017, 508, 18–27 CrossRef CAS; (e) L. Wang, C. Urata, T. Sato, M. W. England and A. Hozumi, Langmuir, 2017, 33, 9972–9978 CrossRef CAS.
- (a) S. K. Nemani, R. K. Annavarapu, B. Mohammadian, A. Raiyan, J. Heil, M. A. Haque, A. Abdelaal and H. Sojoudi, Adv. Mater. Interfaces, 2018, 5, 1801247 CrossRef; (b) E. K. Sam, D. K. Sam, X. Lv, B. Liu, X. Xiao, S. Gong, W. Yu, J. Chen and J. Liu, Chem. Eng. J., 2019, 373, 531–546 CrossRef; (c) Y. Shen, Y. Wu, Z. Shen and H. Chen, Coatings, 2018, 8, 144 CrossRef; (d) H. Zhang, Y. Ma, J. Tan, X. Fan, Y. Liu, J. Gu, B. Zhang, H. Zhang and Q. Zhang, Compos. Sci. Technol., 2016, 137, 78–86 CrossRef CAS; (e) C. H. Xue, Y. R. Li, P. Zhang, J. Z. Ma and S. T. Jia, ACS Appl. Mater. Interfaces, 2014, 6, 10153–10161 CrossRef CAS; (f) K. Tu, X. Wang, L. Kong and H. Guan, Mater. Des., 2018, 140, 30–36 CrossRef CAS.
- (a) M. Z. Ge, C. Y. Cao, J. Y. Huang, X. N. Zhang, Y. X. Tang, X. R. Zhou, K. Q. Zhang, Z. Chen and Y. K. Lai, Nanoscale Horiz., 2018, 3, 235–260 RSC; (b) W. Zhang, N. Liu, Y. Cao, X. Lin, Y. Liu and L. Feng, Adv. Mater. Interfaces, 2017, 4, 1600029 CrossRef; (c) B. Zhang, H. Feng, F. Lin, Y. Wang, L. Wang, Y. Dong and W. Li, Appl. Surf. Sci., 2016, 378, 388–396 CrossRef CAS; (d) C. Gao, Z. Sun, K. Li, Y. Chen, Y. Cao, S. Zhang and L. Feng, Energy Environ. Sci., 2013, 6, 1147–1151 RSC; (e) G. Cao, Y. Wang, C. Wang and S. H. Ho, J. Mater. Chem. A, 2019, 7, 11305–11313 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00519f |
| This journal is © The Royal Society of Chemistry 2020 |