Reversible control of RNA interference by siRNAzos†
Matthew L.
Hammill
,
Golam
Islam
and
Jean-Paul
Desaulniers
 *
*
University of Ontario Institute of Technology, Faculty of Science, 2000 Simcoe Street North, Oshawa, Ontario L1G 0C5, Canada. E-mail: jean-paul.desaulniers@ontariotechu.ca
First published on 25th November 2019
Abstract
In this study, we report the reversible control of RNA interference using siRNAzos, a class of siRNAs that contain azobenzene. Herein, we demonstrate that it is possible to take an active siRNAzo, and inactivate it for up to 24 hours. We also demonstrate reversibility of these siRNAzos within cell culture. For example, active siRNAzos can be inactivated in cell culture with ultraviolet light, and then reactivated with visible light. In addition, we also show that siRNAzos can be activated and inactivated towards the endogenous target gene, BCL2.
The RNA interference (RNAi) pathway is an endogenous defense mechanism that targets viral and parasitic double-stranded RNA (dsRNA), and regulates gene expression in eukaryotes. Fire and Mello published a paper in 1998, identifying double-stranded RNAs as gene-silencing agents in the nematode Caenorhabditis elegans.1 Ever since this discovery, there has been interest in utilizing this pathway for therapeutics and biomolecular tools.2–4 The most recent FDA-approved siRNA is Onpattro, which is used for the treatment of hereditary transthyretin amyloidosis (hATTR).5 Despite this success, siRNAs as therapeutics often come with several known problems, such as poor stability, toxicity, off-target and tissue specific targeting. However, one issue in the field that has largely been overlooked is reversing RNAi activity in the event of adverse siRNA off-target effects. Some recent research shows that short locked nucleic acid-modified oligonucleotides complementary to the seed region of the guide strands can reverse siRNA activity.6 However, despite this study, more research and methods to inactivate siRNA function is needed. In this paper, we describe the utility of photoreversibly controling RNAi using an siRNAzo (azobenzene-containing siRNA).
Several advances over the last decade have led the development of photoresponsive siRNAs, which generally are dsRNAs that are inactive when entered inside of the cell, and then are activated with light. The first example was developed by Friedman and coworkers, in which they used a backbone-modified siRNA labeled with 1-(1-diazoethyl)-4,5-dimethoxy-2-nitrobenzene (DMNPE-N2). Upon exposure with UV light (λ > 320 nm), the groups are removed to yield an active siRNA.7 Heckel and coworkers have used photoresponsive protecting groups on guanine and thymidine nucleotides of the guide strand of siRNAs, which becomes uncaged to UV light.8 Most recently, Mokhir and Meyer developed a 5′-labelled alkoxyanthracenyl siRNA, which becomes uncaged via a singlet oxygen (1O2) photoregenerated photosensitizer on the 3′-end of the guide strand. Uncaging occurs with green or red light to yield an active siRNA.9 Despite these innovative advances, an inactive siRNA complex is irreversibly photoactivated to yield an active siRNA. The process cannot be reversed, and it is not entirely clear what effect the by-products of the caged functional groups when released may have on the cell. Having the ability to reversibly control the activity of an siRNA within the cell would offer greater spatial control of the siRNA. This reversibility could bypass and eliminate the undesired off-target side effects.
Azobenzene is an attractive photoresponsive molecule because of its ability to photo-isomerize in the presence of UV and visible light. This causes a large conformational change in the molecule, which can be used to disrupt biomolecular structures.10 The more stable isomer, trans is the normal resting state of the molecule, but through the addition of energy in the form of UV light between 330–365 nm, photo-isomerization occurs and the molecule adopts a cis isomeric form. The cis form of azobenzene is less stable due to steric hindrance and strain on the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and thus can be converted back to trans with visible light above 450 nm.11 Because of these unique and useful properties, azobenzene has been used in many applications, including its incorporation into oligonucleotides (see Fig. 1) since it is relatively easy to synthesize and has a high quantum yield when photo-switching.12
N bond and thus can be converted back to trans with visible light above 450 nm.11 Because of these unique and useful properties, azobenzene has been used in many applications, including its incorporation into oligonucleotides (see Fig. 1) since it is relatively easy to synthesize and has a high quantum yield when photo-switching.12
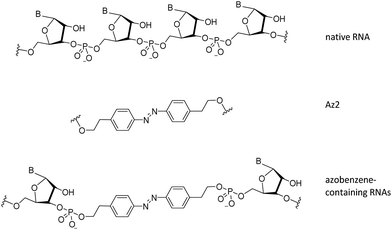 | ||
| Fig. 1 Structural differences between native RNA, and azobenzene-containing RNAs. Az2 corresponds to the azobenzene unit used in this study. | ||
Previous work in our group utilized azobenzene's photo properties in order to make siRNAzos, which are siRNA molecules that contain azobenzene.13,14Fig. 2 highlights the photoswitching properties of the siRNAzo. In the native trans form, the siRNAzo is active. When the siRNAzo is treated with UV light, the azobenzene unit adopts a cis conformation, and thus inactivates the siRNAzo. Restoration of activity can proceed by thermal relaxation and/or visible light.
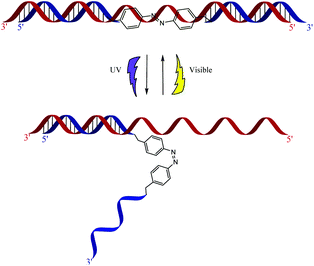 | ||
| Fig. 2 Photoinduced inactivation and reactivation of siRNAzos. The blue strand corresponds to the sense strand, and contains the azobenzene moiety. The red strand corresponds to the antisense strand. | ||
The siRNAzos that we generated contained the azobenzene within the central region of the sense strand (see Table 1). siRNAzo 1 replaces positions 8 and 9 from the 5′-end of the sense strand. Moving this azobenzene unit (Az2) one nucleobase at a time, siRNAzos 2, 3, and 4 replace positions 9 and 10, 10 and 11, and 11 and 12 from the 5′-end of the sense strand, respectively. The siRNAzos 5–7 designed to target the endogenous BCL2 gene also contain the azobenzene unit within the central region of the sense strand. Our previous work showed that not only did the siRNAzos 1–4 have excellent knockdown at picomolar concentrations, but we could also control the siRNAzo's activity through UV inactivation prior to the transfection of the siRNAs.13 We also demonstrated the ability to restore siRNA activity through the application of the broadband visible light greater than 450 nm.
| siRNAzo | siRNAzo duplex | Target |
|---|---|---|
a
![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/b_char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/b_char_0032_0332.gif) corresponds to the azobenzene derivative synthesized from 4-nitrophenylethyl alcohol; the top strand corresponds to the sense strand; the bottom strand corresponds to the antisense strand. In all duplexes, the 5′-end of the bottom antisense strand contains a 5′-phosphate group. corresponds to the azobenzene derivative synthesized from 4-nitrophenylethyl alcohol; the top strand corresponds to the sense strand; the bottom strand corresponds to the antisense strand. In all duplexes, the 5′-end of the bottom antisense strand contains a 5′-phosphate group.
|
||
| wt | 5′-CUUACGCUGAGUACUUCGAtt-3′ | Luciferase |
| 3′-ttGAAUGCGACUCAUGAAGCU-5′ | ||
| 1 | 5′-CUUACGC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) AGUACUUCGAtt-3′ AGUACUUCGAtt-3′ |
Luciferase |
| 3′-ttGAAUGCGACUCAUGAAGCU-5′ | ||
| 2 | 5′-CUUACGCU![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) GUACUUCGAtt-3′ GUACUUCGAtt-3′ |
Luciferase |
| 3′-ttGAAUGCGACUCAUGAAGCU-5′ | ||
| 3 | 5′-CUUACGCUG![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) UACUUCGAtt-3′ UACUUCGAtt-3′ |
Luciferase |
| 3′-ttGAAUGCGACUCAUGAAGCU-5′ | ||
| 4 | 5′-CUUACGCUGA![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) ACUUCGAtt-3′ ACUUCGAtt-3′ |
Luciferase |
| 3′-ttGAAUGCGACUCAUGAAGCU-5′ | ||
| 5 | 5′-GCCUUCU![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) GAGUUCGGUGtt-3′ GAGUUCGGUGtt-3′ |
BCL2 |
| 3′-ttCGGAAGAAACUCAAGCCAC-5′ | ||
| 6 | 5′-GCCUUCUU![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) AGUUCGGUGtt-3′ AGUUCGGUGtt-3′ |
BCL2 |
| 3′-ttCGGAAGAAACUCAAGCCAC-5′ | ||
| 7 | 5′-GCCUUCUUUG![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ![[z with combining low line]](https://www.rsc.org/images/entities/char_007a_0332.gif) ![[2 with combining low line]](https://www.rsc.org/images/entities/char_0032_0332.gif) UUCGGUGtt-3′ UUCGGUGtt-3′ |
BCL2 |
| 3′-ttCGGAAGAAACUCAAGCCAC-5′ | ||
While this work was incredibly promising and useful, we were limited by the azobenzenes propensity to thermally relax back to trans when in the cis conformation. Azobenzene's half life at 37 °C is approximately 4 hours,15 and this limited the durations we could do these experiments at, and consequently limiting their usefulness for longer term experiments.
In this current manuscript, and building from our previous work, we demonstrate that our siRNAzos can be inactivated post-transfection, inside the cells through the controlled application of UV light to the cell culture without damaging the cells. We are also able to reactivate the siRNAzos with visible light. Furthermore, we are able to complete this deactivation/activation cycle up to two times for an siRNAzo. To the best of our knowledge, this marks the first report of using an active siRNA, and inactivating it within cell culture, and then later reversibly activating it. In order to understand the reversible gene-silencing effect observed in our study, we first must understand the RNAi gene-silencing mechanism.
When a siRNA is incorporated into an active antisense-RISC complex, the sense strand is released. This active antisense strand complex can then target its mRNA for cleavage. However, once this active antisense-RISC complex forms, we are not able to inactivate its function reversibly because the azobenzene modification is contained within the sense strand. Thus, within the cell, there is an equilibrium that exists between unbound siRNAs and its bound active antisense-RISC complex. Yet, out of the total siRNA molecules internalized into the cell, it has been shown that only around 4% or less is actively associated with the RISC complex.16 By inactivating unbound siRNAs in the cell, we can prevent further loading to the RISC complex, and prevent the formation of more active antisense-RISC complexes. This in turn would reduce the amount of gene-silencing over time.
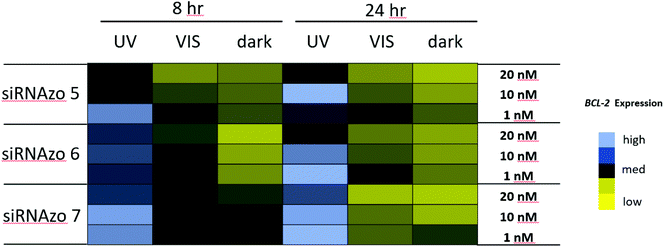
In our previous study, we observed UV-mediated inactivation of the siRNAzo to be effective at 8-hour time points, but at 12 hours the siRNAzo resumed some activity. Although the 8-hour time point is optimal for short assays, many gene expression studies go beyond 8 hours, and we wanted to develop a more robust system that was effective beyond this time window. Using the Dual-Luciferase Reporter® Assay (Promega), we modified our procedure (see ESI† for experimental procedures) to transfect active siRNAzos, in which we could inactivate with UV exposure (45 min dose) two hours post transfection. Within the 8-hour assay time-frame, one exposure of UV light was sufficient to keep the unbound siRNAzos inactive, but in order to extend this level of inactivity to a 24 hours window, we exposed the transfected cells to low repeated doses of UV light (a 45 minutes dose of low intensity 365 nm UV light, repeated every four hours, for a total of six UV exposures). Previous literature reported that this amount of UV light for up to 24 hours was not particularly harmful to the cells.17 We also conducted XTT assays on the cell cultures exposed to UV light, and no loss on cell viability was observed (see ESI S1†). Our gene-silencing results for these two assays are shown in Fig. 3, as a color-coded chart (see Fig. S12–S17 in the ESI† for the corresponding numerical bar graphs).
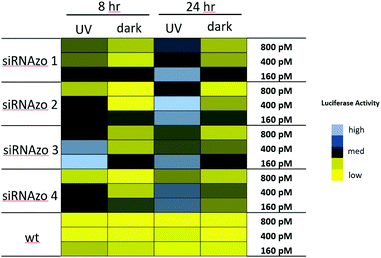 | ||
| Fig. 3 Normalized firefly luciferase activity for siRNAzos 1–4 and wt at 160, 400 and 800 pM in HeLa cells monitored 8- and 24-hours post-transfection. UV corresponds to the siRNA being exposed under a 365 nm UV lamp for inactivation 2 h post transfection for 45 min (8 h), and for an additional 45 min of UV exposure every 4 hours (24 h). Dark corresponds to siRNAs being transfected in HeLa cells in the absence of UV light. 8-Hour fold changes differences in activity for siRNAzos 1–4 (UV vs. dark) for were between 2–55 fold, and 24-hour fold changes were between 1–7 fold. See ESI S1† for details. | ||
High luciferase activity is correlated to the intensity of the blue pixel, whereas low luciferase activity correlates to the intensity of the yellow pixel. There is a clear difference shown in luciferase activity between the UV and dark columns for both time points. Initially, at both 8- and 24-hour time points, luciferase activity is low for the dark-treated samples (dark), an indication of strong RNAi activity. Exposure to UV light causes RNAi activity to diminish and as a result luciferase activity was found to increase as shown by the black and blue pixels in the color-coded chart (UV). More importantly, we were able to keep the unbound siRNAzo inactive for up to 24 hours, without thermal relaxation, which is a significant improvement over our previous study where after 12 hours the siRNAzo was active.13 Overall, the siRNAzos in their active form have good activity, however, we are able to inactivate their activity for up to 24 hours post transfection.
In the next experiment, we examined whether we could not only keep the unbound siRNAzo inactive for 24 hours, but also reactivate the unbound siRNAzos at a desired timepoint. The color-coded chart in Fig. 4 shows this data (see Fig. S18–S27 in the ESI† for the corresponding numerical bar graphs). As before, the UV-treated samples (UV) have only moderate to high luciferase activity, the visible-light treated samples (VIS) have moderate to low luciferase activity, and the no light (dark) samples have low luciferase activity. In all cases, we were able to take an inactive siRNAzo (1–4), and restore its RNAi activity as measured by low luciferase activity after visible light exposure.
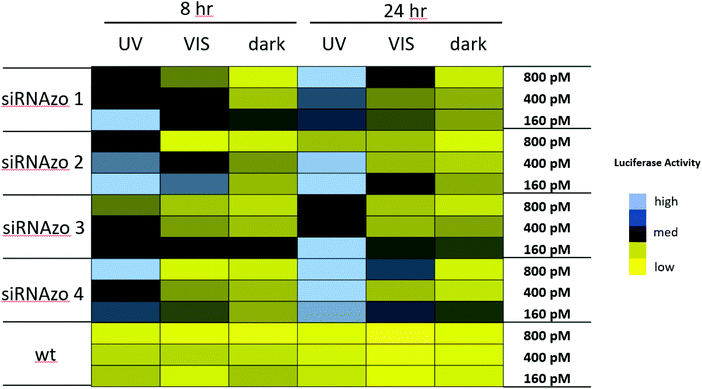 | ||
| Fig. 4 Normalized firefly luciferase expression for siRNAzos 1–4 and wt at 160, 400, and 800 pM in HeLa cells monitored 8- and 24-hours post-transfection. UV corresponds to the siRNA being exposed under a 365 nm UV lamp for inactivation 2 h post transfection for 45 min (8 h), and for an additional 45 min of UV exposure every 4 hours (24 h). VIS corresponds to visible light exposure 4 h after transfection. Dark corresponds to siRNAs being transfected in HeLa cells in the absence of UV light. Fold changes for siRNAs 1–4 (UV vs. dark) for 8 h were between 2 and 14 fold, and 24-hour fold change differences were between 3 and 21 fold. See ESI S1† for details. | ||
We also explored whether the siRNAzo would remain intact after mutliple on/off cycles. The gene-silencing data for this experiment is shown in Fig. 5 where the UV/VIS/UV/VIS bar (grey) shows that knockdown remains robust after two cycles. The first cycle was performed identically to the 8-hour inactivation assay highlighted in Fig. 3 and 4. After 8 hours, we then observed a 2-hour resting period of darkness to let the cells recover after which the cells were re-exposed to UV light for 45 min. After this exposure, the cells were allowed to rest for 1 h 15 min in the dark and then the visible light was utilized again for 30 min to restore the siRNAzo's activity. This second cycle was an important step to show that after one cycle, siRNAzo activity was maintained. At all three concentrations (160, 400, and 800 pM), activity was restored and this is shown with the grey bar in Fig. 5. There is a clear difference in gene-silencing activity between this grey bar and the blue inactivation bar, even after two doses of UV light. Additionally the UV/VIS/UV corresponds to 1.5 cycles (purple bar), and in Fig. 5 the gene-silencing data illustrates that after one inactivation cycle, we can reactivate and then deactivate the siRNAzo and it remains inactive for the remaining 24 h of the assay at 160 and 400 pM. At the higher concentration 800 pM, full inactivity is not fully restored, but is less active than the two full cycles (UV/VIS/UV/VIS) and the dark control (yellow). In addition, to investigate the effect of metabolism on the siRNAzos, we performed a glutathione reduction assay on the sense strand of an siRNAzo and observed minimal degradation for up to 24 hours as monitored by HPLC (see ESI S38† for details).
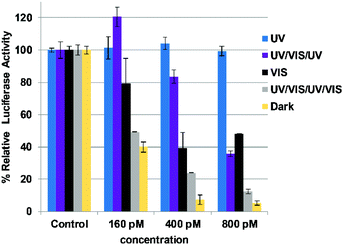 | ||
| Fig. 5 Normalized firefly luciferase activity for siRNAzo 4 at 160, 400 and 800 pM in HeLa cells monitored 24 hours post-transfection. UV corresponds to the siRNAzo being exposed under a 365 nm UV lamp for inactivation 2 h post transfection for 45 min, and for an additional 45 min of UV exposure every 4 hours (six exposures total). UV/VIS/UV corresponds to one and a half consecutive UV/VIS cycles: UV inactivation, 2 hours post-transfection for 45 min, followed by visible light reactivation at 4 hours after transfection for 30 min. A 2-hour rest period of darkness followed by re-exposure to UV light for 45 min, being re-exposed every 4 hours to UV (45 min) afterwards (5 exposures total). VIS corresponds to visible light exposure 4 h after transfection. UV/VIS/UV/VIS corresponds to two consecutive UV/Vis cycles: UV inactivation, 2 hours post-transfection for 45 min, followed by visible light reactivation at 4 hours after transfection for 30 min. A 2-hour rest period of darkness followed by re-exposure to UV light for 45 min, followed by 1 h 15 min in the dark and then the visible light for 30 min (ESI S1† for details). Dark corresponds to siRNAzo 4 being transfected in HeLa cells in the absence of UV light. | ||
In addition, we also examined the inactivation and reactivation of an siRNAzo targeting the endogeneous target, BCL2 (Table 1). This anti-apoptotic endogenous target is of particular interest because it is one of the many oncogenes associated with several cancers that are upregulated.18,19 In a previous study of ours, we observed good knockdown of BCL2 using siRNAs bearing a conformationally-constrained biphenyl spacer within the central region of the sense strand.20 Using our photoresponsive protocol highlighted with the luciferase target, we conducted a similar experiment using real-time polymerase chain reaction (RT-PCR) to quantify the gene-silencing results.
The left half of Fig. 6 shows the results of the BCL2 targeting siRNAzos 5–7 at the 8-hour time point, with the siRNAzos being pre-inactivated prior to transfection. Four hours post-transfection, UV light was administered. As demonstrated in Fig. 6, poor RNAi activity of the siRNAzo is observed as indicated by the presence of high BCL2 expression. In an experiment involving the same transfection setup, visible light was administered four hours post-transfection. As is indicated, excellent gene silencing was observed. The difference between the active and inactive siRNAzo complexes is between two- and five-fold which is to our knowledge the first time an endogenous gene expression silencing profile could be controlled via the RNAi pathway in this manner. A dark control experiment involved transfecting an active BCL2 siRNAzo, and maintaining its active form in the dark. As expected, this experiment showed excellent dose-dependent gene-silencing characteristics.
The right half of Fig. 6 shows the results for the longer 24 hour time point assay, in which we could maintain inactivation of the unbound siRNAzos over the duration of the assay. As done similarly to the luciferase assays, transfections were carried out with the active form of siRNAzos 5–7 and 2 hours later was exposed to repeated doses of 365 nm UV light to inactivate it. The changes in gene silencing activity are between three- and five-fold for the different concentrations. This is consistent with the 8-hour assay. These two experiments show that our siRNAzos are not only effective against endogenous targets, but we can inactivate their activity with UV light.
To account for the reversible gene-silencing observed by the siRNAzos, we must consider both the populations of unbound siRNAzos and siRNAzos bound to RISC in the cell. The azobenzene modification is located on the sense strand of the siRNAzo. When the RISC-antisense strand is activated, the sense strand is no longer part of the complex. Thus, when UV light enters the cell, it is not capable of reversing the antisense strand from the RISC complex. This system differs in its reversibility mechanism compared to the reported high-affinity short oligonucleotides that reversibly target the siRNA guide strand.6 In our system, a population of siRNAzos will be loaded by the RISC complex to form an active RISC-antisense complex, and another population of siRNAzos will remain unbound. Thus, by subjecting the cells to UV light, we hypothesize that this would inhibit the loading of remaining free siRNAzos to the RISC complex. Reactivation of this complex with visible light, would then restore the siRNAzo to its active form, which can then be loaded with more RISC complexes to further gene silence. We believe that this mechanism accounts for the reversible gene-silencing observed.
In conclusion, we are reporting here a significant improvement over prior reports of photoresponsive siRNA technology because we can reversibly control the activity of an exogeneous and endogenous target with light. This newfound ability to reversibly control an endogenous target's activity after deployment into the tissue, would be useful for controlling adverse side effects in susceptible individuals. Many prior siRNAs that targeted a variety of diseases resulted in unexpected side effects.21 Another potential application for these siRNAzos is their use as biomolecular tools to examine the effect of gene expression of complex and/or interrelated genes, in real time. Currently, the simultaneous deployment of multiple siRNAs (siRNA cocktails) is limited because they are concurrently deployed at once post-transfection. Using our siRNAzo technology, we could allow for several different gene-silencing siRNAs to be controlled in real time via light. Our future works include red-shifting the azobenzene's isomerization wavelengths out of the UV portion of the spectrum, thereby allowing us to have unique photochemical control of these siRNAs using specific wavelengths.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Natural Sciences and Engineering Research Council (NSERC) for funding.Notes and references
- A. Fire, S. Q. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver and C. C. Mello, Nature, 1998, 391, 806 CrossRef CAS.
- L. Aagaard and J. J. Rossi, Adv. Drug Delivery Rev., 2007, 59, 75 CrossRef CAS.
- A. L. Hopkins and C. R. Groom, Nat. Rev. Drug Discovery, 2002, 1, 727 CrossRef CAS PubMed.
- X. Shen and D. R. Corey, Nucleic Acids Res., 2017, 46, 1584 CrossRef.
- D. Al Shaer, O. Al Musaimi, F. Albericio and B. G. de la Torre, Pharmaceuticals, 2019, 12, 52 CrossRef CAS.
- I. Zlatev, A. Castoreno, C. R. Brown, J. Qin, S. Waldron, M. K. Schlegel, R. Degaonkar, S. Shulga-Morskaya, H. Xu, S. Gupta, S. Matsuda, A. Akinc, K. G. Rajeev, M. Manoharan and V. Jadhav, Nat. Biotechnol., 2018, 36, 509 CrossRef CAS.
- S. Shah, S. Rangarajan and S. H. Friedman, Angew. Chem., Int. Ed., 2005, 44, 1328 CrossRef CAS.
- V. Mikat and A. Heckel, RNA, 2007, 13, 2341 CrossRef CAS PubMed.
- A. Meyer and A. Mokhir, Angew. Chem., Int. Ed., 2014, 53, 12840 CrossRef CAS.
- A. S. Lubbe, W. Szymanski and B. L. Feringa, Chem. Soc. Rev., 2017, 46, 1052 RSC.
- C. Brieke, F. Rohrbach, A. Gottschalk, G. Mayer and A. Heckel, Angew. Chem., Int. Ed., 2012, 51, 8446 CrossRef CAS PubMed.
- A. A. Beharry and G. A. Woolley, Chem. Soc. Rev., 2011, 40, 4422 RSC.
- M. L. Hammill, C. Isaacs-Trépanier and J.-P. Desaulniers, ChemistrySelect, 2017, 2, 9810 CrossRef CAS.
- M. L. Hammill, A. Patel, M. A. Alla and J.-P. Desaulniers, Bioorg. Med. Chem. Lett., 2018, 28, 3613 CrossRef CAS.
- K. Yamana, K. Kan and H. Nakano, Bioorg. Med. Chem., 1999, 7, 2977 CrossRef CAS.
- Y. I. Pei, P. J. Hancock, H. Zhang, R. Bartz, C. Cherrin, N. Innocent, C. J. Pomerantz, J. Seitzer, M. L. Koser, M. T. Abrams, Y. Xu, N. A. Kuklin, P. A. Burke, A. B. Sachs, L. Sepp-Lorenzino and S. T. Barnett, RNA, 2010, 16, 2553 CrossRef CAS.
- J. D. Stoien and R. J. Wang, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 3961 CrossRef CAS.
- M. L. Cleary, S. D. Smith and J. Sklar, Cell, 1986, 47, 19 CrossRef CAS.
- G. Kroemer, Nat. Med., 1997, 3, 614 CrossRef CAS PubMed.
- J.-P. Desaulniers, G. Hagen, J. Anderson, C. McKim and B. Roberts, RSC Adv., 2017, 7, 3450 RSC.
- M. E. Kleinman, K. Yamada, A. Takeda, V. Chandrasekaran, M. Nozaki, J. Z. Baffi, R. J. C. Albuquerque, S. Yamasaki, M. Itaya and Y. Pan, Nature, 2008, 452, 591 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ob02509j |
| This journal is © The Royal Society of Chemistry 2020 |