Phototherapy with layered materials derived quantum dots
Houjuan
Zhu
*ab,
Nengyi
Ni
a,
Suresh
Govindarajan
 a,
Xianguang
Ding
c and
David Tai
Leong
a,
Xianguang
Ding
c and
David Tai
Leong
 *ad
*ad
aDepartment of Chemical and Biomolecular Engineering, Faculty of Engineering, National University of Singapore, Singapore 117585, Singapore. E-mail: cheltwd@nus.edu.sg; c2dzh@nus.edu.sg
bCentre for Advanced 2D Materials, Graphene Research Centre, National University of Singapore, Singapore 117546, Singapore
cInstitute for Health Innovation and Technology, National University of Singapore, Singapore 117599, Singapore
dNUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, Singapore 117456, Singapore
First published on 4th November 2019
Abstract
Quantum dots (QDs) originating from two-dimensional (2D) sheets of graphitic carbon nitride (g-C3N4), graphene, hexagonal boron nitride (h-BN), monoatomic buckled crystals (phosphorene), germanene, silicene and transition metal dichalcogenides (TMDCs) are emerging zero-dimensional materials. These QDs possess diverse optical properties, are chemically stable, have surprisingly excellent biocompatibility and are relatively amenable to surface modifications. It is therefore not difficult to see that these QDs have potential in a variety of bioapplications, including biosensing, bioimaging and anticancer and antimicrobial therapy. In this review, we briefly summarize the recent progress of these exciting QD based nanoagents and strategies for phototherapy. In addition, we will discuss about the current limitations, challenges and future prospects of QDs in biomedical applications.
1. Introduction
Two-dimensional (2D) nanomaterials have gained tremendous research interest due to the great success achieved by graphene materials in the past few decades.1–3 The commonly reported 2D nanomaterials are graphene,4,5 hexagonal boron nitride (h-BN),3,6,7 graphitic carbon nitride (g-C3N4),8,9 transition metal dichalcogenides (TMDCs),10–16 monoatomic buckled crystals (phosphorene), germanene17,18 and silicene,19–21 all of which exhibit a wide range of applications due to their catalytic, electronic, thermal, optical, chemical, and magnetic properties.22–26In recent years, a growing number of inorganic QDs derived from their larger 2D counterpart have attracted more attention, such as graphene QDs,4,5,27,28 h-BN,29,30 TMD QDs,31–37 g-C3N4 QDs.38–40 This unique class of QDs is endowed with better dispersity in both aqueous and non-aqueous solutions, larger surface-to-volume ratios, easier functionalization, doping, higher tunability in physiochemical properties and fluorescence.41–43 Due to these unique and diverse properties, QDs have been explored to widely apply in various research fields including bioimaging,44–48 cancer therapy,49–52 sensing,44,53–56 optoelectronics,57,58 catalysis and energy storage.59–61
Phototherapy is an attractive non-invasive remotely activated medical modality for the treatment of various diseases.62,63 It can be widely divided into photodynamic therapy (PDT) and photothermal therapy (PTT). During the process of phototherapy, the phototherapeutic agent has to be delivered to the diseased site and subsequently irradiated with light at a particular wavelength; PDT utilizes photosensitizers (PS) that are activated to generate reactive oxygen species (ROS) by light, consequently inducing irreversible damage of cells.64–68 In PTT, the photothermal agent absorbs near infrared (NIR) light to produce heat, directly leading to the ablation of cells.69–71 Nanomaterials driving light-triggered PDT and PTT are promising next generation agents with their excellent specificities that bring about a more defined therapeutic at a much lower applied dose with lowered systemic side effects compared to the traditional radio- and chemotherapies.72–74
To improve the therapeutic efficiency of phototherapies in tumors, a wide variety of PS and PT agents, such as semiconducting polymer quantum dots,72,74,75 gold nanostructures,76,77 metal nanoparticles,78,79 metal sulfide nanoparticles,80–82 two-dimensional materials,16,83 oxide nanoparticles84–86 and carbon derivatives,87 are explored as potential phototherapeutic agents. In comparison with these nanomaterials, QDs possess several key merits as PT agents including widely tunable fluorescence properties, good photostability and chemical stability, excellent solubility and biocompatibility.88
The studies on QDs in many fields are still at the preliminary stage due to the poor understanding and unexploited applications. Although some excellent reviews have comprehensively and comparatively summarized the synthesis, properties and applications of QDs,89,90 a review that specifically focuses on QD based phototherapy is currently missing. This review summarized the recent progress of QDs originating from two-dimensional nanomaterials for phototherapy. The phototherapeutic QD agents in recent literature are summarized (Table 1). The various strategies by which different QDs are applied in PDT and PTT together with insights into their present limitations and challenges will inspire further translation of these QD technologies into biology and medicine.
| System | Light source | Application | PCE for PTT/1O2 QY for PDTa | Analyte | Ref. |
|---|---|---|---|---|---|
| a PCE: photothermal conversion efficiency of PTT agents; 1O2 QY: singlet oxygen quantum yield of PDT agents; N.A: not reported. | |||||
| MoS2 QDs | White light, 0.1 W cm−2 | PDT | N.A. | Human endothelial cells (HMVEC), 3D tumor spheroids | 32 |
| MoS2 QDs | 630 nm laser | Up-conversion and down-conversion bioimaging guided PDT | N.A. | HeLa cells | 49 |
| MoS2-GSH nanodots | 808 nm laser, 0.5 W cm−2 | PTT | N.A. | 4T1 cells, 4T1 tumor-bearing mice | 102 |
| MoSe2 nanodots | 785 nm laser, 2 W cm−2 | PTT | PCE of 46.5% | HeLa cells | 103 |
| MoS2 QD doped disulfide-based SiO2 nanoparticles with hyaluronic acid and Ce6 | 808 nm laser, 1.5 W cm−2; | PDT/PTT | PCE of 22.34% | 4T1 cells, 4T1 tumor-bearing mice | 104 |
| 660 nm laser, 1 W cm−2 | |||||
| MoS2 QDs@polyaniline | 808 nm laser, 1.5 W cm−2 | PA/CT image-guided PTT/RT | PCE of 31.6% | 4T1 cells, 4T1 tumor-bearing mice | 106 |
| MoS2 QDs | Solar light | PDT | S. aureus, E. coli and S. aureus infected wounds | 107 | |
| WS2 QDs | 808 nm laser, 1 W cm−2 | PA/CT image-guided PTT/RT | PCE of 44.3% | HepG2 cells and HeLa cells, BEL-7402 tumor-bearing mice | 50 |
| WS2 nanodot based nanoplatform integrating hyaluronic acid (HA), polyaniline (PANI), and Ce6 | 808 nm laser, 1.5 W cm−2; | FL/PA/CT imaging guided triple-collaborative PTT/RT/PDT | N.A. | 4T1 cells, 4T1 tumor-bearing mice | 105 |
| 670 nm-laser, 1.0 W cm−2 | |||||
| GQDs | White light (400–800 nm), 6.5 mW cm−2 | PDT | 1O2 QY of 1.34 | HeLa cells female, BALB/nu mice with subcutaneous breast cancer (MDA MB-231 green-fluorescent protein) xenografts | 51 |
| Nitrogen doped GQDs functionalized with an amino group | 800 nm excited two-photon excitation (TPE) light, 2.3936 mW, 239.36 nJ per pixel | TPE imaging guided PDT | 0.53 | KB-50 cancer cells | 52 |
| Aptamer-conjugated GQDs/porphyrin derivative | 980 nm laser, 0.72 W cm−2; | Fluorescence-guided PDT/PTT | PCE of 28.58% 1O2 QY of 1.08 | A549 cells | 110 |
| 635 nm laser, 0.16 W cm−2 | |||||
| Folic acid (FA)-functionalized GQDs with loading IR780 iodide | 808 nm laser, 1 W cm−2 | NIR fluorescence imaging guided PTT | PCE of 87.9% | HeLa cells, BALB/c nude mice bearing HeLa tumors. | 119 |
| GQDs | 808 nm laser, 0.5 W cm−2 | PTT/PDT | N.A. | MDA-MB-231 cells | 120 |
| GQDs | 470 nm laser, 1 W | Autophagy-inducing PDT | N.A. | U251 human glioma cells | 121 |
| Upconversion nanoparticle (UCNP) coated GQDs and loading hypocrellin A | 980 nm laser, 0.3 mW | Chemo-PDT | N.A. | HeLa cells | 122 |
| GQDs | 470 nm laser, 1 W | PDT | N.A. | Methicillin-resistant Staphylococcus aureus and Escherichia coli | 123 |
| GQDs | UV light | Gamma irradiated PDT | N.A. | N.A. | 124 |
| GQD immobilized on Mn3O4 coated with polydopamine (PDA) | 670 nm laser, 3 mW cm−2 | MRI and NIR imaging guided PDT | N.A. | A549 cells, A549 tumor-bearing mice | 125 |
| Sulphur doped GQDs and methylene blue | 660 nm LED light, 12 W | PDT | N.A. | MCF-7 cells | 126 |
| GQD-PEG loading Ce6 | 650 nm laser, 20 mW cm−2 | PDT | N.A. | HeLa cells, nude mice bearing HeLa tumor | 131 |
| GQD integrated in MnO2 | 810 nm femtosecond pulses | Two-photon confocal fluorescence guided PTT | N.A. | HeLa | 132 |
| GQDs decorated by silver nanoparticles | 425 ± 10 nm LED light, 3 mW cm−2 | Chemo-PDT | N.A. | HeLa and DU145 cancer cells | 135 |
| GQDs | 800 nm excited TPE light, 2.64 mW, 264 nJ per pixel | TPE guided PDT | N.A. | MDR bacteria | 136 |
| Sulphur doped GQDs combined with methylene blue | 660 nm-LED, 12 W | PDT | N.A. | E. coli and M. Luteus colonies, IMR-90 and A549 cell lines | 137 |
| Nitrogen-doped GQDs | 670 nm laser, 1 W cm−2 | PDT | 1O2 QY of 0.60, 0.49 and 0.41 | E. coli bacteria | 138 |
| N-GQDs integrated in mesoporous silica nanoparticles and loading DOX | 622 nm LED light, | Chemo-PDT | N.A. | MDA-MB-231 cells | 139 |
| 6.8 mW cm−2 | |||||
| Nitrogen-doped GQDs conjugated Rose Bengal | 480 nm-xenon lamp | PDT | N.A. | MCF-7 cells, ear blood vessels of mice | 140 |
| Upconversion nanoparticle (UCNP) coated GQDs and targeted TRITC | 980 nm laser, 1.5 W cm−2 | PDT | N.A. | 4T1 cells and 4T1 tumor bearing mice | 141 |
| Upconversion nanoparticle (UCNP) coated GQDs and targeted folic acid, carboxybutyl triphenylphosphonium | 980 nm laser, 1 W cm−2 | PDT | N.A. | HeLa cells and HeLa tumor bearing nude mice | 142 |
| N-Doped GQDs | 808 nm laser, 2 W cm−2 | PTT | PCE of 62.53% | A549 and HeLa cells | 144 |
| GQDs integrated in magnetic mesoporous silica nanoparticles and loading DOX | 808 nm laser, 2.5 W cm−2 | Magnetic hyperthermia and chemo-PTT | N.A. | 4T1 cells | 145 |
| GQDs integrated in mesoporous silica nanoparticles and loading DOX | 808 nm, 2.5 W cm−2 | Chemo-PTT | N.A. | 4T1 cells | 146 |
| GQDs integrated in silica coated hollow magnetic nanospheres and loading DOX | 671 nm laser, 0.2 W cm−2; 808 nm laser, 0.25 W cm−2 | Magneto-mechanical, chemo-PDT/PTT | PCE of 21.9% | Eca-109 cells | 147 |
| GQDs conjugated by aptamer AS1411 | 808 nm laser, 2 W cm−2 | PTT | N.A. | A549, COS-7, HEK293, HeLa, HepG2, MCF-7 | 148 |
| Hyaluronic acid (HA)-modified and GQD-gated hollow mesoporous carbon nanoparticles (HMCN) and loading DOX | 808 nm laser, 1 W cm−2 | Chemo-PTT | N.A. | HeLa cells and HeLa tumor bearing nude mice | 149 |
| BPQDs; PEGylated BPQDs | 808 nm laser, 1 W cm−2 | PTT | PCE of 28.4% | C6 cells and MCF7 cells | 152 |
| Poly(lactic-co-glycolic acid) (PLGA) nanospheres loaded with BPQDs | 808 nm laser, 1 W cm−2 | PTT | N.A. | MCF7 cells, B16 cells, MCF7 tumor-bearing mice | 153 |
| PEGylated BPQDs; rhodamine-A/PEG-BPQDs | 808 nm laser, 2 W cm−2 | Fluorescence imaging guided PDT/PTT | N.A. | HepG2 cells, 4T1 cells, 4T1 tumor-bearing mice | 155 |
| BPQDs; PEGylated BPQDs | 670 nm light, 0.16 mW cm−2 | PDT | N.A. | HeLa cells, L02 cells, S180 tumor-bearing mice | 156 |
| BPQD-hybridized mesoporous silica framework (BMSF) with in situ synthesized Pt nanoparticles (PtNPs), and decorated with TLS11a aptamer/Mal-PEG-NHS | 670 nm laser, 0.1 W cm−2 | Fluorescence imaging guided PDT | 1O2 QY of 0.142 for BPQD and 0.166 for BMSF | HepG2 cells and HepG2-tumor bearing mice | 157 |
| g-C3N4 QDs | Microwave (MW) light, 5 W | Microwave induced PDT (MIPDT) | N.A. | UMR-106 cells | 158 |
| g-C3N4 QDs: pristine QDs or modified with defects | 800 nm laser, 1.0 W cm−2 | Two-photon imaging (TPI) and TPE guided PDT | N.A. | MCF7 cells | 159 |
| Antimonene quantum dots (AMQDs); PEGylated AM QDs | 808 nm, 1 W cm−2 | PTT | PCE of 45.5% | MCF7 cells, HeLa cells, MCF-7 tumor-bearing mice | 164 |
| Titanium carbide MXene QDs | 808 nm laser, 0.5 W cm−2 | PA imaging guided PTT | PCE of 52.2% | HeLa cells, MCF-7 cells, U251 cells, HEK 293 cells, HeLa tumor-bearing mice | 167 |
| MoO3−x QDs | 880 nm laser, 2 W cm−2 | Photoacoustic (PA) imaging-guided PDT/PTT | PCE of 25.5% | HeLa cells, L02 cells, HeLa tumor-bearing mice | 171 |
2. Transition metal dichalcogenide quantum dot (TMD QD) based phototherapy
Two-dimensional transition metal dichalcogenides (TMDs), due to their comparable structure to graphene, have gained attention recently.91–93 Their generalized formula and structure consist of monoatomic layer thick, stacked layers of repetitive covalently bonded chemical compounds X–M–X, in which M represents transition metals such as Mo, Ru, and W, while X represents chalcogen atoms including S, Se and Te.89,94 To obtain TMD QDs with top-down approaches, the intra-planar X–M–X bonds need to be broken to form smaller dots with abundant edge atoms. Such top-down approaches are not limited to laser pulse ablation,95 free radical etching,96 hydrothermal/solvothermal reaction,97 intercalation assisted exfoliation,98 chemical vapor deposition (CVD) and mechanical exfoliation and milling.99,100 Due to quantum confining effects,55 TMD QDs are found to show a wider bandgap in comparison with their 2D forms.101 Additionally, high surface to volume ratios, small sizes, chemically reactive sites at the edges and direct band gaps contribute to the extensive interest in these uniquely placed TMD QDs for applications in various fields, such as in energy and catalysis.59 In recent years, a few TMD QDs including MoS2 QDs and WS2 QDs are emerging as PTT and PDT agents.Owing to their near infrared (NIR) absorbance, some TMD QDs have been developed as PPT nanoagents to treat cancer. For example, bio-clearable and ultra-small MoS2 nanodots modified by glutathione (GSH) were developed to be a theranostic agent.102 These MoS2 nanodots not only could remarkably photothermally kill cancer cells, but also served as novel photoacoustic (PA) imaging agents for observing efficient tumor accumulation after intravenous (i.v.) injection. Because of their smaller sizes when compared to MoS2 nanosheets, these MoS2 nanodots showed more efficient clearance via urine. The photothermal treatment of tumors using MoS2 nanodots achieved excellent therapeutic efficacy. Similarly, Wang et al. obtained MoSe2 nanodots (NDs) with an ultrasmall size of 2–3 nm through ultrasonication-assisted liquid exfoliation from bulk MoSe2.103 These nanodots absorbed strongly at NIR wavelengths with a PCE of about 46.5% with efficient HeLa cell ablation under NIR laser (785 nm) irradiation and yet induced negligible cytotoxicity without irradiation.
Since MoS2 QDs on their own have limited blood circulation time and are rapidly cleared from the body, Li et al. designed an amino-modified biodegradable agent composed of MoS2 QD doped disulfide-based silica (SiO2) nanoparticles (denoted as MoS2@ssSiO2).104 This MoS2@ssSiO2 obtained by inserting the MoS2 QDs into SiO2 nanoparticles not only could be degraded and excreted through the redox reaction of glutathione (GSH), but also increased the blood circulation time, in turn inducing a high tumor uptake compared with separate MoS2 QDs. Through adsorption of both chlorin e6 (Ce6) and hyaluronic acid (HA) on the outer shell, MoS2@ssSiO2 has a corresponding photodynamic and tumor-targeting effect, and is guided by fluorescence/CT/multispectral optoacoustic tomography (MSOT) combined PTT/PDT of the tumor. Besides, this group further integrated HA, polyaniline (PANI), Ce6, and tungsten sulfide (WS2) nanodots into a single platform (HA-WS2@PANI/Ce6) for fluorescence/PA/X-ray computed tomography (CT) imaging-guided combinational radiation therapy/PTT/PDT of tumors.105 In another study, the same group fabricated versatile MoS2 QDs@polyaniline (MoS2@PANI) based inorganic−organic nanohybrids for PA/CT image guided combinational PTT/radiotherapy (RT) of tumors, achieving better anticancer efficiency.106 Another similar dual-modal PA/CT imaging guided synergetic PTT/RT theranostic nanoagent, which was only composed of WS2 QDs, was reported to achieve better therapeutic efficacy by Zhao and Gu's group.50
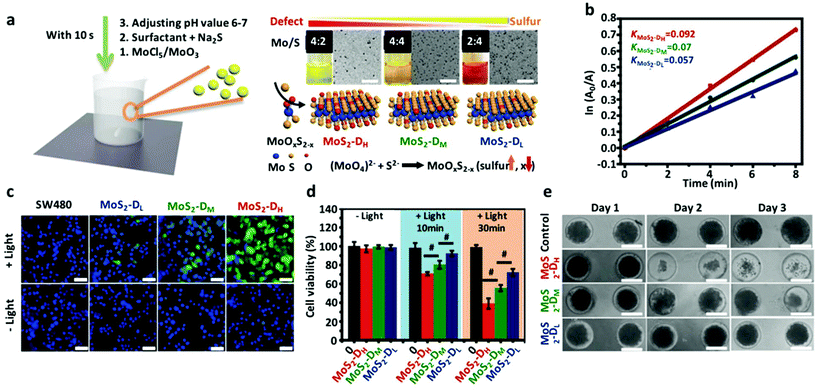
Besides exerting the photothermal effect, some TMD QDs such as MoS2 QDs were demonstrated to be better photosensitizers, which could realize the efficient PDT of tumors through producing ROS under light irradiation. Recently, Leong's group developed a general bottom-up synthesis reaction to synthesize TMD QDs with variable defect states (Ding–Leong reaction). They showed the versatility of this synthesis method by preparing a wider library of TMD QDs (Fig. 1a).32 The reactions were very fast (10–20 s) with very mild conditions such as room temperature, aqueous and atmospheric conditions. As the protocol was bottom-up, the reaction stoichiometric ratio of the precursors can be easily controlled to engineer defects at room temperature. With these defect-variable TMD QDs, it was shown experimentally that on increasing crystallinity defects there was a significant increase in 1O2 QY (Fig. 1b). The positive correlation between the defect degree and photodynamic properties was also verified at the cellular level (Fig. 1c). With these discoveries, MoS2 QDs with more defects showed higher photodynamic killing in cancer cells and in 3D tumor spheroids (Fig. 1d and e). This research provides a simple synthetic strategy to expand the existing TMD QD library and a tool for researchers to investigate the defect effects on their properties for applications in many different fields.
Additionally, Dong and Zhang's group prepared small sized MoS2 QDs of approximately 15 nm with a layered thickness of 2 nm, which presented remarkable down- and up-conversion photoluminescence behaviors. These MoS2 QDs were prepared by ultrasonication of bulk MoS2 powder in tetrabutylammonia through breaking of Mo–S bonds.49 These optical properties make these quantum dots good candidates for imaging probes. Besides, MoS2 QDs demonstrated superior 1O2 generation over protoporphyin (PpIX). In addition to cancer therapy, MoS2 QDs may also have promising antimicrobial application under environmental conditions. Yin's group reported that MoS2 QDs could produce multiple ROS species types under simulated solar light irradiation, leading to remarkably enhanced antibacterial activity. In vivo experiments showed that MoS2 QDs significantly enhanced wound healing.107
3. Graphene quantum dot (GQD) based phototherapy
Graphene quantum dots (GQDs) may consist of one or more graphene layers. Each single atomic thickness layer is made up of carbon atoms packed into a planar honeycomb crystal structure with excellent thermal and chemical stability. GQDs are found to have low cytotoxicity,108 good biocompatibility,109 and the ability to generate reactive oxygen species (ROS) upon photoexcitation and can be used for PDT against bacteria or cancer. Also, GQDs can transfer electrons to conjugated drugs or photothermal agents which can increase anticancer activity.110The outstanding properties of GQDs have inspired surging interest in some fields for example in electronics,111,112 optics,113,114 sensors,115,116 drug delivery,117 bioimaging,118 and photothermal119,120 and photodynamic therapy.121–126 Even though cadmium based semiconductor quantum dots have exploitable optical properties for bioimaging applications, they still suffers from low biocompatibility and high intrinsic toxicity.127,128 The GQDs might be a viable alternative to cadmium based semiconductor quantum dots with their excellent biocompatibility, chemical inertness, wavelength dependent luminescence and easy scalability traits.129
The clinical use of photosensitizer porphyrin derivatives such as Ce6 and verteporfin is hampered by their hydrophobic nature.130 To overcome these solubility issues, GQDs hidden within redox-triggered cleavable PEG shells have been used as a conjugated platform to anchor down the photosensitizer molecules, without affecting their photodynamic efficiency whilst improving their hydrophilicity and therefore avoiding rapid clearance from the system. The planar π conjugated structure of GQDs quenches photoactive Ce6's fluorescence and 1O2 generation in a non-tumor environment. However, when exposed to tumor relevant GSH, the PEG shell undergoes reductive cleavage for a responsive burst release of Ce6. This system showed excellent in vivo/ex vivo imaging coupled with high PDT efficacy and superior biocompatibility.131 Besides this combination, another GQD-based multifunctional two-photon nanoprobe composed of GQD and MnO2 nanosheets was designed for intracellular tumor-related GSH detection (triggered release) and amplified PDT in cancer cells.132 A porphyrin derivative (P) was conjugated with GQDs coated with polyethylene glycol (PEG) to form GQDs-PEG-P which showed good stability, low cytotoxicity and excellent biocompatibility when tested on A549 and MCF-7 cancer cells. Importantly, the nanomaterial displayed a PCE of 28.58% and high QY of 1O2 generation.110
Another nanoassembly of polydopamine stabilized GQDs-Ce6 was developed which effectively induced tumor cell ablation through enhancing photodynamic effects by delivering the nanoassembly to the targeted Toll-like receptor 9 on the cell surface, continuously stimulating to enhance T lymphocyte infiltration.133
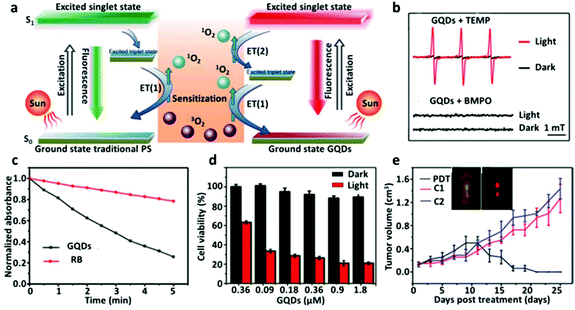
Ge et al.51 reported a GQD based PDT nanoagent which can generate 1O2 through a multistate sensitization process with a high 1O2 QY of ∼1.3, highest among the PDT agents. A new 1O2-generating mechanism increases the QY of GQDs through multistage sensitization. Such a phenomenon is not observed in conventional photosensitizers such as PpIX or phthalocyanines (Fig. 2a). The 1O2 generated by GQDs in the presence of 2,2,6,6-tetramethylpiperidine (TEMP) and 5-tert-butoxycarbonyl 5-methyl-1-pyrroline N-oxide (BMPO) under 532 nm laser irradiation is shown by the Electron Spin Resonance (ESR) spectrum (Fig. 2b). Also, the ability of GQDs to generate 1O2 has been verified through a chemical trapping method using Rose Bengal (RB) as a photosensitizer and disodium 9,10-anthracendipropionic acid (Na2-ADPA) as a trapping agent. This comparison showed that the degradation of Na2-ADPA was larger than that of RB resulting from GQDs under light irradiation (Fig. 2c). The results in HeLa cells showed that the GQDs have low cytotoxicity and biocompatibility in the dark even at 1.8 μM (Fig. 2d). The in vivo experiments in mouse models showed the fluorescence imaging capability of GQDs (Fig. 2e). The mouse group treated with GQDs under light irradiation destroyed tumors and no tumor was observed to regrow after 17 days in the group with GQDs under light irradiation (Fig. 2e).
Thakur et al.120 prepared GQDs from eco-friendly withered leaves of fig trees. The GQDs were tested for in situ labelling probes on cancer cells. When GQDs were taken up by cancer cells and irradiated with an IR laser (808 nm), concentration dependent ROS was generated. These GQDs maintained high photoluminescence stability despite continuous irradiation up to 30 minutes. Passivating nanomaterials with PEG improved their circulation time in vivo and reduced the interaction with macrophages and monocytes in vivo.134
Through PEGylation, a new form of Ag-GQD was prepared and loaded with a chemotherapeutic drug doxorubicin (DOX).135 This nanomaterial system was tested in vitro on HeLa and DU145 cancer cells. When combining the photodynamic effect through the addition of QDs, the combined drug–photosensitizer treatment synergistically increased cell death when irradiated with a 425 nm radiation source. Doping of GQDs could tune their PDT capability. Kuo et al.52 reported amino group functionalized GQDs doped with nitrogen (amino-N-GQDs) with an absorption wavelength of ∼800 nm which had a quantum yield of 33% with strong photoluminescence. The nitrogen doping improved ROS generation and eliminated methicillin-resistant bacteria, Staphylococcus aureus. Their studies revealed that nitrogen doped GQDs exhibited stronger PDT compared with undoped GQDs, which is similar to the photodynamically anti-microbial properties of other GQDs that had been successfully used to kill bacteria.136,137
In another study, it has been reported that increasing nitrogen doping further to 5.1% from 2.9% could increase ROS generation considerably when photo-excited with 670 nm light. This trend can be seen in the quantum yield decreases of N-GQD (5.1% N), N-GQD (2.9% N) and GQD with decreasing defects (0.259, 0.165, and 0.137 respectively).138 Additionally, an efficiency controllable PDT was designed through varying the doping amount of nitrogen atoms.139 Furthermore, dual chemo-photodynamic therapy was achieved by loading a nucleus-targeting drug. Sun et al.140 prepared nitrogen doped GQDs coupled with photosensitizers RB for two photon induced PDT. The material had good photo-stability and biocompatibility. The N-GQDS-RB exhibited high cytotoxicity through irradiation with a one- or two-photon laser. Up-conversion nanoparticles (UCNP) have also been reported to combine with GQDs to enhance the highly efficacious PDT through emitting UV-vis light under NIR excitation to active GQDs for producing 1O2 efficiently.141,142
GQDs have an interesting ability to convert NIR light waves strongly into heat which can kill cancer cells.143 GQDs doped with N increased the PCE to 62.53%.144 GQD capped magnetic mesoporous silica nanoparticles (MMSN) generated heat under a magnetic field or NIR irradiation and concurrently released doxorubicin.145 A multimodal therapeutic system of magneto-mechanical, photothermal, photodynamic and chemo therapies (DOX) was designed for cancer therapy through integrating GQDs into silica coated hollow magnetic nanospheres; similarly DOX loaded mesoporous silica nanoparticle (MSN) capped GQDs (GQDs-MSNs) exhibited pH and temperature responsive drug release behavior, and when irradiated with near-infrared light, the heat generated killed cancer cells.146,147 A multifunctional zeolite imidazolate framework-8 (ZIF-8) was used as a drug carrier with embedded GQDs as photothermal seeds, and the DOX was released under acidic conditions.143
The nature of the photodynamic effect is that it is non-specific and cells within the immediate vicinity at the point of excitement are subjected to the same oxidative damage regardless of whether the cells are the targeted cells or merely spectators. Therefore, targeting becomes an important consideration especially when developing a highly potent photodynamic agent. Wang et al.148 developed active targeting theranostic GQDs based on aptamer AS1411. This specific aptamer acts as a bio-targeting moiety against tumor cells with high specificity and induces cell death even at low dosage. The small size, biocompatibility, stable fluorescence, and NIR responsiveness of GQDs complement the targeting aptamer. Similarly, HA-modified and GQD-integrated hollow mesoporous carbon nanoparticles (HMCN) were used to encapsulate drugs for targeting of CD44 cell surface receptors, overexpressed on cancer cells.149
4. Atomically thin black phosphorus quantum dot (BPQD) based phototherapy
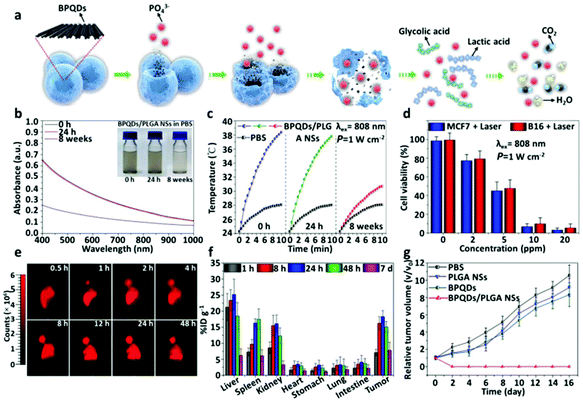
An emerging 2D material, black phosphorus (BP), has notable potential prominence in electronics due to its structural properties.150 BP possesses a layered structure and a layer-dependent bandgap that increases from 0.3 eV for the bulk material to 2.0 eV for the monolayer form.151 Ultrasmall derivatives of BP nanosheets, known as BP quantum dots (BPQDs), possess notably useful properties too. These BPQDs have been reported to be ∼2.6 nm in size and show promising NIR photothermal performance, displaying a large extinction coefficient at 808 nm and a high PCE of 28.4%.152Yu's group further examined the potential of BPQDs as photothermal agents through the synthesis of biodegradable nanospheres from poly(lactic-co-glycolic acid) (PLGA) loaded with black BPQDs for PTT applications.153 The nanospheres (NS), which were ∼100 nm in size, were rationally designed to allow for high PTT efficiency and more precise control of the biodegradation rate (Fig. 3a). Under physiological conditions, the external PLGA shell of the BPQDs/PLGA NS would gradually degrade through hydrolysis into lower molecular weight fragments without (Fig. 3b) or with light excitation (Fig. 3c). The in vivo photothermal killing effect with the NS was studied with MCF7 and B16 cells, where a steady decline in cell viability was dose-dependent on BPQD loading (Fig. 3d). The in vivo biodistribution of the NS was studied through Cyanine 5.5 (Cy5.5) fluorescence imaging of tumors in mice, with observations that the fluorescence intensity increased up to the 24 h timepoint, revealing continuous accumulation of the NS within tumors. The intensity could also be maintained till 48 h (Fig. 3e). Quantitative biodistribution in mice revealed a high concentration of BPQDs/PLGA NS in the tumor, in addition to the liver, spleen and kidneys (Fig. 3f). In vivo photothermal cancer therapy was conducted in mice injected with different treatment agents and their tumor volumes were tracked across time. Mice with the BPQD/PLGA NS treatment revealed gradual shrinking of the tumors, which were eliminated after ∼16 days (Fig. 3g).
In addition to PTT applications, BPQDs are also gaining prominence for their potential role in PDT. BP nanosheets have been reported to allow for efficient generation of 1O2.154 For instance, Liu and colleagues designed a multifunctional BPQD-based system for both bioimaging and combinatory PTT/PDT application.155 The PEGylated BPQDs exhibited strong NIR photothermal performance and yielded cytotoxic 1O2 for PDT. In vitro and in vivo investigations revealed that the combined phototherapy was more effective for cancer cell ablation than either therapy employed on their own. BPQDs also demonstrated high loading of organic fluorescent dyes for tumor imaging purposes. Additionally, Huang's group synthesized BPQDs with a hydrodynamic diameter of 5.4 nm that could be renally cleared as intact particles.156 Recently, Liu's group designed a hepatocellular carcinoma (HCC)-specific targeting aptamer “TLS11a”-modified BPQD-hybridized nanocatalyst to enhance its PDT performance.157 The nanocatalyst was constructed with a BPQD-hybridized mesoporous silica framework (BMSF) with in situ synthesized Pt nanoparticles (Pt NPs), which respectively acted as a photosensitizer to produce ROS and hence served as a catalyst to react with H2O2 to produce O2 that could amplify the PDT effect through positive feedback mechanisms in the hypoxic tumor microenvironment (TME). Thereafter, modification by TLS11a aptamer/Mal-PEG-NHS targets HCC cells. In vivo and in vitro experiments further demonstrated the nanoagent's active targeting property and excellent photodynamic effects in hypoxic TME with minimal side effects.
In general, BP nanosheet- and BPQD-based phototherapy platforms and systems are attractive as phosphorus is a common element within the human body, essential to life at the cellular level158 and hence regarded as likely benign at worst.153 Moreover, ultrasmall BP is expected to yield nontoxic degradation products that exist in the body.158 Like most nanomaterials in consideration for phototherapy, real clinical applications would depend on successful optimization of biocompatibility, bodily clearance and other necessary safety requirements.
5. Graphitic carbon nitride (g-C3N4) antimonene, transition metal oxide (TMO), and MXene quantum dot (QD) based phototherapy
Graphitic carbon nitride (g-C3N4), a metal-free conjugated polymer with semiconductor properties has been well known as a photocatalyst due to its ability to catalyze redox reactions under light irradiation. Additional advantages such as biocompatibility, adjustable band gap and position, and low expected production cost further boost g-C3N4 as an interesting phototherapy materials candidate.159 Recently, g-C3N4 has also been examined as a photosensitizer for PDT applications, where its water-splitting activity was explored as a novel strategy to counter hypoxic tumor microenvironments that resist PDT.160In 2017, Chu et al. used 5 nm g-C3N4 for microwave induced PDT.161 Nanomaterial mediated microwave-induced PDT (MIPDT) was previously developed by the research group as a potential method of addressing deep-seated cancers.162 In the paper by Chu et al., in vitro studies showed the entry of g-C3N4 into UMR-106 osteosarcoma cells with an increase in singlet oxygen generation under microwave induction. Analysis of cell viability revealed the performance of g-C3N4 as a potential agent for MIPDT. In another example, Wu et al. used g-C3N4 QDs with defects as a dual-functional platform that could perform two-photon excited photodynamic therapy (TPE-PDT) and two-photon imaging (TPI).163 Three forms of g-C3N4 QDs were designed. The CN-DPT QDs, synthesized by copolymerizing melamine with a phenyl monomer, showed high performing TPI coupled with efficient generation of ROS for TPE-PDT. Overall, the work demonstrated the emerging potential of g-C3N4 as part of a dual-functional TPI and TPE-PDT therapeutic system.
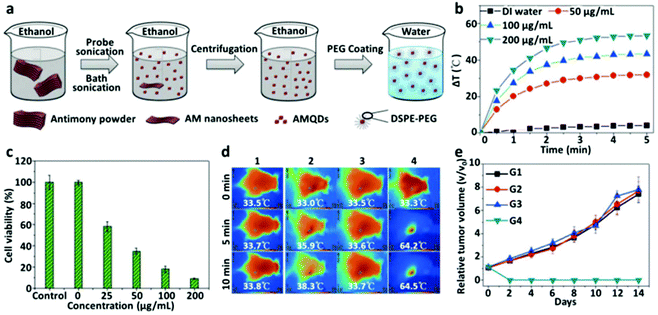
Antimonene (AM) is an emerging 2D material derived through exfoliating bulk antimony, with certain advantageous physical properties over other notable 2D materials, such as BP and graphene.164 Although AM is yet to be explored in detail for therapeutic applications, its similarity to other 2D materials made exploring its potential use in phototherapy a possibility to examine. Recently, Tao et al. synthesized ultrasmall AM quantum dots (AMQDs) and examined their potential as a photothermal agent, obtaining a PCE of 45.5% for the PEG-coated AMQDs, which was higher than that of BP.165 AMQDs were synthesized through liquid exfoliation with a combined sonication method involving an ultrasound probe in an ice-bath, followed by coating with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(poly ethylene glycol)] (DSPE-PEG) to yield PEG-coated AMQDs that were stable in aqueous medium without aggregation (Fig. 4a). After 808 nm laser irradiation, PEG-coated AMQDs generated photothermal heating curves that are concentration-dependent (Fig. 4b). NIR irradiation was further used to investigate the killing effect on MCF7 cancer cells, where the decline in cell viability was also concentration-dependent on PEG-coated AMQDs (Fig. 4c). MCF7 tumor-bearing mice were treated and temperature changes were monitored using IR thermal imaging (Fig. 4d). Growth curves of tumors revealed that only when PEG-coated AMQDs were irradiated with an NIR laser could there be an observable decline in tumors (Fig. 4e). Overall, AM remains a very recent material to be applied in phototherapy and clinical applications will depend on further in vivo characterization. However, the outstanding photothermal conversion efficiency will make AM an important material to consider for phototherapeutics.
Transition metal oxide (TMO) nanomaterials refer to the group of inorganic particles that commonly include iron oxides and molybdenum oxides. The suboxide (oxygen deficient) phase of molybdenum oxide (MoO3−x) is one example of such materials with growing research into their phototherapy applications. MoO3−x QDs were discovered to display high optical absorbance in the NIR region due to a strong localized surface plasmon resonance (LSPR) effect.166 For instance, Ding et al. developed MoO3−x QDs with good bioimaging and combined PDT-PTT capabilities.167 These QDs exhibited a PCE of 25.5% and could simultaneously generate ROS, suggesting their potential as an agent for combined PDT-PTT. The QDs demonstrated killing of cancer cells both in vitro and in vivo, without localized irradiation with an 808 nm laser, and there is no apparent damage to major organs. Liu and Hu's groups synthesized MoO3−x QDs at room temperature under ultraviolet irradiation, deriving QDs with a PCE as high as 40.01%.168 The cytotoxicity and photothermal performance of the QDs were tested in vitro with encouraging results but have yet to progress to in vivo testing.
MXenes are a class of 2D transition metal materials consisting of their carbides, carbonitrides and nitrides, with a hydrophilic nature and high volumetric capacitance that can exceed those of carbon electrodes, resulting in notable interest in their energy storage device applications.169 However, in 2017, MXenes were first reported for their potential in the PTT technology space, where Ti3C2 nanosheets possessed a PCE of 30.3% with ablation of tumors in in vivo studies.170 However, like within the former paper, hydrofluoric acid (HF) was almost always used as the etching agent to yield MXenes from the bulk phase. In an effort to avoid using the strongly corrosive and potentially difficult to fully remove HF, Liu and Yu's group designed a HF-free synthesis method, yielding MXene QDs with aluminum oxoanions on the surface, which possessed a large extinction coefficient of 52.8 L g−1 cm−1 at 808 nm and a high PCE of up to 52.2%.171 The MXene QDs were capable of tumor ablation under irradiation and had no observable toxicities when examined both in vitro and in vivo.
6. Limitations and challenges
In recent years, some exciting achievements on QDs have emerged tending more to the application end of the scientific pipeline. However, the much fundamental research is still in the early stage and some challenges still exist, which are waiting to be tackled for future live translation. Firstly, considering the structure of QDs being dependent on the synthesis strategy it is also important to explore their properties and applications. Thus, the development of synthetic methods to more precisely control the TMD QDs such as their sizes, thicknesses, defect degrees, edge configurations and chemical modifications should be given more attention in the future. For example, due to the relative chemical inertness or fewer physical interaction sites on the surface of QDs, strategies on surface modification are lacking, leading to challenges in their bioapplications. However, this point is limited by some shortcomings in the synthesis of QDs, including the complex procedure, low yield, time-consuming process, and low-scale and high-cost production, in most cases. Therefore, finding ways to optimize the synthetic method of QDs is still a burning problem. In addition, as for the properties of QDs, more relevant studies centered on the electrochemical, catalytic, optical and electrical properties of some QDs, such as TMD QDs, h-BN QDs, phosphorene, MXenes, germanene and silicone, can provide even more clarity on linking their physicochemical makeup to their properties. Until now, the mechanism of photoluminescence (PL) has been incompletely understood. When referring to the PL behaviors of QDs, their relatively low quantum yield and wide emission spectra remain to be a question, limiting their extensive applications in biosensing, bioimaging and nanomedicine. In addition, the in-depth exploration of QDs with NIR fluorescence, which is beneficial for deep-tissue applications, is still lacking in studies. Lastly, QD based PTT/PDT theranostics is still unfortunately constrained by the inherent tissue penetration depth of visible or NIR light.7. Summary and perspectives
As an advanced category of quantum dots which are emerging zero-dimensional materials, QDs are gaining increasing attention because of their outstanding physicochemical properties, such as optical, catalytic, chemical, electrochemical, and electronic characteristics.46,59 The new types of QDs are extended to GQDs, TMD QDs, g-C3N4 QDs, h-BN, phosphorene, MXenes, germanene and silicone, which show their booming applications in a diverse range of fields. These QDs inherited some unique characteristics from their 2D counterparts on catalysis and energy applications. In particular, with the help of additional advantages based on their lower dimensionality, such as highly tunable PL properties, low toxicity, good biocompatibility and molecular size, the QDs have demonstrated their potential capability for biosensors, antibacterial materials, bioimaging probes, and especially PDT/PTT of deep tumors.Despite this excellent characterization, QDs also have some limitations and challenges that we have reported in this review. To address these issues, novel synthetic methods for QDs should be developed: (I) with an easy procedure, high production yield, and large-scale and low-cost production and (II) to find a way to control the sizes, thicknesses, defects and edges, in turn regulating the optical and chemicophysical features. For example, surface modifications on the QDs could be applied to adjust the PL properties, such as enhancing the quantum yield and forming green, even red fluorescent TMD QDs, h-BN, phosphorene, MXenes, germanene and silicone. Additionally, a better understanding of the mechanism of PL from QDs is one main goal to be pursued in the future, which is beneficial for deepening application potential. In particular, novel QDs with strong absorbance in the second NIR region (>950 nm) or high photoluminescence in the NIR region (>700 nm) and high photostability and biocompatibility should be synthesized and developed into QD based nanotheranostics (PDT/PTT based theranostics) to achieve enhanced tissue penetration. To enhance their deep tumor penetrance, total reliance on the EPR may not be wise as the EPR effect is determined by the tumor.172–174 Instead, inducing endothelial leakiness with nanomaterials (NanoEL) at the tumor vasculature could be a more controllable strategy to increase access to the interior of the tumor.172 The various QDs reviewed here are highly suited for inducing Type II (indirect) NanoEL through photoactivatable oxidative stress at the tumor vasculature.172 Moreover with greater control of basic nanomaterials physicochemical parameters such as size, surface charge and density, further fine tuning of both Type I (direct) and Type II (indirect) NanoEL effects can bring about tunability of the degree of accessibility for both nanomedicine and conventional drugs or quick restoration of the endothelial barrier.175–178
Although the development of QDs is essential and still in the early stage, they have proven to be promising for understanding fundamental biology, early diagnosis and theranostics. As the field of 2D QDs expands, more efforts are expected to be dedicated to the design and development of new QDs for future biomedical applications beyond photodynamic and photothermal agents. Successful development for further biological applications can be aided by a better understanding and mitigation of potential toxic effects that could occur.179
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the funding provided by the National Research Foundation, Prime Minister's Office, Singapore, under the Competitive Research Program (Award No. NRFCRP13-2014-03).Notes and references
- Y. Liu, X. Dong and P. Chen, Chem. Soc. Rev., 2012, 41, 2283–2307 RSC.
- X. Wang, G. Sun, P. Routh, D. H. Kim, W. Huang and P. Chen, Chem. Soc. Rev., 2014, 43, 7067–7098 RSC.
- F. Bonaccorso, L. Colombo, G. Yu, M. Stoller, V. Tozzini, A. C. Ferrari, R. S. Ruoff and V. Pellegrini, Science, 2015, 347, 1246501 CrossRef.
- H. J. Zhu, Y. J. Zhang, L. L. Zhang, T. Yu, K. Zhang, H. Jiang, L. J. Wu and S. H. Wang, J. Mater. Chem. C, 2014, 2, 7126–7132 RSC.
- H. J. Zhu, H. D. Xu, Y. H. Yan, K. Zhang, T. Yu, H. Jiang and S. H. Wang, Sens. Actuators, B, 2014, 202, 667–673 CrossRef CAS.
- H. Li, R. Y. Tay, S. H. Tsang, X. Zhen and E. H. Teo, Small, 2015, 11, 6491–6499 CrossRef CAS.
- Z. Lei, S. Xu, J. Wan and P. Wu, Nanoscale, 2015, 7, 18902–18907 RSC.
- Y. Noda, C. Merschjann, J. Tarabek, P. Amsalem, N. Koch and M. J. Bojdys, Angew. Chem., Int. Ed., 2019, 58, 9394–9398 CrossRef CAS.
- J. Liu, Y. Zhang, L. Zhang, F. Xie, A. Vasileff and S. Z. Qiao, Adv. Mater., 2019, 31, e1901261 CrossRef.
- B. L. Li, H. L. Zou, J. K. Tian, G. Chen, X. H. Wang, H. Duan, X. L. Li, Y. Shi, J. R. Chen, L. J. Li, J. L. Lei, H. Q. Luo and N. B. Li, Nano Energy, 2019, 60, 689–700 CrossRef CAS.
- B. L. Li, L. Y. Peng, H. L. Zou, L. J. Li, H. Q. Luo and N. B. Li, Small, 2018, 14, 1703560 CrossRef.
- B. L. Li, H. L. Zou, L. Lu, Y. Yang, J. L. Lei, H. Q. Luo and N. B. Li, Adv. Funct. Mater., 2015, 25, 3541–3550 CrossRef CAS.
- H. Zhang, H. He, X. Jiang, Z. Xia and W. Wei, ACS Appl. Mater. Interfaces, 2018, 10, 30680–30688 CrossRef CAS PubMed.
- X. Zhang, Z. Lai, Q. Ma and H. Zhang, Chem. Soc. Rev., 2018, 47, 3301–3338 RSC.
- M. Chhowalla, H. S. Shin, G. Eda, L. J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef.
- H. Zhu, Z. Lai, Y. Fang, X. Zhen, C. Tan, X. Qi, D. Ding, P. Chen, H. Zhang and K. Pu, Small, 2017, 13, 1604139 CrossRef.
- T. Hartman and Z. Sofer, ACS Nano, 2019, 13, 8566–8576 CrossRef CAS.
- N. Liu, G. Bo, Y. Liu, X. Xu, Y. Du and S. X. Dou, Small, 2019, 15, e1805147 CrossRef.
- H. Lin, W. Qiu, J. Liu, L. Yu, S. Gao, H. Yao, Y. Chen and J. Shi, Adv. Mater., 2019, e1903013 CrossRef.
- A. M. Tokmachev, D. V. Averyanov, O. E. Parfenov, A. N. Taldenkov, I. A. Karateev, I. S. Sokolov, O. A. Kondratev and V. G. Storchak, Nat. Commun., 2018, 9, 1672 CrossRef PubMed.
- W. Zhang, L. Sun, J. M. V. Nsanzimana and X. Wang, Adv. Mater., 2018, 30, e1705523 CrossRef.
- C. N. Rao, H. S. Matte and U. Maitra, Angew. Chem., Int. Ed., 2013, 52, 13162–13185 CrossRef CAS.
- X. Huang, C. Tan, Z. Yin and H. Zhang, Adv. Mater., 2014, 26, 2185–2204 CrossRef CAS PubMed.
- P. Miro, M. Audiffred and T. Heine, Chem. Soc. Rev., 2014, 43, 6537–6554 RSC.
- Y. S. Rim, S. H. Bae, H. Chen, N. De Marco and Y. Yang, Adv. Mater., 2016, 28, 4415–4440 CrossRef CAS.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tian and X. Bao, Nat. Nanotechnol., 2016, 11, 218–230 CrossRef CAS.
- S. H. Lee, D. Y. Kim, J. Lee, S. B. Lee, H. Han, Y. Y. Kim, S. C. Mun, S. H. Im, T. H. Kim and O. O. Park, Nano Lett., 2019, 19, 5437–5442 CrossRef CAS.
- Y. Yan, J. Gong, J. Chen, Z. Zeng, W. Huang, K. Pu, J. Liu and P. Chen, Adv. Mater., 2019, 31, e1808283 CrossRef.
- A. L. Exarhos, D. A. Hopper, R. N. Patel, M. W. Doherty and L. C. Bassett, Nat. Commun., 2019, 10, 222 CrossRef.
- Q. Wang, Q. Zhang, X. Zhao, X. Luo, C. P. Y. Wong, J. Wang, D. Wan, T. Venkatesan, S. J. Pennycook, K. P. Loh, G. Eda and A. T. S. Wee, Nano Lett., 2018, 18, 6898–6905 CrossRef CAS.
- B. L. Li, L. X. Chen, H. L. Zou, J. L. Lei, H. Q. Luo and N. B. Li, Nanoscale, 2014, 6, 9831–9838 RSC.
- X. Ding, F. Peng, J. Zhou, W. Gong, G. Slaven, K. P. Loh, C. T. Lim and D. T. Leong, Nat. Commun., 2019, 10, 41 CrossRef PubMed.
- C. C. Price, N. C. Frey, D. Jariwala and V. B. Shenoy, ACS Nano, 2019, 13, 8303–8311 CrossRef CAS.
- X. Lu, X. Chen, S. Dubey, Q. Yao, W. Li, X. Wang, Q. Xiong and A. Srivastava, Nat. Nanotechnol., 2019, 14, 426–431 CrossRef CAS.
- W. Chen, J. Gu, Q. Liu, R. Luo, L. Yao, B. Sun, W. Zhang, H. Su, B. Chen, P. Liu and D. Zhang, ACS Nano, 2018, 12, 308–316 CrossRef CAS PubMed.
- X. Zhang, Z. Lai, Z. Liu, C. Tan, Y. Huang, B. Li, M. Zhao, L. Xie, W. Huang and H. Zhang, Angew. Chem., Int. Ed., 2015, 54, 5425–5428 CrossRef CAS PubMed.
- B. L. Li, J. P. Wang, Z. F. Gao, H. Shi, H. L. Zou, K. Arigaef and D. T. Leong, Mater. Horiz., 2014, 2019, 563–570 Search PubMed.
- Y. Yu, Y. Yang, J. Ding, S. Meng, C. Li and X. Yin, Anal. Chem., 2018, 90, 13290–13298 CrossRef CAS.
- Q. Cui, J. Xu, X. Wang, L. Li, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2016, 55, 3672–3676 CrossRef CAS.
- Z. Song, T. Lin, L. Lin, S. Lin, F. Fu, X. Wang and L. Guo, Angew. Chem., Int. Ed., 2016, 55, 2773–2777 CrossRef CAS PubMed.
- F. Liu, M. H. Jang, H. D. Ha, J. H. Kim, Y. H. Cho and T. S. Seo, Adv. Mater., 2013, 25, 3657–3662 CrossRef CAS PubMed.
- J. M. Yoo, J. H. Kang and B. H. Hong, Chem. Soc. Rev., 2015, 44, 4835–4852 RSC.
- B. L. Li, M. I. Setyawati, L. Chen, J. Xie, K. Ariga, C. T. Lim, S. Garaj and D. T. Leong, ACS Appl. Mater. Interfaces, 2017, 9, 15286–15296 CrossRef CAS.
- Q. Guan, J. Ma, W. Yang, R. Zhang, X. Zhang, X. Dong, Y. Fan, L. Cai, Y. Cao, Y. Zhang, N. Li and Q. Xu, Nanoscale, 2019, 11, 14123–14133 RSC.
- L. Long, X. Niu, K. Yan, G. Zhou, J. Wang, X. Wu and P. K. Chu, Small, 2018, 14, e1803132 CrossRef PubMed.
- Y. Xu, X. Wang, W. L. Zhang, F. Lv and S. Guo, Chem. Soc. Rev., 2018, 47, 586–625 RSC.
- S. Angizi, A. Hatamie, H. Ghanbari and A. Simchi, ACS Appl. Mater. Interfaces, 2018, 10, 28819–28827 CrossRef CAS PubMed.
- H. Zhu, W. Zhang, K. Zhang and S. Wang, Nanotechnology, 2012, 23, 315502 CrossRef PubMed.
- H. Dong, S. Tang, Y. Hao, H. Yu, W. Dai, G. Zhao, Y. Cao, H. Lu, X. Zhang and H. Ju, ACS Appl. Mater. Interfaces, 2016, 8, 3107–3114 CrossRef CAS PubMed.
- Y. Yong, X. Cheng, T. Bao, M. Zu, L. Yan, W. Yin, C. Ge, D. Wang, Z. Gu and Y. Zhao, ACS Nano, 2015, 9, 12451–12463 CrossRef CAS PubMed.
- J. Ge, M. Lan, B. Zhou, W. Liu, L. Guo, H. Wang, Q. Jia, G. Niu, X. Huang, H. Zhou, X. Meng, P. Wang, C. S. Lee, W. Zhang and X. Han, Nat. Commun., 2014, 5, 4596 CrossRef CAS PubMed.
- W. S. Kuo, Y. T. Shao, K. S. Huang, T. M. Chou and C. H. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 14438–14446 CrossRef CAS PubMed.
- R. Gui, H. Jin, Z. Wang and J. Li, Chem. Soc. Rev., 2018, 47, 6795–6823 RSC.
- P. Zhang, Y. Zhuo, Y. Chang, R. Yuan and Y. Chai, Anal. Chem., 2015, 87, 10385–10391 CrossRef CAS PubMed.
- B. L. Li, M. I. Setyawati, H. L. Zou, J. X. Dong, H. Q. Luo, N. B. Li and D. T. Leong, Small, 2017, 13, 1700527 CrossRef PubMed.
- B. L. Li, J. P. Wang, H. L. Zou, S. Garaj, C. H. Lim, J. P. Xie, N. B. Li and D. T. Leong, Adv. Funct. Mater., 2016, 18, 7034–7056 CrossRef.
- K. Wang, Y. Chen, J. Zheng, Y. Ge, J. Ji, Y. Song and H. Zhang, Nanotechnology, 2019, 30, 415202 CrossRef PubMed.
- S. Han, X. Yang, Y. Zhu, C. Tan, X. Zhang, J. Chen, Y. Huang, B. Chen, Z. Luo, Q. Ma, M. Sindoro, H. Zhang, X. Qi, H. Li, X. Huang, W. Huang, X. W. Sun and Y. Han, Angew. Chem., Int. Ed., 2017, 56, 10486–10490 CrossRef CAS PubMed.
- X. Wang, G. Sun, N. Li and P. Chen, Chem. Soc. Rev., 2016, 45, 2239–2262 RSC.
- T. F. Jaramillo, K. P. Jorgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed.
- A. Moshaverinia, C. Chen, X. Xu, S. Ansari, H. H. Zadeh, S. R. Schricker, M. L. Paine, J. Moradian-Oldak, A. Khademhosseini, M. L. Snead and S. Shi, Adv. Funct. Mater., 2015, 25, 2296–2307 CrossRef CAS PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- K. Liu, X. Liu, Q. Zeng, Y. Zhang, L. Tu, T. Liu, X. Kong, Y. Wang, F. Cao, S. A. Lambrechts, M. C. Aalders and H. Zhang, ACS Nano, 2012, 6, 4054–4062 CrossRef CAS PubMed.
- D. E. J. G. J. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 CrossRef CAS PubMed.
- S. B. Brown, E. A. Brown and I. Walker, Lancet Oncol., 2004, 5, 497–508 CrossRef CAS PubMed.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS.
- P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526–4527 CrossRef CAS PubMed.
- H. Y. Liu, D. Chen, L. L. Li, T. L. Liu, L. F. Tan, X. L. Wu and F. Q. Tang, Angew. Chem., Int. Ed., 2011, 50, 891–895 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. G. Li, J. H. Chen, C. Wang, M. W. Shao, S. T. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385–7390 CrossRef CAS PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Y. Liang, H. L. Wang, H. S. Casalongue, D. Vinh and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- H. Zhu, J. Li, X. Qi, P. Chen and K. Pu, Nano Lett., 2018, 18, 586–594 CrossRef CAS PubMed.
- P. Huang, J. Lin, X. Wang, Z. Wang, C. Zhang, M. He, K. Wang, F. Chen, Z. Li, G. Shen, D. Cui and X. Chen, Adv. Mater., 2012, 24, 5104–5110 CrossRef CAS PubMed.
- H. Zhu, Y. Fang, Q. Miao, X. Qi, D. Ding, P. Chen and K. Pu, ACS Nano, 2017, 11, 8998–9009 CrossRef CAS.
- H. Zhu, P. Cheng, P. Chen and K. Pu, Biomater. Sci., 2018, 6, 746–765 RSC.
- Y. Cheng, A. C. Samia, J. D. Meyers, I. Panagopoulos, B. W. Fei and C. Burda, J. Am. Chem. Soc., 2008, 130, 10643–10647 CrossRef CAS PubMed.
- S. J. Wang, P. Huang, L. M. Nie, R. J. Xing, D. B. Liu, Z. Wang, J. Lin, S. H. Chen, G. Niu, G. M. Lu and X. Y. Chen, Adv. Mater., 2013, 25, 3055–3061 CrossRef CAS PubMed.
- C. P. Wang, X. P. Cai, J. S. Zhang, X. Y. Wang, Y. Wang, H. F. Ge, W. J. Yan, Q. Huang, J. R. Xiao, Q. Zhang and Y. Y. Cheng, Small, 2015, 11, 2080–2086 CrossRef CAS.
- M. Manikandan, N. Hasan and H. F. Wu, Biomaterials, 2013, 34, 5833–5842 CrossRef CAS PubMed.
- X. Yi, K. Yang, C. Liang, X. Y. Zhong, P. Ning, G. S. Song, D. L. Wang, C. C. Ge, C. Y. Chen, Z. F. Chai and Z. Liu, Adv. Funct. Mater., 2015, 25, 4689–4699 CrossRef CAS.
- S. G. Wang, X. Li, Y. Chen, X. J. Cai, H. L. Yao, W. Gao, Y. Y. Zheng, X. An, J. L. Shi and H. R. Chen, Adv. Mater., 2015, 27, 2775–2782 CrossRef CAS.
- J. Liu, X. Zheng, L. Yan, L. Zhou, G. Tian, W. Yin, L. Wang, Y. Liu, Z. Hu, Z. Gu, C. Chen and Y. Zhao, ACS Nano, 2015, 9, 696–707 CrossRef CAS.
- T. Liu, C. Wang, X. Gu, H. Gong, L. Cheng, X. Z. Shi, L. Z. Feng, B. Q. Sun and Z. Liu, Adv. Mater., 2014, 26, 3433–3440 CrossRef CAS.
- G. Tian, X. Zhang, X. P. Zheng, W. Y. Yin, L. F. Ruan, X. D. Liu, L. J. Zhou, L. Yan, S. J. Li, Z. J. Gu and Y. L. Zhao, Small, 2014, 10, 4160–4170 CAS.
- G. S. Song, J. L. Hao, C. Liang, T. Liu, M. Gao, L. Cheng, J. Q. Hu and Z. Liu, Angew. Chem., Int. Ed., 2016, 55, 2122–2126 CrossRef CAS PubMed.
- Q. Chen, L. Z. Feng, J. J. Liu, W. W. Zhu, Z. L. Dong, Y. F. Wu and Z. Liu, Adv. Mater., 2016, 28, 7129–7136 CrossRef CAS PubMed.
- P. Huang, C. Xu, J. Lin, C. Wang, X. S. Wang, C. L. Zhang, X. J. Zhou, S. W. Guo and D. X. Cui, Theranostics, 2011, 1, 240–250 CrossRef CAS PubMed.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- X. Cao, C. Ding, C. Zhang, W. Gu, Y. Yan, X. Shi and Y. Xian, J. Mater. Chem. B, 2018, 6, 8011–8036 RSC.
- K. Huang, Z. Li, J. Lin, G. Han and P. Huang, Chem. Soc. Rev., 2018, 47, 5109–5124 RSC.
- X. Duan, C. Wang, A. Pan and R. Yu, Chem. Soc. Rev., 2015, 44, 8859–8876 RSC.
- F. Yu, Q. Liu, X. Gan, M. Hu, T. Zhang, C. Li, F. Kang, M. Terrones and R. Lv, Adv. Mater., 2017, 29, 1603266 CrossRef PubMed.
- K. K. Liu, W. Zhang, Y. H. Lee, Y. C. Lin, M. T. Chang, C. Y. Su, C. S. Chang, H. Li, Y. Shi, H. Zhang, C. S. Lai and L. J. Li, Nano Lett., 2012, 12, 1538–1544 CrossRef CAS PubMed.
- H. D. Ha, D. J. Han, J. S. Choi, M. Park and T. S. Seo, Small, 2014, 10, 3858–3862 CrossRef CAS PubMed.
- B. Li, L. Jiang, X. Li, P. Ran, P. Zuo, A. Wang, L. Qu, Y. Zhao, Z. Cheng and Y. Lu, Sci. Rep., 2017, 7, 11182 CrossRef PubMed.
- S. J. Xu, D. Li and P. Y. Wu, Adv. Funct. Mater., 2015, 25, 1127–1136 CrossRef CAS.
- W. Gu, Y. Yan, C. Zhang, C. Ding and Y. Xian, ACS Appl. Mater. Interfaces, 2016, 8, 11272–11279 CrossRef CAS PubMed.
- H. Jin, M. Ahn, S. Jeong, J. H. Han, D. Yoo, D. H. Son and J. Cheon, J. Am. Chem. Soc., 2016, 138, 13253–13259 CrossRef CAS PubMed.
- X. Zhao, X. Ma, J. Sun, D. Li and X. Yang, ACS Nano, 2016, 10, 2159–2166 CrossRef CAS PubMed.
- C. Zhu, Y. Huang, F. Xu, P. Gao, B. Ge, J. Chen, H. Zeng, E. Sutter, P. Sutter and L. Sun, Small, 2017, 13, 1700565 CrossRef PubMed.
- L. Lin, Y. Xu, S. Zhang, I. M. Ross, A. C. Ong and D. A. Allwood, ACS Nano, 2013, 7, 8214–8223 CrossRef CAS PubMed.
- T. Liu, Y. Chao, M. Gao, C. Liang, Q. Chen, G. S. Song, L. Cheng and Z. Liu, Nano Res., 2016, 9, 3003–3017 CrossRef CAS.
- L. Yuwen, J. Zhou, Y. Zhang, Q. Zhang, J. Shan, Z. Luo, L. Weng, Z. Teng and L. Wang, Nanoscale, 2016, 8, 2720–2726 RSC.
- P. Li, L. Liu, Q. Lu, S. Yang, L. Yang, Y. Cheng, Y. Wang, S. Wang, Y. Song, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2019, 11, 5771–5781 CrossRef CAS PubMed.
- J. Wang, X. Pang, X. Tan, Y. Song, L. Liu, Q. You, Q. Sun, F. Tan and N. Li, Nanoscale, 2017, 9, 5551–5564 RSC.
- J. Wang, X. Tan, X. Pang, L. Liu, F. Tan and N. Li, ACS Appl. Mater. Interfaces, 2016, 8, 24331–24338 CrossRef CAS PubMed.
- X. Tian, Y. Sun, S. Fan, M. D. Boudreau, C. Chen, C. Ge and J. J. Yin, ACS Appl. Mater. Interfaces, 2019, 11, 4858–4866 CrossRef CAS PubMed.
- S. J. Wang, I. S. Coleb and Q. Li, RSC Adv., 2016, 6, 89867–89878 RSC.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- Y. Cao, H. Dong, Z. Yang, X. Zhong, Y. Chen, W. Dai and X. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 159–166 CrossRef CAS.
- W. W. Liu, Y. Q. Feng, X. B. Yan, J. T. Chen and Q. J. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS.
- F. Yuan, T. Yuan, L. Sui, Z. Wang, Z. Xi, Y. Li, X. Li, L. Fan, Z. Tan, A. Chen, M. Jin and S. Yang, Nat. Commun., 2018, 9, 2249 CrossRef PubMed.
- S. Kim, S. W. Hwang, M. K. Kim, D. Y. Shin, D. H. Shin, C. O. Kim, S. B. Yang, J. H. Park, E. Hwang, S. H. Choi, G. Ko, S. Sim, C. Sone, H. J. Choi, S. Bae and B. H. Hong, ACS Nano, 2012, 6, 8203–8208 CrossRef CAS PubMed.
- D. I. Son, B. W. Kwon, D. H. Park, W. S. Seo, Y. Yi, B. Angadi, C. L. Lee and W. K. Choi, Nat. Nanotechnol., 2012, 7, 465–471 CrossRef CAS PubMed.
- N. Li, A. Than, C. Sun, J. Tian, J. Chen, K. Pu, X. Dong and P. Chen, ACS Nano, 2016, 10, 11475–11482 CrossRef CAS PubMed.
- N. Li, A. Than, X. Wang, S. Xu, L. Sun, H. Duan, C. Xu and P. Chen, ACS Nano, 2016, 10, 3622–3629 CrossRef CAS PubMed.
- S. Some, A. R. Gwon, E. Hwang, G. H. Bahn, Y. Yoon, Y. Kim, S. H. Kim, S. Bak, J. Yang, D. G. Jo and H. Lee, Sci. Rep., 2014, 4, 6314 CrossRef CAS PubMed.
- D. Pan, J. Zhang, Z. Li and M. Wu, Adv. Mater., 2010, 22, 734–738 CrossRef CAS PubMed.
- S. Li, S. Zhou, Y. Li, X. Li, J. Zhu, L. Fan and S. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 22332–22341 CrossRef CAS PubMed.
- M. Thakur, M. K. Kumawat and R. Srivastava, RSC Adv., 2017, 7, 5251–5261 RSC.
- Z. M. Markovic, B. Z. Ristic, K. M. Arsikin, D. G. Klisic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepic, T. K. Kravic-Stevovic, S. P. Jovanovic, M. M. Milenkovic, D. D. Milivojevic, V. Z. Bumbasirevic, M. D. Dramicanin and V. S. Trajkovic, Biomaterials, 2012, 33, 7084–7092 CrossRef CAS PubMed.
- S. Y. Choi, S. H. Baek, S. J. Chang, Y. Song, R. Rafique, K. T. Lee and T. J. Park, Biosens. Bioelectron., 2017, 93, 267–273 CrossRef CAS.
- B. Z. Ristic, M. M. Milenkovic, I. R. Dakic, B. M. Todorovic-Markovic, M. S. Milosavljevic, M. D. Budimir, V. G. Paunovic, M. D. Dramicanin, Z. M. Markovic and V. S. Trajkovic, Biomaterials, 2014, 35, 4428–4435 CrossRef CAS.
- S. P. Jovanovic, Z. Syrgiannis, Z. M. Markovic, A. Bonasera, D. P. Kepic, M. D. Budimir, D. D. Milivojevic, V. D. Spasojevic, M. D. Dramicanin, V. B. Pavlovic and B. M. T. Marković, ACS Appl. Mater. Interfaces, 2015, 7, 25865–25874 CrossRef CAS.
- M. Nafiujjaman, M. Nurunnabi, S. H. Kang, G. R. Reeck, H. A. Khan and Y. K. Lee, J. Mater. Chem. B, 2015, 3, 5815–5823 RSC.
- J. D. Monroe, E. Belekov, A. O. Er and M. E. Smith, Photochem. Photobiol., 2019, 95, 1473–1481 CrossRef CAS PubMed.
- A. M. Derfus, W. C. W. Chan and S. N. Bhatia, Nano Lett., 2004, 4, 11–18 CrossRef CAS.
- H. Zhu, T. Yu, H. Xu, K. Zhang, H. Jiang, Z. Zhang, Z. Wang and S. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 21461–21467 CrossRef CAS PubMed.
- K. Li, W. Liu, Y. Ni, D. Li, D. Lin, Z. Su and G. Wei, J. Mater. Chem. B, 2017, 5, 4811–4826 RSC.
- K. Liu, R. Xing, Q. Zou, G. Ma, H. Mohwald and X. Yan, Angew. Chem., Int. Ed., 2016, 55, 3036–3039 CrossRef CAS PubMed.
- Y. Y. Li, D. Du, H. Dong, D. Shi, Y. Y. Li, Z. Wu, D. Du, H. Dong, D. Shi and Y. Y. Li, RSC Adv., 2016, 6, 6516–6522 RSC.
- H. M. Meng, D. Zhao, N. Li and J. Chang, Analyst, 2018, 143, 4967–4973 RSC.
- C. Wu, X. Guan, J. Xu, Y. Zhang, Q. Liu, Y. Tian, S. Li, X. Qin, H. Yang and Y. Liu, Biomaterials, 2019, 205, 106–119 CrossRef CAS.
- J. V. Jokerst, T. Lobovkina, R. N. Zare and S. S. Gambhir, Nanomedicine, 2011, 6, 715–728 CrossRef CAS PubMed.
- K. Habiba, J. Encarnacion-Rosado, K. Garcia-Pabon, J. C. Villalobos-Santos, V. I. Makarov, J. A. Avalos, B. R. Weiner and G. Morell, Int. J. Nanomed., 2016, 11, 107–119 CrossRef CAS PubMed.
- W. S. Kuo, C. Y. Chang, H. H. Chen, C. L. Hsu, J. Y. Wang, H. F. Kao, L. C. Chou, Y. C. Chen, S. J. Chen, W. T. Chang, S. W. Tseng, P. C. Wu and Y. C. Pu, ACS Appl. Mater. Interfaces, 2016, 8, 30467–30474 CrossRef CAS PubMed.
- K. Kholikov, S. Ilhom, M. Sajjad, M. E. Smith, J. D. Monroe, O. San and A. O. Er, Photodiagn. Photodyn. Ther., 2018, 24, 7–14 CrossRef CAS PubMed.
- W. S. Kuo, H. H. Chen, S. Y. Chen, C. Y. Chang, P. C. Chen, Y. I. Hou, Y. T. Shao, H. F. Kao, C. L. L. Hsu, Y. C. Chen, S. J. Chen, S. R. Wu and J. Y. Wang, Biomaterials, 2017, 120, 185–194 CrossRef CAS PubMed.
- J. Ju, S. Regmi, A. Fu, S. Lim and Q. Liu, J. Biophotonics, 2019, 12, e201800367 CrossRef PubMed.
- J. Sun, Q. Xin, Y. Yang, H. Shah, H. Cao, Y. Qi, J. R. Gong and J. Li, Chem. Commun., 2018, 54, 715–718 RSC.
- D. Zhang, L. Wen, R. Huang, H. Wang, X. Hu and D. Xing, Biomaterials, 2018, 153, 14–26 CrossRef CAS PubMed.
- Y. Liu, Y. Xu, X. Geng, Y. Huo, D. Chen, K. Sun, G. Zhou, B. Chen and K. Tao, Small, 2018, 14, e1800293 CrossRef PubMed.
- Z. Tian, X. Yao, K. Ma, X. Niu, J. Grothe, Q. Xu, L. Liu, S. Kaskel and Y. Zhu, ACS Omega, 2017, 2, 1249–1258 CrossRef CAS PubMed.
- Y. Xuan, R. Y. Zhang, X. S. Zhang, J. An, K. Cheng, C. Li, X. L. Hou and Y. D. Zhao, Nanotechnology, 2018, 29, 355101 CrossRef PubMed.
- X. Yao, X. Niu, K. Ma, P. Huang, J. Grothe, S. Kaskel and Y. Zhu, Small, 2017, 13, 1602225 CrossRef PubMed.
- X. Yao, Z. Tian, J. Liu, Y. Zhu and N. Hanagata, Langmuir, 2017, 33, 591–599 CrossRef CAS PubMed.
- F. Wo, R. Xu, Y. Shao, Z. Zhang, M. Chu, D. Shi and S. Liu, Theranostics, 2016, 6, 485–500 CrossRef CAS PubMed.
- X. Wang, X. Sun, H. He, H. Yang, J. Lao, Y. Song, Y. Xia, H. Xu, X. Zhang and F. Huang, J. Mater. Chem. B, 2015, 3, 3583–3590 RSC.
- J. Fang, Y. Liu, Y. Chen, D. Ouyang, G. Yang and T. Yu, Int. J. Nanomed., 2018, 13, 5991–6007 CrossRef CAS PubMed.
- F. Xia, H. Wang and Y. Jia, Nat. Commun., 2014, 5, 4458 CrossRef CAS PubMed.
- H. Liu, A. T. Neal, Z. Zhu, Z. Luo, X. Xu, D. Tomanek and P. D. Ye, ACS Nano, 2014, 8, 4033–4041 CrossRef CAS PubMed.
- Z. Sun, H. Xie, S. Tang, X. F. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., Int. Ed., 2015, 54, 11526–11530 CrossRef CAS PubMed.
- J. Shao, H. Xie, H. Huang, Z. Li, Z. Sun, Y. Xu, Q. Xiao, X. F. Yu, Y. Zhao, H. Zhang, H. Wang and P. K. Chu, Nat. Commun., 2016, 7, 12967 CrossRef CAS PubMed.
- H. Wang, X. Yang, W. Shao, S. Chen, J. Xie, X. Zhang, J. Wang and Y. Xie, J. Am. Chem. Soc., 2015, 137, 11376–11382 CrossRef CAS PubMed.
- Y. Li, Z. Liu, Y. Hou, G. Yang, X. Fei, H. Zhao, Y. Guo, C. Su, Z. Wang, H. Zhong, Z. Zhuang and Z. Guo, ACS Appl. Mater. Interfaces, 2017, 9, 25098–25106 CrossRef CAS PubMed.
- T. Guo, Y. Wu, Y. Lin, X. Xu, H. Lian, G. Huang, J. Z. Liu, X. Wu and H. H. Yang, Small, 2018, 14, 1702815 CrossRef PubMed.
- S. Lan, Z. Lin, D. Zhang, Y. Zeng and X. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 9804–9813 CrossRef CAS PubMed.
- X. Chu, K. Li, H. Y. Guo, H. B. Zheng, S. Shuda, X. L. Wang, J. Y. Zhang, W. Chen and Y. Zhang, ACS Biomater. Sci. Eng., 2017, 3, 1836–1844 CrossRef CAS.
- X. X. Wu, L. Y. Yang, L. Luo, G. H. Shi, X. B. Wei and F. Wang, ACS Appl. Bio Mater., 2019, 2, 1998–2005 CrossRef CAS.
- D. W. Zheng, B. Li, C. X. Li, J. X. Fan, Q. Lei, C. Li, Z. Xu and X. Z. Zhang, ACS Nano, 2016, 10, 8715–8722 CrossRef CAS PubMed.
- X. Chu, K. Li, H. Y. Guo, H. B. Zheng, S. Shuda, X. L. Wang, J. Y. Zhang, W. Chen and Y. Zhang, ACS Biomater. Sci. Eng., 2017, 3, 1836–1844 CrossRef CAS.
- M. Yao, L. Ma, L. Li, J. Zhang, R. Lim, W. Chen and Y. Zhang, J. Biomed. Nanotechnol., 2016, 12, 1835–1851 CrossRef CAS PubMed.
- X. X. Wu, L. Y. Yang, L. Luo, G. H. Shi, X. B. Wei and F. Wang, ACS Appl. Bio Mater., 2019, 2, 1998–2005 CrossRef CAS.
- J. Ji, X. Song, J. Liu, Z. Yan, C. Huo, S. Zhang, M. Su, L. Liao, W. Wang, Z. Ni, Y. Hao and H. Zeng, Nat. Commun., 2016, 7, 13352 CrossRef CAS PubMed.
- W. Tao, X. Ji, X. Xu, M. A. Islam, Z. Li, S. Chen, P. E. Saw, H. Zhang, Z. Bharwani, Z. Guo, J. Shi and O. C. Farokhzad, Angew. Chem., Int. Ed., 2017, 56, 11896–11900 CrossRef CAS PubMed.
- G. Song, J. Shen, F. Jiang, R. Hu, W. Li, L. An, R. Zou, Z. Chen, Z. Qin and J. Hu, ACS Appl. Mater. Interfaces, 2014, 6, 3915–3922 CrossRef CAS PubMed.
- D. Ding, W. Guo, C. Guo, J. Sun, N. Zheng, F. Wang, M. Yan and S. Liu, Nanoscale, 2017, 9, 2020–2029 RSC.
- H. R. Zu, Y. X. Guo, H. Y. Yang, D. Huang, Z. M. Liu, Y. L. Liu and C. F. Hu, New J. Chem., 2018, 42, 18533–18540 RSC.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS PubMed.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- X. Yu, X. Cai, H. Cui, S. W. Lee, X. F. Yu and B. Liu, Nanoscale, 2017, 9, 17859–17864 RSC.
- J. K. Tee, L. X. Yip, E. S. Tan, S. Santitewagun, A. Prasath, P. C. Ke, H. K. Ho and D. T. Leong, Chem. Soc. Rev., 2019, 48, 5381–5407 RSC.
- F. Peng, M. I. Setyawati, J. K. Tee, X. Ding, J. Wang, M. E. Nga, H. K. Ho and D. T. Leong, Nat. Nanotechnol., 2019, 14, 279–286 CrossRef CAS PubMed.
- M. I. Setyawati, C. Y. Tay, S. L. Chia, S. L. Goh, W. Fang, M. J. Neo, H. C. Chong, S. M. Tan, S. C. Loo, K. W. Ng, J. P. Xie, C. N. Ong, N. S. Tan and D. T. Leong, Nat. Commun., 2013, 4, 1673 CrossRef CAS PubMed.
- M. I. Setyawati, C. Y. Tay, B. H. Bay and D. T. Leong, ACS Nano, 2017, 11, 5020–5030 CrossRef CAS PubMed.
- C. Y. Tay, M. I. Setyawati and D. T. Leong, ACS Nano, 2017, 11, 2764–2772 CrossRef CAS PubMed.
- J. K. Tee, M. I. Setyawati, F. Peng, D. T. Leong and H. K. Ho, Nanotoxicology, 2019, 13, 682–700 CrossRef CAS PubMed.
- J. Wang, L. Zhang, F. Peng, X. Shi and D. T. Leong, Chem. Mater., 2018, 30, 3759–3767 CrossRef CAS.
- E. Tan, B. L. Li, K. Ariga, C. T. Lim, S. Garaj and D. T. Leong, Bioconjugate Chem., 2019, 30, 2287–2299 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
: