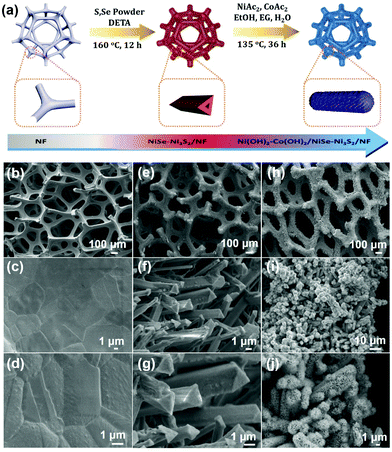
Hierarchical bimetallic hydroxide/chalcogenide core–sheath microarrays for freestanding ultrahigh rate supercapacitors†
Jia
Xu
,
Fenfen
Han
,
Dianlei
Fang
,
Xinlei
Wang
,
Jian
Tang
* and
Weihua
Tang
 *
*
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, P.R. China. E-mail: tangjian@njust.edu.cn; whtang@njust.edu.cn
First published on 3rd December 2019
Abstract
Transition metal compounds (TMCs) either crystalline or amorphous exhibit specific advantages in electrochemical energy storage. To integrate their merits into one electrode, we have herein developed hierarchical bimetallic hydroxide/chalcogenide core–sheath microarrays on nickel foam (NF) for freestanding high-efficiency supercapacitors, wherein interior crystalline metal chalcogenides serve as highly conductive pivots and exterior amorphous bimetallic hydroxides provide rich ion diffusion channels. With the synergic effect of the unique structure and bimetallic composition, the as-prepared Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF electrode displays an ultrahigh areal specific capacitance of 19.01 F cm−2 at 15 mA cm−2, which can be retained as 6.01 F cm−2 even at 125 mA cm−2. To the best of our knowledge, such excellent tolerance of ultrafast ion insertion/extraction at high current density is rare among NF-based free-standing electrodes. The asymmetric supercapacitor by assembling with activated carbon as the negative electrode delivers a volumetric capacitance of 3.93 F cm−3 at 30 mA cm−2, corresponding to an energy density of 13.9 mW h cm−3 at a power density of 200 mW cm−3. A capacitance retention of 82.5% was observed after 4000 cycles, together with an average 97% coulombic efficiency. This work may provide a facile strategy to construct hierarchical microarrays for efficient energy storage devices.
Transition metal compounds (TMCs) have attracted intensive attention for development as electrode materials for high energy density supercapacitors (SCs).1–6 Owing to the surface redox reaction mechanism, this type of electrode material can afford higher specific capacitance than carbon-based counterparts, offering great promise to rival the energy density of conventional batteries. Taking into account high energy density and power density, we must conduct nano-engineering on the electrode structure to promote rapid delivery of sufficient charges.7 However, the nanometer dimension might reduce the mass loading for freestanding electrodes. For practical applications, high areal mass loading (∼10 mg cm−2) is strongly recommended.8–10 The electron and ion transport would be unfavorably damaged by increased mass loading. Accordingly, the electrochemical performance like rate capability is also affected, impelling the unremitting efforts to balance the mass loading and rate capability.11,12
Most of the reported metal-based electrodes for SCs are crystalline compounds that usually demonstrate good electric conductivities and easy preparation processes.13–15 As a comparison, amorphous metal compounds show worse charge transport and limited synthetic strategies.16–19 Owing to the rich electrolyte diffusion channels and isotropic strain and stress, amorphous species are desired for excellent structural stability within a long-term or high-rate charge/discharge duration.17,18 As such, researchers have explored the strategy of surface amorphization to boost the electrochemical performance by preserving the intrinsic crystalline nature. Zhou et al.20 demonstrated that the surface-amorphized TiO2@graphene had a higher specific capacity and superior cycling stability at high rates than crystalline counterpart electrodes. Thin layers of amorphous metal compounds can also be generated by oxygen vacancy engineering, which was able to modulate the electric conductivity and ion intercalation/deintercalation.21,22 It is, therefore, promising to harvest high-performance metal-based electrodes if we can take advantage of synergetic merits of crystalline/amorphous TMC hybrids on energy storage. However, the preparation of hierarchical crystalline/amorphous structured electrodes besides surface engineering has been rarely established.
Inspired by the surface modification strategy, we herein report novel hierarchical core–sheath TMC microarrays composed of interior crystalline metal chalcogenides and exterior amorphous bimetallic hydroxides as freestanding electrodes for high-efficiency SCs. The binary metal ions of Ni2+/Ni3+ and Co2+/Co3+ can greatly enhance the capacitance by taking advantage of mixed valence states to increase the number of active sites and trigger more redox reactions. As shown in Fig. 1a, NiSe–Ni3S2 triangular prism microarrays on nickel foam (NF) were first prepared with an in situ one-pot solvothermal reaction to generate an average mass loading of NiSe–Ni3S2 of ∼8.5 mg cm−2. The sheath layer of amorphous Ni–Co bimetallic hydroxides using foreign Ni and Co sources was further prepared [denoted as Ni(OH)2–Co(OH)2] to uniformly cover the triangular prism surface of NiSe–Ni3S2 with an average mass loading of ∼12 mg cm−2. By taking advantage of the unique structure and synergistic effect between crystalline and amorphous TMCs, the as-prepared Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF exhibited an ultra-high areal specific capacitance (CA) of 19.01 F cm−2 at a current density of 15 mA cm−2, along with an excellent rate capability of 31% at even 125 mA cm−2. To the best of our knowledge, the results represent the highest areal specific capacitance among the freestanding NF-based electrodes. With activated carbon on NF as the negative electrode, we assembled hybrid supercapacitor devices that achieved a high volumetric energy density of 13.9 mW h cm−3 at a power density of 200 mW cm−3. The energy density can be retained as 4.58 mW h cm−3 when the power density was increased to 1720 mW cm−3.
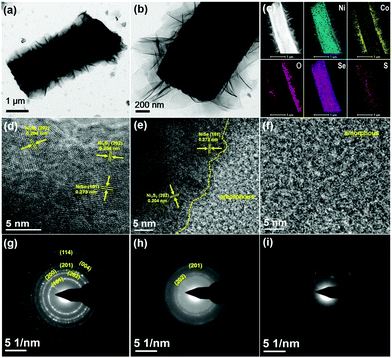
The morphologies of the as-prepared hierarchical electrode were evaluated by using a field emission scanning electron microscope (FE-SEM). Fig. 1b–d show a smooth surface with clear grain boundaries for pristine NF. After the nickel source from NF reacts with mixed S/Se powders, the surface on NF is much rougher than the pristine substrate due to the complete coverage of nickel chalcogenides (Fig. 1e). We can clearly observe vertical microarrays grown on the NF support, together with a rigid triangular prism structure for each nickel chalcogenide subset (Fig. 1f and g). As no additional nickel source is required, these microarrays can be treated as the result of in situ growth of nickel chalcogenides, which is beneficial for the electron extraction from the current collectors. The subsequent hydrothermal growth of Ni(OH)2–Co(OH)2 leads to a much rougher surface for the overall electrode (Fig. 1h–j). The unique triangular prisms serve as the support for the growth of Ni(OH)2–Co(OH)2 nanosheets, without any aggregates between the nickel chalcogenide intervals. It is noteworthy that these metal hydroxides are vertically distributed on triangular prisms with rich space between adjacent nanosheets. As observed in Fig. S1 (ESI†), the NF surface is completely covered by Ni(OH)2–Co(OH)2 flowers, which present two different morphologies (i.e. flower-like lamellar nanostructure on the edge of the NF skeleton and vertical shoulder-to-shoulder nanosheets on the wide plain). The features are consistent with those of previously reported metal hydroxides.23
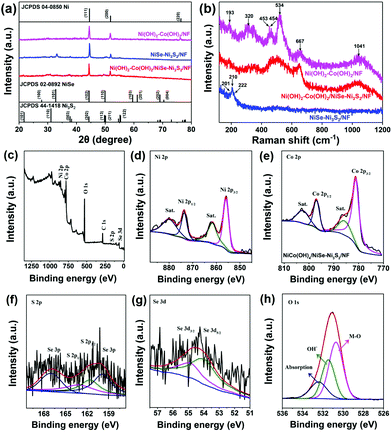
We further used transmission electron microscopy (TEM) to observe the structure of Ni(OH)2–Co(OH)2/NiSe–Ni3S2. As shown in Fig. 2a, the selected Ni(OH)2–Co(OH)2/NiSe–Ni3S2 subset clearly demonstrates a hierarchical structure with a length of 4.5 μm. The hollow feature for nickel chalcogenides is also confirmed from the TEM images of the NiSe–Ni3S2 sample (Fig. S2 in the ESI†). However, the hollow feature can be hardly observed for Ni(OH)2–Co(OH)2/NiSe–Ni3S2 due to the coverage of Ni(OH)2–Co(OH)2 nanosheets. The thin sheet-like structure of Ni(OH)2–Co(OH)2 can be found on the edge (Fig. 2b). TEM elemental mapping images in Fig. 2c also obviously reveal the hierarchical structure of Ni(OH)2–Co(OH)2/NiSe–Ni3S2 microarrays. The Ni, Se and S elements are densely and uniformly distributed in the core of NiSe–Ni3S2 microarrays, while Co and O elements are distributed continuously and uniformly in the sheath of microarrays. The crystallinity of Ni(OH)2–Co(OH)2/NiSe–Ni3S2 is investigated by using high-resolution (HR-TEM) images and selected area electron diffraction (SAED) patterns. For the region selected from the interior rod (Fig. 2d), clear lattice fringes with a d-spacing of 0.273 nm and 0.204 nm are present, which can be assigned to the (101) plane of NiSe and (202) plane of Ni3S2, respectively.24,25 The corresponding SAED image (Fig. 2g) demonstrates clear diffraction rings, suggesting the polycrystalline nature of NiSe–Ni3S2. These ring-like patterns can be reasonably indexed to the (101), (201), and (004) planes of NiSe,1,26 the (202) plane of Ni3S2,27 and the (200) plane of NF, respectively. When the probe is moved from NiSe–Ni3S2 to the exterior, an obvious interface between crystalline NiSe–Ni3S2 and the amorphous phase is shown by the HR-TEM image (Fig. 2e). No lattice fringes can be found in the Ni(OH)2–Co(OH)2 regions (Fig. 2f). Accordingly, the diffraction rings become weaker from the core to sheath (Fig. 2h and i). Therefore, the as-prepared Ni(OH)2–Co(OH)2/NiSe–Ni3S2 possesses a crystalline core and an amorphous sheath.
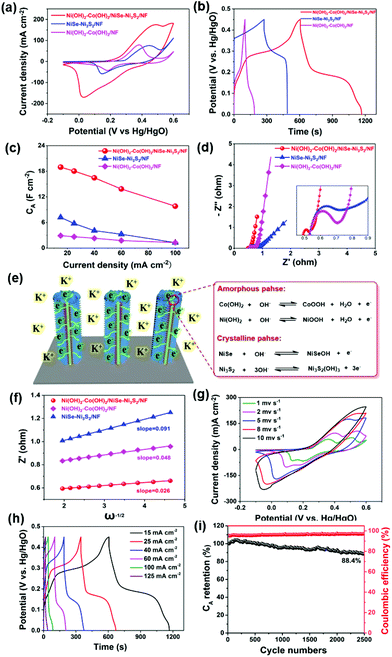
Fig. 3a reveals the characteristic X-ray diffraction (XRD) patterns of Ni(OH)2–Co(OH)2/NF, NiSe–Ni3S2/NF and Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF. Typical diffraction peaks at 44.5°, 51.8°, and 76.4° correspond to the (111), (200), and (220) planes of the nickel substrate (PDF# 04-0850).24 For NiSe–Ni3S2/NF, characteristic peaks at 32.8° (101) and 49.9° (110) from NiSe (PDF# 02-0892) can be clearly observed.24 Meanwhile, the characteristic peaks corresponding to (101), (110), (003) and (113) planes of Ni3S2 (PDF# 02-0892) also confirm the presence of Ni3S2.27 These results indicate that the interior triangle prisms are composed of crystalline NiSe and Ni3S2, which is consistent with the HR-TEM and SAED results. These peaks become weaker when the crystalline NiSe–Ni3S2 core is covered with Ni(OH)2–Co(OH)2 in the case of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF. The average mass loading of Ni(OH)2–Co(OH)2 is about 3.5 mg cm−2, which is relatively high in comparison with that of Ni–Co binary hydroxide (2.8 mg cm−2),28 Ni(OH)2 (1.75 mg cm−2),29 Co(OH)2 (1 mg cm−2),30 and Ni/Co hydroxide (2.3 mg cm−2).31 But Ni(OH)2–Co(OH)2/NF shows just several weak diffractions corresponding to (100), (101), (002), (113) and (103) planes of hydroxides (as shown in Fig. S3 in the ESI†).32 Due to the low intensities of these peaks, we consider that the material has a rather low degree of crystallization and the main form of hydroxides is amorphous. This is consistent with the HR-TEM and SAED results (Fig. 2d–i). The composition of bimetallic hydroxides can be confirmed from Raman spectra (Fig. 3b). In terms of Ni(OH)2–Co(OH)2/NF, three broad peaks at 320, 453, and 534 cm−1 are readily assigned to the symmetric Ni–OH stretching mode, vibrations of the Ni–O stretching mode and mode associated with structural defects of Ni(OH)2, respectively.19,33 The peaks at 193, 454, 667, and 1041 cm−1 are the feature Raman peaks of Co(OH)2.34,35 Two main peaks centered at 454 and 1041 cm−1 can be attributed to the Co–O symmetric stretching mode and OH deformation mode, respectively. As for NiSe–Ni3S2/NF, the main peak at 210 cm−1 corresponds to the Ni–Se stretching mode, and the peaks at 201 and 222 cm−1 are attributed to E vibration modes of Ni3S2.36,37 It is worth noting that the peaks at 453, 454, and 534 cm−1 turn to be one broad peak for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF due to the peak shift. The above characteristics imply the amorphous nature of mixed Ni–Co hydroxides.
X-ray photoelectron spectroscopy (XPS) measurements were further applied to investigate the elemental compositions and valences. As depicted in Fig. 3c, the XPS full survey reveals typical Ni 2p, Co 2p, Se 3d, S 2p, and O 1s peaks for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF. The spin–orbital doublets at 855.9 and 873.5 eV correspond to Ni 2p3/2 and Ni 2p1/2 signals of Ni2+, accompanied by two obvious shakeup satellites (Fig. 3d).13,18Fig. 3d shows two peaks at 781.1 eV (Co 2p3/2) and 796.9 eV (Co 2p1/2) for the Co 2p region, consistent with the +2 valence state of cobalt.14,38,39 The result is also in line with the Co 2p spectrum for Ni(OH)2–Co(OH)2/NF (Fig. S4 in the ESI†). The Se 3d spectrum can be deconvoluted into two states, namely, Se 3d5/2 and Se 3d3/2 signals, corresponding to the binding energy of 54.1 and 55.0 eV, respectively (Fig. 3g). This suggests a valence state of −2 for Se, indicating the presence of NiSe in the sample.24,25 Meanwhile, for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF (Fig. 3d) and NiSe–Ni3S2/NF (Fig. S5†), the peaks at 163.2 and 161.8 eV are associated with S 2p3/2 and S 2p1/2, suggesting the existence of Ni3S2. In detail, the peak at 163.2 eV originates from the sulfur–metal bonds and the binding energy of 161.8 eV is attributed to S2− in low coordination at the surface. These values correlate with previously reported data.24,27 In terms of O 1s peaks, the peaks at 530.7 and 531.4 eV are reasonably assigned to the metal–oxygen bond and OH− groups. Besides, the peak at 532.5 eV arises from the absorption of environmental oxygen.39 In comparison with NiSe–Ni3S2/NF, Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF demonstrates a reduced binding energy gap for Ni 2p and Se 3d with a decrement of 1.4 and 0.9 eV, respectively. These peak shifts may be attributed to the covering of the metal hydroxide sheath on the NiSe–Ni3S2 core. By comparing the spectra of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF before cycling with after 2500 GCD cycling (Fig. S6†) we can see that Ni 2p, Co 2p, and O 1s are basically the same before and after the stability measurement, which indicates that Ni and O elements in the sample are very stable. As for the spectra of S 2p and Se 3d, the Se 3p signal becomes stronger and the Se 3d5/2 signal has a reduced binding energy gap of 1.2 eV. The signals of S 3p1/2 and S 3p3/2 become weaker than the initial state. These can be interpreted as the exposure to air and water for a long time during the three electrode cycling test. In general, the sample retains good stability after the 2500 GCD cycling test.
To investigate the effect of the hierarchical structure on charge storage performance, we compare the electrochemical properties of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF with those of individual component arrays on NF (i.e. NiSe–Ni3S2/NF and Ni(OH)2–Co(OH)2/NF). Fig. 4a shows the comparative cyclic voltammetry (CV) curves at a scan rate of 5 mV s−1. Within a potential window of −0.1 to 0.6 V, Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF demonstrates an obviously larger integrated area, indicating a higher specific capacitance than the two reference electrodes. All CV curves possess redox peaks that are consistent with the voltage platforms in the galvanostatic charge–discharge (GCD) curves (Fig. 4b, Fig. S8, and S9 in the ESI†).40,41 By increasing the scan rate from 1 to 10 mV s−1, we observe linear relationships between logarithm oxidation peak current and logarithm scan rates in for all three electrodes (Fig. S10 in the ESI†). The slope (b value) for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF, NiSe–Ni3S2/NF and Ni(OH)2–Co(OH)2/NF is fitted as 0.49, 0.505 and, 0.651, respectively. Generally, the CV kinetics analysis can be simplified as i(V) = avb, where b indicates the diffusion-controlled contribution (b = 0.5) and capacitive contribution (b = 0.5–1).34 In our case, Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF and NiSe–Ni3S2/NF exhibit a typical diffusion-controlled electrochemical process, while Ni(OH)2–Co(OH)2/NF possesses a dominant diffusion-control and partial capacitive-control process. The longest discharge time at 15 mA cm−2 also endows Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF with the highest CA value of 19.01 F cm−2 compared to that of Ni(OH)2–Co(OH)2/NF (2.9 F cm−2) and NiSe–Ni3S2/NF (7.2 F cm−2). When the current density was increased from 15 to 100 mA cm−2, the capacitance retention was calculated to be 51.6%, 44.6%, and 17.8% for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF, Ni(OH)2–Co(OH)2/NF, and NiSe–Ni3S2/NF (Fig. 4c), respectively. This demonstrates that the unique sheath of amorphous bimetallic hydroxides can efficiently improve the rate capability of crystalline TMC-based electrodes, which is similar to the literature-reported surface amorphization strategy.20
The comparative charge transport (electrons and ions) is further investigated by electrochemical impedance spectrometry (EIS). As shown in Fig. 4d, Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF shows the smallest internal resistance (0.49 Ω) compared to that of Ni(OH)2–Co(OH)2/NF and NiSe–Ni3S2/NF (both are 0.53 Ω). The significantly decreased semicircle diameter for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF indicates its fast electron transport. Meanwhile, Ni(OH)2–Co(OH)2 and NiSe–Ni3S2/NF show similar charge transfer resistances. The longer straight line in the low-frequency region for NiSe–Ni3S2/NF suggests a longer ion diffusion path, which might be caused by its less hollow structure compared to the bimetallic hydroxides. The ion-diffusion impedance can be obtained by plotting the real part of the collected impedance versus the 8 square root of the sampling frequency (Fig. 4f). We find that the ion diffusion impedances are increased in the order of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF (0.026) < Ni(OH)2–Co(OH)2/NF (0.048) < NiSe–Ni3S2/NF (0.091). We further evaluate the specific surface area using BET analysis as shown in Fig. S7.† We find that Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF shows the highest specific surface area (4.6462 m2 g−1) and porosity (0.025389 cm3 g−1) compared to the individual components. Meanwhile, the amorphous shell has structural features (such as vacancies, inner structure disorder and isotropic nature), which can promote the diffusion and reaction of electrolytes.42 This trend is consistent with the as-discussed rate capability; therefore, the rate capability in our case is prominently affected by the ion diffusion ability. We ascribe the excellent electrochemical performance of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF to the following reasons: (i) the rich reversible reaction of multi-components, i.e. transition metal ions react with hydroxyl groups in alkaline aqueous electrolytes27 (Fig. 4e); (ii) the highly conductive metal chalcogenides serving as pivots to promote efficient electron transport from current collectors to the electrode/electrolyte interfaces; (iii) the rich diffusion channels within amorphous layers result in excellent tolerance of ultrafast ion insertion and extraction.
Fig. 4g shows typical CV curves in the potential range of −0.1–0.6 V for Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF as the scan rate increased from 1 to 10 mV s−1. We can observe two couples of prominent redox peaks at low scan rates (1 and 2 mV s−1), which correspond to the faradaic reaction of nickel/cobalt hydroxides and nickel sulfide/selenide as revealed in previous reports.5,24 As the scan rate was increased to higher than 5 mV s−1, the redox peaks shifted due to the polarization effect of electrodes, resulting in the incorporative peaks. GCD curves demonstrate clear voltage platforms in the current density range of 15–125 mA cm−2, suggesting a typical faradaic charge storage (Fig. 4h). Based on the discharge curves, the CA values are calculated to be 19.01, 18.03, 16.45, 13.85, 9.81, and 6.01 F cm−2 at the current densities of 15, 25, 40, 60, 100, and 125 mA cm−2. The long-term stability of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF is evaluated with continuous cyclic GCD measurements at a high current density of 100 mA cm−2. As depicted in Fig. 4i, an average capacitance loss of only 0.00464% per cycle is obtained during 2500 cycles, together with a coulombic efficiency of 96%. The excellent areal capacitances at high current densities of our hierarchical electrode are impressive when compared to representative free-standing electrodes on NF. As summarized in Table S1 (ESI†), the performance of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF (19.01 F cm−2 at 15 mA cm−2, 6.01 F cm−2 at 125 mA cm−2) is much better than that of NiCo2S4@Ni(OH)2@PPy (9.1 F cm−2 at 5 mA cm−2),43 Ni–Tp/PANI (10.3 F cm−2 at 20 mA cm−2),44 Ni@NiO nanowires (4.0 F cm−2 at 8 mA cm−2),45 Co(OH)2/HNNF (3.2 F cm−2 at 5 mA cm−2),30 NiCo2O4@rGO (3.6 F cm−2 at 5 mA cm−2),46 Ni(OH)2–Cu (8.7 F cm−2 at 1 mA cm−2),47 2D-CMO (2.0 F cm−2 at 10 mA cm−2),48 NiCo2S4@PPy (9.781 F cm−2 at 5 mA cm−2),49 and NF/S-Co3O4@NiCo2S4 (5.4 F cm−2 at 15 mA cm−2).50
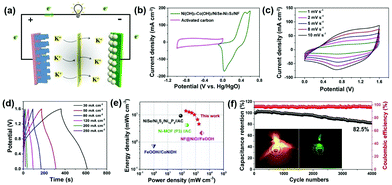
In order to evaluate its potential in practical applications, hybrid supercapacitor (HSC) devices were assembled with Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF as the positive cathode and activated carbon (AC) coated on NF as the negative electrode, while PVA–KOH was used as the gel electrolyte (Fig. 5a). To determine the optimal operating potential window of the Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF//AC HSC devices, the electrochemical properties of AC (∼14 mg cm−2) were first evaluated in a three-electrode system in 3 M KOH aqueous solution. A typical electric double-layer capacitive charge storage is found for AC with a potential range of −1.0 V to 0 V (Fig. S11 in the ESI†). Fig. 5b shows the complementary potential windows of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF and AC, which results in a voltage window of 0–1.6 V for HSC devices. By increasing the scan rates from 1 to 10 mV s−1, the HSC device exhibits pseudocapacitive behavior with a pair of smooth redox peaks (Fig. 5c). The GCD curves acquired at a variety of scan rates from 30 to 250 mA cm−2 present a similar symmetric triangle shape (Fig. 5d). The volumetric specific capacitance is thus calculated to be 39.3, 35.9, 33.3, 28.6, 18.9, and 12.9 F cm−3 at the corresponding current density of 30, 50, 80, 120, 200, and 250 mA cm−2, respectively. Accordingly, the volumetric energy density is calculated as 13.9 mW h cm−3 at a power density of 200 mW cm−3. When the power density is increased to 1720 mW cm−3, the device still delivers an energy density of 4.58 mW h cm−3, implying the excellent rate capability. As presented in the Ragone plot (Fig. 5e), the combination of high energy density and power density is outstanding among representative NF-based free-standing electrodes such as NiSe/Ni3S2/Ni12P5//AC,24 FeOOH//CoNiDH,19 Ni-MOF (P3)//AC,51 and NF@NiO//FeOOH.52 The tolerance toward long-term high-rate cyclic charge/discharge was further evaluated at a current density of 200 mA cm−2. The capacitance retention is as high as 82.5% of its initial value after 4000 cycles, together with an average 97% coulombic efficiency (Fig. 5f). The practical use is verified by lighting different color LED indicators using two tandem HSC devices, as shown in the inset photos in Fig. 5f.
Conclusions
In summary, we have developed hierarchical core–sheath microarrays with amorphous Ni(OH)2–Co(OH)2 nanosheets growing in situ on crystalline NiSe–Ni3S2 triangular prisms supported by nickel foam. With NF as the sole metal source, high mass-loadings of Ni(OH)2–Co(OH)2 and NiSe–Ni3S2 have been constructed via a facile one-pot solvothermal approach. The hybrid composite Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF features porous and uniform distribution of electroactive materials on the skeletons of NF. Owing to the rich components and unique nanostructure, the as-prepared Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF worked as an excellent free-standing electrode, where a maximum areal specific capacitance of 19.01 F cm−2 is achieved at a current density of 15 mA cm−2, together with high rate capability (51.6% at 100 mA cm−2) and good tolerance toward long-term cycling (82.5% capacitance retention after 2500 cycles at 100 mA cm−2). The assembled HSC device with an activated carbon negative electrode delivered the highest volumetric specific capacitance of 13.9 mW h cm−3 at 200 mW cm−3, with 82.5% capacitance retention after 4000 cycles. This work may provide a facile approach to develop structurally well-defined composites as free-standing electrodes for high-energy and durable supercapacitors.Experimental section
Synthesis of NiSe–Ni3S2/NF
A piece of clean nickel foam (NF) (2.5 × 4 cm) was immersed in 30 mL selenium (Se)/sulfur (S) solution in diethylenetriamine (Se: 0.474 g, S: 0.192 g) and ultrasonicated for 20 min. The mixture (50 mL) was then transferred to a Teflon-lined stainless steel autoclave. The autoclave was sealed and heated to 160 °C for 12 h. After cooling down to room temperature, the NF was thoroughly washed with deionized water and ethanol. The dark red NiSe–Ni3S2/NF was obtained after drying under vacuum at 55 °C. The average mass loading of NiSe–Ni3S2 is about 8.5 mg cm−2.Synthesis of Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF
Polyethylene oxide–polypropylene oxide–polyethylene oxide (PEO–PPO–PEO, P123) (1.75 g, 0.3 mmol), nickel(II) acetate tetrahydrate (0.25 g, 1 mmol) and cobalt(II) acetate tetrahydrate (0.25 g, 1 mmol) were added into the mixed solvent composed of ethanol (18 mL), ethylene glycol (15 mL) and deionized water (1 mL). The immediately formed light green solution was allowed to be vigorously stirred for another 5 h. The transparent solution containing one piece of NiSe–Ni3S2/NF (50 mL) was transferred to a Teflon-lined stainless steel autoclave, which was heated to 135 °C for 36 h. The light blue NF was obtained after thoroughly washing with deionized water and ethanol. For comparison, Ni(OH)2–Co(OH)2/NF was prepared through the same procedure with a clean NF as the substrate. The average mass loading of Ni(OH)2–Co(OH)2 is about 3.5 mg cm−2. So the total mass loading of Ni(OH)2–Co(OH)2/NiSe–Ni3S2 is about 12 mg cm−2.Assembly of hybrid supercapacitors (HSCs)
The negative electrode was prepared by coating the slurry of activated carbon (AC), acetylene black and polytetrafluoroethylene (weight ratio is 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on NF (1 × 2 cm), which was dried at 60 °C overnight under vacuum. The average mass loading of AC was 14 mg cm−2. The HSC device was prepared by sandwiching a Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF (1 × 2 cm) positive electrode and an AC/NF negative electrode on two sides of a piece of cellulose paper, and poly(vinyl alcohol)–KOH gel was used as the electrolyte.
1) on NF (1 × 2 cm), which was dried at 60 °C overnight under vacuum. The average mass loading of AC was 14 mg cm−2. The HSC device was prepared by sandwiching a Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF (1 × 2 cm) positive electrode and an AC/NF negative electrode on two sides of a piece of cellulose paper, and poly(vinyl alcohol)–KOH gel was used as the electrolyte.
Electrochemical measurement
The three-electrode electrochemical performance was investigated using a CHI760E electrochemical workstation (Shanghai, China) in 3 M KOH aqueous solutions. Ni(OH)2–Co(OH)2/NiSe–Ni3S2/NF, NiSe–Ni3S2/NF or Ni(OH)2–Co(OH)2/NF was directly used as the working electrode, along with Pt foil as the counter electrode and Hg/HgO as the reference electrode. The HSCs were measured in a two-electrode cell configuration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51861145401) and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.References
- P. Yang, Z. Wu, Y. Jiang, Z. Pan, W. Tian, L. Jiang and L. Hu, Adv. Energy Mater., 2018, 8, 1801392 CrossRef.
- J. Yang, C. Yu, X. Fan, S. Liang, S. Li, H. Huang, Z. Ling, C. Hao and J. Qiu, Energy Environ. Sci., 2016, 9, 1299–1307 RSC.
- S. Wang, L. Li, Y. Shao, L. Zhang, Y. Li, Y. Wu and X. Hao, Adv. Mater., 2019, 31, 1806088 CrossRef PubMed.
- A. VahidMohammadi, M. Mojtabavi, N. M. Caffrey, M. Wanunu and M. Beidaghi, Adv. Mater., 2019, 31, 1806931 CrossRef PubMed.
- Y. Liu, X. Teng, Y. Mi and Z. Chen, J. Mater. Chem. A, 2017, 5, 24407–24415 RSC.
- H. Chen, L. Hu, M. Chen, Y. Yan and L. Wu, Adv. Funct. Mater., 2014, 24, 934–942 CrossRef CAS.
- K. Yu, X. Pan, G. Zhang, X. Liao, X. Zhou, M. Yan, L. Xu and L. Mai, Adv. Energy Mater., 2018, 8, 1802369 CrossRef.
- Z. Pan, H. Zhi, Y. Qiu, J. Yang, L. Xing, Q. Zhang, X. Ding, X. Wang, G. Xu, H. Yuan, M. Chen, W. Li, Y. Yao, N. Motta, M. Liu and Y. Zhang, Nano Energy, 2018, 46, 266–276 CrossRef CAS.
- Z. Huang, Y. Song, D. Feng, Z. Sun, X. Sun and X. Liu, ACS Nano, 2018, 12, 3557–3567 CrossRef CAS PubMed.
- Z. Pan, H. Zhi, Y. Qiu, J. Yang, L. Xing, Q. Zhang, X. Ding and X. Wang, Nano Energy, 2018, 46, 266–276 CrossRef CAS.
- Y. Song, T. Liu, B. Yao, M. Li, T. Kou, Z.-H. Huang, D.-Y. Feng, F. Wang, Y. Tong, X.-X. Liu and Y. Li, ACS Energy Lett., 2017, 2, 1752–1759 CrossRef CAS.
- Y. Song, T. Liu, M. Li, B. Yao, T. Kou, D. Feng, F. Wang, Y. Tong, X.-X. Liu and Y. Li, Adv. Energy Mater., 2018, 8, 1801784 CrossRef.
- J. Zhao, Z. Li, X. Yuan, Z. Yang, M. Zhang, A. Meng and Q. Li, Adv. Energy Mater., 2018, 8, 1702787 CrossRef.
- X. Li, H. Wu, A. M. Elshahawy, L. Wang, S. J. Pennycook, C. Guan and J. Wang, Adv. Funct. Mater., 2018, 28, 1800036 CrossRef.
- D. Zhou, X. Su, M. Boese, R. Wang and H. Zhang, Nano Energy, 2014, 5, 52–59 CrossRef CAS.
- Q. Li, Y. Xu, S. Zheng, X. Guo, H. Xue and H. Pang, Small, 2018, 14, 1800426 CrossRef PubMed.
- H. Li, Y. Gao, C. Wang and G. Yang, Adv. Energy Mater., 2015, 5, 1401767 CrossRef.
- J. Chen, J. Xu, S. Zhou, N. Zhao and C.-P. Wong, Nano Energy, 2016, 21, 145–153 CrossRef CAS.
- H. Li, M. Yu, F. Wang, P. Liu, Y. Liang, J. Xiao, C. Wang, Y. Tong and G. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- T. Zhou, Y. Zheng, H. Gao, S. Min, S. Li, H. Liu and Z. Guo, Adv. Sci., 2015, 2, 1500027 CrossRef PubMed.
- S. Sun, T. Zhai, C. Liang, S. V. Savilov and H. Xia, Nano Energy, 2018, 45, 390–397 CrossRef CAS.
- F. Han, J. Xu, J. Zhou, J. Tang and W. Tang, Nanoscale, 2019, 11, 12477–12483 RSC.
- Y. Li, Y. Shi, S. Wang, J. Liu, J. Lin, Y. Xia, X. Wu, C. Fan, J. Zhang, H. Xie, H. Sun and Z. Su, Adv. Energy Mater., 2019, 9, 1803690 CrossRef.
- K. Tao, Y. Gong and J. Lin, Nano Energy, 2019, 55, 65–81 CrossRef CAS.
- J. Lin, H. Wang, Y. Yan, X. Zheng, H. Jia, J. Qi, J. Cao, J. Tu, W. Fei and J. Feng, J. Mater. Chem. A, 2018, 6, 19151–19158 RSC.
- W. Wei, L. Mi, Y. Gao, Z. Zheng, W. Chen and X. Guan, Chem. Mater., 2014, 26, 3418–3426 CrossRef CAS.
- W. He, C. Wang, H. Li, X. Deng, X. Xu and T. Zhai, Adv. Energy Mater., 2017, 7, 1700983 CrossRef.
- X. Sun, G. Wang, H. Sun, F. Lu, M. Yu and J. Lian, J. Power Sources, 2013, 238, 150–156 CrossRef CAS.
- N. A. Alhebshi, R. B. Rakhi and H. N. Alshareef, J. Mater. Chem. A, 2013, 1, 14897–14903 RSC.
- Z. Yu, Z. Cheng, X. Wang, S. X. Dou and X. Kong, J. Mater. Chem. A, 2017, 5, 7968–7978 RSC.
- L. Ye, L. Zhao, H. Zhang, B. Zhang and H. Wang, J. Mater. Chem. A, 2016, 4, 9160–9168 RSC.
- X. Li, J. Shen, W. Sun, X. Hong, R. Wang, X. Zhao and X. Yan, J. Mater. Chem. A, 2015, 3, 13244–13253 RSC.
- S. Sarkar, M. Pradhan, A. K. Sinha, M. Basu, Y. Negishi and T. Pal, Inorg. Chem., 2010, 49, 8813–8827 CrossRef CAS PubMed.
- J. Yang, H. Liu, W. N. Martens and R. L. Frost, J. Phys. Chem. C, 2010, 114, 111–119 CrossRef CAS.
- H. Sheng, X. Zhang, Y. Ma, P. Wang, J. Zhou, Q. Su, W. Lan, E. Xie and C. J. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 8992–9001 CrossRef CAS PubMed.
- J. S. Chen, C. Guan, Y. Gui and D. J. Blackwood, ACS Appl. Mater. Interfaces, 2017, 9, 496–504 CrossRef CAS PubMed.
- C. Liu, K. Wang, X. Zheng, X. Liu, Q. Liang and Z. Chen, Carbon, 2018, 139, 1–9 CrossRef CAS.
- C. Xu, X. Kong, S. Zhou, B. Zheng, F. Huo and M. Strømme, J. Mater. Chem. A, 2018, 6, 24050–24057 RSC.
- H. Liang, J. Lin, H. Jia, S. Chen, J. Qi, J. Cao, T. Lin, W. Fei and J. Feng, J. Mater. Chem. A, 2018, 6, 15040–15046 RSC.
- Y. Gogotsi and R. M. Penner, ACS Nano, 2018, 12, 2081–2083 CrossRef CAS PubMed.
- M. Forghani and S. W. Donne, J. Electrochem. Soc., 2018, 165, A664–A673 CrossRef CAS.
- C. Huang, Y. Hu, S. Jiang and H. C. Chen, Electrochim. Acta, 2019, 325, 134936 CrossRef CAS.
- M. Liang, M. Zhao, H. Wang, J. Shen and X. Song, J. Mater. Chem. A, 2018, 6, 2482–2493 RSC.
- Q. Chen, S. Lei, P. Deng, X. Ou, L. Chen, W. Wang, Y. Xiao and B. Cheng, J. Mater. Chem. A, 2017, 5, 19323–19332 RSC.
- H. Sun, Z. Ma, Y. Qiu, H. Liu and G. Gao, Small, 2018, 14, 1800294 CrossRef PubMed.
- C. Zhang, X. Geng, S. Tang, M. Deng and Y. Du, J. Mater. Chem. A, 2017, 5, 5912–5919 RSC.
- D. Shi, L. Zhang, X. Yin, T. J. Huang and H. Gong, J. Mater. Chem. A, 2016, 4, 12144–12151 RSC.
- X. Zhang, J. Luo, P. Tang, X. Ye, X. Peng, H. Tang, S.-G. Sun and J. Fransaer, Nano Energy, 2017, 31, 311–321 CrossRef CAS.
- M. Yan, Y. Yao, J. Wen, L. Long, M. Kong, G. Zhang, X. Liao, G. Yin and Z. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 24525–24535 CrossRef CAS PubMed.
- Y. Ouyang, H. Ye, X. Xia, X. Jiao, G. Li, S. Mutahir, L. Wang, D. Mandler, W. Lei and Q. Hao, J. Mater. Chem. A, 2019, 7, 3228–3237 RSC.
- Y. Yan, P. Gu, S. Zheng, M. Zheng, H. Pang and H. Xue, J. Mater. Chem. A, 2016, 4, 19078–19085 RSC.
- Q. Chen, J. Li, C. Liao, G. Hu, Y. Fu, O. K. Asare, S. Shi, Z. Liu, L. Zhou and L. Mai, J. Mater. Chem. A, 2018, 6, 19488–19494 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Materials synthesis and characterization, CV and morphology studies. See DOI: 10.1039/c9nr08418e |
| This journal is © The Royal Society of Chemistry 2020 |