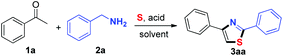Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Brønsted acid-promoted thiazole synthesis under metal-free conditions using sulfur powder as the sulfur source†
Penghui Nia,
Jing Tana,
Rong Lia,
Huawen Huang a,
Feng Zhang*b and
Guo-Jun Deng
a,
Feng Zhang*b and
Guo-Jun Deng *a
*a
aKey Laboratory for Green Organic Synthesis and Application of Hunan Province, Key Laboratory of Environmentally Friendly Chemistry and Application of Ministry of Education, College of Chemistry, Xiangtan University, Xiangtan 411105, China. E-mail: gjdeng@xtu.edu.cn
bCollege of Science, Hunan Agricultural University, Changsha, 410128, China. E-mail: zhangf@iccas.ac.cn
First published on 23rd January 2020
Abstract
A Brønsted acid-promoted sulfuration/annulation reaction for the one-pot synthesis of bis-substituted thiazoles from benzylamines, acetophenones, and sulfur powder has been developed. One C–N bond and multi C–S bonds were selectively formed in one pot. The choice of the Brønsted acid was the key to the high efficiency of this transformation under metal-free conditions.
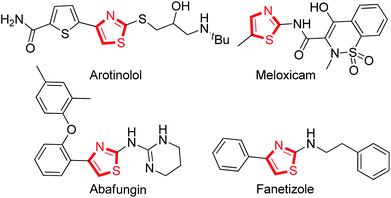
At least 50% of the biologically active compounds have a heterocyclic skeleton.1 Among these, the thiazole ring is an important five-membered aromatic heterocycle with nitrogen and sulfur atoms, and the unique structure has led to many applications in different pharmaceuticals and biological processes.2 For example (Fig. 1), antimicrobial (Abafungin),3 antihypertension (Arotinolol),4 anti-inflammatory (Meloxicam),5 and immunomodulatory (Fanetizole)6 drugs are prevalent among the drugs based on thiazole that have reached the marketplace.7
In view of this, great efforts have been invested in the development of novel synthetic protocols to facilitate the construction of thiazole derivatives. The typical procedure for the synthesis of thiazoles involves the reaction of α-haloketones with thioureas/thioamides using catalysts such as cyclodextrin,8 iodine,9 silica chloride,10 baker's yeast,11 and others12 (Scheme 1a). Besides, Wu13 and co-workers developed a catalyst-free protocol for the construction of polysubstituted thiazoles from α-haloketones and thioureas/thioamides. Togo14 reported the efficient synthesis of thiazoles via a base-promoted 1H-1-(1′-alkynyl)-5-methyl-1,2,3-benziodoxathiole 3,3-dioxide reaction with thioamides. Recently, Kshirsagar15 and co-workers developed NIS-mediated intermolecular cyclization of styrenes and thioamides using water as the solvent. On the other hand, the transition metal-catalyzed direct coupling of pre-existing thiazole compounds provides an alternative approach (Scheme 1b).16 Very recently, Jiao17 and co-workers developed a novel Cu-catalyzed aerobic oxidative approach to obtain thiazoles using elemental sulfur as the sulfur source via a multiple Csp3–H bond cleavage strategy (Scheme 1c). In spite of synthetic efficiency, these methods suffer from limitations with respect to special substrates and transition-metal catalysts. Therefore, the development of efficient methods for the synthesis of thiazoles from simple and readily available substrates under metal-free conditions is highly desirable. It is well-known that the sulfur element is cheap, stable, and easy to handle and thus, it is an ideal sulfur source for C–S bond construction.18 In our continuing efforts on using elemental sulfur for the synthesis of sulfur-containing heterocycles under simple conditions,19 we describe a three-component strategy for thiazole formation from readily available acetophenones, benzylamines, and sulfur powder under metal-free conditions (Scheme 1d).
We commenced our investigation using acetophenone (1a), benzylamine (2a), and sulfur powder as the model system (Table 1). When the reaction was performed using formic acid as the additive at 130 °C in DMSO (dimethyl sulfoxide) for 8 h, the desired product 3aa was obtained in a 24% yield (Table 1, entry 1). Then, a series of Brønsted acid reagents including HOAc, TFA (trifluoroacetic acid), TsOH (p-toluene sulfonic acid), MsOH (methanesulfonic acid), PivOH (trimethylacetic acid), benzoic acid, nicotinic acid, and isonicotinic acid were investigated (Table 1, entries 2–9). Among them, isonicotinic acid was the preferable additive for this reaction to give 3aa in a 66% yield (Table 1, entry 9). A sharp decline in the reaction yield was observed when DMF (N,N-dimethylformamide), DMAc (N,N-dimethylacetamide), NMP (N-methyl pyrrolidone), toluene, PhCl, and 1,4-dioxane were used as the solvents (Table 1, entries 10–15). Increasing the amount of sulfur powder or decreasing the reaction temperature both led to a lower yield of the product (entries 16–17). Meanwhile, the reaction atmosphere, such as Ar and O2, provided the target product in 62% and 34% yields, respectively (entries 18–19). Furthermore, only a 13% yield of the sulfuration product was observed in the absence of acid additives (entry 20).
| Entry | Acid | Solvent | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), acid (0.2 mmol), S (0.4 mmol), solvent (0.6 mL), 130 °C, 8 h, under air atmosphere.b GC yield using dodecane as the internal standard. n.d. means not detected.c S (0.6 mmol, 3 equiv.).d 120 °C.e Under an argon atmosphere.f Under an oxygen atmosphere. | |||
| 1 | Formic acid | DMSO | 24 |
| 2 | HOAc | DMSO | 33 |
| 3 | TFA | DMSO | n.d. |
| 4 | TsOH | DMSO | n.d. |
| 5 | MsOH | DMSO | n.d. |
| 6 | PivOH | DMSO | 45 |
| 7 | Benzoic acid | DMSO | 28 |
| 8 | Nicotinic acid | DMSO | 54 |
| 9 | Isonicotinic acid | DMSO | 66 |
| 10 | Isonicotinic acid | DMF | n.d. |
| 11 | Isonicotinic acid | DMAc | n.d. |
| 12 | Isonicotinic acid | NMP | n.d. |
| 13 | Isonicotinic acid | Toluene | n.d. |
| 14 | Isonicotinic acid | PhCl | n.d. |
| 15 | Isonicotinic acid | 1,4-Dioxane | Trace |
| 16c | Isonicotinic acid | DMSO | 58 |
| 17d | Isonicotinic acid | DMSO | 47 |
| 18e | Isonicotinic acid | DMSO | 62 |
| 19f | Isonicotinic acid | DMSO | 34 |
| 20 | DMSO | 13 | |
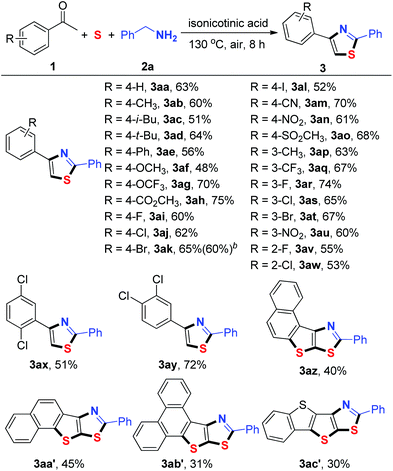
Under the optimized reaction conditions, the generality of the sulfuration/annulation reaction cascade to the synthesized thiazoles was investigated (Table 2). The model reaction of 1a and 2a in the presence of sulfur powder afforded 3aa in a 63% isolated yield. Similar yields were obtained when methyl, butyl, phenyl, and methoxy substituents were located at the para position of the phenyl ring (3ab–3af). The results showed that the substrates with halogen functional groups (F, Cl, Br, and even I) on the phenyl ring were compatible for this transformation (3ai–3al, 3ar–3at, and 3av–3aw). The substrates bearing strong electron-withdrawing groups (–OCF3, –CO2CH3, –CN, –NO2, and –SO2CH3) were also compatible with the reaction conditions, providing the corresponding products in good yields (3ag–3ah, 3am–3ao and 3au). Acetophenones 2x and 2y bearing two substituents reacted smoothly to yield the desired products 3ax and 3ay, respectively. It should be noted that bis-heteroannulation products (3az–3ac′) could be achieved when aromatic fused ring ketones (2z–2c′) were used as the substrates under optimal conditions. In this type of reaction, two sulfur atoms were incorporated into the heterocycles and four C–S bonds were selectively formed in one pot. Aliphatic ketones such as 1-cyclohexylethanone and 3-methylbutan-2-one both failed to afford the desired product under the current reaction conditions. We also used non-methyl ketones such as propiophenone and 1,2-diphenylethan-1-one. However, we did not observe the target products.
Subsequently, various substituted benzylamines were examined under the optimized reaction conditions (Table 3). First, a series of para-substituted benzylamines, including electron-donating groups and electron-withdrawing groups, were converted into the corresponding thiazoles (3ba–3ga) in good yields. Furthermore, meta and ortho-substituted benzylamines were able to give the desired products in moderate to good yields (3ha–3ka). Bis-substituted benzylamines such as 3-chloro-4-fluorobenzylamine (2l) and (3,5-difluorophenyl)methanamine (2m) also successfully participated in this oxidative cyclization process, affording the desired products 3la and 3ma in 51% and 67% yields, respectively. Notably, 4-pyridinemethaneamine (2n) also reacted efficiently to afford the corresponding 3na in a moderate yield. Unfortunately, aliphatic amines failed to afford the desired product.
| a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), S (0.4 mmol), isonicotinic acid (0.2 mmol), DMSO (0.6 mL), 130 °C, 8 h, under an air atmosphere, isolated yield based on 1a. |
|---|
 |
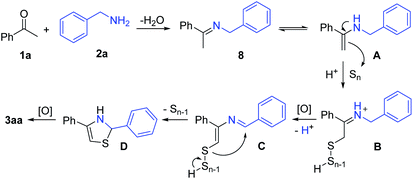
In order to understand the reaction mechanism, several control experiments were designed under different conditions (Scheme 2). The reaction of acetophenone 1a and benzothioamide 5 only yielded a trace amount of the desired product under the optimal conditions. No reaction occurred in the absence of sulfur powder (Scheme 2a). Similarly, the replacement of benzothioamide with benzamide 6 did not give the thiazole product (Scheme 2b). The treatment of N-(1-phenylethyl)benzothioamide 7, (E)-1-phenyl-N-(1-phenylethylidene)methanamine 8 and (E)-N-benzylidene-1-phenylethanamine 9 under the optimal reaction conditions did not afford the target product (Scheme 2c–e). However, imines 8 and 9 under the optimal conditions without isonicotinic acid provided the target product with 37% and trace amount yields, respectively (Scheme 2d and e).
On the basis of the experimental observations and previous reports,17,18f,19d a possible reaction mechanism is proposed (Scheme 3). The dehydrative condensation of acetophenone (1a) and benzylamine (2a) should be the initial step, which affords the imine intermediate 8. The tautomerization of the intermediate 8 generates enamine A. Subsequently, the interaction of A and elemental sulfur delivers the poly-sulfur intermediate B through Willgerodt–Kindler type oxidation.20 Further oxidation and deprotonation of B affords the intermediate C. Then, intramolecular attack occurs to release Sn−1 and generate the intermediate D, which finally furnishes the product 3aa by the oxidation process.
In summary, we have developed a novel Brønsted acid-promoted protocol for the synthesis of 2,4-disubstituted thiazoles from benzylamines, acetophenones, and sulfur powder under metal-free conditions. The cheap and readily available sulfur powder acted as the sulfur source to selectively assemble the thiazole derivatives. This reaction represents effective access to thiazoles from readily available starting materials with good functional group tolerance. Further studies on the mechanism are ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21871226, 21572194), the China Postdoctoral Science Foundation (2018M632976, 2019T120709) and the Hunan Provincial Innovative Foundation for Postgraduate (CX2018B362, CX2018B046).Notes and references
- (a) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed; (b) T. Shiro, T. Fukaya and M. Tobe, Eur. J. Med. Chem., 2015, 97, 397 CrossRef CAS PubMed.
- (a) A. Rouf and C. Tanyeli, Eur. J. Med. Chem., 2015, 911 CrossRef CAS; (b) R. E. Buntrock, J. Chem. Educ., 2012, 89, 1349 CrossRef CAS.
- (a) P. J. Palmer, R. B. Trigg and J. V. Warrington, J. Med. Chem., 1971, 14, 248 CrossRef CAS PubMed; (b) E. M. Willis and K. E. Ross, Aust. Vet. J., 2019, 97, 4 CrossRef CAS.
- W. C. Patt, H. W. Hamilton, M. D. Taylor, M. J. Ryan, D. G. Taylor Jr, C. J. C. Connolly, A. M. Doharty, S. R. Klutchko, I. Sircar, B. A. Steinbaugh, B. L. Bately, C. A. Painchand, S. T. Rapundalo, B. M. Michniewicz and S. C. J. Olzon, J. Med. Chem., 1992, 35, 2562 CrossRef CAS.
- (a) F. Haviv, J. D. Ratajczyk, R. W. DeNet, F. A. Kerdesky, R. L. Walters, S. P. Schmidt, J. H. Holms, P. R. Young and G. W. Carter, J. Med. Chem., 1988, 31, 1719 CrossRef CAS PubMed; (b) F. Clemence, O. L. Marter, F. Delevalle, J. Benzoni, A. Jouanen, S. Jouquey, M. Mouren and R. Deraedt, J. Med. Chem., 1988, 31, 1453 CrossRef CAS PubMed; (c) I. G. Colditz, D. R. Paull, J. B. Lloyd, L. Johnston and A. H. Small, Aust. Vet. J., 2019, 97, 23 CrossRef CAS PubMed.
- (a) C. Geller, S. Fontanay, M. Mourer, H. Massimba Dibama, J.-B. Regnouf-de-Vains, C. Finance and R. E. Duva, Antiviral Res., 2010, 88, 343 CrossRef CAS PubMed; (b) S. A. F. Rostom, I. M. El-Ashmawy, H. A. Abd EI Razik, M. H. Badr and H. M. A. Ashour, Bioorg. Med. Chem., 2009, 17, 882 CrossRef CAS PubMed.
- (a) R. A. Wiley and D. H. Rich, Med. Res. Rev., 1993, 13, 327 CrossRef CAS PubMed; (b) P. C. Kearney, M. Fernandez and J. A. Flygare, J. Org. Chem., 1998, 63, 196 CrossRef CAS PubMed; (c) D. Goff and J. Fernandez, Tetrahedron Lett., 1999, 40, 423 CrossRef CAS.
- (a) M. Narender, M. S. Reddy, R. Sridhar, Y. V. D. Nageswar and K. R. Rao, Tetrahedron Lett., 2005, 46, 5953 CrossRef CAS; (b) M. Narender, M. S. Reddy, V. P. Kumar, B. Srinivas, R. Sridhar, Y. V. D. Nageswar and K. R. Rao, Synthesis, 2007, 3469 CAS; (c) V. P. Kumar, M. Narender, R. Sridhar, Y. V. D. Nageswar and K. R. Rao, Synth. Commun., 2007, 37, 4331 CrossRef CAS.
- H. L. Siddiqui, A. Iqbal, S. Ahmed and G. W. Weaver, Molecules, 2006, 11, 206 CrossRef CAS PubMed.
- H. Karade, M. Sathe and M. P. Kaushik, Catal. Commun., 2007, 8, 741 CrossRef CAS.
- L. D. Khillare, U. R. Pratap, M. R. Bhosle, S. T. Dhumal, M. B. Bhalerao and R. A. Mane, Res. Chem. Intermed., 2017, 43, 4327 CrossRef CAS.
- (a) M. Ueno and H. Togo, Synthesis, 2004, 2673 CAS; (b) X. L. Liu, Q. Y. Wang, S. R. Sheng, C. Xu and M. Z. Cai, Synth. Commun., 2008, 38, 3338 CrossRef CAS.
- D. J. Zhu, J. X. Chen, H. L. Xiao, M. C. Liu, J. C. Ding and H. Y. Wu, Synth. Commun., 2008, 39, 2895 CrossRef.
- Y. Ishiwata and H. Togo, Synlett, 2008, 2637 CAS.
- M. H. Shinde and U. A. Kshirsagar, Green Chem., 2016, 18, 1455 RSC.
- For selected examples: (a) J. Hämmerle, M. Schnürch and P. Stanetty, Synlett, 2007, 2975 Search PubMed; (b) M. Schnürch, J. Hämmerle, M. D. Mihovilovic and P. Stanetty, Synthesis, 2010, 837 CrossRef; (c) J. Hämmerle, M. Schnürch, N. Iqbal, M. D. Mihovilovic and P. Stanetty, Tetrahedron, 2010, 66, 8051 CrossRef; (d) S. Kirchberg, S. Tani, K. Ueda, J. Yamaguchi, A. Studer and K. Itami, Angew. Chem., Int. Ed., 2011, 50, 2387 CrossRef CAS PubMed; (e) S. Tani, T. N. Uehara, J. Yamaguchia and K. Itami, Chem. Sci., 2014, 5, 123 RSC.
- X. Y. Wang, X. Qiu, J. L. Wei, J. Z. Liu, S. Song, W. Wang and N. Jiao, Org. Lett., 2018, 20, 2632 CrossRef CAS PubMed.
- For selected examples: (a) T. B. Nguyen, Adv. Synth. Catal., 2017, 359, 1066 CrossRef CAS; (b) T. B. Nguyen, Asian J. Org. Chem., 2017, 6, 477 CrossRef CAS; (c) X. M. Zhu, Y. Z. Yang, G. H. Xiao, J. X. Song, Y. Liang and G. B. Deng, Chem. Commun., 2017, 53, 11917 RSC; (d) H. Liu and X. F. Jiang, Chem.–Asian J., 2013, 8, 2546 CrossRef CAS PubMed; (e) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (f) J. M. Liu, Y. Y. Zhang, Y. Y. Yue, Z. X. Wang, H. B. Shao, K. L. Zhuo, Q. Z. Lv and Z. G. Zhang, J. Org. Chem., 2019, 84, 12946 CrossRef CAS PubMed; (g) J.-R. Zhang, Y.-Y. Liao, J.-C. Deng, K.-Y. Feng, M. Zhang, Y.-Y. Ning, Z.-W. Lin and R.-Y. Tang, Chem. Commun., 2017, 53, 7784 RSC; (h) J.-C. Deng, J.-H. Chen, J.-R. Zhang, T.-T. Lu and R.-Y. Tang, Adv. Synth. Catal., 2018, 360, 4795 CrossRef CAS; (i) J.-C. Deng, Y.-C. Gao, Z. Zhu, L. Xu, Z.-D. Li and R.-Y. Tang, Org. Lett., 2019, 21, 545 CrossRef CAS PubMed.
- For selected examples: (a) P. H. Ni, B. Li, H. W. Huang, F. H. Xiao and G. J. Deng, Green Chem., 2017, 19, 5553 RSC; (b) B. Li, P. H. Ni, H. W. Huang, F. H. Xiao and G. J. Deng, Adv. Synth. Catal., 2017, 359, 4300 CrossRef CAS; (c) G. Z. Li, H. Xie, J. J. Chen, Y. J. Guo and G. J. Deng, Green Chem., 2017, 19, 4043 RSC; (d) X. Z. Che, J. J. Jiang, F. H. Xiao, H. W. Huang and G. J. Deng, Org. Lett., 2017, 19, 4576 CrossRef CAS PubMed; (e) H. Xie, G. Z. Li, F. Zhang, F. H. Xiao and G. J. Deng, Green Chem., 2018, 20, 827 RSC; (f) J. J. Jiang, G. Z. Li, F. Zhang, H. Xie and G. J. Deng, Adv. Synth. Catal., 2018, 360, 1622 CrossRef CAS; (g) J. J. Jiang, H. W. Huang and G. J. Deng, Green Chem., 2019, 21, 986 RSC; (h) Z. H. Xu, H. W. Huang, H. B. Chen and G. J. Deng, Org. Chem. Front., 2019, 6, 3060 RSC; (i) H. W. Huang, Z. H. Xu, X. C. Ji, B. Li and G. J. Deng, Org. Lett., 2018, 20, 4917 CrossRef CAS PubMed; (j) H. W. Huang, Z. H. Qu, X. C. Ji and G. J. Deng, Org. Chem. Front., 2019, 6, 1146 RSC.
- For the S8-promoted oxidative annulations, see: (a) F. Shibahara, R. Sugiura, E. Yamaguchi, A. Kitagawa and T. Murai, J. Org. Chem., 2009, 74, 3566 CrossRef CASFor the Willgerodt–Kindler reaction, see: (b) D. L. Priebbenow and C. Bolm, Chem. Soc. Rev., 2013, 42, 7870 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09656f |
| This journal is © The Royal Society of Chemistry 2020 |