Open Access Article
Open Access ArticleB12-containing volleyball-like molecule for hydrogen storage†
Jing-Jing Guoa,
Hui-Yan Zhaoa,
Jing Wanga and
Ying Liu *ab
*ab
aDepartment of Physics, Hebei Advanced Thin Film Laboratory, Hebei Normal University, Shijiazhuang 050024, Hebei, China. E-mail: yliu@hebtu.edu.cn
bNational Key Laboratory for Materials Simulation and Design, Beijing 100083, China
First published on 26th February 2020
Abstract
A stable core–shell volleyball-like structure of B12@Li20Al12 has been proposed using first-principles calculations. This structure with Th symmetry is constructed with a core structure of Ih-B12 and a volleyball-like shell of Li20Al12. Frequency analysis and molecular dynamics simulations demonstrate the exceptional stability of B12@Li20Al12. The chemical bonding analysis for B12@Li20Al12 is also conducted to confirm its stability and 46 multi-center two-electron σ bonds are observed, which are widely distributed throughout the core–shell structure. For the hydrogen storage capacity of the B12@Li20Al12, our calculated results indicate that about 58 H2 molecules can be absorbed at most, leading to a gravimetric density of 16.4 wt%. The exceptionally stable core–shell volleyball-like B12@Li20Al12 combined with its high hydrogen storage capacity indicates that it can be one of the outstanding hydrogen storage materials of the future.
Introduction
Since the experimental observation of C60,1 more and more attention has been paid to research of novel cage-like structures, including the Met-Cars,2–4 hollow gold cages,5–9 hollow boron cages,10–17 hollow silicon cages with transition metals embedded18–21 and so on. Recently, a volleyball-shape cage-like structure with an extremely high stability of Sc20C60, called “volleyballene”, was proposed.22 Soon after, another two new volleyballenes of Y20C60 and La20C60 were also reported.23 The theoretical prediction of volleyballenes, on the one hand, enriches the cage-like structure library, and on the other hand, opens a new gate to construct other stable volleyball-shape structures.Among all these cage-like structures, hollow boron cages have drawn our attention due to their unique electronic structure (multi-center two-electron bonds) and widespread potential applications in many fields. Up to now, a large variety of boron cages, like B28,10 B38,11 B39−,12 B40− (ref. 13) and B80,14–16 has been theoretical identified. For the B80 structure, it has been proved that core–shell structure (Ih-B12 containing structure of B12@B68)16 has higher stability than the buckyball-like14 and volleyball-like15 structures. Besides, some other Ih-B12 containing structures of B84 and B98–B102 are also predicted to have high stability.17 For the icosahedral B12, many early literatures have proved that most of the boron allotropes are mainly taking it as a building block. These boron allotropes, accompanied with the B-rich compounds composed by the icosahedral B12, are often called boron-icosahedral cluster solids (B-ICSs).24 Up to now, many B-ICSs have been proposed, such as boron nitride nanostructures,25 B12X2 (X = As, P, O)26–28 and boron carbide.29 These results indicate that Ih-B12 structure is one of promising building blocks to construct varieties of boron-based structures.
Nowadays, with the increasing energy shortage and environmental problems, searching for an alternate energy has become more and more emergent. Hydrogen is one of the promising candidates due to its wide distribution, renewables and environmental protection. However, how to storage hydrogen effectively is still an open problem. Compared with the hydrogen storage technologies of high pressure tank and liquid state storage, which are limited by the large size and weight of the tank and the high cost for liquefaction, the solid state storage has become a popular technology which can storage hydrogen by providing storage mediums and release it without changing the structure of mediums. A large amount of work has proved that carbon nanostructures (including carbon fullerenes, carbon nanotubes and graphyne), which decorated by the alkali metal atoms,30–34 alkaline-earth metal atoms35–38 and transition-metal atoms,39–44 can be the promising hydrogen storage mediums. Similarly, boron nanostructures decorated by the metal atoms can also be applied in hydrogen storage. For example, researches have shown that B80 fullerene decorated by the metal atoms, forming B80M12 (M = Na, K),45 B80Ca12,46 B80Sc12 (ref. 47) and B80Mg12,48 can be applied in hydrogen storage. More recently, two types of Ih-B12 containing structures of B12@Mg20B12 (ref. 49) and B12@Mg20Al12 (ref. 50) with high hydrogen storage capacities have been proposed. The widespread Ih-B12 structures and the superior hydrogen storage performance of boron nanostructures functionalized by the metal atoms motivate us to investigate more metals-decorated Ih-B12 structures and their hydrogen storage capacities.
In this paper, considering the wide existence of the Ih-B12 structure in boron-based structure and the exceptional stability of volleyballenes, we constructed a new stable core–shell volleyball-like B12@Li20Al12 structure. Different from the pentakis dodecahedron of B12@Mg20B12 and B12@Mg20Al12, the B12@Li20Al12 with Th symmetry has a volleyball-like shape, which is similar with the Sc20C60 volleyballene. We further investigated its stability by vibrational frequency analysis and molecular dynamics (MD) simulations. Besides, the chemical bond, which determines the structures and dynamics of molecules, was also explored by chemical bonding analysis to evaluate the stability of the B12@Li20Al12. The interaction between H2 molecules and the B12@Li20Al12 was also investigated to measure the hydrogen storage capacity of B12@Li20Al12.
Calculation details
In our calculations, the exchange–correlation potential was described by the Perdew–Burke–Ernzerhof version (PBE) of the general gradient approximation (GGA).51 The double-numerical basis plus polarized functions (DNP)52 was chosen without spin restrictions. The geometric optimizations were performed with unrestricted symmetry. The core electrons treatment was all-electron. For the MD simulations, we chose the NVE ensemble (NVE: number of particles N, volume V and energy E are constant) to carry out and the total time was 5.0 ps with a time step of 1.0 fs. As for the adsorption of hydrogen molecules on the core–shell B12@Li20Al12, it is necessary to take the van der Waals (vdW) interactions, which are crucial for the formation, stability and function of molecules, into account. Here, we chose hybrid semi empirical dispersion-correction approach of Tkatchenko and Scheffler (TS) scheme53 to describe the vdW interactions. In order to measure the effects of temperature on the hydrogen desorption, we also conducted the MD simulations for the B12@Li20Al12 with H2 absorbed using the NVT ensemble (NVT: number of particles N, volume V and temperature T are constant). The total time was 0.5 ps and the time step was 0.1 fs. The chemical bonding analysis via adaptive natural density partitioning (AdNDP) method54 and localized orbital locator (LOL) were carried out in Gaussian 09 package.55Results and discussions
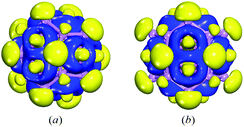
The core–shell volleyball-like B12@Li20Al12 structure was constructed based on the icosahedral Ih-B12 structure. As for the shell structure, it consisted of six Al2Li8 structures, forming the shape of a volleyball. After energy minimization, we got the stable core–shell volleyball-like B12@Li20Al12 structure, as shown in Fig. 1. Taking further consideration into the relationship between core structure and shell structure, it can be found that 12 Al atoms are located above the center of 12 triangular faces of B12 icosahedron, every Al atom connects with three B atoms in B3 triangle. For the 20 Li atoms, there are two different positions: eight top locations above the center of the rest triangular faces of B12 icosahedron (LiI), every LiI atom connects with three B atoms in B3 triangle just like Al atom, and twelve top locations above the B atoms of the B12 icosahedron (LiII), every LiII atom connects with one B atom. As for connections between Li and Al atoms, they can be classified as two types as shown in Fig. S1.† Besides, the detail construction process of the core–shell volleyball-like B12@Li20Al12 is also given in Fig. S1, Tables S1 and S2† also list the average distances of all type of connections and the charge partitioning of the core–shell volleyball-like B12@Li20Al12.To explore the relative stability of the core–shell B12@Li20Al12, we adjusted three different positions of the core B12 icosahedron inside the Li20Al12; besides, we select some low-energy isomers during the MD simulations. After energy minimization, all the structures revert to core–shell volleyball-like B12@Li20Al12 we proposed, as shown in Fig. S2,† indicating that the core–shell volleyball-like B12@Li20Al12 is an energy minimum within the scope of our research.
Considering the similarity between the B12@Li20Al12 and the B80M12 (M = Li, Na, Mg, K, Ca and Sc), the relative stability of them are compared by calculating the average adsorption energy of per metal atom (Eb) and corresponding results are listed in Table S3.† The higher value of Eb, the stronger interaction between metal atoms and B atoms. The calculated results indicate that the Eb of B12@Li20Al12 is up to 2.78 eV, higher than the Eb of B80M12 (M = Li, Na, Mg, K and Ca), except for the extremely high value of Eb (4.06 eV) for B80Sc12. These results indicate that metal atoms have stronger connection with B atoms in B12@Li20Al12.
Vibrational frequency analysis is conducted to check the kinetic stability of the core–shell volleyball-like B12@Li20Al12. The frequency range is between 89.9 cm−1 and 583.1 cm−1 and no imaginary frequencies are found, indicating that this core–shell structure has good kinetic stabilities. Besides, we also list several vibrational frequency modes as shown in Fig. S3,† which can be used to evaluate the distribution of vibration of different atoms at different frequencies. We further simulated the Raman spectrum of the core–shell volleyball-like B12@Li20Al12 and list several peaks, as shown in Fig. 2. This may provide a theoretical basis for future experimental synthesis and observation.
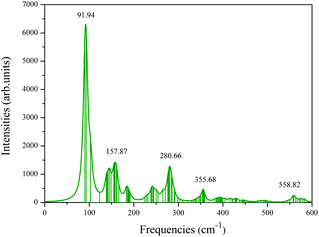 | ||
| Fig. 2 Simulated Raman spectrum for the core–shell volleyball-like B12@Li20Al12. The assumed temperature and incident light are 300 K and 488.00 nm, respectively. | ||
The thermodynamic stability of core–shell volleyball-like B12@Li20Al12 was further checked by ab initio NVE MD simulations. The initial temperatures were set to be 1600 K, 1800 K, 2000 K and 2200 K, corresponding to the effective temperatures of 785 K, 876 K, 930 K and 993 K, respectively, as shown in Fig. S4.† It can be inferred that the core–shell volleyball-like B12@Li20Al12 configuration can be well-kept during the simulations at 2000 K, corresponding to the effective temperature of 930 K. When the initial temperature increases, such as 2200 K, corresponding to the effective temperature of 993 K, the structure collapses and part of the inner B atoms appears in the shell structure.
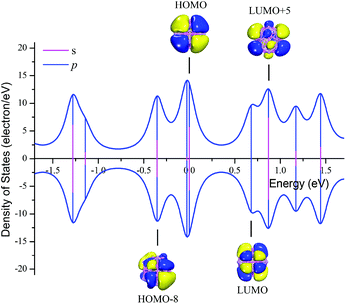
The deformation electron density (Fig. 3) and partial density of states (PDOS) (Fig. 4) were carried out to investigate the electronic structure of core–shell volleyball-like B12@Li20Al12. The deformation electron density, which can be described as the difference between total cluster electron density and the electron density of isolated atoms, can be of great help in indicating the bonds formation. To be specific, the charge depletes on both Al and Li atoms; while the charge gathers into two rings on each Al2Li8 subunit rather than the bond position between two bonding atoms, indicating that there may be multi-center two-electron bonds. Further AdNDP analysis confirm that all the σ bonds found in B12@Li20Al12 are multi-center two-electron bonds. As for the PDOS curve accompanied with some selected frontier molecular orbitals, it is found that the profile of HOMO orbital represents the characters of s–p hybridization, which is also coincident with the PDOS curve. The LUMO orbital is mainly composed of the 2p electrons from the Al atoms in the shell. Besides, both HOMO−8 and LUMO+5 orbitals represent the characters of s–p hybridization, which can also be proved by the PDOS curve.
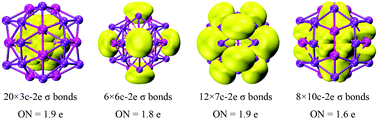
The chemical bonding analysis for core–shell volleyball-like B12@Li20Al12 by AdNDP method is shown in Fig. 5. There are 92 valence electrons in total, forming 46 multi-center two-electron σ bonds. Of all the multi-center two-electron σ bonds, 20 three-center two-electron (3c-2e) σ bonds are observed on the icosahedral B12; 6 six-center two-electron (6c-2e) σ bonds are observed among two inner B atoms, two outer Al atoms and two outer LiII atoms; 12 seven-center two-electron (7c-2e) σ bonds are observed among one inner B atom, one outer Al atom and five outer Li atoms (two LiI atoms and three LiII atoms); 8 ten-center two-electron (10c-2e) σ bonds are observed among three inner B atoms, three outer Al atom and four outer Li atoms (every 10c-2e σ bond located on the region centered on LiI atom with surrounding three LiII atoms, three Al atoms and three inner B atoms). The fully covered σ bonds of the B12@Li20Al12, which are also coincident with the characters of the localized orbital locator (LOL) as shown in Fig. S5,† are the powerful illustration of structural stability.
Before evaluating the hydrogen storage properties of the B12@Li20Al12, we research its stability under ambient conditions. For this purpose, we analysis the electron localization function (ELF) of B12@Li20Al12 as shown in Fig. S5.† It can be concluded that electrons gather on both Li atoms and inner B atoms, indicating Li atoms have strong connection with B atoms. Besides, we also investigated the oxygen absorption of B12@Li20Al12. The results indicate that O atom tends to be absorbed at the bridge position between LiI and Al and connects with three Li atoms and one Al atom with an average adsorption energy of 1.10 eV. After O atom absorbed, the configuration of the B12@Li20Al12 does not change, indicating O atom has little influence on the structure of B12@Li20Al12, which can be proof of its stability under ambient conditions.
The interaction between the core–shell volleyball-like B12@Li20Al12 and hydrogen molecules was investigated to explore the potential application in hydrogen storage. Several possible absorbed locations of H2 molecule are given in Fig. S6,† corresponding distance of H2 to the nearest metal atoms (dH–M), adsorption energies (Ead) and the desorption temperature (Td) of H2 molecule on the eleven specific adsorption locations are also listed in Table S4.† Here, the Ead was defined as Ead = E[B12@Li20Al12(H2)] − E(B12@Li20Al12) − E(H2) and the Td was calculated using Td = (Ead/kB)(ΔS/R − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P)−1 (Van't Hoff equation), where the Ead = adsorption energy, kB = Boltzmann constant, P = pressure (1 atm), R = gas constant and ΔS is the change in H2 entropy from gas to liquid phase. It is found that all absolute values of adsorption energies of absorbed H2 molecule on the eleven specific adsorbed locations lie in the range of 0.10–0.60 eV, which is flexible for hydrogen molecules to be absorbed and released. Especially, the adsorption energy of absorbed H2 molecule on T1 adsorbed location is up to −0.32 eV, corresponding to a desorption temperature of 408 K. Further analysis indicates that the existence of chemical bond between H2 molecule and LiII atom is the main contributor to high adsorption energy. The wide distribution of adsorbed locations suggests that the B12@Li20Al12 may be the potential hydrogen storage material.
P)−1 (Van't Hoff equation), where the Ead = adsorption energy, kB = Boltzmann constant, P = pressure (1 atm), R = gas constant and ΔS is the change in H2 entropy from gas to liquid phase. It is found that all absolute values of adsorption energies of absorbed H2 molecule on the eleven specific adsorbed locations lie in the range of 0.10–0.60 eV, which is flexible for hydrogen molecules to be absorbed and released. Especially, the adsorption energy of absorbed H2 molecule on T1 adsorbed location is up to −0.32 eV, corresponding to a desorption temperature of 408 K. Further analysis indicates that the existence of chemical bond between H2 molecule and LiII atom is the main contributor to high adsorption energy. The wide distribution of adsorbed locations suggests that the B12@Li20Al12 may be the potential hydrogen storage material.
To further explore the chemical bonding properties between H2 molecules and LiII atoms, we evenly added 12 H2 molecules on the top positions of 12 LiII atoms, and calculated deformation electron density of the B12@Li20Al12–12H2 as shown in Fig. S7.† Besides, partial density of states (PDOS) of H atoms in 12 isolated H2 molecules, H atoms in B12@Li20Al12–12H2, Al (Li) atoms in B12@Li20Al12 and Al (Li) atoms in B12@Li20Al12–12H2 were also calculated and shown in Fig. S8.† From the deformation electron density, we can clearly observe that there are charge transfer from LiII atoms to the H2 molecules. The charge analysis indicates that every H atom gets 0.04 e on average. As for the PDOS curve, we can conclude from Fig. S8(a) and (b)† that the PDOS of H atoms in B12@Li20Al12–12H2 almost retains the same shape as that of 12 isolated H2 even though being pushed to lower energy states. Besides, we also observed a tiny peak near Fermi level for the PDOS of H atoms in B12@Li20Al12–12H2, which further indicates that H atoms get electrons. The PDOS of Al atoms is basically unchanged after H2 molecules absorbed, just as shown in Fig. S8(c) and (d),† indicating that there are no interactions between Al and H atoms. For Li atoms, at Fermi level, the PDOS of s electrons decreases to zero and the PDOS of p electrons also decreases after H2 molecules absorbed. All the results are highly consistent with the results of charge analysis.
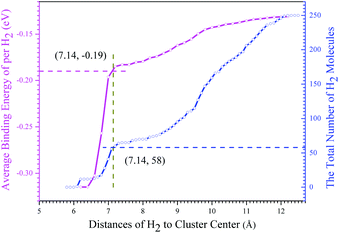
In order to further evaluate the hydrogen storage capacity of the core–shell volleyball-like B12@Li20Al12, 250 H2 molecules were placed around the structure. Subsequently, we counted the distances of different atoms to the cluster center and got the results as shown in Fig. 6 and 7. It can be clearly observed in Fig. 6 that H2 molecules start to occupy the outer space at the distance of about 6.04 Å to the cluster center, corresponding to the distance of about 2.0 Å to the surface LiII atoms, which is also consistent with chemical bond length (about 1.95 Å) between H2 molecule and LiII atom. The first layer of absorbed H2 molecules ends at the distance of about 6.24 Å to the cluster center with 12 H2 molecules absorbed by 12 LiII atoms via forming chemical bonds, leading to a gravimetric density of 3.9 wt%. The average adsorption energy of per H2 for the first layer of absorbed H2 molecules is about −0.30 eV. Based on the Van't Hoff equation, the desorption temperature for the first layer of absorbed H2 molecules is 402 K. The second layer of absorbed H2 molecules is mainly distributed between 6.80 Å and 7.14 Å to the cluster center, about 0.83 Å to the first layer. The distance between the first and second layer of absorbed H2 molecules is much shorter than the balance distance of two free H2 molecules (about 2.70 Å), indicating that the absorption of the second layer of H2 molecules is mainly from the B12@Li20Al12, rather than the first layer of absorbed H2 molecules. After the second layer of H2 molecules being absorbed, there are about 58 absorbed H2 molecules in total, with a gravimetric density of 16.4 wt%. The average adsorption energy of per H2 is −0.19 eV, whose absolute value lies within the range of 0.1–0.6 eV; the desorption temperature is 242 K calculated by the Van't Hoff equation. As for the third layer of absorbed H2 molecules, it is mainly distributed between 9.44 Å and 10.06 Å to the cluster center, about 2.78 Å (close to the 2.70 Å) to the second layer of absorbed H2 molecules, which means that the absorbed H2 molecules, not the cluster, absorb the third layer of H2 molecules. Thus, about 58 H2 molecules can be absorbed around the B12@Li20Al12 with a hydrogen uptake of 16.4 wt% and an average adsorption energy of per H2 of −0.19 eV, corresponding to a desorption temperature of 242 K.
After 58 H2 absorbed by the B12@Li20Al12, it is natural to investigate the mechanism of H2 desorption. To this end, we conducted the MD simulations of the B12@Li20Al12 with both 58 H2 and 12 H2 absorbed. We further counted the distances of the H2 molecules to the cluster center and the results are shown in Fig. S9.† The H2 molecules, whose distances to the cluster center are shorter than 7.14 Å, can be considered as being absorbed by the B12@Li20Al12. Based on the statistical results, there are about 25 H2 molecules absorbed by the B12@Li20Al12 at the temperature of 300 K, leading to a gravimetric density of 7.8 wt%. As the temperature increases, such as 400 K and 500 K as shown in Fig. S9(a),† more and more H2 molecules can be released and there are only 9 H2 molecules being absorbed at the temperature of 600 K. In addition, we also investigated the desorption of the absorbed H2 molecules in the first layer. The 12 H2 molecules, which are absorbed by the B12@Li20Al12 via forming chemical bonds with LiII atoms, can also be released by heating. The results of MD simulations indicate that about 8 H2 molecules can be still absorbed at the temperature of 300 K, while only about 3 H2 molecules can be absorbed at the temperature of 600 K a shown in Fig. S9(b).†
Conclusions
In summary, we constructed a new stable core–shell volleyball-like B12@Li20Al12 structure using the DFT calculations. The structure, which can be regarded as an icosahedron B12 covered by a volleyball-like shell structure of Li20Al12, has a high symmetry of Th. Calculation results demonstrate that the B12@Li20Al12 has good kinetic stability and can maintain its original structure at the effective temperature of 930 K. The chemical bonding analysis of the B12@Li20Al12 shows that the multi-center two-electron σ bonds exist all over the structure, which is of great importance for structural stability. The analysis in the hydrogen storage of the B12@Li20Al12 indicates that about 58 H2 can be absorbed by this structure with a gravimetric of 16.4 wt%, corresponding to an average adsorption energy of per H2 of −0.19 eV, which is flexible for hydrogen molecules to be absorbed and released. All these outstanding results suggest that the new stable core–shell volleyball-like B12@Li20Al12 structure may have potential application in hydrogen storage.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 11274089 and U1331116), the Natural Science Foundation of Hebei Province for Distinguished Young Scholars (Grant No. A2018205174), the Science Foundation of Hebei Education Department for Young Scholar (Grant No. QN2017086) and the Innovation Funding Project for Doctoral Students in Hebei Province (Grant No. CXZZBS2019080). We also acknowledge partially financial support from the 973 Project in China under Grant No. 2011CB606401.References
- H. M. Kroto, J. R. Heath, S. C. O'Brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162–163 CrossRef CAS.
- J. S. Pilgrim and A. Duncan, J. Am. Chem. Soc., 1993, 115, 6958–6961 CrossRef CAS.
- B. C. Guo, S. Wei, J. Purnell, S. Buzza and A. W. Castleman Jr, Science, 1992, 256, 515–516 CrossRef CAS PubMed.
- S. Wei, B. C. Guo, J. Purnell, S. Buzza and A. W. Castleman Jr, J. Phys. Chem., 1992, 96, 4166–4168 CrossRef CAS.
- J. Li, X. Li, H. J. Zhai and L. S. Wang, Science, 2003, 299, 864–867 CrossRef CAS PubMed.
- M. P. Johansson, D. Sundholm and J. Vaara, Angew. Chem., Int. Ed., 2004, 43, 2678–2681 CrossRef CAS PubMed.
- D. Tian, J. Zhao, B. Wang and R. B. King, J. Phys. Chem. A, 2007, 111, 411–414 CrossRef CAS PubMed.
- A. J. Karttunen, M. Linnolahti, T. A. Pakkanen and P. Pyykkö, Chem. Commun., 2008, 465–467 RSC.
- Y. Gao and X. C. Zeng, J. Am. Chem. Soc., 2005, 127, 3698–3699 CrossRef CAS PubMed.
- J. Zhao, X. Huang, R. Shi, H. Liu, Y. Su and R. B. King, Nanoscale, 2015, 7, 15086–15090 RSC.
- J. Lv, Y. C. Wang, L. Zhu and Y. M. Ma, Nanoscale, 2014, 6, 11692–11696 RSC.
- Q. Chen, W. L. Li, Y. F. Zhao, S. Y. Zhang, H. S. Hu, H. Bai, H. R. Li, W. J. Tian, H. G. Lu, H. J. Zhai, S. D. Li, J. Li and L. S. Wang, ACS Nano, 2015, 9, 754–760 CrossRef CAS PubMed.
- H. J. Zhai, Y. F. Zhao, W. L. Li, Q. Chen, H. Bai, H. S. Hu, Z. A. Piazza, W. J. Tian, H. G. Lu, Y. B. Wu, Y. W. Mu, G. F. Wei, Z. P. Liu, J. Li, S. D. Li and L. S. Wang, Nat. Chem., 2014, 6, 727–731 CrossRef CAS PubMed.
- G. N. Szwacki, A. Sadrzadeh and B. I. Yakobson, Phys. Rev. Lett., 2007, 98, 166804 CrossRef PubMed.
- X. Q. Wang, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 153409 CrossRef.
- H. Li, N. Shao, B. Shang, L. F. Yuan, J. Yang and X. C. Zeng, Chem. Commun., 2010, 46, 3878–3880 RSC.
- D. L. V. K. Prasad and E. D. Jemmis, Phys. Rev. Lett., 2008, 100, 165504 CrossRef PubMed.
- H. Kawamura, V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 245433 CrossRef.
- E. N. Koukaras, C. S. Garoufalis and A. D. Zdetsis, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 235417 CrossRef.
- J. Wang, Y. Liu and Y. C. Li, Phys. Chem. Chem. Phys., 2010, 12, 11428–11431 RSC.
- J. Li, J. Wang, H. Y. Zhao and Y. Liu, J. Phys. Chem. C, 2013, 117, 10764–10769 CrossRef CAS.
- J. Wang, H. M. Ma and Y. Liu, Nanoscale, 2016, 8, 11441–11444 RSC.
- J. Wang and Y. Liu, Sci. Rep., 2016, 6, 30875 CrossRef CAS PubMed.
- B. Albert and H. Hillebrecht, Angew. Chem., Int. Ed., 2009, 48, 8640–8668 CrossRef CAS PubMed.
- J. Yin, J. D. Li, Y. Hang, J. Yu, G. A. Tai, X. M. Li, Z. H. Zhang and W. L. Guo, Small, 2016, 12, 2942–2968 CrossRef CAS PubMed.
- T. L. Aselage, D. R. Tallant and D. Emin, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 3122–3129 CrossRef CAS.
- D. Li and W. Y. Ching, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 1451–1454 CrossRef CAS PubMed.
- S. Bakalova, Y. Gong, C. Cobet, N. Esser, Y. Zhang, J. H. Edgar, Y. Zhang, M. Dudley and M. Kuball, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 075114 CrossRef.
- M. M. Balakrishnarajan, P. D. Pancharatna and R. Hoffmann, New J. Chem., 2007, 31, 473–485 RSC.
- G. E. Froudakis, Nano Lett., 2001, 1, 531–533 CrossRef CAS.
- Q. Sun, P. Jena, Q. Wang and M. Marquez, J. Am. Chem. Soc., 2006, 128, 9741–9745 CrossRef CAS PubMed.
- K. R. S. Chandrakumar and S. K. Ghosh, Nano Lett., 2008, 8, 13–19 CrossRef CAS PubMed.
- W. Liu, Y. H. Zhao, Y. Li, Q. Jiang and E. J. Lavernia, J. Phys. Chem. C, 2009, 113, 2028–2033 CrossRef CAS.
- C. R. Luna, P. Bechthold, G. Brizuela, A. Juan and C. Pistonesi, Appl. Surf. Sci., 2018, 459, 201–207 CrossRef CAS.
- M. Yoon, S. Yang, C. Hicke, E. Wang, D. Geohegan and Z. Zhang, Phys. Rev. Lett., 2008, 100, 206806 CrossRef PubMed.
- H. Lee, J. Ihm, M. L. Cohen and S. G. Louie, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 115412 CrossRef.
- S. Seenithurai, R. K. Pandyan, S. V. Kumar, C. Saranya and M. Mahendran, Int. J. Hydrogen Energy, 2014, 39, 11990–11998 CrossRef CAS.
- S. V. Hosseini, H. Arabi and A. Kompany, Int. J. Hydrogen Energy, 2017, 42, 969–977 CrossRef CAS.
- E. Durgun, S. Ciraci and T. Yildirim, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 085405 CrossRef.
- B. Chakraborty, P. Modak and S. Banerjee, J. Phys. Chem. C, 2012, 116, 22502–22508 CrossRef CAS.
- Q. Sun, Q. Wang, P. Jena and Y. Kawazoe, J. Am. Chem. Soc., 2005, 127, 14582–14583 CrossRef CAS PubMed.
- P. Modak, B. Chakraborty and S. Banerjee, J. Phys.: Condens. Matter, 2012, 24, 185505 CrossRef CAS PubMed.
- A. Gangan, B. Chakraborty, L. M. Ramaniah and S. Banerjee, Int. J. Hydrogen Energy, 2019, 44, 16735–16744 CrossRef CAS.
- A. Yadav, B. Chakraborty, A. Gangan, N. Patel, M. R. Press and L. M. Ramaniah, J. Phys. Chem. C, 2017, 121, 16721–16730 CrossRef CAS.
- Y. C. Li, G. Zhou, J. Li, B. L. Gu and W. H. Duan, J. Phys. Chem. C, 2008, 112, 19268–19271 CrossRef CAS.
- M. Li, Y. F. Li, Z. Zhou, P. W. Shen and Z. F. Chen, Nano Lett., 2009, 9, 1944–1948 CrossRef CAS PubMed.
- G. F. Wu, J. L. Wang, X. Y. Zhang and L. Y. Zhu, J. Phys. Chem. C, 2009, 113, 7052–7057 CrossRef CAS.
- J. L. Li, Z. S. Hu and G. W. Yang, Chem. Phys., 2012, 392, 16 CrossRef CAS.
- H. Y. Zhao, L. Y. Ai, H. M. Ma, J. J. Guo, J. L. Qiu, J. Wang and Y. Liu, J. Phys. Chem. C, 2019, 123, 17639 CrossRef CAS.
- J. J. Guo, H. Y. Zhao, L. Y. Ai, J. Wang and Y. Liu, Int. J. Hydrogen Energy, 2019, 44, 28235–28241 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- B. Delley, J. Chem. Phys., 1989, 92, 508–517 CrossRef.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef PubMed.
- D. Y. Zubarev and A. I. Boldyrev, Phys. Chem. Chem. Phys., 2008, 10, 5207–5217 RSC.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.-A. Petersson, et al., Gaussian 09, revision C.01, Gaussian, Inc., Wallingford, CT, 2010 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra10491g |
| This journal is © The Royal Society of Chemistry 2020 |