Highly efficient visible-light-driven oxygen-vacancy-based Cu2+1O micromotors with biocompatible fuels†
Qinglong
Wang‡
,
Renfeng
Dong‡
 *,
Qianxian
Yang
,
Jiajia
Wang
*,
Shuyu
Xu
and
Yuepeng
Cai
*
*,
Qianxian
Yang
,
Jiajia
Wang
*,
Shuyu
Xu
and
Yuepeng
Cai
*
School of Chemistry and Environment, Guangzhou Key Laboratory of Materials for Energy Conversion and Storage, Guangdong Provincial Engineering Technology Research Centre for Materials for Energy Conversion and Storage, South China Normal University, Guangzhou, 510006, China. E-mail: renfengdong@scnu.edu.cn; jjwang@m.scnu.edu.cn; caiyp@scnu.edu.cn
First published on 4th October 2019
Abstract
Photocatalytic light-driven micro/nanomotors have exhibited great potential in various applications ranging from environmental to biomedical fields. However, in order to expand the practicality of synthetic micromotors, overcoming the challenges of efficiently converting visible light energy to mechanical propulsion energy in fully-biocompatible environments has become critically important. Here, we firstly introduce oxygen vacancies into micromotors by a one-pot method without any additional modification and report a highly efficient Cu2+1O light-driven micromotor with simple fabrication, low cost, and excellent motion performance under low intensity, multi-wavelength visible light (from blue to red) and with biocompatible fuels. Under visible light (1/3 light intensity of sunlight), such oxygen vacancy-based micromotors can reach a maximum speed of 18 body length s−1 in pure water which is comparable to that of conventional Pt-based catalytic micromotors fueled by toxic H2O2. In addition, the motors show over 100 body length s−1 at very low concentrations of additional biocompatible fuels (0.2 mM tannic acid) which is comparable to the speed of bubble-driven microrockets. Even under blue light with only 1/44 of the intensity of sunlight, the motors can be propelled at speeds of 10 body length s−1 in water, indicating that they are the most efficient visible-light driven micromotors fueled by pure water to date. These exceptionally high speeds set new records for photocatalytic micromotors operated in fully green environments, and the proposed novel fabrication approach may pave a new way for designing and mass-producing highly efficient, smart micromachines for on-demand operations, motion-based sensing, and enhanced cargo transportation.
New conceptsSynthetic micro/nanomotors represent one of the most exciting challenges in nanotechnology and hold considerable promise for various future applications. High-speeds mean higher power and higher cargo towing abilities, which are essential for artificial micro/nanomotors. Here, we have demonstrated a brand-new fabrication approach which can greatly enhance the propulsion of photocatalytic micromotors with a series of advantages, such as low cost, simple operation and not needing any additional modification. We have prepared novel Cu2+1O micromotors that are capable of achieving super-high speeds in fully green environments (with biocompatible fuels and visible light), by introducing oxygen vacancies into the micromotors in the one-pot preparation method. The proposed novel concept may pave a new way for designing and mass-producing highly efficient, smart micromachines for on-demand operation, motion-based sensing, and enhanced cargo transportation. |
Introduction
At the frontier of nanotechnology research, micro/nanomotors have shown excellent prospects across many fields, such as targeted drug delivery, medicine, water purifications, and sensing applications after more than ten years of development.1 These micro/nanomotors are controllable and intelligent “robots” that have been specially modified to move in microscopic environments and perform specialized tasks.2 Their propulsion can be based on different power sources, such as magnetic fields,3 light,4 acoustic waves,5 electric fields,6 thermal energy,7 or chemical energy.8Photocatalytic micro/nanomotors (PMNMs) which can convert both optical and chemical energy inputs into their mechanical propulsion via photocatalytic reactions are one of the most attractive light-driven micro/nanomotors due to their flexible propulsion regulation.9 However, conventional methods to enhance the photocatalytic activity are accompanied by complex fabrication methods and high costs, such as coating of noble metal layers (including Pt or Au) to form heterostructures, or annealing to form well-ordered crystal structures. Until now, such reported efficient PMNMs either need toxic fuels, such as H2O2,10 or need harmful light sources, such as UV light.11 Other reported PMNMs which can operate in fully green environments (visible light and biocompatible fuels) tend to exhibit weak propulsion (ESI,† Table S1). The last choice for increasing the propulsion of the micromotor is by increasing the visible light intensity which is neither environmentally friendly nor energy saving. Achieving high-speeds enables higher power and higher cargo towing ability, so it is necessary to develop highly efficient light-driven micro/nanomotors which can be operated in fully biocompatible environments, and it is best that the motors can be mass-produced with a cost-efficient method.
Here, we demonstrate novel oxygen vacancy-based Cu2+1O PMNMs which have outstanding propulsion characteristics in a fully biocompatible environment. An oxygen vacancy is one type of crystal defect which binds photogenerated electrons and suppresses electron–hole recombination, thus enhancing the photocatalytic activity of the material.12 By taking advantage of the oxygen vacancy, the synthesis of the Cu2+1O PMNMs can be completed in one-step with exceptionally low costs and without any further modification. And, such Cu2+1O micromotors show excellent propulsion in biocompatible fuels (pure water or low concentration tannic acid, which exists commonly in grape wine) under a wide range of low energy visible light. Here, under blue light of intensity 48.8 mW cm−2, only approximately 1/3 of the average intensity of sunlight (136.8 mW cm−2),13 motor speeds can reach 18 body length s−1 in pure water and over 100 body length s−1 in 0.2 mM tannic acid solution. They are absolutely the most efficient PMNMs either in fuel-free conditions (in pure water) or in biocompatible fuels conditions (with additional chemicals) so far. In addition, these Cu2+1O PMNMs can be efficiently and continuously powered by multiwavelength visible light (blue, green and red), which enhances the flexibility of these micro/nanomotors for practical applications. Such highly efficient oxygen vacancy-based Cu2+1O PMNMs can be not only mass-produced by a simple one-pot method at low cost, but also operated in fully biocompatible environments with excellent motion performance and low energy requirements, they hold considerable promise for future practical applications, especially in environmental or cargo delivery fields.
Results and discussion
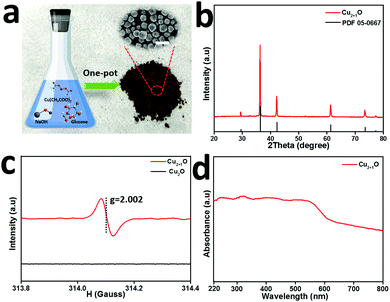
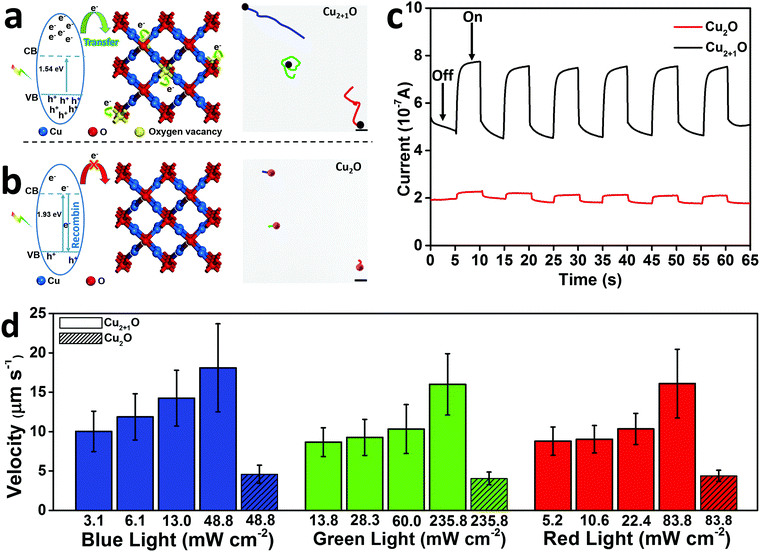
We characterized the Cu2+1O micromotors by scanning electron microscopy (SEM), X-ray diffraction (XRD), electron paramagnetic resonance (EPR) and UV-visible diffuse reflectance (UV-vis DRS) in detail. Fig. 1a shows the preparation process of Cu2+1O micromotors by a simple one-pot method; the color of the Cu2+1O micromotors is dark brown, and the size of the motors is about 1 µm which be confirmed by the SEM. The XRD measurement was conducted to confirm the composition of the prepared samples as shown in Fig. 1b, and the XRD pattern of Cu2+1O could be completely matched with the standard spectrum of PDF 05-0667, which shows that oxygen vacancies have been successfully introduced into the Cu2+1O micromotors.14 We also performed the SEM and XRD patterns of Cu2O, which is fully demonstrated in Fig. S1 (ESI†). The EPR measurement was performed to further confirm the presence of oxygen vacancies. As shown in Fig. 1c, the Cu2O samples have no EPR signal, indicating that there are no local unpaired electrons in the Cu2O sample. In contrast, the Cu2+1O shows a distinct signal peak at g = 2.002, which is attributed to the oxygen vacancies.14 The results of EPR fully demonstrate that we have successfully introduced oxygen vacancies upon Cu2+1O micromotors. The characterization of the UV-vis DRS of the material indicates that Cu2+1O exhibits a broad absorption band of light from 200 to 800 nm (Fig. 1d), and the optical absorption of the Cu2+1O samples under visible light is also strong, which can be attributed to the existence of oxygen vacancies.15 We also confirmed that the band gap energy of Cu2+1O and Cu2O was 1.54 eV and 1.93 eV, respectively (Fig. S2, ESI†), which shows that Cu2+1O has a narrower band gap and higher photoactivity ability. In summary, these characterizations and measurements sufficiently prove that the sample we have prepared is oxygen-vacancy-Cu2+1O micromotors, and has strong absorption capacity for light (220–800 nm), which was consistent with our experimental phenomena.Due to the presence of oxygen vacancies, the Cu2+1O motor with narrower band gap energy could achieve higher speed compared to Cu2O particles under multispectral light in pure water. The propulsion mechanism of the motors involves the redox reaction of the motor with water to generate hydrogen and oxygen under light,16 generating a concentration gradient of photocatalytic products. The formation of a gradient is attributed to the asymmetric surface reaction due to the limited penetration depth of light in semiconductors, and the resulting diffusiophoresis propels the motor. Fig. 2(a) and (b) illustrate the band gap structure and electron transfer mechanism of Cu2+1O and Cu2O, which indicated that the recombination of electron–hole pairs can be greatly reduced by oxygen vacancies. And the Cu2+1O micromotors (average velocity 18.10 µm s−1) travel substantially longer distances over the same time period compared with Cu2O particles (average velocity 4.59 µm s−1), exhibiting a substantial speed acceleration associated with the presence of oxygen vacancies. In order to further explain the effect of oxygen vacancies on the photocatalytic performance of Cu2+1O, the current–time measurement of Cu2O and Cu2+1O electrodes in water with and without blue light irradiation was performed (Fig. 2c). It is obvious that the photocurrent of Cu2+1O is much higher than that of Cu2O, owing to the efficient charge separation by oxygen vacancies under blue light. Additionally, the current of Cu2+1O is also higher than that of Cu2O with blue light off, which is attributed to the excess unpaired electrons caused by oxygen vacancies. According to the results of the current–time measurement, oxygen vacancies could greatly enhance the photocatalytic activity of Cu2+1O micromotors by inhibiting the recombination of electron–hole pairs.17 The speed of Cu2O particles in pure water is only 4.59 µm s−1 (48.8 mW cm−2 blue light), 4.06 µm s−1 (235.8 mW cm−2 green light), and 4.40 µm s−1 (83.8 mW cm−2 red light) respectively (Fig. 2d). Under the same experimental conditions, the velocity of the oxygen-vacancy-Cu2+1O micromotors is substantially increased to 18.10 µm s−1 (blue light), 16.00 µm s−1 (green light), and 16.10 µm s−1 (red light). The speed of Cu2+1O micromotors is increased by factors of 3.9 (blue light), 3.9 (green light), and 3.7 (red light) compared to Cu2O particles, which shows the significant oxygen-vacancy-induced acceleration behavior. The motor speed reaches a maximum of 18.10 µm s−1 under 48.8 mW cm−2 blue light, which is over 11 times faster than a previously reported water-fueled BiOI/Au Janus micromotor18 under similar experimental conditions. In addition, it is worth noting that even under 3.1 mW cm−2 blue light, which is only 1/44 of sunlight energy, the speed of such motors can reach 10 µm s−1 in pure water; this speed is comparable to that of the reported water-fueled SOM-based micromotor which needs over double the light energy.19 On the other hand, these results fully revealed that oxygen vacancies play a decisive role in the improvement of the photocatalytic performance of the Cu2+1O micromotors. As the light intensity increases, the luminous flux (Φ) increases, and more photogenerated holes and electrons in Cu2+1O are generated, resulting in stronger photocatalytic ability. The relationship between the incident light intensity (I) and the luminous flux (Φ) is as follows:
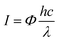 | (1) |
 | ||
| Fig. 2 (a and b) Schematic of the band gap structure of Cu2+1O and Cu2O, and the trajectory of Cu2+1O and Cu2O micromotors for 3 s under 13.0 mW cm−2 blue light in pure water (taken from SI-Video S1, ESI†). Scale bar: 10 µm. (c) Current–time curve of Cu2+1O and Cu2O electrodes with cyclic “on” and “off” blue light (13.0 mW cm−2) in 0.5 M Na2CO3 at a bias voltage of +0 V. (d) The speed of Cu2+1O micromotors and Cu2O particles under different light intensity in pure water (corresponding to SI-Video 2, ESI†). | ||
Interestingly, we observed that such Cu2+1O micromotors exhibit dramatic speed acceleration with super low concentration of a common reducing agent – tannic acid. Tannic acid (C76H52O46) is widely used in anticancer, hemostatic, and anti-aging applications and is biocompatible and safe for organisms.20 The main structural unit of tannic acid is gallic acid and a glucose ring, and the aromatic ring has been hydroxylated and can be easily decomposed by oxidizing substances such as hydroxyl radicals.21 Traditionally, the fuel used in photocatalytic micro/nanomotors is mostly a strong oxidant, such as hydrogen peroxide,22,23 or a neutral solution, like pure water.24 For the first time, we have used a strong reducing substance as the fuel to provide powerful energy for the motor. The tannic acid is photocatalytically decomposed by OH˙, h+, and O2˙− in both oxidation and reduction reaction processes. Thus, the decomposition of the fuel can be accelerated and the motor speed is greatly enhanced (Fig. 3a).19 The Janus structure of the micromotor responsible for diffusiophoretic propulsion is achieved when the motor is exposed to a directional light source (Fig. 3a). When the Cu2+1O micromotors were excited to generate electron–hole pairs under light, the oxygen vacancies could limit electron recombination to promote the photocatalytic activity of the Cu2+1O micromotors. Additionally, tannic acid is photocatalytically decomposed to a large amount of rapidly diffusing small molecules concentrated on the illuminated side of the motor. Therefore, by exploiting the limited depth of light penetration in the Cu2+1O material, we are able to construct asymmetric surface chemical reactions on the Cu2+1O micromotors, building a concentration gradient of photocatalytic products further to propel the motor.25 As shown in the trajectory of Cu2O and Cu2+1O micromotors in 0.2 mM tannic acid (Fig. 3b), under the same experimental conditions, the Cu2+1O micromotors show much longer track lines, which means higher speed of Cu2+1O micromotors. And the corresponding speed of Cu2O and Cu2+1O micromotors is 20.17 and 107.32 µm s−1 in tannic acid, respectively. In particular, the speed of Cu2+1O micromotors is comparable to that of the photocatalytic bubble-driven micromotor at high concentrations of H2O2 (8 wt% ≈ 2.56 × 103 mM) as fuel.22 The results from Fig. 3b further illustrate that the speed acceleration behavior of the motor is attributed to the presence of oxygen vacancies. And we have further tested several other biocompatible organic fuels such as glucose, malic acid, and vitamin C, and all these biocompatible fuels also worked, although the speed of the Cu2+1O micromotors in these biocompatible fuels was much lower than in tannic acid.
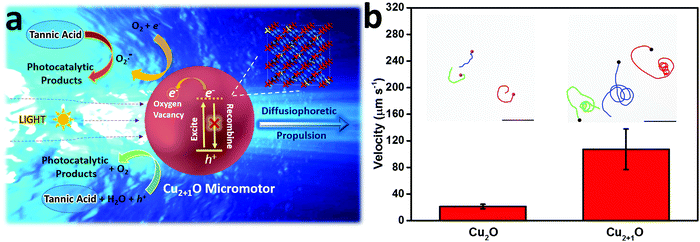 | ||
| Fig. 3 (a) Schematic of the propulsion mechanism of the light-driven Cu2+1O micromotor. (b) The velocity of Cu2O and Cu2+1O micromotors under 48.8 mW cm−2 blue light in 0.2 mM tannic acid; the insets are the trajectory of Cu2O and Cu2+1O micromotors moving for 3 s, respectively (taken from SI-Video S3, ESI†). Scale bar: 50 µm. | ||
As a photocatalytic micromotor, the speed of the motor can be easily regulated by the concentration of tannic acid and the intensity of the light. In 0.05 mM tannic acid solution, the motor speed reaches 65.22 µm s−1 (48.8 mW cm−2 blue light), 55.33 µm s−1 (235.8 mW cm−2 green light), and 54.14 µm s−1 (83.8 mW cm−2 red light), which increased by 3.6, 3.5, and 3.4 times relative to that in pure water under the same conditions (Fig. 4a). It should be noted that the speed of the motor is enhanced when the tannic acid concentration is increased below the 0.2 mM threshold, but when the tannic acid concentration is above 0.2 mM, the speed decreases with increasing acid concentration. The possible reason for this maximum is that the excessively high concentration of tannic acid weakens the concentration gradient around the surface of the Cu2+1O micromotor, causing the speed to decay. By utilizing the ultralow concentration of tannic acid as fuel, the motor speed could be greatly accelerated, which may promote the practical application of light-driven micro/nanomotors.
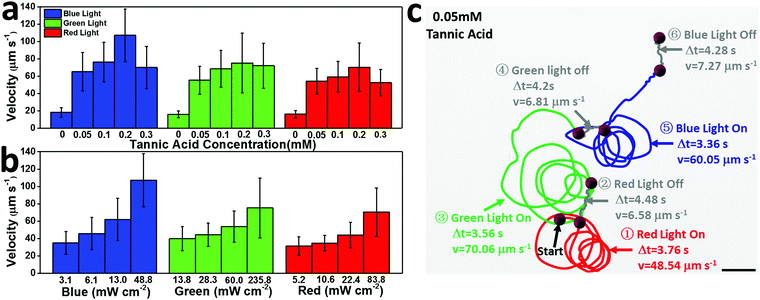 | ||
| Fig. 4 (a) The relationship between Cu2+1O micromotor speed and tannin concentration under 48.8 mW cm−2 blue light, 235.8 mW cm−2 green light, and 83.8 mW cm−2 red light (corresponding to SI-Video 4, ESI†). (b) The relationship between the speed of Cu2+1O micromotors and light intensity in 0.2 mM tannic acid (corresponding to SI-Video 5, ESI†). (c) The trajectory of a Cu2+1O micromotor “stop/go” switch motion under red (83.8 mW cm−2), green (235.8 mW cm−2), and blue (48.8 mW cm−2) light in 0.05 mM tannic acid (taken from SI-Video S6, ESI†). Scale bar: 10 µm. | ||
In addition, we confirmed the effect of light intensity on the motor speed in 0.2 mM tannic acid. Light intensity is one of the key factors affecting the speed of the motor, and the relationship between the incident light intensity (I) and the luminous flux (Φ) is as shown in formula (1). From Fig. 4b, the speed of the motor is positively correlated with the light intensity, and the stronger the light intensity, the greater the motor speed. When the light intensity is increased from 3.1 mW cm−2 (blue light), 13.8 mW cm−2 (green light), and 5.2 mW cm−2 (red light) to 48.8 mW cm−2 (blue light), 235.8 mW cm−2 (green light), and 83.8 mW cm−2 (red light), the corresponding speed of the motor increased significantly from 39.93, 35.18, and 31.50 µm s−1 to 107.32, 75.27, and 70.41 µm s−1 respectively. It is worth noting that the lowest light intensity of blue, green, and red lights here is corresponding to 1/44, 1/10, and 1/26 sunlight intensity, respectively, while the average speeds of the motors are all above 30 µm s−1 under the 3 different light energies with biocompatible and low concentration tannic acid. Such speeds are even faster than classic highly efficient Pt-based catalytic micromotors which need toxic H2O2 fuel.26 It is also worth mentioning that the sample has stronger light absorption in the blue wavelength range (400–500 nm) than green and red light (Fig. 1d), and more blue light energy can be converted into mechanical energy which further propels the motor more efficiently, so the Cu2+1O micromotors show the best performance under blue light no matter whether in pure water or tannic acid fuels. Moreover, the Cu2+1O micromotors also show excellent performance both in pure water and low concentration tannic acid under UV light (Fig. S4, ESI†), however the highest speed of such motors is still achieved under blue light.
Furthermore, the high velocity of the Cu2+1O micromotors can be achieved by continuously switching four different wavelengths of light; as shown in Fig. 4c, in 0.05 mM tannic acid, the motor can be continuously regulated by blue, green and red light, with speeds of 60.05, 70.06, and 48.54 µm s−1, respectively. Once the light source is turned off, the speed of the motor drops sharply, displaying the much weaker directional motion under the background light. Thus, the oxygen-vacancy-Cu2+1O micromotors can not only be efficiently and continuously driven by different wavelengths of visible light (blue, green, red light) with low concentration tannic acid as fuel, but can also be toggled to realize a quick “stop/go” transition by turning the light source on or off. Therefore, the Cu2+1O micromotors could achieve highly efficient self-driven propulsion under multiple spectra, and the speed of the motor could be easily controlled by light intensity and tannic acid concentration. In addition, the speed of the Cu2+1O micromotors shows no obvious change after storing for 1 month, which means that these motors are greatly stable and can be stored for a long time.
Conclusions
In summary, we have demonstrated highly efficient self-propelled micromotors under multi-spectral visible light in a fully biocompatible environment based on the incorporation of oxygen vacancies upon the Cu2+1O micromotor. Such oxygen-vacancy-induced acceleration phenomenon reflects the promoted photocatalytic activity of the motor due to the enhanced charge separation by oxygen vacancies, which is fully confirmed by the results of XRD and ESR characterization and current–time curve measurements. Compared with traditional PMNMs, the Cu2+1O micromotors can be easily prepared by a one-pot method without any further modification, but also show excellent photocatalytic propulsion in biocompatible fuels. In addition, the velocity of Cu2+1O micromotors not only can be efficiently regulated by adjusting the fuel concentration and light intensity as for conventional PMNMs, but has one more flexible regulation route: adjusting the wavelength. Still, the excellent on/off characteristic of motion control has also been well demonstrated. The highly efficient, low-cost and easy prepared oxygen vacancy-based Cu2+1O micromotors may provide a valuable reference for the development of highly efficient smart micro/nanomotors with superior capabilities in various future applications in order to benefit the world.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Natural Science Foundation of China (21805096, 21471061 and 21671071), Natural Science Foundation of Guangdong Province (2018A030313358 and 2017A030310432), Applied Science and Technology Planning Project of Guangdong Province (2015B010135009 and 2017B090917002), Innovation Team Project of Guangdong Ordinary University (2015KCXTD005), and the Great Scientific Research Project of Guangdong Ordinary University (2016KZDXM023).Notes and references
- J. J. Abbott, K. E. Peyer, L. X. Dong and B. J. Nelson, How Should Microrobots Swim?, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011, p. 157 RSC; J. Wang, Nanomachines: Fundamentals and Applications, Wiley, 2013 RSC; S. Campuzano, D. Kagan, J. Orozco and J. Wang, Analyst, 2011, 136, 4621 RSC; Y. Mei, A. A. Solovev, S. Sanchez and O. G. Schmidt, Chem. Soc. Rev., 2011, 40, 2109 RSC; T. E. Mallouk and A. Sen, Sci. Am., 2009, 300, 72 CrossRef CAS; T. Mirkovic, N. S. Zacharia, G. D. Scholes and G. A. Ozin, Small, 2010, 6, 159 CrossRef.
- T. Patiño, X. Arqué, R. Mestre, L. Palacios and S. Sánchez, Acc. Chem. Res., 2018, 51, 2662 CrossRef CAS; K. Kim, J. Guo, Z. Liang and D. Fan, Adv. Funct. Mater., 2018, 28, 1705867 CrossRef; M. Safdar, S. U. Khan and J. Jänis, Adv. Mater., 2018, 30, 1703660 CrossRef; C. Gao, Z. Lin, X. Lin and Q. He, Adv. Therapeut., 2018, 1, 1800056 CrossRef; M. Xuan, R. Mestre, C. Gao, C. Zhou, Q. He and S. Sánchez, Angew. Chem., Int. Ed., 2018, 57, 6838 CrossRef; J. Li, B. Esteban Fernández de Ávila, W. Gao, L. Zhang and J. Wang, Sci. Robot., 2017, 2, eaam6431 CrossRef.
- X.-Z. Chen, M. Hoop, F. Mushtaq, E. Siringil, C. Hu, B. J. Nelson and S. Pané, Appl. Mater. Today, 2017, 9, 37 CrossRef.
- J. Wang, Z. Xiong, J. Zheng, X. Zhan and J. Tang, Acc. Chem. Res., 2018, 51, 1957 CrossRef CAS.
- T. Xu, L.-P. Xu and X. Zhang, Appl. Mater. Today, 2017, 9, 493 CrossRef.
- L. Bouffier, V. Ravaine, N. Sojic and A. Kuhn, Curr. Opin. Colloid Interface Sci., 2016, 21, 57 CrossRef CAS.
- X. Lin, T. Si, Z. Wu and Q. He, Phys. Chem. Chem. Phys., 2017, 19, 23606 RSC.
- A. A. Solovev, S. Sanchez, M. Pumera, Y. F. Mei and O. G. Schmidt, Adv. Funct. Mater., 2010, 20, 2430 CrossRef CAS.
- R. Dong, Y. Cai, Y. Yang, W. Gao and B. Ren, Acc. Chem. Res., 2018, 51, 1940 CrossRef CAS.
- D. Zhou, L. Ren, Y. C. Li, P. Xu, Y. Gao, G. Zhang, W. Wang, T. E. Mallouk and L. Li, Chem. Commun., 2017, 53, 11465 RSC.
- R. Dong, Q. Zhang, W. Gao, A. Pei and B. Ren, ACS Nano, 2016, 10, 839 CrossRef CAS.
- M. Li, Y. Hu, S. Xie, Y. Huang, Y. Tong and X. Lu, Chem. Commun., 2014, 50, 4341 RSC.
- H. Ren, Y. Wu, Y. Li, W. Cao, Z. Sun, H. Xu and X. Zhang, Small, 2013, 9, 3981 CrossRef CAS.
- R. Yang, X. Lu, X. Huang, Z. Chen, X. Zhang, M. Xu, Q. Song and L. Zhu, Appl. Catal., B, 2015, 170–171, 225 CrossRef CAS.
- Z. Wei, J. Sun, Y. Li, A. K. Datye and Y. Wang, Chem. Soc. Rev., 2012, 41, 7994 RSC.
- Q. Zhang, R. Dong, Y. Wu, W. Gao, Z. He and B. Ren, ACS Appl. Mater. Interfaces, 2017, 9, 4674 CrossRef CAS.
- Y. Lv, Y. Liu, Y. Zhu and Y. Zhu, J. Mater. Chem. A, 2014, 2, 1174 RSC; X.-J. Wang, Y. Zhao, F.-t. Li, L.-J. Dou, Y.-P. Li, J. Zhao and Y.-J. Hao, Sci. Rep., 2016, 6, 24918 CrossRef CAS.
- R. Dong, Y. Hu, Y. Wu, W. Gao, B. Ren, Q. Wang and Y. Cai, J. Am. Chem. Soc., 2017, 139, 1722 CrossRef CAS.
- A. Mallick and S. Roy, Nanoscale, 2018, 10, 12713 RSC.
- F. Liu, V. Kozlovskaya, O. Zavgorodnya, C. Martinez-Lopez, S. Catledge and E. Kharlampieva, Soft Matter, 2014, 10, 9237 RSC; M. Shin, J. H. Ryu, J. P. Park, K. Kim, J. W. Yang and H. Lee, Adv. Funct. Mater., 2015, 25, 1270 CrossRef CAS; C. K. Angerhofer, D. Maes, P. U. Giacomoni, Skin Aging Handbook, ed. N. Dayan, William Andrew Publishing, Norwich, NY, 2009, p. 205 Search PubMed.
- B. Ahmed, H. Mohamed, E. Limem and B. Nasr, Ind. Eng. Chem. Res., 2009, 48, 3370 CrossRef CAS; N. L. Kruthika, G. B. Raju and S. Prabhakar, Mater. Sci. Forum, 2013, 734, 117 Search PubMed.
- Y. Li, F. Mou, C. Chen, M. You, Y. Yin, L. Xu and J. Guan, RSC Adv., 2016, 6, 10697 RSC.
- F. Mou, Y. Li, C. Chen, W. Li, Y. Yin, H. Ma and J. Guan, Small, 2015, 11, 2564 CrossRef CAS.
- D. Zhou, Y. C. Li, P. Xu, L. Ren, G. Zhang, T. E. Mallouk and L. Li, Nanoscale, 2017, 9, 11434 RSC.
- C. Chen, F. Mou, L. Xu, S. Wang, J. Guan, Z. Feng, Q. Wang, L. Kong, W. Li, J. Wang and Q. Zhang, Adv. Mater., 2017, 29, 1603374 CrossRef PubMed.
- H. Ke, S. Ye, R. L. Carroll and K. Showalter, J. Phys. Chem. A, 2010, 114, 5462 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods and additional details can be found. See DOI: 10.1039/c9nh00592g |
| ‡ Qinglong Wang and Renfeng Dong contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |