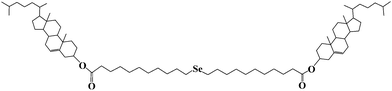
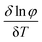A redox-responsive organogel based on a selenium-containing low molecular mass gelator†
Haojie
Zhang
,
Xiuping
Lu
,
Zhao
Chen
,
Jianzhong
Jiang
 * and
Yukai
Chen
* and
Yukai
Chen
The Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu, P. R. China. E-mail: jzjiang@jiangnan.edu.cn
First published on 28th November 2019
Abstract
To develop responsive organogels, a low molecular mass gelator (Chol-Se) with selenium as its redox-active center was designed and synthesized. Chol-Se could form thermally reversible physical gels in several organic solvents. By controlling the redox state of the selenide group in Chol-Se, responsive transformation of Chol-Se in ethyl acetate between the gelation and solution was achieved. Chol-Se will serve as an excellent smart material with potential applications in sensors, recognition, and separation.
Organogels are semi-solid materials with an organic solvent phase entrapped within a three-dimensional network of self-assembled, intertwined gelator fibers.1–7 Low molecular mass organic gelators (LMOGs) have gained increasing interest in recent studies due to their viscoelasticity, non-birefringence, thermoreversibility, thermostability, optical clarity, chirality effects, and biocompatibility.1,2,5 The driving force for the spontaneous formation of the gel could be non-covalent interactions, such as hydrogen bonding, electrostatic interactions, host–guest interactions, π–π stacking, and van der Waals forces.5–7 For example, cholesterol is a lipid-type rigid molecule with a steroid skeleton of four fused rings. In some cholesterol-containing LMOGs, the covalent bond in the cholesteryl group induces self-association through van der Waals interactions.8–11
Recently, stimulus-responsive gels have attracted widespread attention as new functional materials.12–15 Development of these external stimuli responsive gels may lead to novel smart materials, which is highly desirable for advanced applications in drug delivery, sensors, and oil spillage.16 The reversible transformation of the gels could be induced and controlled by heating, light, electricity, ultrasonication, shear stimulus, redox, and so on.17–21
Among the above stimuli, the redox reaction is of particular interest in the application of artificial muscles and electrorheological fluids.22,23 Various redox moieties including tetrathiafulvalene,24,25 ferrocene,26 thiophene,27 disulphide bonds,28 and benzoylpyridinium29 have been successfully inserted into the molecular structures of LMOGs.
Selenium is an essential microelement for humans and its deficiency may result in different types of diseases.30 Very recently, selenium has received widespread attention as a new redox-responsive functional group.30–33 Compared with other redox moieties, selenium-containing molecules are more susceptible to redox stimuli because of lower bond energies (Se–C 244 kJ mol−1 and Se–Se 172 kJ mol−1).33 Therefore, suitable Se-based compounds might be promising redox-responsive LMOGs. Nevertheless, to the best of our knowledge, the LMOGs based on low molecule mass selenium-containing organogelators have been less documented.
In this paper, a new LMOG with selenium as its redox-active center was designed and synthesized. The gelator (bis-(undecanoic acid cholesteryl ester)-selenide, Chol-Se), which consists of a cholesteryl moiety and a selenide group, can gel several organic solvents (Scheme 1). Reversible sol–gel phase transition by redox reaction can be achieved.
Chol-Se was synthesized from selenium, 11-bromoundecanoic acid, and cholesterol through three reaction steps. The detailed procedure is described in the ESI† (Scheme S1). The gelator was characterized by 1H NMR spectroscopy (Fig. S1 and S2, ESI†).
The gelation ability of Chol-Se was evaluated in different organic solvents. The mixtures were initially heated to dissolve the gelator molecules, followed by cooling to room temperature. The gelation behavior was evaluated by the “inverse flow” method. The gelation testing results and the critical gelation concentration (CGC, mg mL−1) data are summarized in Table 1. The results show that Chol-Se is a good gelator. Chol-Se can gelate nonpolar solvents, such as hexane, heptane, octane, and dodecane, polar protic solvents, such as pentanol, hexanol, octanol, and benzyl alcohol, and polar aprotic solvents, such as ethyl acetate (Fig. S3, ESI†).
| Solvent | CGC (mg mL−1) | Solvent | CGC (mg mL−1) |
|---|---|---|---|
| S or P = solution or precipitation; G = gel. | |||
| Methanol | P | Cyclohexane | S |
| Ethanol | P | n-Hexane | G(10) |
| Isopropanol | P | n-Heptane | G(10) |
| Butanol | P | n-Octane | G(10) |
| 1-Pentanol | G(17) | n-Decane | G(9) |
| 1-Hexanol | G(16) | Dodecane | G(8) |
| Benzyl alcohol | G(23) | Benzene | S |
| 1-Octanol | G(11) | Methylbenzene | S |
| Acetonitrile | S | Dioxane | S |
| DMF | P | Tetrahydrofuran | S |
| Ethyl acetate | G(18) | Pyridine | S |
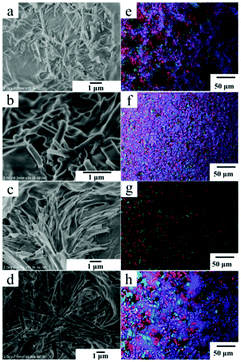
The morphologies of the xerogels of Chol-Se in different alcohol solvents were examined by scanning electron microscopy (SEM). Straight short rob-shaped fibers were observed in pentanol and hexanol (Fig. 1a and b), and a bunch of acicular fibers were formed in benzyl alcohol and octanol (Fig. 1c and d). The increasing carbon number of the alcohol solvent is in favor of longitudinal growth of the fibers because of van der Waals forces. Polarizing microscopes can be used to observe the anisotropy of the gel. As shown in Fig. 1e–h, the gel of Chol-Se formed in the alcohol solutions has typical anisotropic properties with a remarkable polarizing phenomenon. The gel formed in benzyl alcohol has a regular black cross extinction phenomenon compared to the other gels, which indicated the formation of a spherulitic radial arrangement of fibers in benzyl alcohol.
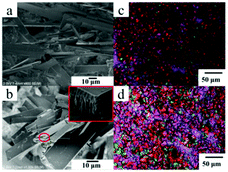
As shown in Fig. 2, lamellar structures with a width of 10–30 μm and thickness of 0.8–1 μm can be formed in n-decane and n-dodecane. In the partial enlargement of the upper right corner of Fig. 2b, there are regular dentate arrangements of aggregates at the fracture of the aggregate, which indicate a regular hollow accumulation of Chol-Se in n-dodecane. Anisotropy and ordered aggregates were observed in both Chol-Se/n-decane and Chol-Se/n-dodecane gel systems (Fig. 2c and d).
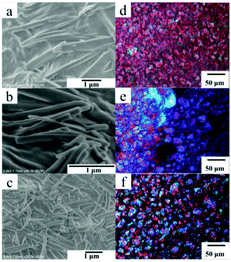
The morphologies of gels formed in ethyl acetate were also examined by SEM and polarizing optical light microscope (POM). The Chol-Se molecules are self-assembled into rod-like nanofibers with a width of 300 nm, which were observed in Fig. 3a–c. With an increase in the gelator concentration, the fibers became closer, and banded aggregates were observed (Fig. 3e and f), which means that the network structures formed by entangled fibers were gradually put in order. As described above, high concentration of gelator led to considerable thermal stability, which is due to the enhancement of the network structure stability. Similar to the literature,34,35 the polarity of the solvent induced regulation of the microstructures of the gel 3D networks from three-dimensional nanofiber structures to lamellar structures as the solvents changed from alcohols and ethyl acetate to alkanes.
The effect of the gelator concentration was also measured by thermal analysis. An effective method of studying thermal stability is to measure the phase transition temperature of the gel, and a higher phase transformation temperature means better thermal stability. As shown in Fig. S4a (ESI†), the relationship between phase transformation temperature (Tg) and the concentration of the gelator was observed. Each point on the curve suggests the lowest gelator concentration to form a network at this temperature. The sol–gel transition temperatures for Chol-Se increased with increasing gelator concentration, and a plateau finally appeared. The gelator tended to crystallize rather than gelate when the environment temperature was higher than the gelation temperature. With an increase in gelator concentration, the three-dimensional network constructed by the gelator will be destroyed at a higher temperature, which suggests that the gel is more stable.
The thermodynamic parameters of gelation were calculated using the following equations:36
ΔG° = RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) φ φ | (1) |
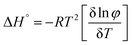 | (2) |
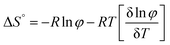 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) φ)/δT] of Fig. S4b (ESI†) was plotted for the gel obtained from ethyl acetate. The thermodynamic parameters of the gel from ethyl acetate were calculated (Table 2). The result suggested the gel formation was a spontaneous process because of the negative value of the Gibbs free energy.
φ)/δT] of Fig. S4b (ESI†) was plotted for the gel obtained from ethyl acetate. The thermodynamic parameters of the gel from ethyl acetate were calculated (Table 2). The result suggested the gel formation was a spontaneous process because of the negative value of the Gibbs free energy.
The inter-planar distance can be determined by XRD. Therefore, the gelator molecular packing model can be judged by comparing the d value and the length of the gelator molecule. As shown in Fig. S5 (ESI†), the XRD spectra shows three strong diffraction peaks at 2θ = 4.3°, 6.5° and 8.0°. Their corresponding d spacings are d1 = 20.56 Å, d2 = 13.62 Å and d3 = 10.89 Å, and the ratio of d values is 1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/3
1/3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/4. This result accounts for the formation of a layered structure of Chol-Se in ethyl acetate in which the layer spacing is 41.12 Å, which is shorter than the molecular length of Chol-Se at 46.64 Å. The ordered aggregation of Chol-Se molecules depends on the van der Waals force provided by the cholesterol groups and the rigid skeleton part of the cholesterol groups that are overlapped with each other.37,38 Therefore, a possible accumulation model of the Chol-Se molecules in ethyl acetate is shown in Fig. 4.
1/4. This result accounts for the formation of a layered structure of Chol-Se in ethyl acetate in which the layer spacing is 41.12 Å, which is shorter than the molecular length of Chol-Se at 46.64 Å. The ordered aggregation of Chol-Se molecules depends on the van der Waals force provided by the cholesterol groups and the rigid skeleton part of the cholesterol groups that are overlapped with each other.37,38 Therefore, a possible accumulation model of the Chol-Se molecules in ethyl acetate is shown in Fig. 4.
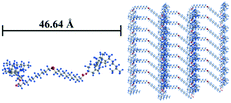 | ||
| Fig. 4 The molecular structure of Chol-Se calculated by Gaussian (left) and the possible accumulation model of the gelator molecules in ethyl acetate (right). | ||
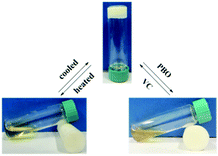
In addition to thermal sensitivity, the organogel of Chol-Se in ethyl acetate has a redox-responsive ability. It is well-known that the selenide group can be transformed to selenoxide via oxidation and then reversible transition via reduction. As shown in Fig. 5, when an equal amount (in moles) of dibenzoyl peroxide (BPO) was added, the organogel gradually turned into a suspension. Afterwards, an equivalent amount (in moles) of ascorbic acid (VC) was added; then, the organogel could be reformed at room temperature after slight heating. Therefore, the sol–gel phase transition of the organogel of Chol-Se in ethyl acetate could occur by redox reaction.
The redox reaction transformation was measured by 77Se-NMR and MS spectroscopy (Fig. 6). The chemical shifts of 77Se-NMR obviously shifted from 160 to 871 ppm because of the formation of selenoxide groups after oxidation. Meanwhile, the molecular ion peak increases from 1187 (M + H+) to 1203 (M + H+) in ESI-MS (Fig. 6b), which also indicated the oxidization of selenides to selenoxides.
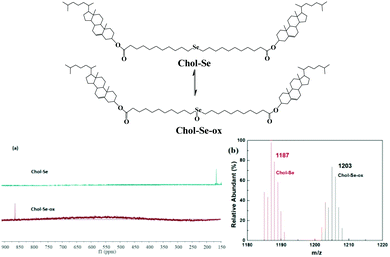 | ||
| Fig. 6 The structure changes of Chol-Se by redox reaction. 77Se NMR (a) and MS (b) spectra of Chol-Se and Chol-Se-Ox. | ||
As shown in Fig. 7, the rod-shaped nanofibers of the Chol-Se gel (Fig. 6a) were transformed to a disordered accumulation of Chol-Se-Ox precipitation (Fig. 7b), which is consistent with the sol–gel transition results after redox reaction.
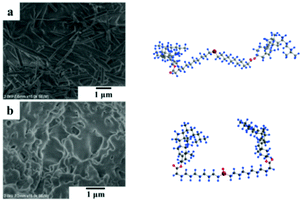 | ||
| Fig. 7 (a) SEM image of the gel of Chol-Se in ethyl acetate, and (b) SEM image of precipitation of Chol-Se-Ox in ethyl acetate. | ||
The space configurations of Chol-Se and Chol-Se-Ox were calculated by Gaussian, and the results are shown in the right corner of Fig. 7. The molecular space configuration of Chol-Se is W-shaped, and for Chol-Se-Ox, it is U-shaped. The reason for these phenomena is that the addition of BPO transformed the selenide group into a selenoxide group and changed the molecular space configuration from W-shaped to U-shaped, which led to a laminated gelator that cannot easily maintain an orderly arrangement after its change in molecular structure. Therefore, the three-dimensional network of the gel collapsed.
In summary, a novel LMOG containing selenide and a cholesterol group was designed and synthesized. The gelator can form gels in several organic solvents. The thermodynamic parameters of the gelation process were calculated, and the results indicate the gel formation is a spontaneous process. The gel formed in ethyl acetate was characterized by DSC, SEM, POM and XRD. The XRD spectrum indicates a layered structure of Chol-Se in ethyl acetate, and the layer spacing is 41.12 Å, which is consistent with the calculation in Gaussian. Moreover, Chol-Se can undergo a reversible sol–gel transition in ethyl acetate by adding VC and BPO. Further studies and the application of Chol-Se in sensors, recognition, and separation are underway.
This work was supported by the National Natural Science Foundation of China (No. 21872064, 21473080, 20901032).
Conflicts of interest
There are no conflicts to declare.Notes and references
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3159 CrossRef CAS PubMed.
- D. J. Abdallah and R. G. Weiss, Adv. Mater., 2000, 12, 1237–1247 CrossRef CAS.
- F. M. Menger and K. L. Caran, J. Am. Chem. Soc., 2000, 122, 11679–11691 CrossRef CAS.
- J. H. van Esch and B. L. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263–2266 CrossRef CAS PubMed.
- M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS PubMed.
- N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- W. Cai, G. T. Wang, P. Du, R. X. Wang, X. K. Jiang and Z. T. Li, J. Am. Chem. Soc., 2008, 130, 13450–13459 CrossRef CAS PubMed.
- E. Ostuni, P. Kamaras and R. G. Weiss, Angew. Chem., Int. Ed. Engl., 1996, 35, 1324–1326 CrossRef CAS.
- L. Lu and R. G. Weiss, Langmuir, 1995, 11, 3630–3632 CrossRef CAS.
- P. Terech, E. Ostuni and R. G. Weiss, J. Phys. Chem., 1996, 100, 3759–3766 CrossRef CAS.
- K. Murata, M. Aoki, T. Suzuki, T. Harada, H. Kawabata, T. Komori, F. Ohseto, K. Ueda and S. Shinkai, J. Am. Chem. Soc., 1994, 116, 6664–6676 CrossRef CAS.
- Y. Nagai, L. D. Unsworth, S. Koutsopoulos and S. Zhang, J. Controlled Release, 2006, 115, 18–25 CrossRef CAS PubMed.
- C. Li, J. Madsen, S. P. Armes and A. L. Lewis, Angew. Chem., Int. Ed., 2006, 45, 3510–3513 CrossRef CAS PubMed.
- J. A. Cox and K. S. Alber, J. Electrochem. Soc., 1996, 143, 126–128 CrossRef.
- S. Tao, Y. Wang and Y. An, J. Mater. Chem., 2011, 21, 11901–11907 RSC.
- M. Fourmigué and P. Batail, Chem. Rev., 2004, 104, 5379–5418 CrossRef PubMed.
- X. Yang, G. Zhang and D. Zhang, J. Mater. Chem., 2012, 22, 38–50 RSC.
- J. O. Jeppesen, J. Perkins, J. Becher and J. Fraser, Angew. Chem., Int. Ed., 2001, 40, 1216–1221 CrossRef CAS PubMed.
- S. R. Jadhav, P. K. Vemula, R. Kumar, S. R. Raghavan and G. John, Angew. Chem., 2010, 122, 7861–7864 CrossRef.
- T. Naota and H. Koori, J. Am. Chem. Soc., 2005, 127, 9324–9325 CrossRef CAS PubMed.
- H. Engelkamp, S. Middelbeek and R. J. M. Nolte, Science, 1999, 284, 785–788 CrossRef CAS PubMed.
- Z. Sun, Q. Huang, T. He, Z. Li, Y. Zhang and L. Yi, ChemPhysChem, 2014, 15, 2421–2430 CrossRef CAS PubMed.
- X. Sui, X. Feng, M. Hempenius and G. J. Vancso, J. Mater. Chem. B, 2013, 1, 1658–1672 RSC.
- C. Wang, D. Q. Zhang and D. B. Zhu, J. Am. Chem. Soc., 2005, 127, 16372–16373 CrossRef CAS PubMed.
- C. Wang, Q. Chen, F. Sun, D. Zhang, G. Zhang, Y. Huang, R. Zhao and D. Zhu, J. Am. Chem. Soc., 2010, 132, 3092–3096 CrossRef CAS PubMed.
- J. Liu, P. He, J. Yan, X. H. Fang, J. X. Peng, K. Q. Liu and Y. Fang, Adv. Mater., 2008, 20, 2508–2511 CrossRef CAS.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS PubMed.
- M. Matteucci, G. Bhalay and M. Bradley, J. Pept. Sci., 2004, 10, 318–325 CrossRef CAS PubMed.
- K. Hatakeyama-Sato, R. Ichinoi, Y. Sasada, Y. Sasaki, K. Oyaizu and H. Nishide, Chem. Lett., 2019, 48, 555–557 CrossRef CAS.
- T. Sun, Y. Jin, R. Qi, S. Peng and B. Fan, Polym. Chem., 2013, 4, 4017–4023 RSC.
- H. P. Xu, W. Cao and X. Zhang, Acc. Chem. Res., 2013, 46, 1647–1658 CrossRef CAS PubMed.
- Y. Zhang, C. Yang, S. Guo, H. Chen and X. Liu, Chem. Commun., 2016, 52, 12717–12720 RSC.
- Y. Zhou, K. Jie and F. H. Huang, Chem. Commun., 2017, 53, 8364–8367 RSC.
- Y. Lan, M. G. Corradini, R. G. Weiss, S. R. Raghavan and M. A. Rogers, Chem. Soc. Rev., 2015, 44, 6035–6058 RSC.
- Z. Zhang, S. Zhang, J. Zhang, L. Zhu and D. Qu, Tetrahedron, 2017, 73, 4891–4895 CrossRef CAS.
- J. Naskar, G. Palui and A. Banerjee, J. Phys. Chem. B, 2009, 113, 11787–11792 CrossRef CAS PubMed.
- M. K. Nayak, B. H. Kim, J. E. Kwon, S. Park, J. Seo, J. W. Chung and S. Y. Park, Chem. – Asian J., 2010, 16, 7437–7447 CAS.
- S. Yagai, Y. Nakano, S. Seki, T. Okubo, T. Isoshima, T. Karatsu, A. Kitamura and Y. Kikkawa, Angew. Chem., 2010, 122, 10186–10190 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nj04248b |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2020 |