Boron clusters as a platform for new materials: composites of nucleic acids and oligofunctionalized carboranes (C2B10H12) and their assembly into functional nanoparticles†
Damian
Kaniowski
 a,
Katarzyna
Ebenryter-Olbinska
a,
Katarzyna
Ebenryter-Olbinska
 a,
Katarzyna
Kulik
a,
Katarzyna
Kulik
 a,
Slawomir
Janczak
b,
Anna
Maciaszek
a,
Slawomir
Janczak
b,
Anna
Maciaszek
 a,
Katarzyna
Bednarska-Szczepaniak
a,
Katarzyna
Bednarska-Szczepaniak
 b,
Barbara
Nawrot
b,
Barbara
Nawrot
 *a and
Zbigniew
Lesnikowski
*a and
Zbigniew
Lesnikowski
 *b
*b
aCentre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Sienkiewicza 112, 90-363 Lodz, Poland. E-mail: bnawrot@cbmm.lodz.pl
bInstitute of Medical Biology, Polish Academy of Sciences, 106 Lodowa St., 92-232 Lodz, Poland. E-mail: zlesnikowski@cbm.pan.pl
First published on 30th October 2019
Abstract
Nucleic acids are key biomolecules in all life forms. These biomolecules can encode and transfer information via Watson-Crick base-pairing interactions and can form double-stranded structures between complementary sequences with high precision. These properties make nucleic acids extremely successful in applications in materials science as nanoconstruction materials. Herein, we describe a method for the automated synthesis of “oligopeds”, which are building blocks based on the boron cluster structure equipped with short DNA adapters; these building blocks assemble into functional nanoparticles. The obtained, well defined, torus-like structures are the first DNA nanoconstructs based on a boron cluster scaffold. The results indicate the potential of boron clusters in DNA nanoconstruction and open the way for the design of entirely new types of buildings blocks based on polyhedral heteroborane geometry and its unique properties. The use of antisense oligonucleotides as DNA adapters illustrates one of the possible applications of the obtained nanoconstructs as vectors for therapeutic nucleic acids.
Introduction
Nucleic acids, while retaining their status as “the molecules of life”, are becoming “molecular wires”, i.e., materials for the construction of molecular structures at the junction between the biological and inorganic worlds.1,2 Natural as well as modified nucleic acids are used in practice. Unmodified nucleic acids are easily accessible because of the availability of automated chemical methods for nucleic acid synthesis, but their properties are ordinary. Searches for nucleic acids with new or improved properties based on modifications of natural sequences are being performed. The two main approaches to nucleic acid modifications focus on two different aims. The first approach centers on improving or modulating the natural features of nucleic acids, such as the binding affinity towards the complementary strand, stability in a biological environment, and performance of specific activities. This approach is primarily used if the modified nucleic acids are intended to perform their natural or nearly natural functions in biological systems, e.g., serving as antisense oligonucleotides, ribozymes, interfering RNAs or primers.3The aim of the second approach is the incorporation of new, “unnatural” properties, which is usually performed by adding suitable labels and modifying units in nucleic acids. These properties include fluorescence, luminescence, specific redox activity, radioactivity, and specific affinity-bearing residues. In most cases, the purpose of these modifications is to equip the nucleic acid molecule that functions as a molecular probe with components that provide detectable signals under designed conditions. In these technological rather than biological applications, modified nucleic acids are often used for the construction of diagnostic tests or biosensors. Recently, unmodified and modified DNA fragments have been extensively explored as building blocks for nanotechnology. In this case, the modifying units often act as scaffolds in the creation of structures with designed two-dimensional (2D) and three-dimensional (3D) DNA assembly.4
One of the original openings in nanoscale construction was derivatives composed of boron cluster (polyhedral boron hydrides) components. These derivatives were used to build molecular rotors,5 nanovehicles,6 nanopincers7 and artificial ion transporters across biological membranes.8
Herein, we propose the use of boron clusters as a platform in the design of a new type of bioinorganic construct functionalized with nucleic acids. Based on our previous studies on nucleic acid–boron cluster conjugates,9–11 we designed “oligopeds”,12 which are conjugates of short pieces of DNA and oligofunctionalized boron clusters (carboranes, C2B10H12) that serve as building blocks for the construction of two- (2D) and three-dimensional (3D) nanoframeworks. The design of these oligopeds is based on a rigid, ball-like structure of boron clusters of the type C2B10H12, which allows for precise spatial organization of the attached DNA oligonucleotides at the molecular level (Fig. S1†). The synthesis of the boron cluster building blocks (“oligopeds”) tethered with heterooligonucleotide adapters, their assembly into self-organized nanoparticles and the study of the obtained nanoconstructs are described. For the oligonucleotide adapter, an antisense oligomer targeting the epidermal growth factor receptor (EGFR) gene, which is overexpressed in several tumor types, was used.
Results and discussion
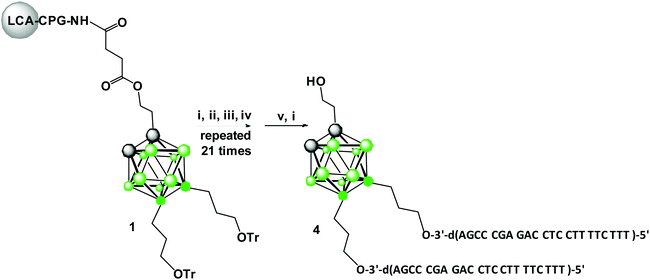
Synthesis of 1,2-dicarba-closo-dodecaborane tripeds 4 and 5 9,12-bis-functionalized with DNA 22-mer oligonucleotides
The boron-cluster-modified solid support 1 (Scheme 1) used for the automated synthesis of building blocks (oligopeds) containing the boron-cluster scaffold functionalized with short pieces of DNA-oligomers (adapters) was prepared as described previously in ref. 12.The improved method for the synthesis of key intermediate 9,12-di(3-O-tritylprop-1-yl)-1,2-dicarba-closo-dodecaborane based on alkylation of 9,12-diiodo-1,2-dicarba-closo-dodecaborane with Grignard reagent, 1-trityloxypropylmagnesium bromide was used (Scheme S1,† details of the synthesis and compound characteristics are described in ESI, procedures S1–S3, Fig. S6–S13†).12–14
Oligonucleotide synthesis on the modified support 1 was performed using standard phosphoramidite chemistry (Scheme 1),15 except that the nucleoside phosphoramidite monomers were base-protected with the easily removable (ultra-mild) acetyl group (Ac) for dC, the phenoxyacetyl group (Pac) for dA and the tert-butylphenoxyacetyl group (tBPac) for dG to avoid potential deboronation of the carborane cluster of the tripeds under standard alkaline deprotection conditions. The deboronation reaction leading to closo-/nido-transformation of 1,2-dicarba-closo-dodecaborane-modified DNA-oligonucleotides was previously observed.9
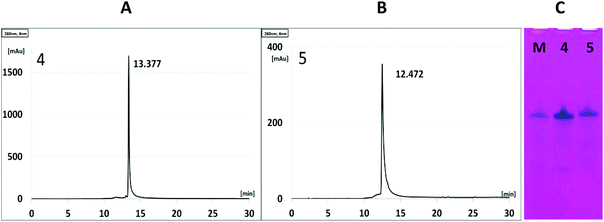
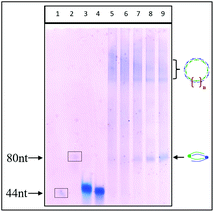
The cleavage of 5′-DMTr-oligonucleotides from the solid support and their deprotection were performed according to two steps “trityl on”/“trityl off” procedure (see Materials and methods). Consequently, triped 4 was obtained bearing two 22-mer oligodeoxyribonucleotide strands with the following sequence 5′-d(TTT CTT TTC CTC CAG AGC CCGA)-3′, which is complementary (antisense) to the mRNA of the EGFR fragment. Analogously, triped 5 (Table 1) was synthesized bearing two oligodeoxyribonucleotide strands with the sequence 5′-d(TCG GGC TCT GGA GGA AAA GAAA)-3′, which is complementary to triped 4. The homogeneity of both tripeds 4 and 5, bis-functionalized with complementary DNA fragments, was confirmed by analytical RP-HPLC (Fig. 1A and B) and PAGE (Fig. 1C), and the integrity of these tripeds was analyzed by UV-VIS and ESI-Q-TOF/MALDI-TOF mass spectrometry (Table 1, Fig. S3†). Though in the present work natural PO-oligonucleotides were used as components of the triped building blocks, one can envision application of the state-of-the-art antisense oligonucleotide backbone modifications such as phosphorothioate (PS) or 2′-O-methyl (2′-OMe) in future, in the case of therapeutic applications of the proposed nanoconstructs.
| Comp. no. | Oligonucleotide sequence | MWcalc. | m/z | Rta [min] | λ max [nm] |
|---|---|---|---|---|---|
| a The RP-HPLC conditions are described in the Materials and methods section. b The m/z ratio of an ion is measured by MALDI-TOF MS. c The m/z ratio of an ion is measured by ESI-Q-TOF MS. | |||||
| 2 | 5′-d(TTT CTT TTC CTC CAG AGC CCGA)-3′ | 6612.3 | 6612.9b | 12.94 | 263 |
| 3 | 5′-d(TCG GGC TCT GGA GGA AAA GAAA)-3′ | 6857.3 | 6856.0b | 12.06 | 256 |
| 4 |
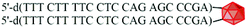
|
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652.9152 652.9152 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653.5508c 653.5508c |
13.38 | 261 |
| 5 |
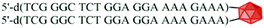
|
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143.2908 143.2908 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143.7510c 143.7510c |
12.47 | 258 |
Thermodynamic properties of duplexes formed by complementary tripeds 4 and 5 and unmodified oligonucleotides 2 and 3
Triped 4, functionalized with two EGFR-antisense strands, and triped 5, containing two complementary strands, which were iso-sequential to the unmodified oligomers 2 and 3, respectively, were annealed at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
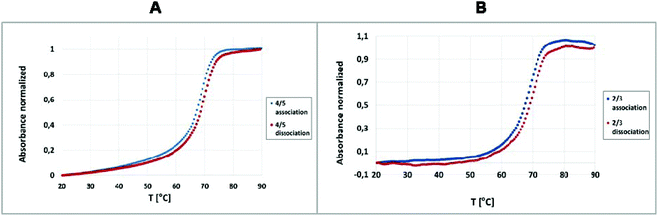
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The duplexes 2/3 and 4/5 (existing as a population of differently sized assembly products, Fig. 3) were subjected to UV-monitored thermal melting experiments at temperatures ranging from 20 °C to 90 °C, followed by backward annealing over the same temperature range (from 90 °C to 20 °C), with a temperature gradient of 0.5 °C min−1. The recorded dissociation and association profiles (Fig. 2) enabled the use of a two-state model to calculate the thermodynamic parameters, including the enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG) of the double-stranded (ds) → single stranded (ss) (dissociation) and ss → ds (association) transitions with the MeltWin program. The thermodynamic data for the dissociation and association transitions, as well as the respective melting temperatures (Tm), are shown in Table S1.† It should be noted that the recorded melting and annealing curves indicate very good cooperativity. It is illustrated by the narrow temperature window of transition between the duplex and single stranded form that does not exceed 15 °C, i.e. the value typical of natural unmodified DNA duplexes. The calculated enthalpy, entropy and free Gibbs energy changes indicate that the thermodynamic stability of the duplex formed by unmodified oligonucleotides 2 and 3 is similar to that noted for that formed by the boron-cluster scaffold functionalized-DNA (4/5) and the stabilizing forces in these duplexes are of similar nature and comparable strength. The duplex formed by tripeds 4 and 5 shows melting temperatures similar to the duplex formed by the parent, unmodified oligonucleotides 2 and 3, with a Tm of 69.5 °C and 69.1 °C, respectively, for the dissociation transition (ΔTm = 0.4 °C). For the association transition, the Tm was 68.7 °C and 68.3 °C, respectively, (ΔTm = 0.4 °C). These results suggest that the boron clusters, linked to the sense and antisense oligonucleotide strands, do not alter the stability of the 4/5 duplex and that thermodynamic parameters of both duplexes are in the same range. The melting curves demonstrate that both the tested duplexes exhibit minimal dissociation–association transition hysteresis. The ΔTm was almost identical for both tested complexes, 4/5 and 2/3 (ΔTm = 0.8 and 0.7 °C, respectively) suggesting that the presence of the boron-cluster cores does not disturb the recognition and binding of the strands.16,17
1. The duplexes 2/3 and 4/5 (existing as a population of differently sized assembly products, Fig. 3) were subjected to UV-monitored thermal melting experiments at temperatures ranging from 20 °C to 90 °C, followed by backward annealing over the same temperature range (from 90 °C to 20 °C), with a temperature gradient of 0.5 °C min−1. The recorded dissociation and association profiles (Fig. 2) enabled the use of a two-state model to calculate the thermodynamic parameters, including the enthalpy (ΔH), entropy (ΔS) and Gibbs free energy (ΔG) of the double-stranded (ds) → single stranded (ss) (dissociation) and ss → ds (association) transitions with the MeltWin program. The thermodynamic data for the dissociation and association transitions, as well as the respective melting temperatures (Tm), are shown in Table S1.† It should be noted that the recorded melting and annealing curves indicate very good cooperativity. It is illustrated by the narrow temperature window of transition between the duplex and single stranded form that does not exceed 15 °C, i.e. the value typical of natural unmodified DNA duplexes. The calculated enthalpy, entropy and free Gibbs energy changes indicate that the thermodynamic stability of the duplex formed by unmodified oligonucleotides 2 and 3 is similar to that noted for that formed by the boron-cluster scaffold functionalized-DNA (4/5) and the stabilizing forces in these duplexes are of similar nature and comparable strength. The duplex formed by tripeds 4 and 5 shows melting temperatures similar to the duplex formed by the parent, unmodified oligonucleotides 2 and 3, with a Tm of 69.5 °C and 69.1 °C, respectively, for the dissociation transition (ΔTm = 0.4 °C). For the association transition, the Tm was 68.7 °C and 68.3 °C, respectively, (ΔTm = 0.4 °C). These results suggest that the boron clusters, linked to the sense and antisense oligonucleotide strands, do not alter the stability of the 4/5 duplex and that thermodynamic parameters of both duplexes are in the same range. The melting curves demonstrate that both the tested duplexes exhibit minimal dissociation–association transition hysteresis. The ΔTm was almost identical for both tested complexes, 4/5 and 2/3 (ΔTm = 0.8 and 0.7 °C, respectively) suggesting that the presence of the boron-cluster cores does not disturb the recognition and binding of the strands.16,17
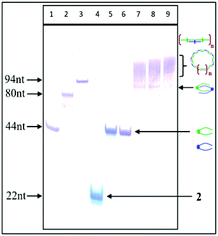
PAGE analysis of the self-assembly of complementary tripeds 4 and 5 and the effect of the ratio of complementary components and the magnesium ion concentration on their association
The effect of the ratio of the complementary components on the formation of double-stranded complexes was investigated by mixing tripeds 4 and 5 at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 20 mM Tris-HCl buffer (pH 8), 50 mM NaCl and 10 mM MgCl2. The mixture was heated for 5 min at 75 °C and then slowly (3 h) cooled to room temperature, followed by an overnight incubation at 4 °C. Then, the samples were analyzed by non-denaturating PAGE (15% polyacrylamide with no urea added) (Fig. 3).
1 in 20 mM Tris-HCl buffer (pH 8), 50 mM NaCl and 10 mM MgCl2. The mixture was heated for 5 min at 75 °C and then slowly (3 h) cooled to room temperature, followed by an overnight incubation at 4 °C. Then, the samples were analyzed by non-denaturating PAGE (15% polyacrylamide with no urea added) (Fig. 3).
PAGE analysis demonstrated that the tripeds 4 and 5, mixed at molar ratios 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, self-assemble into the well-defined dimeric structures containing 88 nt units (Fig. 3, lanes 7 and 8), as well as into a bunch of higher-order complexes containing greater numbers of complementary triped units 4 and 5 (lanes 7–9, Fig. 3). Although the PAGE analysis does not allow assessing the shape of the higher-order complexes, the preferential formation of circular structures for 1
1, self-assemble into the well-defined dimeric structures containing 88 nt units (Fig. 3, lanes 7 and 8), as well as into a bunch of higher-order complexes containing greater numbers of complementary triped units 4 and 5 (lanes 7–9, Fig. 3). Although the PAGE analysis does not allow assessing the shape of the higher-order complexes, the preferential formation of circular structures for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 triped ratio was confirmed by AFM and cryo-TEM (vide infra). Slightly higher molecular weight structures formed at 1
1 triped ratio was confirmed by AFM and cryo-TEM (vide infra). Slightly higher molecular weight structures formed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of tripeds 4 and 5 (see lanes 8 and 9, Fig. 3) represent perhaps linear structures with unpaired “dangling ends”, possibly in equilibrium with the circular ones. The relative fraction of the different types of higher-order complexes depends on various factors, including nucleobase sequences of the specific DNA component, boron cluster geometry and environmental properties.
1 molar ratio of tripeds 4 and 5 (see lanes 8 and 9, Fig. 3) represent perhaps linear structures with unpaired “dangling ends”, possibly in equilibrium with the circular ones. The relative fraction of the different types of higher-order complexes depends on various factors, including nucleobase sequences of the specific DNA component, boron cluster geometry and environmental properties.
Next, we tested the effect of the magnesium ion concentration on the annealing of the complementary tripeds 4 and 5.
Self-assembly of 4 and 5 (used in equimolar amounts of 0.10 OD of 4 and 0.12 OD of 5, in 10 μL) was performed as described above and in the Materials and methods section, with an increasing concentration of MgCl2 (1, 5, 10 and 20 mM). The image of the non-denaturating PAGE slab gel shown in Fig. 4 demonstrates that with higher magnesium ion concentrations, the complex composed of 88 nucleotides (one molecule of 4 and one molecule of 5) prevails, whereas higher-ordered, less mobile complexes are less abundant.
Interactions of magnesium, sodium and potassium ions with DNA and RNA molecules are essential for duplex formation and for nucleic acid molecular biology and their technological applications. Both divalent and monovalent cations bind to nucleic acid molecules and affect their physical properties. For oligonucleotides, magnesium cations stabilize the formation of duplexes, especially at lower concentrations (up to 50 mM), and facilitate the folding of the duplexes into secondary and tertiary structures.18 We hypothesize that the observed preferential formation of dimeric structures containing 88-nt units over higher-order structures with increasing magnesium concentrations (Fig. 4, lanes 5–9) may be due to the stabilization of the original, dimeric form and the lower availability of only partially annealed 4/5 duplexes for further extension.
Atomic force microscopy imaging (AFM) in the semi-contact mode
AFM measurements were performed in the semi-contact (tapping mode) under ambient conditions using a scanning probe BioScope Resolve AFM microscope equipped with a nanosensor silicon cantilever. The sample of nanostructures obtained from tripeds 4 and 5 (annealed at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 0.10 OD of 4 and 0.12 OD of 5, in 10 μL of Tris buffer pH 8 (20 mM Tris-HCl, 10 mM MgCl2, 50 mM NaCl)) was applied to a freshly cleaved mica surface and used for analysis. A ratio of 1
1, 0.10 OD of 4 and 0.12 OD of 5, in 10 μL of Tris buffer pH 8 (20 mM Tris-HCl, 10 mM MgCl2, 50 mM NaCl)) was applied to a freshly cleaved mica surface and used for analysis. A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
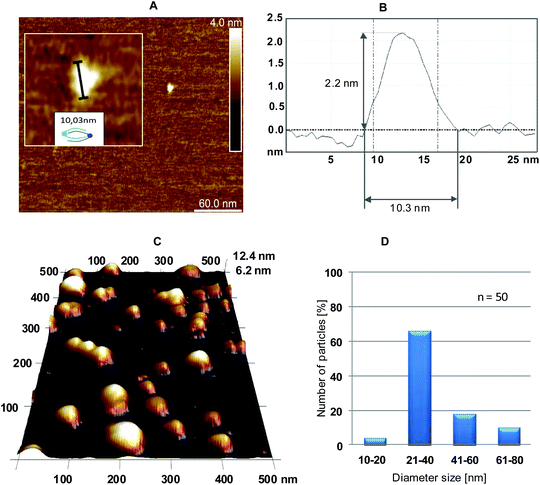
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 4/5 and a magnesium ion concentration of 10 mM were selected based on the above-described optimization of the annealing process. The results of the AFM measurements are shown in Fig. 5. Because the tapping mode was used, only the general features of the formed 4/5 complexes could be observed (i.e., drop-like nanostructures instead of the expected toroid-shape nanostructures). The contact mode led to the destruction of the studied objects.
1 for 4/5 and a magnesium ion concentration of 10 mM were selected based on the above-described optimization of the annealing process. The results of the AFM measurements are shown in Fig. 5. Because the tapping mode was used, only the general features of the formed 4/5 complexes could be observed (i.e., drop-like nanostructures instead of the expected toroid-shape nanostructures). The contact mode led to the destruction of the studied objects.
Fig. 5A shows a small nanostructure, for which the height and diameter were assessed as 2.2 nm × 10.3 nm, respectively (an AFM diagram is shown in Fig. 5B), that fit well to the expected dimensions of a simple dimeric object consisting of one 4 and one 5 triped.
The height was in the expected range for the helical diameter of a double stranded B-type DNA (approximately 2 nm),19 and the diameter corresponded to the size of the nanostructure determined with the IC Measure program (as shown for one droplet in the inset in Fig. 5A).
AFM topographic images suggest a drop-like outline of the nanostructures formed with 4 and 5 tripeds (Fig. 5C). However, because of the tapping mode of AFM the details of the subtle structure of the resultant nanoparticles are not seen. The boundaries of the drops are made of various numbers of complementary tripeds and adopt torus-like form (Fig. 7). The IC Measure analysis of the AFM images collected during the measurement allowed analyzing the size distribution, as shown in Fig. 5D. The obtained data demonstrate that most abundant nanostructures were those with a diameter of 21–40 nm (>60%) and 41–60 nm (approximately 20%). Only approximately 10% of the nanostructures had a diameter in the range of 61–80 nm.
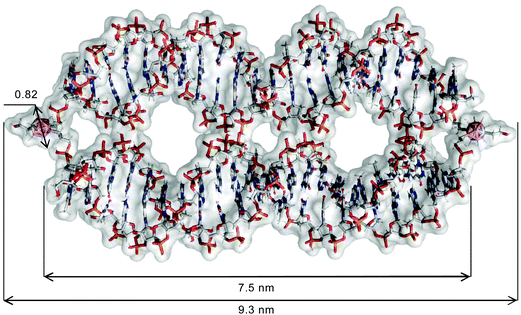
The results of the above-described evaluation are in agreement with the average length of the structure formed by tripeds 4 and 5, which is approximately 9.3 nm, based on the rough scheme of the resultant 4/5 dimer obtained by summing the size of two 1,2-dicarba-closo-dodecaborane clusters (approximately 0.8 nm),20 two propyl linkers (approximately 0.35 nm)21 and the length of one 22-mer DNA duplex (approximately 7.5 nm).22
The in silico assessment is consistent with the estimated size of the complex, which is calculated for 9.3 nm (Fig. 6; for the detailed description of the molecular modeling procedure, please see the Materials and methods section). The obtained result corroborates the data generated by AFM imaging and the IC Measure assessment.
Cryogenic transmission electron microscopy
Samples were prepared for the cryo-TEM measurement in the same manner as for the AFM experiments. Cryo-TEM images were collected for nanostructures 4/5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
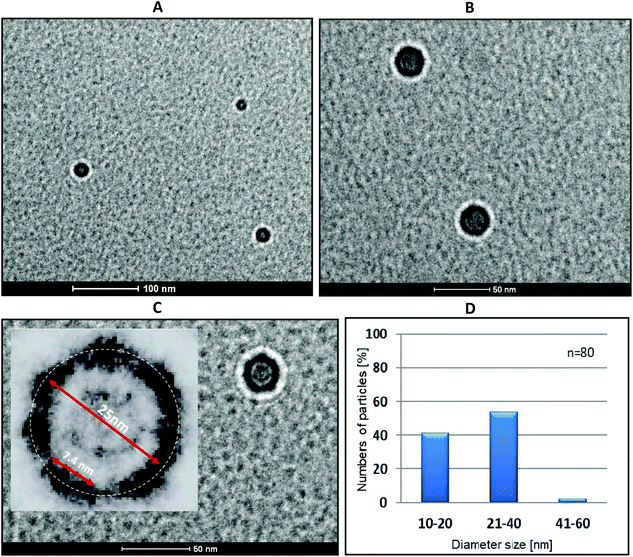
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) (Fig. 7) as well as for the reference samples (buffer, boron cluster (C2B10H12), duplex 2/3 and single-stranded tripeds 4 and 5, Fig. S4 and S5†). In the case of complex 4/5, regular ring nanostructures are formed with various diameters ranging from 10 to 60 nm. The size distribution (Fig. 7D) shows that the major fraction contains ring nanostructures of diameter size of 21–40 nm (approximately 54%) and of 10–20 nm (40%), containing 6–12 and 4–6 pairs of 4 and 5, respectively. By contrast, the reference compounds form either aggregates (Fig. S5B, D and E†) or may exist as single-duplex complexes (Fig. S4C†).
1 ratio) (Fig. 7) as well as for the reference samples (buffer, boron cluster (C2B10H12), duplex 2/3 and single-stranded tripeds 4 and 5, Fig. S4 and S5†). In the case of complex 4/5, regular ring nanostructures are formed with various diameters ranging from 10 to 60 nm. The size distribution (Fig. 7D) shows that the major fraction contains ring nanostructures of diameter size of 21–40 nm (approximately 54%) and of 10–20 nm (40%), containing 6–12 and 4–6 pairs of 4 and 5, respectively. By contrast, the reference compounds form either aggregates (Fig. S5B, D and E†) or may exist as single-duplex complexes (Fig. S4C†).
The cryo-TEM images of triped 4/5 complexes reveal dark-walled rings composed of DNA helices separated by boron clusters (see Fig. 7C, inset, which is a 4× magnification of the nanostructure shown in Fig. 7C, main picture, at a 50 nm scale). These rings are surrounded by light areolae (halos) that appear on the edge of the organic/inorganic complexes and the carbon grid, which are Fresnel fringes.23 The fringes produced outside the specimen appear much brighter and wider compared with those inside of the nanostructure rings, next to the circular carbon grid (Fig. 7C). These findings raised the question of why complexes of 4/5 form cyclic (torus-like) nanostructures, when mixed in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. To answer this question, it must be observed that the angle between two neighboring B–H or C–H bonds in an unsubstituted 1,2-dicarba-closo-dodecaborane cage is 60° (Fig. S1†). This angle is followed by B–C bonds that form between the first carbon atom of the propylene spacers and the boron atoms of the cage in oligopeds 4 and 5. Though one would not expect a high rigidity of the triped structure due to the flexibility of both the linker and the single-stranded DNA adapters, some degree of preorientation is anticipated due to the geometry of the boron cluster. The effect of this geometry is then fixed after the formation of the double-stranded DNA structure that results from the annealing of the strands of complementary tripeds; consequently, torus-like structures are formed. Interestingly, after lightening the image and magnifying it 4-fold one can observe dark elements of approximately 7.5 nm, which is the expected size of a 22-mer DNA duplex (Fig. 7C, inset). We speculate that the most abundant nanostructures (Fig. 7C) are composed of eight DNA helices joined by boron clusters, for which the calculated circumference is approximately 70 nm. This number is a sum of the size of one 22-mer duplex and two propyl linkers joined by one B–B bond of the cluster. The circumference, as measured by IC Measure (white circle), is ca. 78.5 nm. The size distribution of the obtained nanoparticles (Fig. 7D) could have been a result of the freedom of the DNA adapters to adopt an alternative orientation in the space within a limited range.
1 ratio. To answer this question, it must be observed that the angle between two neighboring B–H or C–H bonds in an unsubstituted 1,2-dicarba-closo-dodecaborane cage is 60° (Fig. S1†). This angle is followed by B–C bonds that form between the first carbon atom of the propylene spacers and the boron atoms of the cage in oligopeds 4 and 5. Though one would not expect a high rigidity of the triped structure due to the flexibility of both the linker and the single-stranded DNA adapters, some degree of preorientation is anticipated due to the geometry of the boron cluster. The effect of this geometry is then fixed after the formation of the double-stranded DNA structure that results from the annealing of the strands of complementary tripeds; consequently, torus-like structures are formed. Interestingly, after lightening the image and magnifying it 4-fold one can observe dark elements of approximately 7.5 nm, which is the expected size of a 22-mer DNA duplex (Fig. 7C, inset). We speculate that the most abundant nanostructures (Fig. 7C) are composed of eight DNA helices joined by boron clusters, for which the calculated circumference is approximately 70 nm. This number is a sum of the size of one 22-mer duplex and two propyl linkers joined by one B–B bond of the cluster. The circumference, as measured by IC Measure (white circle), is ca. 78.5 nm. The size distribution of the obtained nanoparticles (Fig. 7D) could have been a result of the freedom of the DNA adapters to adopt an alternative orientation in the space within a limited range.
One can suppose that anchoring DNA adapters in other spatial arrangements to the 1,2-dicarba-closo-dodecaborane core using an ellipsoidal metallacarborane scaffold instead of ball-like carborane or use of the DNA adapters of different lengths would result in a different topology of the formed nanostructures. Studies in this direction are ongoing in our laboratories.
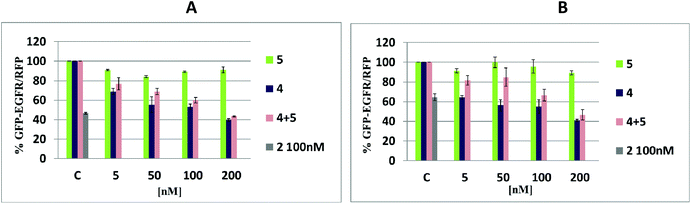
Silencing activity tests of tripeds 4 and 5 and their nanoconstruct 4/5 in MCF-7, A431 and HeLa cells
Analysis of the silencing activity of nanoconstructs 4/5 was performed using a dual fluorescence assay (DFA) (Fig. 8).31 This system is based on the measurement of the expression level of the green fluorescent protein fused with the target protein (GFP-EGFR, expressed from the pEGFP-EGFR plasmid) and of the control red fluorescence protein (RFP, expressed from the pDsRED-N1 plasmid). The assay allows determining the reduction of the level of the GFP-EGFR upon the binding of the antisense oligonucleotide to its complementary segment in the messenger RNA of EGRF, followed either by mRNA degradation or steric blockage. Based on that proven for the iso-sequential to 4 antisense oligonucleotide mechanism of action involving RNase H32 we assume that constructs 4/5 exhibits antisense activity via RNase H activation, too. Three human cell lines (breast adenocarcinoma, MCF-7; squamous carcinoma, A431, and cervical carcinoma, HeLa) in the presence of Lipofectamine 2000 were transfected with two plasmids and nanoconstructs 4/5, or with the reference tripeds 4 and 5 (at a 5–200 nM concentration) and with unmodified ASO 2 (100 nM), and incubated for 48 h, and for total 72 h for HeLa cells (Fig. S14†). The use of nanoconstructs 4/5 resulted in effective reduction of the EGFR level in a concentration-dependent manner in tested tumor cell lines, MCF-7 and A431, relative to the control (100% is the GFP-EGFR/RFP expression level in cells transfected analogously, except that the oligonucleotide component was not applied) (Fig. 8A and B). As expected, triped 4 was an active gene silencer while triped 5 caused only small EGFR expression fluctuations (up to 15%), probably due to the non-specific off-target effects. Slightly lower silencing activity of nanconstructs 4/5 than antisense triped 4 was observed for HeLa cells. The prolonged incubation time (72 h vs. 48 h) resulted in increased silencing activity (Fig. S14†). We assume that although 4/5 due to slightly different metabolism of the HeLa cells compared to many other cancer cells33 requires more time to exert the expected effect (>60% EGFR silencing), its cyclic structure may lead to higher stability against the cellular hydrolytic systems.If our assumption is correct, such cyclic nanoconstructs may have the therapeutic effect superior to the single stranded ASOs 2 and 4.
Metabolic activity of HeLa cells treated with tripeds 4 and 5 and nanoconstructs 4/5
The metabolic activity of HeLa cells after 48 and 72 hours of incubation with nanostructures 4/5 was measured by the colorimetric MTT assay (Fig. S15†), where the metabolic activity of mitochondrial succinate dehydrogenase correlates with the number of viable cells.34 The cells were treated with tripeds 4 and 5 or nanoconstructs 4/5 delivered in a 5–200 nM concentration range, or with the reference oligonucleotide 2 (100 nM). Lipofectamine 2000 was used for transfection. The MTT results indicate that in general the screened oligonucleotides are non-toxic to the cancer cells, and the mitochondrial activity is only slightly lowered in the case of 4/5 used at higher concentrations (100–200 nM, for 48 h). Thus, the nanostructures 4/5 do not interfere with cellular metabolism, but, as discussed earlier, decrease the level of target protein overexpressed in cancer cells.Experimental
Materials
Magnesium chloride was obtained from POCH Poland (now: Avantor Performance Materials Poland S.A; Gliwice, Poland); trityl chloride and Stains-all dye were obtained from Sigma Aldrich (St Louis, MO, USA). Phosphoramidites of dA, dC, T and dG protected at their 5′-hydroxyl with DMTr groups and at their exocyclic amine with phenoxyacetyl (dAPac), tert-butylphenoxyacetyl (dGtBPac) or acetyl (dCAc) protection were obtained from ChemGenes Corporation (Wilmington, MA, USA). The nucleoside-linked LCA–CPG solid support was obtained from Biosearch Technologies (Petaluma, USA). Benzylmercaptotetrazole (0.25 M Hyacinth BMT activator solution in anhydrous CH3CN) was obtained from EMP Biotech (Berlin, Germany).The 5% phenoxyacetic anhydride in tetrahydrofuran/pyridine was obtained from Glen Research (Sterling, VA, USA). Trifluoroacetic acid, ≥99.0%, was obtained from TCI Chemicals (Tokyo, Japan). The C18 SepPak cartridges were obtained from Waters (Milford, Ireland), and ammonium hydroxide (30%) (J.T. Baker brand) was obtained from Avantor Performance Materials (Center Valley, PA, USA). Marker oligonucleotides (M) of 44, 80 or 94 nucleotides (nt) were synthesized in house.
Methods
For the synthesis of tripeds 4 (iso-sequential to 2) and 5 (iso-sequential to 3), the LCA CPG support loaded with triped-shaped 1-(2-O-succinylethyl)-9,12-bis(3-O-tritylprop-1-yl)-1,2-dicarba-closo-dodecaborane was used instead. Synthesis was performed under typical conditions with a benzylmercaptotetrazole (BMT) activator (0.25 M in CH3CN) and a coupling time of 500 s. For the oxidation step, a 0.1 M solution of I2 in THF/H2O/pyridine (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v/v) was used for 5 min. The capping step was performed with 5% phenoxyacetic anhydride in THF/pyridine and a 10% solution of 1-methylimidazol/THF/pyridine (1
1; v/v/v) was used for 5 min. The capping step was performed with 5% phenoxyacetic anhydride in THF/pyridine and a 10% solution of 1-methylimidazol/THF/pyridine (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v/v) for 30 s. The 5′-DMTr-protected oligomers were cleaved from the support and deprotected at their exo-amine functions with a mixture of NH4OH (30%) and EtOH (3
1; v/v/v) for 30 s. The 5′-DMTr-protected oligomers were cleaved from the support and deprotected at their exo-amine functions with a mixture of NH4OH (30%) and EtOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for 180 min. After concentration under reduced pressure, the “DMTr-on” oligonucleotides were purified by reverse-phase HPLC as described below (the HPLC profiles of the crude products are given in Fig. S1†). Subsequent removal of the 5′-DMTr groups was performed on C18 SepPak cartridges by treating the oligomers with 2% TFA, followed by washing the cartridges with water (approximately 10 mL) and eluting the desired product with 30–50% CH3CN in water (approximately 0.5 mL). The resulting unmodified DNA oligonucleotides 2 and 3 and tripeds 4 and 5 bearing two DNA 22-mer adapters were analyzed by ESI-Q-TOF mass spectrometry (4,5), MALDI-TOF (2,3) and UV-VIS spectroscopy (data given in Table 1). The purity of 4 and 5 was analyzed by RP-HPLC and 15% polyacrylamide/7 M urea gel electrophoresis (PAGE, Fig. 2C).
1, v/v) for 180 min. After concentration under reduced pressure, the “DMTr-on” oligonucleotides were purified by reverse-phase HPLC as described below (the HPLC profiles of the crude products are given in Fig. S1†). Subsequent removal of the 5′-DMTr groups was performed on C18 SepPak cartridges by treating the oligomers with 2% TFA, followed by washing the cartridges with water (approximately 10 mL) and eluting the desired product with 30–50% CH3CN in water (approximately 0.5 mL). The resulting unmodified DNA oligonucleotides 2 and 3 and tripeds 4 and 5 bearing two DNA 22-mer adapters were analyzed by ESI-Q-TOF mass spectrometry (4,5), MALDI-TOF (2,3) and UV-VIS spectroscopy (data given in Table 1). The purity of 4 and 5 was analyzed by RP-HPLC and 15% polyacrylamide/7 M urea gel electrophoresis (PAGE, Fig. 2C).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 2
2 or 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in 20 mM Tris-HCl buffer (pH 8) containing 50 mM NaCl and 10 mM MgCl2 in a total volume of 10 μL. For 0.1 optical density unit (OD) of 4, an equivalent amount of 5 was 0.12 OD. The mixture was heated for 5 min at 75 °C, slowly cooled (3 h) to room temperature and then left overnight at 4 °C. The samples were analyzed by non-denaturating (no urea added) 15% polyacrylamide gel electrophoresis (PAGE) (Fig. 4).
1 in 20 mM Tris-HCl buffer (pH 8) containing 50 mM NaCl and 10 mM MgCl2 in a total volume of 10 μL. For 0.1 optical density unit (OD) of 4, an equivalent amount of 5 was 0.12 OD. The mixture was heated for 5 min at 75 °C, slowly cooled (3 h) to room temperature and then left overnight at 4 °C. The samples were analyzed by non-denaturating (no urea added) 15% polyacrylamide gel electrophoresis (PAGE) (Fig. 4).
The effect of the MgCl2 concentration (1, 5, 10 and 20 mM) on the assembly of tripeds 4 and 5 used at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (0.10 OD of 4 and 0.12 OD of 5) was studied under the same non-denaturating PAGE conditions (Fig. 5).
1 molar ratio (0.10 OD of 4 and 0.12 OD of 5) was studied under the same non-denaturating PAGE conditions (Fig. 5).
Electrophoresis was performed at room temperature at a constant voltage of 300 V cm−1 and a current of 6 mA for 3 h. The PAGE slab gels were stained with Stains-all for 30 min, and then, the gels were scanned using a G-Box apparatus (Syngene, Cambridge, UK).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.10 OD of 4 and 012 OD of 5) assembled in 20 mM Tris-HCl buffer, pH 8.0, containing 50 mM NaCl and 10 mM MgCl2 in a total volume of 10 μL according to the procedure described for PAGE shown above was applied on the surface of a freshly cleaved mica, which was left at room temperature for 30 min for sample adsorption and fixation. Then, the mica plate was washed with 1 mL of Milli-Q water and loaded onto the AFM instrument. Nano Scope 1.5 and 9.4 software analyses were used, and PeakForce-Hires was explored (k = 0.4 N m−1). The AFM measurements were performed as complimentary service by Bruker Company Warsaw (Bionanopark Lodz).
1 (0.10 OD of 4 and 012 OD of 5) assembled in 20 mM Tris-HCl buffer, pH 8.0, containing 50 mM NaCl and 10 mM MgCl2 in a total volume of 10 μL according to the procedure described for PAGE shown above was applied on the surface of a freshly cleaved mica, which was left at room temperature for 30 min for sample adsorption and fixation. Then, the mica plate was washed with 1 mL of Milli-Q water and loaded onto the AFM instrument. Nano Scope 1.5 and 9.4 software analyses were used, and PeakForce-Hires was explored (k = 0.4 N m−1). The AFM measurements were performed as complimentary service by Bruker Company Warsaw (Bionanopark Lodz).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.10 OD of 4 and 0.12 OD of 5) assembled in 10 μL of 20 mM Tris-HCl buffer (pH 8), 50 mM NaCl and 10 mM MgCl2 were applied to the grid as a 3 μL droplet and then subjected to blotting with filter paper and rapid freezing in liquid ethane using the fully automated blotting device Vitrobot Mark IV (FEI Company, Hillsboro, Oregon, USA). After preparation, the vitrified specimens were kept under liquid nitrogen until they were inserted into a cryo-TEM-holder Gatan 626 (Gatan, Inc., Pleasanton, USA) and analyzed in the TEM at −178 °C. In order to obtain enhanced contrast and to archive details of the specimen structure the excitation of objective lens was slightly decreased and focused below the specimen (slight underfocus). Therefore a phase-contrast effect is visible around the imaged structures. The cryo-TEM measurements were performed under a contractual service agreement with CMPW PAN in Zabrze.
1 (0.10 OD of 4 and 0.12 OD of 5) assembled in 10 μL of 20 mM Tris-HCl buffer (pH 8), 50 mM NaCl and 10 mM MgCl2 were applied to the grid as a 3 μL droplet and then subjected to blotting with filter paper and rapid freezing in liquid ethane using the fully automated blotting device Vitrobot Mark IV (FEI Company, Hillsboro, Oregon, USA). After preparation, the vitrified specimens were kept under liquid nitrogen until they were inserted into a cryo-TEM-holder Gatan 626 (Gatan, Inc., Pleasanton, USA) and analyzed in the TEM at −178 °C. In order to obtain enhanced contrast and to archive details of the specimen structure the excitation of objective lens was slightly decreased and focused below the specimen (slight underfocus). Therefore a phase-contrast effect is visible around the imaged structures. The cryo-TEM measurements were performed under a contractual service agreement with CMPW PAN in Zabrze.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (2 μL of Lipofectamine 2000 per 1 μg of oligonucleotide component 4, 5, 4/5 or 2) according to the manufacturer's protocol. For the DFA, the HeLa, MCF-7 or A431 cells were transfected with the reporter plasmid pDsRed-N1 (BD Biosciences) (45 ng per well), pEGFP-EGFR plasmid (100 ng per well) and suitable tripeds 4 and 5 and nanostructures 4/5 (5–200 nM) dissolved in 50 μL of OPTI-MEM medium (GIBCO, BRL, Paisley, New York, NY, USA). As a negative control, cells prepared analogously but transfected with Lipofectamine 2000 only were used. Cells prepared analogously but transfected with the non-modified reference 2 in 100 nM concentrations were used as a positive control. After 5 hours of incubation, the transfection mixture was replaced with 200 μL of fresh medium with antibiotics per well. After 48 or 72 hours of incubation at 37 °C under a 5% CO2 atmosphere, the cells were washed two times with PBS buffer (without Ca2+ and Mg2+) and lysed with NP-40 buffer (150 mM NaCl, 1% IGEPAL, 50 mM Tris-HCl pH 7.0, and 1 mM PMSF) overnight at 37 °C. The prepared cell lysates were used for fluorescence measurement.
1 ratio (2 μL of Lipofectamine 2000 per 1 μg of oligonucleotide component 4, 5, 4/5 or 2) according to the manufacturer's protocol. For the DFA, the HeLa, MCF-7 or A431 cells were transfected with the reporter plasmid pDsRed-N1 (BD Biosciences) (45 ng per well), pEGFP-EGFR plasmid (100 ng per well) and suitable tripeds 4 and 5 and nanostructures 4/5 (5–200 nM) dissolved in 50 μL of OPTI-MEM medium (GIBCO, BRL, Paisley, New York, NY, USA). As a negative control, cells prepared analogously but transfected with Lipofectamine 2000 only were used. Cells prepared analogously but transfected with the non-modified reference 2 in 100 nM concentrations were used as a positive control. After 5 hours of incubation, the transfection mixture was replaced with 200 μL of fresh medium with antibiotics per well. After 48 or 72 hours of incubation at 37 °C under a 5% CO2 atmosphere, the cells were washed two times with PBS buffer (without Ca2+ and Mg2+) and lysed with NP-40 buffer (150 mM NaCl, 1% IGEPAL, 50 mM Tris-HCl pH 7.0, and 1 mM PMSF) overnight at 37 °C. The prepared cell lysates were used for fluorescence measurement.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (2 μL of Lipofectamine 2000 per 1 μg of nucleic acid) according to the manufacturer's protocol. The control cells were treated with Lipofectamine 2000 only. The cells were incubated for 48 h or 72 h at 37 °C under a 5% CO2 atmosphere, followed by the addition of MTT solution in PBS (5 mg mL−1) to each well. The cells were then incubated for 3 h at 37 °C under a 5% CO2 atmosphere. Finally, 95 μL of lysis buffer (NP-40, 20% SDS, 50% aqueous dimethylformamide, pH 4.5) was added to each well and cells were incubated overnight at 37 °C. The sample absorbance was measured at two wavelengths: 570 nm and the reference wavelength of 630 nm (colourless walls plate reader, PerkinElmer). The results from the control cells were considered as 100% viability.
1 ratio (2 μL of Lipofectamine 2000 per 1 μg of nucleic acid) according to the manufacturer's protocol. The control cells were treated with Lipofectamine 2000 only. The cells were incubated for 48 h or 72 h at 37 °C under a 5% CO2 atmosphere, followed by the addition of MTT solution in PBS (5 mg mL−1) to each well. The cells were then incubated for 3 h at 37 °C under a 5% CO2 atmosphere. Finally, 95 μL of lysis buffer (NP-40, 20% SDS, 50% aqueous dimethylformamide, pH 4.5) was added to each well and cells were incubated overnight at 37 °C. The sample absorbance was measured at two wavelengths: 570 nm and the reference wavelength of 630 nm (colourless walls plate reader, PerkinElmer). The results from the control cells were considered as 100% viability.
Conclusions
In conclusion, we developed a methodology for automated, solid phase synthesis of heterooligonucleotides on a boron-cluster-modified solid support,24,25 generating boron cluster “oligopeds”, which represent a new generation of building blocks for nanoconstruction. Assembly of the complementary oligopeds led to torus-like nanoparticles that contained both DNA and boron cluster components and preferentially contained 6–12 pairs of tripeds to obtain the designed nanostructure geometry. The silencing activity of the nanoconstructs 4/5 targeting the exogenous EGFR gene was tested using a dual fluorescence assay (EGFR-GFP/RFP) in MCF-7, A431 and HeLa cells. Pronounced silencing of the target gene was observed and the nanoconstruct was non-toxic, as shown in HeLa cells. This proposed technology makes it possible to prepare circularized DNA that merges the properties of nucleic acids and boron clusters for various applications in molecular biology and in vivo studies and serves as therapeutic nucleic acid delivery vectors26–28 as well as carriers of heavy boron-loaded materials for anticancer boron neutron capture therapy (BNCT).29,30Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by the National Science Center, Poland, grant 015/16/W/ST5/00413. Contributions from the Statutory Fund of IMB PAS (S. J., Z. J. L., K. B.-S.) and CMMS PAS (B. N., K. E.-O., K. K., D. K.) are also gratefully acknowledged. The authors thank Łukasz Kowelski (Bruker Company Warsaw) for help in AFM measurements and Aleksander Foryś (CMPW PAN Zabrze) for assistance in Cryo-TEM microscopy and dr Carla Sardo (IMB PAS) for characteristics of intermediates of the synthesis of 1.Notes and references
- N. C. Seeman and H. F. Sleiman, DNA nanotechnology, Nat. Rev. Mater., 2017, 3, 17068 CrossRef.
- Y.-J. Chen, B. Groves, R. A. Muscat and G. Seelig, DNA nanotechnology from the test tube to the cell, Nat. Nanotechnol., 2015, 10, 748 CrossRef CAS , and references cited therein.
- C. Chen, Z. Yang and X. Tang, Chemical modification of nucleic acid drugs and their delivery systems for gene-based therapy, Med. Res. Rev., 2018, 38, 829 CrossRef.
- H. Yang, K. L. Metera and H. F. Sleiman, DNA modified with metal complexes: Applications in the construction of higher order metal–DNA nanostructures, Coord. Chem. Rev., 2010, 254, 2403 CrossRef CAS.
- M. F. Hawthorne, J. I. Zink, J. M. Skelton, M. J. Bayer, C. Liu, E. Livshits, R. Baer and D. Neuhauser, Electrical or Photocontrol of the Rotary Motion of a Metallacarborane, Science, 2004, 303, 1849 CrossRef CAS.
- Y. Shirai, J.-F. Morin, T. Sasaki, J. M. Guerrero and J. M. Tour, Recent progress on nanovehicles, Chem. Soc. Rev., 2006, 35, 1043 RSC.
- A. M. Spokoyny, M. G. Reuter, C. L. Stern, M. A. Ratner, T. Seideman and C. A. Mirkin, Carborane-Based Pincers: Synthesis and Structure of SeBSe and SBS Pd(II) Complexes, J. Am. Chem. Soc., 2009, 131, 9482 CrossRef CAS.
- M. P. Grzelczak, S. P. Danks, R. C. Klipp, D. Belic, A. Zaulet, C. Kunstmann-Olsen, D. F. Bradley, T. Tsukuda, C. Vinas, F. Teixidor, J. J. Abramson and M. Brust, Ion Transport across Biological Membranes by Carborane-Capped Gold Nanoparticles, ACS Nano, 2017, 11, 12492 CrossRef CAS.
- Z. J. Lesnikowski, Boron clusters - A new entity for DNA-oligonucleotide modification, Eur. J. Org. Chem., 2003, 4489 CrossRef CAS.
- Z. J. Lesnikowski, DNA as a platform for new biomaterials. Metal-containing nucleic acids, Curr. Org. Chem., 2007, 11, 355 CrossRef CAS.
- A. B. Olejniczak, B. Nawrot and Z. J. Lesnikowski, DNA modified with boron cluster-metal complexes [M(C2B9H11)2] – Synthesis, properties and applications, Int. J. Mol. Sci., 2018, 19(11), 3501 CrossRef.
- S. Janczak, A. Olejniczak, S. Balabańska, M. K. Chmielewski, M. Lupu, C. Viñas and Z. J. Lesnikowski, The boron clusters as a platform for new materials: synthesis of functionalized o-carborane (C2B10H12) derivatives incorporating DNA fragments, Chem. – Eur. J., 2015, 21, 15118 CrossRef CAS.
- F. Teixidor, R. Sillanp, A. Pepiol, M. Lupu and C. Vinas, Synthesis of Globular Precursors, Chem. – Eur. J., 2015, 21, 12778 CrossRef CAS.
- M. Scholz and E. Hey-Hawkins, Carbaboranes as pharmacophores: Properties, synthesis, application strategies, Chem. Rev., 2011, 111, 7035 CrossRef CAS.
- T. Brown and D. J. S. Brown, Modern machine-aided methods of oligodeoxyribonucleotide synthesis, in Oligonucleotides and analogues. A Practical Approach, ed. F. Eckstein, IRL Press, Oxford, 1991, pp. 1–24 Search PubMed.
- K. Ebenryter-Olbińska, D. Kaniowski, M. Sobczak, B. A. Wojtczak, S. Janczak, E. Wielgus, B. Nawrot and Z. J. Leśnikowski, Versatile method for the site specific modification of DNA with boron clusters – Anti-EGFR antisense oligonucleotide case, Chem. – Eur. J., 2017, 23, 16535 CrossRef PubMed.
- D. Kaniowski, K. Ebenryter-Olbinska, M. Sobczak, B. Wojtczak, S. Janczak, Z. J. Lesnikowski and B. Nawrot, High Boron-loaded DNA-Oligomers as Potential Boron Neutron Capture Therapy and Antisense Oligonucleotide Dual-Action Anticancer Agents, Molecules, 2017, 22, 1393 CrossRef.
- R. Owczarzy, B. G. Moreira, Y. You, M. A. Behlke and J. A. Walder, Predicting Stability of DNA Duplexes in Solutions Containing Magnesium and Monovalent Cations, Biochemistry, 2008, 47, 5336 CrossRef CAS.
- D. W. Ussery, DNA Structure: A-, B- and Z-DNA Helix Families, in Encyclopedia of Life Sciences, Macmillan Publishers Ltd., Nature Publishing Group, 2002 Search PubMed.
- D. A. Morgan, J. Sloan and M. L. H. Green, Direct imaging of o-carborane molecules within single walled carbon nanotubes, Chem. Commun., 2002, 2442 RSC.
- J. Clayden, N. Greeves and S. G. Warren, Organic Chemistry, Oxford University Press, Oxford, 2012 Search PubMed.
- S. Neidle, Principles of nucleic acid structure, Elsevier Inc., 2008 Search PubMed.
- T. D. Nguyen, M. A. O'Keefe, R. Kilaas, R. Gronsky and J. B. Kortright, Effects of Fresnel Fringes on TEM Images of Interfaces in X-Ray Multilayers, in Physics of X-ray Multilayer Structures Technical Digest, 1992, vol. 7, p. 94 Search PubMed.
- A. S. Batsanov, M. A. Fox, J. A. K. Howard, A. K. Hughes, A. L. Johnson and S. J. Martindale, 9,12-diiodo-1,2-dicarba-closo-dodecaborane(12), Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2003, 59, 74 CrossRef.
- R. J. B. H. N. van den Berg, C. G. N. Korevaar, H. S. Overkleeft, G. A. van der Marel and J. H. van Boom, Effective, High-Yielding, and Stereospecific Total Synthesis of d-erythro-(2R,3S)-Sphingosine from d-ribo-(2S,3S,4R)-Phytosphingosine, J. Org. Chem., 2004, 69, 5699 CrossRef CAS.
- L. Wu, Y. Wang, J. Wu, C. Lv, J. Wang and X. Tang, Caged circular antisense oligonucleotides for photomodulation of RNA digestion and gene expression in cells, Nucleic Acids Res., 2013, 41, 677 CrossRef CAS.
- B. K. Ruble, S. B. Yeldell and I. J. Dmochowski, Caged oligonucleotides for studying biological systems, J. Inorg. Biochem., 2015, 150, 182 CrossRef CAS.
- X. J. Tang, M. Su, L. Yu, C. Lv, J. Wang and Z. Li, Photomodulating RNA cleavage using photolabile circular antisense oligodeoxynucleotides, Nucleic Acids Res., 2010, 38, 3848 CrossRef CAS.
- M. Ueno, H. S. Ban, K. Nakai, R. Inomata, Y. Kaneda, A. Matsumura and H. Nakamura, Dodecaborate lipid liposomes as new vehicles for boron delivery system of neutron capture therapy, Bioorg. Med. Chem., 2010, 18, 3059 CrossRef CAS.
- R. F. Barth, P. Mi and W. Yang, Boron delivery agents for neutron capture therapy of cancer, Cancer Commun., 2018, 38, 1 CrossRef.
- K. Sipa, E. Sochacka, J. Kazmierczak-Baranska, M. Maszewska, M. Janicka, G. Nowak and B. Nawrot, Effect of base modifications on structure, thermodynamic stability, and gene silencing activity of short interfering RNA, RNA, 2007, 13, 1301 CrossRef CAS.
- A. K. Petch, M. Sohail, M. D. Hughes, I. Benter, J. Darling, E. M. Southern and S. Akhtar, Messenger RNA expression profiling of genes involved in epidermal growth factor receptor signalling in human cancer cells treated with scanning array-designed antisense oligonucleotides, Biochem. Pharmacol., 2003, 66, 819 CrossRef CAS.
- A. Dlugosz, K. Gach-Janczak, J. Szymanski, D. Deredas, H. Krawczyk, T. Janecki and A. Janecka, Anticancer Properties of a New Hybrid Analog AD-013 Combining a Coumarin Scaffold with an α-methylene-δ-lactone Motif, Anticancer Agents Med. Chem., 2018, 18, 450 CrossRef CAS.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures illustrate the HPLC profiles and ESI-Q-TOF mass spectra of 4 and 5, cryo-TEM images of buffer, boron cluster, duplex 2/3, tripeds 4 and 5, scheme of the boron cluster spatial orientation, the procedure for the synthesis of LCA CPG solid support 1, UV, FT-IR, 1H-NMR spectra of the key intermediates as well as silencing activity and cytotoxicity of 4/5 in HeLa cells. See DOI: 10.1039/c9nr06550d |
| This journal is © The Royal Society of Chemistry 2020 |