Open Access Article
Open Access ArticleMechanism and application of surface-initiated ATRP in the presence of a Zn0 plate†
Rebecca
Faggion Albers
 abcd,
Wenqing
Yan
b,
Matteo
Romio
bc,
Edson R.
Leite
de,
Nicholas D.
Spencer
abcd,
Wenqing
Yan
b,
Matteo
Romio
bc,
Edson R.
Leite
de,
Nicholas D.
Spencer
 b,
Krzysztof
Matyjaszewski
f and
Edmondo M.
Benetti
b,
Krzysztof
Matyjaszewski
f and
Edmondo M.
Benetti
 *bc
*bc
aComplex Materials, Department of Materials, ETH Zürich, Vladimir-Prelog-Weg 1-5/10, CH-8093 Zurich, Switzerland
bLaboratory for Surface Science and Technology, Department of Materials, ETH Zürich, Vladimir-Prelog-Weg 1-5/10, CH-8093 Zurich, Switzerland. E-mail: edmondo.benetti@mat.ethz.ch
cSwiss Federal Laboratories for Materials Science and Technology (Empa), Lerchenfeldstrasse 5, CH-9014, St. Gallen, Switzerland
dDepartment of Chemistry, Federal University of São Carlos, 13565-905 São Carlos, SP, Brazil
eBrazilian Nanotechnology National Laboratory (LNNano), Brazilian Center for Research in Energy and Materials (CNPEM), 13083-970 Campinas, Brazil
fDepartment of Chemistry, Carnegie Mellon University, 4400 Fifth Avenue, Pittsburgh, USA
First published on 23rd October 2020
Abstract
Surface-initiated atom transfer radical polymerization in the presence of a Zn0 plate (SI-Zn0-ATRP) enables the rapid synthesis of chemically diverse polymer brushes from both planar inorganic supports, and fabrics made of natural fibers. This process enables the controlled formation of polymer-brush layers on a variety of substrates, without the need for either inert conditions or lengthy deoxygenation procedures of the reaction mixtures.
The development of oxygen-tolerant, surface-initiated, controlled radical polymerization (SI-CRP) methods has paved the way for the accessible synthesis of polymer-brush coatings on large substrates, circumventing the need for lengthy deoxygenation of reaction mixtures. Following closely the recent advances in the development of oxygen-tolerant CRPs in solution,1 the application of analogous processes to solid surfaces has offered the possibility to translate the synthesis of polymer brushes from fundamental studies into technologically relevant processes.
Similarly to solution-based approaches, the successful and controlled fabrication of polymer brushes under ambient conditions has been accomplished by employing oxygen scavengers. For instance, in the presence of appropriate reducing agents, Cu-based catalysts efficiently consume oxygen through complexation and can still trigger the growth of compositionally different brushes during surface-initiated activators regenerated by electron transfer atom transfer radical polymerization (SI-ARGET ATRP).2–7 Alternatively, in the presence of visible or UV light, oxygen can be consumed by organic or organometallic photocatalysts that are typically employed during photoinduced SI-ATRP,8,9 or photoinduced electron-transfer surface-initiated reversible addition fragmentation chain transfer polymerization (SI-PET RAFT),10 enabling the rapid fabrication of polymer brushes without degassing the reaction mixtures, while keeping polymerization setups exposed to air.
Besides scavengers present in solution, oxygen can be alternatively consumed by applying zerovalent metal (Mt0)-coated plates, which consume oxygen to form oxide layers, and simultaneously act as source of transition-metal catalyst for ATRP when placed on initiator-bearing surfaces previously covered by a thin layer of monomer/ligand mixtures.11–18
Following this strategy, Jordan and co-workers introduced Cu0-mediated SI-ATRP, demonstrating how this process can be applied for the modification of extremely large substrates, by simply keeping them on a lab bench, and using just microliter volumes of polymerization mixtures.11,19 This process enabled the extremely rapid synthesis of compositionally different polymer brushes20 from a variety of inorganic and organic supports.16,21
Relevantly, when Cu0-coated plates were replaced with Fe0-coated surfaces thick brush films could be grown in a biocompatible process—even from supports pre-seeded with mammalian cells, without altering their viability.18
Motivated by the possibility to expand this method to alternative, readily accessible and inexpensive Mt0 plates, in this work we explore the applicability of Zn0 plates to favor the fast and oxygen–tolerant fabrication of thick brush films.
As previously demonstrated in the case of polymerizations performed in solution, Zn0 powder efficiently acts as reducing agent for CuII during supplemental activator and reducing agent (SARA) ATRP,22–24 showing a very high reducing activity when compared to Fe0 or Mg0.25
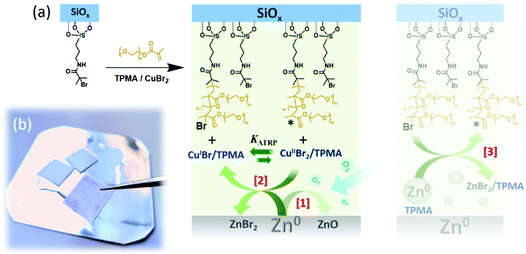
In the case of the synthesis of polymer brushes from planar substrates, polymerization mixtures including monomer, ligand, CuIIBr2 and solvent are sandwiched between an ATRP initiator-modified SiOx substrate, and a Zn0 plate.
The metallic plate was polished just before the experiment (Scheme 1) in order to remove the native oxide layer, and to generate an homogeneous metal surface (ESI†). After this process, a reaction volume of ∼1 μL cm−2 is generated between the two opposing surfaces, and the vertical spacing between the initiator-bearing substrate and the Zn0 plate (d) corresponds to ∼10 μm.17
Under these conditions, oxygen dissolved in the polymerization solution is rapidly consumed through oxidation of Zn0 to ZnIIO (Scheme 1a [1]),26,27 while CuIIBr2/L species are simultaneously reduced to CuIBr/L adducts. These can diffuse in the medium and reach the initiator-functionalized surface, triggering the controlled growth of polymer chains according to the ATRP equilibrium (Scheme 1a [2]).
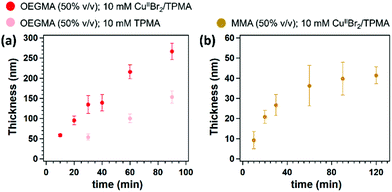
Surface-initiated Zn0-mediated ATRP (SI-Zn0-ATRP) of (oligoethylene glycol)methacrylate (OEGMA) was exemplarily investigated. A 50% (v/v) solution of OEGMA in dimethylformamide (DMF) containing 10 mM CuIIBr2 and 10 mM tris(2-pyridylmethyl)amine (TPMA) was poured on a freshly polished Zn0 plate, and subsequently covered by a SiOx substrate, which had previously been functionalized with a silane-based ATRP initiator. Variable-angle spectroscopic ellipsometry (VASE) was employed to monitor the increment in POEGMA-brush dry thickness (Tdry) ex situ, providing an insight into brush-growth kinetics. As reported in Fig. 1a, SI-Zn0-ATRP enabled the rapid and progressive growth of POEGMA brushes, which reached more than 270 nm of dry thickness after 90 min of reaction. No induction time was recorded, suggesting a very fast reduction of CuIIBr2/L species by Zn0,26 and a rapid diffusion of activators to the initiator sites at the substrate.
It is important to emphasize that the preparation of Zn0 plates by polishing is a standard procedure for treating metal surfaces and requires just 15–20 minutes, and (rather cheap) sandpaper. After this process, 20 × 20 cm2 activated Zn0 plates (typical cost ∼2 €) are obtained. These can be used multiple times and for grafting brushes on a relatively large number of substrates.
Similarly to POEGMA analogues, the growth of poly(methyl methacrylate) (PMMA) brushes by SI-Zn0-ATRP (50% v/v in DMF, with 10 mM CuIIBr2/TPMA) followed an initially fast kinetics, without an induction period (Fig. 1b). However, the rate of PMMA-brush thickening significantly slowed down after ∼60 min, and PMMA films reached a dry thickness of ∼40 nm after two hours of reaction.
Interestingly, when SI-Zn0-ATRP was performed in the absence of CuIIBr2 but in the presence of ligand, significant brush growth could still be recorded. As shown in Fig. 1a, POEGMA brushes reached nearly 150 nm of dry thickness when a mixture including 50% OEGMA and 10 mM TPMA was sandwiched between a Zn0 plate and an ATRP initiator-bearing substrate for 90 min.
This result suggests that Zn0 can act as supplemental activator during the growth of polymer brushes by SI-Zn0-ATRP, in agreement with the mechanism of SARA ATRP studied in solution in the presence of Zn0 particles.25
Considered the physical distance between the Zn0 plate and the ATRP initiator-functionalized substrate within an ideal polymerization setup, we hypothesize that Zn0 micro/nanoparticles produced during the polishing process (Fig. S1†) might diffuse within the polymerization medium and in the presence of ligand can activate surface-bound initiators, triggering brush growth (Scheme 1a [3]). In particular, Zn0/L adducts can act as supplemental activators reacting with alkyl halides and generating radicals that can initiate polymerization from the surface. However, this process simultaneously produces ZnIIBr2/L species, which are very poor deactivators, presumably leading to a redox-initiated free radical surface-initiated polymerization process.25
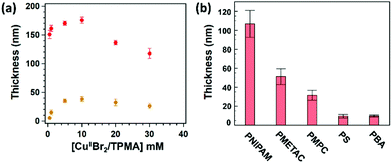
Hence, CuII-based species added in solution are fundamental to guarantee a controlled and steady growth of polymer brushes. The role of CuIIBr2/L during SI-Zn0-ATRP can be elucidated by measuring the dry thickness of POEGMA and PMMA brushes obtained after a fixed polymerization time of 30 min, while varying [CuIIBr2/L] initially added in the mixture. As reported in Fig. 2a, both POEGMA- and PMMA-brush thicknesses significantly increase with [CuIIBr2/L] until a maximum in Tdry is reached, in correspondence to a value of [CuIIBr2/L] ∼ 10 mM. When [CuIIBr2/L] is further increased, the values of Tdry progressively decrease, indicating that an “optimum” concentration of CuII-based species enables the fastest growth of polymer brushes through SI-Zn0-ATRP.
On the one hand, an initial increment in [CuIIBr2/L] results in a concomitant increase in [CuIBr/L] through the rapid reduction by Zn0, determining a faster polymerization and thus the formation of thicker brushes. On the other hand, a further increase in [CuIIBr2/L] beyond 10 mM presumably causes accumulation of deactivators, which necessarily slows down the grafting process and lead to the formation of thinner films.
Hence, the balance between generation of activator species by Zn0-mediated reduction and accumulation of CuII-based deactivators, determines the brush-growth kinetics during SI-Zn0-ATRP.
Under optimized conditions, SI-Zn0-ATRP enabled the synthesis of chemically different polymer brushes by simply performing the grafting process on a Petri dish exposed to air and placed on a lab bench, using just few microliter volumes of reaction solutions, and without the need for their deoxygenation. In this way, poly(methacrylate), poly(acrylate), poly(acrylamide) and poly(styrene) brushes could be fabricated and under full ambient conditions (Fig. 2b). In particular poly(N-isopropylacrylamide) (PNIPAM), poly(2-methacrylolyloxyethyltrimethylammonium chloride) (PMETAC) and poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) brushes with thicknesses ≥30 nm were synthesized following relatively short reaction times. In contrast, poly(styrene) (PS) and poly(butyl acrylate) (PBA) brushes showed just ∼10 nm of dry thickness, presumably due to the relatively low temperature applied during the grafting process.
It is also important to emphasize that with respect to the corresponding grafting process employing Cu0 plates, SI-Zn0-ATRP enables the controlled grafting of polymer brushes while applying reaction environments that could virtually display relatively low toxicity. In order to confirm this hypothesis, besides CuII/CuI species, the rapid growth of POEGMA brushes could be accomplished by applying a Fe-based catalyst. In particular, FeIIIBr3/tertbutylammonium bromide (TBABr) is readily reduced to FeIIBr2/TBABr activators by the Zn0 surface, triggering the growth of more than 30 nm-thick POEGMA brushes in just 60 min of reaction (Fig. S2†). Relevantly, a similar result was previously accomplished by using Fe0 plates as reducing agent for Fe0-mediated SI-ATRP (SI-Fe0-ATRP).19 However, in contrast to SI-Fe0-ATRP, in SI-Zn0-ATRP the grafting process is mediated by metallic plates based on Zn0, which are much cheaper (2 vs. 60 € for a 20 × 20 cm2 plate) and more readily available with respect to Fe0 or Fe0-coated analogues.
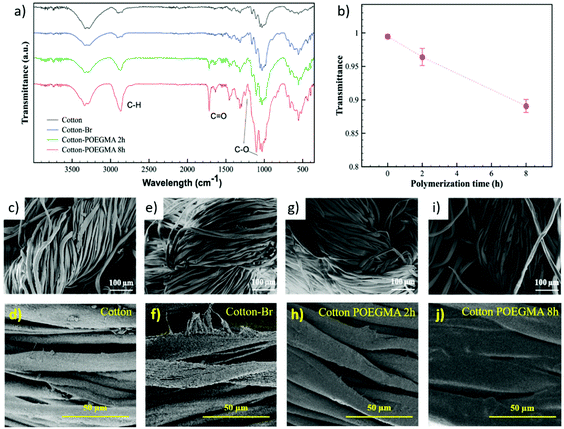
In order to demonstrate the applicability of SI-Zn0-ATRP for the modification of a variety of supports, we subsequently investigated the growth of polymer brushes from cotton-based wound dressings, which had previously been modified with ATRP initiator moieties. 100%-cotton fabrics functionalized with α-bromoisobutyrate functions were initially soaked in a polymerization mixture comprising 50% (v/v) OEGMA in DMF and 10 mM CuIIBr2/TPMA, and subsequently sandwiched between a Zn0 plate and an inert glass slide. Attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR) confirmed the successful grafting of POEGMA brushes from the cotton fibers after 2 and 8 hours of reaction (Fig. 3a), through the appearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band at 1717 cm−1, the C–H band at 2869 cm−1 and the C–O signals at 1248 and 1102 cm−1. In addition, the decrease in the values of transmittance of the C
O stretching band at 1717 cm−1, the C–H band at 2869 cm−1 and the C–O signals at 1248 and 1102 cm−1. In addition, the decrease in the values of transmittance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band with polymerization time suggested the progressive growth of POEGMA grafts. The formation of brush layers on the cotton fibers was additionally confirmed by scanning electron microscopy (SEM). As reported in Fig. 2c–f, the surface morphology of cotton fibers did not show a significant variation after functionalization with ATRP initiator. However, following SI-Zn0-ATRP, a uniform brush film became visible on the cotton structures, and grew thicker after relatively long polymerization times.
O band with polymerization time suggested the progressive growth of POEGMA grafts. The formation of brush layers on the cotton fibers was additionally confirmed by scanning electron microscopy (SEM). As reported in Fig. 2c–f, the surface morphology of cotton fibers did not show a significant variation after functionalization with ATRP initiator. However, following SI-Zn0-ATRP, a uniform brush film became visible on the cotton structures, and grew thicker after relatively long polymerization times.
Conclusions
SI-Zn0-ATRP represents an extremely versatile method to synthesize chemically different polymer brushes under full ambient conditions, using just microliter volumes of reaction solutions, and without the need for their previous deoxygenation. When a polymerization mixture including CuII salts, ligand and monomer is sandwiched between a Zn0 plate and a SiOx substrate functionalized with ATRP initiators oxygen is rapidly consumed via the formation of ZnIIO. Simultaneously, CuII-based species are reduced by the Zn0 plate, yielding CuI activators that in the presence of ligand diffuse to the initiator-bearing substrate, and lead to the rapid growth of polymer grafts, which proceeds according to the ATRP equilibrium. Zn0 micro/nanoparticles leaching from the metallic surface can additionally act as supplemental activators while diffusing through the polymerization mixture. In the absence of CuII species, Zn0/L centers trigger a redox-initiated, free-radical polymerization process, through the simultaneous generation of poor deactivators based on ZnBr2/L adducts.Under optimized experimental conditions, SI-Zn0-ATRP enables the rapid polymerization of different monomer species (acrylates, methacrylates, acrylamides and styrene), it is compatible with both Cu- and Fe-based catalysts, and it could be applied from model inorganic substrates as well as for the modification of three-dimensional fabrics, such as in the case of cotton-based wound dressings.
Experimental section
Materials
Silicon wafers (P/B〈100〉 were purchased from Si-Mat Silicon Wafers (Germany), Zn plates were purchased from Mayitr. 3-(Aminopropyl)triethoxysilane (APTES, ≥98%, Sigma-Aldrich), 2-bromoisobutyryl bromide (BiBB, 98%, Sigma-Aldrich), triethylamine (TEA, ≥99.5%, Sigma-Aldrich), dichloromethane (DCM, dry, ≥99.8%, Acros), cotton fabrics (100%, Rhena® Ideal), tris(2-pyridylmethyl)amine (TPMA, 98%, Sigma Aldrich), 2,2′-bipyridyl (bipy, ≥, Sigma-Aldrich), tris(2-dimethylaminoethyl)amine (Me6TREN, 99%, ABCR), CuIIBr2 (99%, Sigma Aldrich), 2-methacryloyloxyethyl phosphorylcholine (MPC, 97%, Sigma-Aldrich), [2-(methacryloyloxy)ethyl]trimethylammonium chloride solution (METAC, 80% in water, Sigma-Aldrich), and tetrahydrofuran (dry THF, 99.5%, Acros), were used as received. Water used in all experiments was from Millipore Milli-Q. Oligo(ethylene glycol)methacrylate (OEGMA, Mn ∼ 500, Sigma-Aldrich), methyl methacrylate (MMA, 99%, Sigma-Aldrich), styrene (99.5%, Acros Organics) and butyl acrylate (BA, 99%, Acros Organics) were purified by passing them through a basic alumina column. N-Isopropylacrylamide (NIPAM, >98.0%, Tokyo Chemical Industry) was recrystallized from toluene to remove inhibitors.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2SO4
1 H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2) for 30 min, and subsequently washed extensively with ultra-pure water. The substrates were first functionalized with APTES, through vapor deposition, and they were subsequently washed with dry toluene and ultra-pure water. Later on, SiOx-APTES substrates were placed in a flask kept under N2 atmosphere, in which dry DCM, BIBB (1 v%) and TEA (1 v%) were added. The reaction was carried out for one hour, and the substrates were finally washed with chloroform and ethanol, yielding ATRP initiator-functionalized SiOx surfaces.
H2O2) for 30 min, and subsequently washed extensively with ultra-pure water. The substrates were first functionalized with APTES, through vapor deposition, and they were subsequently washed with dry toluene and ultra-pure water. Later on, SiOx-APTES substrates were placed in a flask kept under N2 atmosphere, in which dry DCM, BIBB (1 v%) and TEA (1 v%) were added. The reaction was carried out for one hour, and the substrates were finally washed with chloroform and ethanol, yielding ATRP initiator-functionalized SiOx surfaces.
Following polymerization, brush-modified cotton fabrics were subjected to soxhlet extraction with ethanol as solvent to remove any unreacted monomer/unbound polymer.
The values of dry thickness of polymer brushes were obtained from 4 different substrates analyzed after SI-Zn0-ATRP. At least 3 different points on each substrate were measured by VASE.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Prof André R. Studart, Dr Rafael Libanori (ETH Zürich) and Prof. Katharina Maniura (EMPA) for the fruitful scientific discussions. We acknowledge the funding Brazilian agency Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (2016/14493-7 and 2017/22304-2) and EMPA for the financial support.References
- J. Yeow, R. Chapman, A. J. Gormley and C. Boyer, Chem. Soc. Rev., 2018, 47, 4357–4387 RSC.
- K. Matyjaszewski, H. C. Dong, W. Jakubowski, J. Pietrasik and A. Kusumo, Langmuir, 2007, 23, 4528–4531 CrossRef CAS.
- G. J. Dunderdale, C. Urata, D. F. Miranda and A. Hozumi, ACS Appl. Mater. Interfaces, 2014, 6, 11864–11868 CrossRef CAS.
- T. Sato, G. J. Dunderdale, C. Urata and A. Hozumi, Macromolecules, 2018, 51, 10065–10073 CrossRef CAS.
- N. Li, T. Li, X. Y. Qiao, R. Li, Y. Yao and Y. K. Gong, ACS Appl. Mater. Interfaces, 2020, 12, 12337–12344 CrossRef CAS.
- D. Hong, H. C. Hung, K. Wu, X. J. Lin, F. Sun, P. Zhang, S. J. Liu, K. E. Cook and S. Y. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 9255–9259 CrossRef CAS.
- H. Kang, W. Jeong and D. Hong, Langmuir, 2019, 35, 7744–7750 CrossRef CAS.
- B. Narupai, Z. A. Page, N. J. Treat, A. J. McGrath, C. W. Pester, E. H. Discekici, N. D. Dolinski, G. F. Meyers, J. R. de Alaniz and C. J. Hawker, Angew. Chem., Int. Ed., 2018, 57, 13433–13438 CrossRef CAS.
- W. Q. Yan, S. Dadashi-Silab, K. Matyjaszewski, N. D. Spencer and E. M. Benetti, Macromolecules, 2020, 53, 2801–2810 CrossRef CAS.
- M. Li, M. Fromel, D. Ranaweera, S. Rocha, C. Boyer and C. W. Pester, ACS Macro Lett., 2019, 8, 374–380 CrossRef CAS.
- T. Zhang, Y. Du, F. Muller, I. Amin and R. Jordan, Polym. Chem., 2015, 6, 2726–2733 RSC.
- E. S. Dehghani, Y. H. Du, T. Zhang, S. N. Ramakrishna, N. D. Spencer, R. Jordan and E. M. Benetti, Macromolecules, 2017, 50, 2436–2446 CrossRef CAS.
- Y. J. Che, T. Zhang, Y. H. Du, I. Amin, C. Marschelke and R. Jordan, Angew. Chem., Int. Ed., 2018, 57, 16380–16384 CrossRef CAS.
- T. Zhang, E. M. Benetti and R. Jordan, ACS Macro Lett., 2019, 8, 145–153 CrossRef CAS.
- M. Fantin, S. N. Ramakrishna, J. J. Yan, W. Q. Yan, M. Divandari, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, Macromolecules, 2018, 51, 6825–6835 CrossRef CAS.
- W. Q. Yan, M. Fantin, S. Ramakrishna, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, ACS Appl. Mater. Interfaces, 2019, 11, 27470–27477 CrossRef CAS.
- W. Q. Yan, M. Fantin, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, ACS Macro Lett., 2019, 8, 865–870 CrossRef CAS.
- A. Layadi, B. Kessel, W. Yan, M. Romio, N. D. Spencer, M. Zenobi-Wong, K. Matyjaszewski and E. M. Benetti, J. Am. Chem. Soc., 2020, 142, 3158–3164 CrossRef CAS.
- T. Zhang, Y. H. Du, J. Kalbacova, R. Schubel, R. D. Rodriguez, T. Chen, D. R. T. Zahn and R. Jordan, Polym. Chem., 2015, 6, 8176–8183 RSC.
- W. Q. Yan, M. Fantin, N. D. Spencer, K. Matyjaszewski and E. M. Benetti, ACS Macro Lett., 2019, 8, 865–870 CrossRef CAS.
- K. H. Zhang, W. Q. Yan, R. Simic, E. M. Benetti and N. D. Spencer, ACS Appl. Mater. Interfaces, 2020, 12, 6761–6767 CrossRef CAS.
- K. F. Augustine, T. G. Ribelli, M. Fantin, P. Krys, Y. Cong and K. Matyjaszewski, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 3048–3057 CrossRef CAS.
- D. Konkolewicz, Y. Wang, M. Zhong, P. Krys, A. A. Isse, A. Gennaro and K. Matyjaszewski, Macromolecules, 2013, 46, 8749–8772 CrossRef CAS.
- D. Konkolewicz, Y. Wang, P. Krys, M. J. Zhong, A. A. Isse, A. Gennaro and K. Matyjaszewski, Polym. Chem., 2014, 5, 4396–4417 RSC.
- Y. Zhang, Y. Wang and K. Matyjaszewski, Macromolecules, 2011, 44, 683–685 CrossRef CAS.
- A. Anastasaki, V. Nikolaou, G. Nurumbetov, P. Wilson, K. Kempe, J. F. Quinn, T. P. Davis, M. R. Whittaker and D. M. Haddleton, Chem. Rev., 2016, 116, 835–877 CrossRef CAS.
- E. Liarou, R. Whitfield, A. Anastasaki, N. G. Engelis, G. R. Jones, K. Velonia and D. M. Haddleton, Angew. Chem., Int. Ed., 2018, 57, 8998–9002 CrossRef CAS.
- X. Dong, H. Bao, K. Ou, J. Yao, W. Zhang and J. He, Fibers Polym., 2015, 16, 1478–1486 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0py01233e |
| This journal is © The Royal Society of Chemistry 2020 |