Eupulcherol A, a triterpenoid with a new carbon skeleton from Euphorbia pulcherrima, and its anti-Alzheimer's disease bioactivity†
Chun-Xue
Yu
a,
Ru-Yue
Wang
a,
Feng-Ming
Qi
b,
Pan-Jie
Su
a,
Yi-Fan
Yu
a,
Bing
Li
a,
Ye
Zhao
a,
De-Juan
Zhi
a,
Zhan-Xin
Zhang
 *a and
Dong-Qing
Fei
*a and
Dong-Qing
Fei
 *a
*a
aSchool of Pharmacy, Lanzhou University, Lanzhou 730000, China. E-mail: zhangzhx@lzu.edu.cn; feidq@lzu.edu.cn
bState Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, China
First published on 19th November 2019
Abstract
Eupulcherol A (1), a novel triterpenoid with an unprecedented carbon skeleton, was isolated from Euphorbia pulcherrima. Its structure was determined by comprehensive analysis of spectroscopic data, including HRESIMS and 1D and 2D NMR, and the absolute configuration was defined by single crystal X-ray diffraction analysis. Biological studies showed that compound 1 possessed anti-Alzheimer's disease (AD) bioactivity, which could delay paralysis of transgenic AD Caenorhabditis elegans. A plausible biogenetic pathway for eupulcherol A (1) was also proposed.
Introduction
The genus Euphorbia belongs to the family Euphorbiaceae, which is characterized by the production of white milky latex. Some of its species are considered toxic.1 At the same time, it is one of the largest genera in the Euphorbiaceae family, with about 1600 species. Most of them have been used for the treatment of skin diseases, gonorrhea, migraine, intestinal parasites, and warts.2,3 Numerous chemical studies on the Euphorbia species have been performed in recent decades due to their various types of natural products, including diterpenoids, triterpenoids, flavonoids and steroids.4–7 However, only a few studies on the chemical constituents of Euphorbia pulcherrima have been reported.8–12 These inspired us to investigate the chemical constituents of E. pulcherrima.E. pulcherrima is a perennial herb, widely distributed in the tropical and sub-tropical areas of the world and well known for the treatment of hypermenorrhea, bruises, traumatic hemorrhage, and fracture.12 As part of our continuing study on the structurally unique and anti-Alzheimer's disease (AD) constituents from medicinal plants,13–16 a novel triterpenoid with a new carbon skeleton, namely eupulcherol A (1), was isolated from the whole plant of E. pulcherrima.
Alzheimer's disease (AD) is a neurodegenerative disease characterized by progressive cognitive decline, with abnormal deposits of β-amyloid (Aβ) and aberrant phosphorylation of tau (p-tau) as its pathological hallmarks.17 Aggregated protein and lost neurons and synapses cause a progressive decline in memory and other cognitive functions that ultimately lead to dementia.18 For biological evaluation, we used Caenorhabditis elegans as an AD pathological model to evaluate the bioactivity of the new compound. After being transferred with the human Aβ1–42 gene downstream of the muscle promoter, C. elegans can display Alzheimer's disease (AD)-like symptoms of paralysis induced by Aβ toxicity.19 Herein, we describe the isolation, structure characterization, and anti-AD bioactivity of this compound.
Results and discussion
The air-dried whole plant of E. pulcherrima (20.0 kg) was powdered and extracted with 95% EtOH at room temperature three times (7 days each). Evaporation of ethanol left a crude extract (2.5 kg), which was suspended in H2O, and then extracted with EtOAc to afford the EtOAc soluble fraction (852 g). The EtOAc extract was subjected to column chromatography, and eluted with petroleum ether–acetone (from 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
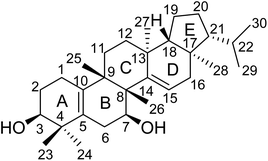
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give six fractions (A–F). Fraction C was further fractionated and chromatographed repeatedly on silica gel and MCI gel columns to give the new compound 1 (Fig. 1).
1) to give six fractions (A–F). Fraction C was further fractionated and chromatographed repeatedly on silica gel and MCI gel columns to give the new compound 1 (Fig. 1).
Eupulcherol A (1), isolated as colorless needles, was found to have a molecular formula of C30H48O2 by analysis of its HRESIMS at m/z 463.3557 [M + Na]+, indicating seven degrees of unsaturation. Its IR spectrum exhibited absorption bands at 3396, 1656 and 1619 cm−1, assignable to hydroxyl groups and two double bonds, respectively. The 1H NMR data (Table 1) showed characteristic signals, including an olefinic proton at δH 5.61 (1H, dd, J = 4.8, 1.8 Hz, H-15), two oxygenated methines at δH 4.21 (1H, dd, J = 10.2, 6.0 Hz, H-7) and 3.53 (1H, dd, J = 5.4, 1.8 Hz, H-3), two secondary methyls at δH 0.92 (3H, d, J = 6.6 Hz, H3-30) and 0.86 (3H, d, J = 4.8 Hz, H3-29), six tertiary methyl groups at δH 1.17 (3H, s, H3-27), 1.06 (6H, s, H3-23 and H3-24), 0.87 (3H, s, H3-26), 0.85 (3H, s, H3-25), and 0.79 (3H, s, H3-28). The 13C NMR, DEPT and HSQC spectra of 1 (Table 1) suggested the presence of 30 carbons, which were sorted into eight methyls, eight methylenes, six methines (including two oxygen-bearing carbons and one olefinic carbon), and eight quaternary carbons (including three olefinic carbons). From the number of carbon resonances in the 13C NMR spectrum, in combination with the molecular formula, it could be inferred that compound 1 could be a triterpenoid. Moreover, deducting two degrees of unsaturation accounted for two double bonds, and the remaining five degrees of unsaturation were indicative of the pentacyclic ring system of 1.
| No. | δ C | δ H (J in Hz) |
|---|---|---|
| 1 | 20.0 | (β)2.12, m |
| (α)1.96, m | ||
| 2 | 25.8 | (β)1.77, m |
| (α)1.84, m | ||
| 3 | 74.9 | 3.53, dd (5.4, 1.8) |
| 4 | 38.9 | |
| 5 | 128.8 | |
| 6 | 30.3 | (β)1.86, m |
| (α)2.36, dd (16.8, 6.0) | ||
| 7 | 66.9 | 4.21, dd (10.2, 6.0) |
| 8 | 48.0 | |
| 9 | 44.1 | |
| 10 | 134.2 | |
| 11 | 31.0 | (β)1.52, m |
| (α)1.75, m | ||
| 12 | 35.7 | (β)1.28, m |
| (α)1.33, m | ||
| 13 | 36.4 | |
| 14 | 144.0 | |
| 15 | 127.0 | 5.61, dd (4.8, 1.8) |
| 16 | 43.8 | (β)2.16, dd (18.0, 1.8) |
| (α)2.30, dd (18.0, 4.8) | ||
| 17 | 40.1 | |
| 18 | 59.7 | 1.60, dd (12.6, 7.8) |
| 19 | 20.2 | (β)1.48, m |
| (α)1.53, m | ||
| 20 | 28.2 | (β)1.88, m |
| (α)1.29, m | ||
| 21 | 59.6 | 1.06, m |
| 22 | 30.7 | 1.48, m |
| 23 | 22.6 | 1.06, s |
| 24 | 27.8 | 1.06, s |
| 25 | 20.4 | 0.85, s |
| 26 | 15.4 | 0.87, s |
| 27 | 20.6 | 1.17, s |
| 28 | 15.7 | 0.79, s |
| 29 | 22.8 | 0.86, d (4.8) |
| 30 | 21.9 | 0.92, d (6.6) |
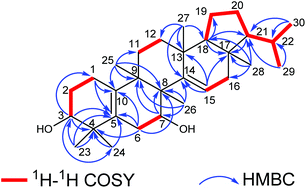
The elucidation of the planar structure of 1 was aided by the results of 2D NMR experiments (Fig. 2). The 1H–1H COSY spectrum of 1 displayed interactions of H2-1/H2-2/H-3 and H2-6/H-7, indicating the connections of C-1/C-2/C-3 and C-6/C-7. These spin systems, in combination with the HMBC correlations of H2-1/C-5 and C-10; H-3/C-1, C-4, C-5 and C-24; H2-6/C-4, C-5, C-8 and C-10; H-7/C-8 and C-9; and H3-25/C-8, C-9 and C-10, suggested the presence of a six-membered ring (ring A) fused to the six-membered ring (ring B) with a Δ5(10) double bond. The C-3 (δC 74.9) and C-7 (δC 66.9) of 1 were substituted with a hydroxyl group respectively which were demonstrated by the chemical shifts of the carbon atoms in the low field, and two tertiary methyl groups (C-23 and C-24) were attached to C-4, which were corroborated by HMBC correlations from H3-23(H3-24) to C-3, C-4 and C-5. Moreover, observations of 1H–1H COSY correlations of H2-11/H2-12 and H-15/H2-16, accompanied by HMBC correlations of H-15/C-13, C-14 and C-17; H3-25/C-8, C-9 and C-11; H3-26/C-7, C-8, C-9 and C-14; H3-27/C-12, C-13, C-14 and C-18; and H3-28/C-16, C-17 and C-18 indicated the presence of six-membered ring C fused to positions C-13 and C-14 on six-membered ring D which were substituted with a Δ14(15) double bond, and four tertiary methyl groups (CH3-25, CH3-26, CH3-27 and CH3-28) at C-9, C-8, C-13 and C-17, respectively. At the same time, ring B was fused with ring C through C-8 and C-9.
For the last ring system, HMBC correlations from H-18 to C-17, C-19 and C-21 and from H3-28 to C-17, C-18 and C-21, in combination with the 1H–1H COSY correlations of H-18/H2-19/H2-20/H-21/H-22/H3-29(H3-30), revealed that a five-membered ring (ring E) existed. Rings D and E composed a bicyclo[4.3.0]nonane ring system. Besides, the HMBC correlations from H3-29 to C-21 and C-22 and from H3-30 to C-21 and C-22 gave an isopropyl moiety at C-21. Thus, the planar structure of 1 was identified as shown in Fig. 1.
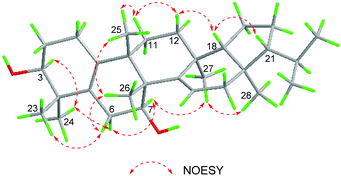
The relative configuration of 1 was determined based on the NOESY data (Fig. 3). In the NOESY experiment, the cross peaks of H-3/H3-24, H3-24/H-6α, H-7/H-6α, H-7/H3-27, and H3-27/H3-28 indicated that H-3, H-6α, H-7, H3-24, H3-27 and H3-28 adopted the same orientation, arbitrarily designated as α-orientation. Likewise, NOESY correlations of H3-23/H-6β, H-6β/H3-26, and H3-25/H3-26 showed that H3-23, H-6β, H3-25, and H3-26 were cofacial, and oriented in the β-direction. There were correlations of H3-25/H-11β, H-11β/H-12β, and H-12β/H-18 indicating that H-18 was in the β-direction. Because of the correlation between H-18 and H-21 in the NOESY experiment, the H-21 in the β-direction can be determined.
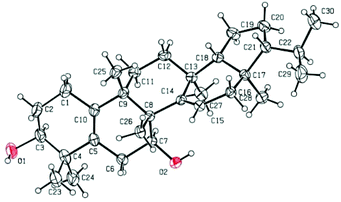
To determine the absolute configuration and confirm the unique structure of 1, a single-crystal X-ray crystallographic experiment was performed by using Cu Kα radiation. The X-ray crystallographic data (Fig. 4) corroborated the planar structure and fully determined the absolute configuration as 3S, 7S, 8R, 9S, 13S, 17R, 18R, 21R with a Flack parameter of 0.03(12).
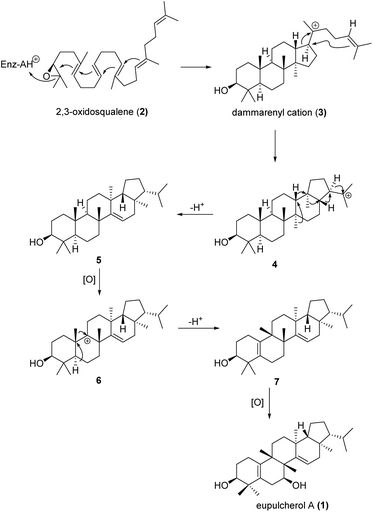
Eupulcherol A (1) bears a unique pentacyclic triterpenoid carbon skeleton, which is a structural feature not previously reported in natural products. A plausible biogenetic route toward the novel skeleton is shown in Scheme 1. Like all triterpene alcohols, 1 seems to be derived from 2,3-oxidosqualene (2).20 Firstly, a type II terpene cyclase catalysed reaction will afford the dammarenyl cation (3), which can give intermediate 4 through simultaneous ring expansion and cyclisation. Next, cation induced cascade migration and deprotonation will afford compound 5. Hydride abstraction of 5 will lead to intermediate 6, which might also be generated by oxidation of compound 5 to a tertiary alcohol followed by elimination of OH−. Then methyl migration and deprotonation of intermediate 6 will afford intermediate 7, which will give eupulcherol A through oxidation.
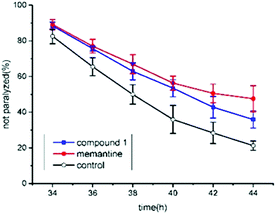
The anti-AD bioactivity of compound 1 was evaluated using transgenic AD Caenorhabditis elegans CL4176 as a model. Previous work has demonstrated that memantine can significantly delay worm paralysis and acts as a disease modifying agent.21 Here, memantine was used as a positive control. The result showed that 50 μM of memantine significantly delayed AD worm paralysis (Fig. 5). Furthermore, 100 μM of compound 1 also significantly alleviated AD-like symptoms (p < 0.05). Although compound 1 exhibited lower anti-AD activity than memantine (p < 0.05), it showed the potential to act as an anti-AD compound candidate. In our previous work, a similar triterpenoid was extracted and identified from Teucrium viscidum and it also has a mild anti-AD activity in C. elegans.15 It is difficult to differentiate which of them had a stronger biological activity by using AD worms. The anti-AD activity of compound 1 should be further evaluated in a murine model of Alzheimer's disease in our future work.
Conclusions
In summary, one new pentacyclic triterpenoid with an uncommon carbon skeleton was identified from the whole plant of E. pulcherrima. The un-methylated AB ring junction is extremely rare and found only in some hopanes from oils and sediments.22 This unique structure further enriches the structural diversity of triterpenoids. Its potential bioactivities and ecological roles are worth unveiling in future research.Experimental section
General experimental procedures
Melting points were determined on an X-4 digital display micromelting point apparatus, and are uncorrected. Optical rotations were measured on a PerkinElmer 341 polarimeter. IR spectra were obtained on a Nicolet NEXUS 670 FT-IR spectrometer. NMR spectra were recorded on a Bruker Avance NEO-600 spectrometer with TMS as the internal standard. HRESIMS data were obtained on a Thermo LTQ Orbitrap Elite mass spectrometer. Silica gel (200–300 mesh) used for column chromatography and silica gel GF254 (10–40 μM) used for TLC were supplied by the Qingdao Marine Chemical Factory, Qingdao, China. MCI GEL CHP 20P (75–150 μM) was purchased from Mitsubishi Chemical Holdings in Japan. Spots were detected on TLC under UV light or by heating after spraying with 5% H2SO4 in C2H5OH (v/v).Plant material
The dried whole plants of Euphorbia pulcherrima were purchased in September 2014 from Hebei Anguo Medicine Market, Anguo, China. The plant material was identified by Dr Jian-Yin Li, School of Pharmacy, Lanzhou University, Lanzhou, China. A voucher specimen (No. 20140821EP) was deposited at the School of Pharmacy, Lanzhou University.Extraction and isolation
The dried whole plants of E. pulcherrima (20.0 kg) were powdered and extracted with 95% ethanol at room temperature three times. Evaporation of ethanol left a crude extract (2.5 kg), which was suspended in H2O, and then extracted with EtOAc to afford the EtOAc soluble fraction (852 g). The EtOAc extract was subjected to column chromatography, and eluted with petroleum ether–Me2CO [from petroleum ether–Me2CO (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to petroleum ether–Me2CO (1
1) to petroleum ether–Me2CO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)] to give six fractions (A–F). Fraction C (62 g) was chromatographed over MCI gel, and eluted by a gradient of EtOH–H2O (from 50% to 100%) to afford three subfractions Ca–Cc. Fraction Cc (8.2 g) was loaded on a chromatography column over silica gel and eluted with petroleum ether–EtOAc (20
1)] to give six fractions (A–F). Fraction C (62 g) was chromatographed over MCI gel, and eluted by a gradient of EtOH–H2O (from 50% to 100%) to afford three subfractions Ca–Cc. Fraction Cc (8.2 g) was loaded on a chromatography column over silica gel and eluted with petroleum ether–EtOAc (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give compound 1 (36 mg). Recrystallization from a mixture of petroleum ether, acetone and MeOH yielded colorless needles.
1) to give compound 1 (36 mg). Recrystallization from a mixture of petroleum ether, acetone and MeOH yielded colorless needles.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); mp 228–230 °C; [α]25D +10 (c 1.10, CH2Cl2); IR (KBr)νmax 3547, 3396, 3054, 2950, 2864, 2305, 1656, 1619, 1463, 1264, 1051 cm−1; 1HNMR (CDCl3, 600 MHz) data, see Table 1; 13CNMR (CDCl3, 150 MHz) data, see Table 1; (+) HRESIMS m/z 463.3557 [M + Na]+ (calcd for C30H48O2, 463.3547).
1); mp 228–230 °C; [α]25D +10 (c 1.10, CH2Cl2); IR (KBr)νmax 3547, 3396, 3054, 2950, 2864, 2305, 1656, 1619, 1463, 1264, 1051 cm−1; 1HNMR (CDCl3, 600 MHz) data, see Table 1; 13CNMR (CDCl3, 150 MHz) data, see Table 1; (+) HRESIMS m/z 463.3557 [M + Na]+ (calcd for C30H48O2, 463.3547).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 164 reflections were measured, 9517 independent reflections (Rint = 0.0268). The final R1 value was 0.0430 (I > 2σ(I)). The final wR(F2) value was 0.1105 ((I > 2σ(I)). The final R1 value was 0.0496 (all data). The final wR(F2) value was 0.1058 (all data). The goodness of fit on F2 was 1.019. The absolute structure parameter was 0.03(12). Crystallographic data for the structure of compound 1 have been deposited at the Cambridge Crystallographic Data Centre (deposition no. CCDC 1955657†).
164 reflections were measured, 9517 independent reflections (Rint = 0.0268). The final R1 value was 0.0430 (I > 2σ(I)). The final wR(F2) value was 0.1105 ((I > 2σ(I)). The final R1 value was 0.0496 (all data). The final wR(F2) value was 0.1058 (all data). The goodness of fit on F2 was 1.019. The absolute structure parameter was 0.03(12). Crystallographic data for the structure of compound 1 have been deposited at the Cambridge Crystallographic Data Centre (deposition no. CCDC 1955657†).
Bioactivity assay for delaying paralysis in transgenic humanized Alzheimer's disease Caenorhabditis elegans
Caenorhabditis elegans strain CL4176 was purchased from the Caenorhabditis Genetics Center (CGC) (University of Minnesota, Minneapolis, MN). Worms were transferred with the human Aβ1–42 gene downstream of the promoter of myo-3 and they were cultured at 16 °C on nematode growth medium (NGM) seeded with E. coli OP50 as their standard food source. About 100 eggs were added onto NGM in the presence of 100 μM of the test compound dissolved in 0.1% DMSO, 50 μM memantine was considered as a positive control and 0.1% DMSO was regarded as a negative control. The worms were kept at 16 °C until they grew into L3 larvae, and then the culture temperature was increased to 25 °C for 34 h. Paralyzed worms were identified and counted under a dissecting stereo-microscope at 2 h intervals until all the worms did not move autonomously. The anti-AD bioactivity of the test compounds was indicated as the percentage of non-paralysis worms in the tested worm population. The more slowly the worms were paralyzed, the higher was the anti-AD bioactivity of the test compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (31670350 and 31870324), the National Key Research and Development Program of China (2018YFC1706300-4), the Natural Science Foundation of Gansu Province, China (17JR5RA201), the Science and Technology Project of Lanzhou City (2018-4-61), and the Fundamental Research Funds for the Central Universities (lzujbky-2018-k13). We gratefully acknowledge Dr Yong-Liang Shao of Lanzhou University for the X-ray single-crystal data measurement. We also thank Prof. Shao-Hua Wang of Lanzhou University for his valuable suggestions on the proposed biosynthetic pathway.Notes and references
- A. Vasas and J. Hohmann, Chem. Rev., 2014, 114, 8579–8612 CrossRef CAS.
- Q. L. Liang, C. C. Dai, J. H. Jiang, Y. P. Tang and J. A. Duan, Fitoterapia, 2009, 80, 514–516 CrossRef CAS.
- L. S. Wan, Y. Nian, X. R. Peng, L. D. Shao, X. N. Li, J. Yang, M. Zhou and M. H. Qiu, Org. Lett., 2018, 20, 3074–3078 CrossRef CAS.
- D. S. Yang, Y. L. Zhang, W. B. Peng, L. Y. Wang, Z. L. Li, X. Wang, K. C. Liu, Y. P. Yang, H. L. Li and X. L. Li, J. Nat. Prod., 2013, 76, 265–269 CrossRef CAS.
- Q. W. Shi, X. H. Su and H. Kiyota, Chem. Rev., 2008, 108, 4295–4327 CrossRef CAS.
- C. Jiang, P. Luo, Y. Zhao, J. L. Hong, S. L. Morris-Natschke, J. Xu, C. H. Chen, K. H. Lee and Q. Gu, J. Nat. Prod., 2016, 79, 578–583 CrossRef CAS.
- S. N. Liu, D. Huang, S. L. Morris-Natschke, H. Ma, Z. H. Liu, N. P. Seeram, J. Xu, K. H. Lee and Q. Gu, Org. Lett., 2016, 18, 6132–6135 CrossRef CAS.
- F. Warnaar, Lipids, 1977, 12, 707–710 CrossRef CAS.
- W. J. Baas, Planta Med., 1977, 32, 1–8 CrossRef CAS.
- X. A. Dominguez, J. G. Delgado, M. De Lourdes Maffey, J. G. Mares and C. Rombold, J. Pharm. Sci., 1967, 56, 1184–1185 CrossRef CAS.
- I. Smith-Kielland, J. M. Dornish, K. E. Malterud, G. Hvistendahl, C. Romming, O. C. Bockman, P. Kolsaker, Y. Stenstrom and A. Nordal, Planta Med., 1996, 62, 322–325 CrossRef CAS.
- Y. Dai, S. N. Liu, J. Xu, C. Zhao and Q. Gu, Fitoterapia, 2019, 134, 355–361 CrossRef CAS PubMed.
- P. Q. Wu, Y. F. Yu, Y. Zhao, C. X. Yu, D. J. Zhi, F. M. Qi, D. Q. Fei and Z. X. Zhang, Org. Biomol. Chem., 2018, 16, 9038–9045 RSC.
- Z. X. Zhang, P. Q. Wu, H. H. Li, F. M. Qi, D. Q. Fei, Q. L. Hu, Y. H. Liu and X. L. Huang, Org. Biomol. Chem., 2018, 16, 1745–1750 RSC.
- Z. Y. Li, F. M. Qi, D. J. Zhi, Q. L. Hu, Y. H. Liu, Z. X. Zhang and D. Q. Fei, Org. Chem. Front., 2017, 4, 42–46 RSC.
- D. Q. Fei, L. L. Dong, F. M. Qi, G. X. Fan, H. H. Li, Z. Y. Li and Z. X. Zhang, Org. Lett., 2016, 18, 2844–2847 CrossRef CAS.
- S. E. Lamb, B. Sheehan, N. Atherton, V. Nichols, H. Collins, D. Mistry, S. Dosanjh, A. M. Slowther, I. Khan, S. Petrou and R. Lall, Br. Med. J., 2018, 361, k1675 CrossRef.
- C. Ballard, S. Gauthier, A. Corbett, C. Brayne, D. Aarsland and E. Jones, Lancet, 2011, 377, 1019–1031 CrossRef.
- S. Abbas and M. Wink, Phytomedicine, 2010, 17, 902–909 CrossRef CAS.
- M. J. Stephenson, R. A. Field and A. Osbourn, Nat. Prod. Rep., 2019, 36, 1044–1052 RSC.
- G. M. Alley, J. A. Bailey, D. M. Chen, B. Ray, L. K. Puli, H. Tanila, P. K. Banerjee and D. K. Lahiri, J. Neurosci. Res., 2010, 88, 143–154 CrossRef CAS.
- K. Shiojima, Y. Arai, T. Kasama and H. Ageta, Chem. Pharm. Bull., 1993, 41, 262–267 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1D and 2D NMR, HRESIMS, and IR spectra, and crystallographic data in CIF. CCDC 1955657. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ob02334h |
| This journal is © The Royal Society of Chemistry 2020 |