Open Access Article
Open Access ArticleRisk assessment of fluoride and arsenic in groundwater and a scenario analysis for reducing exposure in Inner Mongolia
Koyomi Nakazawa a,
Osamu Nagafuchi*a,
Uchralt Otedeb,
Ji-qun Chenc,
Koji Kanefujid and
Ken'ichi Shinozukaa
a,
Osamu Nagafuchi*a,
Uchralt Otedeb,
Ji-qun Chenc,
Koji Kanefujid and
Ken'ichi Shinozukaa
aEnvironmental Research Laboratory, Comprehensive Research Organizations of Fukuoka Institute of Technology, 3-30-1 Wajiro-higashi, Higashi-ku, Fukuoka 811-0295, Japan. E-mail: nagafuchi@bene.fit.ac.jp
bThe College of Asia and Pacific, Australian National University, Canberra, ACT 0200, Australia
cNGO Echoing Steppe, China
dThe Institute of Statistical Mathematics, 10-3 Midori-cho, Tachikawa, Tokyo 190-8562, Japan
First published on 13th May 2020
Abstract
In contrast to Mongolia, family-owned land in Inner Mongolia is separated by fences, preventing the free movement of nomads and leading people to rely heavily on the same source of groundwater for their domestic water needs. Therefore, it is important to clarify groundwater quality and understand the associated human health concerns. To evaluate the risks of drinking groundwater to human health in Inner Mongolia, we examined groundwater quality by field surveys, a human health risk analysis, and a scenario analysis. During the summer of 2015 in Inner Mongolia, we measured the concentrations of major ions, metals, metalloids, and rare earth metals in groundwater samples (n = 32) and river water samples (n = 10), for which there were no known anthropogenic contamination sources. In addition, as part of a scenario analysis, samples of tap water (n = 1), snowmelt (n = 1), and bottled water (n = 1) were also evaluated. We used our analytical results to calculate hazard quotient (HQ) ratios by means of a probabilistic risk assessment method. The results indicated that residents who drank groundwater every day might have risk concerns for F− (mean ± standard deviation, 2.51 ± 1.80 mg L−1; range, 0.07–7.70 mg L−1) and As (6.49 ± 9.64 μg L−1; 0.31–47.0 μg L−1). We observed no relationships between well depth or any geophysical variation and groundwater quality. On the basis of the scenario analysis results, we concluded that using snow as a source of drinking water in winter could reduce health risks associated with using groundwater for this population in Inner Mongolia.
Introduction
In 2015, 71% of the global population (5.2 billion people) had access to a safe drinking water facility, that is located nearby, available when needed, and free from contamination.1 Groundwater, rainwater, and river water are commonly used as sources of drinking water. Securing water of good quality for people who live in remote areas is very important for protecting their health, and many studies have been conducted to characterize groundwater quality worldwide.2–6 In East Asia, the main natural and anthropogenic sources of water pollution are arsenic (As), fluoride (F−), nitrate (NO3−), iron (Fe), and chloride (Cl−). Because about one-third of drinking water is supplied from groundwater,7 it is essential to secure groundwater of sufficiently high quality for consumption.Residents of the Mongolian plateau rely on groundwater for their livelihoods. In Mongolia (Outer Mongolia), nomads move over the land with their livestock, utilizing groundwater, river water, and snow as sources of drinking water. The authors have conducted surveys of groundwater quality on the Mongolian Plateau (Outer Mongolia, South Gobi region) continuously during summer since 2012.8 We have reported groundwater samples that exceed the World Health Organization's (WHO) drinking water quality guidelines for F− (1.5 mg L−1) and NO3− (50 mg L−1).9 In broader groundwater quality surveys outside of the South Gobi, we revealed that the non-carcinogenic human health risks of F− and As from drinking water are a concern.10 In addition, although NO3− was not categorized at the level of being a human health risk concern, some groundwater samples showed high levels of NO3− resulting from contamination by livestock waste. If more nomads and their livestock visit drinking-water wells, NO3− contamination may become a health risk. In general, our previous research in South Gobi,8,10,11 has revealed that some groundwater quality parameters may be high enough to pose a risk to human health, and this risk is likely to be widespread over the entire Mongolian plateau.
Conditions for groundwater usage in the Inner Mongolia Autonomous Region (Inner Mongolia) are different from those of the South Gobi area. In Outer Mongolia, nomads usually move freely, but people living in Inner Mongolia have been settled following establishment of the government's land use system in the 1960s. At that time, land was fenced off, and the residents began to rely heavily and exclusively on groundwater for their daily water needs. In addition, as economic development has progressed, people have begun digging multiple wells on their property, and groundwater contamination has become a serious problem in some areas. Prior to this settlement, local residents usually relied on snow as a source of drinking water in winter. Presently, people usually use groundwater from deep wells year round because the groundwater never freezes.
Preliminary interviews with nomads in the region indicated that the local government has conducted water quality assessments in some areas in Inner Mongolia, but the results of these surveys have never been shared with the nomads. Physical events, such as children's teeth turning black and an increasing number of people with skeletal deformities, are making some residents in Inner Mongolia feel vaguely uneasy about their health.
Many studies have been conducted on groundwater in Inner Mongolia,3,4,12–15 but only a few have included metals and metalloids in their characterization of human health risk.16 Clarifying human health risks is important for not only residents but also decision makers. In addition, industrial companies have begun to operate in Inner Mongolia. It is important to clarify the baseline levels of potentially harmful substances in drinking water to establish the human health risk of drinking water prior to any industrial development projected to occur in the near future.
Our aim in this study was to quantify water quality in Inner Mongolia and to characterize the non-carcinogenic human health risk concerns of F−, NO3−, Hg, As, Al, V, Mn, Co, Ni, Cu, Zn, Se, Mo, Cd, Sb, and Pb in groundwater based on a probabilistic risk assessment method. We also examined the relationship between the concentration of identified potentially harmful substances and geophysical factors (e.g., well depth). In addition, we conducted a scenario analysis to identify strategies for reducing non-carcinogenic human health risk concerns for drinking water. Because Inner Mongolia is vast in area (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 183
183![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2), we focused on a remote area where people live a typical nomadic lifestyle although land are fenced off and no industrial development activity has occurred.
000 km2), we focused on a remote area where people live a typical nomadic lifestyle although land are fenced off and no industrial development activity has occurred.
Materials and methods
Study area
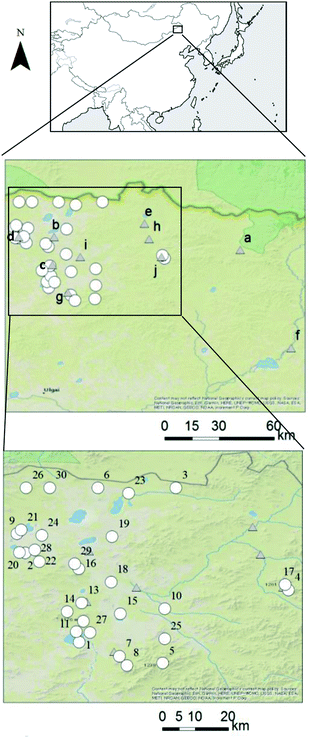
To conduct the risk analysis, we collected and analyzed 32 groundwater samples from wells in Mandahbulag Township, which is located in the northern area of Inner Mongolia (Fig. 1). There are no anthropogenic sources of contamination in this area, such as underground mineral development (mining) projects or industrial plants, so we were able to observe chemical concentrations and evaluate human health risks under a natural (baseline) condition. We informally interviewed area residents to gain background on the area. According to the residents, there are more than 120 wells in the area, and the wells are about 30 minutes (by car) apart from each other. Because of the number of wells and the distance between wells, we could not sample groundwater from all wells. In addition, Mandahbulag Township is almost 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km2 in size, so we discussed suitable wells to sample with the nomads of the township to gain a representative coverage of the wells in the township.
000 km2 in size, so we discussed suitable wells to sample with the nomads of the township to gain a representative coverage of the wells in the township.
To conduct the scenario analysis, river water, tap water, and snow samples were also collected. Ten river water samples were collected around Mandahbulag Township (Fig. 1). One tap water sample was collected in Wuliyasitai Township, located 120 km southwest of Mandahbulag Township. This area was selected because some of the residents temporarily move to Wuliyasitai Township during summer. Also, bottled water which is provided by the hotel in Wuliyasitai Township were collected. In addition, nomads from the area reported that people in Mandahbulag Township also use melted snow as a source of drinking water in winter. Therefore, we collected one snow sample in Mandahbulag Township in December 2015 (aroud St.18). Adding snow as a potential source of drinking water in the scenario analysis could provide additional information about reducing human health risks related to drinking water.
Water sampling and analysis
The sampling was conducted in August 2015, near the end of the growing season. Each property was fenced off where the well was located. We travelled by car (about 2–30 min between the wells by car) to sample groundwater from each well and were able to visit four to five houses each day. Groundwater samples were collected by bucket and stored in a 60 mL polyethylene bottle. Location information of each well was determined by GPS (Garmin Ltd, Montana 650), and the depth of each well was obtained from the owner of well. During the sampling period, the livestock rearing season was almost finished, and we were therefore able to meet with some of the nomads at their houses and informally interview them, often while drinking a traditional tea. Because of their lifestyle, they worry about the environment, and we interviewed them to assess their perceptions of risk parameters.River water samples were collected by bucket or by the placing a 60 mL polyethylene bottle directly into the river from a bridge or the river bank. Sampling locations on several rivers and at several points on one river had been identified by residents as locations where they had collected water in the past. The snow sample was scooped by hand and directly placed into the sample bottle; the collector wore polypropylene gloves to prevent contamination from handling the snow. The tap water sample was collected directly into the sampling bottle from the tap in Wuliyastai Township. Also, bottled water which is provided by the hotel. All water samples were transported to the laboratory in 60 mL polyethylene bottles without filtration.
Degradation of groundwater quality has been a concern on the Mongolian plateau because of increased mining and industrial development. For that reason, we were mainly concerned with substances that might be relevant to those operations (e.g., major ions, metals, and metalloids). The water samples were filtered (Dismic CS-25, Advantec, Tokyo) and then anions (F−, Cl−, SO42−, and NO3−) and cations (NH4+, Na+, K+, Mg2+ and Ca2+) are analyzed with Metrohm 761 Compact IC chromatogram system (Herisau, Switzerland) with Metrosep A Supp 4–250/4.0 and YK-421, respectively. For anion analysis, an isocratic gradient method (1.8 mM Na2CO3 and 1.7 mM NaHCO3) was used with flow rates of 1–1.5 mL min−1. For the cation analysis 4 mM of phosphoric acid was employed as eluent.
The Hg concentration was measured by an RA-3320FG+ mercury analyser (CVAFS, Nippon Instruments Co. Ltd., Osaka, Japan). The samples were prepared by adding a small quantity of BrCl (0.5% of sample volume) to the sample. Then 1.1 mL of 5 N NaOH and 1000 ppm Cu2+ (CuSO4·5H2O) reagent was added per 5 mL sample, followed by 0.3 mL of 10% SnCl2. The detection limit for mercury was 0.15 ng L−1. The quality assurance and quality control (QA/QC) were validated by analysing the standard reference material (NIST SRM 1641e, mercury in water). The recovered value for the target element were 104% (n = 3), indicating the accuracy of the method.
The concentrations of the other trace elements were determined by inductively coupled plasma mass spectrometry (ICP-MS; Elan DRC II, PerkinElmer, Inc. USA). Nitric acid (TAMAPURE-AA10) was added to the water sample so as to obtain 0.4 N nitric acid solution, and heated on a hot plate at 190 °C for 6 hours to extract metal elements. The sample solution was finally made 0.4 N nitric acid concentration.
All containers used in the study were acid cleaned prior to use. Praseodymium was added to the digests as an internal standard (20 ng L−1). The detection limit for the 10 elements ranged from 0.01 ng mL−1 to 0.8 ng mL−1. The detection limit for arsenic was 0.04 ng mL−1. Calibration was achieved using a multi-element Calibration standard 2, 3, 4 and 5 (PerkinElmer Inc. USA) prepared from stock standard made up in a 2% HNO3 solution. The samples were first acidified with 0.4 N HNO3 and then assayed in triplicate. The QA/QC were validated by analysing the standard reference material (NIST SRM 1648, urban particulate matter from National Institute of Standards and Technology, USA). The recovered value for the As were 103% (n = 3), indicating the accuracy of the method. We only present results for elements that are assigned RfD (ingestion reference dose) values by the US EPA.17
Risk assessment
There are three exposure pathways: dermal, oral, and inhalation. In this paper, we focus on human health risk for nomads via chronic metal exposure through drinking water. Because of the implementation of the settlement policy in Inner Mongolia and the ensuing economic growth in the 1990s during which people dug several wells on each property, we first assumed that residents used only groundwater as drinking water. Non-carcinogenic risk was assessed by the US EPA method.18,19 The exposure dose through ingestion of water was calculated according to US EPA:18,20
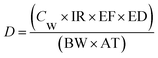 | (1) |
In the scenario analysis, for people who drink from several water sources, the average daily exposure dose (Di) was estimated by using the following formula:
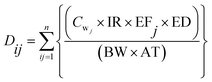 | (2) |
For the scenario analysis (described later), the assumption was made that several water resources were used; Dij was estimated for each concentration i and exposure frequency j and then summed of all exposure source.
The hazard quotient (HQ) was calculated as HQ = D/RfD, where D is the exposure dose through ingestion of water as defined in eqn (1) or (2), and RfD is the value of the ingestion reference dose obtained from the US EPA.17
Families in this region are multi-generational; in many cases, households comprise three generations. To simplify our study, we did not evaluate human health risk for the different generations. Because we were unable to measure most of the items in eqn (1), we used the default values provided by the US EPA:23 IR = 2 L per day, EF = 350 days per year, ED = the 90th-percentile value (i.e., 30 years), BW = 70 kg, and AT = 30 years × 365 days (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 days).
950 days).
We used the RfD value of the total concentration of each chemical constituent (in all oxidation states) for the risk assessment.
Probabilistic risk assessment
To perform the probabilistic risk assessment, we assigned a characteristic distribution (log-normal or normal, depending on fit) to each dataset (F−, NO3−, Al, V, Mn, Co, Ni, Zn, As, Se, Mo, Cd, and Sb in groundwater (Cw in eqn (1))). Using the average and SD values of each dataset, we conducted random Monte Carlo simulations. Simulations were performed with Crystal Ball software (Oracle Co., KKE Inc., Japan), and the Latin hypercube sampling method was used with 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterations to define the probabilistic distributions.21 The US EPA23 recommends that the HQ of individual chemical species should be <1. In our probabilistic risk analysis, we required when the 95th-percentile value of HQ distribution as being the cut-off value for human health risk concerns in drinking water, become HQ of ≤1 to be considered safe.
000 iterations to define the probabilistic distributions.21 The US EPA23 recommends that the HQ of individual chemical species should be <1. In our probabilistic risk analysis, we required when the 95th-percentile value of HQ distribution as being the cut-off value for human health risk concerns in drinking water, become HQ of ≤1 to be considered safe.
Scenario analysis
In addition to the human health risk analysis, we conducted a scenario analysis to see what steps could be taken to reduce risks originating from drinking groundwater. We set the base case as a nomad using groundwater for all water intake, as discussed previously. Using the results of our risk analysis (discussed later), in the baseline case, a F− of 50% and an As of 70% were considered to qualify as a risk concern (i.e., a HQ ratio ≥1 and a cut-off value greater than at the 95th-percentile value of the HQ). Reducing these percentages will reduce the risk level. We therefore set four scenarios in addition to the baseline.Scenarios were set based on water resources available to residents and living patterns indicated by the informal discussions with residents and observed during our fieldwork. Some people temporarily move to Wuliyasitai Township in summer following the very busy livestock breeding season, and some residents stay in the city for summer vacation (another nomad is typically hired to care for the livestock). We set this lifestyle as scenario 1. In this case, it was assumed that the people use groundwater from a well in Mandahbulag Township for 305 days and tap water from Wuliyasitai Township for the remaining 60 days. In scenario 2, the residents use river water for the entire year, but this scenario is admittedly not realistic because the nomads cannot move about freely because of the fences. Therefore, in scenario 3, we assumed that nomads use groundwater for half of the year and river water for the other half of year. In scenario 4, we assumed that melted snow is used for drinking water in winter. For the purposes of this scenario, we set snow for use as drinking water in winter to 90 days and groundwater use for the other 275 days. All other parameters are given in the results.
Results and discussion
Physicochemical characteristics of groundwater and river water samples
The analytical results for groundwater, river water, snowmelt, tap water, and bottled water are summarized in Table 1. The coefficient of variance (CV) of the chemical constituents in groundwater and river water varied from 19% to 140% and 41% to 222%, respectively. The average values for river water tended to be lower than those for groundwater. The lanthanide concentration in particular was high in groundwater, but quite low or not detected in the other samples. The origin of the tap water sample (n = 1) in Wuliyasitai Township is groundwater and most substances were detected. Although bottled water is provided by some hotels in this city, most residents use tap water in their daily life. The snow sample had low or not detectable concentrations of all items. All concentrations in the bottled water and snow samples were below the WHO guideline values.| Parameter | Unit | Groundwater (n = 32) | River water (n = 10) | Snow (n = 1) | Tap water (n = 1) | Bottled water (n = 1) | WHO guideline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Max | CV (%) | Mean | SD | Min | Max | CV (%) | ||||||
| F− | mg L−1 | 2.51 | 1.80 | 0.07 | 7.70 | 140 | 2.79 | 4.68 | 0.11 | 15.7 | 60 | 0.14 | 4.33 | 0.08 | 1.5 |
| Cl− | mg L−1 | 331 | 551 | 8.10 | 2250 | 60 | 51.8 | 59.3 | 2.60 | 167 | 87 | 0.93 | 246 | 7.18 | — |
| NO3− | mg L−1 | 28.9 | 38.6 | 1.06 | 179 | 75 | 0.50 | 0.40 | 0.19 | 1.40 | 124 | 2.05 | 11.5 | 3.42 | 50 |
| SO42− | mg L−1 | 277 | 405 | 7.12 | 1400 | 69 | 81.5 | 84.7 | 4.85 | 273 | 96 | 2.51 | 76.0 | 2.11 | — |
| Na+ | mg L−1 | 354 | 407 | 7.35 | 1630 | 87 | 53.2 | 44.1 | 18.8 | 155 | 121 | — | 67.2 | 11 | 50 |
| NH4+ | mg L−1 | 29.3 | 150 | 0.06 | 851 | 20 | 68.4 | 76.2 | 0.02 | 210 | 90 | — | 146 | 0.2 | — |
| K+ | mg L−1 | 4.98 | 2.82 | 0.14 | 11.5 | 177 | 4.71 | 3.07 | 1.70 | 11.4 | 153 | — | 1.98 | 0.17 | — |
| Mg2+ | mg L−1 | 96.6 | 95.2 | 3.42 | 365 | 102 | 40.4 | 22.6 | 5.72 | 64.8 | 179 | — | 62.8 | 0.8 | — |
| Ca2+ | mg L−1 | 94.7 | 62.1 | 6.71 | 288 | 152 | 51.2 | 23.1 | 18.9 | 87.3 | 222 | — | 36.5 | 0.8 | — |
| Hg | ng L−1 | 6.73 | 32.7 | 0.26 | 186 | 21 | 1.56 | 0.82 | 0.90 | 3.47 | 192 | 0 | 0.75 | 0.44 | 6000 |
| Li | μg L−1 | 104 | 98.0 | 24.1 | 486 | 106 | 45.2 | 26.9 | 5.66 | 93.7 | 168 | — | 50.5 | 1.93 | — |
| Al | μg L−1 | 41.8 | 55.8 | 4.82 | 226 | 75 | 92.1 | 167 | 4.58 | 559 | 55 | 0.01 | 6.31 | 5.31 | — |
| V | μg L−1 | 8.76 | 8.83 | 0.40 | 36.2 | 99 | 2.84 | 3.52 | 0.41 | 11.1 | 81 | — | 6.84 | 0.12 | — |
| Cr | μg L−1 | 1.60 | 4.14 | 0.18 | 23.6 | 39 | 0.36 | 0.32 | 0.08 | 0.97 | 111 | — | 0.24 | 0.13 | — |
| Mn | μg L−1 | 168 | 448 | 0.23 | 2280 | 38 | 56.5 | 136 | 0.33 | 442 | 41 | N.D. | 29.6 | N.D. | — |
| Co | μg L−1 | 0.38 | 0.38 | 0.04 | 1.73 | 100 | 0.25 | 0.14 | 0.06 | 0.59 | 175 | — | 0.67 | 0.01 | — |
| Ni | μg L−1 | 2.20 | 1.80 | 0.33 | 9.93 | 122 | 1.32 | 0.64 | 0.22 | 2.26 | 206 | — | 2.55 | 0.12 | 70 |
| Cu | μg L−1 | 16.9 | 43.2 | 0.89 | 241 | 39 | 1.50 | 1.24 | 0.40 | 4.46 | 120 | — | 1.98 | 1.10 | 2000 |
| Zn | μg L−1 | 13.1 | 27.0 | 0.00 | 134 | 49 | 1.83 | 1.40 | 0.08 | 5.09 | 131 | 0.01 | 178.4 | 1.45 | — |
| As | μg L−1 | 6.49 | 9.64 | 0.31 | 47.0 | 67 | 4.83 | 5.94 | 0.33 | 20.2 | 81 | N.D. | 2.16 | 0.05 | 10 |
| Se | μg L−1 | 2.60 | 2.71 | 0.16 | 10.8 | 96 | 0.23 | 0.12 | 0.08 | 0.44 | 198 | N.D. | 11.02 | 0.02 | 40 |
| Sr | μg L−1 | 2520 | 1960 | 291 | 7740 | 129 | 497 | 398 | 132 | 1380 | 125 | — | 982 | 11.6 | — |
| Mo | μg L−1 | 13.1 | 17.0 | 0.50 | 72.5 | 77 | 2.40 | 5.30 | 0.27 | 17.4 | 45 | — | 18.9 | 0.30 | — |
| Cd | μg L−1 | 0.58 | 0.81 | 0.01 | 3.49 | 72 | 0.14 | 0.28 | 0.03 | 0.92 | 51 | N.D. | 0.95 | 0.02 | 3 |
| In | μg L−1 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | — | N.D. | N.D. | — |
| Sb | μg L−1 | 0.23 | 0.20 | 0.01 | 0.87 | 114 | 0.16 | 0.11 | 0.06 | 0.44 | 143 | — | 0.50 | 0.02 | 20 |
| Te | μg L−1 | 0.05 | 0.06 | 0.00 | 0.27 | 75 | 0.01 | 0.01 | N.D. | 0.02 | 96 | — | N.D. | N.D. | — |
| Ba | μg L−1 | 89.3 | 89.3 | 4.49 | 312 | 100 | 46.9 | 36.1 | 13.8 | 140 | 130 | — | 60.0 | 19.0 | — |
| Pb | μg L−1 | 0.22 | 0.20 | 0.03 | 0.88 | 108 | 0.41 | 0.24 | 0.17 | 0.98 | 173 | N.D. | 0.18 | 0.40 | 10 |
| Fe | μg L−1 | 285 | 1280 | 2.25 | 7140 | 22 | 91.2 | 105 | 6.42 | 374 | 87 | 0.01 | 1.50 | 0.64 | — |
| Bi | μg L−1 | 0.00 | 0.00 | N.D. | 0.02 | 21 | 0.00 | 0.00 | N.D. | 0.01 | 109 | — | 0.01 | N.D. | — |
| Sc | μg L−1 | 2.26 | 4.99 | N.D. | 29.0 | 45 | 1.37 | 0.92 | 0.07 | 2.76 | 149 | — | 1.84 | 0.06 | — |
| Y | μg L−1 | 0.62 | 3.20 | N.D. | 17.9 | 19 | 0.09 | 0.06 | 0.03 | 0.22 | 141 | — | N.D. | N.D. | — |
| La | μg L−1 | 1.55 | 8.47 | N.D. | 47.2 | 18 | 0.09 | 0.11 | N.D. | 0.34 | 86 | — | N.D. | N.D. | — |
| Ce | μg L−1 | 3.74 | 20.5 | N.D. | 114 | 18 | 0.20 | 0.22 | 0.01 | 0.72 | 89 | — | N.D. | N.D. | — |
| Nd | μg L−1 | 0.70 | 3.73 | N.D. | 20.8 | 19 | 0.09 | 0.11 | N.D. | 0.34 | 87 | — | N.D. | N.D. | — |
| Sm | μg L−1 | 0.18 | 0.89 | N.D. | 4.96 | 20 | 0.03 | 0.02 | 0.01 | 0.08 | 154 | — | 0.02 | N.D. | — |
| Eu | μg L−1 | 0.05 | 0.20 | N.D. | 1.12 | 24 | 0.01 | 0.01 | N.D. | 0.02 | 206 | — | 0.02 | N.D. | — |
| Gd | μg L−1 | 0.18 | 0.96 | N.D. | 5.38 | 19 | 0.02 | 0.02 | N.D. | 0.07 | 104 | — | N.D. | N.D. | — |
| Tb | μg L−1 | 0.02 | 0.12 | N.D. | 0.70 | 19 | 0.00 | 0.00 | N.D. | 0.01 | 105 | — | N.D. | N.D. | — |
| Dy | μg L−1 | 0.12 | 0.63 | N.D. | 3.53 | 19 | 0.02 | 0.01 | N.D. | 0.05 | 107 | — | N.D. | N.D. | — |
| Ho | μg L−1 | 0.02 | 0.12 | N.D. | 0.65 | 20 | 0.00 | 0.00 | N.D. | 0.01 | 128 | — | N.D. | N.D. | — |
| Er | μg L−1 | 0.06 | 0.33 | N.D. | 1.83 | 19 | 0.01 | 0.01 | N.D. | 0.03 | 122 | — | N.D. | N.D. | — |
| Tm | μg L−1 | 0.01 | 0.04 | N.D. | 0.24 | 20 | 0.00 | 0.00 | N.D. | 0.00 | 109 | — | N.D. | N.D. | — |
| Yb | μg L−1 | 0.06 | 0.29 | N.D. | 1.63 | 19 | 0.01 | 0.01 | N.D. | 0.02 | 131 | — | N.D. | N.D. | — |
| Lu | μg L−1 | 0.01 | 0.04 | N.D. | 0.22 | 20 | N.D. | N.D. | N.D. | N.D. | 97 | — | N.D. | N.D. | — |
Estimation of risk to human health of drinking water
We first checked the datasets to determine whether the concentrations had log-normal distributions. The frequency of occurrence of each chemical constituent (ions and heavy metals) in groundwater and river water is shown in Fig. 2. Here, each dataset was first log-transformed, and then the Shapiro–Wilk test was applied to confirm that the concentrations of F−, NO3−, Al, V, Mn, Co, Ni, Zn, As, Se, Mo, Cd, and Sb were normally distributed (i.e., the original datasets showed log-normal distributions). After performing the log-transformation of groundwater data and applying the Shapiro–Wilk test to the river water dataset, F−, NO3−, Al, V, Mn, Co, Ni, Zn, As, Se, Mo, Cd, and Sb ere assumed to have a log-normal distribution.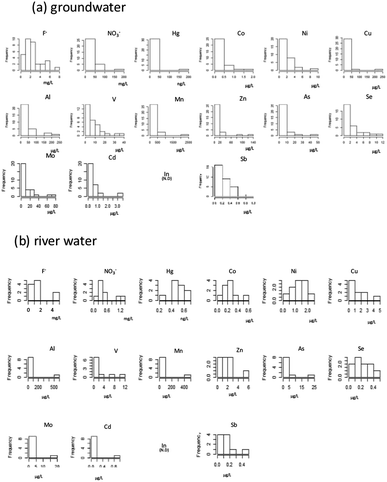 | ||
| Fig. 2 Concentration histograms of each chemical constituent in groundwater ((a), n = 32) and river water ((b), n = 10). Histograms are shown for items in the risk analysis. | ||
To perform the probabilistic analyses, we assumed that the distribution of each chemical constituent in groundwater was log-normal, even if the Shapiro–Wilk test showed it to not have a log-normal distribution. The log-normal distribution is empirically useful because it cannot take negative values just as environmental values cannot have negative values, and this distribution may fit the data well.22 The risk analysis showed that F− and As are considered to be human health risk concerns, as determined by the HQ of ≤1 (Fig. 3).
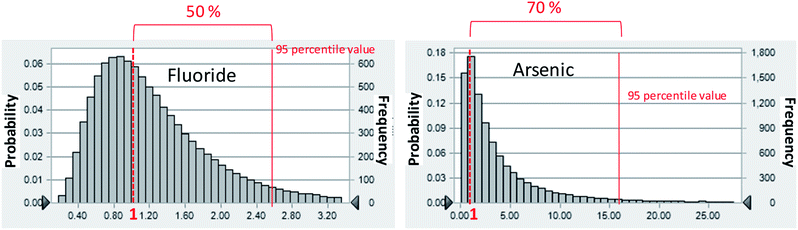 | ||
| Fig. 3 Probabilistic distribution of HQ for F− and As in Mandahbulag vilage. The red line and red dashed lines indicate HQ = 1 and 95 percentile value, respectively. | ||
Geophysical characteristics of F− and As in well water
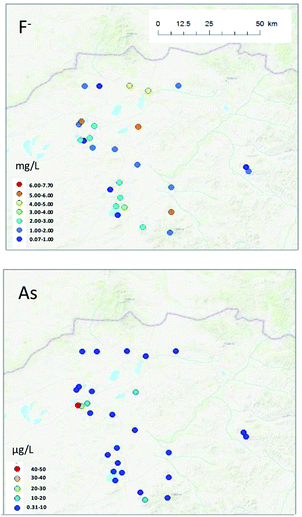
Because of their potential importance to human health, we compared the F− and As concentrations obtained in our study with those from other areas. We also analysed the geophysical characteristics of F− and As in well water in our study as a step toward understanding how to reduce risks from drinking groundwater.The concentration of F− in groundwater in this study varied from 0.07 to 7.70 mg L−1 (mean, 2.51 mg L−1; Table 1). In general, the F− concentration tends to be high in groundwater in the Mongolian Plateau, and fluorosis is endemic in northern China.25 In the middle Loess plateau, the F− concentration varies from 0.20 to 2.70 mg L−1 (mean, 0.84 mg L−1), and in the Hetao Basin of Inner Mongolia, it varies from 0.30 to 2.57 mg L−1 (median, 1.02 mg L−1).24 In Outer Mongolia, the concentration of F− in groundwater in the northern area ranges from 0.37 to 0.90 mg L−1 (mean, 0.62 mg L−1), whereas in South Gobi, which is located 80 km north of the China-Outer Mongolia border, it ranges from ND to 5.46 mg L−1 (mean, 1.54 mg L−1) in Oyu Tolgoi and from 0.38 to 3.78 mg L−1 (mean, 1.56 mg L−1) in Tavan Tolgoi.
To clarify the similarity of F− concentration in groundwater, we conducted a Distance Index (DI)26,27 analysis for anions (F−, Cl−, NO3−, SO42−) and cations (Ca2+, Mg2+, K+, NH4+, Na+).
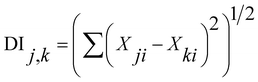 | (3) |
The DI value shows the degree of similarity between two samples. If the composition among the anions (F−, Cl−, NO3−, SO42−) or cations (Ca2+, Mg2+, K+, NH4+, Na+) is the same between wells, then DI is 0. Therefore, a small DI value indicates similarity between the items. After obtaining the DI results, we performed a cluster analysis, but no apparent relationship between the sites was observed in anion and cations (Fig. 4). In addition, there was no apparent relationship between well depth and F− (Fig. 5).
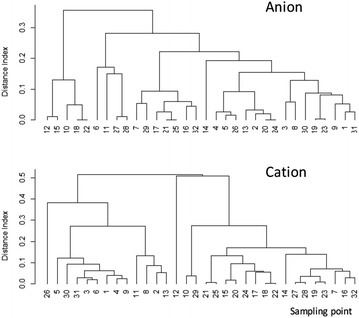 | ||
| Fig. 4 Cluster analysis results of major anion and cation concentrations in groundwater samples. See Fig. 1 for locations of sampling points. Sampling points 31 and 32 do not appear in Fig. 1 due to lack of GPS information. | ||
High As concentrations in groundwater have been reported throughout the world.28–30 In Inner Mongolia, the Tumet Plain (including Huhhot Basin, BaMen, and Bayinao Hexi) have reported groundwater As values ranging from <1 to 2400 μg L−1.28 In Hohhot Basin, the range for shallow groundwater is <1 to 1480 μg L−1 (mean, 2.9 μg L−1) and that for the deep groundwater is <1 to 308 μg L−1 (mean, 128 μg L−1).28 The As concentrations in groundwater in Mandahbulag Township ranged from 0.31 to 47.0 μg L−1 (mean, 6.49 μg L−1), which is lower than many of the other reported values. However, in the northern area of Outer Mongolia, the concentrations range from 0.05 to 1.95 μg L−1 (mean, 0.75 μg L−1), and in Oyu Tolgoi and Tavan Tolgoi (located near Outer Mongolia and China, but in Inner Mongolia), As ranges from 0.19 to 25.8 μg L−1 (mean, 6.63 μg L−1), and 0.23 to 12.2 μg L−1 (mean, 2.57 μg L−1), respectively.11 Overall, it appears that the As concentration range is wider in Inner Mongolia than it is in Outer Mongolia.
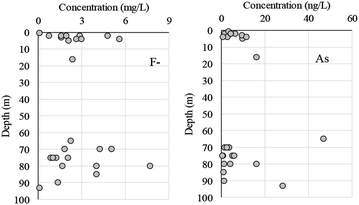
Guo et al.24 reported As concentrations of 0.96 to 720 μg L−1 in groundwater from Hetao Basin, but the concentration was <50 μg L−1 in samples from depths <10 m, which coincides with an interval dominated by yellow-brown clay/silty clay layers. The As concentrations in our research area were all <50 μg L−1. However, As concentrations were relatively lower in shallow (<10 m) wells and higher in deeper wells (Fig. 5), indicating that we should monitor As concentration in deep wells.
No spatial tendency was observed in F− or As concentrations in groundwater in this study (Fig. 6). However, some groundwater samples contained high F− or As concentrations, and a more precise risk analysis is warranted in these areas.
Scenario analysis for reducing risk concern
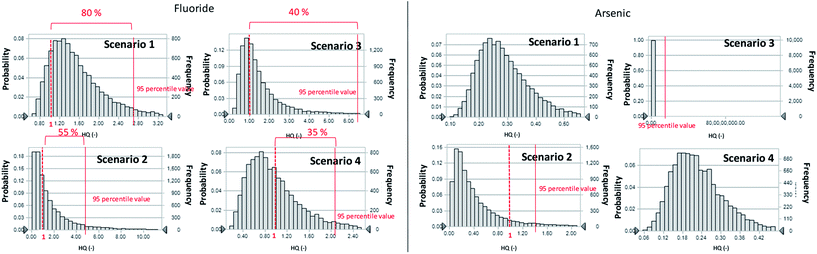
The parameter values used in the scenario analysis are shown in Table 2. The F− and As concentrations in groundwater, river water, tap water, and snow were obtained from the sampling results of this study (Table 1). The frequencies of each HQ ratio obtained from the scenario analysis are presented in Fig. 7. For As, the 95th-percentile value of the HQ ratio did not exceed 1, except for scenario 2 and 3. Therefore, scenarios 1 or 4 are preferred to avoid the human health risk concerns. However, for F−, the 95th-percentile values of the HQ ratio exceeded 1 in all scenarios. The cut offs of HQ ratio exceeded 1 in scenarios 1 to 4 were 80%, 55%, 40%, and 35%, respectively. Therefore, HQ ≥ 1 and the cut off at the 95th-percentile value (40% and 35%) was smaller than the baseline case (45%) in scenario 3 and 4, respectively. It was therefore possible to reduce the level of risk caused by drinking groundwater contaminated with F− in scenario 3 or 4.| F− and As concentration in each water item (Cw) | |||||
|---|---|---|---|---|---|
| Item | Scenario | Concentration (SD) (mg L−1) | Distribution | Remarks | |
| F− | As | ||||
| Ground water | 1, 3, 4 | 1.79 (1.03) | 3.22 (1.18) | Log-normal | Ground water obtained from 32 wells in the study area |
| River water | 2, 3 | 2.79 (4.68) | 4.83 (5.95) | Log-normal | River water obtained from 10 river sampling sites in the study area |
| Snow water | 4 | 0.14 | 0.0008 | Deterministic | Snow was collected from Mandahbulg Township, in December 2015 |
| Tap water in the city | 1 | 4.33 | 2.16 | Deterministic | City tap water collected on a city 120 km from the study area. According to resident, the source of the tap water is groundwater. Sample collected in 2015 |
| Exposure Frequency (EF) | |||
|---|---|---|---|
| Scenario | Days | Distribution | Remarks |
| 1 | Groundwater: 305, tap water in the city: 60 | Deterministic | Some residents stay in the city for summer vacation |
| 2 | River water: 365 | Deterministic | River water is assumed to be a good alternative to groundwater |
| 3 | Groundwater: 182, river water: 183 | Deterministic | Groundwater and river water are assumed to each be used for half of the year |
| 4 | Ground water: 275, snowmelt in winter: 90 | Deterministic | Snowmelt was used historically. Assumed that groundwater is used the rest of the year |
From our research results, we were unable to draw any conclusions about seasonal variations. However, using snow as a source of drinking water during the winter season would most likely reduce the overall consumption of F− and As and thereby lower the associated health risk.
Conclusion
A groundwater risk screening assessment was conducted in a limited number of samples of water from Inner Mongolia. We found that F− and As in groundwater (i.e., drinking water) may pose a risk for human health. No consistent geophysical characteristics were observed to be related to F− or As concentration in groundwater. The scenario analysis revealed that using snowmelt during winter as a source of drinking water could reduce consumption of that F− and As and thereby reduce the associated human health risks.The local government has been digging wells and providing water purification systems for some low-income families in the study area. However, these families generally do not use the water purification systems because they do not have sufficient capacity to provide enough water for daily living. However, if a filter system did work well enough, that would be another way to reduce human health risk concerns from drinking groundwater. In addition, the use of rainwater and bone char filtration are also possible options to reduce risk.31 Residents of Inner Mongolia often eat mutton, and sheep bones could be used as part of a filtration system to reduce the F− concentration in groundwater.31
Local residents said there were more than 120 wells in our study area. Because of the size of the area and the number of wells, we were unable to survey all of the wells in this study. A more complete field survey of groundwater sources is needed to clarify the level of human health risk. Additional epidemiological study is also needed to lower the human health risks associated with drinking groundwater in this area.
Conflicts of interest
The authors declare no conflicts of interest in this research.Acknowledgements
This study was supported in part by the Japan Society for the Promotion of Science KAKENHI Grant No. 26257301 (Grant-in-Aid for Research (A)), the Joint Research Program of Arid Land Research Center Tottori University (29C2010, 31B2001), the Sasagawa Scientific Research Grant from the Japan Science Society (Grant No. 27-607), and the ISM Cooperative Research Program (2016 ISM/CRP-4308, 2046 and 2017 ISM/CRP-4110, 2049). The authors would also like to thank PerkinElmer Japan Co., Ltd., for support with chemical analyses. We also thank local nomads and Yi Jin for assistance with our research.References
- UNICEF WHO 2017, Progress on drinking water, sanitation and hygieneJoint Monitoring Programme 2017 update and SDG baselines, https://www.who.int/water_sanitation_health/publications/jmp-2017/en/, accessed 30 March 2020 Search PubMed.
- D. Raj and E. Shaji, Geosci. Front., 2017, 8, 117–124 CrossRef CAS.
- S. D. Dhiman and K. K. Ashok, Hydrol. Sci. J., 2006, 51(6), 1149–1162 CrossRef CAS.
- H. Guo, Y. Jia, W. B. Richard, J. Yuxiao, W. Zhao, W. Xiu, J. Shen, Y. Li, Y. Cao, W. Yang, D. Zhang, C. Wei, Y. Zhang and W. Cao, Sci. Total Environ., 2016, 541, 1172–1190, DOI:10.1016/j.scitotenv.2015.10.018.
- J. Liu, Z. Chen, L. Wang, Y. Zhang, Z. Li, J. Xu and Y. Peng, Environ. Sci. Pollut. Res., 2016, 23, 15003–15014, DOI:10.1007/s11356-016-6617-1.
- S. Giri and A. K. Singh, Environ. Monit. Assess., 2015, 187, 63, DOI:10.1007/s10661-015-4265-4.
- Groundwater flow-resources and circulation in Monsoon Asia, ed. M. Taniguchi, Kyoritsu Shuppan, Tokyo, 2011 Search PubMed.
- O. Nagafuchi, K. Nakazawa, K. Okano, K. Osaka, Y. Nishida and N. Hishida, Inn. Asia, 2014, 16, 429–443 Search PubMed.
- WHO, Guidelines for Drinking-water Quality, World Health Organization, 4th edn, 2006, http://www.who.int/water_sanitation_health/publications/2011/dwq_guidelines/en/, accessed 10 March 2018 Search PubMed.
- K. Nakazawa, O. Nagafuchi and K. Okano, A case study on Oyu Tolgoi and Tavan Tolgoi in South Gobi, Steppe and Mine: Natural resources development and environmental problems in Mongolia and Tibet, ed. J. Tanase and I. Shimamura, Akashi syoten, Tokyo, 2015, pp. 133–146 Search PubMed.
- K. Nakazawa, O. Nagafuchi, K. Okano, K. Osaka, E. Hamabata, J. Tsogtbaatar and J. Choijil, J. Water Health, 2016, 14(6), 1009–1018 CrossRef PubMed.
- S. Hossain, T. Hosono, H. Yang and J. Shimada, Water Air Soil Pollut., 2016, 227, 385, DOI:10.1007/s11270-016-3089-3.
- H. Guo, S. Yang, X. Tang, Y. Li and Z. Shen, Sci. Total Environ., 2008, 393, 131–144, DOI:10.1016/j.scitotenv.2007.12.025.
- L. Xiao, Z. Jin and F. Zhang, J. Geochem. Explor., 2015, 159, 252–261, DOI:10.1016/j.gexplo.2015.09.018.
- B. Xu, Y. Zhang and G. Wang, Hum. Ecol. Risk Assess., 2018, 1–20 Search PubMed.
- B. Jianmin, W. Yu and Z. Juan, Nat. Hazards, 2015, 77, 1903–1914, DOI:10.1007/s11069-015-1682-1.
- USEPA The screening level (SL) tables, United States Environmental Protection Agency, 2017, http://www.epa.gov/reg3hwmd/risk/human/rb-concentration_table/Generic_Tables/index.htm, accessed 10 March 2018 Search PubMed.
- USEPA, US EPA Risk Assessment Guidance for Superfund (RAGS). Volume I: Human Health Evaluation Manual (Part F, Supplemental Guidance for Inhalation Risk Assessment), United States Environmental Protection Agency, 2010, https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-f, accessed 10 March 2018 Search PubMed.
- US EPA, Exposure factors handbook 2011 edition Search PubMed.
- R. Kolluru, S. Bartell, R. Pitblando and S. Striclff, Risk assessment and management handbook for environmental, health and safety professionals, McGraw-Hill Inc, New York, 1996 Search PubMed.
- US EPA, Risk Assessment Guidance for Superfund: Volume III – Part A Process for Conducting Probabilistic Risk Assessment, 2001, https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-volume-iii-part, accessed 10 March 2018 Search PubMed.
- D. M. Kammen and D. M. Hassenzahl, Should We Risk It?, Princeton University Press, Princeton, New Jersey, 1989 Search PubMed.
- USEPA 1989 Risk Assessment Guidance for Superfund Volume 1 Human Health Evaluation Manual (Part A), United States Environmental Protection Agency, EPA/540/1-89/002, http://www.epa.gov/oswer/riskassessment/ragsa/pdf/preface.pdf, accessed 10 March 2018 Search PubMed.
- H. Guo, Y. Zhang, L. Xing and Y. Jia, Appl. Geochem., 2012, 27, 2187–2196, DOI:10.1016/j.apgeochem.2012.01.016.
- K. Luo and F. Feng, Toxicol. Environ. Chem., 2009, 90, 237–246, DOI:10.1080/02772240701456091.
- R. R. Socal, Syst. Zool., 1961, 10, 71–79 Search PubMed.
- R. P. McIntosh, An index diversity and the relation of certain concepts to diversity, Ecology, 1967, 48, 392–404 CrossRef.
- P. L. Smedley, M. Zhang, G. Zhang and Z. Luo, Appl. Geochem., 2003, 18, 1453–1477, DOI:10.1016/S0883-2927(03)00062-3.
- K. Ohno, T. Yanase, Y. Matsuo, T. Kimura, M. H. Rahman, Y. Magara and Y. Matsui, Sci. Total Environ., 2007, 381, 68–76, DOI:10.1623/hysj.51.6.1149.
- Y. Zhou, Y. Zeng, J. Zhou, H. Guo, Q. Li, R. Jia, Y. Chen and J. Zhao, Appl. Geochem., 2017, 77, 116–125, DOI:10.1016/j.apgeochem.2016.09.005.
- J. Fawell, K. Bailey, J. Chilton, E. Dahi, L. Fewtrel, and Y. Magara, World Health Organization titles with IWA Publishing, Fluoride in drinking-water, 2006, http://www.who.int/water_sanitation_health/publications/fluoride_drinking_water_full.pdf, accessed 10 March 2018 Search PubMed.
| This journal is © The Royal Society of Chemistry 2020 |