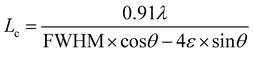Carbon nano-onion-powered optically transparent and economical dye-sensitized solar cells†
Debananda
Mohapatra
 ab,
Siva Sankar
Nemala
ab,
Siva Sankar
Nemala
 *b,
Mostafa Saad
Sayed
a,
Jae-Jin
Shim
*b,
Mostafa Saad
Sayed
a,
Jae-Jin
Shim
 a,
Sudhanshu
Mallick
b,
Parag
Bhargava
b and
Smrutiranjan
Parida
*b
a,
Sudhanshu
Mallick
b,
Parag
Bhargava
b and
Smrutiranjan
Parida
*b
aSchool of Chemical Engineering, Yeungnam University, Gyeongsan, Gyeongbuk 38541, Republic of Korea
bDepartment of Metallurgical Engineering and Materials Science, IIT Bombay, Powai, Mumbai 400-076, India. E-mail: paridasm@iitb.ac.in; sivashankar@iitb.ac.in
First published on 10th July 2020
Abstract
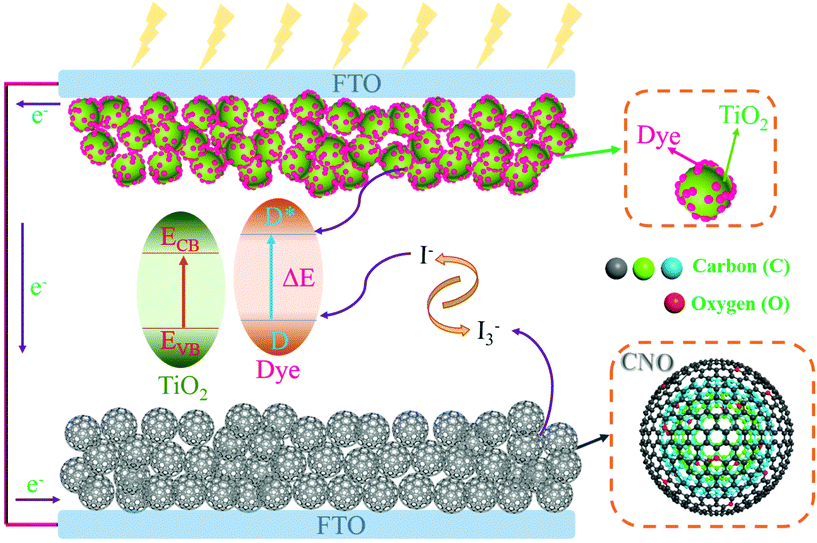
The integration of dye-sensitized solar cells (DSSCs) with building roof panels, windows, and various decorative outdoor installations is presently an important research topic for their immediate commercialization potential because of their power generation capability, sustainability, and aesthetic appearance. For industrial applications, Pt counter electrodes (CEs) need to be replaced with Pt-free CEs because of their limited sources and cost. An ideal CE should be economical, abundant, and have excellent electrochemical stability and activity, with easy processing in bulk. As an alternative practical CE, we introduce for the first time a carbon nano-onion (CNO)-based CE for transparent DSSCs. We developed a simple, energy-efficient one-step synthesis technique for fabricating high-quality CNOs in bulk quantities without any sophisticated instrumentation and expensive nanodiamond precursors. CNO CEs proved to be promising in terms of optical transparency, reasonable electrochemical redox activity for the I−/I3− redox couple, and exchange current density comparable to Pt CEs. CNO-powered DSSCs demonstrated an optical transparency of >55% with a significant solar energy conversion efficiency of 5.17%. The intrinsic hydrophilicity of the as-synthesized CNO eliminates the use of a binder or an additive, unlike in the case of other carbon allotropes. Our results demonstrate the possibility of using CNO-based CEs as promising substitutes for scarce and expensive Pt-based CEs for low-cost DSSCs.
Introduction
The excessive use of fossil fuels has resulted in global climate change, which has necessitated the urgent development of alternative clean renewable energy-based technologies to provide a promising solution to the increasing global energy demands. From economic and technology viewpoints, dye-sensitized solar cells (DSSCs), which directly convert incident solar energy into usable electricity using a simple Grätzel cell, can be considered a green and clean technology for sustainable energy resources.1,2 From marketing and technology perspectives, the estimated cost for the bulk production of DSSCs is approximately $3–4 W p−1, which is comparable to the estimated bulk production cost of silicon-based PV modules (∼$3 W p−1).3,4 The cost and performance of DSSCs primarily depend on their individual components such as the photoanode (semiconducting metal oxide, TiO2), dye (ruthenium complex), and counter electrode (CE)/cathode, which is usually composed of rare and expensive platinum (Pt). Since its discovery and the first DSSC fabrication by O'Regan and Grätzel in 1990,2,5 extensive research has been conducted to enhance the efficiency by reducing the cost of the CE without compromising its high catalytic activity and corrosion stability.6 In the search for ideal alternatives to Pt CEs, many carbonaceous materials7 such as mesoporous carbon,8 carbon black,9 activated carbon,10 CNTs,7,11,12 carbon nitride,13 and graphene1,14–16 have been investigated because of their low cost and earth abundance. The stability of DSSCs is one of the critical factors for achieving eco-sustainable technologies and needs to be improved without lowering the solar conversion efficiency. The CE plays a vital role in and is an indispensable component for the long-term stable operation of DSSCs, and it performs two essential functions: transferring electrons to the redox system (I−/I3−) and redox charge mediation. The CE should have high electrical conductivity, and hence, low charge-transfer resistance and high exchange current density. A low material-processing cost is an added advantage. Although many different carbonaceous materials have been explored, Pt remains the preferred material because of its high catalytic activity and unparalleled exchange current density. The optical transparency of the CEs in DSSCs is essential for certain applications such as real-time transparent windows, roof panels, and various decorative installations. The coupling of optically transparent DSSCs to building-integrated photovoltaics (BIPVs) would provide sustainable power as well as aesthetic appearance.17 The transparency of both the photoanode and the dye can be achieved simply by controlling the size of the semiconducting TiO2 nanoparticles and the colored pigments, respectively.The major challenge is the development of a low-cost Pt-free, optically transparent CE without sacrificing its high electrocatalytic activity and exchange current density. Transparent CEs based on metal selenides and Pt nanoparticles were recently reported for DSSCs.18,19 However, metal selenides are carcinogenic, while Pt is rare and expensive to use on a bulk scale, which limits their commercial production. To the best of our knowledge, there are very few reports on transparent carbonaceous CEs such as carbon nitrides,13 graphenes,1,14,20,21 and CNTs.12,22 A carbon nano-onion (CNO), a multi-shelled concentric structure that physically mimics an onion, is an important member of the fullerene family. The intergraphitic distance between two concentric onion rings is approximately 0.335 nm, which is close to the interplanar graphitic distance of 0.334 nm.23,24 Because of their unique shell-shaped structure, high graphitization, and exohedral porosity, CNOs have recently attracted unprecedented interest for energy storage,23,24 microbial fuel cells,25 and sensing applications.26 Due to the exceptional properties of CNOs such as good electrical conductivity, unique shell-like structure, good electrocatalytic activity, high surface area, etc., they have received substantial attention towards the DSSC application. The difficulty in the large-quantity synthesis of CNOs with reliable quality considerably limits their application horizon; hence, they are one of the least-studied carbon allotropes. Therefore, the application of CNOs depends not only on the synthesis conditions but also on the quality of the resulting product in terms of graphitization, exchange current density, and ultimately the electrocatalytic activity. For industrial applications such as DSSCs, CNOs must be synthesized by energy-efficient, economical, and straightforward means without using reported post-processed toxic and corrosive treatments with a very high-temperature vacuum annealing technique.24 The obtained CNO should be easy-to-adopt solution-processable without sacrificing its intrinsic graphitic nature.
To the best of our knowledge, this is the first report on CNOs as a promising alternative CE material that is not only optically transparent but also electrocatalytically reasonable for the I−/I3− redox couple for transparent (>55%) DSSCs with an impressive solar conversion efficiency of 5.17%. Interestingly, the CNO-based CE for DSSCs does not require any binders/additives because of its inherent high hydrophilicity and strong inter-particulate interaction in a film. Its hydrophilicity (with a zeta potential of ∼60 mV (ref. 27)) means that its coating can be solution-processed quickly without compromising its electrical conductivity; this is in contrast to other carbon allotropes, where the use of binders is necessary, which is responsible for increasing the contact resistance and decreasing the exchange current density, which is a paramount requirement for an ideal Pt-free CE.
Experimental section
Materials
Lithium iodide (LiI, 99.9%), iodine (I2, 99.99%), 4-tert-butylpyridine (4-tbp, 96%), acetonitrile (99.8%), lithium perchlorate (LiClO4, 99.99%) and 1-methyl-3-propylimidazolium iodide (98%) were obtained from Sigma-Aldrich and used as received. TiO2 powder (P 25, Degussa), polyethylene glycol (PEG 600, Merck AR grade), ethanol (Merck AR grade), and Triton X-100 (Merck AR Grade) were also used as received. A conductive transparent electrode, fluorine-doped tin oxide on glass (FTO; sheet resistance, ∼8 Ω cm−2), dye (N719), and Surlyn spacer were procured from Dyesol, Australia. The redox electrolyte solution for DSSC application was prepared using 0.5 M LiI, 0.05 M I2, 0.5 M 4-tbp, and 0.6 M 1-methyl-3-propylimidazolium iodide in acetonitrile.Preparation of water-soluble CNOs
The pristine CNO preparation method has already been shown and discussed in our previously published article.27 Briefly, we adopted a simple and economical one-step flame synthesis process, devoid of any vacuum setups and expensive precursors, for the bulk preparation of CNOs from clarified butter. Clarified butter, also known as “ghee” in India, was used as the precursor material. The as-obtained black carbon CNO nanoparticles were collected and used for the DSSC application without employing any post-processing treatments or purification steps.Preparation of CNO counter electrodes
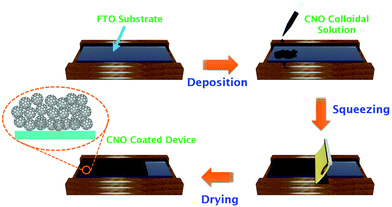
A 2 cm × 2.5 cm as-received FTO substrate was cut and cleaned using a soap solution followed by water and ethanol bath sonication for 20 min to remove unwanted inorganic/organic impurities. The cleaned FTO substrates were then heated at 450 °C for 40 min. Then, a thin uniformly thick CNO layer was coated on the cleaned FTO substrates by the doctor blade method from a freshly prepared CNO suspension, as depicted in Scheme 1. The hydrophilicity of the as-prepared CNO samples was significantly exploited without using any binder additives. The pre-cleaned FTO substrates coated with the CNO layer (∼850 nm) were kept under air for approximately 30 min and then heated at 400 °C for 1 h under an inert atmosphere. Upon cooling, the FTO substrates were characterized and used for DSSC operation.Fabrication of photoanodes and CNO–DSSC devices
The detailed processes of TiO2 slurry preparation and coating on the pre-cleaned FTO substrates by the doctor blade technique are reported elsewhere.28 Briefly, the TiO2 slurry was prepared by mixing TiO2 powder, PEG 600, and ethanol in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with two drops of Triton-X. The mixture was then transferred into a poly-propylene bottle and enough grinding media (3 mm dia) were added into it and kept on a pot mill for 15 days. Later, the prepared TiO2 slurry was coated on the cleaned FTO substrate by the doctor blade technique. The coated films were sintered at 450 °C with a ramping heat rate of 3 °C min−1. The thickness of the sintered TiO2 film was found to be 6 μm (after a single coating), and the active area was 0.25 cm2. The sintered TiO2 films were immersed in a dye solution containing 0.3 mM N719 for 24 h in a dark place at room temperature to facilitate the absorption of the dye molecules and later dried in moisture-free air. The TiO2 photoanode and the transparent CNO CE (Scheme 2) were assembled with a Surlyn spacer placed between them. A drop of iodide/triiodide (I−/I3−) redox electrolyte consisting of 0.5 M LiI, 0.05 M I2, 0.5 M 4-tbp, and 0.5 M 1-butyl-3-methylimidazolium iodide (ionic liquid) in acetonitrile solution was injected into the gap between the electrodes.
3 with two drops of Triton-X. The mixture was then transferred into a poly-propylene bottle and enough grinding media (3 mm dia) were added into it and kept on a pot mill for 15 days. Later, the prepared TiO2 slurry was coated on the cleaned FTO substrate by the doctor blade technique. The coated films were sintered at 450 °C with a ramping heat rate of 3 °C min−1. The thickness of the sintered TiO2 film was found to be 6 μm (after a single coating), and the active area was 0.25 cm2. The sintered TiO2 films were immersed in a dye solution containing 0.3 mM N719 for 24 h in a dark place at room temperature to facilitate the absorption of the dye molecules and later dried in moisture-free air. The TiO2 photoanode and the transparent CNO CE (Scheme 2) were assembled with a Surlyn spacer placed between them. A drop of iodide/triiodide (I−/I3−) redox electrolyte consisting of 0.5 M LiI, 0.05 M I2, 0.5 M 4-tbp, and 0.5 M 1-butyl-3-methylimidazolium iodide (ionic liquid) in acetonitrile solution was injected into the gap between the electrodes.
Physiochemical and electrochemical characterization studies and modeling
The CNO crystallographic analysis was carried out by X-ray diffraction (XRD; PANalytical, X'Pert-PRO MPD) analysis with Cu Kα (1.54 Å) irradiation. Raman spectroscopy (XploRA Plus, Horiba; laser excitation at 532 nm) was performed to confirm the graphitic-layered structure and defects of the CNOs. The surface chemical composition of the as-prepared CNO particles was investigated by X-ray photoelectron spectroscopy (XPS, K-Alpha, Thermo Scientific). The microstructure of the as-prepared CNO particles was analyzed by high-resolution transmission electron microscopy (HR-TEM, JEOL) at an accelerated voltage of 200 kV. Field-emission gun scanning electron microscopy (FEG-SEM, JEOL JSM 7600F) was performed to investigate the top and cross-sectional surfaces of the CNO-based CE. UV–vis spectroscopy (V-650, Jasco) was performed to determine the optical properties of the bare FTO substrate and the CNO–DSSC device. AUTODESK 3DS MAX 2017 and Avogadro version 1.2.0 software were used for the atomistic 3D design, modeling, and visualization of a single CNO particle, CNO CE fabrication, the CNO–DSSC device scheme (Scheme 2), and the graphical abstract.Cyclic voltammetry (CV) measurements were performed using a Metrohm Autolab PGSTAT302N potentiostat electrochemical workstation system and a NEWPORT solar simulator. To understand the electrocatalytic behavior for the reduction of I3− in the redox couple, the CV curves of the standard Pt and CNO-based CEs were recorded in a three-electrode configuration using Ag/AgCl as the reference electrode, Pt as the auxiliary electrode, and Pt/CNO/FTO as the working electrode. The electrolyte solution contained 0.01 M LiI, 0.001 M I2, and 0.1 M LiClO4 in acetonitrile. CV was performed at a scan rate of 100 mV s−1 in the potential range of −0.6 to 1.0 V. Tafel polarization measurements were carried out in a symmetrical cell configuration at a scan rate of 100 mV s−1 to determine the electrocatalytic activity through the exchange current density. The current density–voltage (J–V) measurements were performed using the solar simulator, which had a xenon arc lamp of 150 W and provided one-sun illumination (AM 1.5, 100 mW cm−2). The voltage sweep during the J–V measurements was controlled using a Keithley 2420 source meter.
Results and discussion
CNO microstructure and surface chemical composition
The XRD spectrum of the as-prepared CNOs reveals a predominantly graphitic structure. In the XRD spectrum, Fig. 1a, the strong signature of the graphitic crystallographic orientations (sp2-carbon) in the as-prepared CNOs gave rise to a prominent diffraction peak at 24.7°, which corresponds to the (002) plane reflection of hexagonal graphitic carbon (ICDD File No. 00-041-1487).29 The appearance of a diffraction peak at around 43° corresponding to the (100) and/or (101) reflection planes represents a low concentration of sp3-graphitic defects. The intensity of the peak at 43° is much lower (about 80%) than that of the prominent sp2 peak at 24.7°. This indicates that the hexagonal stacking of graphene layers with ordered parallel graphitic planes is more predominant than turbostratic stacking. However, the (002) graphitic peak is slightly broad, which can be due to some incomplete graphitization (see XPS), the random distribution of individual CNO layers (stacking defects27), and the presence of functional groups.25 The graphitic defects in the CNOs were further examined by Raman spectroscopy.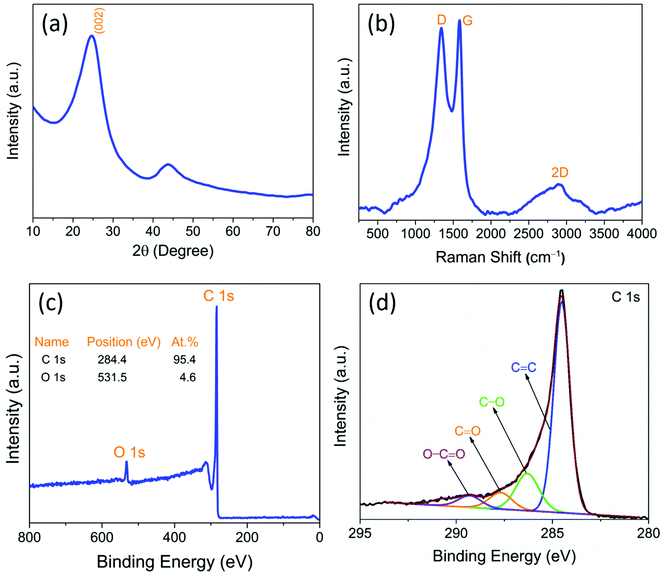 | ||
| Fig. 1 (a) X-ray diffraction spectrum, (b) Raman spectrum (showing distinguishable D, G, and 2D-bands), (c) XPS survey spectrum, and (d) high-resolution core C 1s spectra of the as prepared CNO. | ||
As shown in Fig. 1a, the d-value of the prominent peak corresponding to the (002) reflection plane was found to be 0.34 nm, which is consistent with that determined by HR-TEM (Fig. 2c). The obtained d002 value is comparable to that of the nanodiamond-derived CNO, which requires expensive precursor materials and sophisticated synthesis conditions such as high temperatures (1800 °C) and pressures (10−6 Torr).24 For curvy or stacked graphitic materials, the layer stacking order (Lc) is an important parameter to estimate the presence of concentric graphitic layers. We estimated Lc by applying the William–Hall equation30 to the most prominent graphitic XRD peak ((002) reflection plane) shown in Fig. 1a:
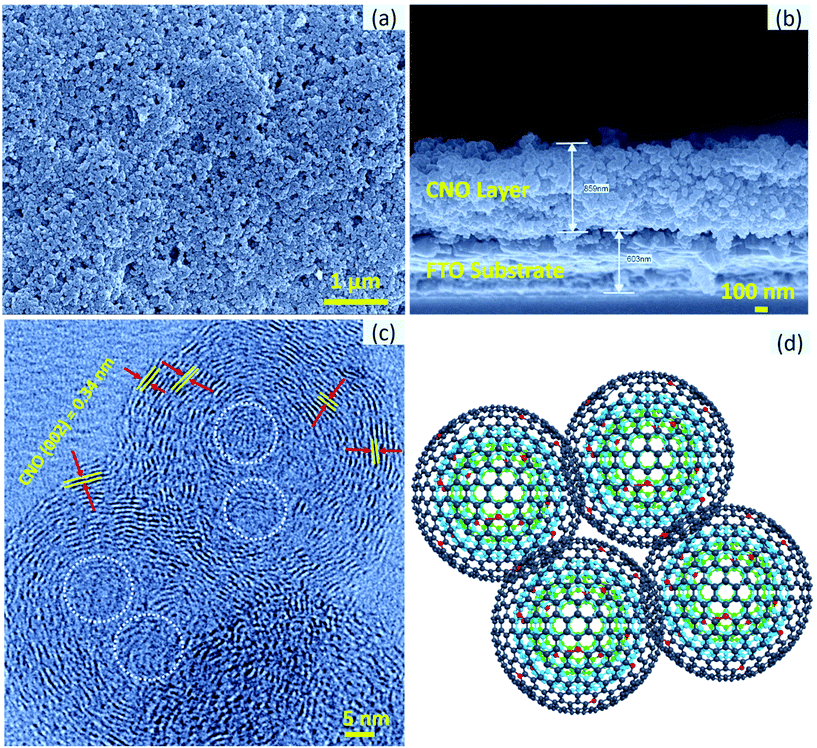
The top-view and cross-sectional images of the CNO-based CE are shown in Fig. 2a. As can be seen, the CNOs are uniformly coated and distributed over the substrate. Moreover, the film is highly porous without any cracks, which is important to achieve a high device performance. The porosity of the film was further confirmed by the cross-sectional image of the CNO-based CE (Fig. 2b). As can be seen, the film is continuous with a thickness of approximately 860 nm and has considerable local porosity without any cracks, which are necessary for electrolyte penetration and maximizing the solid–electrolyte interface.
The as-synthesized CNO particles were investigated in depth by HR-TEM; the HR-TEM images are shown in Fig. 2c. In the as-synthesized CNO, carbon rings are imperfect due to the presence of some graphitic voids or graphene facets. The calculated intergraphitic layer distance for the prominent (002) peak is 0.34 nm, as shown in Fig. 2c, which is close to that of the highly oriented pyrolytic graphite (0.335 nm).31 This evidences the presence of multilayered sp2-graphitized multilayers. The calculated intergraphitic layer distance perfectly matches that of the reported CNOs prepared using expensive diamond precursors requiring sophisticated instrumentation.24 Importantly, the CNO particles are in intimate contact with the interpenetrating graphitic layers in between them, which is preferable for fast electron transfer kinetics. The model of the tight intergraphitic contact between individual CNO particles illustrated in Fig. 2d is consistent with the experimentally observed results shown in Fig. 2c. In addition, distinct multiple cores in the particle indicate distinct growth centers, which resulted in the successful formation of a layered CNO structure, as indicated by the white dotted circle in Fig. 2c. The exposed graphene edges in a graphitic structure act as catalytic centers, in general, contributing to the electrochemical reactivity,16 and hence the enhancement of the electrocatalytic I3−/I− redox process in the case of CNOs.
The relative defect concentration in the as-synthesized CNO particles was estimated by the ID/IG ratio determined from the Raman spectrum shown in Fig. 1b. The spectrum shows mainly three characteristic peaks: the D, G, and 2D bands at 1341, 1578, and 2870 cm−1, respectively. The D band signifies both structural and graphitic defects in sp3-type carbon, whereas the G band signifies the ordered in-plane sp2-carbon atoms in the graphitic structure. Interestingly, the G band for highly oriented pyrolytic graphite (HOPG) typically appears at 1582 cm−1;32 however, in the case of CNOs, it downshifted to 1578 cm−1. The CNO, which is composed of an appropriate pentagon-to-hexagon ratio, is an essential member of the fullerene family. As the geometry of the CNO tends to be spherical, only hexagonal rings are not enough to curl and close to attain the spherical shape. On the other hand, the presence of pentagon atomic blocks helps in curling; however, it results in a strain due to bond length compensation. Since our as-synthesized CNO comprises around 30 nm particles, the quantum confinement effect, and the interfacial surface energy resulted in the downward shift of the G band.31,32 The ID/IG ratio was found to be 0.78, which is lower than that of the reported graphene33 and CNOs prepared from the expensive nanodiamond precursor by a high-vacuum annealing method.24,33 Furthermore, a strong 2D band signifies the presence of stacked concentric graphitic multilayers, which are typically observed in 2D few-layered graphene samples.32,33 Multishell-like graphitic stacking can be discerned in Fig. 2c.
Surface chemistry such as the hydrophilicity of carbonaceous materials plays a significant role in the bulk solution processability of electrodes without using any polymer-based binders/additives. It is crucial to understand the as-synthesized CNO surface chemistry to determine the presence of any functional groups, as the CNOs were prepared by an open-air synthesis method. The surface chemical bonding and composition of the as-prepared CNOs were investigated by XPS. The XPS survey spectrum (Fig. 1c) shows only the presence of carbon and oxygen in 95.4 and 4.6 at%, respectively, without any other impurities. This means that a very high C/O ratio of about 20 highlights its high purity. The strong C 1s peak centered at 284.5 eV, and a weak O 1s peak at 531.5 eV without any other elemental peaks indicate the high purity and homogeneity of the as-synthesized CNOs.34 The deconvolution of high-resolution C 1s spectra (Fig. 1d) confirms the presence of graphitic sp2 carbon and carbon atoms attached to various oxygen-containing surface functional groups. The deconvoluted peaks of C 1s correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.5 eV), C–O (286.3 eV), C
C (284.5 eV), C–O (286.3 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (287.7 eV), and O–C
O (287.7 eV), and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.3 eV), as shown in Fig. 1d.34 This evidences that the as-synthesized CNO contains mostly aromatic sp2 carbon cores (approximately 77%) with surface-adsorbed hydroxyl and carboxylic functional groups, which are responsible for high hydrophilicity in polar solvents. Generally, nanodiamond-derived CNOs are hydrophobic due to their complete graphitization and require chemical surface functionalization post-processing, which results in the uncontrolled loss of graphitization. However, CNOs prepared in this work by a low-temperature ambient air flame synthesis method retain their graphitic character with good hydrophilicity, without requiring any post-synthesis chemical modifications. The structural defects and functional groups are required, to a controlled degree, in the structure to achieve low electrochemical polarization of the redox reaction at the CNO–electrolyte interface.
O (289.3 eV), as shown in Fig. 1d.34 This evidences that the as-synthesized CNO contains mostly aromatic sp2 carbon cores (approximately 77%) with surface-adsorbed hydroxyl and carboxylic functional groups, which are responsible for high hydrophilicity in polar solvents. Generally, nanodiamond-derived CNOs are hydrophobic due to their complete graphitization and require chemical surface functionalization post-processing, which results in the uncontrolled loss of graphitization. However, CNOs prepared in this work by a low-temperature ambient air flame synthesis method retain their graphitic character with good hydrophilicity, without requiring any post-synthesis chemical modifications. The structural defects and functional groups are required, to a controlled degree, in the structure to achieve low electrochemical polarization of the redox reaction at the CNO–electrolyte interface.
The as-synthesized CNOs have distinct characteristics such as high hydrophilicity and surface wettability, which are because of the creation of hydroxyl and carboxylic surface functional groups (Fig. 1d) on the as-prepared CNO surface. As a result, CNOs are highly dispersible in polar solvents and form a homogeneous conductive ink in water, thus preventing the need for any dispersants. The average zeta potential of the as-synthesized CNO suspension in ethanol was found to be −60 mV, as reported previously by our group.27 The magnitude of the zeta potential indicates high dispersion stability against aggregation or sedimentation. The negative zeta potential is due to the presence of hydrophilic oxygen functional groups, which impart a net negative surface charge, hence, the “wettability” of the CNOs. Particle suspension with a zeta potential of ≥±30 mV is typically considered stable against aggregation due to charge stabilization, due to strong electrostatic repulsive forces that prevent coagulation/precipitation.35
CNO–DSSC photovoltaic device performance
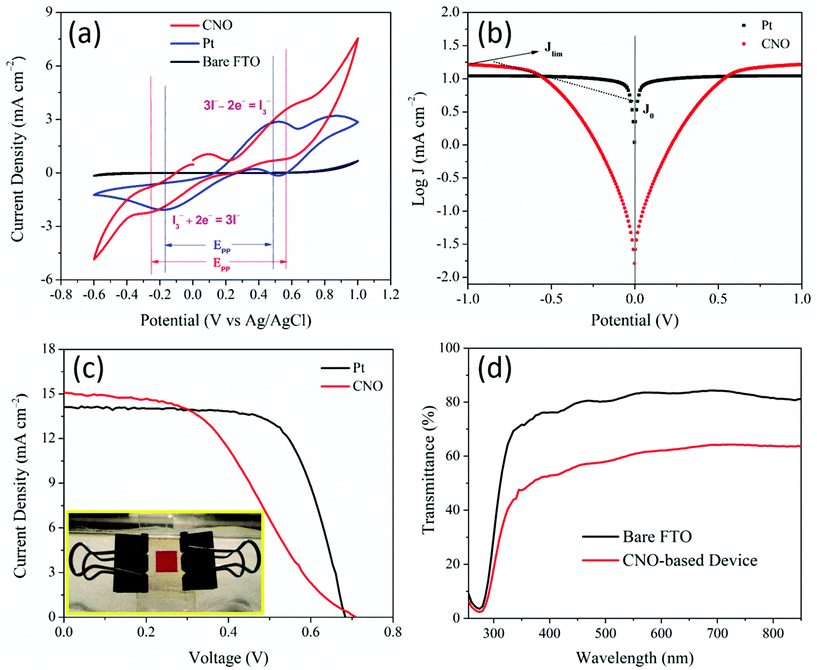
The electrocatalytic activity of the CNO CE towards the I3−/I− redox process was analyzed by cyclic-voltammetry analysis (CV) in a three-electrode configuration. The following reactions occur in the redox couple in an electrochemical cell:| Oxidation: 3I− ⇌ I3− + 2e− | (1) |
| Reduction: I3− + 2e− ⇌ 3I− | (2) |
The measured electrochemical parameters of different CEs are tabulated in Table 1. Furthermore, the peak-to-peak separation (EPP) value of the redox reaction is inversely proportional to the electron transfer rate. Based on Randles–Ševcik theory, which defines the effect of the scan rate on the peak current (IPC), the relationship between the binding sites and the diffusion of iodide ions in a CE is estimated (eqn (3)). The adsorption quantity of the reacting ion (I3−) on the surface of the CEs is estimated using eqn (4).
IPC1 = 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000n1.5ACDn0.5ν0.5 000n1.5ACDn0.5ν0.5 | (3) |
| IPC1 = [(nF)2νψ]/RT | (4) |
| CEs | E ox1 (V) | E red1 (V) | J ox1 (mA cm−2) | J red1 (mA cm−2) | E PP (V) | D n (cm2 s−1) | ψ (mol cm−2) |
|---|---|---|---|---|---|---|---|
| Pt | 0.484 | −0.170 | 2.877 | −2.090 | 0.654 | 8.35 × 10−5 | 1.88 × 10−10 |
| CNO | 0.564 | −0.250 | 3.614 | −2.188 | 0.814 | 7.60 × 10−5 | 0.53 × 10−10 |
Here, IPC1, Dn, and A are the peak current of Red1 (mA), the diffusion coefficient (cm2 s−1), and the electrode area (cm2), respectively. n is the number of electrons involved in the redox reaction (which is 2), C is the concentration of I3 (mol), ν is the scan rate (mV s−1), F is the Faraday constant, R is the universal gas constant, T is the absolute temperature, and ψ is the adsorption quantity of the reacting ion, I3−. The CNO-based CE is comparable to the standard Pt CE in terms of diffusivity and adsorption quantity of the reacting ion (I3−) onto the CE surface (Table 1).
The Tafel plot emphasizes the importance of interfacial charge transfer kinetics occurring at the electrode surface during the redox activity.
The Tafel polarization curves (logarithmic current density vs. applied voltage) are shown in Fig. 3b. A Tafel plot describes two critical parameters, the exchange current density (J0) and the limiting diffusion current density (Jlim) from which the electrocatalytic activity of the electrodes can be estimated using the Tafel and diffusion zones of the Tafel plot (tabulated in Table 2). The J0 value is calculated from the intersection of the extrapolated intercepts of the linear regions of the anodic and cathodic curves at an overpotential of zero. The Tafel curves indicate that the J0 value of the prepared CNO-based CE (5.75 mA cm−2) is lower than that of the Pt CE (9.12 mA cm−2), which indicates the effectiveness of the CNO-based CEs in catalyzing the redox reaction of the I−/I3− couple. The electron transfer rate constant (K0) of the CNO and Pt CEs is estimated using the J0 values from the Tafel plots using eqn (5).
| K0 = J0/CFn | (5) |
| Counter electrode | DSSC parameters | Tafel parameters | |||
|---|---|---|---|---|---|
| R s (Ω) | R ct1 (Ω) | J 0 (mA cm−2) | J lim (mA cm−2) | K 0 (cm s−1) | |
| Pt | 10.4 | 2.6 | 9.12 | 11.02 | 9.39 × 10−5 |
| CNO | 10.3 | 3.1 | 5.75 | 16.59 | 5.92 × 10−5 |
The EIS profiles of the DSSCs fabricated with CNO and Pt-based CEs are shown in Fig. 4 with their modeled equivalent circuit. Fig. 4a shows two semicircles for the two fabricated DSSC devices. The semicircles indicate the charge-transfer resistances, Rct1, and Rct2, at the interface of the CE/liquid electrolyte and photoanode/electrolyte, respectively. The starting point of the curves shows the same resistance value (10.4 Ω) as both devices were fabricated with the same TCO substrates, which indicate the sheet resistance of the TCO substrate. The Rct1 values of the Pt and CNO-based CEs are 2.6 and 3.1 Ω, respectively. The Rct1 value of the CNO-based CE is somewhat higher than that of the Pt-based CE, which reflects on the performance of the DSSCs. The electrochemical impedance parameters obtained by EIS of the DSSCs comprising Pt and CNO-based CEs are tabulated in Table 2 and compared with those reported in the literature in Table 4.
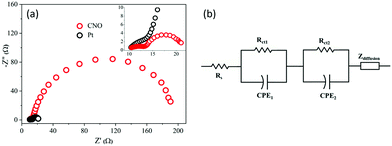 | ||
| Fig. 4 (a) Nyquist plots of CNO and Pt-based DSSC devices and (b) the corresponding equivalent circuit. | ||
The photovoltaic performance of the DSSCs fabricated with CNOs and Pt as the CEs was measured under simulated solar light irradiation with an intensity of 100 mW cm−2 and AM 1.5. The photocurrent–voltage (J–V) characteristics of the DSSCs are shown in Fig. 3c. The photovoltaic conversion efficiency (η) of the fabricated cells was calculated from the fill factor (FF), open-circuit voltage (Voc), and short circuit current density (Jsc) obtained from the J–V curves and the incident optical power (Pin); these parameters are tabulated in Table 3. DSSC cell testing statistics (Fig. S1†), their detailed performance parameters (Table S1†), and stability test for CNO CE and CNO-based complete cells are also provided in Fig. S2.† The low FF of the CNO CE-based DSSC is due to its high series resistance of the device (24 Ω cm−2) compared with that of the Pt-based DSSC.
| CE | J sc (mA cm−2) | V oc (mV) | FF | η (%) | R s (Ω cm−2) | R sh (Ω cm−2) |
|---|---|---|---|---|---|---|
| Pt | 14.1 | 683 | 0.70 | 6.69 | 1.7 | 4080.05 |
| CNO | 15.1 | 701 | 0.49 | 5.17 | 24 | 700.02 |
| No. | Counter electrode | Preparation | Transparency (%) | V oc (mV) | J sc (mA cm−2) | FF | Efficiency | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Transparent graphene | Graphite oxide exfoliation and thermal reduction | ∼70 | 760 | 3.02 | 36.0 | 0.26 | 14 |
| 2 | Graphene | Electrophoretic deposition of GO solution by the modified Hummer's method | ∼85 | 700 | 5.6 | 60.0 | 2.3 | 15 |
| 3 | Moderately rGO | Spin coating of GO solution by the modified Hummer's method | ∼80 | 640 | 16.5 | 26.9 | 2.85 | 20 |
| 4 | Few layer graphene | Dip coating of liquid phase exfoliated graphene | ∼80 | 720 | 12.2 | 38.2 | 3.35 | 16 |
| 5 | rGO | Spin coating of GO solution by the modified Hummer's method | ∼80 | 710 | 14.02 | 38.0 | 3.99 | 21 |
| 6 | Graphene nanoplatelets | Drop cast and purchased GNPs | ∼60 | 673 | 11.0 | 60.0 | 4.38 | 1 |
| 7 | MWCNT | Spray coating of CNT dispersion | ∼40 | 405 | 3.38 | 31.0 | 1.18 | 11 |
| 8 | MWCNT microballs | Spray coating of CNT dispersion | ∼65 | 725 | 12.25 | 55.0 | 4.66 | 12 |
| 9 | SWNTs | Electrophoretic deposition | ∼60 | 715 | 13.0 | 52.3 | 4.94 | 9 |
| 10 | Carbon nitride | Magnetron sputtering | ∼60 | 782 | 12.66 | 13.0 | 1.23 | 13 |
| 11 | Activated carbon | Sol–gel coating | ∼45 | 660 | 8.33 | 40.0 | 2.35 | 10 |
| 12 | Carbon nano-onion | Facile flame synthesis | ∼60 | 701 | 15.1 | 50.0 | 5.17 | This work |
To demonstrate the transparency of the assembled CNO-based DSSC, we recorded the UV–vis spectra of the bare FTO and the entire CNO–DSSC device, which are shown in Fig. 3d. Bare FTO showed approximately 80% transparency, whereas the CNO–DSSC device showed a transparency of approximately 55% in the visible spectral range of 400–700 nm. As a proof of concept, we show the transparent assembled CNO–DSSC (Fig. 3c inset) as a prototype for a commercial transparent DSSC window and its corresponding J–V curves. The transparency of a CE is essential for the fabrication of plastic DSSCs and for achieving tandem solar cell-like operation in practical applications such as windows and roof panels. To the best of our knowledge, this is the first time that a CNO has been explored for transparent DSSC applications with a significant solar conversion efficiency of 5.17% and a reasonable redox activity of the I−/I3− couple.
Conclusions
The present study investigated the possibility of the commercialization of optically transparent DSSCs. For semi-transparent window and roof panel applications, we introduced for the first time a CNO-based CE, which is more cost-effective, electrochemically stable, and active than the standard Pt-based CEs. The CNO-based DSSC demonstrated promising electrocatalytic activity for the I−/I3− redox couple, exhibiting superior exchange current density and charge-transfer resistance. In addition, it delivered a solar power conversion efficiency of 5.17% at an optical transparency of >55%. The combination of a low-cost CE material with a scalable synthesis method and a significant conversion efficiency may accelerate the design and commercialization of BIPVs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the access to experimental facilities at the Centre for Research in Nanotechnology & Science (CRNTS) and Metallurgical Engineering and Materials Science (MEMS) Dept., IIT Bombay. Dr D. Mohapatra acknowledges the financial support received from the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (NRF-2020R1G1A1015243).Notes and references
- L. Kavan, J. H. Yum and M. Grätzel, ACS Nano, 2011, 5, 165–172 CrossRef CAS PubMed.
- B. O'Regan and M. Grätzel, Radiat. Phys. Chem., 1991, 353, 737–740 Search PubMed.
- M. Kouhnavard, N. A. Ludin, B. V. Ghaffari, K. Sopian and S. Ikeda, ChemSusChem, 2015, 8, 1510–1533 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- P. Joshi, Y. Xie, M. Ropp, D. Galipeau, S. Bailey and Q. Qiao, Energy Environ. Sci., 2009, 2, 426–429 RSC.
- E. Ramasamy, W. J. Lee, D. Y. Lee and J. S. Song, Appl. Phys. Lett., 2007, 90, 2005–2008 CrossRef.
- Z. Huang, X. Liu, K. Li, D. Li, Y. Luo, H. Li, W. Song, L. Q. Chen and Q. Meng, Electrochem. Commun., 2007, 9, 596–598 CrossRef CAS.
- G. Wang, W. Xing and S. Zhuo, J. Power Sources, 2009, 194, 568–573 CrossRef CAS.
- H. Kim, H. Choi, S. Hwang, Y. Kim and M. Jeon, Nanoscale Res. Lett., 2012, 7, 1–12 CrossRef PubMed.
- I. Y. Y. Bu and J. Zheng, Mater. Sci. Semicond. Process., 2015, 39, 223–228 CrossRef CAS.
- S. Widodo, G. Wiranto and M. N. Hidayat, Energy Procedia, 2015, 68, 37–44 CrossRef CAS.
- S. I. Cha, B. K. Koo, S. H. Seo and D. Y. Lee, J. Mater. Chem., 2010, 20, 659–662 RSC.
- C. Wu, G. Li, X. Cao, B. Lei and X. Gao, Green Energy Environ., 2017, 2, 302–309 CrossRef.
- X. Wang, L. Zhi and K. Müllen, Nano Lett., 2008, 8, 323–327 CrossRef CAS PubMed.
- H. Choi, S. Hwang, H. Bae, S. Kim, H. Kim and M. Jeon, Electron. Lett., 2011, 47, 281–283 CrossRef CAS.
- S. Nemala, S. Prathapani, P. Kartikay, P. Bhargava, S. Mallick and S. Bohm, IEEE J. Photovolt., 2018, 8, 1252–1258 Search PubMed.
- A. Hinsch, W. Veurman, H. Brandt, R. L. Aguirre, K. Bialecka and K. F. Jensen, Prog. Photovoltaics, 2012, 20, 698–710 CAS.
- Y. Duan, Q. Tang, J. Liu, B. He and L. Yu, Angew. Chem., Int. Ed., 2014, 53, 14569–14574 CrossRef CAS PubMed.
- G. Calogero, P. Calandra, A. Irrera, A. Sinopoli, I. Citro and G. Di Marco, Energy Environ. Sci., 2011, 4, 1838 RSC.
- H. S. Jang, J. M. Yun, D. Y. Kim, D. W. Park, S. I. Na and S. S. Kim, Electrochim. Acta, 2012, 81, 301–307 CrossRef CAS.
- H. S. Jang, J. M. Yun, D. Y. Kim, S. I. Na and S. S. Kim, Surf. Coat. Technol., 2014, 242, 8–13 CrossRef CAS.
- E. Ramasamy, W. J. Lee, D. Y. Lee and J. S. Song, Electrochem. Commun., 2008, 10, 1087–1089 CrossRef CAS.
- D. Mohapatra, G. Dhakal, M. S. Sayed, B. Subramanya, J. J. Shim and S. Parida, ACS Appl. Mater. Interfaces, 2019, 11, 8040–8050 CrossRef CAS PubMed.
- D. Pech, M. Brunet, H. Durou, P. Huang, V. Mochalin, Y. Gogotsi, P.-L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651–654 CrossRef CAS PubMed.
- T. H. Han, D. Mohapatra, N. Mahato, S. Parida, J. H. Shim, A. T. N. Nguyen, V. Q. Nguyen, M. H. Cho and J. J. Shim, J. Ind. Eng. Chem., 2020, 81, 269–277 CrossRef CAS.
- D. Mohapatra, N. S. K. Gowthaman, M. S. Sayed and J. J. Shim, Sens. Actuators, B, 2020, 304, 127325 CrossRef.
- D. Mohapatra, S. Badrayyana and S. Parida, Mater. Chem. Phys., 2016, 174, 112–119 CrossRef CAS.
- S. S. Nemala, K. S. Aneja, P. Bhargava, H. L. M. Bohm, S. Mallick and S. Bohm, Electrochim. Acta, 2018, 285, 86–93 CrossRef CAS.
- M. Zeiger, N. Jäckel, V. Mochalin and V. Presser, J. Mater. Chem. A, 2016, 4, 3172–3196 RSC.
- M. Bystrzejewski, H. Lange, A. Huczko, P. Baranowski, H.-W. Hübers, T. Gemming, T. Pichler, B. Büchner and M. H. Rümmeli, J. Solid State Chem., 2008, 181, 2796–2803 CrossRef CAS.
- D. Ugarte, Nature, 1992, 359, 707–709 CrossRef CAS PubMed.
- M. A. Pimenta, G. Dresselhaus, M. S. Dresselhaus, L. G. Cançado, A. Jorio and R. Saito, Phys. Chem. Chem. Phys., 2007, 9, 1276–1291 RSC.
- E. Obraztsova, M. Fujii, S. Hayashi, V. Kuznetsov, Y. V. Butenko and A. Chuvilin, Carbon, 1998, 36, 821–826 CrossRef CAS.
- H. Ago, T. Kugler, F. Cacialli, W. R. Salaneck, M. S. P. Shaffer, A. H. Windle and R. H. Friend, J. Phys. Chem. B, 1999, 103, 8116–8121 CrossRef CAS.
- M. Mao, S. Chen, P. He, H. Zhang and H. Liu, J. Mater. Chem. A, 2014, 2, 4132–4135 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The solar conversion efficiency chart of each CNO-DSSC device compared with Pt-based DSSCs and their detailed performance parameters, long stability tests for CNO CE and CNO-based complete cells. See DOI: 10.1039/d0nr04382f |
| This journal is © The Royal Society of Chemistry 2020 |