Electrothermal patches driving the transdermal delivery of insulin†
Quentin
Pagneux‡
 ab,
Ran
Ye‡
ab,
Ran
Ye‡
 c,
Li
Chengnan
a,
Alexandre
Barras
c,
Li
Chengnan
a,
Alexandre
Barras
 a,
Nathalie
Hennuyer
a,
Nathalie
Hennuyer
 b,
Bart
Staels
b,
Bart
Staels
 b,
D.
Caina
b,
D.
Caina
 c,
J. I. Avila
Osses
c,
J. I. Avila
Osses
 c,
Amar
Abderrahmani
c,
Amar
Abderrahmani
 a,
Valérie
Plaisance
a,
Valérie
Plaisance
 a,
Valérie
Pawlowski
a,
Valérie
Pawlowski
 a,
Rabah
Boukherroub
a,
Rabah
Boukherroub
 a,
Sorin
Melinte
a,
Sorin
Melinte
 *c and
Sabine
Szunerits
*c and
Sabine
Szunerits
 *a
*a
aUniv. Lille, CNRS, Centrale Lille, Yncréa ISEN, Univ. Polytechnique Hauts-de-France, UMR 8520 – IEMN, F-59000 Lille, France. E-mail: sabine.szunerits@univ-lille.fr
bUniv. Lille, Inserm, CHU Lille, Institut Pasteur de Lille, U1011 – EGID, F-59000 Lille, France
cInstitute of Information and Communication Technologies, Electronics and Applied Mathematics, Université catholique de Louvain, 1348 Louvain-la-Neuve, Belgium. E-mail: sorin.melinte@uclouvain.be
First published on 2nd December 2019
Abstract
Transdermal patches have become a widely used approach for painless delivery of drugs. One major current limitation of these systems remains the restricted skin permeation of proteins and peptides as exemplified by insulin, necessitating different considerations for their successful transdermal delivery. We present a novel patch design based on the integration of nano-engineered heating elements on polyimide substrates for electrothermal transdermal therapy. The results reveal that tuning of the electrical resistivity of an array of gold nanoholes, patterned on polyimide, facilitates a fast-responding electrothermal skin patch, while post-coating with reduced graphene oxide offers capabilities for drug encapsulation, like insulin. Application of insulin-loaded patches to the skin of mice resulted in blood glucose regulation within minutes. While demonstrated for insulin, the skin patches might be well adapted to other low and high molecular weight therapeutic drugs, enabling on-demand electrothermal transdermal delivery.
New conceptsWe developed a new concept based on electrothermal heating for the transdermal delivery of drugs such as insulin. This is different to other heating devices such as photothermal heating or microneedles for insulin delivery as it is based on perforated thin gold films integrated onto wearable patches. With the need of power densities below 200 mW cm−2 to reach steady state temperatures of 52 °C in a few seconds, the transdermal drug delivery device is adapted for self-powering platforms using commercially available handheld battery systems. By using in vitro and in vivo experiments, we studied the efficiency of the designed electrothermal insulin delivery patch. Such on-demand transdermal patches based on simple nanotechnology are clearly a key medical challenge for chronic diseases such as diabetes, where efficient and non-invasive dosing of insulin remains an important challenge. |
Introduction
Transdermal skin patches represent key products from research in materials science. The medical impact of transdermal drug delivery as a non-invasive and painless system is constantly highlighted when compared to intravenous drug delivery routes. The potential to maintain steady-state drug levels in blood after topical application, with the amounts being varied by changing the drug concentration and components in the patch, has been put forward in favor over oral drug delivery.1–5 The first transdermal patches consisted of a combination of a reservoir containing the drug and a rate-controlling membrane.6 Uncontrolled drug delivery and potential drug overdose are the major limitations of these passive transdermal skin patches. A key technology advancement to achieve efficient and controlled delivery of therapeutics is the development of on-demand dosage transdermal skin patches,7–14 with no liquid reservoir, where drug delivery is initiated upon an external stimulus from the drug-incorporated matrix. A catalogue of different transdermal patches is currently developed for actively delivering various therapeutic agents including lipophilic, low-molecular weight to water-soluble macromolecules such as peptides and proteins, which have limited transport across the outermost layer of the epidermis, the stratum corneum.4,5,11,15The use of on-demand transdermal patches is clearly a key medical challenge for chronic diseases such as diabetes. Diabetes is one of the leading causes of early mortality worldwide,16 mainly caused by cardiovascular diseases. Diabetes complications often ensue because of the long-term uncontrolled blood glucose levels, despite the current treatments. All oral antidiabetics are unable to achieve long-term glycemic control, leading clinicians to finally prescribe insulin administration.17 Currently, insulin is delivered by numerous daily subcutaneous needle injections through a pen or catheters connected to pumps. Unfortunately, these methods are both painful and inconvenient, as the invasive multiple injections significantly deteriorate the life quality of patients.18 The discomfort associated with this type of administration often leads diabetic patients to neglect or even give up the therapy and thereby induces variable glycemic control, as well as the development of complications.
Efficient and non-invasive dosing of insulin remains an important challenge with different technical achievements reported over the last few years. The reported methods include inhaling approaches (e.g. rapid-acting inhaled insulin – ExuberaR) and nasal delivery (under phase 2 and 3 clinical trials),19 and transdermal delivery protocols.4,15,20–24
Besides the work of Prausnitz and co-workers, who demonstrated that the removal of full epidermis by microdermabrasion significantly increases insulin skin penetration at levels sufficient to reduce blood glucose level,25,26 insulin delivery using microneedles is certainly one of the most promising transdermal delivery approaches.13,21,22 The first generation of polymer-based microneedles, dissolved during the contact with the skin, are currently being replaced by hydrogel-based microneedles.21 The latter enable hydrophilic drugs to be released by swelling in the dermis layer and withdrawn from the skin owing to their toughness.13 Application of a hydrogel-based microneedle patch carrying 62 insulin units (1 IU = 35 μg) has lowered the glucose level of a diabetic rat by 10% after 2 h and by 63% after 12 h post-administration, respectively.13 Yet, to achieve sufficient insulin delivery iontophoresis-assistance was necessary. In line with this effort, alginate-based microneedle-arrays patches loaded with exendin 4 and glucose oxidase have been recently proposed by Chen and co-workers for type 2 diabetes therapy.2 While the skin iontophoresis principle has generated commercial platforms in the past,27 electrode complexity and corrosion upon storage, as well as stringent requirements on drug formulation to diminish the role of competing ions involved in the process hamper the progress and the hybridization of the technology with other techniques that modulate the stratum corneum to facilitate specific paths for insulin.
Different to the microneedle and iontophoresis systems, heat-assisted microporation enhances transdermal delivery of insulin. Direct laser-based heating is limited to the use of Er:YAG, whose light output is strongly absorbed by water, which results in about 15 times better light absorption by the skin and favored transdermal insulin delivery.28 While the light of near-infrared (NIR) lasers is not readily absorbed by the skin and heating effects are minor, the combination with NIR absorbing materials was proven to be beneficial for insulin permeation through the skin.19 The main current limitation of this approach is the integration of powerful laser diodes into the patches.
Here, we engineer a transdermal patch for insulin delivery using standard components in wearable systems. The key novel aspect of this system is the use of an electrothermal protocol for on-demand release of insulin with enhanced dose delivery through the skin together with the possibility of drug reloading after release. Electrothermal systems are used in a wide range of applications such as window defrosting and outdoor displays, but have not been implemented into transdermal drug delivery devices. Our results unveil that nano-perforated gold thin layers,29 generated onto Kapton sheets (50–125 μm in thickness) using colloidal lithography, present outstanding electrothermal capabilities. The patch configuration requires a power density below 250 mW cm−2 to reach a steady state temperature of 50 °C in a few seconds. It can be further loaded with biological molecules by post-coating the layer of gold nanoholes (Au NHs) with a micrometer-thick reduced graphene oxide (rGO) drug reservoir. The resulting electrothermal properties are similar to those of the state-of-the art graphene aerogels for efficient Joule heating up to 50 °C using 500 mW cm−3,30 free-standing rGO films, attaining 90 °C at 236 mW cm−2 power density,31 chemical vapor deposited graphene on Cu foils (43 Ω sq−1),32 AuCl3 doped and embedded in polyethylene–terephthalate, requiring 200 mW cm−2 to reach a steady state temperature of 100 °C, or rGO thin films on polyimide substrates reaching 50 °C steady-state temperature at an input power density of 250 mW cm−2.33
The reliability of rGO in electrothermal applications34 together with good insulin loading capacity24,35 and insulin release ability, owing to the formation of a thermal gradient weakening non-covalent insulin interactions with rGO, suggest it as an ideal transdermal drug release matrix in connection with electrothermal patches. By using in vitro and in vivo experiments, we study the efficiency of the designed rGO-based electrothermal insulin delivery patch. The electrothermal system achieves highly-controlled insulin delivery and enables efficient management of blood glucose concentrations without pain and stress.
Electrothermal skin patches
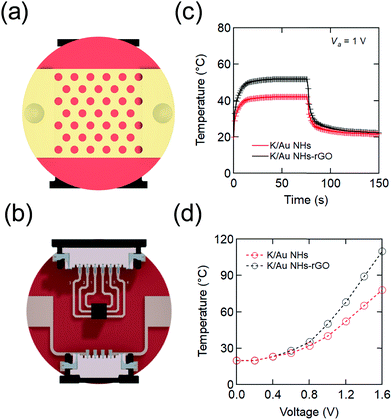
The architecture of the electrothermal skin patch is illustrated in Fig. 1. It is based on the formation of arrays of gold nanoholes (Au NHs) on Kapton, a polymer with a glass transition between 360 and 410 °C, highly suitable for electrothermal systems. Nano-perforated gold thin layers integrated to Kapton (K/Au NHs), obtained by colloidal lithography, were chosen for resistive heating,29 due to easy tuning of the electrical resistance needed by wearable devices (Fig. 1 and Fig. S1, Table S1, ESI†). To facilitate the application of different bias voltages, Ti (2 nm)–Au (40 nm) contacting pads were further deposited on the edges of the patch and circuited to connectors integrated at the back side. Numerical calculations for the designed devices have been carried out assuming Joule heating (ESI†).36 Based on experiments and theory, the best performing K/Au NH electrothermal interface has 300 nm diameter holes. An applied voltage of 1 V was sufficient to raise the temperature to a steady state value of 40 °C in about 10 s (Fig. 1c). Transdermal drug delivery systems are typically occlusive patches, yet the core of our device is a nanoporous metallic layer. Thus, it is mechanically compatible with both nanomesh electronic interfaces, offering air and moisture permeability, and with nanopatterned additive slabs for drug encapsulation generated with cost-effective procedures.37,38 For practical utility, note that polyimide may be sterilized with standard methods.For the controlled release of therapeutics, rGO was post-coated on the K/Au NHs devices due to its excellent drug loading capacity.8,24 Deposition of micrometer-thick (ca. 1 μm) chemically-derived rGO onto K/Au NHs patches results in stable and reproducible electrothermal devices (Fig. 1c and d). Indeed, rGO thickness is a straightforward parameter to control the electrical resistance of the coupled metal-rGO system and thus the magnitude of the dc electrical power required to drive the K/Au NHs-rGO patches to a defined temperature.
Insulin loading and release
Insulin loading was achieved by dropping insulin (100 μg) onto the rGO coating layer of the electrothermal patch. Non-covalent interactions through H-bonding, electrostatic interactions, and π–π stacking have been put forward as coupling mechanisms between rGO and insulin.24,35 The loading capacity of the rGO coating of the electrothermal device for human insulin (Fig. 2a) was determined as reported previously for insulin loaded hydrogels.24 Here it was about 95%, which correlates with about 95 μg (2.7 IU). Generally, to correct high concentrations of blood sugar in humans, 0.3–1.0 IU kg−1 insulin is needed to decrease the blood glucose by 50 mg dl−1.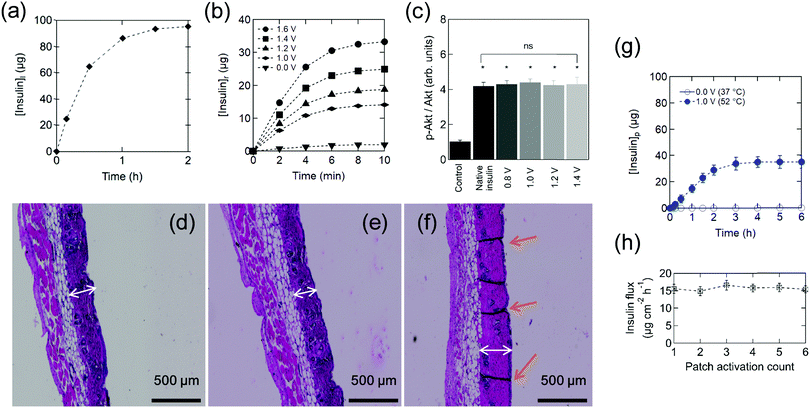 | ||
| Fig. 2 Insulin-loaded electrothermal patches. (a) Human insulin loading [insulin]l on the electrothermal patch. (b) Cumulative in vitro release profile of insulin [insulin]r from the patch in PBS (pH 7.4) maintained at 37 °C upon application of different heating ramps determined by HPLC (Fig. S2, ESI†). Results are presented as the mean ± standard deviation of four experiments. (c) Analysis of Akt phosphorylation in HepG2 cells exposed to insulin released from the electrothermal patch at the indicated bias voltages. Phosphorylation of Akt (p-Akt) over total Akt (Akt) was measured by Western Blotting using rabbit polyclonal antibodies. All the samples were compared to cells treated with vehicle only, dimethyl sulfoxide (negative control). The results are expressed as the mean ± standard deviation of at least 3 independent samples for each group (one-way ANOVA test, ns = not significant, * represents a significant difference at P < 0.05). Hematoxylin and eosin staining showing the histological changes of skin after heating for 10 min: (d) 52 °C (1.0 V), (e) 55 °C (1.05 V), (f) 60 °C (1.1 V). The white arrows indicate the thickness of the epidermis and the orange arrows indicate the canals formed. (g) In vitro cumulative permeation of insulin delivered from an electrothermal patch loaded with 100 μg (2.8 IU) insulin across mouse skin upon continuous application of 1.0 V bias voltage for 10 min (closed symbols) as well as passive delivery (open symbols). (h) Reusability experiments were performed by patch reloading with 2.8 IU followed by application of a 1.0 V bias for 10 min and determination of the insulin flux for 2 h. All data points represent the average ± standard deviation of at least four different measured samples. | ||
Fig. 2b shows the cumulative in vitro release profile of insulin from the electrothermal patch into PBS (pH 7.4) maintained at 37 °C upon the application of a dc electrical bias. To test the biological activity of the electrothermally released insulin, human hepatic HepG2 cells were cultivated in the presence of native or electrothermally released insulin. Insulin acts on HepG2 through its receptor, which after binding induces a signaling cascade leading to protein kinase B phosphorylation (p-Akt). This well-characterized key regulator of hepatic glucose metabolism is used here as a marker for assessing the metabolic activity of insulin. Fig. 2c displays insulin activity (p-Akt/Akt ratio), which increases when HepG2 cells were treated with insulin in comparison to non-treated cells. Applied biases up to 1.6 V have no effect on the biological insulin activity, which was as active as native insulin. For transdermal delivery, the skin temperature at which threshold pain occurs is around 52 °C (1.0 V), indicating that the biological activity of the insulin electrothermally released from the engineered patch is preserved. Next, we tested the potential damage on the skin caused by heating. Damages on skin tissue are caused upon exposure for a prolonged time at temperatures above the physiological limit as previously described.39 Typical hematoxylin eosin stained images of normal skin tissue upon heating exhibit the attributive characteristics of normal dermis stratum, as shown in Fig. 2d–f. No visible tissue damage is observed for both the epidermis and dermis until 55 °C (1.05 V), while at 60 °C (1.1 V) thickening of the epidermis and formation of skin disruption were observed (Fig. 2f). No long-lasting effects on skin integrity, evaluated by staining mouse skin with hematoxylin eosin after application of electrothermal pulses of 1.0 V for 10 min for 6 days (Fig. S22, ESI†), were observed.
Fig. 2g shows a standard permeation profile of insulin from patches loaded with 100 μg insulin. The high molecular weight and hydrophilic character of insulin restrict passive skin permeation and no skin permeation of insulin is detected without electrothermal activation. When mouse skin is subjected to 10 min heating at 1.0 V (∼52 °C), after a lag time of about 20 min, sustained transdermal transport of insulin occurs over a time period of 2 h. The total amount of permeated insulin after 2 h equals to 23 ± 3 μg, outperforming reported permeation percentages for integrated microneedles,13 which correspond to an insulin flux of 15.6 ± 1.3 μg cm−2 h−1 (2.0 ± 0.4 μM cm−2 h−1), comparable with the results of Pillai et al. addressing insulin delivery from poloxame-407 gels using transdermal iontophoresis in the presence of menthone as a skin enhancer.40 The patch can be reused several times without any apparent loss of performance (Fig. 2h). To get a better understanding of the mechanism underlying the electrothermal delivery of insulin, in vitro permeation studies using fluorescent isothiocyanate-labeled insulin (FITC-insulin) were performed. From the fluorescence images taken after 2 h permeation (Fig. S23, ESI†) one can notice that a larger amount of insulin is delivered through micrometer-sized pores. However, smaller pores might have been created temporally as fluorescent insulin is observed in the entire skin.
In vivo pharmacodynamics
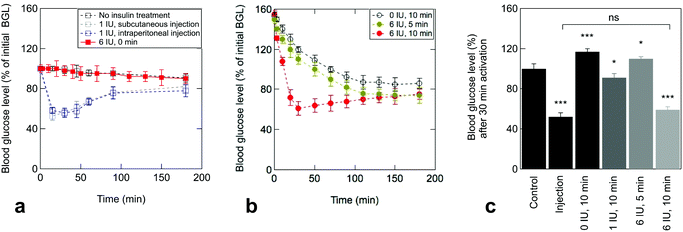
The efficiency of the electrothermal patch to deliver therapeutic insulin quantities was tested by four standard experiments on mice. These groups include (a) no insulin treatment (negative control), (b) subcutaneous injection (1 IU kg−1) as first positive control, (c) intraperitoneal injection (1 IU kg−1) as second positive control and (d) electrothermal delivery of insulin from patches loaded with 6 IU. Prior to drug administration, the basal blood insulin of each mouse was monitored, followed by the attachment of the electrothermal patch to estimate the insulin baseline. In mice with an insulin shot via subcutaneous or intraperitoneal injection (Fig. 3a), the blood glucose level (BGL) declined immediately and reached a minimum at 15 and 30 min, respectively. Control mice (no insulin treatment) showed a negligible change in the BGL over the determined period (Fig. 3a). The same was true for the transdermal skin patch loaded with insulin without activation, confirming that no passive insulin delivery takes place in vivo.The BGLs of mice stimulated with the electrothermal insulin patches are depicted in Fig. 3b. In the case of insulin-free patches, an increased BGL was determined after electrothermal activation at 1.0 V for 10 min. This is due to experimental constraints generated by the anesthesia process, necessary to guarantee the patch adhesion during the stimulation and provoking stress and increase of blood glucose. Application of insulin-loaded patches (6 IU) induces a comparable BGL to the insulin-free ones after treatment. Significantly different to the insulin-free patches, the BGL declined steadily with a minimum at about 30 min after treatment, in line with the time profile of subcutaneous and intraperitoneal injections (Fig. 3a). Activation of 10 min was the required time to reach a therapeutic effect (Fig. 3b), as shorter activation times did not show a rapid drop of the BGL. Among the mice that received insulin via electrothermal patches, those given doses with 6 IU displayed BGLs comparable to those treated by injection (Fig. 3c).
Conclusions
In conclusion, the integration of nano-heaters into transdermal patches loaded with insulin enables efficient release, capable of reducing the blood glucose level in mice in a comparable manner to insulin injection. The core of our device is a nanoporous metallic layer, which is highly mechanically compatible with both nanomesh interfaces for portable data analysis, offering air and moisture permeability, and nano-patterned additive slabs for drug encapsulation. With the need of power densities below 200 mW cm−2 to reach a steady state temperature of 52 °C in a few seconds, the transdermal drug delivery device is adapted for platforms using commercially available handheld battery systems. We provide the proof of concept of a newly engineered transdermal patch with electrothermal control for the delivery of therapeutic agents via incorporation of reduced graphene oxide. For practical utility, we note that polyimide can be sterilized via gamma, electron-beam, and hydrogen peroxide plasma sterilization methods, among others, offering a solid starting point for prototyping more complex drug delivery systems. Our findings join the worldwide efforts devoted to expanding the portfolio of nano-engineered materials for soft electronics and biotechnology. This new patch could answer the promise for wearable on-demand drug delivery to enhance therapy efficacy and to better manage chronic diseases such as diabetes.Methods
Materials
Glycerin, human insulin and FITC-labeled insulin were obtained from Sigma Aldrich. Graphene oxide (GO) was purchased from Graphenea (Spain). Kapton® HN polyimide foils (thickness of 50, 75 and 125 μm) were obtained from DuPont (Circleville, OH, USA). Fluorescein isothiocyanate-labeled insulin (FITC-labeled insulin) was purchased from Sigma-Aldrich. The human insulin ELISA kit was from Invitrogen and shows an analytical sensitivity of 0.17 μIU ml−1 and an assay range of 0.1–250 μIU ml−1.Fabrication of gold nanohole modified Kapton (K/Au NHs)
Kapton HN polyimide foils (rectangular 20 × 10 mm2 and circular pieces with 20 mm diameter) were cleaned with acetone in an ultrasonic water bath for 30 min, followed by isopropanol for 10 min and then dried under a nitrogen flow. A monolayer of 980 nm polystyrene beads (Microparticles GmbH) was first deposited in the middle of the Kapton surface with an area of 8 × 10 or 11 × 11 mm2 by self-assembly. To reduce the size of the particles, SF6 and oxygen plasma etching was employed (6 mTorr). The samples were coated with 2 nm Ti followed by 40 nm Au at a constant deposition rate of 1 Å s−1 using physical vapor deposition. The polystyrene beads on top of the Kapton were removed by dissolution in chloroform (overnight). To facilitate the application of the bias voltage, 2 nm Ti and 40 nm Au contacts were further deposited on the K/Au NHs patches and connected on the back side of the devices with flat flexible connectors (FFCs) and 4 conductor, 1 A, 1 mm FFC jumper cables (Fig. S3, ESI†). Patches can be operated via flexible printed circuit boards as well (Fig. S4, ESI†).Post-coating K/Au NHs with rGO
The rGO was formed by adding hydrazine hydrate (0.50 ml, 32.1 mM) to 5 ml GO aqueous suspension (0.5 mg ml−1) in a round bottom flask and heated in an oil bath at 100 °C for 24 h. During this time, the reduced GO gradually precipitates out of the solution. The product was isolated by filtration over a polyvinylidene difluoride membrane with a 0.45 μm pore size, washed copiously with water (5 × 20 ml) and methanol (5 × 20 ml), and dried in an oven at 60 °C for 6 h. An aqueous suspension of rGO (1 mg ml−1, 5 μl) was drop-cast three times onto K/Au NHs and allowed to dry over night at 70 °C. This resulted in a rGO film of about 5 μm thickness.Insulin loading onto the electrothermal patch
30 μl of insulin (100 μg ml−1 in water) was dropped onto the electrothermal patch and kept for 2 h at 4 °C. Then the patch was washed three times with water to remove any unbound insulin. The concentration of insulin loaded onto the rGO coating of the patch was determined using high-performance liquid chromatography (HPLC). A standard human insulin calibration curve was generated beforehand from a series of insulin solutions ranging from 1 to 100 μg ml−1. The concentration of insulin in the patch was determined according to [insulin]rGO = [insulin]initial − [insulin]supernatant, with [insulin]rGO the concentration of insulin on the rGO matrix (μg ml−1), [insulin]initial the concentration of insulin in solution (100 μg ml−1) and [insulin]supernatant the concentration of insulin in the supernatant (μg ml−1).Electrothermal release of insulin into solution
Release experiments were performed into 1 ml PBS (pH 7.4). Different bias voltages were applied to the electrothermal patch for various time intervals (1–10 min). The quantity of released insulin was determined by assessment of insulin concentration in the supernatant, after activation, by HPLC.Characterization
Scanning Electron Microscopy (SEM) images were obtained using an electron microscope ULTRA 55 (Zeiss) equipped with a thermal field emission emitter and three different detectors (Energy selective Backscattered Detector with filter grid, high efficiency In-lens Secondary Electron Detector and Everhart-Thornley Secondary Electron Detector). Absorption spectra were recorded using a PerkinElmer Lambda UV-Vis 950 spectrophotometer in a 1 cm quartz cuvette. The wavelength range was 200–1100 nm. Micro-Raman spectroscopy measurements were performed on a Horiba Jobin Yvon LabRam high resolution micro-Raman system combined with a 473 nm (1 mW) laser diode as an excitation source. Visible light is focused by a 100× objective. The scattered light is collected by the same objective in backscattering configuration, dispersed by a monochromator with 1800 mm focal length and detected by using a CCD camera. Temperature changes were captured by using an infrared camera (Thermovision A40) and treated using ThermaCam Researcher Pro 2.9 software. High-performance liquid chromatography analysis was performed with a Shimadzu LC2010-HT (Shimadzu, Tokyo, Japan) using a C4 QS Uptisphere® column (300 Å, 250 × 4.6 mm, Interchim, Montluçon, France) heated at 40 °C. The mobile phase (A) is trifluoroacetic acid (0.05%) in water and the eluent (B) is trifluoroacetic acid (0.045%) in acetonitrile (flux: 1 ml min−1). The flux was 100% A for 5 min, a linear gradient from 0–80% of B for 15 min, then 80% B for 5 min. The detector wavelength used was 215 nm. Fluorescence images were obtained on a TS 100 Eclipse Nikon equipped with a GFP (green fluorescence protein; excitation 469/35 and emission 525/39) filter cube and 10× and 40× objectives.Determination of insulin activity
The activity of the electrothermally released insulin was evaluated, in vitro, on a human hepatic HepG2 cell line model using native insulin as a positive control and the same culture without insulin as a negative control as described previously.35 After overnight culture in a serum-free and glucose-free culture medium, HepG2 cells were incubated for 1 h without insulin (negative control), with native insulin (positive control) and with electrothermally released insulin at a physiological active concentration of 200 nM. After 1 h of incubation, cells were lysed and proteins were collected. Expression of p-Akt and Akt proteins was evaluated by Western blotting analysis. For Western blotting experiments, 40 μg of total proteins of each condition were loaded and separated onto a polyacrylamide gel, then transferred onto a nitrocellulose membrane. Immunoblotting for p-Akt and Akt was performed using anti p-Akt specific antibody (Thr-308, Cell signaling), anti-Akt specific antibody (sc-8312, Santa Cruz) and fluorescence-coupled secondary antibodies against both mouse and rabbit primary antibodies. Analysis of the Western blot was performed using the Odyssey revelation process and ImageJ quantification techniques. A one-way ANOVA test was carried out.In vitro skin permeation experiments
Skin permeation studies were performed using fresh mice skin. For this, C57BL/6 mice were anaesthetized with isoflurane, shaved with an electric shaver (Philips Series 7000) and further treated with a depilatory cream (Veet) for 1.5 min. Then, the mice were killed by cervical dislocation and the skin from the back of the mice was cut into pieces of at least 20 mm in diameter and preserved in Dulbecco's modified Eagle medium (DMEM) supplemented with gentamicin (0.4%). The thickness of the skin was determined with a digimatic micrometer (Mitutoyo) and was 600 ± 5 μm. A specially designed static Franz diffusion cell (Proviscin, France, see Fig. S3, ESI†) exhibiting an effective area of 0.78 cm2 was adapted for electrothermally-initiated permeability tests. After filling the receptor compartment with degassed phosphate buffer saline (PBS) with pH = 7.4, the solution was maintained at 32 °C using a circulating bath (Julabo) and stirred with a magnetic stirring bar at around 500 rpm. The mouse skin was carefully clamped between the donor and the receptor compartment (8 ml). Pre-incubation in the receptor compartment medium for 20 min was performed before the electrothermal patch was applied to the skin previously wetted with 30 μl of a glycerin solution (50%) to ensure contact between the patch and the skin. Electrothermal patches loaded with 2.88 IU were used for the permeation studies. Electrothermal activation was performed upon continuous application of 1.0 V for 1 min (corresponding to ∼52 °C). At determined time intervals, 300 μl aliquots of diffused solution were removed from the receptor compartment and analysed using HPLC. After each sampling, an equal volume of fresh diffusion medium was added to the receptor compartment to maintain a constant volume. All experiments were performed in triplicate. The release and permeation profiles were determined by plotting the cumulative amount of insulin in the receptor compartment (Qexp) against time,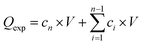 , with Qexp the cumulative amount of insulin diffused through the skin (μg), cn the amount of insulin (μg) determined at the nth sampling interval, and V the volume of the acceptor phase (receptor compartment) (ml). The insulin flux (J) was determined according to J = A/S, where J is the flux of insulin through the skin (μg cm−2 h−1), A the linear slope of the cumulative amount versus time curves in equilibrium conditions (μg h−1), and S the surface of the membrane of the Franz cell (0.78 cm2). To estimate the amount of insulin trapped in the skin, the skin was added into a water/ice mixture for 10 min and sonicated in the presence of ZnO2 beads (4 mm in diameter), before being centrifuged for 30 min at 13
, with Qexp the cumulative amount of insulin diffused through the skin (μg), cn the amount of insulin (μg) determined at the nth sampling interval, and V the volume of the acceptor phase (receptor compartment) (ml). The insulin flux (J) was determined according to J = A/S, where J is the flux of insulin through the skin (μg cm−2 h−1), A the linear slope of the cumulative amount versus time curves in equilibrium conditions (μg h−1), and S the surface of the membrane of the Franz cell (0.78 cm2). To estimate the amount of insulin trapped in the skin, the skin was added into a water/ice mixture for 10 min and sonicated in the presence of ZnO2 beads (4 mm in diameter), before being centrifuged for 30 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 rpm using an ultracentrifuge (Mini Scanfuge ORIGIO). The liquid phase was collected and filtrated through a 0.1 μm Nylon filter (Whatman Puradisc 13 mm) and the amount of insulin was determined.
500 rpm using an ultracentrifuge (Mini Scanfuge ORIGIO). The liquid phase was collected and filtrated through a 0.1 μm Nylon filter (Whatman Puradisc 13 mm) and the amount of insulin was determined.
In vivo studies
Delivery of insulin from the electrothermal patch was investigated in C57BL/6 mice as a model. Before the experiment, the back of the mouse was shaved (Philips Series 7000). The shaved area was further treated with a depilatory cream (Veet) for 1.5 min to eliminate all the remaining hair. The next day the mice fasted for 4 h and the blood glucose level was determined using a glucometer. Before application of the electrothermal patch, the mice are anesthetized with isoflurane for 2 min (level 2, 1 l min−1 air) and the blood glucose level was determined again. Then, the electrothermal patches loaded with 6 IU (210 μg insulin) were applied to the shaved area and fixed with the help of adhesive tape (Sparadrap Micropore, 2133). The patch was activated for 10 min at 1.0 V under anesthesia and then removed. Blood glucose level values determined by a glucometer were plotted against time to obtain the blood glucose level-time profiles. All animal experiments throughout this study were conducted according to the policy of the Federation of European Laboratory Animal Science Association and the European Convention for the protection of vertebrate animals used for experimental and other scientific purposes, with implementation of the principle of the 3Rs (replacement, reduction, refinement).Thermal damage on skin
After C57BL/6 mice were subjected to electrothermal treatment, their skin was excised and immerged into formaldehyde (4% in PBS) for 24 h, followed by dehydration (STP120, Leica) through a graded series of alcohols each for 2 h [70% (2 times), 90% (2 times), 100% (3 times)]. Then, the skin was immersed into xylene (2 times for 2 h) followed by immersion into a paraffin bath at 60 °C (2 times for 2 h). A skin section of 5 μm thickness was cut from the sample (RM2143, Leica) and stained for microscopic examination. Giemsa staining was achieved through the immersion of the skin into: acetone (3 min, 2 times), ethanol (90%, 2 min), ethanol (70%, 2 min), ethanol (50%, 2 min), ethanol (30%, 2 min), water (2 min), PBS (pH 6.8, 0.3 M, 5 min), Giemsa (1/10 in PBS, 45 min), PBS (pH 6.8, 0.3 M, 2 min), acetic acid (0.15%, 20 s), water (2 min).Numerical calculations
By using COMSOL, Joule heating calculations of patches heated by an applied electrical bias were carried out numerically, considering a periodic array of nano-sized holes patterned in the gold thin layer deposited on Kapton. We obtained thus insight into the overall behavior of the electrothermal system (Fig. S5–S21, ESI†).Author contributions
R. Y., D. C., J. I. A. O. and S. M. were involved with the fabrication of the devices. J. I. A. O. carried out numerical calculations. Q. P., N. H. and B. S. carried out in vivo experiments and A. B., L. Q. and R. B. performed studies on thermal damage to skin and in vitro skin permeation experiments. A. A., V. Pawlowski and V. Plaisance determined insulin activity. All authors analyzed the results and wrote the paper. S. S. designed the study.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Financial support from the Centre National de la Recherche Scientifique (CNRS), the University of Lille, the Hauts-de-France region, the CPER “Photonics for Society” and SATT Nord is acknowledged. This work was supported by the Belgian F. R. S. – FNRS in the frame of the research conventions T.1004.14 – ECOSTOFLEX, T.0106.16. – SELFPHON and R.50.02.16.F – FLAG-ERA JTC 2015 – Graphtivity. B. S. is a recipient of an Advanced ERC Grant (694717).Notes and references
- A. C. Anselmo, Y. Gokarn and S. Mitragotri, Nat. Rev. Drug Discovery, 2018, 18, 19–40 CrossRef PubMed
.
- W. Chen, R. Tian, C. Xu, B. C. Yung, G. Wang, Y. Liu, Q. Ni, F. Zhang, Z. Zhaou, J. Wang, G. Niu, Y. Ma, L. Fu and X. Chen, Nat. Commun., 2017, 8, 1777 CrossRef PubMed
.
- J. Kim, A. S. Campbell, B. Esteban-Fernández de Ávila and J. Wang, Nat. Biotechnol., 2019, 37, 389–406 CrossRef CAS PubMed
.
- M. R. Prausnitz, S. Mitragotri and R. Langer, Nat. Rev. Drug Discovery, 2004, 3, 115–124 CrossRef CAS PubMed
.
- D. A. LaVan, T. McGuire and R. Langer, Nat. Biotechnol., 2003, 21, 1184–1191 CrossRef CAS PubMed
.
-
A. Zaffaroni, US Pat., 3598122, Alza Corporation, 1971 Search PubMed
.
- I. Hwang, H. N. Kim, M. Seong, S.-H. Lee, M. Kang, H. Yi, W. G. Bae, M. K. Kwak and H. E. Jeong, Adv. Healthcare Mater., 2018, 7, 1800275 CrossRef PubMed
.
- F. Teodorescu, G. Guéniat, C. Foulon, M. Lecoeur, A. Barras, S. Boulahneche, M. S. Medjram, T. Hubert, A. Abderrahmani, R. Boukherroub and S. Szunerits, J. Controlled Release, 2017, 245, 137–146 CrossRef CAS PubMed
.
- Q. Ouyang, X. Feng, S. Kuang, N. Panwar, P. Song, C. Yang, G. Yang, X. Hemu, G. Zhang, H. S. Yoon, J. P. Tam, B. Liedberg, G. Zhu, K.-T. Yong and Z. L. Wang, Nano Energy, 2019, 62, 610–619 CrossRef CAS
.
- J. G. Hardy, E. Larrañeta, R. F. Donnelly, N. McGoldrick, K. Migalska, M. T. C. McCrudden, N. J. Irwin, L. Donnelly and C. P. McCoy, Mol. Pharmaceutics, 2016, 13, 907–914 CrossRef CAS PubMed
.
- J. Wu, K. S. Paudel, C. Strasinger, D. Hammell, A. L. Stinchcomb and B. J. Hinds, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 11698–11702 CrossRef CAS PubMed
.
- H. Kim, H. Lee, K.-S. Seong, E. Lee, S. Y. Yang and J. Yoon, Adv. Healthcare Mater., 2015, 4, 2071–2077 CrossRef CAS PubMed
.
- R. F. Donnelly, T. R. Sing, M. J. Garland, K. Migalska, R. Majithiya, C. M. McCrudden, P. L. Kole, T. M. Mahmood, H. O. McCarthy and A. D. Woolfson, Adv. Funct. Mater., 2012, 22, 4879–4890 CrossRef CAS PubMed
.
- H. Lee, C. Song, Y. S. Hong, M. S. Kim, H. R. Cho, T. Kang, K. Shin, S. H. Choi, T. Hyeon and D.-H. Kim, Sci. Adv., 2017, 3, e1601314 CrossRef PubMed
.
- J. Yu, Y. Zhang, Y. Ye, R. DiSanto, W. Sun, D. Ranson, F. S. Ligler, J. B. Buse and Z. Gu, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 8260–8265 CrossRef CAS PubMed
.
- A. K. Wright, E. Kontopantelis, R. Emsley, I. Buchan, N. Sattar, M. K. Rutter and D. M. Ashcroft, Diabetes Care, 2017, 40, 338–345 CrossRef PubMed
.
- J. B. Brown, G. A. Nichols and A. Perry, Diabetes Care, 2004, 27, 1535–1540 CrossRef PubMed
.
- M. Peyrot, A. H. Barnett, L. F. Meneghini and P. M. Schumm-Draeger, Diabetic Med., 2012, 29, 682–689 CrossRef CAS PubMed
.
- R. B. Shan, M. Patel, D. M. Maahs and V. N. Shah, Int. J. Pharm. Invest., 2016, 6, 1–9 CrossRef PubMed
.
- X. Hu, J. Yu, C. Qian, Y. Lu, A. R. Kahkoska, Z. Xie, X. Jing, J. B. Buse and Z. Gu, ACS Nano, 2017, 11, 613–620 CrossRef CAS PubMed
.
- S. Liu, M.-N. Jin, Y.-S. Quan, F. Kamiyama, H. Katsumi, T. Sakane and A. Yamamoto, J. Controlled Release, 2012, 161, 933–941 CrossRef CAS PubMed
.
- W. Martanto, S. P. Davis, N. R. Holiday, J. Wang, H. S. Gill and M. R. Prausnitz, Pharm. Res., 2004, 21, 947–952 CrossRef CAS PubMed
.
- K.-Y. Seong, M.-S. Seao, D. Y. Hwang, E. D. O'Cearbhaill, S. Sreenan, J. M. Karp and S. Y. Yang, J. Controlled Release, 2017, 265, 48–56 CrossRef CAS PubMed
.
- F. Teodorescu, Y. Oz, G. Quéniat, A. Abderrahmani, C. Foulon, M. Lecoeur, R. Sanyal, A. Sanyal, R. Boukherroub and S. Szunerits, J. Controlled Release, 2017, 246, 164–173 CrossRef CAS PubMed
.
- Y. C. Kim, J. Park and M. R. Prausnitz, Adv. Drug Delivery Rev., 2012, 64, 1547–1568 CrossRef CAS PubMed
.
- S. Andrews, J. W. Lee, S.-O. Choi and M. R. Prausnitz, Pharm. Res., 2011, 28, 2110–2118 CrossRef CAS PubMed
.
- K. S. Paudel, M. Milewski, C. L. Swadley, N. K. Brogden, P. Ghosh and A. L. Stinchcomb, Ther. Delivery, 2010, 1, 109–131 CrossRef CAS PubMed
.
- J. Y. Fang, W. R. Lee, S. C. Shen, H. Y. Wang, C. L. Fang and C. H. Hu, J. Controlled Release, 2004, 100, 75 CrossRef CAS PubMed
.
- M. Virk, K. Xiong, M. Svedendahl, M. Käll and A. B. Dahlin, Nano Lett., 2014, 14, 3544 CrossRef CAS PubMed
.
- R. Menzel, S. Barg, M. Miranda, D. B. Anthony, S. M. Bawaked, M. Mokhtar, S. A. Al-Thabaiti, S. N. Basahel, E. Saiz and M. S. P. Shaffer, Adv. Funct. Mater., 2015, 25, 28–35 CrossRef CAS
.
- D. Su, Y. Huang, L. Huang, J. Liang, Y. Ma and Y. Chen, Small, 2011, 7, 3186 CrossRef PubMed
.
- J. Kang, H. Kim, K. S. Kim, S.-K. Lee, S. Bae, J.-H. Ahn, Y.-J. Kim, J.-B. Choi and B. H. Hong, Nano Lett., 2011, 11, 5154–5158 CrossRef CAS PubMed
.
- Z. Liu, Z. Li, Z. Xu, Z. Xia, X. Hu, L. Kou, L. Peng and C. Gao, Chem. Mater., 2014, 26, 6789–6795 Search PubMed
.
- D. Janas and K. K. Koziol, Nanoscale, 2014, 6, 3037 RSC
.
- F. Teodorescu, L. Rolland, V. Ramarao, A. Abderrahmani, D. Mandler, R. Boukherroub and S. Szunerits, Chem. Commun., 2015, 51, 14167–14170 RSC
.
- J. J. Bae, S. C. Lim, G. H. Han, Y. W. Jo, D. L. Doung, E. S. Kim, S. J. Chae, T. Q. Huy, N. V. Luan and Y. H. Lee, Adv. Funct. Mater., 2012, 22, 4819 CrossRef CAS
.
- S. Yang, Y.-C. Chen, L. Nicolini, P. Pasupathy, J. Sacks, B. Su, R. Yang, D. Sanchez, Y.-F. Chang, P. Wang, D. Schnyer, D. Neikirk and N. Lu, Adv. Mater., 2015, 27, 6423–6430 CrossRef CAS PubMed
.
- A. Miyamoto, S. Lee, N. F. Cooray, S. Lee, M. Mori, N. Matsuhisa, H. Jin, L. Yoda, T. Yokota, A. Itoh, M. Sekino, H. Kawasaki, T. Ebihara, M. Amagai and T. Someya, Nat. Nanotechnol., 2017, 12, 907–913 CrossRef CAS PubMed
.
- I. T. Im, S. B. Youn and G. K. Dong, in Proc. of the Int. Heat Transfer Conference (IHTC-15), 1057, Begell House Inc., 2014.
- O. Pillai and R. Panchagnula, J. Controlled Release, 2003, 89, 127 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00576e |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2020 |