Open Access Article
Open Access ArticleInvestigation on dispersion properties of CO2 and ester solvent mixtures using in situ FTIR spectroscopy†
Zihao Yang‡
*a,
Taiheng Yin‡ a,
Fengfan Zhanga,
Wei Wua,
Meiqin Lin
a,
Fengfan Zhanga,
Wei Wua,
Meiqin Lin *a,
Zhaoxia Dongab and
Juan Zhang
*a,
Zhaoxia Dongab and
Juan Zhang a
a
aUnconventional Petroleum Research Institute, China University of Petroleum (Beijing), Beijing, 102249, People's Republic of China. E-mail: zihaoyang@cup.edu.cn; linmq@cup.edu.cn
bChina University of Geosciences (Beijing), Beijing, 100083, People's Republic of China
First published on 13th May 2020
Abstract
To study the microscopic dispersion state of CO2 in different ester solvents, the solubility, volume expansion coefficients and in situ Fourier transform infrared (FTIR) spectra of the CO2–ester system were measured. The results show that the solubility and expansion coefficient of CO2 in ester solvents decreases as the hydrocarbon chain increases. As the pressure increases, the infrared absorption peaks of CO2 and the functional groups characteristic of ester molecules shift, indicating that CO2 molecules interact with ester molecules and that CO2 would destroy the interactions between the ester molecules. The hydrocarbon chain length of the ester molecules has a significant effect on the infrared absorption peak of the CO2–ester system. As the hydrocarbon chain length increases, the CO2 absorption peak shift and peak shift of the carbonyl groups in the ester gradually decrease.
1. Introduction
It is easy to prepare supercritical CO2. Supercritical CO2 is very dense, highly soluble, chemically inert, safe and nontoxic and can be separated at room temperature; thus, it has been widely used in supercritical fluid extraction.1–7 Meanwhile, CO2 has been used to improve oil recovery and has played an important role in petroleum production, especially in low permeability oilfields.8–11 The dissolution of CO2 in crude oil and the resulting volume expansion are considered to be the two major mechanisms responsible for improving oil recovery.12–16 It is well known that both supercritical fluid extraction and CO2 flooding are essentially equilibrium processes that occur between CO2 and organic liquid systems under high temperatures and high pressures. Therefore, many researchers have studied the CO2–organic liquid phase and accumulated various experimental data on the phases of CO2 and the organic liquid systems.17–24Our recent research findings have shown that, when CO2 molecules enter an organic liquid, instead of not dissolving in the organic liquid, the CO2 molecules are dispersed in the organic liquid in the form of molecular aggregates.25,26 CO2 molecules are dispersed in the organic liquid and have unique microscale morphologies. Thus, a molecular dynamics technique was used to study the radial distribution function of each molecule in mixed systems containing CO2 and an organic liquid, which included n-hexane, cyclohexane, toluene and ethanol, and simulations were performed for increasing pressures, revealing the microscopic morphology of dispersed CO2 in these four organic liquids. It is believed that, in organic liquids, the dispersion state of the CO2 molecules (including CO2 molecule aggregates and aggregates of CO2 and organic liquid molecules) directly affects the volume expansion of the CO2–organic liquid system. However, molecular dynamics can only simulate parameters such as the radial distribution function, the distortion distribution function and the interaction energy of the molecules in the system to infer the intermolecular interactions, and thus, direct experimental results cannot be obtained.
As the pressure increases in the simulation, the infrared absorption spectra of the functional groups and hydrocarbon chains of the organic molecules will change due to the influence of the CO2 molecules. Similarly, due to the effect of the organic liquid molecules, the infrared absorption spectra of CO2 will also change. Therefore, we can study the microscopic interactions of the molecules in the system with high-temperature and high-pressure in situ infrared technology. However, this technique has mainly been used to study the interactions between CO2 and polymer molecules and between CO2 and organic powders.27–29 The existing experimental method cannot meet the requirements for studying the interaction between CO2 and organic liquid molecules.27–29 Therefore, we have improved the in situ infrared device and previously used the improved device to measure the infrared absorption peaks of CO2 and 2-hexanone, hexanal, and 1-hexanol under high pressures.30
The carbon dioxide + ester systems at high pressure are important in the separation process in a wide variety of field such as food, pharmaceutical and related industries.31,32 Although the volumetric properties and vapor–liquid equilibrium data for carbon dioxide + ester systems at high pressure have been extensively investigated, its microscopic dispersion states are still mostly uncovered.33–37 To further study the CO2–organic liquid dispersion state, this study measured the solubility and volume expansion coefficient of CO2 dispersed in esters with different hydrocarbon chains lengths under different pressures at 308.15 K and examined the effect of the hydrocarbon chain length on the solubility and volume expansion coefficient of CO2. In addition, the in situ infrared absorption spectra of CO2–ester systems under different pressure conditions were obtained to study the effects of the hydrocarbon chain length on the infrared absorption spectra of the CO2–ester systems.
2. Experimental section
2.1. Materials
Carbon dioxide with a purity of 99.8% was purchased from Beijing Jinggao Gases Co., Ltd. Ethyl acetate (99.0%), propyl propionate (98.0%) and butyl butyrate (99.5%) were supplied by Shanghai Aladdin Bio-Chem Technology Co., Ltd.2.2. Experimental instruments and methods
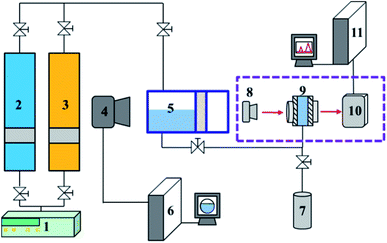
The visible pressure–volume–temperature (PVT) apparatus and in situ FTIR apparatus were used for the experiments. The experimental setup is shown in Fig. 1. The main body of the device included an autoclave, a supercharging system and a sampling system. The internal temperature and pressure of the autoclave were monitored by temperature sensors and pressure sensors with accuracies of ±0.1 °C and ±0.1 MPa, respectively. The volumetric accuracy of the autoclave was ±0.01 mL. The high-pressure in situ infrared liquid sample cell was heated by an internal thermocouple and controlled by an external controller. The maximum operating temperature was 200 °C, and the temperature accuracy was ±0.1 °C. The highest operational pressure was 10 MPa, and the pressure accuracy was 0.1 MPa. Infrared absorption data were collected from 900 cm−1 to 4000 cm−1. The detailed device information and operating procedures can be found in our previously published papers.18,30 Briefly, a known volume (V0 = 30 mL) of ester solvent was first injected into the vacuumed PVT cell, and CO2 was pressurized into the cell. After the system reached equilibrium, the liquid phase volume (V1) was calculated from the image captured by the camera. Then, the mixed liquid was injected slowly into the optical cell, and the infrared spectrum of the samples was obtained by using a Bruker VERTEX 80v infrared spectrometer. Finally, the mixed liquid was taken from the cell and charged into a small steel vessel that has already been vacuumed and weighed (M0), and the pressure of the cell is kept constant by moving the piston when discharging the organic solvent out of the cell. The mass of CO2 dissolved in the ester solvent was calculated using the weight difference method: the mass of the steel vessel containing organic solvents and CO2 (M1) minus the mass of the vessel after releasing CO2 to atmosphere (M2). The solubility (mole fraction) of CO2 was also calculated using these data. The volume expansion coefficient (N) of ester solvent was calculated by N = V1/V0. The measurements are repeated three times, and the results are presented as the average of the replicates.3. Results and discussion
3.1. The solubility and volume expansion coefficient of CO2 in the ester liquids
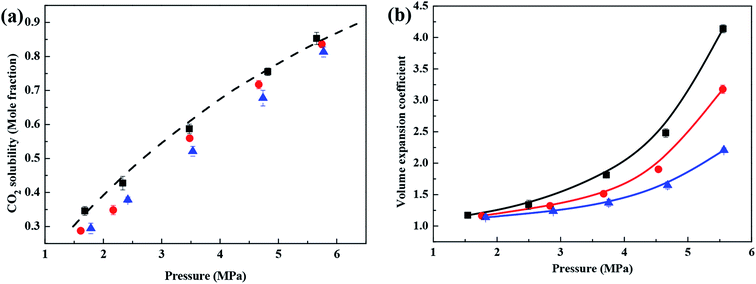
Fig. 2 shows the solubility and volume expansion coefficient of CO2 in ethyl acetate, propyl propionate and butyl butyrate under different pressure conditions at 308.15 K. The CO2 solubility and volume expansion coefficients of CO2 and ethyl acetate, propyl propionate, and butyl butyrate increase with increasing pressure. To our knowledge, literature vapor–liquid equilibrium data for the binary mixture of CO2 and ethyl acetate, propyl propionate, and butyl butyrate at 308.15 K are not available. The predictive Soave–Redlich–Kwong (PSRK) equation of state (EOS) was used to evaluate the vapor–liquid equilibrium behavior of CO2 and ethyl acetate mixtures at 308.15 K.38 There is a good agreement between the experimental data obtained here and the theoretical data obtained using the PRSK equation of state. Under the same experimental conditions, the solubility of CO2 and the volume expansion coefficient of the CO2–ester liquid system with different hydrocarbon chain lengths both decrease in the following order: ethyl acetate > propyl propionate > butyl butyrate. This result indicates that the hydrocarbon chain length has a relatively large impact on the solubility of CO2 in the ester liquids and the volume expansion coefficient of the CO2–ester liquid system.Previous studies have shown that the solubility of CO2 in ester liquids is closely related to the intermolecular forces between CO2 molecules, the intermolecular forces between CO2 and ester molecules and the intermolecular forces between ester molecules.17,18 Ester liquids with longer chain lengths have larger contact areas for intermolecular interactions and stronger intermolecular forces. Under the same pressure and temperature, these stronger intermolecular forces prevent CO2 from entering the ester liquid, leading to CO2 being less soluble in ester liquids with longer hydrocarbon chains. On the other hand, the evaporation enthalpy of a solvent can also indirectly reflect the stability of intermolecular interactions.30,39 The evaporation enthalpy of ethyl acetate, propyl propionate and butyl butyrate are 35.62, 42.14 and 51.12 kJ mol−1 respectively at 298 K.40–43 Under the same conditions, the evaporation enthalpy decreases in the order butyl butyrate > propyl propionate > ethyl acetate, indicating that the molecular interactions of butyl butyrate are the most stable, followed by propyl propionate and ethyl acetate. This result also shows that, under the same conditions, CO2 can more easily enter the liquid phase of ethyl acetate, thus making CO2 more soluble in ethyl acetate than in the other two systems.
Esters with different hydrocarbon chain lengths have different distances between the ester molecules. Under the combined action of intermolecular forces, this difference in distance also causes different microscopic dispersions of CO2 in the ester liquid, which will have a relatively large impact on the volume of the liquid system. Combined with the results of Fig. 2b, this shows that, when the temperature is constant, under the same pressure, the volume expansion is closely related to the magnitude of the intermolecular forces between the molecules of the organic liquid. The stronger the intermolecular force is, the lower the solubility of CO2 and the smaller the expansion coefficient.25,26 The abovementioned results indicate that the molecular structure, intermolecular forces and microscopic dispersion state of the organic liquids are the key factors affecting the solubility of CO2 and the corresponding volume expansion coefficient of the system.
3.2. In situ FTIR absorption spectra of the CO2–ester liquids systems
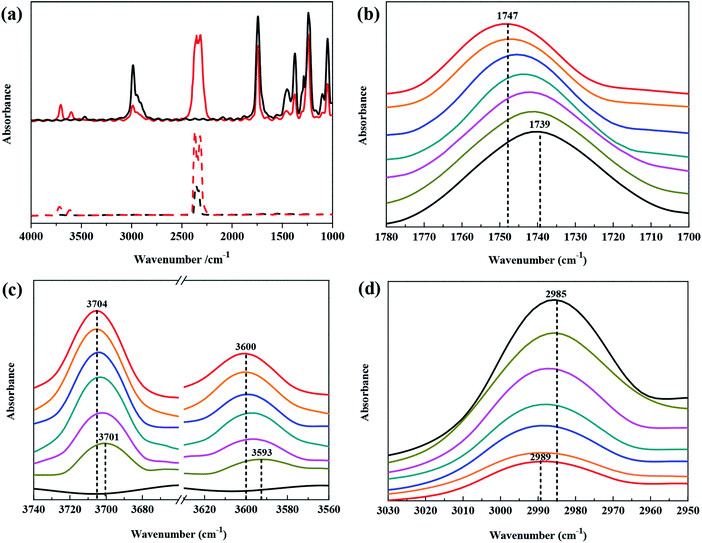
The in situ FTIR technique can be used to detect the sample changes as a function of the environment, time, temperature, and pressure. These influencing factors can cause certain regular patterns of the change in the intermolecular force and force constant of the molecules. These patterns can be reflected by in situ FTIR absorption peak shifts. When the intermolecular interaction is weakens, the force constant of the chemical bond will increase, and the corresponding absorption peak shifts to a higher wavenumber.44,45 In addition, when the concentration of the detected sample is relatively low, the intensity of the absorption peak is weak; when the concentration of the sample is relatively high, the intensity of the absorption peak is high.Fig. 3a shows the FTIR spectra of raw CO2 and the CO2–ethyl acetate system, which were obtained at 308.15 K and at atmospheric pressure and 7.39 MPa. Specifically, the absorption peak at 1745 cm−1 is characteristic of the ethyl acetate carbonyl (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups; the absorption peaks at 3704 cm−1, 3600 cm−1 and 2314 cm−1 are characteristic of CO2;30 the absorption peak at 2989 cm−1 corresponds to the stretching vibration of –CH3; the absorption peaks at 1490 cm−1 and 1350 cm−1 are attributed to the bending vibrations of –CH2 and –CH3. At 7.39 MPa, intense CO2 absorption peaks are observed at 3704 cm−1, 3600 cm−1 and 2314 cm−1 in the CO2–ethyl acetate system and no such peaks are detected at atmospheric pressure. This finding indicates that, when the pressure increases from atmospheric pressure to 7.39 MPa, the CO2 concentration in the liquid phase significantly increases. This also shows that more CO2 is dissolved in ethyl acetate at 7.39 MPa than at atmospheric pressure, which confirms that the solubility of CO2 in ethyl acetate increases with increasing pressure.
O) groups; the absorption peaks at 3704 cm−1, 3600 cm−1 and 2314 cm−1 are characteristic of CO2;30 the absorption peak at 2989 cm−1 corresponds to the stretching vibration of –CH3; the absorption peaks at 1490 cm−1 and 1350 cm−1 are attributed to the bending vibrations of –CH2 and –CH3. At 7.39 MPa, intense CO2 absorption peaks are observed at 3704 cm−1, 3600 cm−1 and 2314 cm−1 in the CO2–ethyl acetate system and no such peaks are detected at atmospheric pressure. This finding indicates that, when the pressure increases from atmospheric pressure to 7.39 MPa, the CO2 concentration in the liquid phase significantly increases. This also shows that more CO2 is dissolved in ethyl acetate at 7.39 MPa than at atmospheric pressure, which confirms that the solubility of CO2 in ethyl acetate increases with increasing pressure.
Fig. 3a also shows that, when the pressure increases from atmospheric pressure to 7.39 MPa, the intensity of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak at 1745 cm−1 and the intensity of the –CH– absorption peak at 2985 cm−1 are significantly weakened. This occurs because, when the pressure rises, more CO2 is dissolved in the ethyl acetate liquid, thus diluting the ethyl acetate liquid, causing the concentration of the ethyl acetate liquid to decrease and the intensity of the ethyl acetate absorption peak to decrease.
O absorption peak at 1745 cm−1 and the intensity of the –CH– absorption peak at 2985 cm−1 are significantly weakened. This occurs because, when the pressure rises, more CO2 is dissolved in the ethyl acetate liquid, thus diluting the ethyl acetate liquid, causing the concentration of the ethyl acetate liquid to decrease and the intensity of the ethyl acetate absorption peak to decrease.
Fig. 3b shows the FTIR spectra of the νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption peak of ethyl acetate in the CO2–ethyl acetate system from atmospheric pressure to 7.39 MPa at 308.15 K. When the liquid system is under atmospheric pressure, the position of the carbonyl absorption peak is 1739 cm−1; when the pressure rises to 7.39 MPa, the position of the carbonyl absorption peak gradually shifts to 1747 cm−1. As the pressure is increased, the position of the carbonyl absorption peak shifts to a high wavenumber by 8 cm−1. A decreased intermolecular force will cause an absorption peak to shift to a high wavenumber in the in situ FTIR spectrum.44–46 Therefore, the abovementioned results indicate that, in the process of injecting CO2 and as the pressure increases, more CO2 molecules enter the ethyl acetate liquid, and the intermolecular force between the ethyl acetate molecules decreases, resulting in an increase in the force constant of the carbonyl bond. As a result, the position of the carbonyl absorption peak shifts toward a high wavenumber.
O absorption peak of ethyl acetate in the CO2–ethyl acetate system from atmospheric pressure to 7.39 MPa at 308.15 K. When the liquid system is under atmospheric pressure, the position of the carbonyl absorption peak is 1739 cm−1; when the pressure rises to 7.39 MPa, the position of the carbonyl absorption peak gradually shifts to 1747 cm−1. As the pressure is increased, the position of the carbonyl absorption peak shifts to a high wavenumber by 8 cm−1. A decreased intermolecular force will cause an absorption peak to shift to a high wavenumber in the in situ FTIR spectrum.44–46 Therefore, the abovementioned results indicate that, in the process of injecting CO2 and as the pressure increases, more CO2 molecules enter the ethyl acetate liquid, and the intermolecular force between the ethyl acetate molecules decreases, resulting in an increase in the force constant of the carbonyl bond. As a result, the position of the carbonyl absorption peak shifts toward a high wavenumber.
Fig. 3c shows the FTIR spectra (the combination modes 2ν2 + ν3 and ν1 + ν3 stretching vibrations of the CO2 molecule) of the CO2–ethyl acetate system at 308.15 K when the pressure increased from atmospheric pressure to 7.39 MPa. Fig. 3c shows that, at 1.89 MPa, the positions of the 2ν2 + ν3 and ν1 + ν3 CO2 absorption peaks are 3701 cm−1 and 3593 cm−1, respectively. When the pressure is increases to 7.39 MPa, the positions of these two absorption peaks shift to 3704 cm−1 and 3600 cm−1. This result indicates that, when the pressure is increased from atmospheric pressure to 7.39 MPa, the absorption peaks of the CO2–ethyl acetate system at 3700 cm−1 and 3600 cm−1 shift to higher wavenumbers by 3 cm−1 and 7 cm−1, respectively. Due to an increase in the concentration, the FTIR absorption peak of pure CO2 will increase with an increase in the pressure, but the position of the peak will not change.47 When the pressure is 7.5 MPa, the absorption peak positions of pure CO2 at these two locations are 3718 cm−1 and 3614 cm−1.30 Therefore, in the CO2–ethyl acetate system, with an increase in the pressure, the interaction between the ethyl acetate molecules and CO2 molecules leads to an increase in the force constant of CO2, and the position of the FTIR CO2 absorption peak shifts to a higher wavenumber.
Fig. 3d shows the absorption peaks (at approximately 2900 cm−1) attributed to the –CH3 groups in ethyl acetate in the CO2–ethyl acetate system at 308.15 K when the pressure increases from atmospheric pressure to 7.39 MPa. These –CH3 peaks are located at 2985 cm−1 and 2989 cm−1 at atmospheric pressure and at 7.39 MPa, respectively. As the pressure increases, the –CH3 absorption peak shifts to a high wavenumber by 4 cm−1. This result indicates that, with increasing pressure, more CO2 enters the liquid molecules of ethyl acetate, which not only affects the interaction between the ethyl acetate molecules but also increases the force constant of the C–H bond in the –CH3 group. Therefore, the infrared absorption peak of –CH3 shifts toward a high wavenumber.
Fig. S1 and S2† show the FTIR absorption spectra of the CO2–propyl propionate system and CO2–butyl butyrate system under different pressure conditions, respectively. The infrared spectra analysis of the three systems under the same conditions shows that, for the same pressure variation range, the length of the hydrocarbon chains has a significant impact on the shift of the absorption peaks of various functional groups of the system, as shown in Table 1. Table 1 shows that, under the same pressure variation condition, as the ester hydrocarbon chain length increases, the shift of the CO2 absorption peak at 3600 cm−1 and the shift of the carbonyl absorption peak at 1700 cm−1 gradually decrease. The solubility and volume expansion analyses show that the ester chain length greatly affects the solubility of CO2 in the ester and the volume expansion coefficient of the CO2–ester system. The longer the chain length, the more difficult it is for CO2 to dissolve and the smaller the volume expansion coefficient. The in situ infrared spectra show that the CO2 absorption peak shift and carbonyl absorption peak shift are smaller in the esters with longer hydrocarbon chains, indicating that the longer the chain length, the more unfavorable it is for CO2 to disperse in the ester liquid and the more difficult it is for CO2 to enter the ester liquid to break the structure between the ester molecules. This also explains the lower solubility of CO2 in long-chain esters under the same conditions and the smaller volume expansion of the CO2-long-chain ester system.
| Ester type | CO2 (3700 cm−1) shift/cm−1 | CO2 (3600 cm−1) shift/cm−1 | –CH3 (2900 cm−1) shift/cm−1 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1700 cm−1) shift/cm−1 O (1700 cm−1) shift/cm−1 |
|---|---|---|---|---|
| Ethyl acetate | 3 | 7 | 4 | 8 |
| Propyl propionate | 3 | 5 | 2 | 5 |
| Butyl butyrate | 1 | 2 | — | 3 |
3.3. Microscopic dispersion state of CO2 in the ester liquids
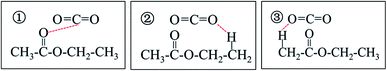
The results of this study show that, as the pressure increases, the infrared absorption peak of CO2 and the absorption peaks characteristic of the ester functional groups shift, which indicates that CO2 is likely to interact with ester molecules. In addition, the interactions between CO2 and ester molecules increase with the increasing of pressure. The CO2 and ester molecules can have many different interaction modes.48–50 The C atoms in the CO2 molecule and the O atom in the carbonyl group of the ester molecule interact via Lewis acid–Lewis base (LA–LB) modes. A proton on the α-C on the alcohol side of the ester molecule and an O atom in CO2 interact in the form of C–H⋯O. A proton on the α-C on the carbonyl side of the ester molecule and an O atom in CO2 interact in the form of C–H⋯O. Taking ethyl acetate as an example, a specific interaction between the CO2 and ethyl acetate molecules is shown in Fig. 4.Based on the in situ infrared data analysis of CO2 + ethyl acetate system under different pressures, the possible dispersion process of CO2 in ethyl acetate can be inferred as follows (Fig. 5). In the ethyl acetate liquid, the ethyl acetate molecules form a network structure with three low-stability interactions (Fig. 5a). As the pressure increases, the solubility of CO2 in the ethyl acetate liquid continuously increases, and the carbonyl absorption peak of the ethyl acetate molecules gradually shifts to a higher wavenumber, and the force constant of the carbonyl group gradually increases, indicating that the interactions between the ethyl acetate molecules gradually weaken. As increasing numbers of CO2 molecules enter into the ethyl acetate liquid, the distance between the ethyl acetate molecules becomes larger. In the microscopic state, the original network structure of the ethyl acetate molecules is destroyed, and some of the ethyl acetate molecules are present as single molecules in the ethyl acetate system (Fig. 5b). CO2 molecules form interactions with ethyl acetate through three non-covalent bonds, furthering disassembling the network structure of ethyl acetate. When supercritical conditions are reached, non-covalent bonds form between the CO2 and ethyl acetate molecules, resulting in a completely dissociated ethyl acetate network structure (Fig. 5c).
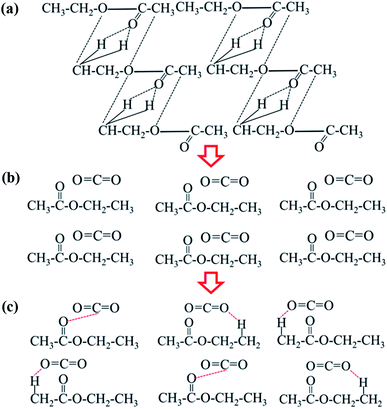 | ||
| Fig. 5 Schematic of intermolecular interaction of CO2 and ethyl acetate from ambient pressure to supercritical conditions: (a) ambient pressure; (b) 2.50 MPa; (c) the supercritical condition of CO2. | ||
4. Conclusions
In this work, the phase equilibrium data and FTIR spectra for the CO2 and ester solvents mixtures under different pressures were obtained with a static analytical method using the PVT instrument and improved in situ infrared spectrometer under different pressure. At the same temperature, the CO2 solubility and volume expansion coefficient of the CO2–ester liquid system increase with increasing pressure. Longer hydrocarbon chains are associated with greater intermolecular forces, which can decrease the solubility of CO2 in the ester liquid and decrease the volume expansion of the system. For the CO2–ester liquid system, as the pressure increases, the infrared absorption peaks of CO2 and the carbonyl group of the ester molecules gradually shift to higher wavenumbers, and the longer the hydrocarbon chain is, the smaller the shift. The microscopic dispersion state of CO2 in ester solvents was further described based on the experimental results from the perspective of intermolecular interactions of the CO2 molecules and ester molecules. Under supercritical CO2 conditions, CO2 molecules form interactions with ethyl acetate through three non-covalent bonds, resulting in the original network structure of the ethyl acetate molecules is destroyed. This study will enhance the understanding of the dispersion properties of CO2 and ester solvents mixtures and facilitate their applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51774302), National Key Technologies R&D Program of China (2017ZX05009-004) and National Natural Science Foundation of China (21503274).References
- H. Sovová, L. Opletal, M. Bártlová, M. Sajfrtová and M. Křenková, Supercritical fluid extraction of lignans and cinnamic acid from Schisandra chinensis, J. Supercrit. Fluids, 2007, 42, 88–95 CrossRef.
- S. Cavero, M. R. García-Risco, F. R. Marín, L. Jaime, S. Santoyo, F. J. Señoráns, G. Reglero and E. Ibañez, Supercritical fluid extraction of antioxidant compounds from oregano chemical and functional characterization via LC-MS and in vitro assays, J. Supercrit. Fluids, 2006, 38, 62–69 CrossRef CAS.
- E. Reverchon and I. De Marco, Supercritical fluid extraction and fractionation of natural matter, J. Supercrit. Fluids, 2006, 38, 146–166 CrossRef CAS.
- R. F. Rodrigues, A. K. Tashima, R. M. S. Pereira, R. S. Mohamed and F. A. Cabral, Coumarin solubility and extraction from emburana (Torresea cearensis) seeds with supercritical carbon dioxide, J. Supercrit. Fluids, 2008, 43, 375–382 CrossRef CAS.
- G. L. Filho, V. V. De Rosso, M. A. A. Meireles, P. T. V. Rosa, A. L. Oliveira, A. Z. Mercadante and F. A. Cabral, Supercritical CO2 extraction of carotenoids from pitanga fruits (Eugenia uniflora L.), J. Supercrit. Fluids, 2008, 46, 33–39 CrossRef CAS.
- C. R. Piantino, F. W. B. Aquino, L. A. Follegatti-Romero and F. A. Cabral, Supercritical CO2 extraction of phenolic compounds from Baccharis dracunculifolia, J. Supercrit. Fluids, 2008, 47, 209–214 CrossRef CAS.
- D. Cossuta, B. Simándi, E. Vági, J. Hohmann, A. Prechl, É. Lemberkovics, Á. Kéry and T. Keve, Supercritical fluid extraction of Vitex agnus castus fruit, J. Supercrit. Fluids, 2008, 47, 188–194 CrossRef CAS.
- P. M. Jarrell, C. E. Fox, M. H. Stein and S. L. Webb, Practical aspects of CO2 flooding, Monograph Series, Society of Petroleum Engineers, 2002 Search PubMed.
- M. Cao and Y. Gu, Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes, Fuel, 2013, 109, 157–166 CrossRef CAS.
- C. Or, K. Sasaki, Y. Sugai, M. Nakano and M. Imai, Swelling and viscosity reduction of heavy oil by CO2-gas foaming in immiscible condition, SPE Reservoir Eval. Eng., 2016, 19, 294–304 CrossRef CAS.
- S. M. Seyyedsar and M. Sohrabi, Visualization observation of formation of a new oil phase during immiscible dense CO2 injection in porous media, J. Mol. Liq., 2017, 241, 199–210 CrossRef CAS.
- M. Lashkarbolooki, M. Riazi and S. Ayatollahi, Experimental investigation of dynamic swelling and Bond number of crude oil during carbonated water flooding; effect of temperature and pressure, Fuel, 2018, 214, 135–143 CrossRef CAS.
- S. Le Van and B. H. Chon, Effects of salinity and slug size in miscible CO2 water-alternating-gas core flooding experiments, J. Ind. Eng. Chem., 2017, 52, 99–107 CrossRef CAS.
- T. Huang, X. Zhou, H. Yang, G. Liao and F. Zeng, CO2 flooding strategy to enhance heavy oil recovery, Petroleum, 2017, 3, 68–78 CrossRef.
- Y. Yang, X. Li, P. Guo, Y. Zhuo and Y. Sha, Improving oil recovery in the CO2 flooding process by utilizing nonpolar chemical modifiers, Chin. J. Chem. Eng., 2016, 24, 646–650 CrossRef CAS.
- A. Rostami, M. Arabloo, M. Lee and A. Bahadori, Applying SVM framework for modeling of CO2 solubility in oil during CO2 flooding, Fuel, 2018, 214, 73–87 CrossRef CAS.
- Z. Yang, W. Wu, Z. Dong, M. Lin, S. Zhang and J. Zhang, Reducing the minimum miscibility pressure of CO2 and crude oil using alcohols, Colloids Surf., A, 2019, 568, 105–112 CrossRef CAS.
- Z. Yang, M. Li, B. Peng, M. Lin and Z. Dong, Dispersion property of CO2 in oil. 2: volume expansion of CO2 + organic liquid at near-critical and supercritical conditions of CO2, J. Chem. Eng. Data, 2012, 57, 1305–1311 CrossRef CAS.
- S. N. Joung, C. W. Yoo, H. Y. Shin, S. Y. Kim, K. Yoo, C. S. Lee and W. S. Huh, Measurements and correlation of high-pressure VLE of binary CO2-alcohol systems (methanol, ethanol, 2-methoxyethanol and 2-ethoxyethanol), Fluid Phase Equilib., 2001, 185, 219–230 CrossRef CAS.
- C. J. Chang, K. Chiu and C. Day, A new apparatus for the determination of P-x-y diagrams and Henry's constants in high pressure alcohols with critical carbon dioxide, J. Supercrit. Fluids, 1998, 12, 223–237 CrossRef CAS.
- H. Lee, S. Yong Mun and H. Lee, High-pressure phase equilibria for the carbon dioxide-2-methyl-1-butanol, carbon dioxide–2-methyl-2-butanol, carbon dioxide–2-methyl-1-butanol–water, and carbon dioxide–2-methyl-2-butanol–water systems, Fluid Phase Equilib., 1999, 157, 81–91 CrossRef CAS.
- K. Gauter, R. A. Heidemann and C. J. Peters, Modeling of fluid multiphase equilibria in ternary systems of carbon dioxide as the near-critical solvent and two low-volatile solutes, Fluid Phase Equilib., 1999, 158–160, 133–141 CrossRef CAS.
- J. Yu, S. Wang and Y. Tian, Experimental determination and calculation of thermodynamic properties of CO2 + octane to high temperatures and high pressures, Fluid Phase Equilib., 2006, 246, 6–14 CrossRef CAS.
- E. N. Lay, V. Taghikhani and C. Ghotbi, Measurement and correlation of CO2 solubility in the systems of CO2 + toluene, CO2 + benzene, and CO2 + n-hexane at near-critical and supercritical conditions, J. Chem. Eng. Data, 2006, 51, 2197–2200 CrossRef CAS.
- Z. Yang, M. Li, B. Peng, M. Lin and Z. Dong, Aggregation of CO2 and organic liquid molecules at near critical and supercritical condition of CO2, J. Dispersion Sci. Technol., 2014, 35, 168–174 CrossRef CAS.
- Z. Yang, M. Li, B. Peng, M. Lin and Z. Dong, Dispersion property of CO2 in oil. Part 3: aggregation of CO2 molecule in organic liquid at near critical and supercritical condition of CO2, J. Dispersion Sci. Technol., 2014, 35, 143–149 CrossRef CAS.
- A. V. Ewing, A. A. Gabrienko, S. V. Semikolenov, K. A. Dubkov and S. G. Kazarian, How do intermolecular interactions affect swelling of polyketones with a differing number of carbonyl groups? An in situ ATR-FTIR spectroscopic study of CO2 sorption in polymers, J. Phys. Chem. C, 2015, 119, 431–440 CrossRef CAS.
- S. P. Nalawade, F. Picchioni, J. H. Marsman and L. P. B. M. Janssen, The FT-IR studies of the interactions of CO2 and polymers having different chain groups, J. Supercrit. Fluids, 2006, 36, 236–244 CrossRef CAS.
- A. Rajendran, B. Bonavoglia, N. Forrer, G. Storti, M. Mazzotti and M. Morbidelli, Simultaneous measurement of swelling and sorption in a supercritical CO2–poly(methyl methacrylate) system, Ind. Eng. Chem. Res., 2005, 44, 2549–2560 CrossRef CAS.
- L. Peng, Z. Yang, M. Li, M. Lin and Z. Dong, Dispersion properties of CO2 and polar organic solvents with the same alkyl chain length mixtures using in situ FTIR spectroscopy, J. Supercrit. Fluids, 2018, 135, 234–244 CrossRef CAS.
- H. S. Byun, M. Y. Choi and J. S. Lim, High-pressure phase behavior and modeling of binary mixtures for alkyl acetate in supercritical carbon dioxide, J. Supercrit. Fluids, 2006, 37, 323–332 CrossRef CAS.
- S. Sima, V. Feroiu and D. Geană, New high pressure vapor-liquid equilibrium data and density predictions for carbon dioxide + ethyl acetate system, Fluid Phase Equilib., 2012, 325, 45–52 CrossRef CAS.
- A. Chrisochoou, K. Schaber and U. Bolz, Phase equilibria for enzyme-catalyzed reactions in supercritical carbon dioxide, Fluid Phase Equilib., 1995, 108, 1–14 CrossRef CAS.
- Y. L. Tian, H. G. Zhu, Y. Xue, Z. H. Liu and L. Yin, Vapor-liquid equilibria of the carbon dioxide + ethyl propanoate and carbon dioxide + ethyl acetate systems at pressure from 2.96 MPa to 11.79 MPa and temperature from 313 K to 393 K, J. Chem. Eng. Data, 2004, 49, 1554–1559 CrossRef CAS.
- A. Kordikowski, A. P. Schenk, R. M. Van Nielen and C. J. Peters, Volume expansions and vapor-liquid equilibria of binary mixtures of a variety of polar solvents and certain near-critical solvents, J. Supercrit. Fluids, 1995, 8, 205–216 CrossRef CAS.
- H. Li, R. Zhu, W. Xu, H. Xu, Z. Dong and Y. Tian, Vapor-liquid equilibrium data of (carbon dioxide + methyl propionate) and (carbon dioxide + propyl propionate) at pressures from (1.00 to 12.00) MPa and temperatures from (313.0 to 373.0) K, J. Chem. Eng. Data, 2009, 54, 1510–1517 CrossRef CAS.
- M. Kato, D. Kodama, M. Sato and K. Sugiyama, Volumetric behavior and saturated pressure for carbon dioxide + ethyl acetate at a temperature of 313.15 K, J. Chem. Eng. Data, 2006, 51, 1031–1034 CrossRef CAS.
- Y. C. Tien, C. S. Su, L. H. Lien and Y. P. Chen, Recrystallization of erlotinib hydrochloride and fulvestrant using supercritical antisolvent process, J. Supercrit. Fluids, 2010, 55, 292–299 CrossRef CAS.
- A. K. Baev, Specific intermolecular interactions of organic compounds, Springer Science & Business Media, 2012 Search PubMed.
- I. A. Vasil'ev and V. M. Petrov, Thermodynamic properties of oxygen-containing organic compounds, Khimiya, Leningrad, 1984 Search PubMed.
- E. Mosconi, C. Quarti, T. Ivanovska, G. Ruani and F. De Angelis, Structural and electronic properties of organo-halide lead perovskites: a combined IR-spectroscopy and ab initio molecular dynamics investigation, Phys. Chem. Chem. Phys., 2014, 16, 16137–16144 RSC.
- B. E. Poling, J. M. Prausnitz and J. P. O'connell, The properties of gases and liquids, Mcgraw-hill, New York, 2001 Search PubMed.
- Y. Lebedev and E. A. Miroschnichenko, Thermochemistry of the vaporization of organic compounds. The vaporization and sublimation enthalpy and saturated pressure, Nauka, Moscow, 1981 Search PubMed.
- P. S. Makashir and E. M. Kurian, Spectroscopic and thermal studies on the decomposition of l,3,5-triamino-2,4,6-trinitrobenzene (TATB), J. Therm. Anal., 1996, 46, 225–236 CrossRef CAS.
- M. Pravica, B. Yulga, S. Tkachev and Z. Liu, High-pressure far- and mid-infrared study of 1,3,5-triamino-2,4,6-trinitrobenzene, J. Phys. Chem. A, 2009, 113, 9133–9137 CrossRef CAS PubMed.
- G. Herzberg, Molecular spectra and molecular structure II. Infrared and Raman spectra of polyatomic molecules, Van Nostrand, New York, 1945 Search PubMed.
- L. Peng, Dispersion property of CO2 in heavy crude oil and polar organic liquids, Doctoral thesis, China University of Petroleum (Beijing), China, 2017.
- Y. Yuan and A. S. Teja, Quantification of specific interactions between CO2 and the carbonyl group in polymers via ATR-FTIR measurements, J. Supercrit. Fluids, 2011, 56, 208–212 CrossRef CAS.
- Y. Akiyama, S. Fujita, H. Senboku, C. M. Rayner, S. A. Brough and M. Araia, An in situ high pressure FTIR study on molecular interactions of ketones, esters, and amides with dense phase carbon dioxide, J. Supercrit. Fluids, 2008, 46, 197–205 CrossRef CAS.
- C. Drohmann and E. J. Beckman, Phase behavior of polymers containing ether groups in carbon dioxide, J. Supercrit. Fluids, 2002, 22, 103–110 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra00326c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |