 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Transition metal-free aminofluorination of β,γ-unsaturated hydrazones: base-controlled regioselective synthesis of fluorinated dihydropyrazole and tetrahydropyridazine derivatives
Juan
Zhao
,
Min
Jiang
* and
Jin-Tao
Liu
*
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: jtliu@sioc.ac.cn; jiangmin@sioc.ac.cn; Fax: +(86)-21-64166128
First published on 11th May 2020
Abstract
Correction for ‘Transition metal-free aminofluorination of β,γ-unsaturated hydrazones: base-controlled regioselective synthesis of fluorinated dihydropyrazole and tetrahydropyridazine derivatives’ by Juan Zhao et al., Org. Chem. Front., 2018, 5, 1155–1159, DOI: 10.1039/C7QO01105A.
The authors regret that Table 2 was duplicated as Table 3 in the original article. The correct Table 3 is presented below.
| a Reaction conditions: 1 (0.2 mmol), Selectfluor (0.24 mmol), NaHCO3 (0.4 mmol), CH3CN (4 mL), 100 °C, under a nitrogen atmosphere, 1 h. Isolated yields of 3. Ratio determined by 19F NMR spectroscopy. |
|---|
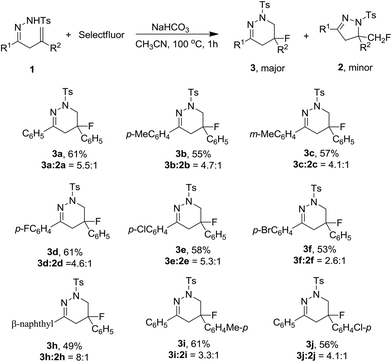
|
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Partner Organisations 2020 |
