Open Access Article
Open Access ArticleFractions of arsenic and selenium in fly ash by ultrasound-assisted sequential extraction†
Kai-Qiang Hea,
Chun-Gang Yuan *ab,
Meng-Dan Shia,
Yang-Hong Jianga and
Su-Juan Yuc
*ab,
Meng-Dan Shia,
Yang-Hong Jianga and
Su-Juan Yuc
aHebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, Department of Environmental Science & Engineering, North China Electric Power University, Baoding 071000, China. E-mail: cgyuan@ncepu.edu.cn
bMOE Key Laboratory of Resources and Environmental Systems Optimization, College of Environmental Science and Engineering, North China Electric Power University, Beijing 102206, China
cState Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China
First published on 4th March 2020
Abstract
Sequential extraction has been validated as an effective method to assess the fractions of elements in fly ash. However, the time consumption and high labor costs limit the application of the conventional sequential extraction (CSE) for fast screening of elemental fractions in fly ash. In this study, two ultrasound-assisted sequential extraction (UASE) methods were developed for fast analysis of arsenic (As) and selenium (Se) fractions in fly ash (FA). The parameters of UASE were optimized by comparing the results of As and Se obtained from UASE with those values observed via CSE. The operation time of sequential extraction procedures for As and Se were shortened from 24.5 h to less than 90 min. A certified reference material (CRM, GBW08401) and real fly ash samples were applied to validate the developed UASE. The recoveries of As and Se were found in the range of 82.3% to 114%. For all fractions, the performance of UASE was faster than CSE with the acceptable uncertainties. The analytical results demonstrated that the concentration of As in F3 was found to be higher than other fractions, while the main forms of Se were found to be in F1 and F3 in the fly ash samples. Based on the advantages of high efficiency and easy operation, the developed UASE procedures can be applied for fast screening of the mobility and bioavailability of As and Se in FA from coal fired power plants.
Introduction
Coal-fired power plants with a total installed capacity of about 692 GW have been built in China during the past ten years, which is two fold higher than the total installed capacity of the other countries in the world. In China, nearly half the total amount of consumed coal was used to generate power in coal-fired power plants.1,2 All of the coal-fired power plants were required to install the fly ash removal devices to meet the national environmental standards. Therefore, huge amount of fly ash would be produced every day in China. The fly ash can be beneficially used in many fields such like construction, paving, soil remediation, etc. However, the trace heavy metals in coal can be released and recondensed into fly ash during the coal combustion process.3,4 The heavy metals in fly ash can pose environmental risks during its storage or beneficial utilization. It is very necessary to screen the potential leaching behaviours of heavy metals in fly ash before utilization or treatment. Compared with the other trace elements, As and Se in fly ash attracted more attention for its volatility and enrichment ability in fly ash.5,6 The environmental effects of As and Se are determined not only based on their total amount but also on their fractions and bioavailability. Hence, it is highly desirable to identify their fractions in fly ash.The bioavailability of As and Se in fly ash can be evaluated by fractions with sequential extraction methods.7,8 The elements with various mobility in solid samples can be differentiated into different fractions using the specific reagents.9 Among the published sequential extraction methods, the procedures proposed by BCR and Tessier have been popularly used.10,11 These schemes are practical and effective for cations but not for anions such as As and Se.12,13 A five-step sequential extraction procedure developed by Wenzel had been proved suitable for the extraction of As in different fractions in solid samples.14 Using this scheme, As can be characterized into five fractions: (1) non-specifically sorbed fraction (F1); (2) specifically sorbed fraction (F2); (3) amorphous and poorly-crystalline hydrous Fe and Al oxides fraction (F3); (4) well-crystallized hydrous Fe and Al oxides fraction (F4); and (5) residual fraction (F5). This scheme has been widely used in many studies.15–17
Many researchers are trying to improve efficiency (time-saving) and reproducibility of the conventional procedures.18,19 Ultrasound assistant technique is regarded as an effective approach to accelerate the process of sequential extraction.10 Leśniewska et al. successfully applied ultrasound to extract Cd, Pb and Ni in soil.20 Compared with the conventional BCR method, the total time of ultrasound-assisted procedure was shortened from 48 h to 27 min.21 One study observed that the recoveries of Cu, Cr, Ni, Pb and Zn in all fractions in sludge were in the range of 96–100% with ultrasound treatment.22 The literature also revealed that the ultrasound extraction efficiency of different elements was dependent on the sample matrix,23 which indicates that it is very necessary to develop ultrasound-assisted methods for the samples with different matrix.
Considering the huge production of fly ash in power plants every day and high enrichment of As and Se in fly ash, it is urgent to monitor the fractions of As and Se in fly ash before it is transported into the environment. Therefore, it is critical to develop efficient fraction analysis method for fast screening the fractions of As and Se in fly ash. However, to the best of our knowledge, there is no study published about ultrasound assistant sequential extraction method for As and Se in fly ash. In this study, two new sequential extraction procedures for As and Se fraction were proposed and the experimental parameters which potentially affect the extraction efficiency were investigated and optimized. The proposed methods were validated by analyzing As and Se in fly ash from different coal fired power plants.
Materials and methods
Reagents and material
Ultrapure water was obtained from an EASYpure LF System D7382-33 (Barnstead Thermolyne, USA) and used throughout the work. Ammonium sulfate ((NH4)2SO4), ammonium dihydrogen phosphate (NH4H2PO4) and ammonium oxalate ((NH4)2C2O4) were obtained from Guangfu Chemical Company (Tianjin, China). Oxalic acid (H2C2O4), ascorbic acid (C6H8O6), hydrofluoric acid (HF), nitric acid (HNO3), hydrogen peroxide (H2O2), hydrochloric acid (HCl), sodium hydroxide (NaOH), potassium borohydride (KBH4) and thiourea (CH4N2S) were purchased from Kermel Chemical Company (Tianjin, China). All chemicals were of analytical grade or better. All plasticwares and glasswares were previously treated in 10% HNO3 for 24 h before use. Standard solution of As(V) and Se(IV) were prepared by dilution from a certified standard solution (1000 mg L−1). A certified reference material (fly ash, CBW08401) was used for quality control during the whole procedure.Instrumentation
The ultrasound assistant sequential extraction was carried out using an ultrasonic processor KQ-250B (Shumei Ultrasonic Instruments Co., Ltd, Kunshan, China), programmable for temperature, ranging from 20 to 80 °C with intensification frequency 40 kHz and power of 250 W. A centrifugate (TDZ5-WS, Hexi Co., Ltd., Hunan, China) was used to separate the supernatant from the solution. Total digestion was carried out by a microwave digestion instrument, MWD-800 (Metash Instruments Co., Ltd., Shanghai, China). The concentrations of As and Se were measured using an atomic fluorescence spectrometer (AFS-933, Titan Instruments Co., Ltd., Beijing, China). The measurement conditions of the AFS were described in Table S1 (ESI†).Sample collection and pre-treatment
The fly ash (FA) samples were collected from six power plants in Hebei province, China. 1.0 kg of fly ash was collected from the sampling points of electrostatic precipitators each time. Then the samples collected from same power plant were subsequently homogenized. The details of sampling sites and sampling process were described in Text S1.† All the samples were ground, freeze-dried and sieved through 100 mesh for use. The prepared FA samples were named as Sample 1–6 in turn. The characteristics of the FA samples were listed in Table S2 in the ESI.†Sequential extraction procedure
The conventional sequential extraction (CSE) procedure is based on our previous work.17 The details of these two procedures (CSE and UASE) are listed in Table 1. All ultrasound assisted steps were strictly operated at 20 °C. The UASE scheme can be described in brief as following process.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25)
25)
| Fraction | Reagent | Operational conditions | ||
|---|---|---|---|---|
| Ultrasound assisted procedure | Conventional procedure | |||
| As | Se | As/Se | ||
| Non-specifically sorbed fraction (F1) | 0.05 M (NH4)2SO4, 25 mL | 12 min at 20 °C | 9 min at 20 °C | 4 h shaker agitation at room temperature |
| Specifically sorbed fraction (F2) | 0.05 M NH4H2PO4, 25 mL | 24 min at 20 °C | 20 min at 20 °C | 16 h shaker agitation at room temperature |
| Amorphous and poorly-crystalline hydrous oxides Fe and Al fraction (F3) | 0.2 M (NH4)2C2O4, pH 3.25, 25 mL | 3 min at 20 °C in dark | 28 min at 20 °C in dark | 4 h in dark at room temperature |
| Well-crystallized hydrous oxides of Fe and Al fraction (F4) | 0.2 M (NH4)2C2O4 + 0.1 M ascorbic acid, pH 3.25, 25 mL | 30 min in water basin at 96 ± 3 °C in the light | ||
| Residual fraction (F5) | HNO3 + H2O2 + HF | Microwave digestion, 46 min | ||
Non-specifically sorbed fraction (F1): 25 mL of 0.05 M (NH4)2SO4 solution was added to 1 g of fly ash and sonicated for 12 min for As and 9 min for Se. As and Se in specifically-sorbed fraction (F2): 25 mL of 0.05 M NH4H2PO4 solution was added to the residue obtained from step 1 and sonicated for 24 min and 20 min for As and Se, respectively. 25 mL of 0.2 M NH4-oxalate solution (pH 3.25) was used to extract the As and Se in amorphous Fe and Al oxide-bound fraction (F3) from the residue after step 2 and sonicated in the dark for 3 min and 28 min. The As and Se bound to well-crystallized hydrous Fe and Al oxides (F4) was extracted by a mixed solution with 0.2 M NH4-oxalate and 0.1 M ascorbic acid through water basin for 30 min. The residues from step 4 were digested by HNO3, HF, and H2O2 at 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using the microwave digestion program within 46 min for the extraction of residual fraction (F5). The microwave digestion procedure was also employed for the determination of total amount (As and Se). The microwave digestion program were shown in Table S3 (ESI†).
1 using the microwave digestion program within 46 min for the extraction of residual fraction (F5). The microwave digestion procedure was also employed for the determination of total amount (As and Se). The microwave digestion program were shown in Table S3 (ESI†).
In addition, 12.5 mL of NH4-oxalate (pH 3.25) was used to clean F3 and F4 As/Se after extracting program by shaking for 10 min.
Quality control
The recoveries of As and Se were verified by comparing the sum of different fractions to the results of the total digestion. For the conventional method, the recoveries of As and Se in CRM were 116 ± 8.4% and 81.3 ± 10.7%, respectively. The recoveries of CSE for FA samples were in the range of 86.8–127% with an average of 97.7% for As and 81.7–111% with an average of 85.2% for Se.Results and discussion
It was reported that sonication time and temperature were the important factors affecting the ultrasound-assisted extraction efficiency during the sequential extraction procedure.24,25 The possible effects of temperature and sonication time were investigated for the method development. The concentrations of As and Se in each fraction obtained by the conventional procedure (Wenzel scheme) were used as reference values for the optimization by ultrasound-assisted procedure. Sample 6 was chosen as the analyte in the following experiments. All the extractions were repeated five times.Non-specifically sorbed fraction (F1)
The elements in this component are regarded as the most active fraction and can be easily migrated into the environment. In this study, As and Se in F1 were extracted by 0.05 M (NH4)2SO4 solution. The dissociation of (NH4)2SO4 could provide strong ion exchanging ability for soluble As and Se. As and Se in this faction indicate the possible leachable As and Se in FA by water under natural conditions.The effects of sonication time on the extraction of As/Se were investigated in Fig. 1(a and b). Arsenic in F1 was found to be more susceptible to ultrasound time than Se. The recoveries of As in this fraction kept increasing with sonication time before 12 min. Dabek et al. also found that 15 min of sonication time was the optimal time for the elements in exchangeable fraction.26 For Se in F1, the recoveries could reach as high as 101% at 9 min, which indicated that Se in F1 should be easier to be extracted than As with ultrasound assistance.
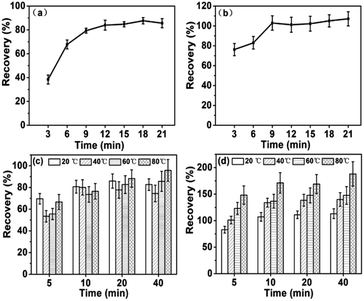 | ||
| Fig. 1 Effects of sonication time on the extraction of As (a) and Se (b) in F1 at 20 °C, and effects of sonication temperature on F1 As (c) and Se (d). | ||
The effects of temperature were shown in Fig. 1(c and d). The extraction efficiency increased with sonication time at different temperatures for both As and Se. With short sonication time (less than 15 min), temperature didn't show obvious effects on the recoveries of As. The recoveries of about 70–85% were obtained at all testing temperatures (20 °C, 40 °C, 60 °C, 80 °C). Comparing with As, temperature showed obvious effects on the recoveries of Se. It is clear that the extraction efficiencies can be beneficially affected by temperature. Unfortunately, this property is not good for the method development because Se in F1 can be excessively extracted at elevated temperatures (40 °C, 60 °C, 80 °C). The recoveries higher than 100% indicate the ultrasound-assisted method excessively extracted Se from the next fraction at these temperature points. Se was much more sensitive to temperature than As and lower temperature was beneficial for Se extraction in F1.
It was concluded that the optimal sonication time was 12 min and 9 min for As and Se, respectively. The sonication temperature was chosen at 20 °C.
Specifically-sorbed fraction (F2)
As and Se in this fraction were extracted by 25 mL of 0.05 M NH4H2PO4 solution. Phosphate ions could actively compete for the adsorption sites of As and Se on particle surfaces due to the smaller size and higher charge density.27 The specifically-sorbed fraction provides binding information of As and Se with mineral surface. This fraction may be potentially mobilized due to changes in pH or phosphate addition.14Fig. 2(a and b) showed the effects of sonication time on the recoveries of As and Se in F2. A recovery of 86.9% was obtained at 24 min for As, and a recovery of 94.6% was obtained at 20 min for Se. The recoveries of As decreased when the sonication time was longer than 25 min. The phenomenon may result from re-adsorption of leached elements and agglomeration of fine particles.24,28 It will be discussed in the following text.
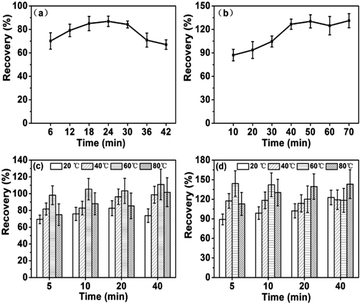 | ||
| Fig. 2 Effects of sonication time on the extraction of As (a) and Se (b) in F2 at 20 °C, and effects of sonication temperature on F2 As (c) and Se (d). | ||
Normally, fly ash consists of cluster particles with rough surface, which enables the particles to be favourable to re-adsorb the released elements in solution. To investigate the variation of As concentration in solution with sonication time, As standard solution was spiked into the suspension at 24 min and a final concentration of 20 ng mL−1 As was achieved. The concentration of As in the solution was detected at different time points. It can be seen from Fig. 3(a) that the detected As concentration was obviously lower than the expected concentration. The result indicated that re-adsorption occurred during sonication. The previous researches proved that trace metals extracted from fly ash could be influenced by its particle size.13,29,30 Therefore, the variation of FA particle size with sonication time was also investigated in this study. The results were shown in Fig. 3(b). There were two distinct peaks (one of them was split) for each curve. The first peak was located at about 0.36–60.9 μm, and the second peak was located at about 63.5–112 μm. The similar particle size span of fly ash was also reported in the literature.31 Li et al. demonstrated that condensation and nucleation of inorganic components were responsible for the first peak, while the second peak resulted from fusion and coalescence of inorganic components.32 As the ultrasound time increased, the size of FA gradually dwindled before 24 min. Arsenic mainly attached to the inner surface of the particle was simultaneously released into the solution with the fragmentation of particles. However, the particle size got larger again after 24 min. In this period, As may be combined with the adsorption site of the internal particle surface and gathering with FA particles, resulting in a decrease of As in the extract. Ultrasonic treatment could accelerate the splitting procedure of large particles before 24 min. The concentration of smaller particles (0.36–60.9 μm) in the solution increased with time going on and reached its maximum until 24 min. After that, the smaller particles were converted into the larger particles through collision again at 42 min.
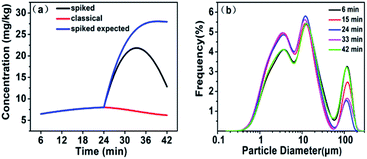 | ||
| Fig. 3 The effects of sonication time on As adsorption (a) and change of particle size with sonication time (b). | ||
Temperature is one of the major factors affecting the balance between adsorption and re-adsorption.33 In general, high temperature could expedite to transfer the equilibrium into the desorption process for As in F2 (Fig. 2(c)). Re-adsorption phenomenon was also found for Se at 40 °C and 60 °C (Fig. 2(d)). The recoveries of Se at 20 °C were mostly near 100%. Although high temperature could significantly improve the extraction efficiency for As and Se in F2, higher deviations were validated during the further study. Mason et al. also found that elevated temperature was not beneficial to get stable sonochemical effects during sonication.34 Therefore, ultrasound at room temperature (20 °C) to extract the As and Se in F2 was recommended.
In summary, 24 min and 20 min at 20 °C were confirmed to be the optimal conditions to extract As and Se in F2, respectively.
Amorphous and poorly-crystalline hydrous oxides of Fe and Al (F3)
Fly ash contain a large amount of mullite (3Al2O3·2SiO2), kaolinite (Al2Si2O5(OH)4), illite (KAl2(AlSi3O10)(OH)2), muscovite (KAl2(OH,F)2(AlSi3O10)), magnetite (FeFe2O3), hematite (Fe2O3) and montmorillonite (Al2Si4O10(OH)2·H2O). The XRD data indicated that over 65% of the materials in fly ash were amorphous.35,36 Depending on these characteristics, oxalate reagent is an effective agent for the extraction of amorphous Fe and Al oxide-bound fraction in FA.14 Oxalate ions would be stronger than NH2OH·HCl solution which had been normally used in BCR and Tessier schemes to compete with As/Se for adsorption sites in fly ash. In addition, the reductive dissolution of Fe(III) (hydr)oxides by oxalate would not occur rapidly in the absence of light.37,38 Therefore, ultrasound in dark could avoid excessive extraction during this step. Although F3 was with lower bioavailability than that of both F1 and F2, they can also provide an important indication of As/Se which can be potentially mobilized due to anion exchange.For As/Se in F3, the recoveries showed a downward trend after a slight rise in Fig. 4(a and b). It can be seen that As and Se bound to F3 were easy to extract in a short time under ultrasound treatment. For As, the optimal sonication time was 3 min with a recovery of 99.2%. A satisfactory recovery (107%) was obtained at 20 °C with 28 min sonication time for Se in F3.
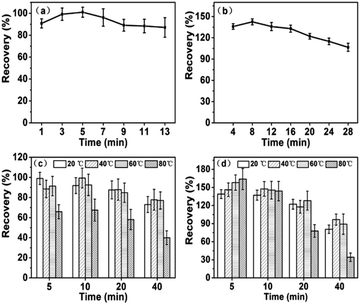 | ||
| Fig. 4 Effects of sonication time on the extraction of As (a) and Se (b) in F3 at 20 °C, and effects of sonication temperature on F3 As (c) and Se (d). | ||
As shown in Fig. 4(c and d), serious re-sorption were exhibited for As/Se in F3 at various temperatures. In general, the recoveries firstly increased and then decreased for As, whereas the recoveries of Se were continuously decreased. The recoveries of As/Se in F3 were similar whether at 20 °C, 40 °C, 60 °C. It is notable that the recoveries for both As and Se became much lower at 80 °C than that at the other temperatures. The phenomenon indicated that the elevated temperature was not beneficial for the extraction of As and Se in amorphous Fe and Al oxide-bound fraction. Hence, 20 °C was used for this step.
The above results illustrated that 3 min and 28 min at 20 °C were suitable to extract As and Se in F3.
Well-crystallized hydrous oxides of Fe and Al (F4) and residual fraction (F5)
The characteristics of As and Se in F4 are more stable than that in F3 in the environment. However, the redox state can be changed under certain conditions, which may cause the degradation of organic matter and the release of complexed metals.39 Ascorbate was applied to extract As and Se in F4. Ascorbate could exchange electrons with Fe(III) to form a surface Fe(II)–O bond, which is more labile than a Fe(III)–O bond, and therefore more easily cleaved from the surface to the further extraction of oxide-bound fraction in the light.40 Owing to only 30 min for F4 in CSE, the sonication was not applied for this step.In general, the residual fraction is the most stable fraction. During the combustion, the trace metals will be released from coal into flue gas, further cooled down and agglomerated with fly ash together. As and Se in F5 are strongly bound to the crystalline structures of the minerals.41 They would not be released in natural conditions. Only strong acid could destroy the structure of As/Se bound to minerals in this fraction.42 Hence, HNO3, HF and H2O2 were used for digestion during this step.
Application of the UASE method
Considering the different behaviours of As and Se under ultrasound-assisted extraction conditions, it is not possible to propose one effective ultrasound-assisted procedure to simultaneously extract As and Se in fly ash. Therefore, two schemes with different ultrasound parameters were proposed for the sequential extraction of As and Se, respectively. The repeatability and reproducibility of the proposed schemes (UASE) were validated by analyzing real FA samples and standard materials for twenty times. Assuming the content of As/Se in five fractions obtained by the conventional method as the referenced value for various fractions, the accuracy of As/Se in fractions obtained by UASE was tested. The results were shown in Tables 2 and 3. Under the optimal conditions, it was observed that the average total recoveries were 92.1% and 101% for As in FA and CRM, 101% and 105% for Se in the corresponding substance by the UASE method. The recoveries of different fractions were higher than 96.3% for Se and 82.3% for As. The recoveries of As (90.5%) in CRM were better than that in FA. During the verification experiment, there were high % RSD values obtained by UASE, but acceptable compared with CSE, especially for F4 As (up to 18.8%) and F3 Se (up to 16.7%) in CRM. High RSD values also occurred in CSE scheme for these two fractions and might result from the low concentration of As and Se in these two fractions.| Fraction | FA samples | GBW08401 | ||||
|---|---|---|---|---|---|---|
| Conventional | Ultrasound | Average recovery | Conventional | Ultrasound | Average recovery | |
| Mean/SD | Mean/SD | Mean/SD | Mean/SD | |||
| RSD | RSD | RSD | RSD | |||
| F1 | 2.82 ± 0.32 | 2.32 ± 0.20 | 82.3% | 4.55 ± 0.53 | 4.12 ± 0.52 | 90.5% |
| 11.3% | 8.6% | 11.6% | 12.6% | |||
| F2 | 9.04 ± 0.91 | 7.98 ± 0.82 | 88.3% | 4.41 ± 0.52 | 4.77 ± 0.43 | 108% |
| 10.1% | 10.3% | 11.8% | 9.0% | |||
| F3 | 22.6 ± 1.36 | 20.6 ± 1.63 | 90.9% | 3.43 ± 0.17 | 3.60 ± 0.25 | 105% |
| 6.0% | 7.9% | 5.0% | 6.9% | |||
| F4 | 0.83 ± 0.12 | 0.92 ± 0.11 | 111% | 0.14 ± 0.03 | 0.16 ± 0.03 | 114% |
| 14.5% | 12.0% | 21.4% | 18.8% | |||
| F5 | 3.42 ± 0.35 | 3.86 ± 0.37 | 113% | 1.86 ± 0.21 | 1.90 ± 0.24 | 102% |
| 10.2% | 9.6% | 11.3% | 12.6% | |||
| Total | 38.72 | 35.64 | 92.1% | 14.39 | 14.55 | 101% |
| Fraction | FA samples | GBW08401 | ||||
|---|---|---|---|---|---|---|
| Conventional | Ultrasound | Average recovery | Conventional | Ultrasound | Average recovery | |
| Mean/SD | Mean/SD | Mean/SD | Mean/SD | |||
| RSD | RSD | RSD | RSD | |||
| a LOD: limit of detection. | ||||||
| F1 | 1.93 ± 0.32 | 1.86 ± 0.24 | 96.4% | 0.27 ± 0.04 | 0.26 ± 0.03 | 96.3% |
| 16.6% | 12.9% | 14.8% | 11.5% | |||
| F2 | 1.11 ± 0.12 | 1.21 ± 0.18 | 109% | <LODa | <LODa | — |
| 10.8% | 14.9% | |||||
| F3 | 1.71 ± 0.25 | 1.92 ± 0.22 | 112% | 0.11 ± 0.02 | 0.12 ± 0.02 | 109% |
| 14.6% | 11.5% | 18.2% | 16.7% | |||
| F4 | <LODa | <LODa | — | <LODa | <LODa | — |
| F5 | 3.80 ± 0.33 | 3.66 ± 0.41 | 96.3% | 0.59 ± 0.05 | 0.64 ± 0.06 | 109% |
| 8.7% | 11.2% | 8.5% | 9.4% | |||
| Total | 8.55 | 8.65 | 101% | 0.97 | 1.02 | 105% |
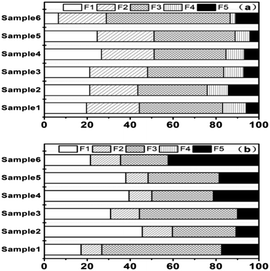
The performance of developed scheme was as good as CSE scheme. The FA samples (from six power plants) were analyzed with the proposed method and the results of fraction distributions were shown in Fig. 5. The total concentration of As was in the range of 17.8–127 mg kg−1. Although the contents of As in FA were different, the bioavailable fractions (F1–F3) which could be released into environmental consisted of the main fractions (75.9–88.9%). In real FA samples, the highest fraction was F3 (32.4–57.7%), indicating higher binding of As with Fe and Al hydrous oxides in amorphous and poorly-crystalline. The content of Se (in the range 4.58–22.9 mg kg−1) was much lower than As in FA. F1 (17.1–45.6%) and F3 (22.2–55.8%) were the main forms for Se. The percentages of Se in bioavailable fractions (F1–F3) to the total amount ranged from 57.7% to 90%.
The total extraction time of As and Se in F1–F4 could be shortened to 69 and 87 min, respectively. This indicates that the proposed UASE procedures are promising for the accelerated screening of bioavailable metal fractions in fly ash.
Validation parameters
The accuracy of sequential extraction procedure can be significantly affected by the instrument conditions and the operators. It is necessary to assess the analytical parameters and the uncertainty of proposed UASE procedure including limit of detection (LOD), limit of quantity (LOQ), and precision.20 The LOD was calculated as three times the standard deviation of eleven replicates of the blank extract-solution, while the LOQ were calculated as ten times the standard deviation.43 The precision for each fraction was evaluated by the RSD values of six successive analysis of one same fly ash sample. The main analytical parameters were shown in Table 4. By using UASE in this study, the LODs were in the range of 2.7–9.6 ng g−1 for As, whereas in the range of 1.8–11.1 ng g−1 for Se in all of the fractions. As to LOQ, the values for As and Se were lower than 31.7 ng g−1. The RSDs were between 3.2% and 5.6%.| Fraction | LOD (ng g−1) | LOQ (ng g−1) | RSD (%) | |
|---|---|---|---|---|
| F1 | As | 3.0 | 10.3 | 3.9 |
| Se | 3.1 | 10.5 | 4.8 | |
| F2 | As | 3.5 | 12.8 | 4.3 |
| Se | 2.3 | 8.2 | 3.6 | |
| F3 | As | 6.3 | 16.3 | 4.4 |
| Se | 1.8 | 5.8 | 5.6 | |
| F4 | As | 2.7 | 11.8 | 3.2 |
| Se | 4.5 | 14.3 | 5.3 | |
| F5 | As | 9.6 | 30.3 | 4.1 |
| Se | 11.1 | 31.7 | 4.4 | |
To elevate the trueness of the UASE procedure, the guide to the expression of uncertainty in measurement using a modelling approach was applied.21 The basis on the absence standard reference material for each fraction, the contents obtained by CES were considered as the reference indexes. The uncertainty (uc(CMe)/CMe) of As and Se in various fractions can be given by the following equation:
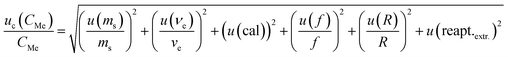 | (1) |
| Fraction | As (FA) | As (CRM) | Se (FA) | Se (CRM) |
|---|---|---|---|---|
| F1 | 14.8 | 17.5 | 21.4 | 18.1 |
| F2 | 14.4 | 14.0 | 19.2 | — |
| F3 | 9.6 | 10.9 | 20.1 | 24.3 |
| F4 | 18.1 | 26.7 | — | — |
The analytical parameters demonstrated that the method was reliable, precise and practical enough for fraction analysis of As and Se in fly ash.
Conclusions
Two fast ultrasound-assisted sequential extraction procedures were developed to assess the mobility and bioavailability of As and Se in FA. The proposed methods were much faster than the conventional procedure. When the traditional shaking was replaced with ultrasound, the total operation time was shortened from more than 24.5 h to less than 70 min and 90 min for As and Se, respectively. The proposed UASE schemes were verified by analyzing the real FA samples and CRM. The recoveries of As and Se in five fractions were in the range of 82.3% to 114%. The most majority of As (32.4–57.7%) was present in amorphous and poorly-crystalline hydrous oxides of Fe and Al (F3), while most of Se were mainly in F1 (17.1–45.6%) and F3 (22.2–55.8%) in fly ash. The UASE procedure can be used to fast screen the bioavailability of As and Se in FA with good precision and reliability.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was kindly funded by the National Key R&D Program of China (no. 2018YFB0605101) and the Fundamental Research Funds for the Central Universities (no. 2017ZZD07; no. 2018QN088).References
- S. Deng, Y. Shi, Y. Liu, C. Zhang, X. Wang, Q. Cao, S. Li and F. Zhang, Fuel Process. Technol., 2014, 126, 469–475 CrossRef CAS.
- Y. Zhao, J. Yang, S. Ma, S. Zhang, H. Liu, B. Gong, J. Zhang and C. Zheng, Int. Geol. Rev., 2017, 60, 639–670 Search PubMed.
- S. M. Swanson, M. A. Engle, L. F. Ruppert, R. H. Affolter and K. B. Jones, Int. J. Coal Geol., 2013, 113, 116–126 CrossRef CAS.
- Y. Zhang, P. Shang, J. Wang, P. Norris, C. E. Romero and W. P. Pan, Fuel, 2017, 208, 647–654 CrossRef CAS.
- B. Johanna, M. H. Khan and K. Alexander, Int. J. Environ. Res. Public Health, 2009, 6, 1609–1619 CrossRef PubMed.
- J. O. Nriagu and J. M. Pacyna, Nature, 1988, 333, 134–139 CrossRef CAS PubMed.
- C. Yuan, L. Yin, S. Liu and B. He, Fresenius Environ. Bull., 2010, 19, 221–225 CAS.
- V. J. Zatka, J. S. Warner and D. Maskery, Environ. Sci. Technol., 1992, 26, 138–144 CrossRef CAS.
- P. Richter, P. Grino, I. Ahumada and A. Giordano, Atmos. Environ., 2007, 41, 6729–6738 CrossRef CAS.
- C. M. Davidson and G. Delevoye, J. Environ. Monit., 2001, 3, 398–403 RSC.
- A. Tessier, P. G. C. Campbell and M. Bisson, Anal. Chem., 1979, 51, 844–851 CrossRef CAS.
- K. Müller, B. Daus, P. Morgenstern and R. Wennrich, Water, Air, Soil Pollut., 2007, 183, 427–436 CrossRef.
- A. Violante, V. Cozzolino, L. Perelomov, A. G. Caporale and M. Pigna, J. Soil Sci. Plant Nutr., 2010, 10, 266–290 Search PubMed.
- W. W. Wenzel, N. Kirchbaumer, T. Prohaska, G. Stingeder, E. Lombi and D. C. Adriano, Anal. Chim. Acta, 2001, 436, 309–323 CrossRef CAS.
- W. Liu, Y. Zhu, Y. Hu, P. Williams, A. Gault, A. A. Meharg, J. Charnock and F. Smith, Environ. Sci. Technol., 2006, 40, 5730–5736 CrossRef CAS PubMed.
- J. J. Xie, C. G. Yuan, Y. W. Shen, J. Xie, K. Q. He, H. T. Zhu and K. G. Zhang, Ecotoxicol. Environ. Saf., 2019, 169, 487–495 CrossRef CAS PubMed.
- C. G. Yuan, Y. Jin and S. Wang, Fresenius Environ. Bull., 2013, 22, 884–889 CAS.
- I. Lavilla, B. Perez-Cid and C. Bendicho, Int. J. Environ. Anal. Chem., 1998, 72, 47–57 CrossRef CAS.
- A. Väisänen and A. Kiljunen, Int. J. Environ. Anal. Chem., 2005, 85, 1037–1049 CrossRef.
- B. Leśniewska, M. Krymska, E. Świerad, J. Wiater and B. Godlewska-Żyłkiewicz, Environ. Sci. Pollut. Res., 2016, 23, 25093–25104 CrossRef PubMed.
- B. Leśniewska, K. Kisielewska, J. Wiater and B. Godlewska-Żyłkiewicz, Environ. Monit. Assess., 2016, 188, 29 CrossRef PubMed.
- B. Pérez-Cid, I. Lavilla and C. Bendicho, Anal. Chim. Acta, 1998, 360, 35–41 CrossRef.
- G. M. Greenway and Q. J. Song, J. Environ. Monit., 2002, 4, 950–955 RSC.
- S. Canepari, E. Cardarelli, S. Ghighi and L. Scimonelli, Talanta, 2005, 66, 1122–1130 CrossRef CAS PubMed.
- T. G. Kazi, M. K. Jamali, A. Siddiqui, G. H. Kazi, M. B. Arain and H. I. Afridi, Chemosphere, 2006, 63, 411–420 CrossRef CAS PubMed.
- E. Dabek-Zlotorzynska, M. Kelly, H. Chen and C. L. Chakrabarti, Anal. Chim. Acta, 2003, 498, 175–187 CrossRef CAS.
- R. Melamed, J. Jurinak and L. Dudley, Soil Sci. Soc. Am. J., 1995, 59, 1289–1294 CrossRef CAS.
- I. Pumure, J. Renton and R. Smart, Chemosphere, 2010, 78, 1295–1300 CrossRef CAS PubMed.
- Y. Jin, C. Yuan, W. Jiang and L. Qi, J. Mater. Cycles Waste Manage., 2013, 15, 516–521 CrossRef CAS.
- T. Su, H. Shi and J. Wang, Energy Fuels, 2011, 25, 3514–3521 CrossRef CAS.
- H. Tian, K. Liu, J. Zhou, L. Lu, J. Hao, P. Qiu, J. Gao, C. Zhu, K. Wang and S. Hua, Environ. Sci. Technol., 2014, 48, 3575–3582 CrossRef CAS PubMed.
- Z. Li, Y. Ji, H. Ma, P. Zhao, X. Zeng, S. Liu, Y. Jiang, L. Wang, A. Liu and H. Gao, Aerosol Air Qual. Res., 2017, 17, 1105–1116 CrossRef CAS.
- J. Deng, X. Feng and X. Qiu, Chem. Eng. J., 2009, 152, 177–182 CrossRef CAS.
- T. J. Mason, Sonochemistry, 1999, 357, 355–369 CAS.
- B. G. Kutchko and A. G. Kim, Fuel, 2006, 85, 2537–2544 CrossRef CAS.
- A. M. Tasić, I. D. Sredović-Ignjatović, L. M. Ignjatović, I. B. Anđelković, M. P. Antić and L. V. Rajaković, J. Serb. Chem. Soc., 2016, 81, 403–417 CrossRef.
- C. M. Davidson, A. S. Hursthouse, D. M. Tognarelli, A. M. Ure and G. J. Urquhart, Anal. Chim. Acta, 2004, 508, 193–199 CrossRef CAS.
- G. Kirk, Eur. J. Soil Sci., 1999, 50, 369–378 CrossRef CAS.
- P. Fedotov and M. Miró, Wiley-Jupac series., 2007, vol. 1, pp. 467–520 Search PubMed.
- C. Gleyzes, S. Tellier and M. Astruc, Trac. Trends Anal. Chem., 2002, 21, 451–467 CrossRef CAS.
- C. G. Yuan, Microchim. Acta, 2009, 165, 91–96 CrossRef CAS.
- H. Wang, Q. Song and Q. Yao, Spectrosc. Spectral Anal., 2012, 32, 1662–1665 CAS.
- L. A. Currie, Anal. Chim. Acta, 1999, 391, 127–134 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra08481a |
| This journal is © The Royal Society of Chemistry 2020 |