Open Access Article
Open Access ArticleComparative assessment of physicochemical and antioxidative properties of mung bean protein hydrolysates
Zhaojun Zheng ,
Man Wang,
Jiaxin Li,
Jinwei Li and
Yuanfa Liu
,
Man Wang,
Jiaxin Li,
Jinwei Li and
Yuanfa Liu *
*
State Key Laboratory of Food Science and Technology, School of Food Science and Technology, National Engineering Research Center for Functional Food, National Engineering Laboratory for Cereal Fermentation Technology, Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, Jiangnan University, 1800 Lihu Road, Wuxi 214122, Jiangsu, People's Republic of China. E-mail: yfliu@jiangnan.edu.cn; Fax: +86-510-85876799; Tel: +86-510-85876799
First published on 14th January 2020
Abstract
Two commercial plant proteases namely ficin and bromelain, were acquired to hydrolyze mung bean protein over 300 min hydrolysis, and the physicochemical and antioxidative properties of the obtained hydrolysates were investigated. Bromelain-treated mung bean protein hydrolysates presented a higher degree of hydrolysis in comparison with ficin-treated hydrolysates, further modifying their physicochemical and emulsifying properties. All mung bean protein hydrolysates exhibited 50% scavenging of DPPH radical (IC50) in the concentration range from 8.67 to 16.22 μg mL−1. Our results also showed that strong metal ion-chelating activity was found in the ficin- (higher activity) and bromelain-treated protein hydrolysates. In addition, oxidative stability of linoleic acid was significantly enhanced by two selected protein hydrolysates, particularly the bromelain-treated hydrolysate with the highest inhibition effect of linoleic acid oxidation (94.55 ± 0.10%). Interestingly, both of these two hydrolysates could effectively retard lipid oxidation of sunflower oil and sunflower oil-in-water emulsion, while the ficin-treated hydrolysate showed slightly better performance. Therefore, mung bean protein hydrolysates showed potential to inhibit lipid oxidation, which could be advantageous in the food industry for producing fortified food.
1. Introduction
With the worsening of environmental pollution, environmental-friendly foods have been attracting a lot of well-deserved attention lately. Tilman and Clark1 found that ruminant meats have greenhouse gas emissions per gram of protein about 250 times those of legumes. Given that animal-based foods contribute to more negative environmental impacts than plant-based foods, protein from legume sources is a wise alternative to replace protein from animal sources.2,3 Mung bean (Vigna radiata L.) is a leguminous seed with a protein content ranging from 20 to 33%, which is significantly higher than the levels found in cereal grains and conventional root crops.4 The protein in mung bean contains plenty of essential amino acids, comparing favorably with that of FAO/WHO reference protein.5 Accordingly, mung bean protein is recognized as a replacement for animal proteins in product formulations.In addition to the fundamental nutritional role, beans protein may be capable to produce varied peptide sequences with specific biological properties.6 Since legume protein are more economical and practical in comparison with animal proteins,7 legume-derived peptides are attracting wide attention and interest of researchers for producing antioxidant peptides. For instance, Evangelho et al.8 found that black bean protein treated with alcalase digestion showed strong activities on scavenging free radicals and enhancing emulsion stability. Reports over the past decade also evidenced that protein hydrolysates from other legume like pea, Phaseolus lunatus and Phaseolus vulgaris seeds7,9 could effectively scavenge free radicals and chelate metal ion, presenting their excellent antioxidant capacity. Generally speaking, the antioxidant peptides remain latent as inactive sequences within their parent proteins and are released by proteolysis. Therefore, the antioxidant capacity of protein hydrolysates is mainly based on the extent of hydrolysis, which in turn plays a crucial role in their physicochemical properties.
Enzymatic hydrolysis is widely recognized to possess the capacity of translating proteins into free amino acids, peptides or polypeptides, yielding the hydrolysate with varying physicochemical properties.10 These properties are directly responsible for the action and bioactivity of protein hydrolysates when they interact with other components of food such as oil and water.11 In a similar way, physicochemical properties of the resulting hydrolysate are greatly affected by hydrolysis conditions and protease employed. Enzyme type generally dictates the cleavage patterns of the peptide bonds, thereby impacting the properties of resultant hydrolysate. Commercial enzymes ficin and bromelain were reported to produce protein hydrolysates with varied physicochemical properties and different antioxidant activity, such as the reported in fresh water carp protein hydrolysates,12 egg-white protein hydrolysates,13 and peptide hydrolysates from deer, sheep, pig and cattle red blood cell fractions.14 However, to date, limited consideration has been given to the systematic and comparative influence of protease specificity and reaction conditions on physicochemical and antioxidant properties of the resulting hydrolysates.
Taken all together, hydrolysis involved in enzyme types play a determinant role in the physicochemical and antioxidative characteristics of protein hydrolysates. Cysteine proteases ficin and bromelain from fig and pineapple respectively, are currently well-known plant proteases used in food processing. Nevertheless, the influences of these two cysteine proteases on the physicochemical properties and antioxidative activity of mung bean protein hydrolysates remain unclear. Therefore, this work aimed at investigating not only the physicochemical changes of mung bean protein hydrolyzed by ficin and bromelain at different hydrolysis time, but also their abilities on scavenging radicals and chelating metal ion. To further confirm their antioxidant activity and practicability, the oxidative stability of lipid products such as linoleic acid, sunflower oil and sunflower oil-in-water emulsion was assessed with the addition of the corresponding hydrolysates.
2. Materials and methods
2.1 Materials
Mung bean was obtained from Shiyuedaotian Co. Ltd (Beijing, China). Ficin and bromelain were purchased from J&K Scientific Co. Ltd (Shanghai, China). Sunflower oil (Arawana Brand, Jiali Food Ltd., Shanghai, China) and corn oil (Longevity Flower Co., Ltd., Jinan, Shandong, China) were purchased from local supermarket in China. Dithiothreitol (DTT), 8-anilino-1-naphthalenesulfonic acid (ANS) and linoleic acid were purchased from Macklin Co. Ltd. (Shanghai, China). 1,1-Diphenyl-2-picrylhydrazyl radical 2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazyl (DPPH) and ferrozine were purchased from Sigma-Aldrich. All other chemicals used were of analytical reagent grade.2.2 Preparation of mung bean protein isolate
Mung bean protein isolate was prepared according to the method of alkaline extraction and acid precipitation.15 Briefly, the defatted mung bean flour was suspended in water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 flour/water, w/v) and adjusted to pH 9.0 with 15 mM NaOH. After stirring for 60 min at room temperature, the sample was centrifuged at 10
8 flour/water, w/v) and adjusted to pH 9.0 with 15 mM NaOH. After stirring for 60 min at room temperature, the sample was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 15 min at 4 °C in a centrifuge (ThermoFisher, Germany). The supernatant was collected and adjusted to pH 4.0 with 2 M HCl, and then centrifuged at 10
000 × g for 15 min at 4 °C in a centrifuge (ThermoFisher, Germany). The supernatant was collected and adjusted to pH 4.0 with 2 M HCl, and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min. The precipitation was washed with distill water and lyophilized.
000 × g for 10 min. The precipitation was washed with distill water and lyophilized.
2.3 Preparation of mung bean protein hydrolysates
Mung bean protein powder was treated with pilot-scale Short-wave Infrared Radiation equipment (Senttech Infrared 94 Technology Co. Ltd, Taizhou, China) at 100 °C for 20 min. According to our previous study,16 after cooling down at room temperature, sample was dispersed in deionized water to obtain a 10% solution (w/v), which was divided into two aliquots. One aliquot was incubated with ficin (2%, w/w) at pH 5.7 and 65 °C, and the second one was incubated with bromelain (2%, w/w) at pH 7.0 and 55 °C. The respective control was prepared under the same incubation conditions without enzyme addition. One aliquot was collected every 60 min and heated at 90 °C for 15 min to inactive the enzymes. After cooling down at room temperature, the pH of sample was adjusted to neutral. Finally, the solutions were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min and the supernatants were collected and lyophilized.
000 × g for 10 min and the supernatants were collected and lyophilized.
2.4 Determination of degree of hydrolysis
Degree of hydrolysis (DH) was determined using the o-phthaldialdehyde (OPA) method as described by Nielsen et al.17 Briefly, 1.5 mL OPA reagent and 200 μL sample (or control) were mixed and incubated at room temperature for exactly 2 min. Then the absorbance of the mixture was measured at 340 nm in a UV-visible spectrophotometer (GE Healthcare, USA).2.5 SDS-PAGE electrophoresis
SDS-PAGE was carried out to estimate the molecular weight profiles of protein and hydrolysates by using the SDS-PAGE Preparation kit (Sangon Biotech, Shanghai, China). Protein sample and loading buffer (Beyotime, Shanghai, China) were mixed in a 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and incubated in boiling water bath for 10 min. Aliquots of 10 μL were loaded into the gels (5% stacking gel and 15.5% separating gel). The electrophoresis was performed in a Mini-PROTEAN Tetra Cell system (Bio-Rad, USA).
1 ratio and incubated in boiling water bath for 10 min. Aliquots of 10 μL were loaded into the gels (5% stacking gel and 15.5% separating gel). The electrophoresis was performed in a Mini-PROTEAN Tetra Cell system (Bio-Rad, USA).
2.6 Structural characterization
2.7 Surface charge (ζ-potential)
According to the method of Teh et al.,18 ζ-potential of protein and hydrolysates was measured by using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). An aliquot (1.4 mL) of each sample was added into a visibly clear disposable zeta cell without any air bubbles. The equilibrium time was 1 min.2.8 Emulsifying properties
The emulsifying activity index (EAI) and emulsion stability index (ESI) of mung bean protein hydrolysates were measured by the method of Suppavorasatit et al.19 3 mL of sample (2 mg mL−1) and 1 mL of corn oil were homogenized at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 60 s using IKA T25 homogenizer (Staufen, Germany). A 25 μL aliquot of emulsion was immediately mixed with 2.5 mL of SDS solution (0.1%, w/v) and 60 min after homogenization. The absorbance of the mixture was measured at 500 nm by a UV-visible spectrophotometer (GE Healthcare, USA).
000 rpm for 60 s using IKA T25 homogenizer (Staufen, Germany). A 25 μL aliquot of emulsion was immediately mixed with 2.5 mL of SDS solution (0.1%, w/v) and 60 min after homogenization. The absorbance of the mixture was measured at 500 nm by a UV-visible spectrophotometer (GE Healthcare, USA).
2.9 DPPH radical scavenging activity
DPPH radical scavenging activity of protein hydrolysates were determined according to the method of Li et al.20 with a slight modification. Briefly, two-fold serial dilutions of protein hydrolysates were prepared, 50 μL aliquot was mixed with 50 μL of 95% ethanol containing 0.1 mM DPPH. The mixture was allowed to stand in darkness for 30 min, and the absorbance was measured at 517 nm with a microplate reader (SpectraMax 190, Molecular Devices, USA).2.10 Metal-chelating activity
Metal-chelating activity was tested on basis of a previous work.21 Two-fold serial dilutions of protein hydrolysates (50 μL) were prepared in a 96-well plate and mixed with 100 μL of 20 μM FeCl2. Then, the mixture was incubated with 100 μL of 0.5 mM ferrozine at room temperature for 10 min. The control used was deionized water. Absorbance of the resulting solution was measured at 562 nm with a microplate reader (SpectraMax 190, Molecular Devices, USA).2.11 Inhibition of lipid oxidation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min, and then emulsified by a two-stage high-pressure homogenizer (150/50 bar) with an AH-2010 homogenizer (ATS Engineering Inc., Ontario, Canada). Protein hydrolysate was added into emulsion in a final protein concentration of 400 μg mL−1. According to the method of Caetano-Silva et al.,23 the malonaldehyde (MDA) production in emulsion was measured on days 0, 1, 2, 4, 6 and 8. 1.5 mL emulsions were incubated with 1 mL of 1% thiobarbituric acid (w/v) and 2.5 mL of 10% trichloroacetic acid (w/v) for 30 min in a boiling water bath. After cooled down at room temperature, 2.5 mL of solution was mixed with equivalent volume of trichloromethane, and centrifuged at 3000 × g for 10 min. The absorbance of supernatants at 532 nm were measured in a plate reader (SpectraMax 190, Molecular Devices, USA). Moreover, the microstructure of emulsion was observed by using a polarized light microscope (Leica, Germany) installed with a Leica DFC450 video camera.
000 rpm for 2 min, and then emulsified by a two-stage high-pressure homogenizer (150/50 bar) with an AH-2010 homogenizer (ATS Engineering Inc., Ontario, Canada). Protein hydrolysate was added into emulsion in a final protein concentration of 400 μg mL−1. According to the method of Caetano-Silva et al.,23 the malonaldehyde (MDA) production in emulsion was measured on days 0, 1, 2, 4, 6 and 8. 1.5 mL emulsions were incubated with 1 mL of 1% thiobarbituric acid (w/v) and 2.5 mL of 10% trichloroacetic acid (w/v) for 30 min in a boiling water bath. After cooled down at room temperature, 2.5 mL of solution was mixed with equivalent volume of trichloromethane, and centrifuged at 3000 × g for 10 min. The absorbance of supernatants at 532 nm were measured in a plate reader (SpectraMax 190, Molecular Devices, USA). Moreover, the microstructure of emulsion was observed by using a polarized light microscope (Leica, Germany) installed with a Leica DFC450 video camera.2.12 Statistical analysis
All experiments were carried out at least in duplicate, and results were expressed as mean ± standard deviation. One-way analysis of variance (ANOVA) combined with Student's t test were conducted by using Graphpad prism 7 (Graphpad Software Inc., San Diego, USA). The resulting data were analyzed graphically using OriginPro 2016 (OriginLab, Northampton, MA).3. Results and discussion
3.1 Degree of hydrolysis (DH)
The mung bean protein was subjected to hydrolysis by ficin and bromelain under different time, and DH were presented in Fig. 1A and B, respectively. Compared with the slight changed DH values of control without ficin or bromelain digestion (P < 0.05), hydrolysis progress with two plant proteases increased in a time-dependent manner. This indicated pH and temperature did not provide any significant influence on DH of protein, corresponding to the observation by Evangelho et al.8 In terms of enzymatic hydrolysis, bromelain promoted a rapid growth in the DH during the first 5 h of reaction, achieving the value of 15.04 ± 0.33%. Similar trend was also found in the ficin hydrolysis, which presented much lower rate of hydrolysis on the mung bean protein. The difference for the DH by ficin and bromelain might be accounted for partly by protein conformation, which contains less cleavage sites for ficin to act on (Fig. 1C). On the other hand, the DH difference might result in the varied structural and functional properties of ficin and bromelain hydrolysates. Overall, both of mung bean protein hydrolysates obtained from ficin and bromelain reached the highest DH values (11.45 ± 0.02% and 15.04 ± 0.33%, respectively) after 300 min of hydrolysis. Therefore, mung bean proteins hydrolyzed for up to 300 min were used for further study.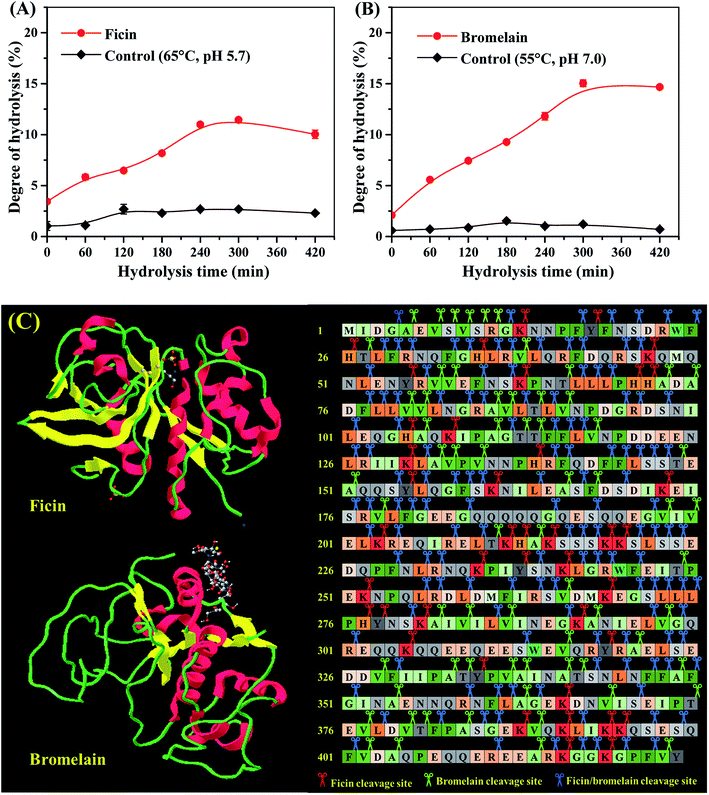 | ||
| Fig. 1 Hydrolysis curves of mung bean protein treated with ficin (A) and bromelain (B); (C) the predicted cleavage sites of ficin and bromelain on the major seed storage protein of mung bean (PDB: 2CV6). | ||
3.2 Molecular mass distribution of hydrolysate
To further compare ficin and bromelain hydrolysis, SDS-PAGE was conducted to describe the molecular weight distribution of mung bean proteins as a function of hydrolysis time. Fig. 2 shows the molecular weight distribution of mung bean protein (control) with very intense broad bands in the range of 37 to 75 kDa, which probably corresponds to the 8S vicilin.24,25 32 and 25 kDa peptide might be 8S vicilin subunit, and the distinct bands of approximately 20 kDa most likely corresponded to 11S fractions or basic 7S subunit of seed storage protein.26 However, large molecular weight bands of mung bean proteins decreased gradually with the increasing hydrolysis time of ficin treatment. An obvious shift from higher molecular weight bands to lower molecular mass units was observed in all ficin hydrolysates, particularly hydrolysates prepared with ficin for 4 and 5 h. Similarly, the intensity of the higher molecular weight bands of bromelain hydrolysates decreased and even disappeared as a function of reaction time. Regardless of hydrolysis time, all bromelain-treated hydrolysates showed the explicit and uniform band around 15 kDa, which was different with that of ficin hydrolysates. Nevertheless, both ficin and bromelain caused the obvious downward shifts to increased percentages of the small peptides (<10 kDa), indicating the bioactive properties of mung bean protein hydrolysates.273.3 Structural characterization of mung bean protein hydrolysates
3.4 Surface charge (ζ-potential) and emulsifying properties
The frequently-used indicator for electrostatic interaction of protein hydrolysates is ζ-potential, which can be influenced by different factors such as particle composition.18,34 Thus, we evaluated the ζ-potential of mung bean protein treated with ficin or bromelain under different reaction time. As shown in Table 1, the ζ-potential values of mung bean protein and hydrolysates were all negative charges, demonstrating that particles of these samples stay apart to avoid molecular aggregation.18 Obviously, the negative charges on ficin hydrolysates were superior to that of untreated mung bean protein. Interestingly, similar trends toward ζ-potential of ficin- and bromelain-treated hydrolysates were also observed as the increasing of hydrolysis time. More specifically, the ζ-potential values of ficin hydrolysates decreased gradually over the hydrolysis time of 240 min, excluding 120 min, where the lowest value was observed. At the end of ficin hydrolysis, the resultant hydrolysate displayed the highest ζ-potential value of −9.35 ± 0.57 mV, whereas the lowest values were observed in the bromelain hydrolysates obtained at 240 and 300 min of hydrolysis. These differences might be correlated to the molecular mass distribution of ficin and bromelain hydrolysates (Fig. 2), which influenced the numbers of anionic and cationic groups they contain.35| Hydrolysis time (min) | Ficin hydrolysate | Bromelain hydrolysate | ||||
|---|---|---|---|---|---|---|
| ζ-Potential (mV) | EAI (m2 g−1) | ESI (h) | ζ-Potential (mV) | EAI (m2 g−1) | ESI (h) | |
| a Results having the same letter in each column are not significantly different (P > 0.05). | ||||||
| 60 | −11.94 ± 1.00 c | 50.66 ± 0.81 a | 1.59 ± 0.04 c | −11.88 ± 0.33 b | 23.34 ± 0.09 d | 2.28 ± 0.02 b |
| 120 | −21.62 ± 0.57 f | 28.97 ± 2.78 c | 2.76 ± 0.63 b | −13.30 ± 0.73 c | 22.99 ± 0.08 d | 0.93 ± 0.00 b |
| 180 | −14.44 ± 0.41 d | 16.73 ± 0.00 d | 2.74 ± 0.00 b | −11.06 ± 0.30 ab | 52.45 ± 0.08 b | 78.11 ± 18.51 a |
| 240 | −19.62 ± 0.60 e | 15.62 ± 0.00 de | 2.62 ± 0.00 b | −16.82 ± 0.32 d | 80.94 ± 0.08 a | 1.50 ± 0.01 b |
| 300 | −9.35 ± 0.57 a | 13.37 ± 0.00 e | 6.48 ± 0.00 a | −15.96 ± 1.88 d | 82.07 ± 0.14 a | 2.43 ± 0.02 b |
| Control | −10.50 ± 0.29 b | 43.60 ± 2.74 b | 1.43 ± 0.19 c | −10.50 ± 0.29 a | 43.60 ± 2.74 c | 1.43 ± 0.19 b |
The emulsifying activity index (EAI) and emulsion stability index (ESI) for mung bean protein and hydrolysates as a function of increasing hydrolysis time are shown in Table 1. Hydrolysis time of ficin was found to exert a negative effect on its emulsifying activity, which declined from 50.66 ± 0.81 to 13.37 ± 0.00 m2 g−1 (P < 0.05). On the contrary, ESI values of ficin hydrolysates were superior to control group, presenting a tendency to grow over hydrolysis time. Similar results were reported by Thaiphanit et al.,36 where coconut protein hydrolysates in sunflower oil-in-water system showed the decreasing EAI and increasing ESI values as the increasing time. Unlikely, the EAI of bromelain hydrolysate was markedly increased by protein hydrolysis (P < 0.05), mainly in the second half of reaction time, possibly attributing to the molecular flexibility of polypeptides and the exposure of hydrophobic areas.29 However, no obvious fluctuation of ESI was observed in emulsion stabilized by bromelain hydrolysates along with the progress of hydrolysis, excluding 180 min hydrolysis time. The highest ESI value of 78.11 h was found in the 180 min-bromelain-treated hydrolysate, which might be due to its high hydrophobicity and secondary structure. Intriguingly, all hydrolysates exhibited the ESI values more than 55 min, which were higher than those of fish protein hydrolysates,37 porcine plasma protein hydrolysates38 and wheat gluten hydrolysates.39 Overall, the emulsifying properties of bromelain hydrolysates were superior to ficin hydrolysates.
3.5 DPPH radical scavenging activity
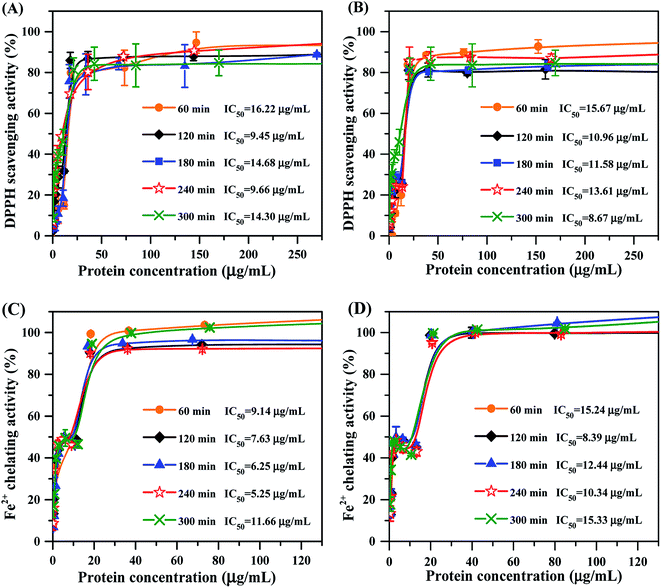
Apart from the physicochemical characteristics of mung bean protein hydrolysates, we also measured their capacity on scavenging DPPH radical. As the concentrations increased, DPPH radical scavenging activity of each hydrolysate increased gradually until a steady state was achieved (Fig. 4A and B). Mung bean hydrolysates obtained from ficin showed strong DPPH radical scavenging activity with the IC50 values ranging from 9.45 to 16.22 μg mL−1, and the lowest and highest values were respectively observed at 120 min and 60 min of hydrolysis (Fig. 4A). In terms of bromelain hydrolysates, excellent ability for capturing the DPPH radical was found at 300 min of hydrolysis, with IC50 value of 8.67 μg mL−1 (Fig. 4B), which were lower than those of documented protein hydrolysates, such as black bean hydrolysate (IC50 = 21.23 μg mL−1)16 and gelatin protein hydrolysate (IC50 = 660 μg mL−1).40 Our results indicated that enzyme type and hydrolysis time greatly influence the DPPH radical scavenging activity of mung bean protein hydrolysates, going along with the previous reports that antioxidant activity of protein hydrolysates relied on multiple factors, especially protein substrate, the specificity of proteases and hydrolysis conditions employed.22,413.6 Metal ion-chelating activity
Ferrous ion (Fe2+) is well-known to catalyze generation of free radicals, leading to oxidative damage for biomacromolecules.42 So, we evaluated the ability of mung bean protein hydrolysates on chelating Fe2+. As depicted in Fig. 4C and D, Fe2+-chelating activity of all hydrolysates were enhanced with increase in the protein concentration up to approximately 40 μg mL−1, but further increases in concentration affected their activity slightly. Mung bean protein hydrolysates obtained from ficin hydrolysis presented prominent capacity to chelate metal ion with IC50 values ranging from 5.25 to 11.66 μg mL−1 (Fig. 4C), which were significantly lower than these of previous reports.43,44 The IC50 values of bromelain hydrolysate were between 8.39 and 15.33 μg mL−1 over the range of hydrolysis time studied. Obviously, hydrolysates prepared with bromelain showed much weaker Fe2+-chelating activity than the hydrolysates obtained from ficin hydrolysis at the same reaction time. This result was coincided with the molecular weight distribution of bromelain-treated hydrolysates, which presented higher molecular weight bands. Accordingly, we reason that ficin hydrolysis was favorable to the release of acidic and alkaline amino acids, thereby promoted the resultant hydrolysates to chelate Fe2+ and thus to retard the oxidation reaction.453.7 Inhibition of lipid oxidation
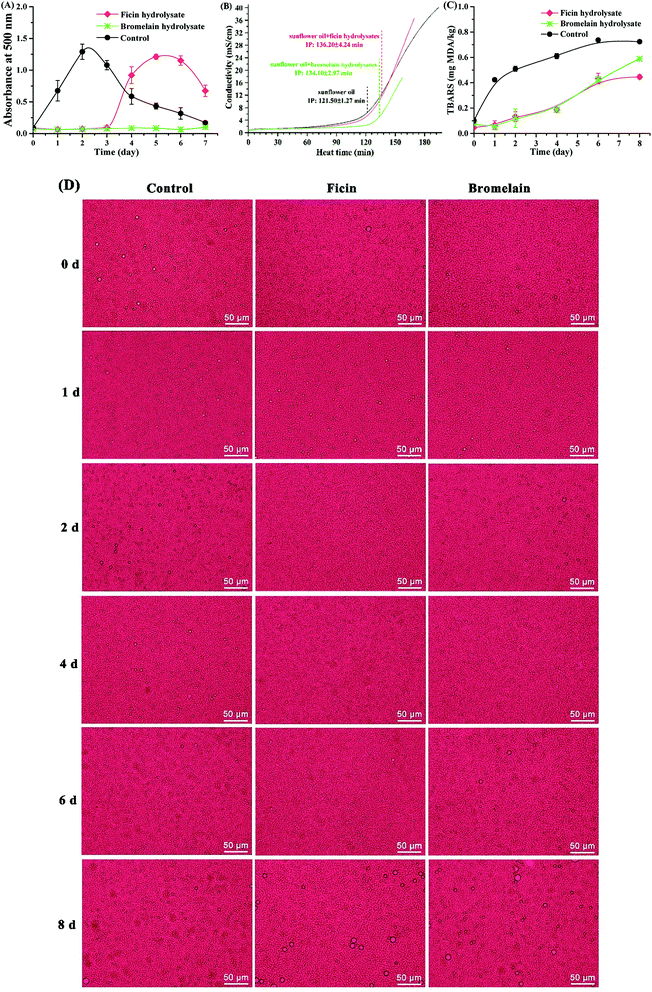
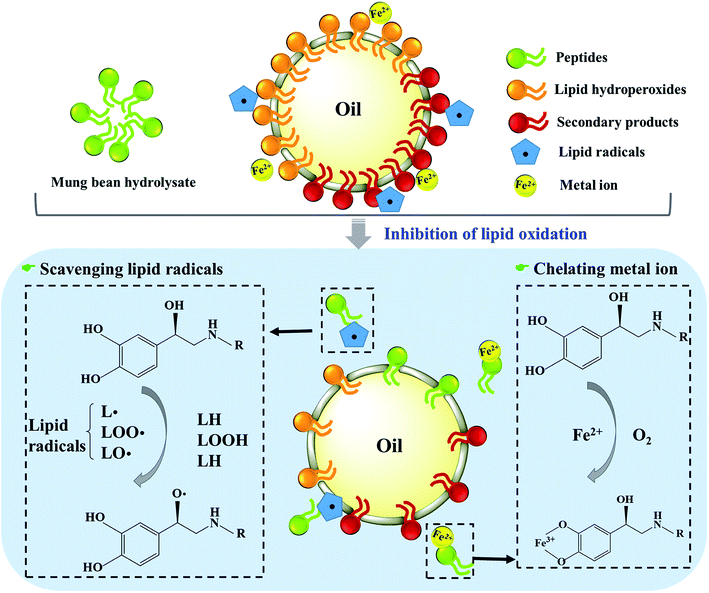
Taken together, mung bean protein treated with ficin and bromelain could inhibit the formation of primary and secondary products during lipid oxidation. As Fig. 6 described, peptides derived from mung bean protein hydrolysates could effectively scavenge radicals by means of donating hydrogen to lipid radicals, further retarding the radical-mediated oxidative chain reactions so as to enhance the oxidative stability of lipid products.53 Also, the hydrolysates showed excellent capacity on chelating metal ions, which could catalyze the generation of free radicals.54 Therefore, lipid oxidation was suppressed by mung bean hydrolysates, particularly the bromelain hydrolysate.
4. Conclusions
In summary, we compared the physicochemical and antioxidative properties of mung bean protein hydrolysates obtained by proteolytic treatments with two plant proteases (ficin and bromelain). Mung bean proteins treated with ficin and bromelain under different hydrolysis time showed different physicochemical properties, but bromelain hydrolysate was superior in DH and emulsifying properties. All protein hydrolysates prepared with ficin and bromelain exhibited excellent DPPH radical scavenging activity with the lowest IC50 values of 9.66 and 8.67 μg mL−1, respectively. Also, Fe2+-chelating activity of each hydrolysate prepared with ficin was stronger than that treated by bromelain at the identical hydrolysis time. Ficin- and bromelain-treated hydrolysates with the highest DPPH radical activity were further evidenced to enhance the oxidative stability of linoleic acid, sunflower oil and O/W emulsion, and the latter appeared to be more effective. Therefore, mung bean protein hydrolysates treated with ficin and bromelain favored oil preservation, since these hydrolysates could potentially scavenge DPPH radicals, chelate metal ions and inhibit lipid oxidation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by Natural Science Foundation of China (31901730, 31671786), Natural Science Foundation of Jiangsu Province (BK20190587), China Postdoctoral Science Foundation (2018M632235) and National Key R&D Program of China (2016YFD0401404).References
- D. Tilman and M. Clark, Nature, 2014, 515, 518 CrossRef CAS PubMed.
- M. D. Kristensen, N. T. Bendsen, S. M. Christensen, A. Astrup and A. Raben, Food Nutr. Res., 2016, 60, 32634 CrossRef PubMed.
- E. H. Temme, I. B. Toxopeus, G. F. Kramer, M. C. Brosens, J. M. Drijvers, M. Tyszler and M. C. Ocke, Public Health Nutr., 2015, 18, 2433–2445 CrossRef PubMed.
- T. G. Kudre, S. Benjakul and H. Kishimura, J. Sci. Food Agric., 2013, 93, 2429–2436 CrossRef CAS PubMed.
- Food and Agriculture Organization of the United Nations, FAO Food Nutr. Pap., 2013, 92, 1–66 Search PubMed.
- A. Connolly, M. B. O'Keeffe, C. O. Piggott, A. B. Nongonierma and R. J. FitzGerald, Food Chem., 2015, 176, 64–71 CrossRef CAS PubMed.
- T. L. Pownall, C. C. Udenigwe and R. E. Aluko, J. Agric. Food Chem., 2010, 58, 4712–4718 CrossRef CAS PubMed.
- J. A. Evangelho, N. L. Vanier, V. Z. Pinto, J. J. Berrios, A. R. Dias and E. R. Zavareze, Food Chem., 2017, 214, 460–467 CrossRef CAS PubMed.
- J. Torruco-Uco, L. Chel-Guerrero, A. Martínez-Ayala, G. Dávila-Ortíz and D. Betancur-Ancona, LWT--Food Sci. Technol., 2009, 42, 1597–1604 CrossRef CAS.
- R. Pachecoaguilar, M. A. Mazorramanzano and J. C. Ramírezsuárez, Food Chem., 2008, 109, 782–789 CrossRef CAS PubMed.
- S. He, C. Franco and W. Zhang, Food Res. Int., 2013, 50, 289–297 CrossRef CAS.
- K. Elavarasan, V. N. Kumar and B. A. Shamasundar, J. Food Process. Preserv., 2014, 38, 1207–1214 CrossRef CAS.
- D. Y. Cho, K. Jo, S. Y. Cho, J. M. Kim, K. Lim, H. J. Suh and S. Oh, Korean Journal for Food Science of Animal Resources, 2014, 34, 362–371 CrossRef PubMed.
- C. S. F. Bah, A. Carne, M. A. McConnell, S. Mros and A. E. D. A. Bekhit, Food Chem., 2016, 202, 458–466 CrossRef CAS PubMed.
- M. Du, J. Xie, B. Gong, X. Xu, W. Tang, X. Li, C. Li and M. Xie, Food Hydrocolloids, 2018, 76, 131–140 CrossRef CAS.
- Z. Zheng, J. Li, J. Li, H. Sun and Y. Liu, Food Hydrocolloids, 2019, 97, 105222 CrossRef.
- P. M. Nielsen, D. Petersen and C. Dambmann, J. Food Sci., 2001, 66, 642–646 CrossRef CAS.
- S.-S. Teh, A. E.-D. A. Bekhit, A. Carne and J. Birch, Food Chem., 2016, 203, 199–206 CrossRef CAS PubMed.
- I. Suppavorasatit, E. G. De Mejia and K. R. Cadwallader, J. Agric. Food Chem., 2011, 59, 11621–11628 CrossRef CAS PubMed.
- C. Li, P. Sun, H. Yu, N. Zhang and J. Wang, RSC Adv., 2017, 7, 1869–1876 RSC.
- A. Mohan, M. C. Udechukwu, S. R. C. K. Rajendran and C. C. Udenigwe, RSC Adv., 2015, 5, 97400–97407 RSC.
- A. Bougatef, N. Nedjar-Arroume, L. Manni, R. Ravallec, A. Barkia, D. Guillochon and M. Nasri, Food Chem., 2010, 118, 559–565 CrossRef CAS.
- M. E. Caetano-Silva, L. R. B. Mariutti, N. Bragagnolo, M. T. B. Pacheco and F. M. Netto, J. Agric. Food Chem., 2018, 66, 1981–1989 CrossRef CAS PubMed.
- C. H. Tang and X. Sun, J. Agric. Food Chem., 2010, 58, 6395–6402 CrossRef CAS PubMed.
- Z. Yi-Shen, S. Shuai and R. FitzGerald, Food Nutr. Res., 2018, 62 DOI:10.29219/fnr.v62.1290.
- E. M. Mendoza, M. Adachi, A. E. Bernardo and S. Utsumi, J. Agric. Food Chem., 2001, 49, 1552–1558 CrossRef CAS PubMed.
- Z. J. Zheng, Y. Huang, R. J. Wu, L. M. Zhao, C. F. Wang and R. J. Zhang, Poult. Sci., 2014, 93, 2641–2650 CrossRef CAS PubMed.
- R. He, A. Alashi, S. A. Malomo, A. T. Girgih, D. Chao, X. Ju and R. E. Aluko, Food Chem., 2013, 141, 153–159 CrossRef CAS PubMed.
- S. B. Zhang and Q. Y. Lu, Food Hydrocolloids, 2015, 47, 51–60 CrossRef CAS.
- N. A. Avramenko, N. H. Low and M. T. Nickerson, Food Res. Int., 2013, 51, 162–169 CrossRef CAS.
- J. Zhao, Y. L. Xiong and D. H. McNear, J. Food Sci., 2013, 78, C152–C159 CrossRef CAS PubMed.
- A. Achouri and Z. Wang, Food Res. Int., 2001, 34, 507–514 CrossRef CAS.
- M. Mohammadian and A. Madadlou, Food Hydrocolloids, 2016, 52, 221–230 CrossRef CAS.
- E. da Rosa Zavareze, A. C. Telles, S. L. Mello El Halal, M. da Rocha, R. Colussi, L. Marques de Assis, L. A. Suita de Castro, A. R. Guerra Dias and C. Prentice-Hernández, LWT--Food Sci. Technol., 2014, 59, 841–848 CrossRef CAS.
- X. Xu, J. Zhong, J. Chen, C. Liu and D. J. Mcclements, Food Chem., 2016, 213, 700–707 CrossRef CAS PubMed.
- S. Thaiphanit, G. Schleining and P. Anprung, Food Hydrocolloids, 2016, 60, 252–264 CrossRef CAS.
- Y. Liu, X. Li, Z. Chen, J. Yu, F. Wang and J. Wang, Food Chem., 2014, 151, 459–465 CrossRef CAS PubMed.
- Q. Liu, B. H. Kong, Y. L. L. Xiong and X. F. Xia, Food Chem., 2010, 118, 403–410 CrossRef CAS.
- W. He, R. Yang and W. Zhao, Food Chem., 2019, 277, 655–663 CrossRef CAS PubMed.
- D.-H. Ngo, Z.-J. Qian, B. Ryu, J. W. Park and S.-K. Kim, J. Funct. Foods, 2010, 2, 107–117 CrossRef CAS.
- V. Klompong, S. Benjakul, D. Kantachote and F. Shahidi, Food Chem., 2007, 102, 1317–1327 CrossRef CAS.
- Y. Shi, J. Kovacs-Nolan, B. Jiang, R. Tsao and Y. Mine, J. Funct. Foods, 2014, 10, 35–45 CrossRef CAS.
- R. He, A. T. Girgih, S. A. Malomo, X. Ju and R. E. Aluko, J. Funct. Foods, 2013, 5, 219–227 CrossRef CAS.
- Q. Zhang, X. Tong, B. Qi, Z. Wang, Y. Li, X. Sui and L. Jiang, J. Funct. Foods, 2018, 42, 298–305 CrossRef CAS.
- S. Dong, M. Zeng, D. Wang, Z. Liu, Y. Zhao and H. Yang, Food Chem., 2008, 107, 1485–1493 CrossRef CAS.
- H. T. Balaydın, İ. Gülçin, A. Menzek, S. Göksu and E. Şahin, J. Enzyme Inhib. Med. Chem., 2010, 25, 685–695 CrossRef PubMed.
- P. M. Angelo and N. Jorge, J. Am. Oil Chem. Soc., 2008, 85, 1045–1049 CrossRef CAS.
- K. Wang, Z. Zheng, C. Liu, Y. Wang, J. Li and Y. Liu, LWT--Food Sci. Technol., 2020, 118, 108726 CrossRef.
- N. Cheetangdee and S. Benjakul, J. Sci. Food Agric., 2015, 95, 1461–1468 CrossRef CAS PubMed.
- Y. Cheng, Y. L. Xiong and J. Chen, Food Chem., 2010, 120, 101–108 CrossRef CAS.
- W. Dridi, W. Essafi, M. Gargouri, F. Leal-Calderon and M. Cansell, Food Chem., 2016, 202, 205–211 CrossRef CAS PubMed.
- K. Nakaya, H. Ushio, S. Matsukawa, M. Shimizu and T. Ohshima, Lipids, 2005, 40, 501–507 CrossRef CAS PubMed.
- S. Maqsood and S. Benjakul, Food Chem., 2010, 119, 123–132 CrossRef CAS.
- C. L. Salcedo and M. A. Nazareno, RSC Adv., 2015, 5, 45878–45887 RSC.
| This journal is © The Royal Society of Chemistry 2020 |