Open Access Article
Open Access ArticleSynthesis, photo and acidochromic properties of spiropyran-containing methanofullerenes†
A. A. Khuzin *a,
A. R. Tuktarov
*a,
A. R. Tuktarov a,
V. A. Barachevsky
a,
V. A. Barachevsky b,
T. M. Valovab,
A. R. Tulyabaeva and
U. M. Dzhemileva
b,
T. M. Valovab,
A. R. Tulyabaeva and
U. M. Dzhemileva
aInstitute of Petrochemistry and Catalysis, Ufa Federal Research Center of the Russian Academy of Sciences, 141 Prospekt Oktyabrya, Ufa, 450075, Russia. E-mail: artur.khuzin@gmail.com
bPhotochemistry Center, Russian Academy of Science, 7a, bld.1, ul. Novatorov, Moscow, 119421, Russia
First published on 21st April 2020
Abstract
Spiropyran-containing methanofullerenes able to rapidly and reversibly respond to optical and chemical stimuli were synthesized for the first time by the Bingel–Hirsch reaction and catalytic cycloaddition of diazo compounds to carbon clusters. The effects of substituent structure in the new hybrid molecule and the mode of spiropyran attachment to fullerene on the spectral kinetic properties and photo- and acidochromic behavior of the synthesized fullerene derivatives was established.
Introduction
A highly important trend in modern organic chemistry is the synthesis, design and study of new photochromic molecules able to reversibly isomerize under the action of various types of stimuli. The enhanced interest in these compounds is caused by their applicability for the development of promising materials and devices with unique characteristics: memory elements,1–3 optoelectronic and holographic devices,4,5 and sensors.6–11Spiropyrans are the best known and unique representatives of photochromic compounds, since they can be easily prepared and structurally modified and because of the possibility of targeted change of their spectral kinetic characteristics over a wide range. The isomers of spiropyrans considerably differ in their physical and optical properties, which inspired a number of studies on the use of these compounds as dynamic materials.12 An important place is occupied by studies directed towards the development of nanomaterials with controlled properties, e.g., light controlled carbon nanomolecules, fullerenes, nanotubes and graphenes.13–15
Recently,16 we performed for the first time the successful chemical bonding of C60 fullerene to spiropyrans via the Prato reaction and found17 that the photochromic properties of pyrrolidinofullerenes are substantially affected by the nature of the electron-withdrawing group in the pyran ring.
In order to pursue these studies directed towards the development of efficient methods for chemical bonding of fullerenes to photochromic spiro derivatives, to obtain new dynamic materials for light-controlled field effect transistors,18 and to study the effects of the fullerene core and spiropyran structure in the new hybrid molecule on the response to external stimuli, we synthesized spiropyran-containing methanofullerenes and studied their photo- and acidochromic behavior.
Experimental section
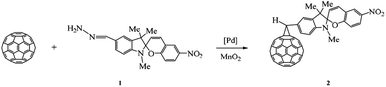
The target methanofullerenes were synthesized using the Bingel–Hirsch reaction and catalytic cycloaddition of diazo compounds to carbon clusters, which gave the possibility to study the effect of the mode of spiropyran attachment to fullerene on the physicochemical properties of new hybrid molecules.The first stage of our study was concerned with chemical bonding of spiropyrans to C60 fullerene upon catalytic cycloaddition of diazo compounds to carbon clusters.19 The reaction of C60 with a diazoalkane generated in situ by oxidation of spiropyran hydrazone 1 with MnO2 in the presence of three-component Pd(acac)2-2PPh3-4Et3Al catalyst (20 mol%) afforded methanofullerene 2 in 55% yield (Scheme 1).
The formation of 6,6-closed cycloadduct in the catalytic reaction between C60 and diazoalkanes is in good agreement with published data20 and is caused by the presence of a heterocyclic moiety in the molecule of the starting spiropyran hydrazone 1.
In order to study the effect of the mode of attachment of the spiro photochrome to the carbon cluster on the physicochemical properties of a new synthesized hybrid molecule, in the next stage of our study, we performed chemical bonding of C60 fullerene to spiropyran via the chromene moiety.
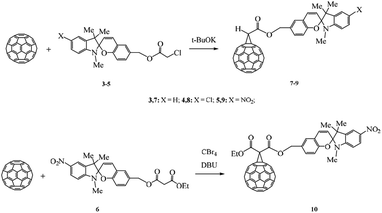
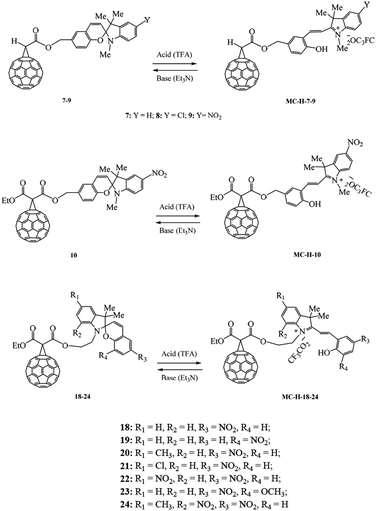
This aim was pursued using the nucleophilic addition of α-halo carbanions to C60 (Bingel–Hirsch reaction). The anions were generated in situ by the reaction of spiropyran chloroacetic 3–5 and malonic 6 acid esters with bases to give the corresponding methanofullerenes 7–10 in 60–70% yields (Scheme 2).
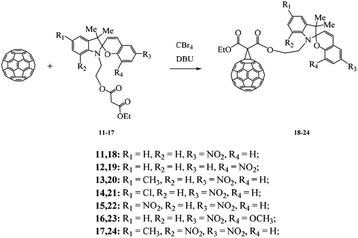
A similar approach was used to prepare hybrid molecules 18–24 in which fullerene was linked to the photochrome through the indole nitrogen atom of the starting spiropyran (Scheme 3). This approach to the preparation of C60 fullerene–spiropyran hybrid molecules gave hope, according to published data,21,22 that the prepared methanofullerenes 18–24 would possess negative photochromism (Scheme 3).
All of the C60- and spiropyran-based hybrid molecules 2, 7–10, 18–24 were isolated from the reaction mixture by column chromatography or preparative HPLC with ∼100% purity.
The structures of hybrid compounds 2, 7–10, 18–24 were reliably established by 1D (1H and 13C) and 2D (H-HCOSY, HSQC, HMBC) NMR spectroscopy and MALDI TOF/TOF mass spectrometry (see ESI†).
Results and discussion
In view of high sensitivity of initial spiropyrans to a broad range of external stimuli10,23–30 and to pursue our research aimed at the development of efficient synthetic routes to new molecular switches based on fullerenes and photochromic compounds,14–16,31–33 we investigated photochromic and acidochromic behaviors of methanofullerenes 2, 7–10, 18–24.The results of studying photochromic properties of the obtained compounds are presented in Table 1 (the Table 1 gives only characteristics of compounds that exhibit photochromic properties, namely, 2, 18–23).
| Compound | λmaxAa (nm) | εb (M−1 cm−1) | λmaxBa (nm) | ΔDmaxBc/DmaxAc | τphdgr1/2d, s |
|---|---|---|---|---|---|
| a λmaxA, λmaxB – are absorption maxima of the initial and photoinduced forms, respectively.b ε – molar extinction coefficient.c DmaxA, ΔDmaxB are, respectively, the optical density at the absorption maximum of the initial form and change of the optical density after UV irradiation.d τphdgr1/2 – the time during which the optical density of the photo-induced form is reduced by half under the influence of unfiltered radiation. | |||||
| 2 | 330 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
615 | 0.4 | 185 |
| 431 | |||||
| 18 | 328 | 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
608 | 0.30 | 260 |
| 428 | |||||
| 608 | |||||
| 19 | 328 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
605 | 0.15 | 135 |
| 428 | |||||
| 472 | |||||
| 20 | 330 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
610 | 0.30 | 205 |
| 428 | |||||
| 21 | 330 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
615 | 0.31 | 170 |
| 428 | |||||
| 465 | |||||
| 22 | 330 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
615 | 0.005 | 200 |
| 428 | |||||
| 482 | |||||
| 23 | 330 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
605 | 0.34 | 235 |
| 428 | |||||
| 462 | |||||
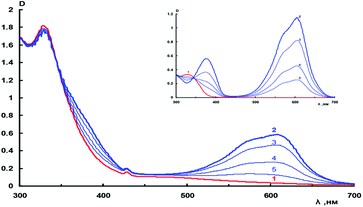
Fig. 1 (inset) depicts the photoinduced spectral changes of the starting spiropyran 11 in toluene; it can be seen that the absorption spectrum of the photoinduced merocyanine form shows an absorption maximum in the visible region at 605 nm. During dark relaxation, the intensity of photoinduced absorption bands gradually decreases. Photoinduced spectral changes of this type are also manifested for hybrid compound 18 in toluene. In this case, the long-wavelength absorption maximum occurs at 608 nm.
The decrease in the amplitude of intensity changes of the absorption bands of the photoinduced merocyanine form of spiropyran in methanofullerene 18 indicates that the latter undergoes photochromic transformations with gradual photodecomposition (Fig. 2).
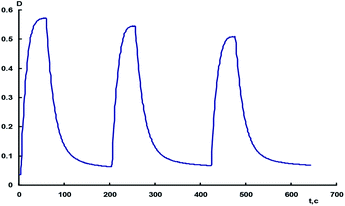 | ||
| Fig. 2 Kinetics of the alternating photocoloration of methanofullerene 18 in toluene measured at 608 nm under irradiation through a UFS-1 light filter and during bleaching in the dark. | ||
A comparison of the curves presented in Fig. 1 demonstrates the absence of significant spectral differences between nitro-substituted spiropyran 11 and spiropyran–fullerene hybrid compound 18, which is apparently indicative of weak interaction between the spiropyran and fullerene moieties. A difference is observed for light sensitivity defined by the ΔDphotB/DmaxA ratio (Table 1). This ratio reflects the photoinduced change in the absorbance at the absorption maximum of the merocyanine form normalized to the maximum absorption intensity of the starting spiro form, which absorbs the activating radiation. It follows from Table 1 that light sensitivity of hybrid compounds 2, 18–23 is lower than that of their precursors. This difference is quite significant for methanofullerene 22 and precursor 15. It is characterized by photoinduced spectral changes for spiropyran 15 and minor changes in the absorption spectra of hybrid 22 in the long-wavelength spectral region.
A considerable difference was found for the photodegradation of photochromic methanofullerenes and the starting spiro photochromes. Hybrid compounds are more stable against irreversible phototransformations than the starting spiropyrans (see ESI†).
Simultaneously, it was found that unlike the starting spiropyrans, methanofullerenes 7–10, 24 do not possess photochromism. This may be due not only to the nature of the substituent and its position in the aromatic ring, but also to the effect of the fullerene core, which destabilizes the merocyanine form and, as a consequence, leads to high thermal relaxation rate and formation of spirocyclic forms.
The results provide the conclusion that the light sensitivity, the rate constant of dark relaxation of the photoinduced merocyanine form, and stability to photodegradation of hybrid molecules are affected by both the nature of substituents in the indoline and chromene moieties and the proper fullerene core.
Recently, it was shown34 that spiropyran-containing pyrrolidinofullerenes undergo reversible isomerization not only on exposure to UV light, but also upon the acid–base switching. Therefore, we studied the acidochromic behavior of methanofullerenes 2, 7–10, 18–24 (Scheme 4).
The experiments showed that on treatment with equimolar amounts of trifluoroacetic acid (TFA), cycloadducts 2, 7–10, 18–24 are converted to protonated merocyanines MC-H-2, 7–10, 18–24 (Fig. 3 and ESI†), which are characterized by hyperchromic effect in the 365–445 nm range of absorption spectra. Treatment of these compounds in solutions with a base (Et3N) restores the initial hybrids 2, 7–10, 18–24 as a result of TFA neutralization. According to published data,35 the conversion of protonated merocyanine to spiropyran involves the formation of neutral merocyanine via deprotonation of phenolic hydroxyl. The intermediate formation of merocyanine upon amine treatment of protonated open form of spiropyran MC-H-18 is indicated, for example, by the absorption maximum at 603 nm in the absorption spectrum of this compound (Fig. 3, inset, curve 3).
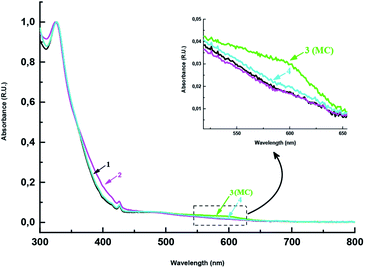 | ||
| Fig. 3 Absorption spectra of methanofullerene 18 in chloroform before (1) and after addition of TFA (2) and after the subsequent neutralization with triethylamine (3 and 4). | ||
The acid-induced opening of spiropyran addends in the synthesized hybrid molecules 2, 7–10, 18–24 was demonstrated not only by UV spectroscopy, but also using NMR spectroscopy. TFA-induced spiropyran ring opening in 18 was indicated by disappearance of both the singlets at 1.31 and 1.19 ppm (see ESI†), corresponding to two methyl protons of the indole moiety, and the multiplets for the two spacer methylene groups at 3.69, 4.51, 4.59 and 4.73 ppm from the 1H NMR spectra of the hybrid molecules. After TFA neutralization with an equimolar amount of Et3N, the initial form of the 1H NMR spectrum is restored (see ESI†). Similar results were obtained in our previous study for photochromic pyrrolidinofullerenes.34
The acid-induced opening of spiropyran addends in the synthesized hybrid molecules 2, 7–10, 18–24 was demonstrated not only by UV spectroscopy, but also using NMR spectroscopy. TFA-induced spiropyran ring opening in 18 was indicated by disappearance of both the singlets at 1.31 and 1.19 ppm (see ESI†), corresponding to two methyl protons of the indole moiety, and the multiplets for the two spacer methylene groups at 3.69, 4.51, 4.59 and 4.73 ppm from the 1H NMR spectra of the hybrid molecules. After TFA neutralization with an equimolar amount of Et3N, the initial form of the 1H NMR spectrum is restored (see ESI†). Similar results were obtained in our previous study for photochromic pyrrolidinofullerenes.34
Quite unexpected results were obtained for spiropyrans and their fullerene hybrids containing two NO2 groups. Indeed, the starting spiropyrans 15 and 17 and methanofullerenes 22 and 24 do not undergo acid-induced isomerization to the corresponding protonated merocyanines even in the presence of a large excess of the acid (see ESI†). Apparently, the presence of two electron-withdrawing groups in the spiropyran molecule makes oxygen at the spiro atom electron-deficient and thus not susceptible to protonation.34,35
Conclusions
Thus, we synthesized for the first time cyclopropane derivatives of C60 fullerene containing spiropyran addends and studied them for photo- and acidochromic behaviours. The effect of structural factors on the spectral and kinetic characteristics of the photochromic transformations of the synthesized fullerene hybrids was established. It was found that the photochromic and acidochromic behaviors of the resulting hybrid molecules are affected by the substituent nature and position in the chromene or indole aromatic rings and by the fullerene to spiropyran binding mode.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financial supported by the Russian Science Foundation (project no. 19-73-00082), RFBR (no. 18-33-20027) and Budget Theme (no. AAAA-A17-117012610065-2). The structural studies were performed using the equipment of Collective Usage Centre ‘Agidel’ (Institute of Petrochemistry and Catalysis, UFSC RAS).Notes and references
- H. Bouas-Laurent and H. Dürr, Pure Appl. Chem., 2001, 73, 639 CAS.
- E. Orgiu and P. Samori, Adv. Mater., 2014, 26, 1827 CrossRef CAS PubMed.
- V. A. Barachevsky, G. I. Dashkov and V. A. Tsehomsky, Photochromism and its application, ed. M. Himija, 1997, p. 280 Search PubMed.
- M. Tomasulo, I. Yildiz and F. M. Raymo, Inorg. Chim. Acta, 2007, 360, 938 CrossRef CAS.
- R. Ramos-Garcia, R. Delgado-Macuil, D. Iturbe-Castillo, E. G. de los Santos and F. S. Corral, Opt. Quantum Electron., 2003, 35, 641 CrossRef CAS.
- J. P. Phillips, A. Mueller and F. Przystal, J. Am. Chem. Soc., 1965, 87, 4020 CrossRef CAS.
- K. Kimura, Coord. Chem. Rev., 1996, 148, 41 CrossRef CAS.
- I. Willner, Acc. Chem. Res., 1997, 30, 347 CrossRef CAS.
- L. Evans, G. E. Collins, R. E. Shaffer, V. Mechelet and J. D. Winkler, Anal. Chem., 1999, 71, 5315 CrossRef PubMed.
- V. I. Minkin, Chem. Rev., 2004, 104, 2751 CrossRef CAS PubMed.
- M. M. Krayushkin, A. M. Bogacheva, K. S. Levchenko, O. I. Kobeleva, T. M. Valova, V. A. Barachevskii, J.-L. Pozzo, M. I. Struchkova, P. S. Shmelin, M. A. Kalik, T. K. Baryshnikova and V. N. Charushin, Mendeleev Commun., 2013, 23, 78 CrossRef CAS.
- R. Klajn, Chem. Soc. Rev., 2014, 43, 148 RSC.
- X. Zhang, L. Hou and P. Samori, Nat. Commun., 2016, 7, 11118 CrossRef CAS PubMed.
- A. R. Tuktarov, A. A. Khuzin and U. M. Dzhemilev, Russ. Chem. Rev., 2017, 86, 474 CrossRef CAS.
- D. I. Galimov, A. R. Tuktarov, D. S. Sabirov, A. A. Khuzin and U. M. Dzhemilev, J. Photochem. Photobiol., A, 2019, 375, 64 CrossRef CAS.
- A. R. Tuktarov, A. A. Khuzin, A. R. Tulyabaev, O. V. Venidictova, T. M. Valova, V. A. Barachevsky, L. M. Khalilov and U. M. Dzhemilev, RSC Adv., 2016, 6, 71151 RSC.
- V. A. Pomogaev, V. A. Barachevsky, A. R. Tuktarov, P. V. Avramov and V. Y. Artyukhov, J. Phys. Chem. A, 2018, 122, 505 CrossRef CAS PubMed.
- A. R. Tuktarov, R. B. Salikhov, A. A. Khuzin, N. R. Popod'ko, I. N. Safargalin, I. N. Mullagaliev and U. M. Dzhemilev, RSC Adv., 2019, 9, 7505 RSC.
- A. R. Tuktarov and U. M. Dzhemilev, Russ. Chem. Rev., 2010, 79, 585 CrossRef CAS.
- A. R. Tuktarov, A. A. Khuzin, V. V. Korolev and U. M. Dzhemilev, Russ. J. Org. Chem., 2012, 48, 99 CrossRef CAS.
- V. A. Barachevsky, Rev. J. Chem., 2017, 7, 334 CrossRef CAS.
- M. J. Feeney and S. W. Thomas, Macromolecules, 2018, 51, 8027 CrossRef CAS.
- J. L. Bahr, G. Kodis, L. de la Garza, S. Lin, A. L. Moore and T. A. Moore, et al., J. Am. Chem. Soc., 2001, 123, 7124 CrossRef CAS PubMed.
- X. Song, J. Zhou, Y. Li and Y. Tang, J. Photochem. Photobiol., A, 1995, 92, 99 CrossRef CAS.
- S. Yagi, S. Nakamura, D. Watanabe and H. Nakazumi, Dyes Pigm., 2009, 80, 98 CrossRef CAS.
- T. A. Darwish, R. A. Evans, M. James, N. Malic, G. Triani and T. L. Hanley, J. Am. Chem. Soc., 2010, 132, 10748 CrossRef CAS PubMed.
- T. A. Darwish, R. A. Evans, M. James and T. L. Hanley, Chem.–Eur. J., 2011, 17, 11399 CrossRef CAS PubMed.
- J. T. C. Wojtyk, A. Wasey, N.-N. Xiao, P. M. Kazmaier, S. Hoz and C. Yu, et al., J. Phys. Chem. A, 2007, 111, 2511 CrossRef CAS PubMed.
- D. A. Davis, A. Hamilton, J. Yang, L. D. Cremar, D. Van Gough, S. L. Potisek, M. T. Ong, P. V. Braun, T. J. Martínez, S. R. White, J. S. Moore and N. R. Sottos, Nature, 2009, 459, 68 CrossRef CAS PubMed.
- Y. Shiraishi, M. Itoh and T. Hirai, Phys. Chem. Chem. Phys., 2010, 12, 13737 RSC.
- A. R. Tuktarov, A. A. Khuzin, A. R. Akhmetov, V. A. Barachevsky, O. V. Venidiktova and U. M. Dzhemilev, Tetrahedron Lett., 2015, 56, 7154 CrossRef CAS.
- A. R. Tuktarov, A. A. Khuzin, A. R. Akhmetov, L. M. Khalilov, A. R. Tulyabaev, V. A. Barachevsky, O. V. Venediktova and U. M. Dzhemilev, Mendeleev Commun., 2016, 26, 143 CrossRef CAS.
- A. R. Tuktarov, A. R. Akhmetov, A. A. Khuzin, O. V. Venidiktova, V. A. Barachevsky and U. M. Dzhemilev, Russ. J. Org. Chem., 2017, 53, 891 CrossRef CAS.
- A. R. Tuktarov, A. A. Khuzin and U. M. Dzhemilev, Mendeleev Commun., 2019, 29, 229 CrossRef CAS.
- N. Darwish, A. C. Aragones, T. Darwish, S. Ciampi and I. Diez-Perez, Nano Lett., 2014, 14, 7064 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00217h |
| This journal is © The Royal Society of Chemistry 2020 |