Open Access Article
Open Access ArticleGreen pathway to a new fuel extender: continuous flow catalytic synthesis of butanol/butyl butyrate mixtures
Nicola Scotti a,
Nicoletta Ravasio
a,
Nicoletta Ravasio a,
Federica Zaccheria
a,
Federica Zaccheria *a,
Adrian Irimescub and
Simona Silvia Merola
*a,
Adrian Irimescub and
Simona Silvia Merola b
b
aIstituto di Scienze e Tecnologie Chimiche “G. Natta”, c/o Dipartimento di Chimica, CNR, Via Golgi 19, 20133 Milano, Italy. E-mail: federica.zaccheria@scitec.cnr.it
bIstituto Motori, CNR, Via G. Marconi 4, 80125 Napoli, Italy
First published on 17th January 2020
Abstract
The preparation of a butanol/butyl butyrate mixture was performed in one-step under continuous flow conditions with a CuO/ZrO2 catalyst. The catalytic system allows one to directly obtain up to 40–42% of butyl butyrate starting from butanol via a dehydrogenative coupling reaction without using solvent or additives. The obtained mixture was tested in a direct injection spark ignition engine as a blend of 70%vol gasoline and 30%vol butanol/butyl butyrate mixture. One of the main goals was to evaluate overall performance and whether knock tendency increased compared to the reference condition that featured gasoline only fueling. Exhaust gas pollutants were evaluated as well, so as to give a more complete picture of environmental impact effects. Overall engine performance and emissions were found to be comparable to those obtained for the reference case, with negligible increase in knocking characteristics.
Introduction
The depletion of fossil sources combined with the need to reduce greenhouse gases prompted the search for sustainable alternatives for the fuel sector in the last decades. In particular the possibility of using waste biomass or agro industrial residues to obtain platform molecules for fuel blends appears to be the most convenient way.1 Thus the possibility of dipping into the lignocellulosic biomass stream makes oxygenated molecules available that offer several advantages in terms of environmental impact, namely in reducing greenhouse gas emissions, due to their low content of pollutants like sulfur, and in ensuring complete combustion reducing particulate formation.2Among these, several alcohols, esters and ethers have been proposed as both additives and blends and the presence of oxygen atoms could significantly alter polarity and affect energy density depending on the substrate.
In particular, alcohols lead to corrosion and degradation of engine and fuel line components as a result of solubilizing water from the air and incompatibility with the materials present in conventional vehicles. In this respect the use of butanol, instead of ethanol (the most common alcohol employed as biofuel), provides some advantages in terms of reduced polarity and higher energy density, due to an increased C/O ratio.3 The properties of butanol make it a better alternative to ethanol as they are closer to gasoline.4 Moreover it could be produced through a fermentation process (the Acetone–Butanol–Ethanol process, ABE) that especially in the past few years has been under continuous improvement, being now an economically viable and competitive route, with a global market of butanol expected to reach 17.78M USD by 2022.5
Also pentanol is a promising molecule, with a greater potential as a diesel blending component because of its higher energy density, higher cetane number, better blend stability and less hygroscopic nature compared to lower alcohols. The production of renewable bio-pentanol is currently under research through biological pathways like natural microbial fermentation, using engineered micro-organisms and bio-synthesis from glucose using Escherichia coli.6
On the other hand, also different esters have been proposed as candidates for fuel utilization, due to their lower polarity and generally higher boiling point.7
Long chain esters derived from oils and fats find suitable application in diesel manufacturing, while they are not appropriate for gasoline blend. The application of biodiesel suffers from the variability of the resulting fuel depending on the source and growing conditions, the oxidative instability of most biodiesel fuels and the poor low temperature behaviour.8
Levulinate and valerate esters have variable properties depending on the chain length coming from the alcoholic counterpart, making them suitable for gasoline or diesel blending9 and they can be produced starting from levulinic acid that in turn is obtained from cellulose and/or hemicellulose upgrading.10–12
Also HMF and furfuryl esters possess high energy density, that is close to the one of gasoline.11,12
Among the esters, butyl butyrate, is completely miscible with gasoline. In addition it has a flash point above the minimum required in the aviation standard and a melting point far lower than kerosene making it a potential biofuel for this application.8 Moreover it has a higher boiling point than the alcohols and a more similar distillation profile to Jet-A-1.13 Thus, butyl butyrate is a very promising compound, that could be produced starting from bio-butanol. In this scenario to find an efficient process for its production is an interesting point to look at.
Unfortunately the traditional esterification methods on large scale mainly rely on the use of the corresponding carboxylic acids as the reagent. In particular, Fischer esterification promoted by homogeneous acid catalysts is mainly employed. Some interesting biocatalytic approaches have been proposed by coupling the fermentative production of butanol with the esterification14 or by carrying out the alcohol/acid condensation with Novozyme 435 directly in the fuel, thus obtaining the final blend at the end of the reaction.15
Different strategies could be used, as is the case of the O-alkylation of an alkene with a carboxylic acid or the Tishchenko reaction (which is an aldehyde homocoupling promoted by Al(OR)3), for the production of ethyl acetate.
Anyhow, the use of carboxylic acids entails their preparation from the corresponding alcohols by oxidation, that is not yet a mature process from a sustainability point of view, as most current technologies employ stoichiometric, toxic and expensive oxidants, with the production of a large quantity of wastes.
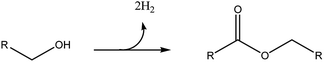
Therefore, when having in hand bioalcohols, as in the case of ethanol or butanol, their direct transformation into the corresponding symmetric esters could be a very significant improvement. In this respect, the dehydrogenative coupling (DHC) reaction is a very attractive green strategy. In this process two molecules of alcohols are straight combined to form the ester, together with the release of two molecules of H2 (Scheme 1).
With respect to the traditional esterification of an acid with an alcohol, DHC shows a very high atom economy and it does not require oxidation of the alcohol into the acid, resulting in both reduction of wastes and profitable process intensification.
Some of us already reported that a CuO/ZrO2 catalyst with high Cu–Zr interdispersion is a very active and selective material for the DHC process under solvent-free batch conditions, without the use of O2 or additives. This process was optimized to obtain butyl butyrate in 98% yield after 24 h by H2 removal, while in short reaction times a 40% yield was reached.16
Here we wish to report on the preparation of a butanol/butyl butyrate mixture under continuous flow conditions with the same synthetic approach and on its use as a fuel. As already mentioned, butyl-butyrate is considered as an option for aviation or diesel engines,8,13 applications for which auto-ignition quality is looked-for as a property. On the contrary, for spark ignition (SI) power units auto-ignition is to be avoided, as it can incur knocking events, phenomena that can cause severe engine damage. This was actually one of the main reasons of concern and constituted the basis for organizing the engine tests, with related operating conditions.
To evaluate the end-use of the butanol/butyl butyrate mixture as a fuel, measurements were performed on a direct injection (DI) SI engine. Its architecture allowed optical accessibility through the piston crown, and can be considered as representative for automotive size units. A blend of 70%vol RON95 gasoline and 30%vol butanol/butyl butyrate mixture was compared to the pure commercial fuel in medium load conditions.
Overall engine output was evaluated (indicated power and variability), as well as regulated exhaust emissions. With an emphasis on knock tendency, three different spark timing settings were tested, with wide open throttle (WOT) and stoichiometric fueling. The optical data obtained in cycle-resolved mode allowed detailed characterization of diffusive flames and these results were correlated to exhaust gas opacity measurements. In this way, apart from an overall parameter (i.e. opacity, directly correlated to the concentration of particles), more insight was obtained into the process of soot formation.
Experimental
Materials
All chemicals were obtained from Sigma-Aldrich, except for 1-butanol (>99.8%) that was purchased from Delchimica. Silica-doped ZrO2 (ZrO2, SiO2 = 3.5% by weight, MELCat XZO 1521) was obtained from MEL Chemicals.Methods
CuO/ZrO2 catalyst was prepared by the chemisorption–hydrolysis (CH) method by adding the support to an aqueous [Cu(NH3)4]2+ solution prepared by dropping NH4OH to a Cu(NO3)2·3H2O solution until pH 9 has been reached. After 20 min under stirring, the slurry, held in an ice bath at 0 °C, was diluted with water. The solid was separated by filtration with a Büchner funnel, washed with water, dried overnight at 120 °C, and calcined in air at 350 °C. The copper loading was 16 wt%.The DHC reaction was carried out in a trickle-bed reactor (Vinci Technologies). In a typical run 1.2 g of catalyst were packed into the tubular reactor and dehydrated in situ at 270 °C under N2 flow (24 mL min−1, 40 min). Then the temperature was raised to 350 °C and the reaction was performed using a 1-butanol flow of 0.150 mL min−1, a N2 flow of 8 mL min−1 and a pressure of 3 atm. The liquid hourly space velocity (LHSV) calculated as F/V, was 7.0 h−1, where F is the feed rate of butanol and V the catalyst volume.
Each catalytic run lasted 80–100 h in which 700–900 mL of 1-butanol/butyl butyrate mixture were produced. The reaction mixture was analyzed by 1H NMR with dimethylformamide as internal standard. The butyl butyrate yield ranged between 45–55% and few amount of butanal (Y = 0–5%) and dibutyl ether (0–2%) were detected. The composition of the final mixture was: 1-butanol = 52%; butyl butyrate = 42%; butanal = 4%; dibutyl ether = 2%.
Carbon balance was evaluated by measuring the weight of the stream solution with respect to the fed one, obtaining a value superior to 97%. In a typical measurement, a sample of the stream solution was collected for 60 min and weighed and the value compared to the theoretical one, calculated on the basis of the flow rate.
Thermogravimetric analysis (TGA) was conducted on a PerkinElmer 7 HT thermobalance. Analyses were performed on the used catalyst by heating the sample from 50 to 1000 °C under air with a temperature ramp of 5 °C min−1.
Engine tests
Combustion experiments were performed in a single cylinder SI engine that featured a Bowditch design,17 which ensured optical accessibility through the piston crown. This setup allowed operation with the two fuel types in conditions relevant for automotive applications, with detailed information on the combustion related processes, including their in-cylinder distribution. More details on engine specifics and procedures applied for data processing are available in previous publications.18–21 Apart from the intent of providing an overview of indicated power output and effects on emissions, one cause of concern was the possibility of knock occurrence.22 For this reason, the influence of spark timing was one of the main parameters that was scrutinized, with three settings, at 5, 10 and 15 crank angle degrees (CAD) before top dead center (bTDC). This allowed different values of maximum amplitude of pressure oscillation23 (MAPO) to be compared for the two fuels. Engine speed was maintained at 2000 rpm with an electric motor/brake that allowed the SI unit to be motored prior to combustion trials. Continuous firing was employed and 200 consecutive cycles were recorded for each operating point, after reaching a quasi-steady thermal regime. Wide open throttle (WOT) was employed for all tests, load condition representative for medium indicated mean effective pressure (IMEP) levels. In-cylinder pressure was measured with an accuracy of 1% and resolution of 0.2 CAD, using a piezo-electric transducer flush-mounted to the cylinder head. Stoichiometric air–fuel ratio was employed, controlled by using the readings of an exhaust gas oxygen sensor with an accuracy of 1%. Differences in stoichiometric air–fuel ratio (AFR) was compensated for the blend by increasing the duration of injection by 2 CAD. To give an idea into the difference with respect to gasoline and ethanol, AFRmixture is 10.84 at a density of 830 kg m−3 and lower heating value (LHV) of 33.42 MJ kg−1; this compares to 14.69 at 740 kg m−3 and LHV 44.9 MJ kg−1 (ref. 24) for the commercial fuel and 8.95 at 790 kg m−3 with 27.4 MJ kg−1 for the short-chain alcohol. With these values (calculated based on C/H/O content), the blend containing 30% of the new compound features an AFR of 13.44, density of 770 kg m−3 and LHV 41.2 MJ kg−1, and thus required an increase of the injection time of around 5%. It should be noted that the difference between the LHV of stoichiometric air–fuel ratios is less than 0.5%, with 2.86 MJ kg−1 for gasoline and 2.85 MJ kg−1 for the blend, due to the increase of fuel so as to maintain the same AFR.25 The actual value of 30% volumetric concentration was chosen based on previous experience with gasoline–butanol blends,26 that were found to feature good performance up to this level, mainly due to evaporative issues.27 As previously mentioned, knock was another reason of concern, and the 30% threshold was chosen for further safety margin. No compatibility issues with the fuel system were encountered during the measurements and overall stability of the blends with gasoline was deemed as high, as no visible separation of the liquids was noted for a period of around 2 months.The start of injection was kept the same for both fuels, at 300 CAD bTDC, during the intake stroke. This point of fuel delivery was found to be the best compromise between available jet penetration and fluid velocity for gasoline, as well as alcohols.19
Exhaust gas measurements were performed using a gas analyzer capable of measuring CO, unburned hydrocarbons (HC) intended as n-hexane equivalent, NOx (mostly NO) and oxygen. Smoke opacity was also evaluated using an opacimeter working with a light source–detector combination based on the Beer–Lambert law. All these measurements featured an accuracy around 3%.28
Optical data recorded in cycle-resolved mode (100 frames per cycle, step 2.4 CAD, during 30 consecutive cycles) was used for characterizing in detail the distribution and size of diffusive flames. These sites were the result of liquid fuel film oxidation and given their low air–fuel ratio, are prone to formation of carbonaceous particles. The analysis was performed by applying a procedure developed with NI Vision Assistant that allowed to extract the luminous layer specific for this oxidation phenomenon at the surface of the liquid film. More details on the procedure are available in ref. 19 and ref. 21 as well as an explanation of the difference with respect to the methodology used for evaluating flame front propagation.
Results and discussion
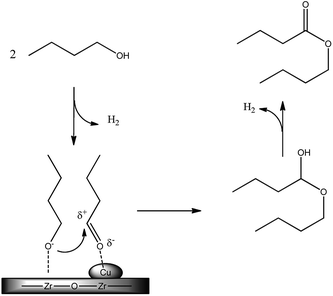
A CuO/ZrO2 catalyst was already reported by some of us to be the most performing one for butanol to butyl butyrate DHC reaction under batch conditions without the use of solvent or additives.16In the previous study, the very high activity and selectivity observed were ascribed to the high interspersion of copper on zirconia, as shown by STEM analysis. Actually the catalyst prepared by copper deposition over ZrO2 with the chemisorption–hydrolysis method presents a very high dispersion of the metallic phase, although the high loading, and an intimate contact with ZrO2 phase, thus resulting in a synergic activity. The presence of basic sites of ZrO2 probably mainly promotes the formation of the alkoxide, that in turns attacks the aldehyde formed and activated over copper sites, as shown in Scheme 2.
The use of flow conditions is recognized to be in many cases a key point forward green engineering due to important advantages related with the enhanced heat and mass transfer, precise residence time control, shorter times, increased safety, reproducibility and easy scalability.29
Therefore the zirconia supported copper catalyst was used under flow conditions by varying the catalyst loading, the pressure and the flow in order to reach the best balance between selectivity and activity.
We studied the possibility to develop and optimize the DHC process with a continuous trickle-bed reactor to produce a 1/1 butanol/butyl butyrate mixture that was studied on a real engine.
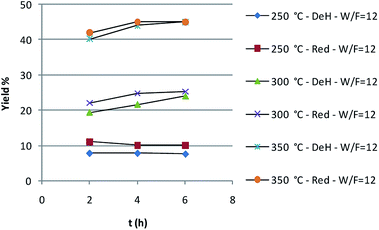
As shown in Fig. 1, temperature has a dramatic effect on the butyl butyrate yield, that from an 8% at 250 °C rises to 55% at 350 °C. The need to work at a temperature higher with respect to the batch reactor is due to operational conditions, that do not allow to reproduce the same contact time.
This aspect also affects somehow the selectivity. In fact, some amounts of aldehyde and of dibutyl ether were detected, while obtaining very good yield in the ester and a final mixture with the following composition: 1-butanol = 50–52%; butyl butyrate = 40–42%; butanal = 0–4%; dibutyl ether = 0–2%.
On the other hand the pre-reduction of the catalyst before the catalytic run does not lead to dramatic effects.
The catalysts resulted to be stable and effective up to more than 90 hours on stream as shown in Fig. 2. The robustness of the catalyst is moreover witnessed by analysis carried out after flow reaction.
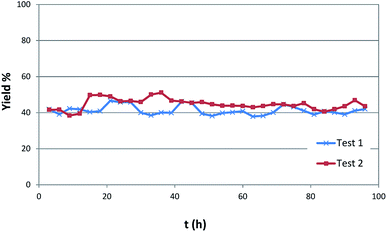 | ||
| Fig. 2 Yield of butyl butyrate vs. time in two different catalytic runs under the same conditions (350 °C, 0.150 mL min−1 of 1-butanol, 8 mL min−1 of N2, P = 3 atm). | ||
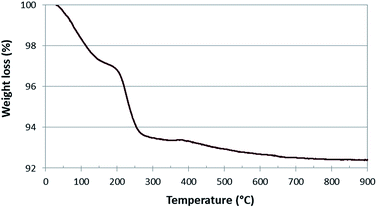
The very few amount of carbon deposit observed confirms the scarce tendency of the solid systems to poisoning. Thus, elemental analysis shows the presence of carbon amount lower than 3%.
TGA experiment carried out on the used catalyst, shows a weight loss of about 3% due to the carbon deposited in the temperature range 200–300 °C (Fig. 3). This value is in agreement with the one obtained by elemental analysis. Moreover the low temperature of the process (with a maximum at 225 °C on the basis of DTA-not reported) indicates the presence of an easy removable carbon, while the presence of amorphous or graphitic carbon oxidizing at high temperature, can be excluded.30,31
Productivity obtained in terms of converted moles with respect to catalyst weight put in light the advantages offered by the flow process. Thus we obtained a productivity of 20 molconv gcat−1 vs. 4.4 molconv gcat−1 obtained under the condition previously described.16
As previously noted, engine measurements were aimed at an overall evaluation of performance and environmental impact. In brief, the main idea was to implement the characterization of fuel effects on engine operation with an emphasis on indicated output, emissions and possible side-effects such as an increase of tendency towards the occurrence of knock, phenomena characterized by pressure oscillations that can cause engine damage.22 This was of particular concern, given the relatively elongated molecular structure of the butyl butyrate and dibutyl ether components. Several studies have identified long chain structures as more prone to auto-ignition, events that can cause significant engine damage and increased heat loss. Even small concentrations exert important effects on the occurrence of knocking episodes.32 On the other hand, a well-placed ether group can be beneficial, given that relatively weak carbon–oxygen bonds tend to break up the fuel into shorter fragments.33
The combustion tests showed that engine performance, evaluated through the indicated mean effective pressure (IMEP), was comparable for the two fuel types. For brevity and smoother flow of discussion, only an overview of the results is presented, with an emphasis on knock tendency and formation of particulate matter. Nonetheless, the main conclusion was that overall power output was around 7 bar IMEP (7.41 bar for gasoline and 7.46 for the blend, with 10 CAD bTDC spark timing); relative differences were below 1%, thus completely comparable when partially substituting gasoline. Adding the butanol/butyl butyrate mixture (identified as butyl30 in the legend of each figure) resulted in a slight improvement of combustion stability. For example, in the case of 10 CAD bTDC spark timing, a value of 1.56% was recorded for the coefficient of variation (COVimep) compared to 1.87% for gasoline (as reference, the upper limit for this parameter is around 5%22). No clear conclusion could be drawn based on these data, but mixing alcohols with gasoline up to a certain concentration could improve evaporative properties and thus induce more homogenous fuel distribution within the combustion chamber.34
As previously mentioned, one of the main goals of the study was to evaluate whether the addition of the butanol/butyl butyrate mixture would have an influence of knocking intensity. The two advanced ignition settings (i.e. 10/15 CAD bTDC) show situations slightly retarded/advanced with respect to the point of maximum brake torque. These operating conditions give an idea of the spark timing related to a “safe” zone (i.e. 10 CAD bTDC) and the outlier of the knock limited ignition advance region (i.e. 15 CAD bTDC).35
For more detailed evaluation, the in-cylinder pressure data was post-processed for evaluating the MAPO parameter (by applying a 5 kHz high pass filter). Evidently, higher values are correlated to increased possibility of knock occurrence, hence augmented risk of engine damage. Given that the data was averaged over the 200 consecutive cycles that were recorded (i.e. shown values are the average of MAPO calculated for individual cycles contained in each set), it combined the intensity and frequency of pressure oscillations. Only a minor increase of the MAPO parameter was observed for the butyl 30 fuel type (Fig. 4) with spark timing around MBT. As expected, an increasing trend was recorded when advancing ignition. The 5 CAD bTDC setting with gasoline is an exception, but given the relatively low MAPO values, no definite conclusion could be drawn.
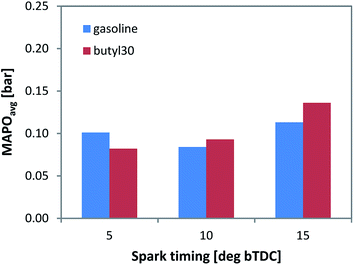 | ||
| Fig. 4 Average MAPO recorded for the two fuels during 200 consecutive firing cycles, with three spark timing settings. | ||
Nonetheless, the data confirm that adding the butanol/butyl butyrate mixture did not cause an increase in knock tendency for the chosen concentration of 30%vol. Just to give an idea into the single event magnitude, the peak MAPO value was 0.35 bar for gasoline and 0.38 bar for the butyl 30 blend; to put things into perspective, the 5% of the peak motored pressure threshold generally used for identifying knocking cycles36 is around 0.9 bar. Of course, in commercial engines, higher peak pressure and MAPO values are to be expected, but the relative difference between the two fuels suggests that 30% of gasoline can be safely replaced with the butanol/butyl butyrate mixture.
For brevity, CO, HC and NOx concentrations are not shown. The influence of using the butyl30 blend compared to gasoline was minimal, again suggesting trouble-free substitution of gasoline with the proposed fuel. A positive aspect is that NOx emissions were reduced by 5–10%, more consistently for the condition with advanced ignition.
Opacity measurements allowed an overall evaluation of the influence on particle emissions. Values recorded for gasoline were 0.24, 0.26 and 0.22 for 5, 10 and 15 CAD bTDC ignition timing respectively; using the butyl30 blend showed an overall reduction of opacity by around 10%, with the same trend as that recorded for the commercial fuel. This is in line with previous studies performed by the authors that showed lower tendency for the formation of soot when blending butanol with gasoline.37 On the other hand, increasing the concentration of alcohol may actually result in higher soot emissions, due to evaporation issues.19 For this reason, a more detailed analysis was performed by using the optical data recorded in cycle-resolved mode.
Fig. 5 shows an overview of the distribution obtained with the two fuel types. Each pair of x–y coordinates was associated with the size of the flame identified by the post-processing procedure (i.e. the larger the object, the larger the bubble size). As expected, most bright spots were located near the intake valves, close to the injector.20
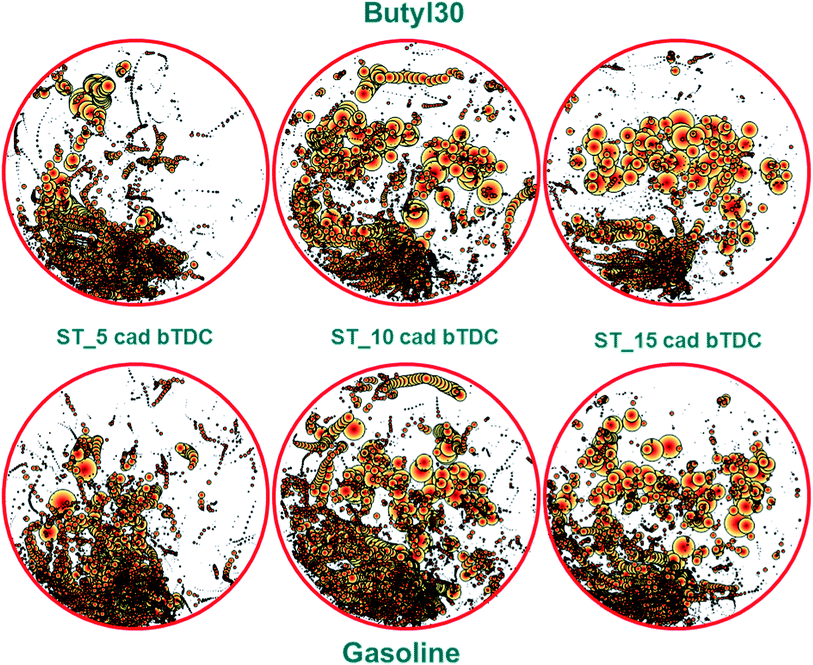 | ||
| Fig. 5 Bubble chart illustrating the distribution of diffusive flames for the investigated conditions; the orientation of each image is consistent with that shown in Fig. 1 (i.e. intake valves and injector on the bottom side and exhaust valves on top). | ||
The two fuels featured similar spread of diffusive flames, suggesting only minor modifications in evaporative properties and consequent distribution of liquid film on the piston crown. An interesting observation is that a common shift of the overall location of diffusive flames was noted towards the exhaust side as ignition was advanced. This effect is most likely correlated to the spark ignited flame–liquid film interaction; more to the point, for retarded ignition there was less unevaporated fuel at the time the normally propagating flame hit the piston surface, thus resulting in less regions where locally rich oxidation occurred. Nonetheless, it can be concluded that higher quantity of liquid film was present in the region closer to the injector and that these sites persisted longer than those close to the exhaust valves (Fig. 5).
To resume the analysis of soot generating sites location and size, the overall area of diffusive flame for each case confirm the values recorded with the opacimeter, with larger soot generating areas for gasoline. An interesting observation is that the optical data features the same trend as that of the opacity, with a peak for both fuel types when using the 10 CAD bTDC ignition setting. No clear explanation for this trend could be identified, but the most likely reason behind the observed effects is the change in the interaction between the normally propagating flame and the liquid film on the piston crown.
Conclusions
A material based on copper supported over zirconia demonstrated to be an efficient and stable catalyst for the production of a mixture of butanol/butyl butyrate via a dehydrogenative coupling reaction under no solvent flow conditions. The catalytic system proved to be active up to 90 hours without significant loss in selectivity and productivity, thus exploiting the advantages of a continuous reactor.The obtained mixture was tested in a direct injection spark ignition engine blended at 30%vol with gasoline and it showed comparable performance with respect to gasoline in terms of power output and safety with respect to knock tendency. Moreover the addition of butanol/butyl butyrate mixture was found to reduce the duration of the initial stage of combustion, resulting in a slight peak pressure increase. Another positive aspect is that NOx concentrations were lower by 5–10% compared to gasoline, and opacity values were reduced by 10%. The latter result was also confirmed by the optical data, which also provided important information on the distribution of particle generating sites.
All these evidences show that the mixture prepared with a catalytic one-step process from butanol is a valid and sustainable choice for gasoline replacement.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is based upon work from COST Action FP1306 Valorisation of lignocellulosic biomass side streams for sustainable production of chemicals, materials & fuels using low environmental impact technologies, supported by COST (European Cooperation in Science and Technology) E-mail: www.cost.eu.Notes and references
- M. H. Kamani, I. Eş, J. M. Lorenzo, F. Remize, E. Rossellò-Soto, F. J. Barba, J. Clark and A. M. Khaneghah, Green Chem., 2019, 21, 3213–3231 RSC.
- K. Nithyanandan, J. Zhang, Y. Li, H. Wu, T. H. Lee, Y. Lin and C. F. Lee, Fuel, 2016, 174, 333–343 CrossRef CAS.
- R. W. Jenkins, C. M. Moore, T. A. Semelsberger, C. J. Chuck, J. C. Gordon and A. D. Sutton, ChemSusChem, 2016, 9, 922–931 CrossRef CAS PubMed.
- A. W. Bhutto, K. Qureshi, R. Abro, K. Harijan, Z. Zhao, A. A. Bazmi, T. Abbase and G. Yu, RSC Adv., 2016, 6, 32140–32170 RSC.
- S. B. Bankar, S. A. Survase, H. Ojamo and T. Granström, RSC Adv., 2013, 3, 24734–24757 RSC.
- B. R. Kumar and S. Saravanan, Renewable Sustainable Energy Rev., 2016, 60, 84–115 CrossRef.
- G. P. Lange, R. Price, P. M. Ayoub, J. Louis, L. Petus, L. Clark and H. Gosselink, Angew. Chem., Int. Ed., 2010, 49, 4479–4483 CrossRef PubMed.
- R. W. Jenkins, M. Munro, S. Nash and C. J. Chuck, Fuel, 2013, 103, 593–599 CrossRef CAS.
- E. Ahmad, Md. I. Alam, K. K. Pant and M. A. Haider, Green Chem., 2016, 18, 4804–4823 RSC.
- F. D. Pileidis and M.-M. Titirici, ChemSusChem, 2016, 9, 562–582 CrossRef CAS PubMed.
- R. Mariscal, P. Maireles-Torres, M. Ojeda, I. Sádaba and M. L. Granados, Energy Environ. Sci., 2016, 9, 1144–1189 RSC.
- M. J. Climent, A. Corma and S. Iborra, Green Chem., 2014, 16, 516–547 RSC.
- C. C. J. Chuck and J. Donnelly, Appl. Energy, 2014, 118, 83–91 CrossRef CAS.
- C. van den Berg, A. S. Heeres, L. A. M. van der Wielen and A. J. J. Straathof, Biotechnol. Bioeng., 2013, 110, 137–142 CrossRef CAS PubMed.
- M. Sjöblom, P. Risberg, A. Filippova, O. G. W. Öhrman, U. Rova and P. Christakopoulos, ChemCatChem, 2017, 9, 4529–4537 CrossRef.
- N. Scotti, F. Zaccheria, C. Evangelisti, R. Psaro and N. Ravasio, Catal. Sci. Technol., 2017, 7, 1386–1393 RSC.
- F. Bowditch, SAE Tech. Pap. Ser., 1961, 610002, DOI:10.4271/610002.
- S. S. Merola, A. Irimescu, S. Di Iorio and B. M. Vaglieco, Energies, 2017, 10(7), 832, DOI:10.3390/en10070832.
- A. Irimescu, S. S. Merola, S. Di Iorio and B. M. Vaglieco, Fuel, 2018, 216, 121, DOI:10.1016/j.fuel.2017.11.116.
- A. Irimescu, S. Merola and S. Martinez, SAE Int. J. Engines, 2018, 11(6), 1343, DOI:10.4271/2018-01-1421.
- S. Martinez, S. S. Merola and A. Irimescu, Appl. Sci., 2019, 9(3), 449, DOI:10.3390/app9030449.
- J. B. Heywood, Internal combustion engine fundamentals, McGraw Hill, NewYork, USA, 1988 Search PubMed.
- W. Zhia, L. Hui and R. D. Reitz, Prog. Energy Combust. Sci., 2017, 61, 78, DOI:10.1016/j.pecs.2017.03.004.
- S. Hosokai, K. Matsuoka, K. Kuramoto and Y. Suzuki, Fuel Process. Technol., 2016, 152, 399, DOI:10.1016/j.fuproc.2016.06.040.
- A. Irimescu, P. Romanian Acad. A, 2010, 11(4), 322 Search PubMed.
- A. Irimescu, Energy, 2011, 36, 3030, DOI:10.1016/j.energy.2011.02.047.
- A. Irimescu, Appl. Energy, 2012, 96, 477, DOI:10.1016/j.apenergy.2012.03.012.
- A. Irimescu, G. Vasiu and G. T. Tordai, Appl. Energy, 2014, 121, 196, DOI:10.1016/j.apenergy.2014.01.078.
- D. Dallinger and C. O. Kappe, Curr. Opin. Green Sustain. Chem., 2017, 7, 6–12 CrossRef.
- S. Wang and G. Q. Lu, J. Chem. Technol. Biotechnol., 2000, 75, 589–595 CrossRef CAS.
- Z. L. Zhang and X. E. Verykios, Catal. Today, 1994, 21, 589–595 CrossRef CAS.
- S. Gersen, M. van Essen, G. van Dijk and H. Levinsky, Combust. Flame, 2014, 161(10), 2729, DOI:10.1016/j.combustflame.2014.03.019.
- M. D. Boot, M. Tian, E. J. M. Hensen and S. M. Sarathy, Prog. Energy Combust. Sci., 2017, 60, 1, DOI:10.1016/j.pecs.2016.12.001.
- V. F. Andersen, J. E. Anderson, T. J. Wallington, S. A. Mueller and O. J. Nielsen, Energy Fuels, 2010, 24(6), 3647, DOI:10.1021/ef100254w.
- D. A. Splitter, A. Gilliam, J. Szybist and J. Ghandhi, Proc. Combust. Inst., 2019, 37(4), 4893, DOI:10.1016/j.proci.2018.05.145.
- S. S. Merola, C. Tornatore and A. Irimescu, Energy Convers. Manage., 2016, 127, 380, DOI:10.1016/j.enconman.2016.09.035.
- S. S. Merola, L. Marchitto, C. Tornatore, G. Valentino and A. Irimescu, SAE Int. J. Engines, 2013, 6(4), 1953, DOI:10.4271/2013-01-2638.
| This journal is © The Royal Society of Chemistry 2020 |