Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comprehensive metabolomics analysis based on UPLC-Q/TOF-MSE and the anti-COPD effect of different parts of Celastrus orbiculatus Thunb.
Na Yanga,
Han Wanga,
Hongqiang Lina,
Junli Liua,
Baisong Zhoua,
Xiaoling Chena,
Cuizhu Wangab,
Jinping Liu *ab and
Pingya Li*a
*ab and
Pingya Li*a
aSchool of Pharmaceutical Sciences, Jilin University, Fujin Road 126, Changchun 130021, Jilin, China. E-mail: liujp@jlu.edu.cn; lipy@jlu.edu.cn; Tel: +86-431-85619803
bResearch Center of Natural Drug, Jilin University, Changchun 130021, Jilin, China
First published on 26th February 2020
Abstract
The root, stem and leaf of Celastrus orbiculatus Thunb. (COT) have all been used as Chinese folk medicine. Aiming at revealing the secondary metabolites and screening the anti-COPD effect of COT, the comprehensive phytochemical and bioassay studies were performed. Based on the ultra-high performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry (UPLC-Q/TOF-MSE), the screening analysis of components in COT was conducted with the UNIFI platform, the metabolomics of the three parts were analyzed with multivariate statistical analysis. Cigarette smoke extract (CSE)-stimulated inflammatory model in A549 cells was used to investigate the biological effect of the three parts. A total of 120 compounds were identified or tentatively characterized from COT. Metabolomics analysis showed that the three parts of COT were differentiated, and there were 13, 8 and 5 potential chemical markers discovered from root, stem and leaf, respectively. Five robust chemical markers with high responses could be used for further quality control in different parts of COT. The root, stem and leaf of COT could evidently reduce the levels of pro-inflammatory factors in a dose-dependent way within a certain concentration range. The stem part had a stronger anti-COPD effect than root and leaf parts. This study clarified the structural diversity of secondary metabolites and the various patterns in different parts of COT, and provided a theoretical basis for further utilization and development of COT.
1. Introduction
Celastrus orbiculatus Thunb. (COT), belonging to the family Celastraceae and the genus Celastrus L., is widely distributed throughout China.1 The parts including root, stem and leaf all could been used as Chinese folk medicine to treat rheumatoid arthritis, vomiting, abdominal pain, and snakebites.2,3 The root of COT was reported to possess anti-tumor,4 antiviral,5 bacteriostatic6 and lipid-lowering1 activities, and about 50 compounds, including triterpenes, sesquiterpenoids, steroids and organic acids, were isolated from the root or root bark.3,7–9 The stem was also reported to have anti-inflammation,10 anti-cancer11 and fatty liver amelioration12 effects, and nearly 100 compounds including triterpenes, diterpenoids, steroids, flavonoids, phenolics and benzoquinone were isolated.13–16 For the leaf part, previous studies have found that the extract of it had insecticidal effect and hypoglycemic effect,17 while only a few flavonoids were isolated.18 It was revealed that there were significant variation for the contents of celastrol or total alkaloids in different parts of COT.19,20 Along with continuous expansion of the folk and clinical application of COT, an in-depth study on the chemical constituents in different parts of COT has attracted more and more attention. However, the comprehensive comparative study on the chemical composition between root, stem and leaf parts of COT has not been reported so far.Recently, the UPLC-Q/TOF-MS method has been innovatively used for screening and identifying chemical components in herbal medicines and traditional Chinese medicine. And the global profiling of various metabolites were reported. As part of these research works, we reported this method to detect some natural products including Platycodon grandiflorum and Ginseng root.21,22 Our research results showed that this method is high throughput, comprehensive, simple and efficient. As far as we know, the UPLC-Q/TOF-MS method has not been reported to identify the components in COT. So, the study in this paper comparatively analyzes the phytochemicals of root, stem and leaf parts of COT by using the UPLC-Q/TOF-MS method for the first time and finds out the similarities and differences between them.
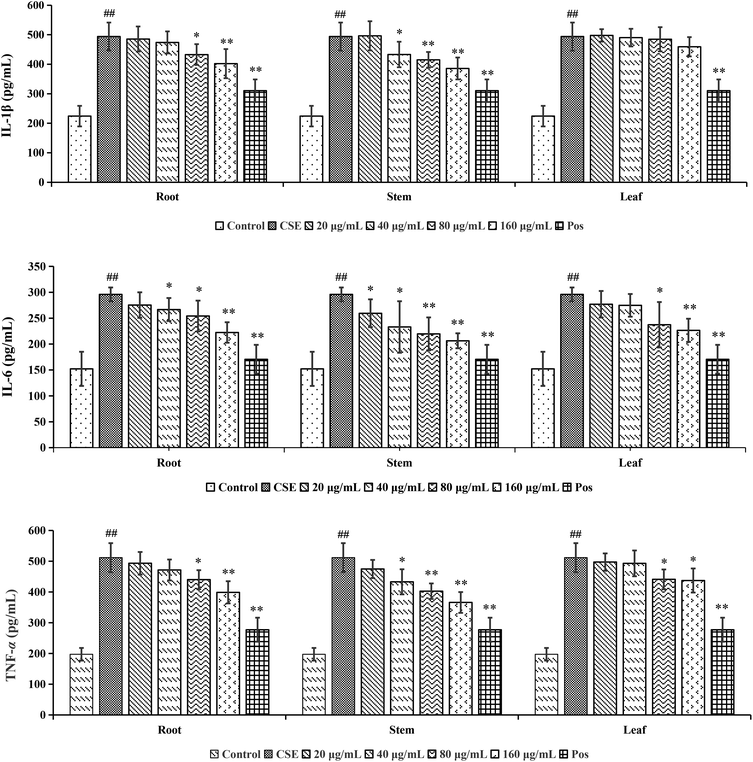
Chronic Obstructive Pulmonary Disease (COPD), predicted to rank as the third leading cause of death in the world,23 is mainly caused by significant exposure to harmful gases or particles.24 Cigarette smoking was the leading environmental risk factor for COPD around the world, and cigarette smokers were more likely to develop respiratory symptoms and had a higher COPD mortality rate. Along with the progressive lung inflammation, some pro-inflammatory mediators such as IL-1β, IL-6 and TNF-α participated in the occurrence and development of COPD.25 Although the COT had been used in treating various inflammatory diseases, the effect on the cigarette smoke extract (CSE)-induced inflammatory reaction has not been reported so far.
In the present study, the main medicinal parts of COT (root, stem and leaf) were chosen as the test sample. On one hand, the similarities and differences of phytochemicals in three parts were analyzed by using UNIFI platform and untargeted metabolomics based on UPLC-Q/TOF-MSE. The components and potential chemical markers to profile diverse classifications of metabolites of three parts were investigated. On the other hand, the effects on CSE-induced inflammatory reaction of these three parts were explored in A549 cells. The anti-COPD activity of different parts was preliminarily discussed. This comprehensive study could reveal the structural diversity of secondary metabolites and the different patterns of main medicinal parts of COT, and provide the data for further clinical application in anti-COPD. The study on the phytochemistry and the pharmacological activity of various parts were both significantly valuable to the research and development of COT.
2. Experiment
2.1. Materials and reagents
A total of 10 batches of fresh COT were collected from different growth areas in China (Table 1). All herbs were authenticated by the authors according to Hunan Province Local Standard for Traditional Chinese Medicine (2009 edition) for “Celastrus orbiculatus Thunb.”. The corresponding specimens had been deposited in the Research Center of Natural Drug, Jilin University, China.| No. | Source | Collection time |
|---|---|---|
| COT 01 | Yichun city, Heilongjiang province, China | 12th August, 2017 |
| COT 02 | Changchun city, Jilin province, China | 3rd August, 2017 |
| COT 03 | Huhhot city, Inner Mongolia autonomous region, China | 10th August, 2017 |
| COT 04 | Lanzhou city, Gansu province, China | 13th August, 2017 |
| COT 05 | Taian city, Shandong province, China | 5th July, 2017 |
| COT 06 | Xi'an city, Shanxi province, China | 14th July, 2017 |
| COT 07 | Zhengzhou city, Henan province, China | 15th July, 2017 |
| COT 08 | Chengdu city, Sichuan province, China | 7th July, 2017 |
| COT 09 | Changsha city, Hunan province, China | 27th July, 2017 |
| COT 10 | Jinhua city, Zhejiang province, China | 2nd July, 2017 |
Methanol and acetonitrile were of LC/MS grade purchased from Fisher Chemical Company. Formic acid was bought from Sigma-Aldrich Company, St. Louis, MO, USA. Deionized water was purified by Millipore water purification system (Millipore, Billerica, MA, USA). All other chemicals were analytically pure. Cigarettes for bioassay analysis were Xiongshi cigarette (China Tobacco Zhejiang Industrial Co., Ltd, Hangzhou, China), each cigarette contained 11 mg of tar, 0.7 mg of nicotine, and 13 mg of carbon monoxide. Human lung carcinoma A549 cells were obtained from the Department of Pathogen Biology, Basic Medical College, Jilin University. ELISA kits were bought from Nanjing Jiancheng Bio-engineering Institute.
2.2. Sample preparation of three parts of COT
Took the whole fresh COT, and separated the root, stem and leaf part respectively to get 30 test samples including root part (R1–R10) samples, stem part (S1–S10) samples and leaf part (L1–L10) samples. The aforementioned parts were air-dried, grinded and sieved with Chinese National Standard Sieve No. 3, R40/3 series to obtain the homogeneous powder respectively. Each powder was weighted (2.0 g) accurately and extracted thrice (3 hours per time) with 100 mL of 80% methanol at 80 °C, cooled, filtered, collected and combined the filtrate of each sample, concentrated and evaporated to dryness.For metabonomics analysis, each residue (all approximately 2.0 mg) was dissolved in 1.0 mL of 80% methanol respectively, after being filtered with a syringe filter (0.22 μm), 30 test solutions (RM1–RM10, SM1–SM10 and LM1–LM10) were obtained, which was injected into the UPLC system directly. Furthermore, to ensure the suitability consistency and the stability of MS analysis, a sample for quality control (QC) was prepared by pooling 20 μL from every test solution, namely containing all of the constituents in this analysis.
For screening analysis, the test solutions of root part (RS), stem part (SS) and leaf part (LS) were prepared by pooling 100 μL from RM1–RM10, SM1–SM10 and LM1–LM10 solutions, respectively.
For bioassay analysis, the test samples (Rbio, Sbio and Lbio) of root part, stem part and leaf part were prepared by combining each residue of R1–R10, S1–S10 and L1–L10, respectively. Then, Rbio, Sbio and Lbio were dissolved in water at the concentration of 3.2 mg mL−1 to get the stock solutions stored in 4 °C.
2.3. Ultra-high performance liquid chromatography combined with quadrupole time-of-flight tandem mass spectrometry (UPLC-Q/TOF-MSE)
The separation and detection of components were performed on the UPLC system combined with Xevo G2-XS Q/TOF mass spectrometer (Waters Co., Milford, MA, USA) with an electrospray ionization (ESI) interface.The ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) was bought from Waters Corporation (Milford, MA, USA). The moving phrase was consisted of eluent A (0.1% methanoic acid in water, v/v) and eluent B (0.1% methanoic acid in acetonitrile, v/v) in a liner gradient program (0–2 min, 10% B; 2–26 min, 10 → 90% B; 26–28 min, 90% B; 28–28.1 min, 90 → 10% B; 28.1–40 min, 10% B) with a flow rate of 0.4 mL min−1. Set the temperature of column and the sample manager at 30 °C and 15 °C, respectively. 10% and 90% acetonitrile in aqueous solution were used as weak and strong wash solvents respectively.
The optimized MS parameters were as follows: source temperature (150 °C), desolvation temperature (400 °C), cone voltage (40 V), capillary voltage at 2.6 kV (ESI+) and 2.2 kV (ESI−), cone gas flow (50 L h−1) and desolvation gas flow (800 L h−1). MSE mode was chosen with low energy of 6 V and high energy of 20–40 V.26,27 The mass spectrometer was calibrated with sodium formate in the range of 100 to 1200 Da in order to ensure the mass reproducibility and accuracy. Leucine enkephalin (m/z 556.2771 in ESI+ and 554.2615 in ESI−) was used as external reference for Lock Spray™ injected at a constant flow of 10 μL min−1. The QC sample was injected randomly 4 times throughout the whole work list. All of the volume injection of the samples and QC was 5 μL per run. During data acquisition, the data for screening analysis was performed in MSE continuum mode, the data for metabolomics analysis was performed in MSE centroid mode. Data recording was performed on MassLynx V4.1 workstation (Waters, Manchester, UK).
2.4. Screening analysis of components in three parts of COT by UNIFI platform
UNIFI 1.7.0 software (Waters, Manchester, UK) was used for data analysis.28,29Firstly, in addition to the internal Traditional Medicine Library on UNIFI platform, the chemical constituent investigation was conducted. As the result, a self-built database of chemical compounds isolated from the genus of Celastrus L. was established by searching the online databases including Web of Science, Medline, PubMed, ChemSpider and China National Knowledge Infrastructure (CNKI). The compound name, molecular formula and chemical structure of components were obtained in the database.
Secondly, the raw data obtained from Masslynx workstation were compressed by Waters Compression and Archival Tool v1.10, then were imported into the UNIFI software.
Thirdly, the compressed data were processed by the streamlined work flow of UNIFI software in order to quickly identify the chemical compounds which were matched the criteria with Traditional Medicine Library and self-built database. The main parameters of processed method were as follow: 2D peak detection was set to 200 as the minimum peak area. In the 3D peak detection, the peak intensity of high energy and low energy was taken more than 200 and 1000 times as the parameter respectively. Selected +H and +Na as positive adducts and +COOH and –H as negative adducts. Leucine enkephalin was used as reference compound in order to get exact mass accuracy, with [M + H]+ 556.2766 for positive ion and [M − H]− 554.2620 for negative ion. As a result, the comprehensive chemical constituents screening list was accomplished.
Finally, a filter was set to refine the results, with the mass error between −5 and 5 ppm and response value over 5000. Each compound was verified by compared with the characteristic MS fragmentation patterns reported in literature or the retention time of the reference substances.
2.5. Metabolomics analysis of three parts of COT
The raw data acquired by Masslynx workstation was processed on MakerLynx XS V4.1 software (Waters, Milford, CT, USA). Firstly, the raw data were processed with alignment, deconvolution, and data reduction, etc. The main parameters of the process method were as follows: retention time (0–28 min), retention time window (0.20), mass (100–1200 Da), mass tolerance (0.10), mass window (0.10), minimum intensity (5%), marker intensity threshold (2000 counts) and noise elimination (level 6). As a result, the list with mass and retention time corresponded to the responses based on all the detected peaks from each data file were shown in Extended Statistics (XS) Viewer. Secondly, multivariate statistical analysis, both principle component analysis (PCA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA), were performed on the MakerLynx software to analyze the resulting data.PCA, a classical unsupervised low dimensional pattern recognition model, was used to show pattern recognition and maximum variation, and the overview and classification were obtained. OPLS-DA was used to obtain the maximum separation between two different groups. S-plots, which could provide visualization of the OPLS-DA predictive results, were created to explore the potential chemical markers which contributed to the differences.
Meanwhile, metabolites with VIP value > 4.0 and p-value < 0.001 were considered as potential chemical markers.30,31 Futhermore, permutation test was also performed to provide a reference distribution with the R2/Q2 values to indicate statistical significance. Finally, the analysis results were shown in Simca 15.0 software (Umetrics, Malmö, Sweden).
2.6. Bioassay analysis of three parts of COT
3. Results and discussion
3.1. Screening analysis of components of three parts of COT
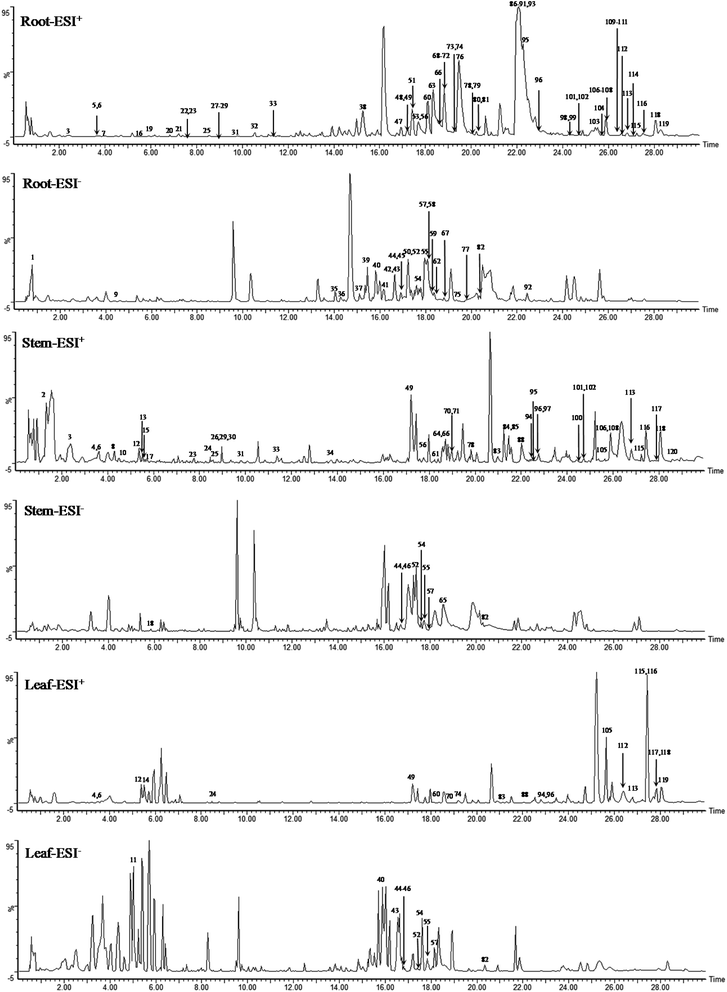
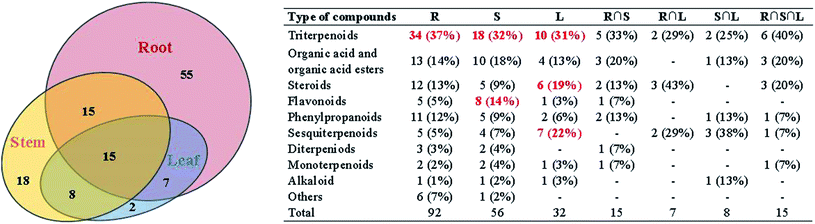
A total of 120 compounds, including 91 in ESI+ mode and 29 in ESI− mode, were identified or tentatively characterized from three parts of COT (Table 2). The base peak intensity (BPI) chromatograms were shown in Fig. 1. The chemical structures were shown in Fig. 2, the results showed that COT was rich in natural components with various structural patterns. On one hand, according to the reference, there were nearly 50, 100, 10 compounds were reported from the root, stem, leaf parts of COT, respectively. While in this study, there were 92, 56 and 32 components were identified or tentatively characterized from root part, stem part and leaf part of COT, respectively. And most of the components were identified from COT for the first time. Various kinds of structures, including triterpeniods, sesquiterpenoids, steroids, flavonoids, organic acid and organic acid esters, phenylpropanoids, diterpeniods, monoterpenoids, alkaloid and others, were contained in each part of COT. The numbers (% of the total identified components in each part) and structural types of compounds identified from root, stem and leaf of COT were shown in Fig. 3. There were 34, 18 and 10 triterpeniods identified from root, stem and leaf, respectively, accounted for 37%, 32% and 31% of the total components in each part. So, it was concluded that triterpeniods were the major constituents in three parts of COT. Moreover, according to each percentage, the root part of COT was also rich in organic acid and organic acid esters, steroids and phenylpropanoids. The stem part was also rich in organic acid and organic acid esters, and flavonoids. The leaf part was also rich in steroids, and sesquiterpenoids. The percentage of flavonoids in the total identified components in stem was higher than the percentages in root part or in leaf part. The percentages of sesquiterpeniods and steroids in the total identified components in leaf was higher than the percentages in root part or in stem part. On the other hand, the shared components (30 for root and stem, 22 for root and leaf, 23 for stem and leaf, 15 for root, stem and leaf) were also found in our study. As shown in Fig. 3, the structures of shared components were various, while triterpeniods held the majority. Celastrol, one of the triterpeniods, was shown to distribute in root, stem and leaf, which was consistent with the ref. 19. So our research work could provide the scientific data to clarify the chemical composition of COT, particularly for the root and the leaf parts.| No. | tR (min) | Formula | Calculated mass (Da) | Theoretical mass (Da) | Mass error (ppm) | MSE fragmentation | Identification | Source | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a * Characteristic component in root; # characteristic component in stem; ※ characteristic component in leaf; a: compared with spectral data obtained from Wiley Subscription Services, Inc. (USA); b: compared with NIST Chemistry WebBook; s: compared with the reference compounds; R: the root of Celastrus orbiculatus Thunb.; S: the stem of Celastrus orbiculatus Thunb.; L: the leaf of Celastrus orbiculatus Thunb. | |||||||||
| 1 | 0.59 | C29H28O8 | 504.1828 | 504.1843 | −2.7 | 549.1810 [M + H − COO]−, 382.1400 [M − H − C7H5O2]−, 311.0857 [M − H − C11H12O3]− | Interiotherin A | R | 33 |
| 2# | 1.23 | C19H20O6 | 344.1256 | 344.1260 | −1.1 | 367.1486 [M + Na]+, 327.1185 [M + H − H2O]+, 315.0707 [M + H − 2 × CH3]+, 238.0591 [M + H − C7H7O]+, 224.0341 [M + H − C8H9O]+ | 5,7-Dihydroxy-6,8-dimethyl-3(S)-3-(3-methoxy-4′-hydroxybenzyl)chroman-4-one | S | — |
| 3 | 2.43 | C9H12O4 | 184.0728 | 184.0736 | −4.4 | 185.0791 [M + H]+, 167.0584 [M + H − H2O]+, 136.0593 [M + H − H2O − CH2OH]+ | Eucommidiol | R, S | 34 |
| 4 | 3.60 | C16H18N2O3 | 286.1328 | 286.1317 | 3.5 | 309.1220 [M + Na]+, 242.0841 [M + H − 3 × CH3]+, 225.0989 [M + H − 2 × OCH3]+ | Picrasidine B | S, L | a |
| 5 | 3.64 | C22H28O11 | 468.1616 | 468.1632 | −3.4 | 469.1660 [M + H]+, 290.1036 [M + H-Glu]+, 232.0691 [M + H − C3H7O-Glu]+ | Cimicifuga glycoside | R | 35 |
| 6 | 3.66 | C19H30O8 | 386.1925 | 386.1941 | −4.2 | 409.1811 [M + Na]+, 226.0124 [M + H − C6H9O5]+, 178.0837 [M + H − 2 × CH3-Glu]+, 190.1255 [M + H − H2O-Glu]+ | Roseoside | R, S, L | 36 |
| 7 | 3.97 | C25H28O6 | 424.1901 | 424.1886 | 3.6 | 447.1812 [M + Na]+, 407.1226 [M + H − H2O]+, 303.0539 [M + H − C9H14]+, 176.0533 [M + H − H2O − C9H14 − C6H5O2]+ | Norkurarinone | R | 37 |
| 8 | 4.17 | C19H20O6 | 344.1244 | 344.1260 | −4.7 | 345.1316 [M + H]+, 222.0530 [M + H − CH3 − C7H7O]+, 153.0511 [M + H − C11H12O3]+ | 2,3-Dihydro-5,7-dihydroxy-8-methoxy-3-[(4-methoxyphenyl)methyl]-6-methyl-4H-1-benzopyran-4-one | S | 38 |
| 9 | 4.44 | C15H18O8 | 326.0993 | 326.1002 | −2.9 | 325.0870 [M − H]−, 128.0426 [M − H − H2O-Glu]−, 100.0409 [M − H − HCOOH-Glu]−, 75.0570 [M − H − C3H3O2-Glu]− | Glucosido-p-coumaric acid | R | s |
| 10 | 4.60 | C20H20O5 | 340.1295 | 340.1311 | −4.5 | 341.1355 [M + H]+, 256.0641 [M + H − C5H9O]+, 238.0692 [M + H − H2O − C5H9O]+, 193.0713 [M + H − C9H7O2]+ | Psorachalcone A | S | — |
| 11※ | 5.02 | C32H34O12 | 610.2048 | 610.2050 | −0.4 | 609.1446 [M − H]−, 541.2011 [M − H − O − C2H3O]−, 371.1898 [M − H − O − 2 × C5H3O3]− | Orbiculin I | L | — |
| 12 | 5.28 | C27H36O13 | 568.2139 | 568.2156 | −2.8 | 591.2031 [M + Na]+, 359.1459 [M + H − CH2OH-Glu]+, 345.1251 [M + H − CH3 − C11H13O4]+ | Citrusin B | S, L | s |
| 13 | 5.39 | C15H10O7 | 302.0415 | 302.0427 | −4.1 | 303.0484 [M + H]+, 178.0320 [M + H − C6H5O3]+, 108.0286 [M + H − H2O − C9H5O4]+ | Isoetin | S | s |
| 14※ | 5.53 | C27H30O16 | 610.1539 | 610.1534 | 0.9 | 611.1613 [M + H]+, 432.1040 [M + H-Glu]+, 253.0388 [M + H − 2 × Glu]+ | Luteolin 7,4′-diglucoside | L | 39 |
| 15 | 5.65 | C24H32O10 | 480.2007 | 480.1996 | 2.3 | 503.1899 [M + Na]+, 386.1517 [M + H − 2 × H2O − COOCH3]+, 360.1426 [M + H − C8H9O]+, 191.0715 [M + H − C8H9O − C9H13O3]+ | Ilexin L3 | S | — |
| 16 | 5.66 | C20H24O7 | 376.1505 | 376.1522 | −4.5 | 399.1388 [M + Na]+, 316.1217 [M + H − 2 × CH3 − CH2OH]+, 310.1320 [M + H − 2 × H2O − CH3O]+, 138.0597 [M + H − C12H15O5]+ | Vladinol C | R | 40 |
| 17 | 5.74 | C15H10O7 | 302.0418 | 302.0427 | −2.9 | 303.0491 [M + H]+, 285.0363 [M + H − H2O]+, 110.0281 [M + H − C9H5O5]+ | Quercetin | S | b |
| 18 | 5.87 | C19H18O4 | 310.1193 | 310.1205 | −3.8 | 309.1266 [M − H]−, 279.0821 [M − H − 2 × CH3]−, 278.0977 [M − H − OCH3]−, 173.0532 [M − H − OCH3 − C8H9]−, 104.0703 [M − H − C11H9O4]− | 6,7-Dimethoxy-2-phenethylchromone | S | s |
| 19 | 5.96 | C10H10O3 | 178.0623 | 178.0630 | −3.9 | 179.0687 [M + H]+, 164.0374 [M + H − CH3]+, 120.0491 [M + H − COOCH3]+, 86.0307 [M + H − C6H5O]+ | Methyl-p-coumarate | R | 41 |
| 20 | 6.75 | C17H14O8 | 346.0688 | 346.0689 | −0.1 | 347.0761 [M + H]+, 329.0629 [M + H − H2O]+, 298.0688 [M + H − H2O − OCH3]+, 219.0460 [M + H − H2O − C6H5O2]+ | Aksilarin | R | s |
| 21 | 7.22 | C14H16O4 | 248.1040 | 248.1049 | −3.5 | 249.1105 [M + H]+, 206.0877 [M + H − CH3CO]+, 193.1007 [M + H − C3H4O]+ | Evodinnol | R | 42 |
| 22 | 7.58 | C21H22O6 | 370.1410 | 370.1416 | −1.7 | 371.1483 [M + H]+, 220 [M + H − C9H11O2]+, 152 [M + H − C12H11O4]+ | (+)-7,8-Didehydroarctigenin | R | 43 |
| 23 | 7.73 | C17H20O4 | 288.1360 | 288.1362 | −0.5 | 311.1252 [M + Na]+, 274.1090 [M + H − CH3]+, 238.0842 [M + H − 2 × H2O − CH3]+ | (+)-Celaphanol A | R, S | s |
| 24 | 8.47 | C34H44O14 | 676.2738 | 676.2731 | 1.0 | 677.2811 [M + H]+, 585.2390 [M + H − H2O − CH3 − C2H3O2]+, 556.2650 [M + H − C7H5O2]+, 441.1997 [M + H − 4 × C2H3O2]+ | Celangulin IV | S, L | — |
| 25 | 8.57 | C22H24O7 | 400.1407 | 400.1522 | −3.8 | 401.1559 [M + H]+, 383.0623 [M + H − H2O]+, 365.2118 [M + H − 2 × H2O]+, 234.0763 [M + H − C9H11O3]+ | Aschantin | R, S | 44 |
| 26 | 8.83 | C20H20O5 | 340.1295 | 340.1311 | −4.5 | 341.1355 [M + H]+, 326.0641 [M + H − CH3]+, 299.0562 [M + H − C3H7]+, 150.0562 [M + H − C4H7 − C8H7O2]+ | Corylifol B | S | 45 |
| 27 | 8.94 | C20H18O5 | 338.1143 | 338.1154 | −3.3 | 339.1209 [M + H]+, 218.0945 [M + H − C8H7O]+, 176.0735 [M + H − H2O − C9H7O2]+ | Demethoxycurcumin | R | 46 |
| 28 | 8.95 | C20H20O6 | 356.1247 | 356.1260 | −3.6 | 357.1320 [M + H]+, 245.0820 [M + H − CH3 − C6H5O2]+, 96.0346 [M + H − H2O − C14H11O4]+ | Leachianone G | R | s |
| 29 | 8.96 | C18H20O5 | 316.1317 | 316.1311 | 1.8 | 339.1209 [M + Na]+, 251.0624 [M + H − 2 × H2O − 2 × CH3]+, 174.0900 [M + H − 2 × H2O − C7H7O]+ | (3R-cis)-3,4-Dihydro-3,4-diol-7-methoxy-3-[(4-methoxyphenyl)methyl]-2H-1-benzopyran | R, S | — |
| 30# | 8.98 | C22H28O8 | 420.1799 | 420.1784 | 3.4 | 443.1696 [M + Na]+, 205.0843 [M + H − 2 × CH2OH − C8H9O3]+, 167.0664 [M + H − C13H18O5]+ | (+)-Lyoniresinol | S | 47 |
| 31 | 9.81 | C24H26O8 | 442.1611 | 442.1628 | −3.8 | 443.1675 [M + H]+, 384.1466 [M + H − C2H3O2]+, 381.1268 [M + H − 2 × CH3O]+, 307.0731 [M + H − CH3 − 2 × CH3O − C2H3O2]+ | Interiorin C | R, S | — |
| 32 | 10.35 | C20H18O4 | 322.1218 | 322.1205 | 4.0 | 323.1291 [M + H]+, 268.1004 [M + H − C4H7]+, 254.0834 [M + H − C5H9]+ | Neobavaisoflavone | R | 48 |
| 33 | 11.39 | C18H34O5 | 330.2392 | 330.2406 | −4.0 | 353.2284 [M + Na]+, 277.2129 [M + H − 3 × H2O]+, 150.1100 [M + H − 2 × H2O − C8H17O2]+, 81.0569 [M + H − H2O − C4H7O2 − C8H17O2]+ | 9-Octadecenoic acid | R, S | s |
| 34 | 13.75 | C18H20O4 | 300.1348 | 300.1362 | −4.3 | 323.1240 [M + Na]+, 138.0556 [M + H − C10H11O2]+, 107.0465 [M + H − CH3O − C10H11O2]+ | trans-3,3′,5,5′-Tetramethoxystilbene | S | — |
| 35* | 14.06 | C30H48O6 | 504.3448 | 504.3451 | −0.6 | 549.3417 [M + HCOO]−, 263.3423 [M − H − C14H24O3]−, 239.3239 [M − H − C16H24O3]− | Virgaureagenin G | R | 49 |
| 36 | 14.33 | C28H42O5 | 458.3053 | 458.3032 | 4.6 | 457.2981 [M − H]−, 344.3321 [M − H − 2 × H2O − HCOOH − CH2OH]−, 233.2957 [M − H − C13H20O3]−, | 3β,4β,23-Trihydroxy-24,30-dinorolean-12,20(29)-dien-28-oic acid | R | 50 |
| 37 | 15.19 | C28H46O5 | 462.3349 | 462.3345 | 0.8 | 461.3276 [M − H]−, 425.3270 [M − H − 2 × H2O]−, 193.2396 [M − H − C17H32O2]− | Polyporusterone F | R | 51 |
| 38 | 15.26 | C39H54O6 | 618.3926 | 618.3920 | 1.0 | 641.3857 [M + Na]+, 533.3344 [M + H − C3H4 − HCOOH]+, 398.2486 [M − 2 × CH3 − C3H4 − C8H7O3]+ | Lup-20(29)-en-28-oic-3β-yl caffeate | R | s |
| 39* | 15.47 | C30H40O4 | 464.2934 | 464.2927 | 1.5 | 509.2551 [M + HCOO]−, 386.2693 [M − H − H2O − C2H3O2]−, 199.2228 [M − H − C17H28O2]− | Pristimerin | R | 52 |
| 40※ | 15.84 | C33H38O9 | 578.2535 | 578.2516 | 3.3 | 577.2683 [M − H]−, 561.2680 [M − H − O]−, 547.2187 [M − H − 2 × CH3]−, 534.2176 [M − H − C2H3O]− | Orbiculin A | R, L | s |
| 41 | 16.28 | C30H46O4 | 470.3399 | 470.3396 | 0.6 | 469.3326 [M − H]−, 454.3157 [M − H − CH3]−, 451.3303 [M − H − H2O]−, 342.3522 [M − H − C8H15O]− | 11-Oxokansenonol | R | 53 |
| 42 | 16.52 | C30H46O6 | 502.3315 | 502.3294 | 4.1 | 547.3326 [M + HCOO]−, 455.3236 [M − H − HCOOH]−, 401.3505 [M − H − 3 × H2O − HCOOH]− | Esculentic acid | R | s |
| 43 | 16.66 | C31H44O6 | 512.3118 | 512.3138 | −3.9 | 511.3045 [M − H]−, 495.2929 [M − H − O]−, 467.2412 [M − H − CO2]−, 233.2535 [M − H − C17H26O3]− | Paeonenolide G | R, L | 54 |
| 44 | 16.79 | C32H40O8 | 552.2717 | 552.2723 | −1.1 | 597.2699 [M + HCOO]−, 487.2250 [M − H − H2O − CH3 − CH3O]−, 193.1065 [M − H − C21H26O5]− | Saucerneol A | R, S, L | 55 |
| 45 | 16.80 | C27H44O8 | 496.3055 | 496.3036 | 3.8 | 541.3050 [M + HCOO]−, 461.3026 [M − H − 2 × H2O]−, 378.2876 [M − H − C6H13O2]− | Turkesterone | R, L | 56 |
| 46 | 16.81 | C30H50O2 | 442.3907 | 442.3811 | −0.9 | 441.3713 [M − H]−, 423.3286 [M − H − H2O]−, 233.3456 [M − H − C14H24O]− | Oleanolic alcohol | S, L | 57 |
| 47 | 17.06 | C20H34O4 | 338.2461 | 338.2457 | 1.1 | 361.2353 [M + Na]+, 267.2239 [M + H − 4 × H2O]+, 262.1673 [M + H − CH3 − 2 × CH2OH]+, 278.0124 [M + H − C3H5O2]+ | Hallol | R | a |
| 48 | 17.22 | C18H28O2 | 276.2086 | 276.2089 | −1.0 | 277.2141 [M + H]+, 248.2197 [M + H − C2H5]+, 222.1520 [M + H − C4H7]+, 67.0579 [M + H − C2H5 − C11H17O2]+ | 6,9,12,15-Octadecatetraenoic acid | R | 58 |
| 49 | 17.23 | C16H30O2 | 254.2249 | 254.2246 | 1.2 | 277.2141 [M + Na]+, 125.1160 [M + H − C4H9 − C3H5O2]+, 112.0804 [M + H − H2O − C9H17]+ | Oleopalmitic acid | R, S, L | 59 |
| 50* | 17.25 | C30H44O4 | 468.3225 | 468.3240 | −3.2 | 467.2441 [M − H]−, 434.2905 [M − H − H2O − CH3]−, 423.3320 [M − H − CO2]− | 3β-Hydroxy-2-oxoolean-12-ene-22,29-actone | R | — |
| 51* | 17.30 | C27H40O6 | 460.2833 | 460.2825 | 1.7 | 483.2751 [M + Na]+, 361.2355 [M + H − C6H12O]+, 417.2282 [M + H − CO2]+, 319.2480 [M + H − C8H14O2]+ | Lucidenic acid N | R | 60 |
| 52# | 17.38 | C27H44O4 | 432.3223 | 432.3240 | −3.8 | 431.2217 [M − H]−, 397.3428 [M − H − O − H2O]−, 395.3258 [M − H − 2 × H2O]−, 249.1019 [M − H − C11H18O2]+ | Chlorogenin | R, S, L | a |
| 53 | 17.56 | C20H22O4 | 326.1515 | 326.1518 | −0.8 | 327.1588 [M + H]+, 312.1295 [M + H − CH3]+, 256.1067 [M + H − 2 × CH3 − C3H5]+, 203.2237 [M + H − C7H8O2]+ | Dehydrodiisoeugenol | R | 61 |
| 54※ | 17.62 | C30H48O5 | 488.3523 | 488.3502 | 4.4 | 487.2917 [M − H]−, 469.3240 [M − H − H2O]−, 433.3297 [M − H − 3 × H2O]−, 411.1033 [M − H − 2 × CH3 − HCOOH]+, 405.3029 [M − H − 2 × H2O − HCOOH]− | Orthosphenic acid | R, S, L | a |
| 55 | 17.80 | C35H40O7 | 572.2788 | 572.2774 | 2.4 | 571.2738 [M − H]−, 555.2826 [M − H − O]−, 331.2334 [M − H − O − C6H5 − C9H7O2]− | Celastrine B | R, S, L | — |
| 56 | 17.84 | C30H46O3 | 454.3445 | 454.3447 | −0.5 | 455.3492 [M + H]+, 408.3435 [M + H − H2O − CHO]+, 325.2291 [M + H − CH3 − C6H11O]+ | (13α,14β,17α,20S,24Z)-Lanosta-26-hydroxy-3-oxo-7,24-dien-21-al | R, S | a |
| 57* | 18.02 | C27H44O7 | 480.3106 | 480.3087 | 4.0 | 479.2439 [M − H]−, 464.2903 [M − H − CH3]−, 461.2934 [M − H − H2O]−, 414.2779 [M − H − H2O − C2H7O]−, 202.0239 [M − H − C11H22O3]+ | Viticosterone | R, S, L | 62 |
| 58 | 18.11 | C30H46O3 | 454.3464 | 454.3447 | 3.8 | 453.3414 [M − H]−, 435.3608 [M − H − H2O]−, 409.3319 [M − H − CO2]−, 376.3716 [M − H − H2O − CH3 − CO2]−, 223.2100 [M − H − C15H18O2]− | Wilforlide A | R | s |
| 59 | 18.20 | C28H48O2 | 416.3636 | 416.3654 | −4.1 | 461.3618 [M + HCOO]−, 265.2591 [M − H − C9H10O2]−, 149.2309 [M − H − C19H38]− | γ-Tocopherol | R | 63 |
| 60 | 18.24 | C30H38O11 | 574.2423 | 574.2414 | 1.5 | 597.2315 [M + Na]+, 395.1897 [M + H − C2H3O2 − C7H5O2]+, 339.1724 [M + H − 4 × C2H3O2]+ | Ejap 3 | R, L | a |
| 61# | 18.38 | C16H32O2 | 256.2393 | 256.2402 | −3.7 | 279.2303 [M + Na]+, 199.1787 [M + H − C4H9]+, 69.0553 [M + H − 2 × CH3 − C9H17O2]+ | Methyl (12S)-12-methyltetradecanoate | S | a |
| 62 | 18.41 | C32H44O8 | 556.3016 | 556.3036 | −3.6 | 555.2910 [M − H]−, 463.2604 [M − H − H2O − CH3 − C2H3O2]−, 313.2285 [M − H − H2O − C7H13O2 − C5H3O2]− | 14,15-Epoxy-14H-cyclopenta [a]phenanthrene, bufa-20,22-dienolide deriv. | R | — |
| 63* | 18.46 | C30H42O7 | 514.2931 | 514.2931 | 0.1 | 515.3017 [M + H]+, 469.2859 [M + H − HCOOH]+, 417.2608 [M + H − C6H10O]+, 377.2297 [M + H − C9H14O]+ | Ganoderenic acid A | R | 60 |
| 64# | 18.67 | C30H48O3 | 456.3583 | 456.3604 | −4.7 | 457.3675 [M + H]+, 439.3520 [M + H − H2O]+, 424.2688 [M + H − H2O − CH3]+, 330.2465 [M + H − C8H15O]+ | Kansenonol | S | 64 |
| 65 | 18.69 | C16H32O2 | 256.2394 | 256.2402 | −2.7 | 301.2376 [M + HCOO]−, 97.0991 [M − H − C6H13 − C3H5O2]−, 68.0554 [M − H − CH3 − C8H17 − C2H3O2]− | 4,8,12-Trimethyltridecanoic acid | S | 65 |
| 66 | 18.69 | C18H30O2 | 278.2233 | 278.2246 | −4.5 | 279.2292 [M + H]+, 261.2353 [M + H − H2O]+, 72.0554 [M + H − C11H19 − C2H3O2]+ | Gamolenic acid | R, S | 66 |
| 67 | 18.74 | C30H46O4 | 470.3399 | 470.3396 | 0.6 | 469.3326 [M − H]−, 454.3157 [M − H − CH3]−, 451.3303 [M − H − H2O]−, 407.3726 [M − H − H2O − CO2]−, 261.4102 [M − H − C14H24O]− | 3β-Hydroxy-11α,12α-epoxy-13β,28-ursolide | R | a |
| 68 | 18.83 | C28H36O7 | 484.2458 | 484.2461 | −0.6 | 469.2597 [M − OH]+, 363.2350 [M + H − C7H6O2]+, 345.1852 [M + H − H2O − C7H6O2]+, 218.1708 [M + H − 2 × H2O − C7H6O2 − C6H5O2]+ | Scutellone I | R | 67 |
| 69 | 18.94 | C30H46O4 | 470.3399 | 470.3396 | 0.6 | 471.3326 [M + H]+, 425.3157 [M + H − HCOOH]+, 289.3241 [M + H − C11H18O2]+, 249.3383 [M + H − C14H22O2]+ | Hederagonic acid | R | 68 |
| 70 | 18.97 | C30H48O3 | 456.3606 | 456.3604 | 0.5 | 457.3678 [M + H]+, 439.2231 [M + H − H2O]+, 249.3437 [M + H − C14H24O]+, 203.1653 [M + H − C14H24O − HCOOH]+ | Ursolic acid | R, S, L | 69 |
| 71 | 19.00 | C18H30O3 | 294.2184 | 294.2195 | −3.6 | 317.2060 [M + Na]+, 277.2115 [M + H − H2O]+, 172.0863 [M + H − C9H15]+, 124.1081 [M + H − C9H15O3]+ | 9-Oxooctadeca-10,12-dienoic acid | R, S | 70 |
| 72 | 19.13 | C30H42O7 | 514.2931 | 514.2931 | 0.1 | 515.3004 [M + H]+, 454.2660 [M + H − CH3 − HCOOH]+, 415.2676 [M + H − C6H12O]+, 321.2393 [M + H − C11H14O3]+ | Ganoderenic acid B | R | 60 |
| 73* | 19.30 | C11H14O2 | 178.1001 | 178.0994 | 4.2 | 201.0886 [M + Na]+,164.0739 [M + H − CH3]+, 107.0589 [M + H − CH3O − C3H5]+, 76.0507 [M + H − 2 × CH3O − C3H5]+ | Isohomogenol | R | 71 |
| 74 | 19.35 | C29H48O2 | 428.3675 | 428.3654 | 4.7 | 451.3568 [M + Na]+, 411.2449 [M + H − H2O]+, 274.2205 [M + H − C10H19O]+, 256.2260 [M + H − H2O − C10H19O]+ | Seringosterol | R, L | 72 |
| 75 | 19.46 | C30H44O7 | 516.3080 | 516.3087 | −1.3 | 515.3007 [M − H]−, 500.2865 [M − H − CH3]−, 497.3383 [M − H − H2O]−, 322.1767 [M − H − 2 × H2O − C8H13O3]− | Ganoderic acid A | R | 73 |
| 76 | 19.72 | C22H30O4 | 358.2132 | 358.2144 | −3.3 | 381.2024 [M + Na]+, 316.2063 [M + H − C2H3O]+, 273.1648 [M + H − C3H7 − C2H3O2]+ | (3S,4aS,10aS)-3-(Acetyloxy)-2,3,4,4a,10,10a-hexahydro-6-hydroxy-1,1,4a-trimethyl-7-(1-methylethyl)-9(1H)-phenanthrenone | R | s |
| 77 | 19.73 | C30H50O6 | 506.3632 | 506.3607 | 4.9 | 505.3559 [M − H]−, 43.3909 [M − H − 4 × H2O]−, 431.2834 [M − H − CH3 − C3H7O]− | 13β,17β-Epoxyalisol A | R | s |
| 78 | 19.89 | C30H46O3 | 454.3438 | 454.3447 | −2.0 | 455.3492 [M + H]+, 409.3435 [M + H − HCOOH]+, 391.1517 [M + H − H2O − HCOOH]+, 249.3317 [M + H − C14H22O]+ | Oleanonic acid | R, S | 74 |
| 79 | 20.05 | C30H46O5 | 486.3334 | 486.3345 | −2.4 | 487.3406 [M + H]+, 441.3344 [M + H − HCOOH]+, 413.3436 [M + H − H2O − CH3 − HCOOH]+, 395.3278 [M + H − 2 × HCOOH]+, 249.5358 [M + H − C14H22O3]+ | Ceanothic acid | R | a |
| 80* | 20.24 | C28H42O6 | 474.2986 | 474.2981 | 1.0 | 497.2908 [M + Na]+, 457.2342 [M + H − H2O]+, 411.2324 [M + H − H2O − CH3 − CH3O]+, 285.0399 [M + H − H2O − C9H16O3]+ | Methyl lucidenate Q | R | 75 |
| 81 | 20.36 | C29H34O8 | 510.2256 | 510.2254 | 0.3 | 533.2148 [M + Na]+, 410.1760 [M + H − C2H3O − C3H6O]+, 268.1455 [M + H − C2H3O − C7H5O − C5H3O2]+ | Orbiculin B | R | — |
| 82 | 20.36 | C30H48O3 | 456.3617 | 456.3604 | 3.0 | 455.3544 [M − H]−, 391.3440 [M − H − H2O − HCOOH]−, 149.3707 [M − H − H2O − C18H27O2]− | Oleanolic acid | R, S, L | 74 |
| 83 | 21.14 | C19H38O4 | 330.2770 | 330.2770 | 0.1 | 353.2632 [M + Na]+, 313.2160 [M + H − H2O]+, 300.0486 [M + H − CH3O]+, 240.0679 [M + H − C3H7O3]+ | β-Monopalmitin | S, L | 76 |
| 84# | 21.46 | C39H54O6 | 618.3949 | 618.3920 | 4.6 | 619.4002 [M + H]+, 565.4126 [M + H − 3 × H2O]+, 472.3557 [M + H − C9H7O2]+, 456.3443 [M + H − C9H7O3]+, 411.1356 [M + H − C14H24O]+ | 27-p-E-Coumaroyloxyursolic acid | S | 77 |
| 85# | 21.49 | C30H46O2 | 438.3485 | 438.3498 | −2.9 | 439.3559 [M + H]+, 421.3432 [M + H − H2O]+, 390.1126 [M + H − H2O − CH3O]+, 250.3443 [M + H − C13H17O]+ | 5-Dehydrokarounidiol | S | — |
| 86* | 21.72 | C13H12O2 | 200.0834 | 200.0831 | −3.3 | 201.0886 [M + H]+, 186.0597 [M + H − CH3]+, 165.0996 [M + H − 2 × H2O]+, 140.0533 [M + H − C2H5O2]+ | Safynol | R | 78 |
| 87* | 22.03 | C12H16O2 | 192.1157 | 192.1150 | 3.7 | 215.1045 [M + Na]+, 163.0490 [M + H − 2 × CH3]+, 150.1739 [M + H − C3H7]+, 135.2173 [M + H − CH3 − C3H7]+, 119.0695 [M + H − CH3 − C2H3O2]+, 91.0556 [M + H − C3H7 − C2H3O2]+ | Carvacryl acetate | R | a |
| 88* | 22.04 | C29H38O4 | 450.2766 | 450.2770 | −0.9 | 451.2844 [M + H]+, 433.2591 [M + H − H2O]+, 405.2771 [M + H − HCOOH]+, 215.3621 [M + H − C15H24O2]+, 200.0917 [M + H − CH3 − C15H24O2]+ | Celastrol | R, S, L | 79 |
| 89 | 22.05 | C9H10O | 134.0737 | 134.0732 | 3.4 | 157.0632 [M + Na]+, 120.1173 [M + H − CH3]+, 106.0704 [M + H − C2H5]+, 77.0477 [M + H − C2H5 − CHO]+ | 4-Ethylbenzaldehyde | R | 80 |
| 90* | 22.22 | C29H36O4 | 448.2609 | 448.2614 | −1.0 | 449.2679 [M + H]+, 434.2548 [M + H − CH3]+, 403.2606 [M + H − HCOOH]+, 267.3315 [M + H − C12H6O2]+ | 25-(9→8)Abeo-24-nor-friedelan-2,3-dioxo-1(10),4,6,9(11)-tetraen-29-oic acid | R | — |
| 91 | 22.46 | C38H44N2O6 | 624.3200 | 624.3220 | 3.2 | 647.3113 [M + Na]+, 503 [M + H − C8H9O]+, 314.1395 [M + H − CH3 − C18H16NO2]+, 297 [M + H − C7H7O − C11H17O2N]+, 266.0790 [M + H − CH3 − C8H9O − C13H18NO2]+ | Neferin | R | 81 |
| 92 | 22.78 | C32H48O5 | 512.3486 | 512.3502 | −3.1 | 511.3413 [M − H]−, 493.2436 [M − H − H2O]−, 465.1023 [M − H − HCOOH]+, 434.2379 [M − H − H2O − C2H3O2]−, 370.2491 [M − H − C8H13O2]− | Ganoderic acid X | R | 82 |
| 93 | 22.80 | C12H16O2 | 192.1159 | 192.1150 | 4.2 | 215.1052 [M + Na]+, 178.0893 [M + H − CH3]+, 107.5147 [M − C5H11O]+, 78.0571 [M + H − C6H11O2]+ | Amyl benzoate | R | s |
| 94 | 22.81 | C30H52O3 | 460.3920 | 460.3917 | 0.8 | 483.3813 [M + Na]+, 407.3593 [M + H − H2O]+, 368.3012 [M + H − H2O − C3H7O2]+, 253.0154 [M + H − C14H24O]+ | Olibanumol H | S, L | 83 |
| 95 | 22.82 | C30H48O4 | 472.3543 | 472.3553 | −2.1 | 473.3615 [M + H]+, 455.3483 [M + H − H2O]+, 427.3561 [M + H − HCOOH]+, 333.1527 [M + H − C9H16O]+, 290.0146 [M + H − CH3 − C11H20O]+ | Echinocystic acid | R, S | 84 |
| 96 | 22.83 | C16H32O2 | 256.2390 | 256.2402 | −4.5 | 279.2276 [M + Na]+, 155.1230 [M + H − C5H11 − CH3O]+, 85.0488 [M + H − C10H21 − OCH3]+ | Methyl pentadecanoate | R, S, L | s |
| 97 | 23.02 | C22H36O4 | 364.2601 | 364.2614 | −3.7 | 365.2665 [M + H]+, 266.2190 [M + H − C6H11O]+, 219.1749 [M + H − H2O − C2H3O2 − C5H9O]+ | Vitetrifolin E | S | 85 |
| 98 | 24.29 | C12H16O2 | 192.1159 | 192.1150 | 4.2 | 215.1052 [M + Na]+, 164.0893 [M + H − CH3]+, 136.0587 [M + H − C4H9]+, 78.0571 [M + H − C6H11O2]+ | Isopentyl benzoate | R | s |
| 99 | 24.31 | C11H14O2 | 178.1003 | 178.0994 | 4.7 | 201.0895 [M + Na]+, 147.0441 [M + H − CH3]+, 136.0491 [M + H − C2H3O]+, 122.0477 [M + H − C3H5O]+, 77.0146 [M + H − CH3O − C4H7O]+ | Anisylacetone | R | 86 |
| 100# | 24.51 | C30H52O3 | 460.3898 | 460.3917 | −4.1 | 483.3682 [M + Na]+, 425.3325 [M + H − 2 × H2O]+, 336.2443 [M + H − C9H17]+, 295.0357 [M + H − C12H22]+ | Neopanaxadiol | S | 87 |
| 101 | 24.71 | C30H48O2 | 440.3656 | 440.3654 | 0.3 | 441.3729 [M + H]+, 423.3611 [M + H − H2O]+, 259.1687 [M + H − C11H18O2]+, 219.0312 [M + H − C14H22O2]+ | Daturanolone | R, S | 88 |
| 102 | 24.94 | C30H48O | 424.3701 | 424.3705 | −1.0 | 425.3774 [M + H]+, 407.3633 [M + H − H2O]+, 392.3459 [M + H − H2O − CH3]+, 218.1926 [M + H − C14H23O]+ | Olean-9(11),12-dien-3β-ol | R, S | s |
| 103* | 25.48 | C21H24O4 | 340.1672 | 340.1675 | −0.8 | 363.1573 [M + Na]+, 189.0711 [M + H − CH3 − C8H9O2]+, 173.0939 [M + H − CH3O − C8H9O2]+, 107.0494 [M + H − CH3O − C13H15O2]+ | Dehydrodiisoeugenol methyl ether | R | 89 |
| 104 | 25.67 | C30H46O4 | 470.3372 | 470.3396 | −4.1 | 471.3445 [M + H]+, 456.2016 [M + H − CH3]+, 398.5107 [M + H − C3H5O2]+, 379.0317 [M + H − 2 × HCOOH]+, 312.2370 [M + H − H2O − C8H13O2]+, 234.4581 [M + H − CH3 − C3H5O2 − C8H13O2]+ | Nigranoic acid | R | a |
| 105 | 25.70 | C37H42O13 | 694.2801 | 694.2778 | 3.6 | 695.2873 [M + H]+, 515.2201 [M + H − 3 × C2H4O2]+, 393.2228 [M + H − 3 × C2H4O2 − C7H6O2]+, 331.0167 [M + H − 2 × C2H4O2 − C7H6O2 − C7H5O2]+ | Celahin D | S, L | s |
| 106 | 25.91 | C28H48O | 400.3698 | 400.3705 | −1.6 | 423.3591 [M + Na]+, 386.2638 [M + H − CH3]+, 326.2823 [M + H − H2O − CH3 − C3H7]+, 254.2266 [M + H − H2O − C8H17O]+, 213.2252 [M + H − H2O − C11H22O]+ | Fungisterol | R, S | a |
| 107 | 25.93 | C27H44O | 384.3390 | 384.3392 | −0.6 | 385.3463 [M + H]+, 367.0417 [M + H − H2O]+, 256.2547 [M + H − H2O − C8H15]+, 215.2415 [M + H − H2O − C11H20]+ | (E)-22-Dehydrocholesterol | R | 90 |
| 108 | 25.96 | C29H48O | 412.3686 | 412.3705 | −4.6 | 413.3746 [M + H]+, 260.2204 [M + H − CH3 − C10H19]+, 257.2151 [M + H − H2O − C10H19]+ | 28-Isofucosterol | R, S | a |
| 109 | 26.22 | C30H48O3 | 456.3588 | 456.3604 | −3.5 | 457.3651 [M + H]+, 439.2142 [M + H − H2O]+, 411.3584 [M + H − HCOOH]+, 265.1812 [M + H − C13H20O]+ | Maytenoic acid | R | s |
| 110 | 26.28 | C29H48O | 412.3702 | 412.3705 | −0.8 | 413.3775 [M + H]+, 395.3676 [M + H − H2O]+, 256.2275 [M + H − H2O − C10H19]+, 218.7218 [M + H − CH3 − C13H24]+ | Chondryllasterol | R | 91 |
| 111 | 26.31 | C18H36O2 | 284.2724 | 284.2715 | 2.7 | 307.2616 [M + Na]+, 140.1312 [M + H − 2 × CH3 − C6H11O2]+, 70.0583 [M + H − 2 × CH3 − C11H21O2]+ | Hexadecanoic acid | R | s |
| 112 | 26.62 | C30H48O | 424.3686 | 424.3705 | −4.4 | 425.3747 [M + H]+, 410.3584 [M + H − CH3]+, 367.3099 [M + H − CH3 − C3H7]+, 273.3120 [M + H − C11H20]+ | Fernenone | R, L | 92 |
| 113 | 26.83 | C30H48O2 | 440.3656 | 440.3654 | 0.3 | 441.3729 [M + H]+, 423.3611 [M + H − H2O]+, 412.0147 [M + H − CHO]+, 233.1890 [M + H − C14H24O]+, 204.3167 [M + H − CHO − C14H24O]+ | Oleanolic aldehyde | R, S, L | a |
| 114 | 27.08 | C28H48O2 | 416.3667 | 416.3654 | 2.9 | 439.3559 [M + Na]+, 192.1212 [M + H − C16H33]+, 151.0731 [M + H − C19H38]+ | Cumotocopherol | R | 93 |
| 115 | 27.25 | C24H38O4 | 390.2752 | 390.2770 | −4.3 | 413.2644 [M + Na]+, 179.0809 [M + H − C2H5 − C4H9 − C8H17O]+, 149.1469 [M + H − C8H17 − C8H17O]+, 77.0216 [M + H − 2 × C9H17O2]+ | Fleximel | R, S, L | b |
| 116 | 27.52 | C30H48O | 424.3709 | 424.3705 | 0.9 | 425.3782 [M + H]+, 410.3572 [M + H − CH3]+, 259.2141 [M + H − C11H18O]+, 221.1939 [M + H − C14H20O]+ | β-Amyron | R, S, L | 94 |
| 117※ | 27.84 | C36H44O9 | 620.3003 | 620.2985 | 2.9 | 621.3083 [M + H]+, 562.2851 [M + H − C2H3O2]+, 461.2303 [M + H − C2H3O2 − C5H9O2]+, 291.2088 [M + H − C2H5 − C2H3O2 − 2 × C7H5O2]+ | Celafolin D-3 | S, L | s |
| 118 | 27.88 | C30H48O2 | 440.3656 | 440.3654 | 0.3 | 441.3729 [M + H]+, 423.3611 [M + H − H2O]+, 398.5359 [M + H − C3H7]+, 288.2288 [M + H − C11H21]+, 247.0117 [M + H − C14H26]+ | Polasterol A | R, S, L | a |
| 119 | 28.10 | C29H46O | 410.2535 | 410.2549 | −3.4 | 411.3599 [M + H]+, 393.3696 [M + H − H2O]+, 272.2261 [M + H − C10H19]+, 231.2084 [M + H − C13H24]+ | Corbisterin | R, L | 95 |
| 120 | 28.49 | C30H50O2 | 442.3813 | 442.3811 | 0.5 | 465.3705 [M + Na]+, 425.3647 [M + H − H2O]+, 291.3580 [M + H − C10H16O]+, 251.5133 [M + H − C13H20O]+ | 3-Oxo-11β-hydroxyfriedelane | S | 96 |
Although the study provided evidences to elucidate the chemical composition of COT, there were still some unresolved issues. For example, as shown in BPI chromatograms, there were some unidentified components. Further research should be carried on the identification of these unknown compounds.
3.2. Metabolomics analysis of three parts of COT
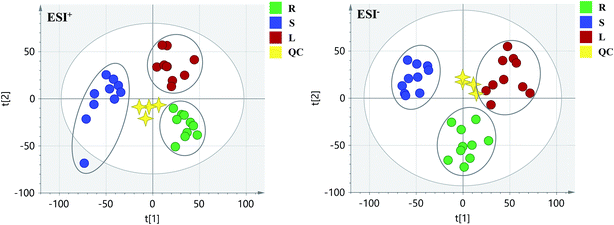
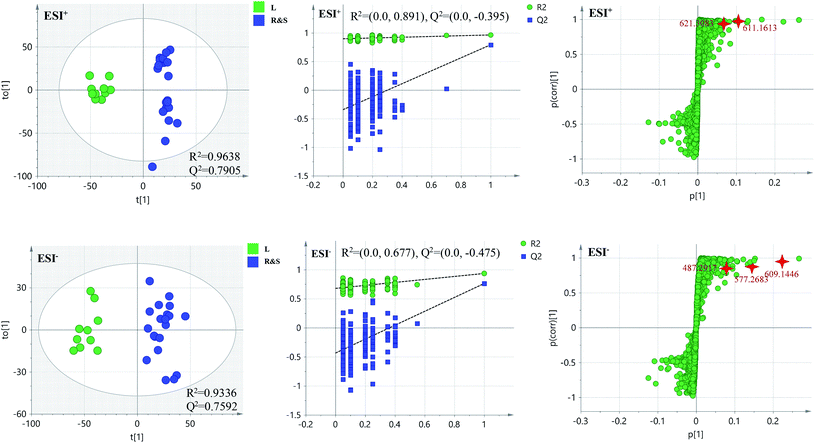
PCA score 2D plots in both ESI+ and ESI− were established as shown in Fig. 4. The QC samples were clustered tightly and were in the middle of the three groups in PCA, which indicated the system had satisfactory stability. The samples from root of COT were clearly gathered together, which indicated there was a good similarity among them, and this phenomenon was also observed in stem and leaf of COT. Meanwhile, the root, stem and leaf groups were easily divided into three clusters, indicating that these three parts of COT could be differentiated in both ESI+ and ESI−.In order to further distinguish one part from the other two parts, OPLS-DA plots, S-plots, permutation tests, and VIP values were obtained to see which variables were responsible for sample separation97 (Fig. 5–7). In OPLS-DA plots, each spot represented a sample. From the perspective of OPLS-DA, one part was clearly separated from the other two parts. The parameters such as R2 and Q2 indicated the model had good ability of prediction and reliability in both ESI+ and ESI− modes. The permutation plots showed the original point on the right was clearly higher than all Q2-values (blue) on the left, which indicated the original models were valid. To identify the metabolites contributing to the discrimination, S-plots were generated under OPLS-DA model. Each spot in S-plots represented a variable. The variables with VIP > 4 and p < 0.001 were considered as potential chemical markers. The possible molecular formula of the markers were calculated by high-accuracy quasi-molecular ion with mass error between ±5 ppm. A total of 26 robust known chemical markers (marked in Table 2) enabling the differentiation between one part with the other two parts were identified and marked in S-plots.
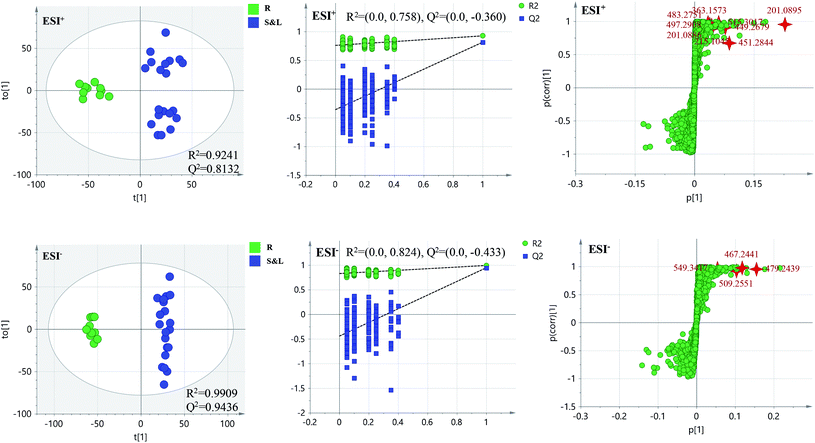 | ||
| Fig. 5 OPLS-DA plots/permutation tests/S-plots between R and S&L. R: the root of Celastrus orbiculatus Thunb.; S: the stem of Celastrus orbiculatus Thunb.; L: the leaf of Celastrus orbiculatus Thunb. | ||
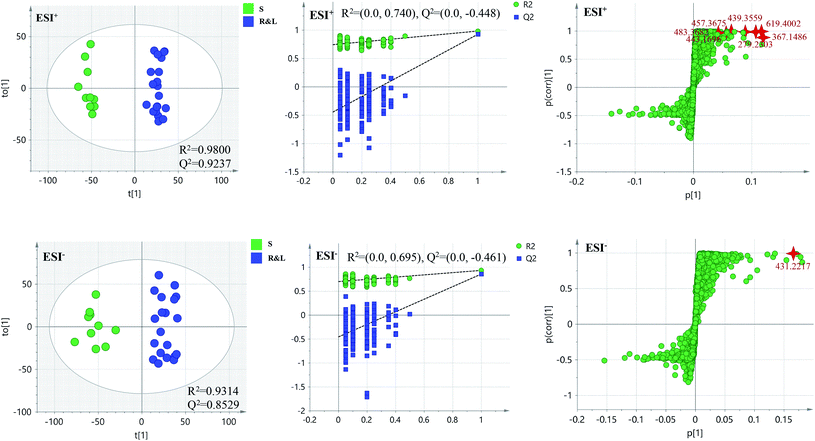 | ||
| Fig. 6 OPLS-DA plots/permutation tests/S-plots between S and R&L. R: the root of Celastrus orbiculatus Thunb.; S: the stem of Celastrus orbiculatus Thunb.; L: the leaf of Celastrus orbiculatus Thunb. | ||
 | ||
| Fig. 7 OPLS-DA plots/permutation tests/S-plots between L and R&S. R: the root of Celastrus orbiculatus Thunb.; S: the stem of Celastrus orbiculatus Thunb.; L: the leaf of Celastrus orbiculatus Thunb. | ||
According to the reference, it was revealed that there was significant variation for the contents of celastrol or total alkaloids in different parts of COT. While in this study, there were 13, 8 and 5 potential chemical markers including celastrol discovered from root, stem and leaf, respectively. The markers in root including 8 triterpenoids (35, 39, 50, 51, 63, 80, 88, 90), 1 steroids (57), 1 organic acid esters (87), 1 phenylpropanoids (103) and 2 other compounds (73, 86). The markers in stem including 4 triterpenoids (64, 84, 85, 100), 1 flavonoids (2), 1 phenylpropanoids (30), 1 steroids (52) and 1 organic acid esters (61). The markers in leaf including 3 sesquiterpenoids (11, 40, 117), 1 flavonoids (14) and 1 triterpenoids (54). Additionally, among these potential chemical markers, the contents of 57 and 88 in root, 52 in stem, 40, 54 and 117 in leaf were much higher than in the other two parts (p < 0.001). While components 35, 39, 50, 51, 63, 73, 80, 86, 87, 90 and 103 were detected only in root, components 2, 30, 61, 64, 84, 85 and 100 were detected only in stem, components 11 and 14 were detected only in leaf part under the detect condition.
The detected result of 88 (celastrol) in our study, with much higher contents in root than in stem and leaf, was consistent with the ref. 19. While there were a few differences between our results and the references. In the present study, compound 39 (pristimerin) was only detected in root, and 11 (orbiculin I) was only detected in leaf. According to the reports, 39 was once isolated from stem14 though mainly from root,98,99 and 11 isolated from root.3 The reason was the concentrations of them were lower than the lowest detection limits. It was worth mentioning that some chemical markers with high responses in UPLC-MS, two triterpenoids (39 and 88) in root, one flavonoids (2) in stem and two sesquiterpenoids (11 and 40) in leaf, could be used for further quality control of three parts of COT respectively.
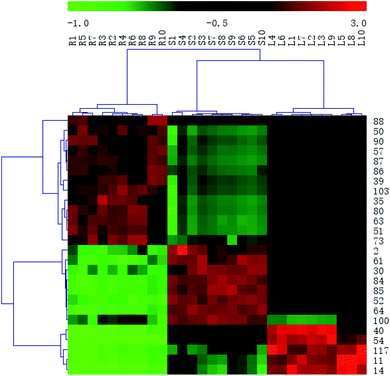
In order to systematically evaluate the chemical markers, a heat-map was generated. The hierarchical clustering heat map, intuitively visualizing the difference level of potential chemical markers in different parts, was shown in Fig. 8. The higher values were indicated by red squares, the lower values were indicated by green squares.
3.3. Bioactivity evaluation
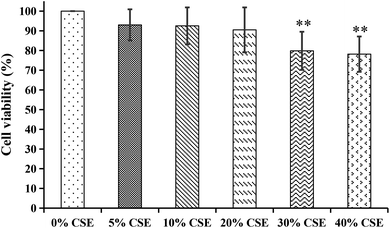 | ||
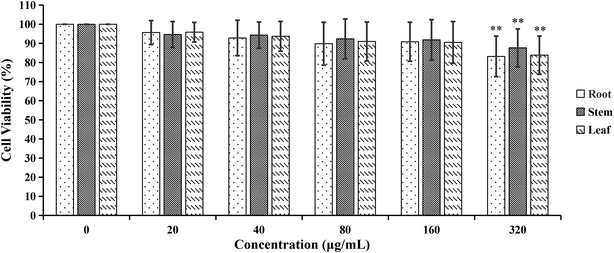
| Fig. 9 The cytotoxicity effects of different concentrations of cigarette smoke extract (CSE) on A549 cells. **p < 0.01, compared with 0% CSE group. | ||
4. Conclusions
In conclusion, for screening analysis, a total of 120 compounds (15 shared components), including 92 from root, 56 from stem and 32 from leaf, were identified or tentatively characterized from COT. Each part of COT was rich in various kinds of structures, especially triterpeniods held the majority. For metabolomic analysis, the root, stem and leaf of COT were differentiated in both ESI+ and ESI− modes. There were 13, 8 and 5 potential chemical markers identified from root, stem and leaf, respectively. Among the above robust markers, 5 robust chemical markers with high responses in UPLC-MS, 2 triterpenoids (pristimerin and celastrol) in root, 1 flavonoids {5,7-dihydroxy-6,8-dimethyl-3(S)-3-(3-methoxy-4′-hydroxybenzyl) chroman-4-one} in stem and 2 sesquiterpenoids (orbiculin I and orbiculin A) in leaf, could be used for further quality control in three parts of COT respectively. For bioassay analysis, the root, stem and leaf of COT could evidently reduce the levels of pro-inflammatory factors in a dose-dependent way within a certain range of 20–160 μg mL−1 in CSE-induced A549 cells. The results showed that the stem part had a stronger anti-COPD effect than root and leaf parts. The different activities might be caused by the various phytochemicals in these three parts of COT. This comprehensive phytochemical study revealed both the structural diversity of secondary metabolites and the different distributions in different parts of COT. It could provide a theoretical basis for further utilization and development of COT. And the identification of anti-COPD components from COT will be explored deeply in the future based on the current results of this study.Conflicts of interest
The authors declare no conflicts of interests.References
- Y. Zhang, Y. H. Si, L. Zhai, S. D. Guo, J. L. Zhao, H. Sang, X. F. Pang, X. Zhang, A. B. Chen and S. C. Qin, Celastrus orbiculatus Thunb. reduces lipid accumulation by promoting reverse cholesterol transport in hyperlipidemic mice, Lipids, 2016, 51, 677–692 CrossRef CAS PubMed.
- M. R. Wang, Research progress of a Chinese herb Celastrus on anti-tumor effect, J. Chin. Med., 2010, 6, 1055–1057 Search PubMed.
- H. Z. Jin, B. Y. Hwang, H. S. Kim, J. H. Lee, Y. H. Kim and J. J. Lee, Antiinflammatory constituents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production, J. Nat. Prod., 2002, 65, 89–91 CrossRef CAS PubMed.
- D. Q. Hou, N. Bai, Z. C Wei, B. Li, G. X. Tang and Y. F. Gao, Celastrus orbiculatus Celastraceae Thunb extracts inhibit proliferation and migration of oral squamous cell carcinoma cells by blocking NF-κB pathway, Trop. J. Pharm. Res., 2019, 18, 1259–1264 CAS.
- Z. Q. Liu, R. X. Zhou and M. Q. Wu, Study on antiviral components of Celastrus orbiculatus Thunb., Acta Acad. Med. Jiangxi, 1983, 1–5 Search PubMed.
- X. G. Tao, Bacteriostatic effect of Celastrus root skin extraction fluid on Staphylococcus aureus, Hunan Agric. Univ., 2012 Search PubMed.
- H. Y. Ni, Z. H. Zhang, W. J. Song and L. F. Guo, Chemical constituents of root bark of Celastrus orbiculatus Thunb., Chin. Pharmaceut. J., 2014, 49, 21 Search PubMed.
- Y. Jiang, H. Li and S. Q. Luo, Study on the chemical constituents of Celastrus orbiculatus Thunb., Chin. Tradit. Herb. Drugs, 1996, 27, 73–89 CAS.
- J. Wu, 1. Preparation and biological activity of cucurbitacin derivatives. 2. Study on the chemical constituents and biological activities of the root bark of Celastrus orbiculatus Thunb., Dissertation, University of Chinese Academy of Sciences, 2011.
- Y. D. Zhu, L. Liu, L. Hu, W. Q. Dong, M. Zhang, Y. Q. Liu and P. Li, Effect of Celastrus orbiculatus in inhibiting Helicobacter pylori induced inflammatory response by regulating epithelial mesenchymal transition and targeting miR-21/PDCD4 signaling pathway in gastric epithelial cells, BMC Complementary Altern. Med., 2019, 19, 91 CrossRef PubMed.
- H. B. Wang, L. D. Tao, T. Y. Ni, H. Gu, F. Jin, X. J. Dai, J. Feng, Y. B. Ding, W. M. Xiao, S. Y. Guo, T. D. S. Hisamitsud, Y. Y. Qian and Y. Q. Liu, Anticancer efficacy of the ethyl acetate extract from the traditional Chinese medicine herb Celastrus orbiculatus against human gastric cancer, J. Ethnopharmacol., 2017, 205, 147–157 CrossRef CAS PubMed.
- Y. Zhang, Y. H. Si, L. Zhai, N. N. Yang, S. T. Yao, H. Sang, D. D. Zu, X. Xu, S. C. Qin and J. H. Wang, Celastrus orbiculatus Thunb. ameliorates high-fat diet-induced non-alcoholic fatty liver disease in guinea pigs, Pharmazie, 2013, 68, 850–854 CAS.
- B. Y. Hwang, H. S. Kim, J. H. Lee, Y. S. Hong, J. S. Ro, K. S. Lee and J. J. Lee, Antioxidant benzoylated flavan-3-ol glycoside from Celastrus orbiculatus, J. Nat. Prod., 2001, 64, 82–84 CrossRef CAS PubMed.
- J. Li, Discovery of natural products with lipid-lowering activity and their mechanism of action, Dissertation, University of Chinese Academy of Sciences, 2018.
- J. J. Li, J. Yang, F. Lu, Y. T. Qi, Y. Q. Liu, Y. Sun and Q. Wang, Chemical constituents from the stems of Celastrus orbiculatus, Chin. J. Nat. Med., 2012, 10, 279–283 CrossRef CAS.
- X. Q. Chen, K. Zan and Q. Wang, Olean-type triterpenes of Celastrus orbiculatus, Biochem. Syst. Ecol., 2012, 44, 338–340 CrossRef CAS.
- Y. W. Liu, Efficacy test of crude ethanol extracts of Celastrus orbiculatus Thunb. and Ginkgo biloba leaves on Agrolimax agrestis Linnaeus, J. Anhui Agric. Sci., 2011, 39, 19176–19185 Search PubMed.
- X. X. Yu, T. T. Zhang and D. Y. Wang, Chemical constituents in hypoglycemic active fraction of Celastrus orbiculatus leaf, J. Chin. Med. Mater., 2014, 6, 998–1000 Search PubMed.
- Y. Zhang, Y. J. Cui, S. D. Guo, N. N. Yang, Y. H. Si and S. C. Qin, Using response surface methodology for optimization of extraction process followed by capillary zone electrophoresis for determination of celastrol in Celastrus orbiculatus Thunb, Chin. J. Hosp. Pharm., 2014, 34, 704–709 CAS.
- Y. H. Zhang, L. H. Yang and C. M. Li, Determination of general alkaloids from Celastrus orbiculatus Thunb. in Guilin area, Acad. J. Second Mil. Med. Univ., 2004, 25, 1012 Search PubMed.
- C. Z. Wang, N. Q. Zhang, Z. Z. Wang, Z. Qi, B. Z. Zheng, P. Y. Li and J. P. Liu, Rapid characterization of chemical constituents of Platycodon grandiflorum and its adulterant Adenophora stricta by UPLC-QTOF-MS/MS, J. Mass Spectrom., 2017, 52, 643–656 CrossRef CAS.
- H. Q. Lin, H. L. Zhu, J. Tan, C. Z. Wang, Q. H. Dong, F. L. Wu, H. Wang, J. L. Liu, P. Y. Li and J. P. Liu, Comprehensive investigation on metabolites of wild-simulated american Ginseng root based on ultra-high performance liquid chromatography-quadrupole time-of-flight mass spectrometry, J. Agric. Food Chem., 2019, 67, 5801–5819 CAS.
- W. S. Tang, H. Y. Peh, W. P. Liao, C. H. Pang, T. K. Chan, S. H. Lau, V. T. Chow and W. S. Wong, Cigarette smoke-induced lung disease predisposes to more severe infection with nontypeable haemophilus influenzae: protective effects of andrographolide, J. Nat. Prod., 2016, 79, 1308–1315 CrossRef.
- L. M. Fabbri, F. Luppi, B. Beghe and K. F. Rabe, Update in chronic obstructive pulmonary disease 2005, Am. J. Respir. Cell Mol. Biol., 2006, 175, 1056–1065 Search PubMed.
- X. Liang, J. Wang, R. J. Guan, L. Zhao, D. F. Li, Z. Long, Q. Yang, J. Y. Xu, Z. Y. Wang, J. K. Xie and W. J. Lu, Limax extract ameliorates cigarette smoke-induced chronic obstructive pulmonary disease in mice, Int. J. Immunopharmacol., 2018, 54, 210–220 CrossRef CAS PubMed.
- H. Q. Lin, H. L. Zhu, J. Tan, H. Wang, Q. H. Dong, F. L. Wu, Y. H. Liu, P. Y. Li and J. P. Liu, Non-targeted metabolomic analysis of methanolic extracts of wild-simulated and field-grown American Ginseng, Molecules, 2019, 24, 1053 CrossRef CAS PubMed.
- Y. R. Wang, C. Z. Wang, H. Q. Lin, Y. H. Liu, Y. M. Li, Y. Zhao, P. Y. Li and J. P. Liu, Discovery of the potential biomarkers for discrimination between Hedyotis diffusa and Hedyotis corymbosa by UPLC-QTOF/MS metabolome analysis, Molecules, 2018, 23, 1525 CrossRef PubMed.
- H. L. Zhu, H. Q. Lin, J. Tan, C. Z. Wang, H. Wang, F. L. Wu, Q. H. Dong, Y. H. Liu, P. Y. Li and J. P. Liu, UPLC-QTOF/MS-based nontargeted metabolomic analysis of mountain- and garden-cultivated Ginseng of different ages in northeast China, Molecules, 2019, 24, 33 CrossRef PubMed.
- H. Q. Lin, H. L. Zhu, J. Tan, H. Wang, Z. Y. Wang, P. Y. Li, C. F. Zhao and J. P. Liu, Comparative analysis of chemical constituents of Moringa oleifera leaves from China and India by ultra-performance liquid chromatography coupled with quadrupole-time-of-flight mass spectrometry, Molecules, 2019, 24, 942 CrossRef.
- C. Z. Wang, N. Q. Zhang, Z. Z. Wang, Z. Qi, H. L. Zhu, B. Z. Zheng, P. Y. Li and J. P. Liu, Nontargeted metabolomic analysis of four different parts of Platycodon grandiflorum grown in northeast China, Molecules, 2017, 22, 1280 CrossRef PubMed.
- Z. J. Zou, Z. H. Liu, M. J. Gong, B. Han, S. M. Wang and S. W. Liang, Intervention effects of puerarin on blood stasis in rats revealed by a 1H-NMR-based metabonomic approach, Phytomedicine, 2015, 22, 333–343 CrossRef CAS PubMed.
- L. Chen, Q. Ge, G. Tjin, H. Alkhouri, L. H. Deng, C. A. Brandsma, I. Adcock, W. Timens, D. Postma, J. K. Burgess, J. L. Black and B. G. G. Oliver, Effects of cigarette smoke extract on human airway smooth muscle cells in COPD, Eur. Respir. J., 2014, 44, 634–646 CrossRef CAS PubMed.
- R. S. Coleman, S. R. Gurrala, S. Mitra and A. Raao, Asymmetric total synthesis of dibenzocyclooctadiene lignan natural products, J. Org. Chem., 2005, 70, 8932 CrossRef CAS PubMed.
- H. M. Hua, H. Q. Yin, B. Q. Li and Y. H. Pei, Study on the constituents of the bark of Eucommia ulmoides, Mol. Plant Breed., 2003, 1, 801–803 CAS.
- Z. G. Zheng, R. S. Wang, H. Q. Cheng, T. T. Duan, B. He, D. Tang, F. Gu and Q. Zhu, Isolated perfused lung extraction and HPLC-ESI-MS(n) analysis for predicting bioactive components of Saposhnikoviae Radix, J. Pharm. Biomed. Anal., 2011, 54, 614–618 CrossRef CAS PubMed.
- C. Masuoka, M. Ono, Y. Ito and T. Nohara, Antioxidative, antihyaluronidase and antityrosinase activities of some constituents from the aerial part of Piper elongatum VAHL, Food Sci. Technol. Res., 2003, 9, 197–201 CrossRef CAS.
- S. Sato, J. Takeo, C. Aoyama and H. Kawahara, Na+-glucose cotransporter (SGLT) inhibitory flavonoids from the roots of Sophora flavescens, Bioorg. Med. Chem., 2007, 15, 3445–3449 CrossRef CAS PubMed.
- Y. N. Lin, D. N. Zhu, J. Qi, M. J. Qin and B. Y. Yu, Characterization of homoisoflavonoids in different cultivation regions of Ophiopogon japonicus and related antioxidant activity, J. Pharm. Biomed. Anal., 2010, 52, 757–762 CrossRef CAS PubMed.
- S. Schwaiger, C. Seger, B. Wiesbauer, P. Schneider, E. P. Ellmerer, S. Sturm and H. Stuppner, Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium Cass.) constituents, Phytochem. Anal., 2006, 17, 291–298 CrossRef CAS PubMed.
- M. J. Chang, T. M. Hung, B. S. Min, J. C. Kim, M. H. Woo, J. S. Choi, H. K. Lee and K. Bae, Lignans from the fruits of Forsythia suspensa (Thunb.) Vahl protect high-density lipoprotein during oxidative stress, J. Agric. Chem. Soc. Jpn., 2008, 72, 2750–2755 CAS.
- S. I. Falcao, M. Vilas-Boas, L. M. Estevinho, C. Barros, M. Domingues and S. M. Cardoso, Phenolic characterization of northeast Portuguese propolis: usual and unusual compounds, Anal. Bioanal. Chem., 2010, 396, 887–897 CrossRef CAS PubMed.
- V. Kumar, V. Karunaratne, M. R. Meegalle and K. Sanath, 1-[2′,4′-dihydroxy-3′,5′-di-(3′′-methylbut-2′′-enyl)-6′-methoxy] phenylethanone from Acronychia pedunculata, root bark, Phytochemistry, 1989, 28, 1278–1279 CrossRef CAS.
- T. Matsumoto, K. Hosono-Nishiyama and H. Yamada, Antiproliferative and apoptotic effects of butyrolactone lignans from Arctium lappa on leukemic cells, Planta Med., 2005, 72, 276–278 CrossRef PubMed.
- T. J. Schmidt, S. Hemmati, E. Fuss and A. W. Alfermann, A combined HPLC-UV and HPLC-MS method for the identification of lignans and its application to the lignans of Linum usitatissimum L. and L. bienne Mill, Phytochem. Anal., 2006, 17, 299–311 CrossRef CAS PubMed.
- S. Yin, C. Q. Fan, Y. Wang, L. Dong and J. M. Yue, Antibacterial prenylflavone derivatives from Psoralea corylifolia, and their structure-activity relationship study, Bioorg. Med. Chem., 2004, 12, 4387–4392 CrossRef CAS PubMed.
- H. L. Jiang, A. Somogyi, N. E. Jacobsen, B. N. Timmermann and D. R. Gang, Analysis of curcuminoids by positive and negative electrospray ionization and tandem mass spectrometry, Rapid Commun. Mass Spectrom., 2006, 20, 1001–1012 CrossRef CAS PubMed.
- Q. H. Ye, W. M. Zhao and G. W. Qin, Lignans from Dendrobium chrysanthum, J. Asian Nat. Prod. Res., 2004, 6, 39–43 CrossRef CAS PubMed.
- X. Y. Guan, H. F. Li, W. Z. Yang, C. H. Lin, C. Sun, B. R. Wang, D. A. Guo and M. Ye, HPLC-DAD-MSn analysis and HPLC quantitation of chemical constituents in Xian-ling-gu-bao capsules, J. Pharm. Biomed. Anal., 2011, 55, 923 CrossRef CAS PubMed.
- H. C. Beatriz, C. P. Miriam, V. C. Claudia, L. O. Jesus F, A. G. Agustin and J. N. Pedro, New saponins from Sechium mexicanum, Magn. Reson. Chem., 2009, 11, 994–1003 Search PubMed.
- S. J. Xu, L. Yang, X. Zeng, M. Zhang and Z. T. Wang, Characterization of compounds in the Chinese herbal drug Mu-Dan-Pi by liquid chromatography coupled to electrospray ionization mass spectrometry, Rapid Commun. Mass Spectrom., 2010, 20, 3275–3288 CrossRef PubMed.
- Y. Y. Zhao, X. L. Cheng, Y. M. Zhang, Y. Zhao, R. C. Lin and W. J. Sun, Simultaneous determination of eight major steroids from Polyporus umbellatus by high-performance liquid chromatography coupled with mass spectrometry detections, Biomed. Chromatogr., 2010, 24, 222–230 CrossRef CAS PubMed.
- C. Niampoka, R. Suttisri, R. Bavovada, H. Takayama and N. Aimi, Potentially cytotoxic triterpenoids from the root bark of Siphonodon celastrineus Griff, Arch. Pharmacal Res., 2005, 28, 546–549 CrossRef CAS PubMed.
- L. Y. Wang, N. L. Wang, X. S. Yao, S. Miyata and S. Kitanaka, Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus, J. Nat. Prod., 2003, 66, 630–633 CrossRef CAS PubMed.
- X. Li, S. H. Li, J. X. Pu, S. X. Huang and H. D. Sun, Chemical constituents from Paeonia anomala subsp.veitchii (Paeoniaceae), Acta Bot. Yunnanica, 2007, 29, 259–262 CrossRef CAS PubMed.
- C. F. Hossain, Y. P. Kim, S. R. Baerson, L. Zhang, R. K. Bruick, K. A. Mohammed, A. K. Agarwal, D. G. Nagle and Y. D Zhou, Saururus cernuus lignans-potent small molecule inhibitors of hypoxia-inducible factor-1, Biochem. Biophys. Res. Commun., 2005, 333, 1026–1033 CrossRef CAS PubMed.
- F. M. Darwish and M. G. Reinecke, Ecdysteroids and other constituents from Sida spinosa L., Phytochemistry, 2003, 62, 1179–1184 CrossRef CAS PubMed.
- N. Sanchez-Avila, F. Priego-Capote, J. Ruiz-Jimenez and M. D. Luque de Castro, Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction, Talanta, 2009, 78, 0–48 CAS.
- M. A. Alamsjah, S. Hirao, F. Ishibashi and Y. Fujita, Isolation and structure determination of algicidal compounds from Ulva fasciata, J. Agric. Chem. Soc. Jpn., 2005, 69, 2186–2192 CAS.
- T. Sugiura, S. Nakane, S. Kishimoto, K. Waku, Y. Yoshioka and A. Tokumura, Lysophosphatidic acid, a growth factor-like lipid, in the saliva, J. Lipid Res., 2002, 43, 2049–2055 CrossRef CAS PubMed.
- C. R. Cheng, M. Yang, Z. Y. Wu, Y. Wang, F. Zeng, W. Y. Wu, S. H. Guan and D. A. Guo, Fragmentation pathways of oxygenated tetracyclic triterpenoids and their application in the qualitative analysis of Ganoderma lucidum by multistage tandem mass spectrometry, Rapid Commun. Mass Spectrom., 2011, 25, 1323–1335 CrossRef CAS PubMed.
- R. Bortolomeazzi, G. Verardo, A. Liessi and A. Callea, Formation of dehydrodiisoeugenol and dehydrodieugenol from the reaction of isoeugenol and eugenol with DPPH radical and their role in the radical scavenging activity, Food Chem., 2009, 118, 256–265 CrossRef.
- M. B. Gallo, W. C. Rocha, U. S. da Cunha, F. A. Diogo, F. C. da Silva, P. C. Vieira, J. D. Vendramim, J. B. Fernandes, M. F. da Silva and L. G. Batista-Pereira, Bioactivity of extracts and isolated compounds from Vitex polygama (Verbenaceae) and Siphoneugena densiflora (Myrtaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae), Pest Manage. Sci., 2006, 62, 1072–1081 CrossRef CAS PubMed.
- K. Nagy, C. Courtet, C. Marie, B. Holst and M. Kussmann, Comprehensive analysis of vitamin E constituents in human plasma by liquid chromatography-mass spectrometry, Anal. Chem., 2007, 79, 7087–7096 CrossRef CAS PubMed.
- L. Y. Wang, N. L. Wang, X. S. Yao, S. Miyata and S. Kitanaka, Euphane and Tirucallane Triterpenes from the Roots of Euphorbia kansui and their in vitro Effects on the Cell Division of Xenopus, J. Nat. Prod., 2003, 66, 630–633 CrossRef CAS PubMed.
- H. Simone and V. Walter, Exploring the fatty acids of vernix caseosa in form of their methyl esters by off-line coupling of non-aqueous reversed phase high performance liquid chromatography and gas chromatography coupled to mass spectrometry, J. Chromatogr. A, 2010, 52, 8270–8278 Search PubMed.
- C. Pettinella, S. H. Lee, F. Cipollone and I. A. Blair, Targeted quantitative analysis of fatty acids in atherosclerotic plaques by high sensitivity liquid chromatography/tandem mass spectrometry, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 850, 168–176 CrossRef CAS PubMed.
- Y. L. Lin and Y. H. Kuo, Four new neoclerodane-type diterpenoids, scutellones B, G, H, and I, from aerial parts of Scutellaria rivularis, Chem. Pharm. Bull., 1989, 37, 582–585 CrossRef CAS.
- X. A. Wen, J. Liu, L. Y. Zhang, P. Z. Ni and H. B. Sun, Synthesis and biological evaluation of arjunolic Acid, bayogenin, hederagonic acid and 4-epi-hederagonic acid as glycogen phosphorylase inhibitors, Chin. J. Nat. Med., 2010, 8, 441–448 CrossRef CAS.
- R. Tundis, B. Deguin, F. Menichini and F. Tillequin, Iridoids from Putoria calabrica, Biochem. Syst. Ecol., 2002, 30, 689–691 CrossRef CAS.
- X. Li, P. Gao, B. Gjetvaj, N. Westcott and M. Y. Gruber, Analysis of the metabolome and transcriptome of Brassica carinata seedlings after lithium chloride exposure, Plant Sci., 2009, 177, 68–80 CrossRef CAS.
- K. Kuroda and A. Nakagawaizumi, Analytical pyrolysis of lignin: products stemming from β-5 substructures, Org. Geochem., 2006, 37, 665–673 CrossRef CAS.
- S. E. Ayyad, S. Z. Sowellim, M. S. El-Hosini and A. Abo-Atia, The structural determination of a new steroidal metabolite from the brown alga Sargassum asperifolium, Z. Naturforsch., C: J. Biosci., 2003, 58, 333–336 CAS.
- H. W. Seo, T. M. Hung, M. K. Na, H. J. Jung, J. C. Kim, J. S. Choi, J. H. Kim, H. K. Lee, I. S. Lee, K. H. Bae, M. S. Hattori and B. S. Min, Steroids and triterpenes from the fruit bodies of Ganoderma lucidum and their anti-complement activity, Arch. Pharmacal Res., 2009, 32, 1573–1579 CrossRef CAS PubMed.
- T. H. Lee, J. L. Chiou, C. K. Lee and Y. H. Kuo, Separation and determination of chemical constituents in the roots of Rhus Javanica L. Var. Roxburghiana, J. Chin. Chem. Soc., 2005, 52, 833–841 CrossRef CAS.
- K. Iwatsuki, T. Akihisa, H. Tokuda, M. Ukiya, M. Oshikubo, Y. Kimura, T. Asano, A. Nomura and H. Nishino, Lucidenic acids P and Q, methyl lucidenate P, and other triterpenoids from the fungus Ganoderma lucidum and their inhibitory effects on Epstein-Barr virus activation, J. Nat. Prod., 2003, 66, 1582 CrossRef CAS PubMed.
- C. A. Luo, W. N. Zhang, C. Q. Sheng, C. J. Zheng, J. Z. Yao and Z. Y. Miao, Chemical composition and antidiabetic activity of Opuntia Milpa Alta extracts, Chem. Biodiversity, 2010, 7, 2869–2879 CrossRef CAS PubMed.
- B. S. Siddiqui, U. Ghani, S. T. Ali, S. B. Usmani and S. Begum, Triterpenoidal constituents of the leaves of Carissa carandas, Nat. Prod. Lett., 2003, 17, 153 CrossRef CAS PubMed.
- L. Liu, Y. Cheng and H. Zhang, Phytochemical Analysis of Anti-atherogenic Constituents of Xue-Fu-Zhu-Yu-Tang using HPLC-DAD-ESI-MS, Chem. Pharm. Bull., 2004, 52, 1295–1301 CrossRef CAS PubMed.
- D. Q. Luo, H. Wang, X. Tian, H. J. Shao and J. K. Liu, Antifungal properties of pristimerin and celastrol isolated from Celastrus hypoleucus, Pest Manage. Sci., 2005, 61, 85–90 CrossRef CAS PubMed.
- M. Skoumal, R. M. Rodriguez, P. L. Cabot, F. Centellas, J. A. Garrido, C. Arias and E. Brillas, Electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton degradation of the drug ibuprofen in acid aqueous medium using platinum and boron-doped diamond anodes, Electrochim. Acta, 2009, 54, 2077–2085 CrossRef CAS.
- J. Yang and K. L. Zhou, NMR spectroscopic analysis of neferine and isoliensinine, Magn. Reson. Chem., 2004, 42, 994–997 CrossRef CAS PubMed.
- C. H. Li, P. Y. Chen, U. M. Chang, L. S. Kan, W. H. Fang, K. S. Tsai and S. B. Lin, Ganoderic acid X, a lanostanoid triterpene, inhibits topoisomerases and induces apoptosis of cancer cells, Life Sci., 2005, 77, 0–265 Search PubMed.
- M. Yoshikawa, T. Morikawa, H. Oominami and H. Matsuda, Absolute Stereostructures of olibanumols A, B, C, H, I, and J from olibanum, gum-resin of Boswellia carterii, and inhibitors of nitric oxide production in lipopolysaccharide-activated mouse peritoneal macrophages, Cheminform, 2009, 57, 957 CAS.
- E. H. Joh and D. H. Kim, A sensitive liquid chromatography-electrospray tandem mass spectrometric method for lancemaside A and its metabolites in plasma and a pharmacokinetic study in mice, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 1875–1880 CrossRef CAS PubMed.
- G. Alam, I. G. Gandjar, L. Hakim, H. Timmerman, R. Verpoorte and S. Wahyuono, Tracheospasmolytic activity of vitetrifolin-E isolated from the leaves of Vitex trifolia L, Maj. Farm. Indones., 2003, 14, 188–194 CAS.
- M. Meier, B. Kohlenberg and N. Braun, Isolation of anisyl acetone from Agarwood Oil, J. Essent. Oil Res., 2003, 15, 54–56 CrossRef CAS.
- L. N. Tao, Q. Y. Meng, Y. Jian, R. Xing and H. R. Guo, A new panaxadiol from the acid hydrolysate of Panax ginseng, Chin. Chem. Lett., 2009, 20, 687–689 CrossRef CAS.
- K. Wang, H. Sun, B. Wu and Y. Pan, Two Novel Olean triterpenoids from Celastrus hypoleucus, Helv. Chim. Acta, 2010, 88, 990–995 CrossRef.
- G. Li, C. S. Lee, M. H. Woo, S. H. Lee, H. W. Chang and J. K. Son, Lignans from the bark of Machilus thunbergii and their DNA topoisomerases I and II inhibition and cytotoxicity, Biol. Pharm. Bull., 2004, 27, 1147–1150 CrossRef CAS PubMed.
- L. P. Ponomarenko, I. V. Stonik, N. A. Aizdaicher, T. Y. Orlova, G. I. Popovskaya, G. V. Pomazkina and V. A. Stonik, Sterols of marine microalgae Pyramimonas cf.cordata (Prasinophyta), Attheya ussurensis sp. nov. (Bacillariophyta) and a spring diatom bloom from Lake Baikal, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2004, 138, 65–70 CrossRef CAS PubMed.
- J. Wandji, F. Tillequin, D. A. Mulholland, J. C. Shirri, N. Tsabang, E. Seguin, P. Verite, F. Libot and Z. T. Fomum, Pentacyclic triterpenoid and saponins from Gambeya boukokoensis, Phytochemistry, 2003, 64, 845–849 CrossRef CAS PubMed.
- F. Wu, K. Koike, T. Nikaido, K. Ishii, T. Ohmoto and K. Ikeda, Terpenoids and flavonoids from Arenaria kansuensis, Chem. Pharm. Bull., 1990, 38, 2281–2282 CrossRef CAS.
- C. R. Chen, L. H. Chao, M. H. Pan, Y. W. Liao and C. I. Chang, Tocopherols and Triterpenoids from Sida acuta, J. Chin. Chem. Soc., 2007, 54, 41–45 CrossRef CAS.
- C. M. Sandison, R. Alexander, R. I. Kagi and C. J. Boreham, Early diagenetic transformation of organic matter in a marine-influenced lignite, Org. Geochem., 2003, 34, 1081–1102 CrossRef CAS.
- C. Johannes and R. L. Lorenz, Preparation and mass spectrometry of 14 pure and 18O2-labeled oxidation products from the phytosterols b-sitosterol and stigmasterol, Anal. Biochem., 2004, 325, 107–116 CrossRef CAS PubMed.
- M. X. Chen, D. Y. Wang and J. Guo, ChemInform abstract: 3-Oxo-11β-hydroxyfriedelane from the roots of Celastrus monospermus, Cheminform, 2010, 34, 114–117 CAS.
- X. W. Chang, B. R. Wang, T. Wang, D. K. Li, Y. Zhao, Y. L. Zhang, D. Z. Zhou and Z. L. Ye, Plant metabolomics approach for age discrimination of mountain cultivated ginseng using UPLC-Q-TOF/MS, China J. Chin. Mater. Med., 2016, 41, 3609–3614 Search PubMed.
- J. Wu, Y. Zhou, L. Y. Wang, J. P. Zuo and W. M. Zhao, Terpenoids from root bark of Celastrus orbiculatus, Phytochemistry, 2012, 7, 159–168 CrossRef PubMed.
- L. Zhang, Z. J. Xu, Y. J. Feng and D. Y. Wang, Chemical constituents of roots of Celastrus orbiculatus, J. Chin. Med. Mater., 2013, 36, 569 CAS.
| This journal is © The Royal Society of Chemistry 2020 |