Large-area transparent flexible guanidinium incorporated MAPbI3 microstructures for high-performance photodetectors with enhanced stability†
Gundam Sandeep
Kumar
a,
Piyush Kanti
Sarkar
a,
Bapi
Pradhan
a,
Mozakkar
Hossain
ab,
K. D. M.
Rao
 *ab and
Somobrata
Acharya
*ab and
Somobrata
Acharya
 *a
*a
aSchool of Applied & Interdisciplinary Sciences, Indian Association for the Cultivation of Science, Jadavpur, Kolkata-700032, India. E-mail: camsa2@iacs.res.in
bTechnical Research Centre, Indian Association for the Cultivation of Science, Jadavpur, Kolkata-700032, India. E-mail: trckdmr@iacs.res.in
First published on 17th January 2020
Abstract
Unveiling the transparency and flexibility in perovskite-based photodetectors with superior photoresponse and environmental stability remains an open challenge. Here we report on guanidinium incorporated metal halide perovskite (MA1−xGuaxPbI3, x = 0 to 0.65) random percolative microstructure (RPM) fabrication using an ultra-fast spray coating technique. Remarkably, RPMs over a large area of 5 × 5 cm2 on flexible substrates with a transparency of ∼50% can be achieved with enriched environmental stability. Transparent photodetectors based on MA1−xGuaxPbI3 (x = 0.12) RPMs manifest excellent performance with a responsivity of 187 A W−1, a detectivity of 2.23 × 1012 Jones and an external quantum efficiency of 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115%. Additionally, the photodetectors exhibited superior mechanical flexibility under a wide range of bending angles and large number of binding cycles. Integrating features including transparency, high performance, stability, flexibility and scalability within a photodetector is unmatched and holds potential for novel applications in transparent and wearable optoelectronic devices.
115%. Additionally, the photodetectors exhibited superior mechanical flexibility under a wide range of bending angles and large number of binding cycles. Integrating features including transparency, high performance, stability, flexibility and scalability within a photodetector is unmatched and holds potential for novel applications in transparent and wearable optoelectronic devices.
New conceptsDevelopment of flexible, transparent optoelectronic devices has received enormous interest owing to their potential applications in smart windows and wearable electronics. Mechanical flexibility of the photodetectors can further inflate the possible range of applications towards wearable electronics and adaptability to curved surfaces. Traditional photodetectors are rigid, brittle, completely opaque and confined to small areas with limited spectral response. Hence, the development of large-area transparent photodetectors with excellent mechanical flexibility and high photoresponse over a broad spectral range can meet the increasing demands of high-performance optoelectronic devices. Here, we fabricate guanidinium incorporated metal halide perovskite (MA1−xGuaxPbI3, x = 0 to 0.65) random percolative microstructures (RPMs) using a single step ultra-fast spray coating technique, which shows transparency of ∼50%, and greatly enriched photoresponse and stability. Furthermore, the RPM based photodetectors displayed excellent mechanical flexibility under a wide range of bending angles and large number of binding cycles. Combining all these features including high performance, stability, transparency and flexibility in a photodetector is unmatched and the performance is second to none. |
Transparent photodetectors have attracted tremendous research attention in recent years owing to their potential applications in next generation optoelectronic devices.1–3 Mechanical flexibility of the photodetectors can further inflate the possible range of applications towards wearable electronics and adaptability to curved surfaces.4–8 Traditional photodetectors are rigid, brittle, completely opaque and confined to small areas with limited spectral response.9–12 Hence, the development of large-area transparent photodetectors with excellent mechanical flexibility and high photoresponse over a broad spectral range is essential to meet the increasing demands of high performance optoelectronic devices.5 However, combining high photoresponsivity, mechanical flexibility and transparency features all together in a single device along with large-area scalability remains an uphill task.
Recently, organic–inorganic hybrid perovskites have emerged as excellent candidates for high performance optoelectronic devices.13–19 A variety of compositions for the cations (MA, FA, BA) and halides (Cl, Br, I) have been used to design superior organic–inorganic hybrid perovskites.20–23 The pure phases of organic–inorganic perovskites are synthesized using mechanochemistry and solution processed methods.24 However, a continuous perovskite thin film is opaque in the visible region, which is not compatible for transparent electronics. However, transparent photodetectors have been realized through patterning perovskites without compromising the optoelectronic properties. Song et al. have shown the fabrication of perovskite network-based photodetectors with limited transparency and photoresponse.3 In addition to the patterning of perovskites and improvement in the device efficiencies, the ambient stability issue remains a great challenge. The decomposition of perovskites due to sensitivity towards oxygen and moisture25 and intrinsic halide segregation26,27 create a major hurdle for the stability. To overcome the stability issue, all-inorganic perovskites negating volatile organic cations have been proposed with promising optoelectronic properties.28–32 However, organic alternatives with an appropriate ionic radius to accommodate within the inorganic Pb–I framework are limited primarily because of the mismatch in the size to maintain an adequate Goldschmidt tolerance factor.33,34 Recently, organic cation guanidinium (C(NH2)3+, Gua) has been incorporated into the pre-existing 3D perovskite lattice of MAPbI3.35 Gua containing a larger ionic radius than MA, resulting in a higher limit of Goldschmidt tolerance factor, can insert into the crystal unit of MAPbI3 to form mixed cation perovskite MA1–xGuaxPbI3 (0 < x < 0.25).33,35 An enhanced thermal and environmental stability along with longer charge carrier lifetime compared to pure MAPbI3 have been obtained upon Gua incorporation in a lower limit. These findings were beneficial for achieving MA1−xGuaxPbI3 based solar cells with enhanced performance.35–38 However, there have been no attempts to fabricate transparent perovskite photodetectors with mixed cations to our knowledge to date.
Here we developed a simple, solution processed and scalable single-step spray coating route for the formation of random percolative microstructures (RPMs) of MA1−xGuaxPbI3 perovskites with ∼50% transparency. Fabrication of transparent photodetectors using the RPMs leads to record high figure-of-merits (FOMs) exhibiting photosensitivity of ∼41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500%, responsivity of ∼187 A W−1, detectivity of ∼2.23 × 1012 Jones and EQE of ∼44
500%, responsivity of ∼187 A W−1, detectivity of ∼2.23 × 1012 Jones and EQE of ∼44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115%. These FOMs are superior in comparison to the reported opaque mixed cationic perovskite-based photodetectors and commercially available silicon-based photodetectors. We have thoroughly investigated the stability of RPMs of MA1−xGuaxPbI3 by aging the devices under ambient conditions (humidity levels ∼45–55%) over 30 days. A large enhancement in the stability of photodetectors fabricated with RPMs of MA1–xGuaxPbI3 is observed in comparison to the MAPbI3 based photodetectors. Furthermore, we demonstrated the mechanical flexibility of the photodetectors under a wide range of bending angles and number of bending cycles.
115%. These FOMs are superior in comparison to the reported opaque mixed cationic perovskite-based photodetectors and commercially available silicon-based photodetectors. We have thoroughly investigated the stability of RPMs of MA1−xGuaxPbI3 by aging the devices under ambient conditions (humidity levels ∼45–55%) over 30 days. A large enhancement in the stability of photodetectors fabricated with RPMs of MA1–xGuaxPbI3 is observed in comparison to the MAPbI3 based photodetectors. Furthermore, we demonstrated the mechanical flexibility of the photodetectors under a wide range of bending angles and number of bending cycles.
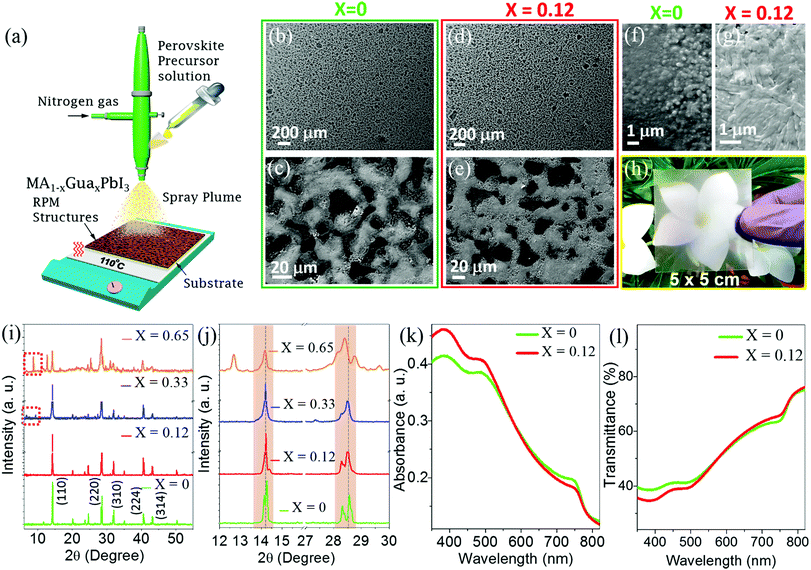
Guanidinium cation incorporated MAPbI3 precursor solutions are prepared by dissolving GuaI, MAI and PbI2 at different molar ratios in anhydrous N,N-dimethylformamide (DMF) (Experimental methods for details, ESI†). The precursor solutions are spray coated on glass or PET substrates as shown in Fig. 1a. Optimal trade-off between the transparency and interconnectivity of perovskite microstructures is achieved by controlling various spray coating parameters (Experimental methods, ESI†). In brief, spray coating is performed for 4 seconds on a pre-heated (110 °C) substrate, which instantaneously formed RPMs of a perovskite. Optical microscope images reveal perforated microstructures of perovskite over a large substrate area (Fig. S1, ESI†). Apparently macroscopic images confirm that the RPMs formed through the spray coating process are independent of the Gua cation composition (Fig. S2, ESI†). An excellent interconnectivity of the RPMs is obtained for both the MAPbI3 and MA1−xGuaxPbI3 (Fig. 1b–e and Fig. S3, ESI†). However, higher resolution scanning electron microscopic (SEM) images reveal different grain morphologies of the RPMs of MAPbI3 and MA1−xGuaxPbI3 with variation of packing (Fig. 1f and g). Pristine MAPbI3 RPMs show corrugations (Fig. 1f) whereas MA0.88Gua0.12PbI3 RPMs show a smooth surface morphology and improved interconnectivity (Fig. 1g). Interconnected platelets with voids are observed upon increasing the Gua content in the mixed cation perovskites (Fig. S3c, ESI†). Fig. 1h shows a photograph of the MA0.88Gua0.12PbI3 RPMs on a PET sheet with a large area of 5 × 5 cm2, which clearly manifests the uniformity and transparency. Therefore, the spray coating process implemented here is not only an ultra-rapid process but also scalable to large area.
We carried out X-ray diffraction (XRD) measurements on pristine MAPbI3 and MA1−xGuaxPbI3 RPMs. XRD reveals tetragonal phase of the MAPbI3 with (110), (220), (310), (224), and (314) reflections at 2θ of 14.2, 28.6, 32, 40.6 and 43.24, respectively (Fig. 1i). The tetragonal phase of MAPbI3 is retained after incorporation of Gua cations but to a lower extent (0 < x ≤ 0.25) by direct substitution of MA, forming a mixed MA1−xGuaxPbI3 phase.35,38 A closer inspection of the (110) and (220) reflections discloses a gradual shift towards lower angles upon incorporation of Gua in MAPbI3, as reported previously (Fig. 1j).35,38 The observed shift of diffraction peaks is attributed to expansion of the unit-cell volume due to the substitution of MA with larger sized Gua cations in the MAPbI3 crystal structure.38 Notably, for x = 0.33 Gua content, additional XRD peaks appear at 2θ values of 6.72 and 9.33 belonging to pure GuaPbI3 and on further increase of the Gua content (x = 0.65), the reflection peak at 9.33 is shifted to 8.71 (see red square boxes in Fig. 1i and Fig. S4, ESI†). Incorporation of Gua in MAPbI3 causes local distortion in the crystal lattice due to the mismatch in size with respect to the MA cation which is manifested by a broadening and shifting of the peaks with different Gua contents. Earlier reports demonstrated formation of phase-separated GuaPbI3 upon incorporation of a higher content of Gua cations.35,38 Our XRD and SEM observations also suggest formation of phase-separated GuaPbI3 with a larger Gua content, which is corroborated by the reported literature reports.38
Since a larger content of Gua gradually leads to the GuaPbI3 phase, we have designed photodetectors with a low content of Gua (MA0.88Gua0.12PbI3) and compared them with the pristine MAPbI3. Comparison of UV-vis absorption spectra (Fig. 1k) and transparency (Fig. 1l) of MAPbI3 and MA0.88Gua0.12PbI3 RPMs clearly demonstrates insignificant change in the shape of the spectra. However, a slight decrease of the absorption edge position of MA0.88Gua0.12PbI3 suggests an increase in the bandgap, which is attributed to the local distortions at the Gua neighboring positions owing to the formation of new bonds and expansion of the crystal unit.35,38 The similarity in the absorption spectra reveals that the substitution of MA by Gua effectively and stabilizes the 3D perovskite phase of MAPbI3. Average visible transmittance (AVT) is defined as the mean transmittance between 370 nm and 740 nm in the visible region.39,40 We have estimated the AVT values for pristine MAPbI3 and MA0.88Gua0.12PbI3 RPMs prepared on glass substrates to be ∼50%, which is consistent with the calculated average fill factor of ∼56% measured from optical microscope images (Fig. S5, ESI†). The UV-vis absorption spectrum and transparency with higher content of Gua also showed similar spectra (Fig. S6, ESI†). Therefore, the fabricated perovskite RPMs are transparent and are independent of mixed cation composition.
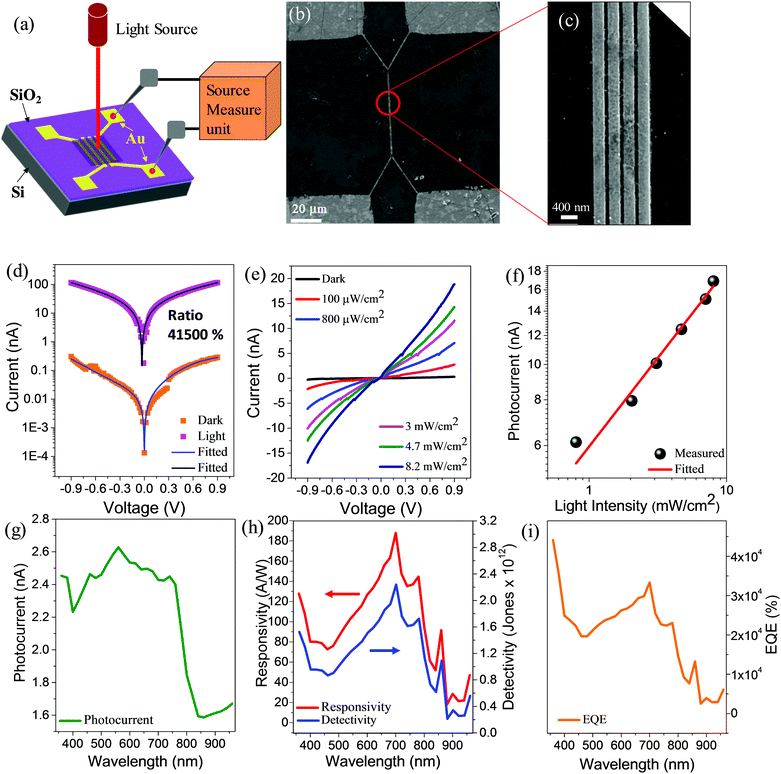
We have measured the photoresponse of MA0.88Gua0.12PbI3 RPMs under the illumination of light and in the dark. The schematic representation of the photodetector device structure and measurement setup is shown in Fig. 2a. The SEM image shows a spacing of 100 nm of the Au electrodes deposited on top of the SiO2/Si substrate (Fig. 2b and c). Photosensitivity (on/off ratio) defined by (Ilight − Idark)/Idark, is estimated to be ∼41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500% at a low operating bias voltage of ∼0.9 V (Fig. 2d). The photocurrent of MA0.88Gua0.12PbI3 RPMs is increased by three orders of magnitude under the illumination of light (Fig. 2d). The measured photosensitivity is higher compared to reported opaque mixed cation perovskite-based photodetectors (Table S1, ESI†).41–43 Furthermore, we studied the photoresponse behavior of the MA0.88Gua0.12PbI3 devices with various light intensities ranging from ∼100 μW cm−2 to ∼8.2 mW cm−2 (Fig. 2e). The photoresponse indicates a linear dependency of light intensity (Fig. 2f), which is essential for reliable photodetector applications. The plot is fitted with the power law expression I = αPβ where α is a constant for a specific wavelength, P is the illuminated light intensity, and the exponent β is the response of the photocurrent to the incident light intensity. The best fitting curve gives a linear behavior with exponent value β = 0.58 (standard error 0.0236). This fractional power dependence (0.5 < β < 1.0) indicates the existence of electron–hole recombination and trapping of charge carriers.44,45 The photoresponse of the MA0.88Gua0.12PbI3 RPMs measured under the illumination of monochromatic light at various wavelengths exhibits photosensitivity in the entire visible region (Fig. 2g). The performance of the photodetector is further characterized by measuring the FOMs such as responsivity (R), detectivity (D), and external quantum efficiency (EQE) (Fig. S7, ESI†) at a low operating voltage of 0.9 V. The responsivity of the photodetector determines the generated photocurrent for a defined intensity of light. MA0.88Gua0.12PbI3 RPM based photodetectors demonstrate a robust spectral responsivity in the entire visible region with a maximum responsivity of 187 A W−1 at a wavelength of 700 nm (Fig. 2h). A maximum detectivity of 2.23 × 1012 Jones at wavelength 700 nm is obtained from the MA0.88Gua0.12PbI3 photodetector (Fig. 2h). We measured the EQE of transparent mixed cation MA0.88Gua0.12PbI3 RPM photodetectors to be 44
500% at a low operating bias voltage of ∼0.9 V (Fig. 2d). The photocurrent of MA0.88Gua0.12PbI3 RPMs is increased by three orders of magnitude under the illumination of light (Fig. 2d). The measured photosensitivity is higher compared to reported opaque mixed cation perovskite-based photodetectors (Table S1, ESI†).41–43 Furthermore, we studied the photoresponse behavior of the MA0.88Gua0.12PbI3 devices with various light intensities ranging from ∼100 μW cm−2 to ∼8.2 mW cm−2 (Fig. 2e). The photoresponse indicates a linear dependency of light intensity (Fig. 2f), which is essential for reliable photodetector applications. The plot is fitted with the power law expression I = αPβ where α is a constant for a specific wavelength, P is the illuminated light intensity, and the exponent β is the response of the photocurrent to the incident light intensity. The best fitting curve gives a linear behavior with exponent value β = 0.58 (standard error 0.0236). This fractional power dependence (0.5 < β < 1.0) indicates the existence of electron–hole recombination and trapping of charge carriers.44,45 The photoresponse of the MA0.88Gua0.12PbI3 RPMs measured under the illumination of monochromatic light at various wavelengths exhibits photosensitivity in the entire visible region (Fig. 2g). The performance of the photodetector is further characterized by measuring the FOMs such as responsivity (R), detectivity (D), and external quantum efficiency (EQE) (Fig. S7, ESI†) at a low operating voltage of 0.9 V. The responsivity of the photodetector determines the generated photocurrent for a defined intensity of light. MA0.88Gua0.12PbI3 RPM based photodetectors demonstrate a robust spectral responsivity in the entire visible region with a maximum responsivity of 187 A W−1 at a wavelength of 700 nm (Fig. 2h). A maximum detectivity of 2.23 × 1012 Jones at wavelength 700 nm is obtained from the MA0.88Gua0.12PbI3 photodetector (Fig. 2h). We measured the EQE of transparent mixed cation MA0.88Gua0.12PbI3 RPM photodetectors to be 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115% (Fig. 2i), which is the best among the transparent photodetectors and relatively higher in comparison to the other photodetectors (Tables S1 and S2, ESI†).41–43 On the other hand, the responsivities of pristine MAPbI3 and MA0.67Gua0.33PbI3 RPM based photodetectors are 48 A W−1 and 135 A W−1 respectively (Table 1, and Fig. S7, ESI†). Moreover, MAPbI3 and MA0.67Gua0.33PbI3 RPMs display a lower detectivity of 6.78 × 1011 Jones and 1.61 × 1012 Jones and EQE of 12
115% (Fig. 2i), which is the best among the transparent photodetectors and relatively higher in comparison to the other photodetectors (Tables S1 and S2, ESI†).41–43 On the other hand, the responsivities of pristine MAPbI3 and MA0.67Gua0.33PbI3 RPM based photodetectors are 48 A W−1 and 135 A W−1 respectively (Table 1, and Fig. S7, ESI†). Moreover, MAPbI3 and MA0.67Gua0.33PbI3 RPMs display a lower detectivity of 6.78 × 1011 Jones and 1.61 × 1012 Jones and EQE of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 396% and 30
396% and 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737%, respectively compared to the MA0.88Gua0.12PbI3 RPM photodetectors (Table 1, and Fig. S7, ESI†). Hence, the RPMs photodetectors fabricated with MA0.88Gua0.12PbI3 showed significantly improved photoresponse compared to MAPbI3 and adequate improvement compared to MA0.67Gua0.33PbI3 devices. To the best of our knowledge, the performance of the MA0.88Gua0.12PbI3 based transparent photodetectors reported here is the highest among the mixed cation perovskite-based photodetectors and transparent perovskite photodetectors (Tables S1 and S2, ESI†).46–49
737%, respectively compared to the MA0.88Gua0.12PbI3 RPM photodetectors (Table 1, and Fig. S7, ESI†). Hence, the RPMs photodetectors fabricated with MA0.88Gua0.12PbI3 showed significantly improved photoresponse compared to MAPbI3 and adequate improvement compared to MA0.67Gua0.33PbI3 devices. To the best of our knowledge, the performance of the MA0.88Gua0.12PbI3 based transparent photodetectors reported here is the highest among the mixed cation perovskite-based photodetectors and transparent perovskite photodetectors (Tables S1 and S2, ESI†).46–49
| Parameter at 0.9 V | MAPbI3 | MA0.88Gua0.12PbI3 | MA0.67Gua0.33PbI3 |
|---|---|---|---|
| Responsivity (A W−1) | 48 | 187 | 135 |
| Detectivity (Jones) | 6.78 × 1011 | 2.23 × 1012 | 1.61 × 1012 |
| EQE (%) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 396 396 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 737 737 |
The robust photodetector performance can be explained due to the formation of a mixed-cation phase in MA0.88Gua0.12PbI3 where MA interacts with the Gua cations. Recent studies suggested incorporation of Gua into 3D perovskite lattices despite the size mismatch between Gua and MA forming a mixed phase.35,38 However, incorporation of Gua occurs until a low limited concentration because of the saturation of the 3D PbI6 octahedra to accommodate larger Gua cations. Hence, a phase separation takes place in the form of GuaPbI3 when a larger content of Gua is used.35,38 Gua cations undergo rapid reorientation on the picosecond time scale, an order of magnitude faster than MA, imparting charge carrier stabilization through the electron–rotor interaction which leads to a long charge carrier lifetime.34,50 Density functional theory calculations revealed an enhanced stability of the mixed cation perovskite upon Gua incorporation exhibiting a highly negative formation enthalpy.38 Additionally, the introduction of Gua increases the number of hydrogen bonds in comparison to MA imposing the structural stability. Incorporation of Gua imparts strain on the crystal lattice due to the bulky nature of Gua cations. Hence, a change in the lattice structure occurs to accommodate Gua cations, which is reflected in the XRD measurements (Fig. 1i and j) showing a small shift with respect to the main reflection of pure MAPbI3. In addition to the stabilization effect provided by rapid Gua reorientation, the additional strain may adversely affect charge carrier stability. The improved stability and long charge carrier lifetime, diminishing the recombination rate and trap state density upon Gua incorporation have significant implications for the observed robust performance of the MA0.88Gua0.12PbI3 photodetector.36,38,51–54 This observation is in-line with the improved performance of mixed cation solar cells upon Gua incorporation.36,38,51–54
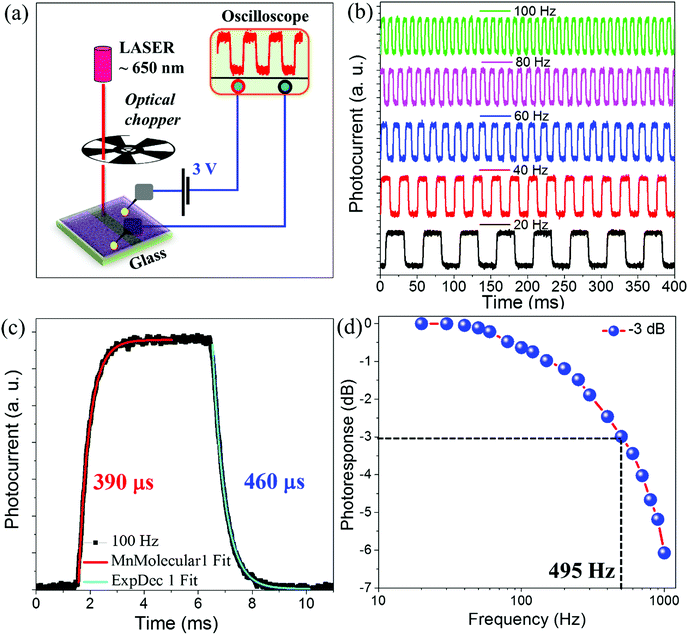
Fig. 3a illustrates the schematic representation of the response time measurement setup along with a laser (650 nm, 3 mW) and optical chopper. We have measured the transient photoresponse of MA0.88Gua0.12PbI3 RPMs based devices using an optical chopper with frequencies ranging from 20 Hz to 1000 Hz at 3 V applied bias. Fig. 3b shows the photoresponse of MA0.88Gua0.12PbI3 RPMs under modulated chopper frequencies of 20 Hz, 40 Hz, 60 Hz, 80 Hz, and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hz respectively. The photoresponse clearly manifests a sharp rise/fall and saturation of the photocurrent when the device is exposed periodically to light (light ON) and dark (light OFF) states. The photoswitching is successfully performed for 800 ON/OFF cycles at 100 Hz frequency (Fig. S8, ESI†). Fig. 3c represents a single ON/OFF cycle, where the rise time (tr) and fall time (tf) are estimated by fitting the photoresponse curve with exponential functions (ESI†).55 The rise and fall time of the MA0.88Gua0.12PbI3 photodetector is measured to be tr ∼ 390 μs and tf ∼ 460 μs respectively. Furthermore, we measured the normalized photoresponse as a function of frequency ranging from 20 to 1000 Hz (Fig. 3d). The cut-off frequency (f−3dB), the frequency at which the photoresponse becomes half of its initial value, is estimated to be ∼495 Hz. Such a higher value of f−3dB is beneficial for increasing the data extraction speed and to enhance the capture capacity of transient signals.
Hz respectively. The photoresponse clearly manifests a sharp rise/fall and saturation of the photocurrent when the device is exposed periodically to light (light ON) and dark (light OFF) states. The photoswitching is successfully performed for 800 ON/OFF cycles at 100 Hz frequency (Fig. S8, ESI†). Fig. 3c represents a single ON/OFF cycle, where the rise time (tr) and fall time (tf) are estimated by fitting the photoresponse curve with exponential functions (ESI†).55 The rise and fall time of the MA0.88Gua0.12PbI3 photodetector is measured to be tr ∼ 390 μs and tf ∼ 460 μs respectively. Furthermore, we measured the normalized photoresponse as a function of frequency ranging from 20 to 1000 Hz (Fig. 3d). The cut-off frequency (f−3dB), the frequency at which the photoresponse becomes half of its initial value, is estimated to be ∼495 Hz. Such a higher value of f−3dB is beneficial for increasing the data extraction speed and to enhance the capture capacity of transient signals.
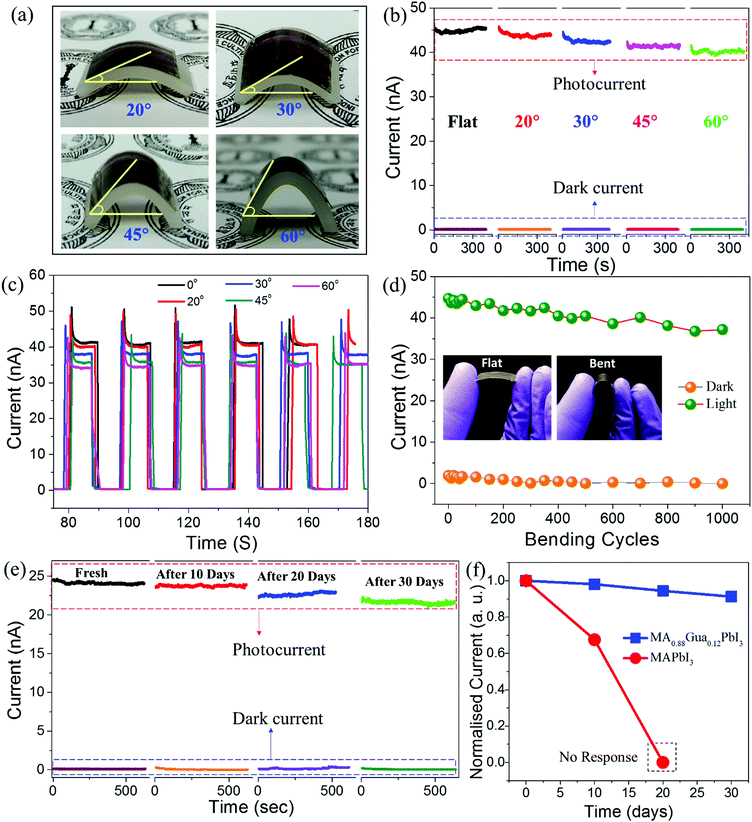
The mandate of next generation photodetectors is to sense light with excellent mechanical flexibility and environment stability. In order to realize a photoresponse under flexible conditions, we spray coated MA0.88Gua0.12PbI3 RPMs on flexible PET substrates with pre-fabricated Ag electrodes (Fig. S9, ESI†). The photodetector fabricated on PET is shaped into curved surfaces with various bending angles of 20°, 30°, 45° and 60° for photodetection (Fig. 4a). The photocurrent measurements are carried out in flat and bending conditions under the continuous illumination of 650 nm laser light (3 mW) at an applied bias voltage of 3 V for testing the mechanical flexibility of the devices. The photocurrent and dark current in flat and various bending conditions displayed a minimal change (Fig. 4b). This behavior is attributed to the strain tolerance of the perovskite RPMs under deformation. Furthermore, we evaluated the photoswitching reversibility at different bending angles, where current is recorded under ON/OFF cycles of light at 3 V (Fig. 4c). The device demonstrates excellent photoswitching reversibility over multiple ON/OFF cycles irrespective of the bending angles. The reliability and robustness of the devices is tested by performing light ON/OFF cycles at 60° bending angle for 1000 cycles. The corresponding dark/photocurrents are recorded on regular intervals and plotted as a function of number of bending cycles (Fig. 4d). The photoresponse is persistent and nearly unchanged under both the flat and bent conditions, which proves the robustness and mechanical flexibility of MA0.88Gua0.12PbI3 RPM based photodetectors. The performance characteristics of the photodetectors fabricated with a larger electrode gap of 100 μm on PET substrates were measured and calculated. A maximum responsivity of ∼275 mA W−1, detectivity of ∼2.43 × 1011 Jones and EQE of ∼68.2% were obtained. These values are lower compared to the devices fabricated with electrode gaps of 100 nm. Such a decrease of the photodetector performance parameters is expected owing to the longer channel length of the device.56,57 Furthermore, we have tested the environmental stabilities of MA0.88Gua0.12PbI3 and MAPbI3 RPMs based photodetectors over a period of 30 days under ambient conditions. The photocurrent is measured under the illumination of a white light source (∼8.2 mW cm−2) at a bias voltage of 0.9 V. The devices fabricated with MA0.88Gua0.12PbI3 showed a considerable stable response over 30 days under dark and light illumination (Fig. 4e). In comparison, MAPbI3 based photodetectors tested under similar conditions show a significantly decreased photoresponse after 10 days and the photoresponse completely degraded in 20 days (Fig. 4f and Fig. S10, ESI†). These measurements demonstrate the stability and mechanical flexibility along with excellent photodetection capabilities of the transparent MA0.88Gua0.12PbI3 RPM based perovskite photodetectors.
In summary, transparent and flexible photodetectors based on perovskite MA1−xGuaxPbI3 RPMs are fabricated for the first time. The RPMs fabricated over a large area of 5 × 5 cm2 using an ultra-fast spray coating method resulted in ∼50% transparency. Photodetectors fabricated with MA0.88Gua0.12PbI3 RPMs showed excellent FOMs with photosensitivity ∼41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500%, responsivity ∼187 A W−1, detectivity ∼2.23 × 1012 Jones, EQE ∼ 44
500%, responsivity ∼187 A W−1, detectivity ∼2.23 × 1012 Jones, EQE ∼ 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115% and response time of 390 μs. To the best of our knowledge, these FOMs are the best among the reported photodetectors fabricated with mixed cation perovskites. The RPM photodetectors displayed matching photoresponse for ∼1000 bending cycles over a wide range of bending angles demonstrating the robust mechanical flexibility. Furthermore, the Gua incorporated perovskites displayed prominently enhanced environment stability in comparison to the pristine MAPbI3. Unmatched performance, enhanced stability and mechanical flexibility combined with transparency of the MA1−xGuaxPbI3 photodetectors could open up new applications in transparent windows, security gadgets and wearable electronics. The preparation of transparent RPMs demonstrated here may be extended towards the fabrication of transparent smart windows for solar energy harvesting.
115% and response time of 390 μs. To the best of our knowledge, these FOMs are the best among the reported photodetectors fabricated with mixed cation perovskites. The RPM photodetectors displayed matching photoresponse for ∼1000 bending cycles over a wide range of bending angles demonstrating the robust mechanical flexibility. Furthermore, the Gua incorporated perovskites displayed prominently enhanced environment stability in comparison to the pristine MAPbI3. Unmatched performance, enhanced stability and mechanical flexibility combined with transparency of the MA1−xGuaxPbI3 photodetectors could open up new applications in transparent windows, security gadgets and wearable electronics. The preparation of transparent RPMs demonstrated here may be extended towards the fabrication of transparent smart windows for solar energy harvesting.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by SERB projects “EMR/2014/000664 & ECR/2017/003264”. We acknowledge the Technical Research Centre of IACS. G. S. K. and P. K. S. acknowledge the DST INSPIRE Programme for the fellowship. M. H. acknowledges the UGC for the fellowship.References
- Y. Ning, Z. Zhang, F. Teng and X. Fang, Small, 2018, 14, 1703754 CrossRef PubMed.
- M. Hossain, G. S. Kumar, S. N. B. Prabhava, E. D. Sheerin, D. McCloskey, S. Acharya, K. D. M. Rao and J. J. Boland, ACS Nano, 2018, 12, 4727–4735 CrossRef CAS PubMed.
- H. Deng, X. Yang, D. Dong, B. Li, D. Yang, S. Yuan, K. Qiao, Y.-B. Cheng, J. Tang and H. Song, Nano Lett., 2015, 15, 7963–7969 CrossRef CAS PubMed.
- X. Luo, F. Zhao, L. Du, W. Lv, K. Xu, Y. Peng, Y. Wang and F. Lu, npj Flexible Electron., 2017, 1, 6 CrossRef.
- R. Dong, C. Lan, F. Li, S. P. Yip and J. C. Ho, Nanoscale Horiz., 2019, 4, 1342–1352 RSC.
- X. Hu, X. Zhang, L. Liang, J. Bao, S. Li, W. Yang and Y. Xie, Adv. Funct. Mater., 2014, 24, 7373–7380 CrossRef CAS.
- S. Lim, M. Ha, Y. Lee and H. Ko, Adv. Opt. Mater., 2018, 6, 1800615 CrossRef.
- S. Lim, D. S. Um, M. Ha, Q. Zhang, Y. Lee, Y. Lin, Z. Fan and H. Ko, Nano Res., 2017, 10, 22–36 CrossRef CAS.
- Z. Wang, R. Yu, X. Wen, Y. Liu, C. Pan, W. Wu and Z. L. Wang, ACS Nano, 2014, 8, 12866–12873 CrossRef CAS PubMed.
- T. Yu, F. Wang, Y. Xu, L. Ma, X. Pi and D. Yang, Adv. Mater., 2016, 28, 4912–4919 CrossRef CAS PubMed.
- Y. Li, Z. Shi, W. Liang, L. Wang, S. Li, F. Zhang, Z. Zhuang Ma, Y. Wang, Y.-Z. Tian, D. Wu, X. Li, Y. Zhang, C.-X. Shan and X. Fang, Mater. Horiz., 2020 10.1039/c9mh01371g.
- Y. Zhang, W. Xu, X. Xu, J. Cai, W. Yang and X. Fang, J. Phys. Chem. Lett., 2019, 10, 836–841 CrossRef CAS PubMed.
- N. K. Kumawat, A. Swarnkar, A. Nag and D. Kabra, J. Phys. Chem. C, 2018, 122, 13767–13773 CrossRef CAS.
- A. H. Proppe, M. H. Elkins, O. Voznyy, R. D. Pensack, F. Zapata, L. V. Besteiro, L. N. Quan, R. Quintero-Bermudez, P. Todorovic, S. O. Kelley, A. O. Govorov, S. K. Gray, I. Infante, E. H. Sargent and G. D. Scholes, J. Phys. Chem. Lett., 2019, 10, 419–426 CrossRef PubMed.
- A. Sarkar, P. Acharyya, R. Sasmal, P. Pal, S. S. Agasti and K. Biswas, Inorg. Chem., 2018, 57, 15558–15565 CrossRef CAS PubMed.
- M. Saliba, J.-P. Correa-Baena, C. M. Wolff, M. Stolterfoht, N. Phung, S. Albrecht, D. Neher and A. Abate, Chem. Mater., 2018, 30, 4193–4201 CrossRef CAS.
- G. S. Kumar, B. Pradhan, T. Kamilya and S. Acharya, Bull. Chem. Soc. Jpn., 2018, 91, 1241–1248 CrossRef CAS.
- C. H. Lin, B. Cheng, T. Y. Li, J. R. D. Retamal, T. C. Wei, H. C. Fu, X. Fang and J. H. He, ACS Nano, 2019, 13, 1168–1176 CAS.
- J. Miao and F. Zhang, J. Mater. Chem. C, 2019, 7, 1741–1791 RSC.
- Z.-K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y. (Michael) Yang, M. Wei, B. R. Sutherland, E. H. Sargent and J. You, Nat. Commun., 2017, 8, 15640 CrossRef CAS PubMed.
- L. K. Ono, E. J. Juarez-Perez and Y. Qi, ACS Appl. Mater. Interfaces, 2017, 9, 30197–30246 CrossRef CAS PubMed.
- F. Xu, T. Zhang, G. Li and Y. Zhao, J. Mater. Chem. A, 2017, 5, 11450–11461 RSC.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Acc. Chem. Res., 2019, 52, 3233–3243 CrossRef CAS PubMed.
- B. Conings, J. Drijkoningen, N. Gauquelin, A. Babayigit, J. D’Haen, L. D’Olieslaeger, A. Ethirajan, J. Verbeeck, J. Manca, E. Mosconi, F. D. Angelis and H.-G. Boyen, Adv. Energy Mater., 2015, 5, 1500477 CrossRef.
- P. Gratia, G. Grancini, J.-N. Audinot, X. Jeanbourquin, E. Mosconi, I. Zimmermann, D. Dowsett, Y. Lee, M. Grätzel, F. De Angelis, K. Sivula, T. Wirtz and M. K. Nazeeruddin, J. Am. Chem. Soc., 2016, 138, 15821–15824 CrossRef CAS PubMed.
- E. T. Hoke, D. J. Slotcavage, E. R. Dohner, A. R. Bowring, H. I. Karunadasa and M. D. McGehee, Chem. Sci., 2014, 6, 613–617 RSC.
- J.-P. Correa-Baena, A. Abate, M. Saliba, W. Tress, T. J. Jacobsson, M. Grätzel and A. Hagfeldt, Energy Environ. Sci., 2017, 10, 710–727 RSC.
- B. Pradhan, G. S. Kumar, S. Sain, A. Dalui, U. K. Ghorai, S. K. Pradhan and S. Acharya, Chem. Mater., 2018, 30, 2135–2142 CrossRef CAS.
- Z. Zheng, Q. Hu, H. Zhou, P. Luo, A. Nie, H. Zhu, L. Gan, F. Zhuge, Y. Ma, H. Song and T. Zhai, Nanoscale Horiz., 2019, 4, 1372–1379 RSC.
- Y. Zhang, S. Li, W. Yang, M. K. Joshi and X. Fang, J. Phys. Chem. Lett., 2019, 10, 2400–2407 CrossRef CAS PubMed.
- T. Yang, F. Li and R. Zheng, ACS Appl. Electron. Mater., 2019, 1, 1348–1366 CrossRef CAS.
- G. Kieslich, S. Sun and A. K. Cheetham, Chem. Sci., 2014, 5, 4712–4715 RSC.
- V. M. Goldschmidt, Naturwissenschaften, 1926, 14, 477–485 CrossRef CAS.
- D. J. Kubicki, D. Prochowicz, A. Hofstetter, M. Saski, P. Yadav, D. Bi, N. Pellet, J. Lewiński, S. M. Zakeeruddin, M. Grätzel and L. Emsley, J. Am. Chem. Soc., 2018, 140, 3345–3351 CrossRef CAS PubMed.
- N. De Marco, H. Zhou, Q. Chen, P. Sun, Z. Liu, L. Meng, E.-P. Yao, Y. Liu, A. Schiffer and Y. Yang, Nano Lett., 2016, 16, 1009–1016 CrossRef PubMed.
- O. Nazarenko, M. R. Kotyrba, S. Yakunin, M. Aebli, G. Rainò, B. M. Benin, M. Wörle and M. V. Kovalenko, J. Am. Chem. Soc., 2018, 140, 3850–3853 CrossRef CAS PubMed.
- A. D. Jodlowski, C. Roldán-Carmona, G. Grancini, M. Salado, M. Ralaiarisoa, S. Ahmad, N. Koch, L. Camacho, G. de Miguel and M. K. Nazeeruddin, Nat. Energy, 2017, 2, 972–979 CrossRef CAS.
- W. Yu, X. Jia, Y. Long, L. Shen, Y. Liu, W. Guo and S. Ruan, ACS Appl. Mater. Interfaces, 2015, 7, 9920–9928 CrossRef CAS PubMed.
- K.-S. Chen, J.-F. Salinas, H.-L. Yip, L. Huo, J. Hou and A. K.-Y. Jen, Energy Environ. Sci., 2012, 5, 9551–9557 RSC.
- W. Wang, Y. Ma and L. Qi, Adv. Funct. Mater., 2017, 27, 1603653 CrossRef.
- L. Dou, Y. (Micheal) Yang, J. You, Z. Hong, W.-H. Chang, G. Li and Y. Yang, Nat. Commun., 2014, 5, 5404 CrossRef CAS PubMed.
- T. Zhang, J. Wu, P. Zhang, W. Ahmad, Y. Wang, M. Alqahtani, H. Chen, C. Gao, Z. D. Chen, Z. Wang and S. Li, Adv. Opt. Mater., 2018, 6, 1701341 CrossRef.
- S. C. Kung, W. E. van der Veer, F. Yang, K. C. Donavan and R. M. Penner, Nano Lett., 2010, 10, 1481–1485 CrossRef CAS PubMed.
- Y. Li, Z. F. Shi, S. Li, L. Z. Lei, H. F. Ji, D. Wu, T. T. Xu, Y. T. Tian and X. J. Li, J. Mater. Chem. C, 2017, 5, 8355–8360 RSC.
- D. G. Georgiadou, Y.-H. Lin, J. Lim, S. Ratnasingham, M. A. McLachlan, H. J. Snaith and T. D. Anthopoulos, Adv. Funct. Mater., 2019, 29, 1901371 CrossRef.
- F. Yao, P. Gui, C. Chen, B. Li, R. Li, C. Tao, Q. Lin and G. Fang, ACS Appl. Mater. Interfaces, 2019, 11, 39875–39881 CrossRef CAS PubMed.
- Y. Wang, X. Zhang, D. Wang, X. Li, J. Meng, J. You, Z. Yin and J. Wu, ACS Appl. Mater. Interfaces, 2019, 11, 28005–28012 CrossRef CAS PubMed.
- S. Tong, C. Gong, C. Zhang, G. Liu, D. Zhang, C. Zhou, J. Sun, S. Xiao, J. He, Y. Gao and J. Yang, Appl. Mater. Today, 2019, 15, 389–397 CrossRef.
- J. Gong, M. Yang, X. Ma, R. D. Schaller, G. Liu, L. Kong, Y. Yang, M. C. Beard, M. Lesslie, Y. Dai, B. Huang, K. Zhu and T. Xu, J. Phys. Chem. Lett., 2016, 7, 2879–2887 CrossRef CAS PubMed.
- K. Poorkazem and T. L. Kelly, Sustainable Energy Fuels, 2018, 2, 1332–1341 RSC.
- R. J. Stoddard, A. Rajagopal, R. L. Palmer, I. L. Braly, A. K. Y. Jen and H. W. Hillhouse, ACS Energy Lett., 2018, 3, 1261–1268 CrossRef CAS.
- S. Wang, Y. Zhu, W. Sun, X. Miao, Z. Ma, C. Yang, B. Liu, S. Li, R. Ma and C. Wang, Sol. Energy, 2018, 176, 118–125 CrossRef CAS.
- N. D. Pham, C. Zhang, V. T. Tiong, S. Zhang, G. Will, A. Bou, J. Bisquert, P. E. Shaw, A. Du, G. J. Wilson and H. Wang, Adv. Funct. Mater., 2019, 29, 1806479 CrossRef.
- A. Sharma, B. Bhattacharyya, A. K. Srivastava, T. D. Senguttuvan and S. Husale, Sci. Rep., 2016, 6, 19138 CrossRef CAS PubMed.
- Y. Peng, F. Guo, H. Xia, W. Lv, L. Sun, S. Xu, H. Zhu, X. Chen, C. Liu, Y. Wang and F. Lu, Appl. Opt., 2019, 58, 1319–1326 CrossRef CAS PubMed.
- Y. Wang, Z. Xia, S. Du, F. Yuan, Z. Li, Z. Li, Q. Dai, H. Wang, S. Luo, S. Zhang and H. Zhou, Nanotechnology, 2016, 27, 175201 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional methods, tables and figures. See DOI: 10.1039/c9nh00774a |
| This journal is © The Royal Society of Chemistry 2020 |