One pot synthesis of pyrrolo[3,2,1-de]phenanthridines from 7-phenylindoles via tandem C–H olefination/aza-Michael addition†
Yumeng
Yuan
,
Guoshuai
Pan
,
Xiaofeng
Zhang
and
Qiufeng
Huang
 *
*
Fujian Key Laboratory of Polymer Materials, College of Chemistry & Materials Science, Fujian Normal University, Fuzhou, Fujian 350007, P.R. China. E-mail: qiufenghuang@fjnu.edu.cn
First published on 7th November 2019
Abstract
An unprecedented one-pot C–H olefination/aza-Michael addition tandem process has been developed for the synthesis of pyrrolo[3,2,1-de]phenanthridines from 7-phenylindoles and alkenes using a [Cp*RhCl2]2/AgOAc/Me4NOAc catalytic system. A relatively wide range of functional groups are tolerated, and a variety of pyrrolo[3,2,1-de]phenanthridines are obtained in good to excellent yields.
Introduction
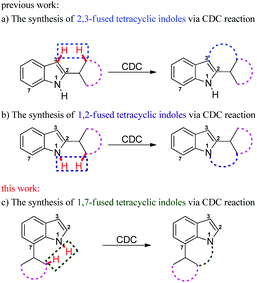
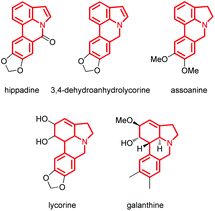
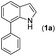
The indole skeleton is unarguably one of the most important structural moieties in innumerable natural and unnatural compounds.1 In particular, fused tetracyclic ring systems containing indole units attracted synthetic chemists due to their prevalent occurrence in many biologically active molecules.2 As the synthesis of these fused tetracyclic indoles usually requires multistep procedures,3 efficient, simple, and divergent production of these molecules remains an important challenge for synthetic organic chemists.In recent years, the transition-metal-catalyzed cross-dehydrogenative-coupling (CDC) has emerged as an attractive and ideal strategy to synthesize complex heterocyclic molecules, as it permits the building of C–C bonds by directly connecting two C–H bonds. The benefits of CDC include atom and step economy, lower cost, and less waste.4 CDC tools were realized for the preparation of 2,3-fused (Scheme 1a) or 1,2-fused tetracyclic indoles (Scheme 1b). For example, the CDC reaction of 2-arylindoles with alkynes, alkenes, ketenes, diazo compounds, and carbon monoxide leading to indolo[2,1-a]isoquinolines,5 indole-indolones,6 benzo[a]carbazoles,7 5H-benzo[a]carbazol-5-ones8and 6H-isoindolo[2,1-a]indol-6-ones9 has been developed. The synthesis of indolo[3,2-c]coumarins,10 indolo[3,2-c]quinolinones,11 and indole[3,2-c]pyrones12via the intramolecular CDC reaction was also uncovered. Greaney and co-workers disclosed a palladium-catalyzed intramolecular CDC reaction between the aromatic ring and the indolyl moiety producing a set of indole-containing seven-and eight-membered rings.13 Inspired by those advances and as part of our continued interest in the NH-indole-directed C–H functionalization,9,14 we herein present a protocol for the rhodium-catalyzed C–H alkenylation and subsequent intramolecular aza-Michael reaction of 7-arylindoles with alkenes leading to diverse biologically important pyrrolo[3,2,1-de]phenanthridines (Fig. 1).15 To the best of our knowledge, the synthesis of such attractive 1,7-fused tetracyclic indoles via the CDC reaction has yet to be described (Scheme 1c).
Results and discussion
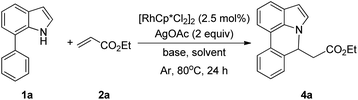
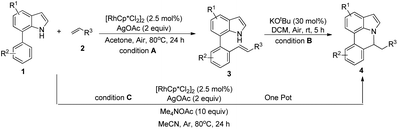
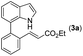
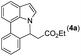
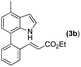
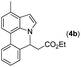
Our initial studies involved the intermolecular Fujiwara–Moritani reaction of 7-phenyl-1H-indol (1a) with ethyl acrylate (2a) employing the conditions of [Cp*RhCl2]2 (2.5 mol%) and AgOAc (2 equiv.) in ethyl acetate (EtOAc) at 100 °C. This leads to the generation of mono-ortho-alkenylated product 3a in 35% (Table S1,† entry 1) with complete E-stereoselectivity. No di-alkenylated or C3-alkenylated product was found in the reaction mixture. We were delighted to find that changing the solvent from EtOAc to acetone could lead to a dramatic increase of the yield of 3a to 84% (Table S1,† entry 2). The optimum reaction temperature is 80 °C, thus affording near quantitative yield (Table S1,† entry 8). Notably, this transformation can also be performed under an air atmosphere; the isolated yield of 3a is 97% (Scheme 2, eqn (1)). Treating 3a with 30 mol% KOtBu in dichloromethane (DCM) resulted in an intramolecular aza-Michael reaction. The aza-Michael product 7H-pyrrolo[3,2,1-de]phenanthridine 4a is obtained in quantitative yield in pure form directly after workup without the need for column chromatography (Scheme 2, eqn (2)). Replacing KOtBu with other bases such as KOH, DMAP, Et3N, KOAc, NaOAc, CsOAc, and LiOAc failed to furnish the desired products, and starting material 3a was recovered (Table S2†). Encouraged by the above results, one-pot synthesis of 7H-pyrrolo[3,2,1-de]phenanthridine 4a by combining intermolecular C–H olefination and intramolecular aza-Michael addition of 7-phenyl-1H-indole (1a) with ethyl acrylate (2a) was surveyed (Table 1). KOtBu had a detrimental effect on the [Cp*RhCl2]2/AgOAc catalyst system, and the starting material 1a was recovered totally (Table 1, entry 1). Other bases such as DBU, DMAP, KOH, and KPF6 also gave unsatisfactory results (entries 2–5). Interestingly, employing quaternary ammonium salts such as nBu4NOAc and Me4NOAc in acetone at 80 °C for 24 h, the desired product 4a can be obtained in 35% and 28% yields, respectively (entries 6 and 7). When the amount of Me4NOAc was increased from 2 equiv. to 10 equiv., the yield of 4a improved to 58% NMR yield (entry 9). No significant change in the yield of 4a was observed on using 10 equiv. of nBu4NOAc (entry 8). When 10 equiv. of other acetate salts such as KOAc, NaOAc, CsOAc, and LiOAc was employed, the reaction stopped after olefination and intramolecular aza-Michael addition product 4a was not formed totally. After an extensive screen of solvents, we found that the use of CH3CN gave the best result of 99% yield of 4a after 24 h (entry 15, 97% isolated yield). MeOH as the solvent gave 3a selectively, and other solvents such as EtOH, DCE, DCM, 1,4-dioxane, and EtOAc led to complete reaction shutdown. Finally, a decrease in the loading of Me4NOAc (5 equiv.) resulted in a lower yield of 4a (88%, Table 1, entry 17).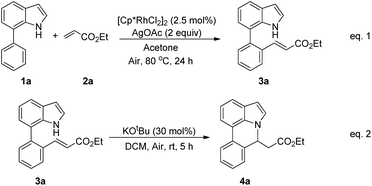 | ||
| Scheme 2 Rhodium-catalyzed ortho C–H olefination of 7-arylindoles (eqn (1)); KOtBu-catalyzed intramolecular aza-Michael addition (eqn (2)). | ||
| Entry | Base | Solvent | Yieldb (%) |
|---|---|---|---|
| a Conditions: 1a (0.3 mmol), 2a (0.75 mmol), [Cp*RhCl2]2(2.5 mol%), AgOAc (2 equiv.), base, solvent(2 mL), 80 °C, 24 h. b 1H NMR Yield on the basis of the amount of 1a used; the number in parentheses is the isolated yield. c Other acetate salts = KOAc, NaOAc, CsOAc, and LiOAc. | |||
| 1 | KOtBu (2 equiv.) | Acetone | Trace |
| 2 | DBU (2 equiv.) | Acetone | Trace |
| 3 | DMAP (2 equiv.) | Acetone | Trace |
| 4 | KOH (2 equiv.) | Acetone | Trace |
| 5 | KPF6 (2 equiv.) | Acetone | Trace |
| 6 | nBu4NOAc (2 equiv.) | Acetone | 35 |
| 7 | Me4NOAc (2 equiv.) | Acetone | 28 |
| 8 | nBu4NOAc (10 equiv.) | Acetone | 30 |
| 9 | Me4NOAc (10 equiv.) | Acetone | 58 |
| 10c | Other acetate salts (10 equiv.) | Acetone | 0 |
| 11 | Me4NOAc (10 equiv.) | Xylene | Trace |
| 12 | Me4NOAc (10 equiv.) | DCE | Trace |
| 13 | Me4NOAc (10 equiv.) | DCM | Trace |
| 14 | Me4NOAc (10 equiv.) | 1,4-Dioxane | Trace |
| 15 | Me4NOAc (10 equiv.) | EtOAc | Trace |
| 16 | Me 4 NOAc (10 equiv.) | MeCN | 99 (97) |
| 17 | Me4NOAc (5 equiv.) | MeCN | 88 |
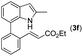
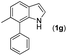
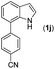
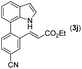
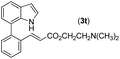
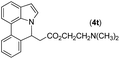
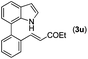
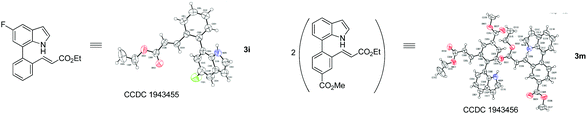
With optimized reaction conditions in hand, we then investigated the scope and generality of the present processes (Table 2). Under condition A, a broad range of 7-phenylindoles could be transformed to the ortho-mono-alkenylated products with up to 97% yield. Many functional groups, such as fluoro (3i), cyano (3j), nitro (3k), chloro (3l, 3o), ester (3m), methoxy (3n), and naphthyl (3p), were well tolerated. The positions of substituents plays an important role in the reaction efficiency and regioselectivity. 7-Phenyl-1H-indoles with the substituents on 4,5-positions of the indole ring or meta- and para-positions of the benzene ring were transformed easily to ortho-alkenylated 7-phenyl-1H-indoles in excellent yields (3b–3d, 3i–3n). However, the reaction of 3-methyl-7-phenyl-1H-indole 1e with 2a provided the corresponding product 3e in a low yield (30%), while 2-methyl-7-phenyl-1H-indole 1f led to complete reaction shutdown. When 6-methyl-7-phenyl-1H-indole (1g) or 7-(o-tolyl)-1H-indole (1h) was employed, a low yield of the 3-alkenylated 7-phenyl-1H-indole was formed selectively (3g, 16%; 3h, 13%). No ortho-alkenylated product was formed. On the other hand, the experimental results showed that the electronic properties of the substituents had no discernible effect on the reaction efficiency. As expected, other acrylates such as 2-methoxyethyl acrylate (2b), tert-butyl acrylate (2c), n-butyl acrylate (2d), and 2-(dimethylamino)ethyl acrylate (2e) all smoothly reacted with 1a to afford the desired products 3q–3t in good yields. Furthermore, ethyl vinyl ketone (2f), N,N-dimethylacrylamide (2g), or acrylonitrile (2h) also efficiently reacted with 1a yielding ortho-alkenylated products 3u–3w in 78%, 56%, and 64% yields, respectively. However, methyl methacrylate is less reactive than ethyl acrylate 2a in this system. Almost no desired product 3 was obtained.
| Entry | 7-Aryl-1H-indole 1 | Alkene 2 | Product 3 | Yield of 3b (%) | Product 4 | Yield of 4b (%) | |
|---|---|---|---|---|---|---|---|
| Condition B | Condition C | ||||||
| a Conditions A: 1a (0.3 mmol), 2a (0.75 mmol), [Cp*RhCl2]2 (2.5 mol%), AgOAc (2 equiv.), acetone (2 mL), 80 °C, air, 24 h; Conditions B: 3a (0.2 mmol), KOtBu (30 mmol%), DCM (2 mL), rt, air, 5 h; Conditions C: 1a (0.3 mmol), 2a (0.75 mmol), [Cp*RhCl2]2 (2.5 mol%), AgOAc (2 equiv.), Me4NOAc (10 equiv.), MeCN (2 mL), 80 °C, Ar, 24 h. b Isolated yields reported. | |||||||
| 1 |
|
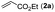
|
|
97 |
|
100 | 99 |
| 2 |
|
2a |
|
75 |
|
93 | 80 |
| 3 |
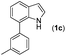
|
2a |
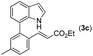
|
78 |
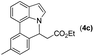
|
92 | 76 |
| 4 |
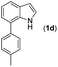
|
2a |
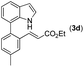
|
79 |
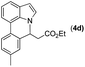
|
90 | 80 |
| 5 |
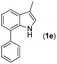
|
2a |
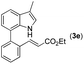
|
30 |
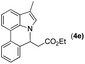
|
87 | 45 |
| 6 |
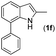
|
2a |
|
NR | |||
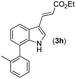
| 7 |
|
2a |
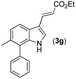
|
16 | |||
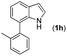
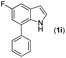
| 8 |
|
2a |
|
13 | |||
| 9 |
|
2a |
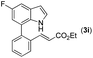
|
88 |
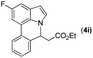
|
93 | 82 |
| 10 |
|
2a |
|
73 |
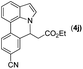
|
93 | 80 |
| 11 |
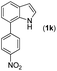
|
2a |
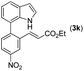
|
73 |
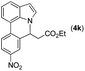
|
92 | 72 |
| 12 |
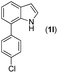
|
2a |
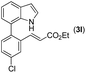
|
64 |
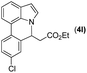
|
91 | 90 |
| 13 |
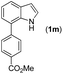
|
2a |
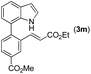
|
80 |
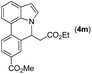
|
72 | 77 |
| 14 |
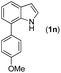
|
2a |
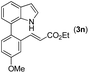
|
77 |
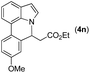
|
95 | 74 |
| 15 |
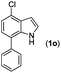
|
2a |
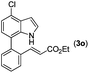
|
79 |
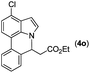
|
93 | 81 |
| 16 |
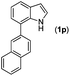
|
2a |
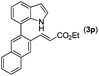
|
87 |
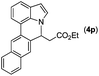
|
91 | 89 |
| 17 | 1a |
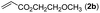
|
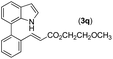
|
92 |
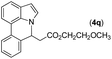
|
90 | 93 |
| 18 | 1a |
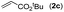
|
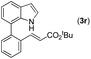
|
78 |
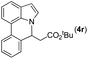
|
88 | 97 |
| 19 | 1a |
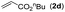
|
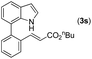
|
81 |
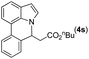
|
89 | 73 |
| 20 | 1a |
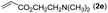
|
|
75 |
|
90 | 80 |
| 21 | 1a |

|
|
78 |
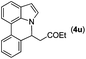
|
72 | 63 |
| 22 | 1a |
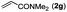
|
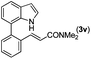
|
56 |
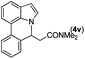
|
87 | 67 |
| 23 | 1a |
|
|
64 |
|
86 | 63 |
|
|||||||
Next, we turned our attention to the synthesis of annulated product 7H-pyrrolo[3,2,1-de]phenanthridines. To our delight, under condition B, the KOtBu-catalyzed intramolecular aza-Michael reaction proceeded quite well to give the desired products 4 in high yield. The reaction seems to be insensitive to the electronic and steric effects of substituents. Under condition C, a great variety of 7-phenyl-1H-indoles can be converted into the corresponding 7H-pyrrolo[3,2,1-de]phenanthridines through a one-pot C–H olefination/aza-Michael addition sequence. The yield of the product mainly depends on the efficiency of the C–H olefination step. It is worth noting that the first equilibrium reaction (C–H olefination) will be shifted to the right, according to Le Châlelier's principle, as intermediate 3 undergoes the aza-Michael reaction efficiently. Consequently, the yield of the one-pot reaction is higher than the overall yield of the two-step procedure. For example, 3-methyl-7-phenyl-1H-indole 1e provided the product 4e in a synthetically useful yield via the one-pot procedure (45%). In contrast, the two-step route to 4e was hampered by the low yielding step and provided 4e in only 29% overall yield. In addition, when compound 4a is resubjected to reaction conditions B and C, no reaction occurred, and compound 4a was recovered. This result likely indicates that the intramolecular aza-Michael addition is irreversible.
Conclusions
In conclusion, we have successfully developed [Cp*RhCl2]2/AgOAc-catalyzed regioselective ortho C–H olefination of 7-phenylindoles and KOtBu-catalyzed intramolecular aza-Michael addition leading to pyrrolo[3,2,1-de]phenanthridines. More importantly, the tandem C–H olefination/aza-Michael addition can proceed in one-pot under mild reaction conditions using the [Cp*RhCl2]2/AgOAc/Me4NOAc catalytic system. A relatively wide range of functional groups were tolerated, and a variety of pyrrolo[3,2,1-de]phenanthridines were obtained in good to excellent yields. The developed efficient and straightforward synthesis of pyrrolo[3,2,1-de]phenanthridines will be useful for the establishment of compound libraries for drug discovery.Experimental
General
7-Phenyl-1H-indoles were synthesized from 7-bromo-1H-indoles and phenylboronic acid via Suzuki coupling.161H NMR spectra and 13C NMR spectra were recorded at 400 MHz and 100 MHz, respectively. 1H chemical shifts (δ) were referenced to TMS, and 13C NMR chemical shifts (δ) were referenced to internal solvent resonance. ESI-HRMS spectra were recorded by using a Q-TOF mass spectrometer. Data collection and structural analysis of the crystal were performed on a Single Crystal Diffractometer equipped with graphite monochromatic Cu Kα radiation (λ = 1.54184 Å).General procedure for the ortho C–H olefination reaction (condition A)
Under an air atmosphere, 7-phenyl-1H-indoles 1 (0.3 mmol), alkenes 2 (0.75 mmol, 2.5 equiv.), [Cp*RhCl2]2 (4.7 mg, 0.0075 mmol, 2.5 mol%), AgOAc (100.1 mg, 0.6 mmol, 2 equiv.), and acetone (2 mL) were placed in a 50 mL sealed tube. The mixture was heated in an oil bath at 80 °C for 24 h and then cooled to room temperature. The mixture was diluted with CH2Cl2 to 5 mL, filtered through a Celite pad, and then washed with CH2Cl2. The volatiles were removed under reduced pressure, and the residue was subjected to silica gel column chromatography [eluting with petroleum ether/ethyl acetate] to afford the corresponding product (3a–3w).General procedure for the aza-Michael reaction (condition B)
Under an air atmosphere, compound 3 (0.2 mmol) and KOtBu (0.06 mmol, 6.8 mg, 30 mol%) were dissolved in CH2Cl2 (2 mL), and the mixture was stirred at room temperature for 5 h. The mixture was diluted with 10 mL of CH2Cl2, filtered through a Celite pad and then washed with CH2Cl2. The organic phases were washed with saturated NH4Cl solution (3 × 10 mL) and dried over anhydrous Na2SO4. The organic solvent was removed under reduced pressure to furnish compounds which were identified as pyrrolo[3,2,1-de]phenanthridines (4a–4w).General procedure for the one pot synthesis of Pyrrolo[3,2,1-de]phenanthridines (condition C)
Under an Ar atmosphere, 7-phenyl-1H-indoles 1 (0.3 mmol), alkenes 2 (0.75 mmol, 2.5 equiv.), [Cp*RhCl2]2 (4.7 mg, 0.0075 mmol, 2.5 mol%), AgOAc (100.1 mg, 0.6 mmol, 2 equiv.), Me4NOAc (3 mmol, 400 mg), and CH3CN (2 mL) were placed in a 50 mL sealed tube. The mixture was heated in an oil bath at 80 °C for 24 h and then cooled to room temperature. The mixture was diluted with CH2Cl2 to 5 mL, filtered through a Celite pad, and then washed with CH2Cl2. The volatiles were removed under reduced pressure, and the residue was subjected to silica gel column chromatography [eluting with petroleum ether/ethyl acetate] to afford the corresponding product (4a–4w).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Yunbin Li and Prof. Shengchang Xiang (Fujian Normal University) for obtaining X-ray crystal diffraction data. Financial support from the NSFC (Grant No. 21872028), the Natural Science Foundation of Fujian Province (Grant No. 2017J01572), the Foundation of Fujian Educational Committee (Grant No. JZ160424), and the Fujian Province University Fund for New Century Excellent Talents is greatly acknowledged.Notes and references
- (a) E. Stempel and T. Gaich, Acc. Chem. Res., 2016, 49, 2390–2402 CrossRef CAS PubMed; (b) N. Chadha and O. Silakari, Eur. J. Med. Chem., 2017, 134, 159–184 CrossRef CAS PubMed; (c) T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS; (d) M.-Z. Zhang, Q. Chen and G.-F. Yang, Eur. J. Med. Chem., 2015, 89, 421–441 CrossRef CAS; (e) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489–4497 CrossRef CAS; (f) W. Zi, Z. Zuo and D. Ma, Acc. Chem. Res., 2015, 48, 702–711 CrossRef CAS; (g) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742–778 RSC; (h) S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108–7149 CrossRef CAS PubMed.
- (a) T. Föster, S. López-Tosco, S. Ziegler, A. P. Antonchick and H. Waldmann, ChemBioChem, 2017, 18, 1098–1108 CrossRef; (b) W. Yu, L. Tong, B. Hu, B. Zhong, J. Hao, T. Ji, S. Zan, C. A. Coburn, O. Selyutin, L. Chen, L. Rokosz, S. Agrawal, R. Liu, S. Curry, R. McMonagle, P. Ingravallo, E. Asante-Appiah, S. Chen and J. A. Kozlowski, J. Med. Chem., 2016, 59, 10228–10243 CrossRef CAS PubMed; (c) N. Yadav, T. Khanam, A. Shukla, N. Rai, K. Hajela and R. Ramachandran, Org. Biomol. Chem., 2015, 13, 5475–5487 RSC; (d) S. Li, A. N. O. Lowell, S. A. Newmister, F. Yu, R. M. Williams and D. H. Sherman, Nat. Chem. Biol., 2017, 13, 467–470 CrossRef CAS; (e) V. Psarra, M. A. Fousteris, L. Hennig, M. Bantzi, A. Giannis and S. S. Nikolaropoulos, Tetrahedron, 2016, 72, 2376–2385 CrossRef CAS; (f) E. Yamuna, R. A. Kumar, M. Zeller and K. J. R. Prasad, Eur. J. Med. Chem., 2012, 47, 228–238 CrossRef CAS PubMed.
- (a) A. Suárez, M. Gohain, M. A. Fernández-Rodríguez and R. Sanz, J. Org. Chem., 2015, 80, 70421–10430 Search PubMed; (b) S.-L. Ding, Y. Ji, Y. Su, R. Li and P. Gu, J. Org. Chem., 2019, 84, 2012–2021 CrossRef CAS; (c) M. Taguchi, Y. Tokimizu, S. Qishi, N. Fujii and H. Ohno, Org. Lett., 2015, 17, 6250–6253 CrossRef CAS PubMed; (d) C. C. Forneris, Y.-P. Wang, G. Mamaliga, T. P. Willumstad and R. L. Danheiser, Org. Lett., 2018, 20, 6318–6322 CrossRef CAS; (e) C. Adouama, M. E. Budén, W. D. Guerra, M. Puiatti, B. Joseph, S. M. Barolo, R. A. Rossi and M. Médebielle, Org. Lett., 2019, 21, 320–324 CrossRef CAS; (f) A. M. Kulkarni, K. Sriniwas, M. V. Deshpande and C. V. Ramana, Org. Chem. Front., 2016, 3, 43 RSC; (g) J.-Q. Chen, Y. Mi, Z.-F. Shi and X.-P. Cao, Org. Biomol. Chem., 2018, 16, 3801 RSC; (h) Y. Wang, F. Xie, B. Lin, M. Chen and Y. Liu, Chem. – Eur. J., 2018, 24, 14302–14315 CrossRef CAS; (i) J. Park, S.-Y. Kim, J.-E. Kim and C.-G. Cho, Org. Lett., 2014, 16, 178–181 CrossRef CAS; (j) P. Pérez-Galán, H. Waldmann and K. Kumar, Tetrahedron, 2016, 72, 3647–3652 CrossRef.
- (a) B. V. Varun, J. Dhineshkumar, K. R. Bettadapur, Y. Siddaraju, K. Alagiri and K. R. Prabhu, Tetrahedron Lett., 2017, 58, 803–824 CrossRef CAS; (b) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787–8863 CrossRef CAS; (c) H. Kim and S. Chang, ACS Catal., 2016, 6, 2341–2351 CrossRef CAS; (d) M. K. Laksman and P. K. Vuram, Chem. Sci., 2017, 8, 5845–5888 RSC; (e) C. Liu, J. Yuan, M. Gao, S. Tang, W. Li, R. Shi and A. Lei, Chem. Rev., 2015, 115, 12138–12204 CrossRef CAS PubMed; (f) W. Ma, P. Gandeepan, J. Li and L. Ackermann, Org. Chem. Front., 2017, 4, 1435–1467 RSC; (g) M. C. Henry, M. A. B. Mostafa and A. Sutherland, Synthesis, 2017, 49, 4586–4598 CrossRef CAS; (h) L. Zheng and R. Hua, Chem. Rec., 2018, 18, 556–569 CrossRef CAS.
- (a) K. Morimoto, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2010, 12, 2068–2071 CrossRef CAS; (b) L. Ackermann, L. Wang and A. V. Lygin, Chem. Sci., 2012, 3, 177 RSC; (c) Y.-Q. Xia and L. Dong, Org. Lett., 2017, 19, 2258–2261 CrossRef CAS.
- X. Wang, Z. Li, S. Cao and H. Rao, Adv. Synth. Catal., 2016, 358, 2059–2065 CrossRef CAS.
- (a) S.-S. Li, Y.-Q. Xia, F.-Z. Hu, C.-F. Liu, F. Su and L. Dong, Chem. – Asian J., 2016, 11, 3165–3168 CrossRef CAS; (b) Q. Li, B. Li and B. Wang, Chem. Commun., 2018, 54, 9147–9150 RSC; (c) B. Li, B. Zhang, X. Zhang and X. Fan, Chem. Commun., 2017, 53, 1297–1300 RSC; (d) Z. Zhang, K. Liu, X. Chen, S.-J. Su, Y. Deng and W. Zeng, RSC Adv., 2017, 7, 30554–30558 RSC.
- X. Yang, Y. Li, L. Kong and X. Li, Org. Lett., 2018, 20, 1957–1960 CrossRef CAS.
- Q. Huang, Q. Han, S. Fu, Z. Yao, L. Su, X. Zhang, S. Lin and S. Xiang, J. Org. Chem., 2016, 81, 12135–12142 CrossRef CAS.
- (a) A. Dey, M. S. Ali, S. Jana, S. Samanta and A. Hajra, Tetrahedron Lett., 2017, 58, 313–316 CrossRef CAS; (b) D. Ding, G. Zhu and X. Jiang, Angew. Chem., Int. Ed., 2018, 57, 9028–9032 CrossRef CAS; (c) C. Cheng, W.-W. Chen, B. Xu and M.-H. Xu, Org. Chem. Front., 2016, 3, 1111–1115 RSC; (d) J. Wu, J. Lan, S. Guo and J. You, Org. Lett., 2014, 16, 5862–5865 CrossRef CAS.
- (a) L. Kong, Z. Zheng, R. Tang, M. Wang, Y. Sun and Y. Li, Org. Lett., 2018, 20, 5696–5699 CrossRef CAS; (b) T.-Y. Zhang, C. Liu, C. Chen, J.-X. Liu, H.-Y. Xiang, W. Jiang, T.-M. Ding and S.-Y. Zhang, Org. Lett., 2018, 20, 220–223 CrossRef CAS.
- C. Cheng, W.-W. Chen, B. Xu and M.-H. Xu, J. Org. Chem., 2016, 81, 11501–11507 CrossRef CAS.
- D. G. Pintori and M. F. Greaney, J. Am. Chem. Soc., 2011, 133, 1209–1211 CrossRef CAS.
- (a) Q. Huang, Q. Song, J. Cai, X. Zhan and S. Lin, Adv. Synth. Catal., 2013, 355, 1512–1516 CrossRef CAS; (b) Q. Han, S. Fu, X. Zhang, S. Lin and Q. Huang, Tetrahedron Lett., 2016, 57, 4165–4169 CrossRef CAS; (c) X. Guo, Q. Han, Z. Tang, L. Su, X. Zhang, X. Zhang, S. Lin and Q. Huang, Tetrahedron Lett., 2018, 59, 1568–1572 CrossRef CAS; (d) Q. Han, X. Guo, Z. Tang, L. Su, Z. Yao, X. Zhang, S. Lin, S. Xiang and Q. Huang, Adv. Synth. Catal., 2018, 360, 972–984 CrossRef CAS.
- (a) Y. Miki, H. Umemoto, M. Dohshita and H. Hamamoto, Tetrahedron Lett., 2012, 53, 1924–1927 CrossRef CAS; (b) K. Suzuki, H. Iwasaki, R. Domasu, N. Hitotsuyanagi, Y. Wakizaka, M. Tominaga, N. Kojima, M. Ozeki and M. Yamashita, Tetrahedron, 2015, 71, 5513–5519 CrossRef CAS; (c) A. Emir, C. Emir, B. Bozkurt, M. A. Onur, J. Bastida and N. U. Somer, Phytochem. Lett., 2016, 17, 167–172 CrossRef CAS; (d) A. Monaco, B. R. Szulc, Z. X. Rao, M. Barniol-Xicota, M. Sehailia, B. M. A. Borges and S. T. Hilton, Chem. – Eur. J., 2017, 23, 4750–4755 CrossRef CAS; (e) S.-L. Ding, Y. Ji, Y. Su, R. Li and P. Gu, J. Org. Chem., 2019, 84, 2012–2021 CrossRef CAS; (f) R. Rocaboy, D. Dailler and O. Baudoin, Org. Lett., 2018, 20, 772–775 CrossRef CAS; (g) W. L. Yu, T. Nunns, J. Richardson and K. I. Booker-Milburn, Org. Lett., 2018, 20, 1272–1274 CrossRef CAS; (h) S. Z. Tasker, A. E. Cowfer and P. J. Hergenrother, Org. Lett., 2018, 20, 5894–5898 CrossRef CAS; (i) M. H. Wahl, C. Jandl and T. Bach, Org. Lett., 2018, 20, 7674–7678 CrossRef CAS; (j) J. Wang, J. Li, X. Shen, C. Dong, J. Lin and K. Wei, Org. Chem. Front., 2017, 4, 1149 RSC; (k) U. V. Mentzel, D. Tanner and J. E. Tøneder, J. Org. Chem., 2006, 71, 5807–5810 CrossRef CAS; (l) H. S. Kim, M. G. Banwell and A. C. Willis, J. Org. Chem., 2013, 78, 5103–5109 CrossRef CAS; (m) M. D. Ganton and M. A. Kerr, Org. Lett., 2005, 7, 4777–4779 CrossRef CAS; (n) C. G. Hartung, A. Fecher, B. Chapell and V. Snieckus, Org. Lett., 2003, 5, 1899–1902 CrossRef CAS; (o) C. Tsukano, N. Muto, I. Enkhtaivan and Y. Takemoto, Chem. – Asian J., 2014, 9, 2628–2634 CrossRef CAS.
- S. Caccchi, G. Fabrizi, A. Goggiamani and A. Lazzetti, Tetrahedron, 2015, 71, 9346–9356 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, copies of NMR spectra. CCDC 1943455 and 1943456. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qo01135h |
| This journal is © the Partner Organisations 2020 |