Open Access Article
Open Access ArticleEffects of ultrasound irradiation on the properties of apricot kernels during accelerated debitterizing
Qing-An Zhang *ab,
Fang-Fang Shia,
Jian-Li Yaoa and
Ning Zhanga
*ab,
Fang-Fang Shia,
Jian-Li Yaoa and
Ning Zhanga
aInstitute of Food & Physical Field Processing, School of Food Engineering and Nutrition Sciences, Shaanxi Normal University, Xi'an, 710062 Shaanxi Province, PR China. E-mail: qinganzhang@snnu.edu.cn
bShaanxi International Science and Technology Cooperation Bases: Cereal Science International Joint Research Center, Xi'an, 710062 Shaanxi Province, PR China
First published on 12th March 2020
Abstract
In this paper, studies were conducted to investigate the effects of ultrasonically accelerated debitterizing on the physicochemical properties of apricot kernels, such as color, texture, oil content, protein characteristics and amino acids, with UV-Vis spectroscopy, synchronous fluorescence spectroscopy, circular dichroism, electrophoresis, environmental scanning electron microscopy, and thermal property analysis. The results indicate that the novel debitterizing technique has insignificant influences on the oil and protein contents of apricot kernels; meanwhile, the color, texture and activity of beta-glucosidase were substantially improved, greatly contributing to the quality modification and shortening the debitterizing time. In addition, ultrasound greatly influenced the amino acid contents and compositions, the fluorescence spectra and the thermal properties of the apricot kernel proteins. In a word, all these results greatly contribute to our understanding of the debitterizing mechanism mediated by ultrasound irradiation and further prove the feasibility of this novel debitterizing technique in the practical processing of apricot kernels.
1. Introduction
An apricot kernel is the fruit seed of apricots, which belongs to the genus Prunus of the subfamily Prunoideae in the family Rosaceae, and it is rich in many nutrients essential to the human body. To be specific, its oil content, mainly unsaturated fatty acids, is approximately 50.0% (g g−1);1 its protein content is 23.0–27.0%, which provides 15% of the total energy of the seeds, and is composed of 8 different essential amino acids, wherein the proportion of umami amino acids is larger.2 In addition, the contents of the total soluble sugars and dietary fibers account for about 5.5% and 12.2% of the whole seed weight, respectively.3 Based on these components, apricot kernels are considered to be one of the most nutritionally balanced foods in the human diet due to the benefits of these nutrients to human health, especially in controlling or preventing diseases such as cough, asthma, and cardiovascular diseases as well as weight maintenance.4 Amygdalin is a characteristic compound in apricot kernels; although its content is not the highest (2–5%),5 it is very important to the product development and function evaluation of apricot kernels. Amygdalin, also called vitamin B17 or nitriloside, is considered to be nontoxic by itself; however, when ingested, it is easily hydrolyzed by beta-glycosidase to yield one molecule of hydrogen cyanide (HCN), two molecules of glycose, and one molecule of benzaldehyde,6 and these metabolic products are potentially toxic. Cyanide can cause sudden death because it is rapidly taken up into cells and blocks mitochondrial electron transport within seconds. Due to their high amygdalin content and relatively facile release of HCN, apricot seeds have drawn more attention from toxicologists than other seeds.7 Generally, the toxicity depends on the intake dose of the seeds. In other words, a lower intake dose is beneficial to health, while a higher dose is toxic (the lethal dose of HCN is 0.5 to 3.5 mg kg−1 body weight).8 Therefore, the debitterizing operation is an indispensable step during the processing of apricot kernels, through which the content of amygdalin is reduced to safe limits, resulting in the removal of the bitterness and potential toxicity of apricot kernels.9The commonly employed methods for debitterizing apricot kernels include two categories: conventional and novel methods. The former comprises cold water debitterizing,10 hot water debitterizing,11 acid solution debitterizing and acid-base alternative debitterizing, while the latter includes vacuum debitterizing,12 ultrasound-assisted debitterizing and microwave-assisted debitterizing.13 Regarding conventional debitterizing methods, the main disadvantages are high energy consumption, time waste (from 6 to 7 hours to 6 to 7 days), labor-intensiveness, high pollution and high loss of nutritional compounds in apricot kernels. For instance, the contents of proteins, carbohydrates, phenols, flavonoids and amygdalin have been detected in the debitterizing water concentrate (DWC) generated from conventional industrial debitterizing, and the total loss of weight is about 17% of the apricot kernels after debitterizing.14 In comparison, novel techniques are relatively rapid, eco-friendly, low-cost and readily industrialized. Among these, ultrasound is regarded as the most promising technique for accelerating the debitterizing of apricot kernels.15 As a non-thermal processing technology, ultrasound irradiation usually influences the components and physicochemical properties of food by generating high-frequency vibrations and acoustic cavitation. In our previous studies, ultrasound was proved to greatly reduce the debitterizing time to 1 to 2 h, and the water consumption could also be decreased greatly;15 meanwhile, little information is available about the effects of ultrasound irradiation on the physicochemical properties of apricot kernels, such as color, protein and oil, during rapid debitterizing, which determines the quality of the debitterized apricot kernels to a great extent.
Ultrasonically accelerated debitterizing is basically regarded as the extraction of amygdalin driven by ultrasound irradiation from the apricot seeds into the debitterizing solution. As a novel technology, ultrasound-assisted extraction (UAE) has attracted much attention and has been widely used in the fields of natural products extraction in recent years, such as the extraction of antioxidant compounds,16 polyphenols,17 and ginsenosides.18 Generally, the mechanism of UAE is attributed to the synergistic performance of the cavitation, mechanical and thermal effects of the ultrasound, which can destroy the cell walls, reduce particle sizes and enhance the mass transfer, resulting in increased extraction efficiency.19 Also, the acoustic cavitation produced from the propagation of ultrasound waves is usually considered to be the main effect. In addition, ultrasound irradiation can cause greater penetration of the solvent into the sample matrix through mechanical effects, improving the contact area between the solid and the liquid; as a result, the target compounds rapidly diffuse from the solid into the solvent.20 Great attention has been paid to the mechanical and physical aspects of UAE in order to explain its mechanism; meanwhile, its effects on non-target compounds in the sample are often neglected, which is exactly what we are concerned with. That is to say, the effects of ultrasound irradiation on the quality of apricot kernels should be seriously considered during accelerated debitterizing.
The aim of this study was to investigate the effects of ultrasonically accelerated debitterizing on the physicochemical properties of apricot kernels, including their color, texture, chemical composition, proteins and amino acids, to understand the mechanism of debitterizing by ultrasound and its effects on the quality of the seeds. All these results provide a theoretical base to evaluate the feasibility of ultrasonically accelerated debitterizing in the practical production of apricot kernels.
2. Materials and methods
2.1. Materials and reagents
Apricot kernels were purchased from the Northwest Herb Market in the city of Xi'an (Shaanxi Province, China). The water content of the apricot kernels is about 4.84 g water/100 g seeds. The beta-glucosidase activity assay kit, BCA protein assay kit (50 T) and SDS-PAGE gel preparation kit were all purchased from Solarbio Co. Ltd. (Beijing, China). Amygdalin (98.42% pure) was purchased from Chengdu Preferred Bio-Technology Co. Ltd (Chengdu, Sichuan Province, China). HPLC-grade methanol (99.9% pure) was bought from Thermo Fisher Scientific Co. Ltd (Shanghai, China). All other reagents were of analytical grade, including sodium hydroxide, sodium carbonate, glycerol, bovine serum protein, G-250, 3,5-dinitrosalicylic acid, glucose, hydrochloric acid, Folin–Ciocalteu reagent, and sodium chloride. These reagents were purchased from Tianli Chemical Reagent Co. Ltd (Tianjin, China). Ultrapure water was prepared using a Millipore Milli-Q purification system.2.2. Preparation of debitterized apricot kernels by ultrasound irradiation
360 g of apricot kernels peeled with hot water were placed in a 5000 mL beaker containing 4320 mL water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, g
12, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL), which was sealed with Parafilm to avoid evaporation of the solution. Debitterizing was conducted under the conditions of 55 °C, 59 kHz and 300 W, with the beaker fixed at a designated position in the ultrasonic bath (SB-500DTY, Ningbo Xinzhi Biotechnology Co. Ltd., Ningbo City, Zhejiang Province, China).15,21 During the operation, samples were collected at intervals; each time, 30 g apricot kernels and 360 mL debitterizing solution were respectively taken out until the debitterizing was complete. After that, the physicochemical properties of the collected samples were investigated, including amygdalin, color, texture, and proteins, to understand the effects of the ultrasonically accelerated debitterizing on the quality of the apricot kernels.
mL), which was sealed with Parafilm to avoid evaporation of the solution. Debitterizing was conducted under the conditions of 55 °C, 59 kHz and 300 W, with the beaker fixed at a designated position in the ultrasonic bath (SB-500DTY, Ningbo Xinzhi Biotechnology Co. Ltd., Ningbo City, Zhejiang Province, China).15,21 During the operation, samples were collected at intervals; each time, 30 g apricot kernels and 360 mL debitterizing solution were respectively taken out until the debitterizing was complete. After that, the physicochemical properties of the collected samples were investigated, including amygdalin, color, texture, and proteins, to understand the effects of the ultrasonically accelerated debitterizing on the quality of the apricot kernels.
2.3. Determination of moisture content
The moisture content was determined for the above-collected samples after heating them an oven at a temperature of 105 °C to a constant weight.132.4. Determination of the amygdalin and beta-glucosidase activities of the apricot kernels
A certain amount of each collected sample was weighed and powdered; then, the amygdalin was extracted from the powder of apricot kernels with methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, g
10, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL) under the conditions of ultrasound power (300 W), 59 kHz (frequency), 35 °C (temperature) and 45 min (ultrasound time).13 Subsequently, the amygdalin in the extraction solution was determined according to the reference with a slight modification.11 To be specific, the amygdalin was separated by an HPLC system (Dalian Elite Analytical Instruments Co. Ltd., Liaoning, China) consisting of two P230II pumps, a UV230II ultraviolet visible detector and a ZW230II column oven. A column of TC-C18 (250 mm × 4.6 mm, 5 μm) was placed in the column with the oven set at 35 °C. The mobile phase consisted of water and methanol (90
mL) under the conditions of ultrasound power (300 W), 59 kHz (frequency), 35 °C (temperature) and 45 min (ultrasound time).13 Subsequently, the amygdalin in the extraction solution was determined according to the reference with a slight modification.11 To be specific, the amygdalin was separated by an HPLC system (Dalian Elite Analytical Instruments Co. Ltd., Liaoning, China) consisting of two P230II pumps, a UV230II ultraviolet visible detector and a ZW230II column oven. A column of TC-C18 (250 mm × 4.6 mm, 5 μm) was placed in the column with the oven set at 35 °C. The mobile phase consisted of water and methanol (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, mL
10, mL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL, degassed by ultrasound for 20 min), and the flow rate was 1 mL min−1. The detection wavelength was 215 nm, and the injection volume was 10 μL. The amygdalin was identified by comparing the retention time with that of the corresponding injected standard, and its content was calculated according to the working curve constructed by the external standard method.
mL, degassed by ultrasound for 20 min), and the flow rate was 1 mL min−1. The detection wavelength was 215 nm, and the injection volume was 10 μL. The amygdalin was identified by comparing the retention time with that of the corresponding injected standard, and its content was calculated according to the working curve constructed by the external standard method.
Considering that beta-glucosidase greatly contributes to the debitterizing of apricot kernels, the beta-glucosidase activity was investigated for the samples according to the ref. 15.
For ease of comparison, the contents are all expressed as the percentage of the dry base weight with subtraction of the moisture content.
2.5. Determination of oil content
The apricot kernels were dried and ground into powder with a cyclone mill, then placed into a conical flask (150 mL) with petroleum ether as the solvent. Finally, the oil was ultrasonically extracted according to the literature,11 and the defatted powder was stored at −18 °C until further analysis.2.6. Color and texture analysis of the apricot kernels
The color of the apricot kernel powder was measured by an automatic color difference meter (SC-80C, Beijing Kangguang Optical Instrument Co., Ltd., China) and the parameters of L*, a*, b* and ΔE were recorded.The texture of the apricot kernels was measured by a texture analyzer (TA.XTPlus, Stable Micro Systems, United Kingdom), including the hardness, fracturability, adhesiveness, springiness, cohesiveness, gumminess, chewiness and resilience of the apricot kernels, to understand the effects of ultrasound irradiation on the organoleptic properties of the debitterized apricot kernels.
2.7. Effects of the ultrasonically accelerated debitterizing on the physicochemical properties of proteins in apricot kernels
The defatted powder and deionized water were mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (g mL−1), followed by adjusting the value of pH to 9.0 with 1 mol L−1 NaOH solution and stirring for 1 h at 40 °C. Subsequently, the mixture was centrifugated and the pH of the collected supernatant was adjusted with 1 mol L−1 HCl solution to 4.5, followed by centrifugation at 4000 rpm for 20 min; the precipitate was finally obtained, frozen under vacuum and stored for further use.
25 (g mL−1), followed by adjusting the value of pH to 9.0 with 1 mol L−1 NaOH solution and stirring for 1 h at 40 °C. Subsequently, the mixture was centrifugated and the pH of the collected supernatant was adjusted with 1 mol L−1 HCl solution to 4.5, followed by centrifugation at 4000 rpm for 20 min; the precipitate was finally obtained, frozen under vacuum and stored for further use.
2.8. Statistical analysis
All the results were statistically analyzed by calculating the mean and standard deviation and are presented as the mean ± standard deviation of three determinations. Analysis of variance (ANOVA) was conducted using SPSS statistics software, version 20.0 (SPSS Inc. Chicago, IL, USA).3. Results and discussion
3.1. Changes in amygdalin during the ultrasonically accelerated debitterizing of apricot kernels
As shown in Fig. 1, the content of amygdalin remaining in the apricot kernels quickly decreased with increasing ultrasound time; the residues were less than 0.91 mg g−1, which is a threshold value to evaluate whether the apricot kernel is bitter.13 That is to say, the apricot kernels had no bitter taste when the debitterizing time reached 90 min or longer. Compared with conventional hot-water debitterizing, ultrasonically accelerated debitterizing could greatly shorten the required time from 7 to 8 h to about 1.5 h,11 suggesting the feasibility of this novel technique. Generally, two reasons can explain the high efficiency of ultrasonically accelerated debitterizing. Firstly, the mechanical and cavitation effects of ultrasound waves can produce a large shear force and many microbubbles; this breaks the boundary layer, strengthens the surface erosion, and increases the contact and collision between the materials and the liquids, resulting in rapid mass transfer or degradation of the amygdalin in the apricot kernels.24 Secondly, the ultrasound cavitation can also activate the activity of beta-glucosidase,15 which subsequently accelerates the degradation of amygdalin, disrupts the original balance and increases the dissolution of the amygdalin, resulting in shortening of the debitterizing time. Furthermore, it is worth mentioning that the aim of debitterizing is not to completely remove all the amygdalin from the apricot kernels, but to allow them to retain a certain amount of amygdalin at which the bitterness cannot be tasted; in this way, the positive bioactive function of the amygdalin can also be protected. Considering this, the apricot kernels ultrasonically debitterized for 90 min were regarded as completely debitterized; this sample of debitterized apricot kernels was used in the following experiments.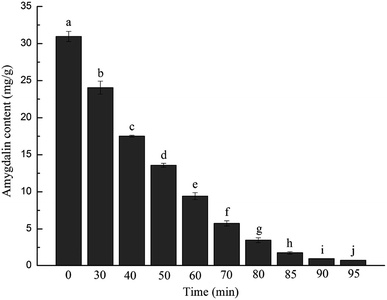 | ||
| Fig. 1 Changes in amygdalin content during the ultrasonically accelerated debitterizing of apricot kernels. | ||
3.2. Color changes of apricot kernels before and after debitterizing
The CIELAB system with the parameters of L*, a*, b* has been widely employed for measuring color differences in the food industry; L* indicates lightness (0 to 100), with 0 being black and 100 being white, the coordinate a* is for red (+) and green (−), and the coordinate b* is for yellow (+) and blue (−). Furthermore, the limits of a* and b* are approximately + or −80.25 The values of a* were 9.14 ± 0.39 and 5.44 ± 0.22 and the values of b* were 22.55 ± 3.60 and 13.14 ± 0.77 before and after debitterizing; it is obvious that significant changes in the values of a* and b* occurred for the apricot kernels after being ultrasonically debitterized, while there is no significant difference in the L* values (94.52 ± 0.01 and 95.80 ± 0.80). To be specific, the a* and b* values significantly decreased after debitterizing, suggesting that the red and yellow colors decreased, while the brightness of the apricot kernels was not greatly influenced. ΔE is the comprehensive color difference deviation, and the theoretical limit of perception of the human eye (ΔE = 3.0) has been suggested as an absolute color discrimination threshold for food. Regarding the parameters of a*, b* and ΔE, it can be concluded that the debitterizing can change the color of apricot kernels by decreasing the levels of red and yellow; this variation can be recognized by the naked human eye because the ΔE value of 10.27 is much higher than the threshold value of 3.0. Also, these variations can be attributed to the transfer or removal of phenolic compounds from the external surface of the peeled apricot kernel, which is driven by ultrasound.263.3. Texture variations of apricot kernels after debitterizing
Generally, texture can reflect the taste of food and must regarded as an important indicator to evaluate the sensory properties of food; a texture analyzer is usually employed to determine the texture characteristics of food.27 As shown in Table 1, the hardness of the ultrasonically debitterized apricot kernels was lower than that of the untreated apricot kernels, and similar changes occurred in the cohesiveness, gumminess, chewiness and resilience. In contrast, the fracturability, adhesiveness and springiness of the ultrasonically treated samples were higher than those of the untreated apricot kernels. All these results greatly contribute to explain the difference in the organoleptic properties between the untreated and debitterized apricot kernels. Overall, ultrasound can not only accelerate the debitterizing but can also influence the sensory properties of apricot kernels, in turn affecting their edible and commercial value.| Parameter | Apricot kernels | Debitterized apricot kernels |
|---|---|---|
| a Different letters in the same row indicate significant differences at p < 0.05. | ||
| Hardness/Ha (×104) | 3.21 ± 0.15a | 2.71 ± 0.05b |
| Fracturability/Fr (×104) | 0.93 ± 0.03a | 1.12 ± 0.18a |
| Adhesiveness/Ad | −105.42 ± 6.72a | −16.80 ± 2.29b |
| Springiness/Sp | 0.51 ± 0.04a | 0.57 ± 0.02a |
| Cohesiveness/Co | 0.51 ± 0.05a | 0.33 ± 0.04b |
| Gumminess/Gu (×104) | 1.33 ± 0.03a | 0.64 ± 0.03b |
| Chewiness/Ch (×103) | 6.30 ± 0.29a | 3.69 ± 0.30b |
| Resilience/Re | 0.35 ± 0.02a | 0.17 ± 0.02b |
3.4. Changes in the moisture, protein and oil contents, and beta-glucosidase activity of apricot kernels
As shown in Fig. 2(A), the water content of the apricot kernels increased significantly after debitterizing; this can enable the amygdalin to be easily dissolved, transferred and degraded, resulting in accelerated debitterizing and texture variation of the apricot kernels. In the meantime, the moisture content can also be used to monitor the drying procedure and calculate the dry weight of the components. The activity of beta-glucosidase was greatly improved after ultrasonically accelerated debitterizing, as shown in Fig. 2(B); this can be attributed to the effects of ultrasound cavitation on the conformation and secondary structure of the enzymes15 and the increased water content in the apricot kernels because the activity of beta-glucosidase will increase once the enzyme contacts the water in the seed. Apricot kernels contain high amounts of recommended monounsaturated oleic acids, a moderate amounts of linoleic acid and low amounts of saturated fatty acids; thus, its fatty acid composition may be more favorable than that of olive oil. Additionally, the oils contain active compounds such as vitamin E.28 Regarding the contents of protein and oil, there were no significant differences after debitterizing, as shown in Fig. 2(C) and (D); i.e. the ultrasonic debitterizing did not cause great loss of these components, which is different from the results obtained from traditional hot-water debitterizing,11 illustrating the advantage of ultrasonically accelerated debitterizing.29 In addition, ultrasonic treatment is different from microwave treatment of apricot kernels, which changes their oil content and fatty acid composition.30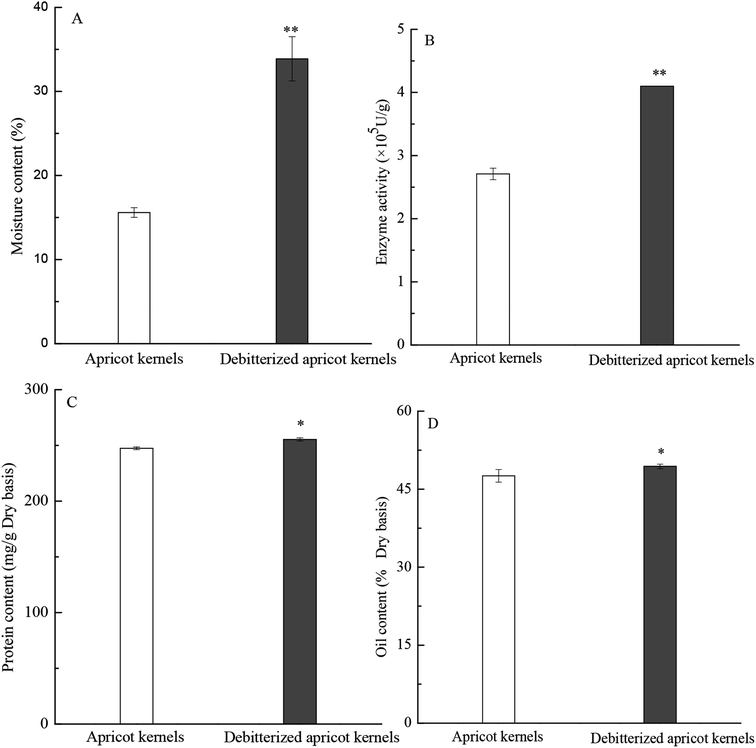 | ||
| Fig. 2 (A) Moisture, (B) the beta-glucosidase activity, (C) protein contents and (D) oil of apricot kernels before and after debitterizing. | ||
3.5. Effects of ultrasonically accelerated debitterizing on the physicochemical properties of proteins in apricot kernels
| Secondary structure | Apricot kernel protein | Debitterized apricot kernel protein |
|---|---|---|
| α-Helix (%) | 15.40 | 16.00 |
| Antiparallel (%) | 17.70 | 16.20 |
| Parallel (%) | 16.70 | 17.80 |
| β-Turn (%) | 21.30 | 20.10 |
| Random coil (%) | 49.10 | 49.50 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times by ESEM. Overall, the differences in the morphology of the protein aggregations of the two samples were significant. In the pictures magnified 1000 times, the proteins of the untreated apricot kernels are closely clustered together, while the proteins of the ultrasonically-accelerated debitterized apricot kernels are slightly dispersed. In the images magnified 5000 times, the surface of the proteins can be observed to contain many pores. Furthermore, the protein surface of the debitterized samples was slightly damaged in comparison with the sample without debitterizing.35 With further magnification of 10
000 times by ESEM. Overall, the differences in the morphology of the protein aggregations of the two samples were significant. In the pictures magnified 1000 times, the proteins of the untreated apricot kernels are closely clustered together, while the proteins of the ultrasonically-accelerated debitterized apricot kernels are slightly dispersed. In the images magnified 5000 times, the surface of the proteins can be observed to contain many pores. Furthermore, the protein surface of the debitterized samples was slightly damaged in comparison with the sample without debitterizing.35 With further magnification of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times, there is no significant difference in the morphology of the proteins; however, the morphology of the sample became slightly more porous and was slightly damaged after debitterizing by ultrasound irradiation. In a word, the morphology of the proteins was not greatly influenced by the novel ultrasonically accelerated debitterizing of apricot kernels, which is consistent with some of the results obtained above by other analyses.
000 times, there is no significant difference in the morphology of the proteins; however, the morphology of the sample became slightly more porous and was slightly damaged after debitterizing by ultrasound irradiation. In a word, the morphology of the proteins was not greatly influenced by the novel ultrasonically accelerated debitterizing of apricot kernels, which is consistent with some of the results obtained above by other analyses.| Amino acid | Apricot kernel protein | Debitterized apricot kernel protein |
|---|---|---|
| a Different letters in the same row indicate significant differences at p < 0.05. | ||
| Aspartic acid/Asp | 8.54 ± 0.40a | 9.56 ± 0.25b |
| Threonine*/Thr | 2.26 ± 0.09a | 2.51 ± 0.08a |
| Serine/Ser | 2.64 ± 0.11a | 3.51 ± 0.11b |
| Glutamic acid/Glu | 15.28 ± 0.12a | 19.32 ± 0.45b |
| Proline/Pro | 6.42 ± 0.45a | 6.07 ± 1.21a |
| Glycine/Gly | 3.62 ± 0.28a | 4.20 ± 0.14b |
| Alanine/Ala | 3.83 ± 0.29a | 3.99 ± 0.10a |
| Cystine/Cys | 1.38 ± 0.07a | 1.69 ± 0.05a |
| Valine*/Val | 3.55 ± 0.02a | 3.57 ± 0.10a |
| Methionine*/Met | 0.42 ± 0.05a | 0.13 ± 0.02b |
| Isoleucine*/Ile | 3.12 ± 0.11a | 3.66 ± 0.06a |
| Leucine*/Leu | 5.38 ± 0.12a | 6.08 ± 0.19b |
| Tyrosine/Tyr | 1.98 ± 0.04a | 2.45 ± 0.08a |
| Phenylalanine*/Phe | 4.24 ± 0.01a | 4.81 ± 0.21b |
| Lysine*/Lys | 2.55 ± 0.18a | 2.66 ± 0.12a |
| Histidine/His | 2.14 ± 0.06a | 2.39 ± 0.17a |
| Arginine/Arg | 7.28 ± 0.18a | 8.47 ± 0.44b |
| Total amino acids/TAA | 74.62 ± 0.05a | 85.06 ± 3.78b |
| Essential amino acids/EAA | 21.50 ± 0.03a | 23.41 ± 0.79a |
| Children essential amino acids/CEAA | 9.42 ± 0.24a | 10.85 ± 0.61b |
| Non-essential amino acids/NEAA | 53.12 ± 0.05a | 61.65 ± 3.00b |
| Hydrophobic (non-polar) amino acids/NAA | 26.95 ± 0.80a | 20.30 ± 1.89b |
| Hydrophilic (polar) amino acids/PAA | 8.26 ± 0.13a | 10.16 ± 0.32b |
| Basic amino acids/BAA | 11.97 ± 0.06a | 13.51 ± 0.73b |
| Acidic amino acids/AAA | 23.82 ± 0.52a | 28.88 ± 0.70b |
| Aromatic amino acids/ARAA | 6.21 ± 0.05a | 7.26 ± 0.29b |
| Sulphur amino acids/SAA | 1.80 ± 0.03a | 1.82 ± 0.08a |
| EAA/NEAA | 0.40 ± 0.00a | 0.38 ± 0.00a |
| EAA/TAA | 0.29 ± 0.00a | 0.28 ± 0.00a |
| CEAA/TAA | 0.13 ± 0.00a | 0.13 ± 0.00a |
4. Conclusions
Ultrasonically accelerated debitterizing did not significantly influence the oil and protein contents of apricot kernels, with only a very small weight loss caused by ultrasound during the debitterizing; meanwhile, the color, texture and the activity of beta-glucosidase were greatly improved, which greatly contributes to the quality modification and shortens the debitterizing time. Considering the effects of debitterizing on the properties of apricot kernel proteins, the results indicate that ultrasound did greatly influence the amino acid contents and compositions, the fluorescence spectrum, and the thermal properties of the apricot kernel proteins, i.e. ultrasound affected the molecular and structural characteristics of the proteins. In a word, all these results greatly contribute to the understanding of the debitterizing mechanism mediated by ultrasound irradiation and further proved the feasibility of this novel debitterizing technique in practical processing of apricot kernels. However, modulation of this technique should be further investigated to reduce the loss of weight and nutrients and increase the improvement of the quality and efficiency in the actual production of apricot kernels.Conflicts of interest
The authors of the present work declare no conflict of interest.Acknowledgements
This study was supported by the National Natural Science Foundation of China [Nos. 31101324, 31972206] and the Key Research Development Program of Shaanxi Province, China [No. 2018ZDXM-NY-086].References
- G. Egea, M. M. González-Real, A. Baille, P. A. Nortes, P. Sánchez-Bel and R. Domingo, Agric. Water Manag., 2009, 96, 1605–1614 CrossRef.
- D. P. Richardson, A. Astrup, A. Cocaul and P. Ellis, Food Sci. Technol. Bull. Funct. Foods, 2009, 6, 41–50 CrossRef.
- S. Yada, K. Lapsley and G. W. Huang, J. Food Compos. Anal., 2011, 24, 469–480 CrossRef CAS.
- S. G. Li, F. Geng, P. Wang, J. K. Lu and M. H. Ma, J. Sci. Food Agric., 2016, 96, 3351–3357 CrossRef CAS.
- I. F. Bolarinwa, C. Orfila and M. R. Morgan, Food Chem., 2014, 152, 133–139 CrossRef CAS PubMed.
- Z. Zdrojewicz, A. Otlewska, P. Hackemer and A. Otlewska, Pol. Merkuriusz Lek., 2015, 38, 300–303 Search PubMed.
- N. Karsavuran, M. Charehsaz, H. Celik, B. M. Asma, C. Yakìncì and A. Aydìn, Toxicol. Environ. Chem., 2014, 96, 1564–1570 CrossRef CAS.
- I. F. Bolarinwa, C. Orfila and M. R. A. Morgan, Food Chem., 2015, 170, 437–442 CrossRef CAS PubMed.
- S. Sahin, J. Health Med. Inf., 2011, 2, 106–107 Search PubMed.
- A. Silem, H. O. Günter, J. Einfeldt and A. Boualia, Int. J. Food Sci. Technol., 2006, 41, 201–213 CrossRef CAS.
- Y. Song, Q.-A. Zhang, X. H. Fan and X. Y. Zhang, J. Food Process. Preserv., 2018, 42, 1–8 Search PubMed.
- Q. H. Zhang, J. Li, J. H. Pang, J. Cui and J. Z. Wang, Sci. Technol. Food Ind., 2014, 35, 248–252 CAS.
- N. Zhang, X. Y. Zhang, X. H. Fan and Q.-A. Zhang, Food & Machinery, 2018, 34, 189–194 Search PubMed.
- Q.-A. Zhang, C. X. Wei, X. H. Fan and F. F. Shi, CyTA - J. Food, 2018, 16, 422–428 CrossRef CAS.
- X. H. Fan, X. Y. Zhang, Q.-A. Zhang, W. Q. Zhao and F. F. Shi, Ultrason. Sonochem., 2019, 52, 468–476 CrossRef CAS PubMed.
- M. B. Hossain, N. P. Brunton, A. Patras, B. Tiwari, C. P. O'Donnell, A. B. Martin-Diana and C. Barry-Ryan, Ultrason. Sonochem., 2012, 19, 582–590 CrossRef CAS PubMed.
- S. S. Teh and E. J. Birch, Ultrason. Sonochem., 2014, 21, 346–353 CrossRef CAS.
- H. M. Lin, Y. G. Zhang, M. Han and L. M. Yang, Ultrason. Sonochem., 2013, 20, 680–684 CrossRef CAS.
- S. R. Shirsath, S. H. Sonawane and P. R. Gogate, Chem. Eng. Process, 2012, 53, 10–23 CrossRef CAS.
- B. Kumari, B. K. Tiwari, M. B. Hossain, N. P. Brunton and D. K. Rai, Food Bioprocess Technol., 2018, 11, 223–241 CrossRef CAS.
- N. Zhang, Q.-A. Zhang, J. L. Yao and X. Y. Zhang, Ultrason. Sonochem., 2019, 58, 104614 CrossRef PubMed.
- S. G. Li, S. Chu, J. K. Lu, P. Wang and M. H. Ma, J. Food Process. Preserv., 2018, 42, 1–7 CrossRef PubMed.
- R. Shibue, T. Sasamoto, M. Shimada, B. Zhang, A. Yamagishi and S. Akanuma, Sci. Rep., 2018, 8, 1–8 CrossRef CAS PubMed.
- Q.-A. Zhang, H. Shen, X. H. Fan, Y. Shen, X. Wang and Y. Song, Ultrason. Sonochem., 2015, 22, 149–154 CrossRef CAS PubMed.
- S. Suzanne Nielsen, Food analysis laboratory manual, Springer, New York, Dordrecht, Heidelberg, London, 2nd edn, 2010, DOI:10.1007/978-1-4419-1463-7.
- L. Feng, M. Zhang, B. Adhikari and Z. M. Guo, Food Bioprocess Technol., 2018, 11, 1328–1338 CrossRef CAS.
- F. Hamedi, M. Mohebbi, F. Shahidi and E. Azarpazhooh, Food Bioprocess Technol., 2018, 11, 1061–1074 CrossRef CAS.
- B. Matthaus and M. M. Özcan, J. Food Lipids, 2009, 16, 187–199 CrossRef.
- B. M. Iqdiam, H. Mostafa, R. Goodrich-Schneider, G. L. Baker, B. Welt and M. R. Marshall, Food Bioprocess Technol., 2018, 11, 634–644 CrossRef CAS.
- F. A. l. Juhaimi, M. Musa Ozcan, K. Ghafoor and E. E. Babiker, Food Chem., 2018, 243, 414–419 CrossRef PubMed.
- Q.-A. Zhang, X. Z. Fu and J. F. García Martín, Ultrason. Sonochem., 2017, 37, 405–413 CrossRef CAS PubMed.
- P. F. Qin, R. T. Liu, X. R. Pan, X. Y. Fang and Y. Mou, J. Agric. Food Chem., 2010, 58, 5561–5567 CrossRef CAS PubMed.
- D. J. Li, Y. Wang, J. J. Chen and B. M. Ji, Spectrochim. Acta, Part A, 2011, 79, 680–686 CrossRef CAS PubMed.
- J. Rosa-Millán, J. L. Orona-Padilla, V. M. Flores-Moreno and S. O. Serna-Saldívar, Int. J. Food Sci. Technol., 2018, 53, 1414–1424 CrossRef.
- A. B. Stefanović, J. R. Jovanović, M. B. Dojčinović, S. M. Lević, V. A. Nedović, B. M. Bugarski and Z. D. Knežević-Jugović, Food Bioprocess Technol., 2017, 10, 1224–1239 CrossRef.
- R. Jaenicke, Eur. J. Biochem., 1991, 202, 715–728 CrossRef CAS.
- S. N. Swain, K. K. Rao and P. L. Nayak, Polym. Int., 2005, 54, 739–743 CrossRef CAS.
- R. Ram, R. V. Chand, A. Forrest and P. C. Southgate, LWT--Food Sci. Technol., 2017, 86, 261–269 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |