Open Access Article
Open Access ArticleCharacteristics and hazards of the cinnamaldehyde oxidation process
Chang Yu ,
Yuan-Lin Li
,
Yuan-Lin Li ,
Min Liang,
Su-Yi Dai,
Li Ma,
Wei-Guang Li,
Fang Lai and
Xiong-Min Liu*
,
Min Liang,
Su-Yi Dai,
Li Ma,
Wei-Guang Li,
Fang Lai and
Xiong-Min Liu*
School of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China. E-mail: xmliu1@gxu.edu.cn; Tel: +86 138 7713 6730
First published on 20th May 2020
Abstract
Pressure and temperature behavior of the cinnamaldehyde oxidation process was determined using a custom-designed mini closed pressure vessel test (MCPVT), which is a new method to investigate the stability and hazard assesment of the cinnamaldehyde oxidation reaction. The oxidation products were analyzed by gas chromatography-mass spectrometry (GC-MS). The results showed that cinnamaldehyde was stable under nitrogen atmosphere but very unstable under oxygen atmosphere. The initial oxidation products were analyzed by iodimetry and the cinnamaldehyde peroxide value could reach 139.44 mmol kg−1 when the oxidation temperature was 308 K. The oxidation kinetics of cinnamaldehyde were studied by using the pressure versus time (P–t) curves obtained from the MCPVT process. The reaction is a second-order reaction, the kinetic equation is ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −2233.66 × (1/T) + 11.19, and the activation energy Ea is 18.57 kJ mol−1 at 308–338 K. The explosion of the cinnamaldehyde oxidation reaction was observed by MCPVT, in which the onset temperature was 373 K. The main products of cinnamaldehyde oxidation are acetaldehyde, benzaldehyde, phenylacetaldehyde, acetophenone, 2-hydroxyphenyl acetone, cinnamaldehyde epoxide, benzoic acid, and cinnamic acid. Oxidation is a three-step process: (1) cinnamaldehyde reacts with oxygen to form peroxides; (2) complex oxidation reactions are caused by the thermal decomposition of peroxides; (3) rapid oxidation and thermal decomposition lead to explosion hazard.
k = −2233.66 × (1/T) + 11.19, and the activation energy Ea is 18.57 kJ mol−1 at 308–338 K. The explosion of the cinnamaldehyde oxidation reaction was observed by MCPVT, in which the onset temperature was 373 K. The main products of cinnamaldehyde oxidation are acetaldehyde, benzaldehyde, phenylacetaldehyde, acetophenone, 2-hydroxyphenyl acetone, cinnamaldehyde epoxide, benzoic acid, and cinnamic acid. Oxidation is a three-step process: (1) cinnamaldehyde reacts with oxygen to form peroxides; (2) complex oxidation reactions are caused by the thermal decomposition of peroxides; (3) rapid oxidation and thermal decomposition lead to explosion hazard.
Introduction
Cinnamon is a special natural plant resource in Guangxi, China. Cinnamaldehyde is the main component in cinnamon oil, which is extracted from cinnamon by steam distillation. As an important spice and organic intermediate, it is widely used in medicine and food due to its strong antibacterial, antiviral, and antiseptic properties.1–5 Cinnamaldehyde, as a kind of biomass compound, is composed of a benzene ring, an unsaturated double bond, and an aldehyde group. Cinnamaldehyde shows high oxidation reactivity6 in the presence of oxidants. Many studies on cinnamaldehyde oxidation have been reported, which mainly focus on the reaction conditions of different oxidants and catalysts. Cinnamaldehyde is particularly oxidized by air in the presence of the N-heterocyclic carbene catalyst.7 The catalytic oxidation of cinnamaldehyde using oxygen, NaClO, and H2O2 as the oxidants with manganese porphyrin and β-cyclodextrin as the catalysts showed the characteristics of free radical reaction.8–10 Yang11 studied the selective oxidation of cinnamaldehyde to benzaldehyde by H2O2 with β-cyclodextrin polymer as the catalyst in weak alkaline conditions. Iridium trichloride-cerium sulfate has a strong catalytic activity for cinnamaldehyde oxidation in acidic solution.12 However, cinnamaldehyde can be oxidized without a catalyst when exposed to air at room temperature due to the unstable conjugated bonds and aldehyde group in its molecular structure. This restricts the application of cinnamaldehyde. For instance, cinnamaldehyde is widely used as a food additive. After being oxidized, cinnamaldehyde loses its sterilization and anti-corrosion functions, which can accelerate the quality deterioration of food.The oxidation of cinnamaldehyde is of concern at low temperatures. Due to the inevitable contact with air in the actual production, transportation, storage, and application, cinnamaldehyde forms organic peroxides,13 which seriously reduce its quality. Organic peroxides are hazardous materials, which are prone to thermal decomposition and explosion.14 The explosion in Brussels on March 22, 2016 was caused by triacetone triperoxide (TATP), which is a unstable peroxide and prone to explosion.15 The structure of cinnamaldehyde has a double bond and an aldehyde group, which is easy to oxidize. In order to reduce or avoid the oxidation reaction of cinnamaldehyde, Schreck et al. reported the molecular encapsulation of cinnamaldehyde with cyclodextrin as an effective method to improve the photostability and thermostability of cinnamaldehyde.16
The complexation of cinnamaldehyde with β-cyclodextrin can prevent cinnamaldehyde oxidation,17 which extends its shelf life. Therefore, it is necessary to study the characteristics and hazard assessment of the oxidation process for cinnamaldehyde production, storage, and transportation.
In the present work, the pressure and temperature behavior of the cinnamaldehyde oxidation process was determined using MCPVT (mini closed pressure vessel test). The characteristics, kinetics, and thermal stability of cinnamaldehyde oxidation were investigated. It is very important to explore the evaluation method and the possible explosion conditions of the oxidation process of cinnamaldehyde. The study of the oxidation products and reaction pathways is needed because it helps to understand the chemical properties and thermal stability of cinnamaldehyde. The purpose of this study is to supply the basic data so as to improve the safety of production, storage, and transportation of cinnamaldehyde, and to provide research methods for the organic synthesis and oxidation mechanism of cinnamaldehyde.
Materials and experimental procedures
Materials
Cinnamaldehyde (99%, Shanghai Medical Technology Co., LTD, China), N2, O2 (99.99%, Nanning Zhong Yi Chuang Gas Co., LTD, China), KI (99.50%, Aladdin Industrial Corporation, China), and Na2S2O3 (99.95%, Aladdin Industrial Corporation, China) were used in the experiments.Cinnamaldehyde oxidation in MCPVT
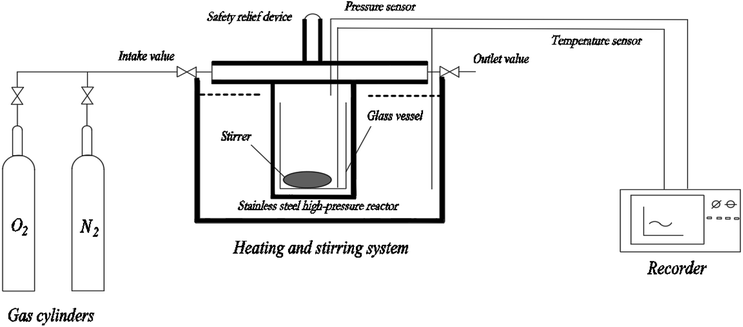
A mini closed pressure vessel test (MCPVT, vessel volume: 35 mL) was designed, as shown in Fig. 1. The main components of MCPVT are: (a) a pressure sensor, made in Japan; (b) a temperature sensor, made in Japan; (c) a recorder, made in Japan; (d) a stainless steel high-pressure reactor, self-designed; (e) a gas cylinder, made in China; (f) a heating and stirring system; the system is a DF-101S collector type constant temperature heating magnetic stirrer, made in China, the heating medium is heat conductive oil (glycerol). MCPVT could thus be used for evaluating the oxidation reaction of cinnamaldehyde. Whether cinnamaldehyde oxidation takes place after the thermal decomposition of cinnamaldehyde or whether it directly reacts with oxygen is worth studying. For understanding the characteristics of oxidation and the order of the thermal decomposition reaction, it is necessary to study the pressure effects of cinnamaldehyde under oxygen and nitrogen atmosphere. In order to avoid the influence of metals on cinnamaldehyde oxidation, 1 g cinnamaldehyde was put into a test tube (glass material) and a magnetic stirrer was put into the test tube, which was then set into the stainless steel reactor of MCPVT. The initial pressure was 0.57 MPa. The oxidation experiments were carried out at isothermal conditions below the boiling point of cinnamaldehyde (524 K) under stirring.The pressure and temperature changes were recorded by the recorder and the signal conditioner. After the reaction, the products were cooled to room temperature and collected. Under the same experimental conditions, nitrogen instead of oxygen was used in a comparative experiment. In order to reduce the experimental error, three parallel experiments were conducted at each temperature.
Analysis of peroxide value
In order to detect the formation of cinnamaldehyde peroxides, a proper amount of cinnamaldehyde was reacted with oxygen in a three-neck flask under open conditions. The reaction was conducted at different temperatures for 8 h with sampling after every 2 h. The peroxide value were determined by iodimetry18 at different temperatures and times. About 0.1 g of the sample obtained from cinnamaldehyde oxidation was added to aqueous potassium iodide. The peroxides were reduced by potassium iodide (eqn (I)). An equivalent amount of iodine was liberated with starch and made the solution blue. Then, the solution was titrated with standardized sodium thiosulfate solution until the blue disappeared (eqn (II)). The experiment was carried out three times in parallel to obtain the average value. The results were quantified as milligrams per kilogram (mmol kg−1) of peroxide.| 2KI + ROOH + H2O → I2 + 2KOH + ROH | (I) |
| I2 + 2Na2S2O3 → Na2S4O6 + 2NaI | (II) |
Analysis of the oxidation products
GC-MS gas (chromatography-mass spectrometry) is an effective means to analyse the oxidation products of cinnamaldehyde. It is of significance for the safe use of cinnamaldehyde, the analysis of the oxidation mechanism, and thermal decomposition characteristics. The GC-MS instrument (GCMS-QP2010 Ultra, Shimadzu Corp.) for the analysis of the oxidation products contained an electron impact ionization detector (EID: 70 eV) and a DB-WAX fused silica capillary column (with an inner diameter of 30 m × 0.25 mm and a film thickness of 0.25 mm; J&W Scientific Inc.). The following temperature program was used: maintained 343.15 K for 3 min, first increased to 450.15 K (8 K min−1) and then increased to 473.15 K (5 K min−1), and finally increased to 513.15 K (20 K min−1). The carrier gas was high-purity helium gas, the column flow rate was 1.53 mL min−1, the injection volume was 0.2 μL, the split ratio was 80.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the inlet temperature was 523.15 K, and the interface temperature was 503.15 K. The mass spectrometer used an EI ion source with an electron energy of 0.8 kV and a mass spectrometer scanning range of 14–500 m/z in the full scan mode.
1, the inlet temperature was 523.15 K, and the interface temperature was 503.15 K. The mass spectrometer used an EI ion source with an electron energy of 0.8 kV and a mass spectrometer scanning range of 14–500 m/z in the full scan mode.
Results and discussion
Pressure behavior of the cinnamaldehyde oxidation process
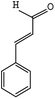
Cinnamaldehyde is a compound with conjugated double bonds. Its structure is shown in Fig. 2. According to the structure of cinnamaldehyde, one of its chemical properties is its high reactivity. Therefore, it is necessary to investigate its thermal stability. First, we investigated the thermal stability of cinnamaldehyde. The MCPVT experiment was carried out under nitrogen atmosphere. The temperature vs. time (T–t) and pressure vs. time (P–t) plots of cinnamaldehyde are shown in Fig. 3 (line 1) and Fig. 4 (line 1), respectively. Then, we investigated the stability of cinnamaldehyde under oxygen atmosphere. Cinnamaldehyde oxidation is shown in reaction (III):| Cinnamaldehyde (l) + O2 (g) → Products | (III) |
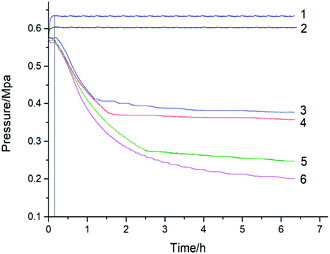 | ||
| Fig. 3 Pressure versus time for cinnamaldehyde, 1 – N2, 353 K; 2 – N2, 308 K; 3 – O2, 308 K; 4 – O2, 318 K; 5 – O2, 328 K; 6 – O2, 338 K. | ||
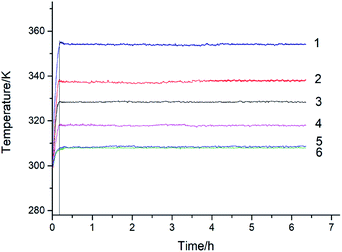 | ||
| Fig. 4 Temperature versus time for cinnamaldehyde, 1 – N2, 353 K; 2 – O2, 338 K; 3 – O2, 328 K; 4 – O2, 318 K; 5 – O2, 308 K; 6 – N2, 308 K. | ||
Cinnamaldehyde is a liquid (l) at room temperature and its boiling point is 524 K. At the room temperature of ∼353 K and 1 MPa pressure, the vapor pressure of cinnamaldehyde is so low that it can be ignored. Therefore, when using MCPVT to investigate reaction (III), it can be considered that the pressure behavior of the oxidation process presents a change in the oxygen (g) content. The cinnamaldehyde oxidation experiments were conducted at 308 K, 318 K, 328 K, and 338 K in oxygen atmosphere. The experimental results are shown in Fig. 3. Line 1 represents the nitrogen atmosphere, which was used as the comparative experiment.
Fig. 3 shows that the pressure remained constant after the temperature was increased to 353 K under nitrogen atmosphere, which indicated that no gas consumption or thermal decomposition reaction occurred. By contrast, the pressure dropped rapidly after heating to 308 K, 318 K, 328 K, and 338 K under oxygen atmosphere. These results showed that oxidation took place with the consumption of a large amount of oxygen. The cinnamaldehyde oxidation process is a gas–liquid reaction. Cinnamaldehyde absorbed oxygen to form non-gaseous products, causing a decrease in the pressure in the MCPVT. Before 0.2 h, cinnamaldehyde absorbed oxygen to form cinnamaldehyde peroxide and the pressure changed slowly. Then, the thermal decomposition of cinnamaldehyde peroxides produced a great quantity of free radicals, causing the fast radical oxidation of cinnamaldehyde and the pressure showed almost a linear decrease. Oxygen consumption increased with the increasing temperature. Hence, the first two stages of cinnamaldehyde oxidation process can be described as follows: (1) cinnamaldehyde reacts with oxygen to form peroxides; (2) complex oxidation reactions are caused by the thermal decomposition of peroxides.
In order to slow down the deterioration of cinnamaldehyde, contact with oxygen should be prevented during the process of production, storage, and transportation.
Kinetics of cinnamaldehyde oxidation
Chemical kinetics is an important method to study the reactivity, stability, and hazard of compounds. Cinnamaldehyde is stable under nitrogen atmosphere but it is unstable under oxygen atmosphere. Therefore, it is necessary to study the oxidation kinetics of cinnamaldehyde at low temperature. It is an attempt to determine the oxidation kinetics of cinnamaldehyde using MCPVT. It may include the kinetics of oxygen absorption and the chemical reaction because it cannot distinguish between the control steps in the mass transfer of the oxygen absorption process and the oxidation reaction in MCPVT. It is valuable for understanding the characteristics, thermal stability, and risks of cinnamaldehyde oxidation.It was assumed that cinnamaldehyde oxidation occurred as follows:
| Cinnamaldehyde (l) + O2 (g) → Products | |||
| t = 0 | CA0 | CB0 | 0 |
| t = t | CA0 − x | CB0 − x | x |
Kinetic equation (eqn (1)):
| dx/dt = k(CA0 − x)α(CB0 − x)β | (1) |
If we make the following assumptions:
(1) The oxidation kinetics is a second-order reaction (t = 0.2–1.2 h).
(2) Cinnamaldehyde vapor pressure is p = 0.
(3) Oxygen is an ideal gas, the remaining molar number of oxygen can be calculated by the ideal gas equation: nt = (B − x) = PV/RT, (P is the pressure, R is the gas constant, and T is the temperature).
So, eqn (2) is simplified by eqn (1)
| dx/dt = k(CA0 − x)(CB0 − x) | (2) |
For the experiment, nA0 = nB0 = 0.007955 mol. The initial temperatures were 308 K, 318 K, 328 K, and 338 K, respectively.
Then, eqn (3) can be obtained by the integral of eqn (2):
| 1/(CA0 − x) = kt + q | (3) |
Fig. 5 showed the plots of (1/CA) versus t at 308 K, 318 K, 328 K, and 338 K, which indicated that ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k had a good linear relationship with t. The initial oxidation reaction of cinnamaldehyde is a second-order reaction.
k had a good linear relationship with t. The initial oxidation reaction of cinnamaldehyde is a second-order reaction.
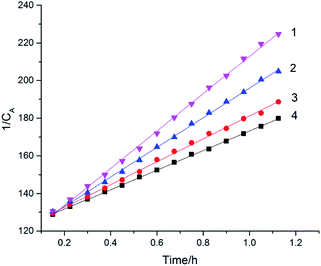 | ||
| Fig. 5 Plots of (1/CA) versus time at different temperatures, 1 – 338 K; 2 – 328 K; 3 – 318 K; 4 – 308 K. | ||
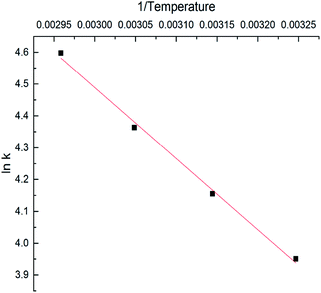
Fig. 6 represents the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k versus (1/T) curve. The fitted linear equation is given as:
k versus (1/T) curve. The fitted linear equation is given as:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k = −2233.66 × (1/T) + 11.19 k = −2233.66 × (1/T) + 11.19
| (4) |
The correlation coefficient (R2) is 0.995. The activation energy (Ea) of cinnamaldehyde oxidation obtained from the linear slope is 18.57 kJ mol−1. Table 1 showed that the reaction rate increased with the increasing temperature at low temperature. This could be attributed to the formation of reactive radicals via the absorption of oxygen to form the peroxides.19 Because of the low activation energy, cinnamaldehyde is easily oxidized even at 308 K. As a traditional spice, the low temperature oxidation of cinnamaldehyde leads to its deterioration during storage, transportation, and usage.
| T (K) | k (h−1) | R2 |
|---|---|---|
| 308 | 99.23977 | 0.9996 |
| 318 | 78.456743 | 0.9982 |
| 328 | 63.75072 | 0.9989 |
| 338 | 51.96077 | 0.9985 |
Peroxide value of cinnamaldehyde oxidation
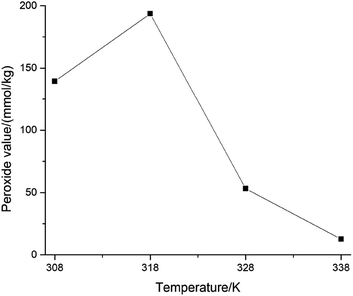
The MCPVT results have shown that oxygen was absorbed by cinnamaldehyde. It is worth noting that the initial oxidation reaction could form some products at low temperature. Does the initial oxidation reaction produce the hazardous substance peroxide? Peroxide is a kind of hazardous compound, which is prone to thermal decomposition and is difficult to separate and characterize due to its instability. In order to verify the formation of the peroxide and investigate the effect of oxidation temperature and time on the peroxide value, the peroxide concentrations were determined by iodimetry, which are expressed by the peroxide value. The effect of temperature on the cinnamaldehyde peroxide value was evaluated at 308 K, 318 K, 328 K, and 338 K.The variation in the peroxide value with temperature under high pressure is shown in Fig. 7. The peroxide value reached the maximum of 193.61 mmol kg−1 at 318 K. This is a strong evidence for the formation of peroxide by cinnamaldehyde oxidation. The peroxide value increased with the increase in the temperature below 318 K, which indicated the accumulation of the peroxide. Following this, the peroxide value decreased with the rising temperature from 318 K to 338 K, which demonstrated that the decomposition rate of peroxide was higher than its formation rate. Peroxide as the intermediate product is easy to decompose by heat,20 resulting in the deep oxidation of cinnamaldehyde. Organic peroxides ought to be stored at low temperature21 as the self-decomposition reaction of peroxide occurs when it exceeds the critical temperature. The heat released from the reaction accelerates its decomposition, where the decomposition temperature depends on the difficulty of its decomposition. The self-accelerated decomposition temperatures of stable peroxide are in the range of 333–343 K, and the unstable ones are 293 K or even lower. So, the peroxide value first increased then decreased with the rising temperature from 308 K to 338 K. The cinnamaldehyde peroxides are more easily accumulated at a lower temperature.
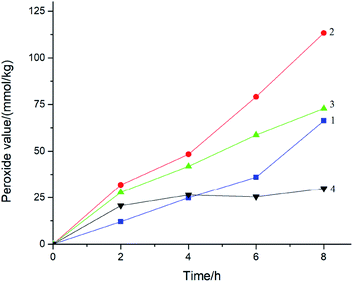
The effect of reaction time on cinnamaldehyde peroxide under normal pressure was evaluated at 308 K, 318 K, 328 K, and 338 K. The variation in the peroxide value with reaction time at different temperatures is shown in Fig. 8. The result showed that the peroxide value increased constantly on prolonging the reaction time and reached the maximum value of 113.29 mmol kg−1 at 318 K within 8 h. Cinnamaldehyde peroxide was continuously formed and accumulated as the reaction time increased at low temperature. The longer the reaction time, the higher was the cinnamaldehyde peroxide value. Therefore, the optimal temperature for cinnamaldehyde peroxide formation was 318 K.
When the temperature was higher than 318 K, cinnamaldehyde peroxide was greatly decomposed. The peroxide value reached a maximum value when the reaction time was 8 h. Cinnamaldehyde peroxide can affect the quality of cinnamaldehyde and increase the thermal instability of cinnamaldehyde. So, it is necessary to inhibit the formation of cinnamaldehyde peroxide and study the possible thermal hazards of the cinnamaldehyde oxidation process.
The hazards of the cinnamaldehyde oxidation process
Cinnamon oil (about 80% cinnamaldehyde) is obtained from the bark and leaves of cinnamon by steam distillation. Also, natural cinnamaldehyde is obtained by distillation of cinnamon oil. In the process of production, storage, and transportation, cinnamaldehyde may react with oxygen. Rapid oxidation reactions are hazardous and can be converted into combustion or explosion reactions. Therefore, it is important to study the oxidation of cinnamaldehyde to avoid safety-related accidents.There are many methods to evaluate the risk of chemical reaction processes, such as Reaction Calorimetry (RC1) and Advanced Reactive System Screening Tool (ARSST). Yang and Leveneur et al.22 reported the risk assessment of hot air in the hydrogenation of levulinic acid to γ-pentalactone using ARSST. It is interesting to study the risk of cinnamaldehyde oxidation by MCPVT because it is a cheap and simple method.
The thermal decomposition violence assessment of organic peroxide was studied by MCPVT (mini closed pressure vessel test).23 MCPVT has shown its superiority24 in measuring the acceleration decomposition temperature and the material kinetic parameters for thermal decomposition. The device has been widely applied in assessing the thermal stability of hazardous compounds.25 Liu26,27 evaluated the thermal stability of nine organic peroxides by MCPVT. Schreck16 used MCPVT to study the explosive risk of mixtures of hydrogen peroxides and alcohols. Therefore, the hazards of cinnamaldehyde oxidation were studied using MCPVT in this section. The gas–liquid oxidation was carried out with 1 g cinnamaldehyde under 0.72 MPa pressure of oxygen by MCPVT, which included a recorder and a signal regulator to monitor the changes in the temperature and pressure of the system. The curves of T–t (temperature versus time) and P–t (pressure versus time) are shown in Fig. 9(a) and (b), respectively.
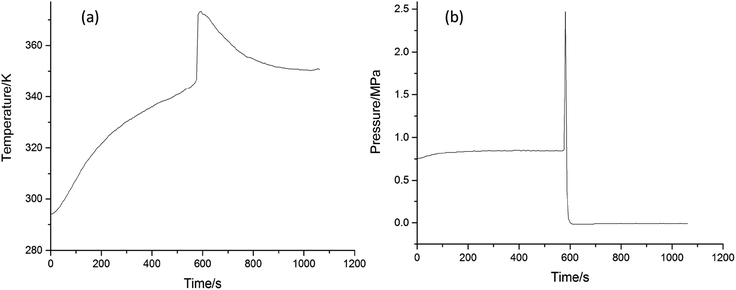 | ||
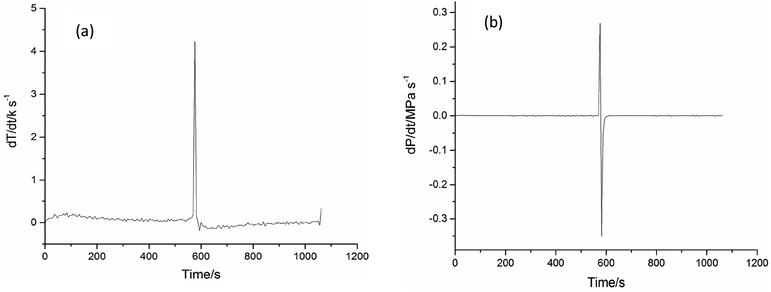
| Fig. 9 Explosion studies of cinnamaldehyde oxidation (a) temperature versus time (b) pressure versus time. | ||
According to Fig. 9, a violent thermal hazard was found in cinnamaldehyde oxidation when the oxidation temperature was raised to 345 K, accompanied by a sharp rise in the system pressure. It indicated that the accumulation and rapid decomposition of cinnamaldehyde peroxides released a large amount of heat, which caused an increase in the system temperature from 345 K to 373 K. The bond energy of O–O of the peroxides is only 43 kcal mol−1, which is easy to break.28 When the system temperature exceeds the self-decomposition temperature of the peroxides, the thermal decomposition reaction of the peroxide will take place to produce small gaseous molecules and active free radicals.
Also, it will lead to the free radical oxidation reaction, which is one of the main reasons for the explosion.29 The free radical concentration and reaction chains increase exponentially when the large exothermic heat of the reaction system accumulates and the total reaction rate is continuously accelerated. A large amount of heat is released by the reaction cycle and leads to an uncontrolled reaction process.30 Organic peroxide has strong decomposition characteristics, it produces a large amounts of heat and gas after decomposition, and can lead to runaway reactions.31 Peroxide decomposition generates a large number of reactive free radicals, which can initiate rapid oxidation reactions.32,33 The cinnamaldehyde oxidation reaction is a remarkable exothermic reaction with a rapid rate at high temperature.
The first derivatives of P–t and T–t are calculated to obtain the dP/dt–t and dT/dt–t curves, as shown in Fig. 10(a) and (b), respectively. When the (dT/dt) or (dP/dt) is positively associated with the reaction rate, it can be used for evaluating the hazards of the oxidation process.26 Liu evaluated the safety of ethers, abietic acid, rosin, and dimethoxymethane oxidation reactions by this method.34–36 The maximum rise rate temperature (dT/dt)max and the maximum rise rate pressure (dP/dt)max are critical parameters to evaluate the stability of the reactant.37 As shown in Table 2, dP/dt and dT/dt reached the maximum value of 0.26 MPa s−1 and 2.25 K s−1, respectively, when the system temperature reached 373 K. This indicates that cinnamaldehyde oxidation can transform to an explosion easily.
| Cinnamaldehyde mass | T0 (K) | Tmax (K) | (dT/dt)max (K s−1) | (dP/dt)max (MPa s−1) |
|---|---|---|---|---|
| 1 g | 345 | 373 | 2.25 | 0.26 |
An explosion reaction occurred due to rapid oxidation when the system temperature was increased to 373 K. The explosion-proof material, as a safety device in MCPVT, can take a maximum pressure of 10 MPa, which was broken inside the autoclave and turned black, and the glass container carrying the reactants was crushed into pieces by the enormous explosive power, as shown in Fig. 11. It is a strong evidence of the violent explosion. The pressure sensor, which could withstand a maximum pressure of 20 MPa, was broken. The actual maximum pressure could not be recorded due to the extremely rapid explosion process. In addition, the explosion was aggravated by the presence of sufficient amount of oxygen. The mixtures of cinnamaldehyde and the peroxide have high energy content. Cinnamaldehyde oxidation is a dangerous process when the peroxide decomposition releases a large amount of heat. Therefore, the thermal decomposition of cinnamaldehyde peroxide and the mixtures would lead to the thermal hazards of cinnamaldehyde oxidation, which can further be converted to an explosion. Also, the hazardous thermal process could be described as the third stage of cinnamaldehyde oxidation.
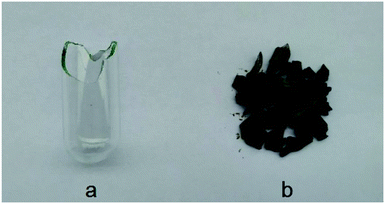 | ||
| Fig. 11 The aftermath of cinnamaldehyde oxidation (a) round bottom flask (b) broken flask after explosion. | ||
Products of cinnamaldehyde oxidation
The oxidation reaction products are indispensable for understanding the characteristics of the oxidation process, the reaction pathway and mechanism, and the reaction hazards. The initial oxidation products of cinnamaldehyde are important to elucidate the mechanism and stability of the chemical reaction.In order to study the initial oxidation products of cinnamaldehyde oxidation and explore the pathways of oxidation, the oxidation products of cinnamaldehyde at 308 K, 318 K, 328 K, and 338 K for 6.5 h were analyzed by GC-MS. The results are shown in Table 3.
| No. | Product | Molecular formula | Reactive content | ||||
|---|---|---|---|---|---|---|---|
| N2 | 308 K | 318 K | 328 K | 338 K | |||
| 1 | Water | H2O | — | 0.05 | 0.09 | 0.19 | |
| 2 | Carbon dioxide | CO2 | 0.17 | 0.19 | 0.25 | 0.8 | |
| 3 | Acetaldehyde | C2H4O | 0.3 | 0.37 | 0.26 | 0.68 | |
| 4 | Formic acid | CH2O2 | 0.3 | 0.51 | 1.09 | 1.87 | |
| 5 | Acetic acid | C2H4O2 | 0.03 | 0.02 | 0.03 | — | |
| 4 | Benzaldehyde | C7H6O | 7.91 | 11.27 | 16.94 | 22 | |
| 5 | Phenylacetaldehyde | C8H8O | 2.89 | 3.75 | 4.31 | 5.8 | |
| 6 | Acetophenone | C8H8O | 0.26 | 0.82 | 1.37 | 1.89 | |
| 7 | Phenylglyoxylic acid | C8H8O2 | 0.31 | 0.46 | 0.65 | 0.98 | |
| 8 | Ethyl benzoate | C9H10O2 | 0.21 | 0.46 | 0.38 | 0.46 | |
| 9 | Benzoic acid | C7H6O2 | 0.12 | 0.97 | 1.85 | 3.76 | |
| 10 | 3-Phenyloxirane-2-carbaldehyde (epoxide) | C9H8O2 | 1.27 | 4.36 | 7.86 | 17.28 | |
| 11 | Ethyl phenylacetate | C10H12O2 | 0.18 | 0.29 | 0.26 | 0.32 | |
| 12 | 2-Hydroxyacetophenone | C8H8O2 | 0.94 | 1.42 | 1.89 | 2.7 | |
| 13 | Cinnamaldehyde | C9H8O | 99.57 | 78.76 | 67.95 | 53.03 | 30.25 |
| 14 | Ethyl cinnamate | C11H12O2 | 1.32 | 1.98 | 3.14 | 4.6 | |
| 15 | Cinnamic acid | C9H8O2 | 3.58 | 4.21 | 5.39 | 5.32 | |
| 16 | Unknown component | 1.3 | 0.92 | 1.21 | 1.1 | ||
According to the results of GC-MS, the similarity of each component was retrieved from the mass spectrometry library. The content of cinnamaldehyde was consistent with that of the raw material in the contrast experiment and the other components were not detected. It indicates that cinnamaldehyde shows no reactivity under nitrogen atmosphere. On the contrary, cinnamaldehyde is easy to oxidize under oxygen atmosphere. The double bond outside the benzene ring was broken to produce the epoxide of cinnamaldehyde (3-phenyloxirane-2-carbaldehyde)6 with the three-ring space configuration and the epoxide continued to react with oxygen to generate phenylacetaldehyde, benzaldehyde, benzoic acid, 2-hydroxyacetone, and other oxidation products. The oxygen consumption and the cinnamaldehyde oxidation depth added to the increase in the oxidation temperature. The degree of allyl oxidation was deepened to form aldehydes, ketones, and alcohols, which continued to react with oxygen to produce deep oxidation products such as benzoic acid and cinnamic acid. The cinnamaldehyde conversion increased with the rising temperature. The reaction amount of cinnamaldehyde increased from 30% to 75% with an increase in the temperature rising from 308 K to 338 K under high pressure, which confirmed the process of rapid oxygen consumption at 338 K. It indicates that both temperature and oxygen concentration are important factors in the cinnamaldehyde oxidation reaction.
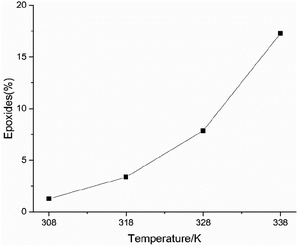
The yield of the epoxide increased with rising temperature, as shown in Fig. 12. Peroxide is a transitional molecule38 in the formation of the epoxide. A higher temperature generates more peroxide and intense peroxide decomposition triggers free radical reactions to produce more epoxide. The results of the peroxide value showed that more peroxide and epoxide were produced under high pressure. The formation of epoxide also reflects the thermal decomposition of peroxide. The peroxide decomposition reaction is a rapid exothermic process and releases small gaseous substances, and it has a high thermal hazard. For the safety of cinnamaldehyde production, storage, and transportation, it is important to study the pathway of cinnamaldehyde oxidation with cinnamaldehyde oxidation products.
Pathways of cinnamaldehyde oxidation
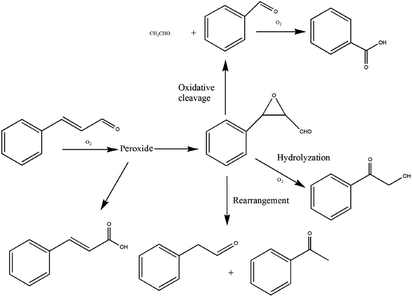
The reaction pathway is very important to understand the oxidation activity, stability, and hazards of cinnamaldehyde. Friedman39 et al. reported the mechanism of the postulated transformation of cinnamaldehyde to benzaldehyde and glyoxal. The postulated mechanism involves the transformation of cinnamaldehyde to the ring-opening peroxide. The peroxide biradical then dimerizes to a cyclic dioxetane, which then undergoes the indicated elimination to form the postulated products. Benzaldehyde, formaldehyde,40 or glyoxal were formed by ring-opening.From the analysis of peroxide and the oxidation products and the mechanism reported in the literature, we proposed the possible oxidation reaction pathway of cinnamaldehyde in MCPVT, which is shown in Scheme 1. Oxygen was absorbed by cinnamaldehyde and the highly reactive double bond outside the ring was destroyed to form the peroxide. A series of free radical reactions was initiated by the peroxide to produce epoxide with tricyclic configuration. The protonated epoxide was hydrolyzed and oxidized selectively to 2-hydroxyacetone.41 The aldehyde group of epoxide was rearranged to produce phenylacetaldehyde or acetophenone. Benzaldehyde was formed by the oxidative cleavage of epoxide and continued to be oxidized to benzoic acid. Furthermore, cinnamaldehyde easily reacted with oxygen to produce cinnamic acid through the stage of peroxide formation.13 Based on the results of the previous research and the above experiment, the unstable conjugated group in cinnamaldehyde molecule is the main factor affecting its oxidative instability. Thus, enough attention should be paid to the instability of cinnamaldehyde with conjugated double bonds. It is significant to isolate the oxidants and add inert gases for the sake of cinnamaldehyde safety.
Conclusion
The characteristics and hazards of the cinnamaldehyde oxidation process were studied by MCPVT. The conclusions are as follows:(1) The thermal stability of cinnamaldehyde was investigated and it was found to be stable under nitrogen atmosphere. Also, it was very unstable under oxygen atmosphere. The oxidation reaction was detected even at 308 K.
(2) A new experimental method for the oxidation kinetics of cinnamaldehyde was proposed. The initial oxidation kinetics is second order and Ea is 18.57 kJ mol−1.
(3) The initial oxidation of cinnamaldehyde to form peroxides was analyzed by iodimetry, where the peroxide concentration is related to the reaction temperature and time. It is important to elucidate the stability, oxidation reaction pathway, and hazard evaluation of cinnamaldehyde.
(4) The explosion hazard of cinnamaldehyde oxidation was observed by MCPVT. This is very noteworthy for the safety of cinnamaldehyde production and use.
(5) The oxidation products of cinnamaldehyde were analyzed by GC-MS and the reaction pathway was speculated. The cinnamaldehyde oxidation process can be divided into three stages: first, cinnamaldehyde reacts with oxygen to form peroxides; second, the complex oxidation reactions are caused by the thermal decomposition of peroxides; third, rapid oxidation and thermal decomposition lead to an explosion hazard.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21776050), National Institute of Advanced Industrial Science and Technology Fellowship of Japan, Major Science and Technology Special Project in Guangxi (AA17204087-20).Notes and references
- S. Viazis, M. Akhtar, J. Feirtag and F. Diez-Gonzalez, Food Microbiol., 2011, 28, 149–157 CrossRef PubMed.
- Y. Wang, K. Feng, H. Yang, Y. Yuan and T. Yue, RSC Adv., 2018, 8, 5806–5815 RSC.
- K. Feng, P. Wen, H. Yang, N. Li, W. Y. Lou, M. H. Zong and H. Wu, RSC Adv., 2017, 7(3), 1572–1580 RSC.
- C. W. Chang, W. L. Chang, S. T. Chang and S. S. Cheng, Water Res., 2008, 42, 278–286 CrossRef CAS PubMed.
- S. B. Fang, H. Y. Ko, S. T. Huang, C. H. Huang, L. T. Li, C. C. Chen, K. C. Wang, C. P. Pai, H. C. Lee and H. W. Fang, RSC Adv., 2015, 5, 22097–22105 RSC.
- H. Chen and H. Ji, Chin. J. Chem. Eng., 2011, 19, 972–977 CrossRef CAS.
- P. C. Chiang and J. W. Bode, Org. Lett., 2011, 13, 2422–2425 CrossRef CAS PubMed.
- H. Chen and H. Ji, AIChE J., 2010, 56, 466–476 CrossRef CAS.
- H. Chen, H. Ji, X. Zhou, J. Xu and L. Wang, Catal. Commun., 2009, 10, 828–832 CrossRef CAS.
- L. Wang, Y. She, W. Xu, Y. Zhang and H. Ji, Prog. Chem., 2005, 17, 678–685 CAS.
- Z. J. Yang, H. Zeng, X. T. Zhou and H. B. Ji, Supramol. Chem., 2013, 25, 233–245 CrossRef CAS.
- P. K. Tandon, M. Purwar, S. Singh, N. Srivastava and M. Srivastava, J. Mol. Catal. A: Chem., 2008, 293, 39–44 CrossRef CAS.
- A. W. Pound and J. R. Pound, J. Phys. Chem., 1934, 38, 1045–1049 CrossRef CAS.
- Y. D. Tian, Study on Thermal Decomposition Characteristics of Organic Peroxides, Nanjing University of Science & Technology, 2013 Search PubMed.
- M. Jacoby, C&EN Global Enterprise, 2016, 94(15), 17–18 Search PubMed.
- A. Schreck, A. Knorr, K. D. Wehrstedt, P. A. Wandrey, T. Gmeinwieser and J. Steinbach, J. Hazard. Mater., 2004, 108, 1–7 CrossRef CAS PubMed.
- M. Davaatseren, Y. J. Jo, G. P. Hong, H. J. Hur, S. Park and M. J. Choi, Molecules, 2017, 22, 1–12 CrossRef PubMed.
- V. Fábos, G. Koczó, H. Mehdi, L. Boda and I. T. Horváth, Energy Environ. Sci., 2009, 2, 767–769 RSC.
- D. Russo, D. Spasiano, M. Vaccaro, R. Andreozzi, G. Li Puma, N. M. Reis and R. Marotta, Chem. Eng. J., 2016, 283, 243–250 CrossRef CAS.
- Y. Li, X. Xu, M. Niu, J. Chen, J. Wen, H. Bian, C. Yu, M. Liang, L. Ma, F. Lai and X. Liu, Energy Fuels, 2019, 33(11), 11200–11209 CrossRef CAS.
- D. E. Clark, Chem. Health Saf., 2001, 8, 12–22 CrossRef CAS.
- Y. Wang, L. Vernières-Hassimi, V. Casson-Moreno, J. P. Hébert and S. Leveneur, Org. Process Res. Dev., 2018, 22(9), 1092–1100 CrossRef CAS.
- X. M. Liu, K. Hasegawa and M. Tamura, J. Nat. Disasters, 2003, 12, 122–126 Search PubMed.
- X. M. Liu and K. Hasegawa, 23th International Pyrotechnics Seminar, Tsukuba, Japan, October 1–3, 1997, pp. 313–325 Search PubMed.
- United Nations, UN Recommendations on the Transport of Dangerous Goods – Manual of Tests and Criteria: Section 38.3, Manual of Tests and Criteria, 2016 Search PubMed.
- X. Liu, S. Ito and Y. Wada, Energy, 2015, 82, 184–192 CrossRef CAS.
- X. Liu, Q. Zhang, S. Ito and Y. Wada, Fuel, 2016, 165, 513–525 CrossRef CAS.
- A. S. Tomlin, T. Turányi and M. J. Pilling, Low-Temperature Combustion and Autoignition, 1997 Search PubMed.
- S. Ding, Huagong Xuebao, 1999, 50(3), 386–391 CAS.
- F. Battin-Leclerc, O. Herbinet, P. A. Glaude, R. Fournet, Z. Zhou, L. Deng, H. Guo, M. Xie and F. Qi, Angew. Chem., Int. Ed., 2010, 49, 3169–3172 CrossRef CAS PubMed.
- H. Jiang, S. Yan and T. Wei, Huagong Xuebao, 2011, 62, 1290–1295 CAS.
- X. M. Liu, P. X. Huang, K. Katoh, Y. J. Wada and M. Tamura, Huagong Xuebao, 2008, 2634–2637 CAS.
- Q. Zhang, Y. F. Zheng, X. M. Liu, B. Wang, L. Ma, F. Lai and X. D. Zhou, Energy Fuels, 2017, 31, 8162–8170 CrossRef CAS.
- X. Liu, Q. Zhang, S. Ito and Y. Wada, Fuel, 2016, 165, 513–525 CrossRef CAS.
- P. Liu, X. Liu, S. Kubota, P. Huang and Y. Wada, J. Therm. Anal. Calorim., 2019, 138, 479–488 CrossRef CAS.
- B. Wang, X. M. Liu, X. N. Fu, Y. L. Li, P. X. Huang, Q. Zhang, W. G. Li, L. Ma, F. Lai and P. F. Wang, Fuel, 2018, 234, 207–217 CrossRef CAS.
- K. D. Wehrstedt, A. Knorr and P. Schuurman, J. Loss Prev. Process Ind., 2003, 16, 523–531 CrossRef.
- P. Wright and J. Abbot, Int. J. Chem. Kinet., 1993, 25, 901–911 CrossRef CAS.
- M. Friedman, N. Kozukue and L. A. Harden, J. Agric. Food Chem., 2000, 48, 5702–5709 CrossRef CAS PubMed.
- R. B. Silverman, X. Lu, J. J. P. Zhou and A. Swihart, J. Am. Chem. Soc., 1994, 116, 11590–11591 CrossRef CAS.
- S. Antoniotti and E. Duñach, Synthesis, 2003, 2753–2762 CAS.
| This journal is © The Royal Society of Chemistry 2020 |