Open Access Article
Open Access ArticleA recyclable heterogeneous–homogeneous–heterogeneous NiO/AlOOH catalysis system for hydrocarboxylation of acetylene to acrylic acid†
Yakun Li ab,
Lifang Yana,
Qiaofei Zhangc,
Binhang Yan*a and
Yi Cheng
ab,
Lifang Yana,
Qiaofei Zhangc,
Binhang Yan*a and
Yi Cheng *a
*a
aDepartment of Chemical Engineering, Tsinghua University, Beijing 100084, PR China. E-mail: binhangyan@tsinghua.edu.cn; yicheng@tsinghua.edu.cn
bDepartment of Material and Chemical Engineering, Zhengzhou University of Light Industry, Zhengzhou, Henan 450001, PR China
cCollege of Chemistry, Chemical and Environmental Engineering, Henan University of Technology, Zhengzhou, Henan 450001, PR China
First published on 8th January 2020
Abstract
Concerns about the high-valued utilization of coal- and natural gas-based acetylene has provided particular impetus for exploration of acrylic acid (AA) production via one-step hydrocarboxylation reaction. Motivated by simple recovery, recycling and reuse of the catalyst, we report a high-performance NiO/AlOOH catalyst with AA space-time-yield of 412 gAA gcat.−1 h−1, obtainable by a simple incipient wetness impregnation method. Detailed kinetic and controlled experiments confirmed that nickel species on such a solid catalyst provide a heterogeneous–homogeneous–heterogeneous catalytic cycle where the chelates formed between CO and leached nickel act as the active species. The thorough recovery of leached nickel species improves the catalyst stability greatly. These preliminary findings indicate further prospects for new heterogeneous catalyst design in traditional homogeneous catalytic systems.
1. Introduction
With the depletion of petroleum reserves, it is highly desired to study sustainable alternatives. Meanwhile, due to the discovery of huge reserves of shale gas by fracking techniques as well as the breakthrough development of coal- and natural gas-based acetylene, the diverse utilization of acetylene to produce high value-added chemicals has received renewed interest.1,2 As an ideal atom economy reaction, the one-step hydrocarboxylation of acetylene to acrylic acid (C2H2 + CO + H2O → C3H4O2) provides a promising and alternative route to replace the partial oxidation of propene method, especially in coal- or gas-rich areas.2,3 However, the commercial production of AA using the homogeneous NiBr2–CuBr2 catalyst system developed by BASF Co. remains problematic due to the difficulty in product-catalyst separation, usage of expensive and sensitive ligands, and corrosivity of acid assistants. Tremendous efforts were made in recent years to solve these problems, such as new nickel or palladium complexes,4,5 water-soluble ligands6 and even ligand free catalyst,7 but the outcomes were still in controversy. Normally, heterogeneous catalysis is an effective and efficient method to overcome the above problems due to the facial separation and recovery of catalysts. Bhattacharyya firstly investigated the acetylene carbonylation reaction with the aid of metal halide/silica gel catalysts in detail, but unfortunately, these supported catalysts showed very low AA yield (only 14.2%) even under optimal conditions.8 Recently, Shi and coworkers studied the synthesis of AA via hydrocarboxylation of acetylene over Ni- and Cu-exchanged Y-zeolite with the presence of cupric salt and nickel salt as promoter respectively.3,9 They found that the catalytic activity showed a pronounced dependence of the reaction conditions and Ni2+ and Cu+ ions in the zeolites were responsible for the catalytic activity, being similar to the homogeneous catalysts.Significantly, question arises simultaneously when using solid catalysts in the liquid phase (liquid–solid or gas–liquid–solid system) because the active transition metals may leach from the catalysts surface into the liquid.10,11 As a consequence, it is very difficult to clarify the catalytically active species whether homogeneous or heterogeneous, even if there is only trance amount of active metals in the liquid.12–14 For example, the argument about the actual active species (palladium atoms or complexes in solution,15 or palladium nanoparticles16,17) in heterogeneous Heck and Suzuki coupling reactions has still been going on. Up to now, however, there are no data concerning the problem of leaching about heterogeneous catalysts for the hydrocarboxylation of acetylene and thereby not clear whether this reaction is homogeneous or heterogeneous. Correspondingly, it is highly desired not only to develop new heterogeneous catalysts with excellent performance but also to clarify the actual active species whether homogeneous or heterogeneous.
In this work, a pseudoboehmite (AlOOH) supported nickel-based catalyst (denoted as NiO/AlOOH) synthesized by a simple incipient wetness impregnation was tested. Such a catalyst showed a high AA space-time-yield (STY) of 412 gAA gcat.−1 h−1 and AA yield of 65.6%. During the hydrocarboxylation reaction, the dissolution of nickel species was observed due to the induction of CO and CuBr2. The leached nickel species underwent a heterogeneous–homogeneous–heterogeneous catalytic cycle.
2. Experimental section
2.1 Catalysts preparation
The NiO/AlOOH catalysts were prepared using a simple incipient wetness impregnation by impregnating pseudoboehmite (AlOOH) with an aqueous solution of nickel nitrate (Ni(NO3)2·6H2O) followed by calcining in air at 300 °C for 2 h. Both AlOOH (purchased from Zibo Jiu Ru Industrial and Trading Co., Ltd) and nickel nitrate (purchased from Sinopharm Chemical Reagent Co., Ltd) were used as received without further purification or drying. Notably, nickel nitrate was dissolved in an acetone solution (C3H6O, purchased from Beijing Tong Guang Fine Chemicals Company).For comparison, some other supports with high surface area like silicon dioxide (SiO2, purchased from Sinopharm Chemical Reagent Co., Ltd), alumina (γ-Al2O3, purchased from Sinopharm Chemical Reagent Co., Ltd), MCM-41 zeolite (purchased from Tianjin Nanhua Catalyst Co., Ltd) and Mg–Al layered double hydroxides (Mg–Al LDHs, purchased from Shanghai Macklin Reagent Co., Ltd) were also investigated by same method.
2.2 Characterization
The actual nickel content of NiO/AlOOH catalysts and the nickel content in residue after reactions are measured by inductively coupled plasma-optical emission spectrometry (ICP-OES, Varian Vista RL spectrometer). Specific surface areas of NiO/AlOOH catalysts are determined by nitrogen adsorption carried out at 77 K on a Quantachrome Autosorb-6B analyzer. The data are calculated by multipoint BET analysis method in the pressure range of P/P0 = 0.05–0.30. Prior to the measurement, the samples are degassed in vacuum at 300 °C for 2 h. X-ray diffraction (XRD) is performed on a Bruker D8 Advance equipment with Cu Kα radiation (35 kV and 25 mA). 2θ scans are run from 20 to 80 at a rate of 0.5 degree per minute. The spectra are identified with JCPDS database (Joint Committee of Powder Diffraction Standards) and the ICSD database (Inorganic Crystal Structure Database). The morphology of NiO/AlOOH catalysts are characterized by JEOL JEM2010 high-resolution transmission electron microscopy (HR-TEM) equipped with an energy dispersive X-ray fluorescence spectrometer (EDX).2.3 Reactivity tests
The catalytic reactions were carried out in a 0.5 L stainless steel-316 autoclave reactor with a mechanical stirring device and temperature controller. In a typical experiment, known quantities of catalyst powders (0.1 g), CuBr2 (0.12 mM L−1), H2O (15 mL), and acetone (150 mL) were charged into the stirred pressure reactor. The reactor was firstly flushed with nitrogen for several times and subsequently pressurized with acetylene to 0.5 MPa and then to 4.5 MPa of initial total pressure with CO (both controlled by mass flowmeter) at room temperature. The molar ratio of CO/C2H2 was 1.75. The reactor was then heated to 250 °C and kept for 30 min under constant agitation (800 rpm). After reaction, the autoclave was quickly cooled to room temperature and the tail gas and liquid were collected, calculated and analyzed. For recycling experiments, the catalyst used in the previous run was separated by centrifugation and washed with acetone for several times and reused after drying in air.The qualitative and quantitative analysis of the reactants and products was performed by a Shimadzu GC 2014 gas chromatograph equipped with a P–N packed column for identifying C2H2, C2H4, CO and CO2 and a Stabilwax capillary column for C2H2, aldehyde, acrylic acid, acetone and tetrahydrofuran (THF). The conversion of acetylene, selectivity and yield of acrylic acid and the space time yield (STY) are defined as follows:
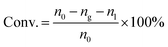 | (1) |
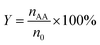 | (2) |
| S = Y/conv. | (3) |
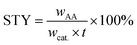 | (4) |
2.4 Kinetic investigation
To explore the nature of catalysis, a detailed kinetic experiment was carefully conducted. In a typical experiment, known quantities of catalyst powders (0.1 g), CuBr2 (0.12 mM L−1), H2O (15 mL), and acetone (150 mL) were charged into the stirred pressure reactor. The reactor was firstly flushed with nitrogen for several times and subsequently pressurized with acetylene to 0.5 MPa and then to 4.5 MPa of initial total pressure with CO (both controlled by mass flowmeter) at room temperature. The molar ratio of CO/C2H2 was 1.75. The reactor was then heated to set temperature under constant agitation (800 rpm). Then, the autoclave was quickly cooled to room temperature and the liquid was collected and the nickel content was analyzed by ICP measurement.3. Results and discussion
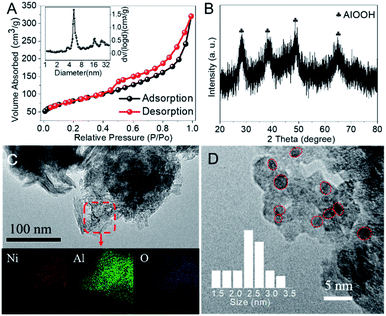
In general, heterogeneous catalysts are composed of one or more catalytically active components dispersed on functional supports with high surface area, in which the physico-chemical properties of the supports and catalytic components, as well as the interaction between them can strongly affect the catalytic performance. Therefore the effect of different supports (i.e., SiO2, γ-Al2O3, AlOOH, MCM-41 and Mg–Al LDHs) with high surface area was investigated and the results were collected in Table S1.† Clearly, all the supports except Mg–Al LDHs exhibited similar AA yield while NiO/AlOOH showed highest STY. It is reported that the abundant hydroxyl groups on the surface of support (e.g., AlOOH) is beneficial for the dispersion of active metals, which is crucial in CO coupling reactions.10 Furthermore, the hydroxyl groups were also reported to promote the adsorption and activation of CO.18,19 Thus, in the following investigations we will focus on discussing the NiO/AlOOH catalyst with 11.5 wt% Ni loading, which was measured by inductively coupled plasma-optical emission spectroscopy.Fig. 1 shows the structural features and morphology of the NiO/AlOOH catalyst. The as-prepared catalyst presents a specific surface area of 289 m2 g−1 and the hysteresis loop indicates a mesoporous feature with an average pore diameter of approximately 6 nm (Fig. 1A). According to the X-ray diffraction pattern shown in Fig. 1B, the NiO/AlOOH catalyst is dominated by the phases of γ-AlOOH while no NiO or Ni signals are observed, indicating the high dispersion of NiO or Ni nanoparticles.20 Furthermore, the transmission electron microscopy and energy-dispersive X-ray spectroscopy element mappings also reveal the homogeneously dispersed NiO species (Fig. 1C). Unsurprisingly, uniform Ni nanoparticles with an average diameter of 2.5 nm are formed on the NiO/AlOOH sample after pre-reduction in H2 at 400 °C for 1 h (Fig. 1D).
The performance of the NiO/AlOOH catalyst was initially tested for the hydrocarboxylation of acetylene. As expected, such a catalyst delivered a high acetylene conversion of 76% and AA yield of 65.6% due to the high dispersion of Ni species. The STY was calculated to be as high as 412 gAA gcat.−1 h−1, which is much higher than other reported heterogeneous catalysts (Table S2†).3,9,21 Besides the high activity, stability is another important factor for commercial process. After reaction the used catalyst was recovered by a simple centrifugal separation and then tested again without additional treatments. Disappointingly, the performance of recovered NiO/AlOOH catalyst was poor (Fig. S1†), which may be caused by the leaching of nickel as discussed above. Then, special attention was paid to the nickel leaching phenomenon and the results of ICP-OES showed that a small amount of nickel ions indeed existed in the liquid media after reaction. Next, of tremendous interest is to clarify the following two questions: (a) Whether the catalytically active species are homogeneous or heterogeneous? (b) What is the leaching mechanism and actual active species?
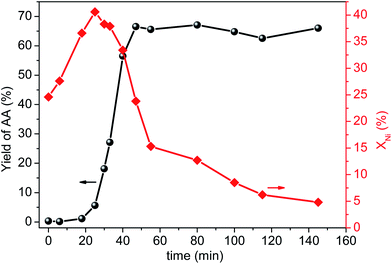
To clarify the catalytically active species are whether homogeneous or heterogeneous, we firstly examined catalytic activity of the filtrate. After centrifugal separation, the filtrate, promoter (1.2 mM L−1 CuBr2) and reaction gases (CO/C2H2 molar ratio of 1.75) were charged into the stirred pressure reactor and the result was shown in Fig. S2.† Clearly, the filtrate exhibited even a little higher catalytic activity than the fresh catalyst, indicating that the reaction was most likely to be homogeneous. Then, we employed nickel nitrate as nickel resource to test its catalytic performance (Fig. S3, details in ESI†). Notably, both the nickel salt alone and nickel salt with clean AlOOH showed much lower catalytic activity than the fresh NiO/AlOOH catalyst, suggesting that the actual active species might be nickel complexes rather than nickel ion. In order to provide more evidence for this speculation, a detailed kinetic experiment was carefully conducted by correlating the yield of AA and the amount of leached nickel to the reaction time, which has been reported to be a direct and unambiguous method to explore the nature of catalysis.10,15,22 As clearly shown in Fig. 2, the whole process can be divided into three stages. First, large amounts of nickel species were quickly dissolved from the surface of solid catalyst, while the yield of AA was very low (less than ∼1%) during the heating stage (time of 0–20 min). Second, in the initial reaction stage (time of 20–25 min) the amount of leached nickel in solution reached a maximum of ∼41% but the reaction rate was still slow. After that (time of 25–47 min), the reaction rate accelerated dramatically accompanied by a gradual decrease of nickel leaching content in solution which illustrated that the leached nickel species re-deposited onto the support. Clearly, the changes of nickel concentration in solution synchronized with the reaction rates. Third, after the AA yield reached a maximum of ∼66% (time of 47–150 min), the re-deposition rate of dissolved nickel species reduced obviously while the yield of AA almost remained unchanged. Thus, a reliable correlation between the yield of AA and leaching content of nickel species was obtained. Combined with the results of filtrate and nickel salt experiments, we believe that the catalytic activity originated from the dissolved Ni species, in the form of nickel complex.
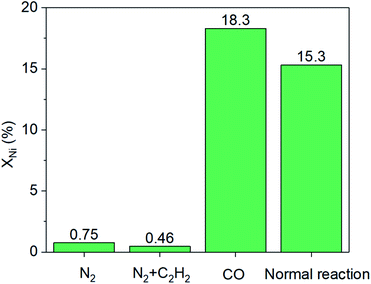
Furthermore, a series of comparison experiments (ESI† for details) were designed to further clarify the mechanism of how the leaching process took place, with the results as shown in Fig. 3. Firstly, the effect of gas atmosphere on the nickel leaching was investigated. The amount of nickel in solution was very low under different gas atmospheres (i.e., N2, CO or CO–C2H2 mixture) without the addition of CuBr2, indicating no obvious phenomenon of nickel leaching. Then, the effect of CuBr2 additive under different gas atmospheres was studied. When CuBr2 was added into the N2–C2H2 mixture, no leaching enhancement was observed with a nickel content of only ∼0.5%. As a contrast, the addition of CuBr2 in the presence of CO led to remarkable nickel leaching with the nickel content increasing from ∼1.5% to ∼14.5% in the solution. These results implied that both CO and CuBr2 played a crucial role in the leaching process. To further study the doubt that whether Cu2+ or Br− promoted the leaching of nickel, we employed Cu(NO3)2 and KBr as additives respectively under the atmosphere of CO. Interestingly, both Cu2+ and Br− could promote the leaching process. The nickel content in solution was ∼8.9% and ∼10.5% respectively when Cu(NO3)2 and KBr were used, which was slightly lower than the case of CO–CuBr2 (∼14.5%). As is reported, Cu2+ can active acetylene and favor the formation of alkyne−Ni-complex intermediates (e.g., CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–Ni(CO)mLn, L-ligand)9,23,24 while CuBr2 is conducive to high CO solubility.4 According to the above results, it can be concluded that only in the presence of CO and CuBr2, large amount of nickel species would dissolve into the solution.
CH–Ni(CO)mLn, L-ligand)9,23,24 while CuBr2 is conducive to high CO solubility.4 According to the above results, it can be concluded that only in the presence of CO and CuBr2, large amount of nickel species would dissolve into the solution.
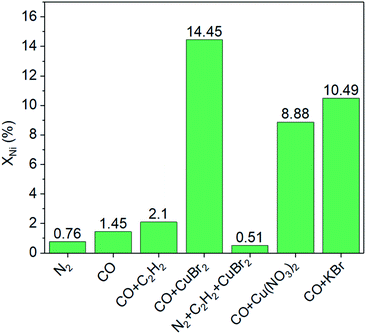 | ||
| Fig. 3 Effects of gas atmosphere and additives on nickel leaching (XNi). Conditions: 150 mL acetone, 100 mg NiO/AlOOH catalyst, initial pressure of 4.5 MPa, temperature of 250 °C for 30 minutes. | ||
To obtain further insight into the active intermediate species, we designed a gas replacement experiment described as follows. During stage-1, the same conditions as a normal reaction were conducted at first (as shown in the caption of Fig. 4). In order to obtain large amount of leached nickel species, the reactor underwent a rapid temperature-rising and -cooling operation in which the temperature followed the tracks of increasing reaction temperature gradually to 250 °C and decreasing temperature immediately from 250 °C to room temperature. During the stage-2, the reaction gas was replaced with N2, N2–C2H2 or CO respectively, with the temperature increasing to 250 °C again and keeping for 30 minutes. According to the ICP results, the content of leached nickel in solution was ∼41% at the end of stage-1 while a normal reaction for 30 minutes led to a nickel amount of ∼15.3%. However, the amount of nickel in solution was very low (<1%) at the end of stage-2 when the reaction gas was replaced with N2 or N2–C2H2 (Fig. 4), indicating the leached nickel redeposited onto the solid catalyst. In contrast, nickel leaching was still pronounced in the case of CO replacement with a nickel content of ∼18.3%, illustrating that the leached nickel species coordinated with CO to form stable chelates like the form of NiH(CO)mBr.25 Combining with the above results of contrast experiments, we can see that NiO was gradually dissolved from catalyst surface and then coordinated with CO at the initial stage of the reaction. With the consumption of CO during the reaction, the leached nickel species redeposited onto solid catalyst again, which was also demonstrated by the kinetic experiment. Therefore, we can conclude a heterogeneous-homogeneous-heterogeneous catalytic cycle for this reaction over the NiO/AlOOH catalyst. More importantly, the stability of NiO/AlOOH catalyst was improved significantly by the means of inert gas replacement. After three recycles, a high acetylene conversion of 82% and AA yield of 46% still can be reached (Fig. S4†). It is worth noting that the decrease of selectivity of AA is related to the particle agglomeration during the re-deposition process (Fig. S5†). Hence, inert gas replacement is a facile and efficient method to recycle the leached nickel, which delivered useful reference to the development of new heterogeneous catalysts for traditional homogeneous liquid reactions. These preliminary findings will open up further prospects for the hydrocarboxylation of acetylene to AA.
4. Conclusions
In summary, a highly active NiO/AlOOH catalyst with AA space-time-yield of 412 gAA gcat.−1 h−1 was developed for the hydrocarboxylation of acetylene to AA. During the reaction, large amount of NiO dissolved from solid catalyst surface into the solution with the induction of CuBr2 and CO. Detailed kinetic and controlled experiments confirmed a heterogeneous–homogeneous–heterogeneous catalytic cycle of the nickel species while the chelates formed between CO and leached nickel act as the actual active species for the hydrocarboxylation of acetylene. The recovery of leached nickel onto the solid catalyst by the means of inert gas replacement can dramatically improve the stability of NiO/AlOOH catalyst.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (NSFC) (21776156 and 21707028), China Postdoctoral Science Foundation (2017M610912) and Research Fund of Zhengzhou University of Light Industry (2018BSJJ025).References
- Nexant Incorporation, Multiclient Prospectus-Chemicals from acetylene - back to the future?, Nexant, 2007, http://www.nexant.com/ Search PubMed.
- H. Schobert, Chem. Rev., 2014, 114, 1743–1760 CrossRef CAS.
- H. Xie, D. Yi, L. Shi and X. Meng, Chem. Eng. J., 2017, 313, 663–670 CrossRef CAS.
- C. M. Tang, Y. Zeng, P. Cao, X. G. Yang and G. Y. Wang, Catal. Lett., 2009, 129, 189–193 CrossRef CAS.
- C. M. Tang, Y. Zeng, X. G. Yang, Y. C. Lei and G. Y. Wang, J. Mol. Catal. A: Chem., 2009, 314, 15–20 CrossRef CAS.
- A. H. M. de Vries, F. J. Parlevliet, L. Schmieder van der Vondervoort, J. H. M. Mommers, H. J. W. Henderickx, M. A. M. Walet and J. G. de Vries, Adv. Synth. Catal., 2002, 344, 996–1002 CrossRef CAS.
- M. Beller, J. G. E. Krauter and A. Zapf, Angew. Chem., Int. Ed., 1997, 36, 772–774 CrossRef CAS.
- S. K. Bhattacharyya and A. K. Sen, Ind. Eng. Chem. Process Des. Dev., 1964, 3, 169–176 CrossRef CAS.
- T. J. Lin, X. Meng and L. Shi, Appl. Catal., A, 2014, 485, 163–171 CrossRef CAS.
- S. S. Soomro, F. L. Ansari, K. Chatziapostolou and K. Köhler, J. Catal., 2010, 273, 138–146 CrossRef CAS.
- R. A. Sheldon, M. Wallau, I. W. C. E. Arends and U. Schuchardt, Acc. Chem. Res., 1998, 31, 485–493 CrossRef CAS.
- I. Sádaba, M. L. Granados, A. Riisager and E. Taarning, Green Chem., 2015, 17, 4133–4145 RSC.
- I. W. Davies, L. Matty, D. L. Hughes and P. J. Reider, J. Am. Chem. Soc., 2001, 123, 10139–10140 CrossRef CAS PubMed.
- J. A. Widegren, M. A. Bennett and R. G. Finke, J. Am. Chem. Soc., 2003, 125, 10301–10310 CrossRef CAS PubMed.
- K. Köhler, S. Pröckl and W. Kleist, Curr. Org. Chem., 2006, 10, 1585–1601 CrossRef.
- M. Lamblin, L. Nassar-Hardy, J. C. Hierso, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS.
- P. J. Ellis, I. J. S. Fairlamb, S. F. J. Hackett, K. Wilson and A. F. Lee, Angew. Chem., Int. Ed., 2010, 49, 1820–1824 CrossRef CAS.
- Y. Zhai, D. Pierre, R. Si, W. Deng, P. Ferrin, A. U. Nilekar, G. Peng, J. A. Herron, D. C. Bell, H. Saltsburg, M. Mavrikakis and M. Flytzani-Stephanopoulos, Science, 2010, 329, 1633–1636 CrossRef CAS PubMed.
- C. Z. Wang, L. P. Han, Q. F. Zhang, Y. K. Li, G. F. Zhao, Y. Liu and Y. Lu, Green Chem., 2015, 17, 3762–3765 RSC.
- H. M. T Galvis, J. H. Bitter, C. B. Khare, M. Ruitenbeek, A. I. Dugulan and K. P. de Jong, Science, 2012, 335, 835–838 CrossRef PubMed.
- H. Xie, T. J. Lin, L. Shi and X. Meng, RSC Adv., 2016, 6, 97285–97292 RSC.
- S. S. Pröckl, W. Kleist, M. A. Gruber and K. Köhler, Angew. Chem., Int. Ed., 2004, 43, 1881–1882 CrossRef PubMed.
- T. J. Lin, X. Meng and L. Shi, Ind. Eng. Chem. Res., 2013, 52, 14125–14132 CrossRef CAS.
- T. J. Lin, X. Meng and L. Shi, J. Mol. Catal. A: Chem., 2015, 396, 77–83 CrossRef CAS.
- F. De Angelis and A. Sgamellotti, Organomet, 2000, 19, 4104–4116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra09737f |
| This journal is © The Royal Society of Chemistry 2020 |